1. Introduction
Chiral semiconductor nanostructures and nanoparticles have attracted tremendous interest due to a variety of intriguing properties [
1,
2,
3]. Due to the chiral shape of the nanostructures and/or the chirality of the molecular orbitals forming excited states in semiconductor nanostructures, excitons acquire mirror asymmetry, which can be observed using circular dichroism (CD, the difference in absorbance between left- and right-handed circularly polarized light) and circularly polarized luminescence (CPL, emitting left- and right-handed circularly polarized light). Such nanostructures with chiral excitation are promising candidates for biosensing, stereoselective reactions, and enantioselective separation [
4,
5,
6]. Moreover, a manifestation of chirality in solid state is the chirality-induced spin selectivity effect [
7], which leads to the spin polarization of electrons scattered on a dissymmetric center, that opens up new prospects for creating spintronics devices [
8,
9].
One of the most common approaches to the synthesis of chiral semiconductor nanostructures and nanoparticles is the attachment of enantiomeric ligands to the semiconductor core of the nanoparticle [
10,
11,
12,
13,
14,
15], which results in the appearance of circular dichroism. Among other nanoparticles, a new class of colloidal semiconductor nanoparticles are cadmium chalcogenide nanoplatelets (NPLs) with 2D electronic structure. [
16]. Due to quantum confinement in one dimension and atomically precise thickness, these NPLs demonstrate pronounced and record narrow excitonic bands among other nanoparticles, which promises applications in light-emitting devices [
17,
18], lasers [
19,
20], and white-light generation [
21,
22]. Pronounced circular dichroism of excitonic bands was shown for colloidal CdSe NPLs capped with enantiomeric ligands [
23,
24]. For atomically thin CdSe NPLs with only 2 monolayer (ML) thickness, record values of circular dichroism have been demonstrated, exceeding those for other types of semiconductor nanoparticles, which is due to the maximum interaction of excitons in the 2D core of the NPL and chiral ligands [
25].
To induce chiral excitons, coordination of ligands on the nanoparticle surface plays an important role. [
26]. It was shown that L/D cysteine and N-acetylcysteine ligands induce different signs of circular dichroism at the same absolute configuration for both 0D CdSe quantum dots and 2D CdSe nanoplatelets [
27,
28]. The change in the sign of the CD is due to a change in ligand coordination when replacing cysteine with acetylcysteine, leading to a change in the orientation of the molecular dipole and the exciton dipole in the core. Also, different signs of circular dichroism were shown for L/D of cysteine on CdSe nanoplatelets with different crystal structures of wurtzite and zinc blende due to differences in ligand coordination [
23]. However, almost all studies on the induction of chirality in nanoparticles concern thiol-containing enantiomeric ligands, usually thiolated amino acids (cysteine and its derivatives), which is not always the best ligand for practical applications, since the thiolate group quenches luminescence. Research on thiol-free ligands is quite limited; there is data on the use of chiral carboxylic acids coordinated by the carboxyl group on the surface of CdSe quantum dots, which enhances circular dichroism [
29].
Here, we report a study of the induction of chirality using thiol-free ligands on the surface of atomically thin CdSe nanoplatelets. We chose the model thiol-free amino acids L-alanine and L-phenylalanine as thiol-free ligands. We analyzed ultimately thin 2 ML thick (thicknesses of 0.6 nm) nanoplatelets grown by the colloidal method to maximize the influence of the ligand on induced chirality and achieve strong circular dichroism. We have developed a novel two-step approach to completely exchange the native oleic acid ligand for chiral amino acids at the basal planes of NPLs. We analyzed the composition and coordination of ligands in detail by Fourier-transform infrared spectroscopy (FTIR). The optical and chiroptical properties of chiral atomically thin CdSe NPLs have been studied by absorption, luminescence, luminescence excitation, and circular dichroism (CD) spectroscopy.
2. Materials and Methods
Cadmium acetate dihydrate (Cd(CH3COO)2∙2H2O, ≥98%), selenium powder (Se, 99.99%), trioctylphosphine (TOP, 90%), oleic acid (OA, 90%), 1-octadecene (ODE, 90%), L-alanine (Ala, ≥98%), L-phenylalanine (Phe, ≥98%), acetic acid (AcA, ≥99.7%) and solvents were purchased from Sigma-Aldrich. The cadmium acetate dihydrate used was previously recrystallized to avoid hydrolysis.
The growth of the initial two-dimensional 2.5 ML thick CdSe nanoparticles coated with oleic acid (CdSe394OA) was carried out by the colloidal method in the octadecene - cadmium acetate - oleic acid system according to the method adapted from [
25]. Shortly, 160 μl of oleic acid, 0.26 g of freshly recrystallized cadmium acetate dihydrate were added to 20 ml of octadecene and the resulting mixture was degassed under argon flow for 45 minutes at a temperature of 160-170 °C. After that, the mixture was cooled to the injection temperature (120 °C) and under vigorous stirring 0.2 ml of 1M Se solution in TOP diluted to 0.5 ml by ODE was injected rapidly into the reaction mixture initiating nanosheet nucleation. The growth of nanoparticles was carried out for 5 hours, gradually increasing the temperature to 140 °C. To complete the synthesis, 2 ml of oleic acid was injected and the resulting solution was cooled to room temperature. The final nanoparticles were precipitated by centrifugation with acetone as a precipitator at 5000 rpm for 10 min. Finally, the sample was dissolved in 2 ml of hexane.
Ligand exchange with L-alanine (Ala) and L-phenylalanine (Phe) ligands the exchange was carried out according to the newly introduced method with an intermediate substitution for acetic acid. Briefly, 500 μl of acetic acid was added to a solution of 2 ml of the original CdSe394OA nanoparticles coated with oleic acid in 6 ml of hexane, after which the mixture was intensively mixed and left at room temperature for 24 hours. The resulting precipitate (CdSe394AcA) was centrifuged, after which it was washed with pure hexane. Centrifugation was repeated several times to wash off the remnants of oleic acid. The resulting nanoparticles were redispersed in 2 ml of hexane. Further exchange for L-alanine and L-phenylalanine was carried out in dioxane. For this purpose, the solvent was previously degassed. To 10 ml of dioxane in the flask, 2 ml of a solution of nanoparticles in hexane and 50 mg of acid were added. The exchange, in which the distillation of acetic acid took place, was carried out for 40 minutes at 80 degrees. The resulting nanoparticles were precipitated by centrifugation with acetone as a precipitator. The final samples named CdSe394Ala and CdSe394Phe were dispersed in 1 ml of toluene.
Transmission electron microscopy (TEM) images were obtained on the LEO19 AB OMEGA microscope operated at 100 kV. Fourier-transform infrared spectroscopy (FTIR) spectra were registered on a Perkin-Elmer Frontier FTIR spectrometer in the 400-4000 cm-1 wavenumber range at room temperature. Samples for analysis were prepared by mixing a drop of NPLs solution or reference Ala or Phe solution in toluene with KBr powder followed by pressing into tablets after solvent evaporation. Absorption spectroscopy was carried out in the 200-800 nm wavelength range with scanning speed 100 nm/min on the Carry50 (Varian) spectrophotometer. Photoluminescence spectra were collected with a LS 55 (Perkin-Elmer) fluorescence spectrometer. Circular dichroism (CD) spectra were recorded on spectropolarimeter Сhirascan (Applied Photophysics) in 300-500 nm wavelength range with scanning speed 10 nm/min and 1 nm step (integration time 3s). Optical measurements were carried out at room temperature from colloidal solutions of NPLs in toluene or methanol diluted to an optical density <1. Quartz cuvettes (Hellma Analytics) with 0.2 cm optical path length were used. Dissymmetry g-factor was calculated as g = ΔA/A = (AL − AR)/A, where AL and AR are the absorbances of circularly polarized left-handed and right-handed light, respectively, and A is the absorbance of unpolarized light.
3. Results
3.1. Atomically thin nanoplatelet growth and ligand exchange
Atomically thin CdSe 2.5 ML NPLs were synthesized by the colloidal growth method. To obtain oleic acid (OA) covered CdSe nanoparticles with zinc blende crystal structure and ultimately thin thickness, a modification of the standard synthesis protocol [
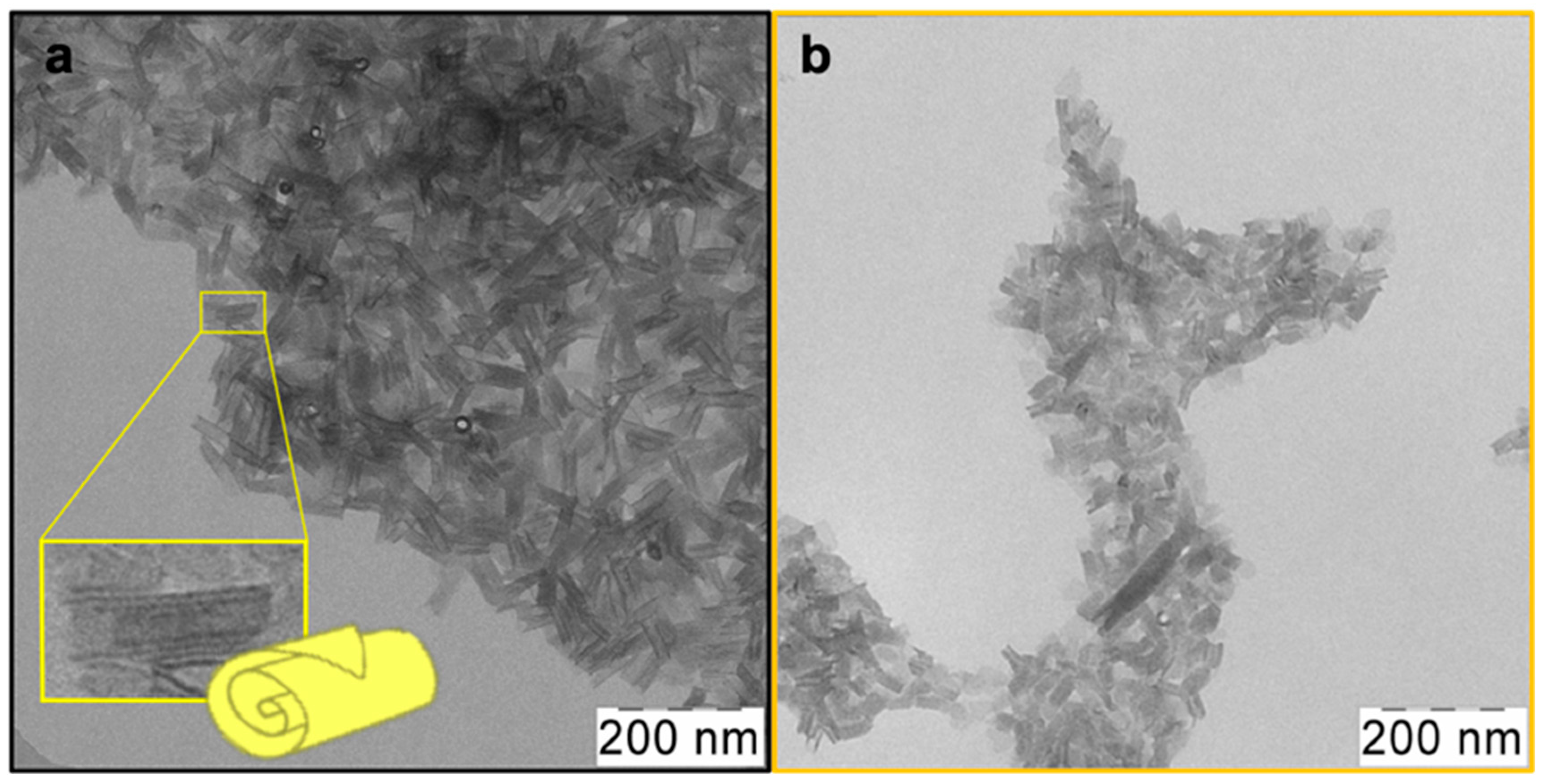
25] was made for the case of low temperatures. As a result, CdSe NPLs with a thickness of 2.5 ML or 0.6 nm were obtained. Its lower-energy exciton transitions occur at a wavelength of 394 nm, so we denoted it CdSe394OA. According to TEM, sufficiently uniform in shape and size as-synthesized nanoparticles were obtained (
Figure 1a). One can see an ensemble of nanoplatelets, which roll into nanoscrolls due to the spontaneous folding effect [
30]. The average length of such scrolls in this case is about 80 nm.
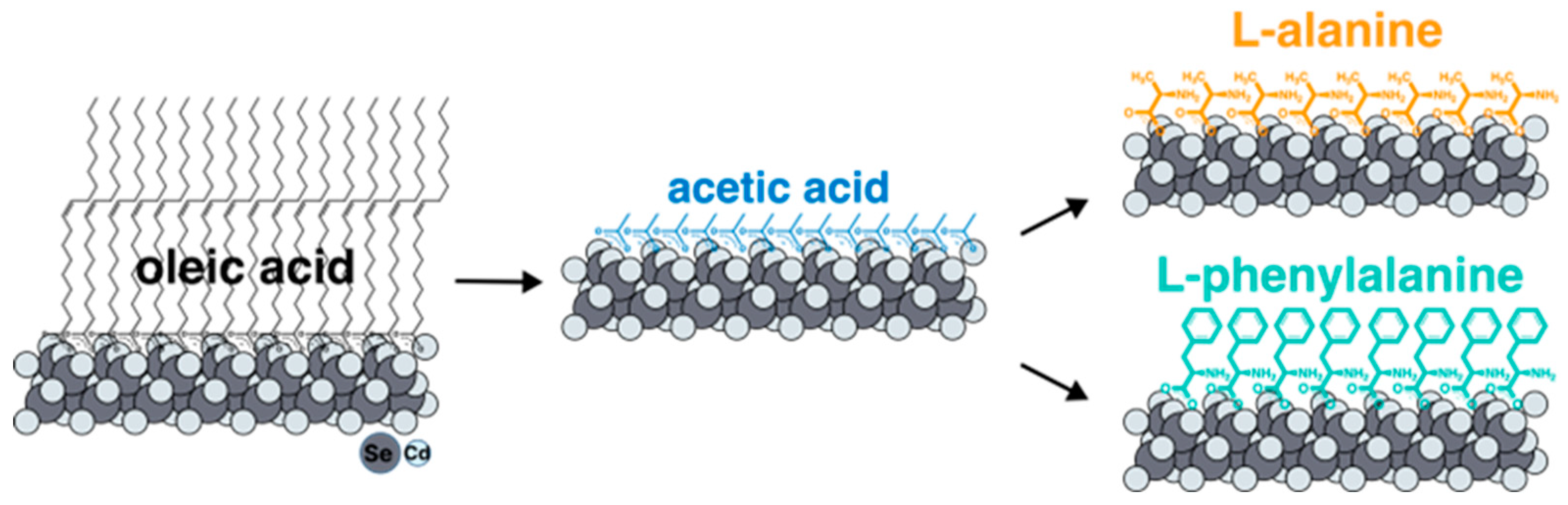
To exchange the ligands of native oleic acid on the surface of nanoparticles for amino acids, it turned out to be necessary to develop a new technique. The all approaches described in the literature for ligand replacement on the basal planes of CdSe NPLs suggest the substitution for the carboxylate group of oleic acid for sulfhydryl group of cysteine or another thiol-containing ligand, which has a greater affinity for cadmium surface cation [
31]. It easily can be realized with use of the phase-transfer method and organic solvent method [
25,
32]. In the same time, substitution for amino acid ligands involves replacing one carboxyl group with another, which means it is not feasible according to the earlier-reported synthesis protocols. For such an exchange, we have developed for the first time a new technique with intermediate substitution for short-chain acetic acid (
Figure 2). Such substitution is thermodynamically advantageous and occurs at room temperature due to the precipitation of nanoparticles as a result of a gradual decrease in solubility in non-polar solvents when the surface is coated with acetic acid. The short-chain acetic ligand was then replaced with the target amino acids at elevated temperature, removing free acetic acid by distillation to shift the equilibrium. The completeness of such an exchange with an intermediate stage of substitution for acetic acid was confirmed by the FTIR spectra (
Figure S1).
After the exchange of oleic acid on the surface of nanoparticles for the alanine ligand (
Figure 1b), the scroll-like morphology is preserved with some loss of uniformity. This may be due to the multistep nature of the exchange process and requires further optimization of synthesis conditions.
3.2. FTIR analysis of ligand coordination
The completeness of amino acid ligands substitution on the basal surface of CdSe nanoparticles and their coordination on it were analyzed using the FTIR method.
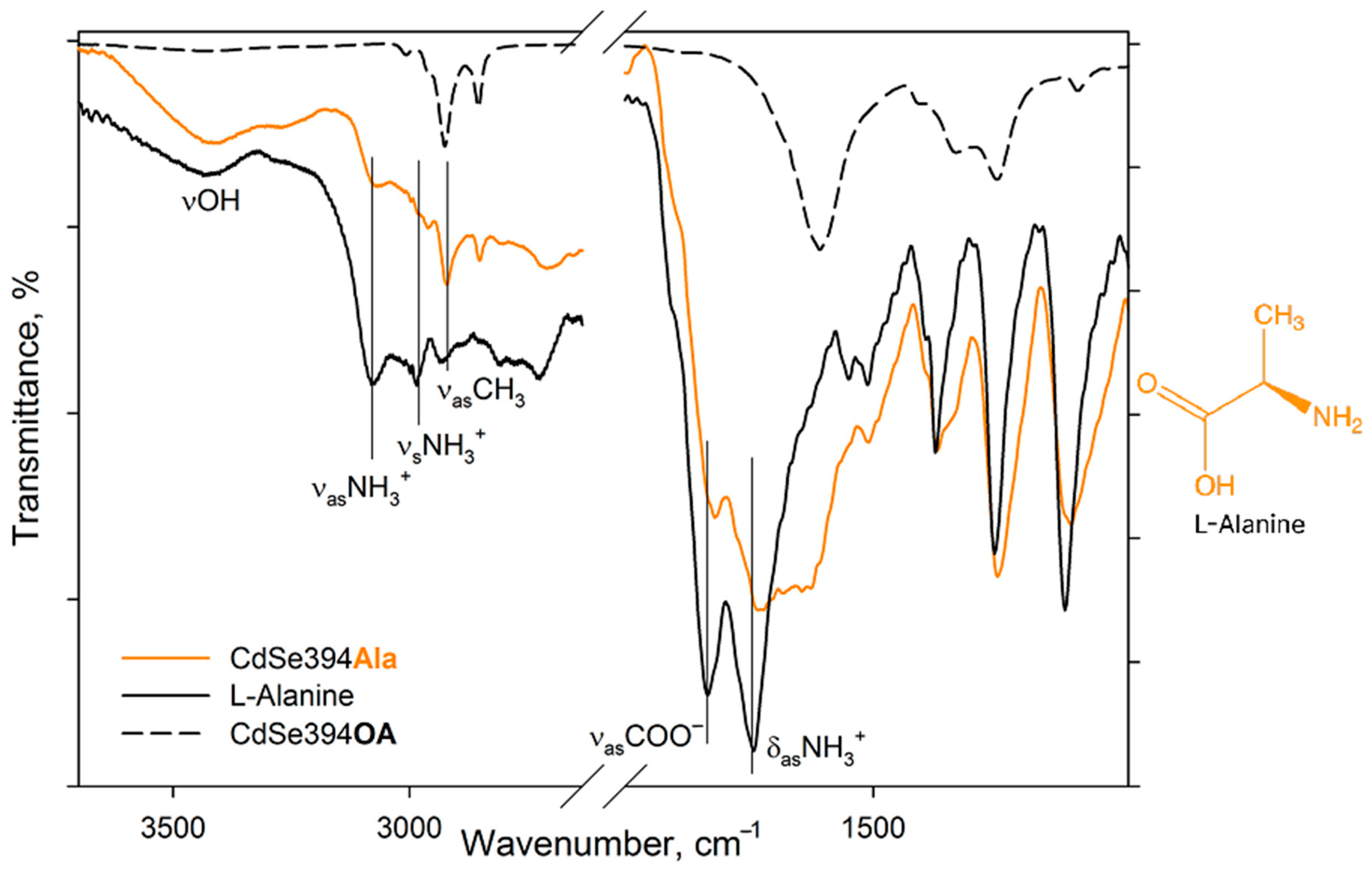
Figure 3 shows the FTIR spectra for pure L-Alanine, a sample coated with the L-Alanine ligand, and an initial sample with oleic acid on the surface.
In the spectrum of pure alanine (
Figure 3, black solid line), a band of about 3440 cm
−1 refers to -OH stretching vibrations of hydroxyl group, which can occur both from the partially uncharged state of the alanine molecule and from water absorbed by the tablet from the air during measurements. The 3290 cm
−1 band is attributed to valence vibrations of the NH
2 group, the presence of which also indicates the presence of a partially uncharged state of the L-Alanine molecule [
33]. But the pronounced bands at 3087 and 3000 cm
−1 are attributed in the literature to asymmetric and symmetrical oscillations of the charged NH
3+ group in the zwitterionic state [
33,
34,
35]. The charged state is indicated by the presence of a band of valence asymmetric COO− vibrations at 1620 cm
−1 and deformation asymmetric NH
3+ vibrations at 1587 cm
−1 [
33,
34,
36]. In the C-H deformation region, asymmetric (1455 cm
−1) and symmetric (1410 cm
−1) deformation vibrational modes of CH
3 are defined.
When switching to the CdSe394Ala sample capped with the L-Alanine ligand on the surface spectrum (
Figure 3, orange line), one can observe the preservation of the main vibrational band’s characteristic of L-Alanine, but with a shift in their position and broadening of the bands. Thus, it is possible to observe the broadening of the band of asymmetric vibrations of the carboxylate group as a result of its attachment to the surface of nanoparticles. It is also worth noting the presence of a minor contribution of vibrational bands from unwashed oleic acid on the surface when compared with CdSe394OA, the presence of which is also visible at the previous stage of exchange with acetic acid (see
Figure S1).
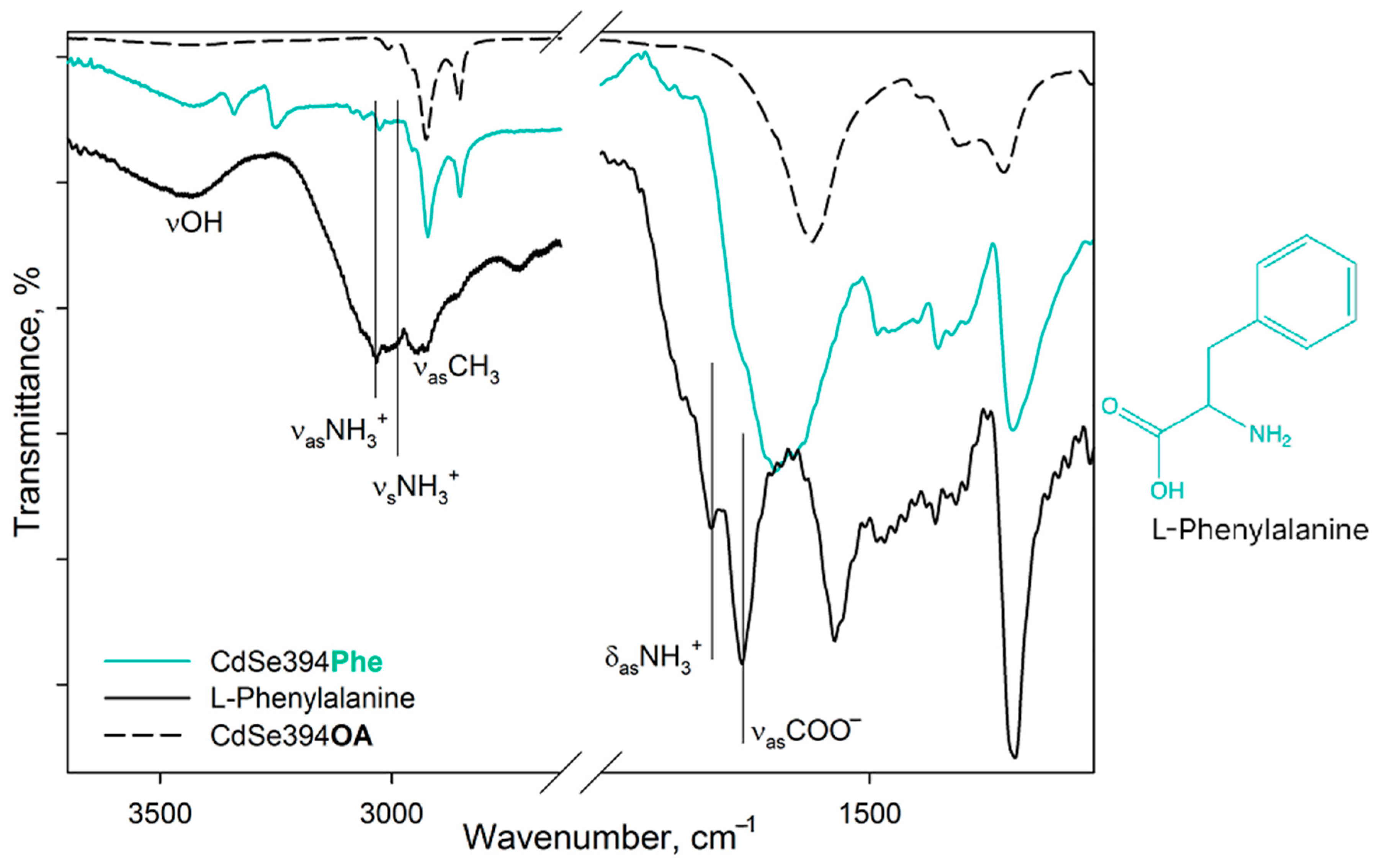
Figure 4 shows FTIR spectra for pure L-Phenylalanine, a sample coated with the L-Phenylalanine ligand, and an initial sample with oleic acid on the surface. In the spectrum of pure L-Phenylalanine, mainly the same bands are observed as in the spectrum of the previously described L-Alanine. In the spectrum of pure L-Phenylalanine are pronounced bands: vibrations of the hydroxyl group (3443 cm
−1), asymmetric (3068 cm
−1) and symmetric (3034 cm
−1) stretching vibrations of the charged NH
3+ group of the zwitterion form [
33,
35], alkyl vibrations (2964-2940 cm
−1), asymmetric (1608 cm
−1) and symmetric (1525 cm
−1) deformation vibrations of the NH
3+ group and asymmetric vibrations of the carboxylate group COO
− (1587 cm
−1) of the zwitterion form. In addition, there is a small contribution of aromatic C=C stretching vibrations in the form of a shoulder at 1625 cm
−1 [
37].
In the case of CdSe394Phe sample capped with the L-Phenylalanine ligand on the surface the FTIR spectrum (
Figure 4) gives at first sight the same bands of the hydroxyl group (3449 cm
−1) and the NH
3+ group (3035 cm
−1) as for pure L-Phenylalanine, however, the latter are noticeably less pronounced, while distinct bands at 3349/3259 cm
−1 attributed to the NH
2 group appear [
35]. Thus, it can be concluded that L-Phenylalanine exists in anionic form on the surface of nanoparticles [
35]. For asymmetric vibrations of the carboxyl group (1567 cm
−1), a shift to the region of lower energies is observed, which also corresponds to the anionic form and indicates attachment to the surface. At the same time, it is worth noting the presence of unsubstituted oleic acid, from which the vibrations of the hydrocarbon chains and the contribution to the fluctuations of the carboxyl group are visible.
For convenience, all of the main vibration assignments from the FTIR Spectra of L-Alanine, CdSe394Ala, L-Phenylalanine and CdSe394Phe shown in
Figure 3 and
Figure 4 are presented in
Table 1.
3.3. Analysis of optical and chiroptical properties
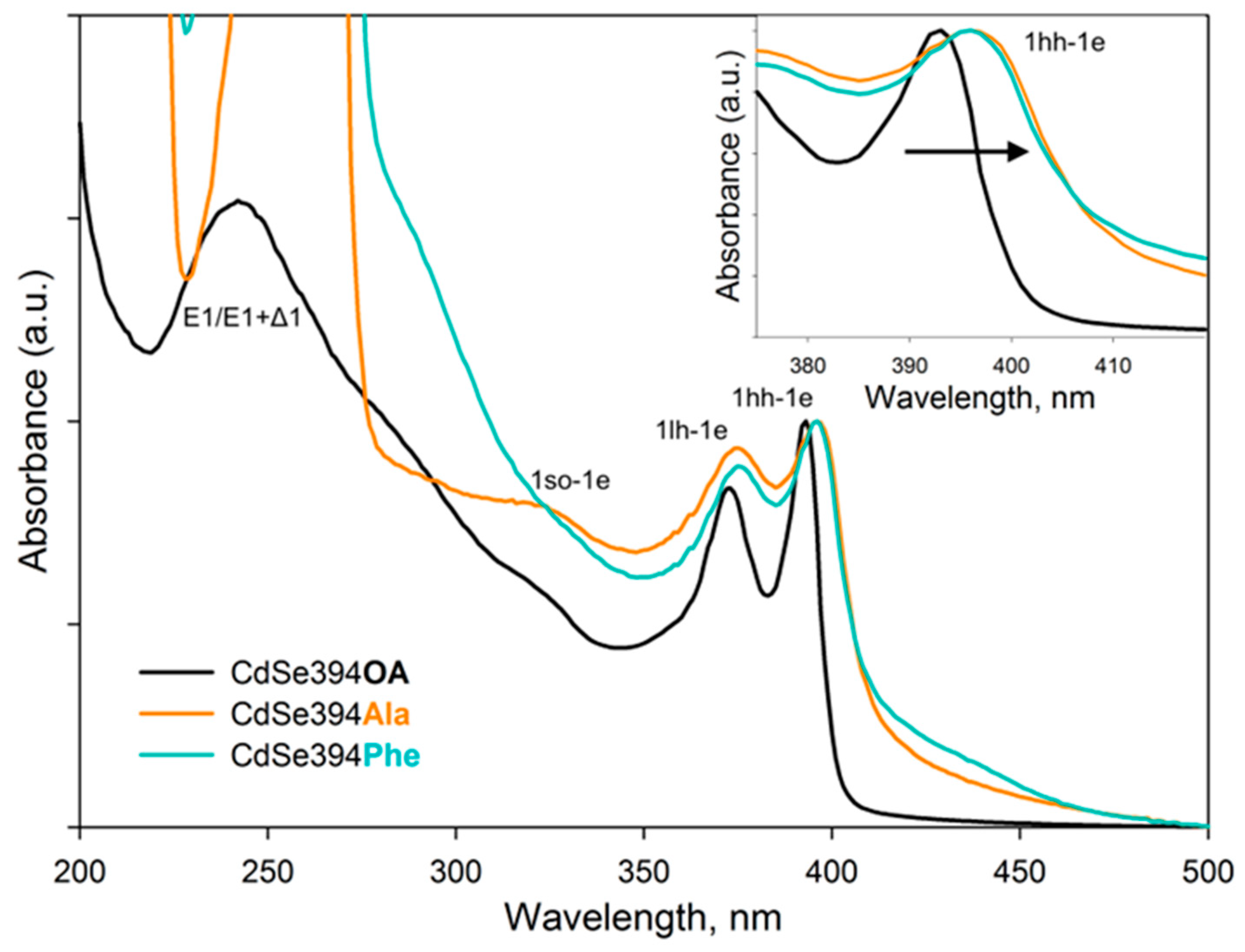
The optical properties of nanoparticles coated with amino acids were analyzed using absorption and luminescence spectroscopy. The obtained absorption spectra are shown in
Figure 5. For the initial CdSe394OA NPLs, standard exciton transitions from the zones of heavy holes (hh), light holes (lh) and spin-orbital (so) to the conduction band at wavelengths of 394, 366 and 320 nm, respectively, as previously noted in the literature [
25,
38] were observed. The wide absorption band at 240 nm refers to the transitions at the boundary of the 2D Brillouin zones (L point) [
39].
The absorption spectra of CdSe NPLs coated with L-alanine and L-phenylalanine ligands are similar to each other and retain exciton transitions of the initial sample, but the positions of these bands are shifted to the long-wavelength region. This shift may be in favor of the successful exchange of ligands on the surface and the resulting deformation of the crystal structure. As described earlier, CdSe NPLs collapse due to the effect of spontaneous folding in case of deformations of the discrepancy between the volume of the ligand and the available seat on the surface of the nanoparticle [
30]. So therefore, the replacement of the ligand itself inevitably leads to changes in the density of the surface coating and affects the electronic properties of such an atomically thin system. It also should be noted, that it is not possible to distinguish the absorption band from the transitions at the border of the Brillouin zones, since the absorption of organic ligands is superimposed.
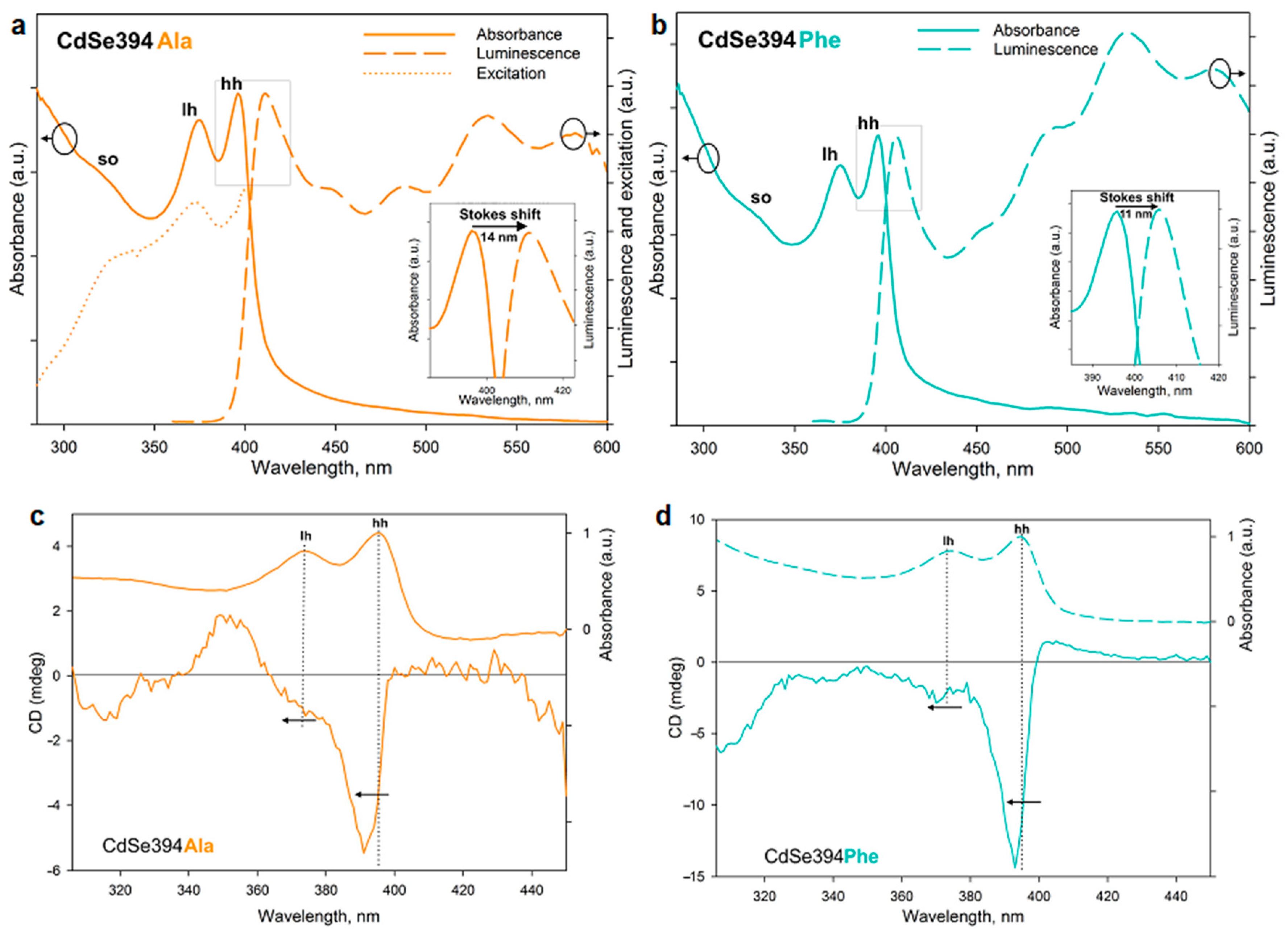
Figure 6a,b shows the absorption, luminescence and luminescence excitation spectra (for CdSe394Ala) of the samples obtained after ligand exchange. The luminescence excitation spectrum (
Figure 6a) confirms the excitonic nature of the first luminescence band. The luminescence band corresponds to the lower exciton transition in terms of energy for both L-alanine (
Figure 6a) and L-phenylalanine (
Figure 6b), however, a bigger Stokes shift is observed for the former, probably from the broadening of the bands due to inhomogeneous surface coating with L-alanine. A wide absorption band at about 530 nm, also noticed for the initial CdSe394OA NPLs (
Figure S2), is attributed to defective luminescence and was ascribed by [
38] as non-passivated atoms on the surface. It can also be noted that for CdSe394Ala, the intensity of exciton luminescence is comparable to the defective one, whereas for CdSe394Phe, the latter prevails (this fact results in a turquoise luminescence of CdSe394Phe, see
Figure S3).
Chiral ligands on the surface of the NPL are able to transfer their mirror asymmetry to the excitations in the semiconductor core NPL, thus inducing chirality of the entire system. This phenomenon has been called chirality transfer and has been actively discussed recently [
2,
3,
25,
28]. The chiroptical properties of NPLs acquired in this way and the efficiency of chirality transfer in the case of chiral amino acid ligands were investigated by CD spectroscopy and demonstrated in
Figure 6c,d. The typical CD spectrum for NPLs coated with the L-alanine ligand CdSe394Ala represents signs alternating bands in the UV-visible region from 300 to 450 nm, which confirms the influence of ligands on different interactions of excitons with right- and left-handed circularly polarized light. The position of the maxima corresponds with the transition bands in the absorption spectra and indicates the excitonic nature of CD transitions. Unlike the sulfur-containing ligands described earlier [
25,
28], CD bands corresponding to heavy and light holes have the same sign, which is similar to the case described for zinc blende with an L-cysteine ligand on the surface [
23,
24]. In passing from L-alanine to L-phenylalanine, the signal intensity increases for CdSe394Phe, which is quite unexpected in view of a more complete substitution in the first case, but may indicate the stronger effect of chirality induction for L-phenylalanine. The positions of the maxima are kept identical, but shifted in both cases from the position of exciton transitions in the absorption spectra.
The rotational strength of exciton transitions in CD spectra was analyzed using the g-factor of dissymmetry given in
Table 2 for the main exciton transitions for both CdSe394Ala and CdSe394Phe.
4. Conclusions
To summarize, we have studied the induction of chirality in ultimately thin CdSe nanoplatelets capped with enantiomers of thiol-free amino acids using the example of L-alanine and L-phenylalanine, which resulted in chiral excitations in the 2D semiconductor core. We successfully grew colloidal atomically thin CdSe nanoplatelets of only 2 monolayers thick with lateral dimensions of the order of 100 nm and zinc blende structure, coated with native oleic acid ligands. To exchange the native oleic acid ligand for chiral thiol-free amino acids, we developed a new two-step approach using exchange for short-chain acetic acid followed by high-temperature exchange for amino acids with distillation of acetic acid. According to FTIR analysis, both L-alanine and L-phenylalanine coordinate cadmium cations with the deprotonated carboxyl group on the basal Cd-rich (001) planes of nanoplatelets. The replacement of native oleic acid with amino acids preserves the electronic structure of HH, LH and SO excitons, and there is a slight red shift in the spectral position of all excitonic bands due to changes in mechanical stresses on the surface, as we assume. After the exchange, the nanoplatelets retain bright excitonic luminescence, which is due to the absence of the quenching thiolate group in the ligands. Thiol-free amino acids successfully induce pronounced chirality of exciton excitations in nanoplatelets, as shown by circular dichroism analysis, with sufficiently high dissymmetry g-factor up to –3.4×10–3 achieved for HH excitons in the case of L-phenylalanine. Thus, our work expands the variety of optically active ligands for chirality induction in cadmium chalcogenide nanoplatelets to the case of enantiomers of thiol-free compounds, and the developed native oleic acid ligand exchange method can be applied to a wide class of ligands with coordination through the carboxyl group.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: FTIR spectra of as-synthesized CdSe394OA (black solid line) and of intermediate exchanged CdSe394AcA (blue solid line). Figure S2: Typical luminescence spectra of CdSe394OA NPLs (black solid line) and its modification after ligand exchange with Ala (CdSe394Ala, orange solid line) and Phe (CdSe394Phe, turquoise solid line) ligands. Figure S3: Photo of CdSe394Phe NPLs sample (a) under ambient light and (b) luminescent under excitation of 370 nm.
Author Contributions
Conceptualization, R.V.; methodology, R.V., D.K.; formal analysis, D.K. and V.Z; investigation, D.K. and V.Z.; data curation, D.K. and V.Z.; writing—original draft preparation, R.V. and D.K.; writing—review and editing, R.V. and D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 22-13-00101 (https://rscf.ru/project/22-13-00101/).
Acknowledgments
FTIR experiments were carried out using the equipment purchased by funds from the Lomonosov Moscow State University Program of the Development. The CD measurements were carried out on the equipment of the Shared-Access Equipment Centre “Industrial Biotechnology” of Federal Research Center “Fundamentals of Biotechnology” Russian Academy of Sciences.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kotov, N. A.; Liz-Marzán, L. M.; Weiss, P. S. Chiral Nanostructures: New Twists. ACS Nano 2021, 15, 12457−12460. [CrossRef]
- Ma, W.; Xu, L.; de Moura, A. F.; Wu, X.; Kuang, H.; Xu, C.; Kotov, N. A. Chiral Inorganic Nanostructures. Chem. Rev. 2017, 117, 8041−8093. [CrossRef]
- Cho, N.H.; Guerrero-Martínez, A.; Ma, J.; Bals, A.; Kotov, N.A.; Liz-Marzán, L. M.; Nam, K.T. Bioinspired chiral inorganic nanomaterials. Nat Rev Bioeng. 2023, 1, 88–106. [CrossRef]
- Banerjee-Ghosh, K.; Ben Dor, O; Tassinari, F.; Capua, E.; Yochelis, S.; Capua, A.; Yang, S.H.; Parkin, S.S.P.; Sarkar, S.; Kronik, L.; Baczewski, L.T.; Naaman, R.; Paltiel, Y. Separation of enantiomers by their enantiospecific interaction with achiral magnetic substrates. Science 2018, 360, 1331–1334. [CrossRef]
- Warning, L.A.; Miandashti, A.R.; McCarthy, L.A.; Zhang, Q.; Landes, C.F.; Link, S. Nanophotonic Approaches for Chirality Sensing. ACS Nano 2021, 15 (10), 15538-15566. [CrossRef]
- Fan, J.; Kotov, N.A. Chiral Nanoceramics. Adv. Mater. 2020, 32, 1906738. [CrossRef]
- Yang, SH.; Naaman, R.; Paltiel, Y.; Parkin, S.S.P. Chiral spintronics. Nat. Rev. Phys. 2021, 3, 328–343. [CrossRef]
- Chen, C.; Gao, L.; Gao, W.; Ge, C.; Du, X.; Li, Z.; Yang, Y.; Niu, G.; Tang, J. Circularly Polarized Light Detection Using Chiral Hybrid Perovskite. Nat. Commun. 2019, 10 (1), 1927. [CrossRef]
- Kim, Y. H.; Zhai, Y.; Lu, H.; Pan, X.; Xiao, C.; Gaulding, E. A.; Harvey, S. P.; Berry, J. J.; Vardeny, Z. V.; Luther, J. M.; Beard, M. C. Chiral-Induced Spin Selectivity Enables a Room-Temperature Spin Light-Emitting Diode. Science2021, 371 (6534), 1129–1133. [CrossRef]
- Moloney, M.P.; Gun’ko, Y.K.; Kelly, J.M. Chiral Highly Luminescent CdS Quantum Dots. Chem. Commun. 2007, 3900– 3902. [CrossRef]
- Nakashima, T.; Kobayashi, Y.; Kawai, T. Optical Activity and Chiral Memory of Thiol-Capped CdTe Nanocrystals. J. Am. Chem. Soc. 2009, 131 (30), 10342– 10343. [CrossRef]
- Gallagher, S.A.; Moloney, M.P.; Wojdyla, M.; Quinn, S. J.; Kelly, J. M.; Gun’ko, Y.K. Synthesis and Spectroscopic Studies of Chiral CdSe Quantum Dots. J. Mater. Chem. 2010, 20, 8350−8355. [CrossRef]
- Tohgha, U.; Varga, K.; Balaz, M. Achiral CdSe Quantum Dots Exhibit Optical Activity in the Visible Region Upon Post-Synthetic Ligand Exchange with D- or L-Cysteine. Chem. Commun. 2013, 49, 1844−1846. [CrossRef]
- Ben Moshe, A.; Szwarcman, D.; Markovich, G. Size Dependence of Chiroptical Activity in Colloidal Quantum Dots. ACS Nano 2011, 5 (11), 9034–9043. [CrossRef]
- Gao, X.; Zhang, X.; Deng, K.; Han, B.; Zhao, L.; Wu, M.; Shi, L.; Lv, J.; Tang, Z. Excitonic Circular Dichroism of Chiral Quantum Rods. J. Am. Chem. Soc. 2017, 139 (25), 8734– 8739. [CrossRef]
- Diroll, B.T.; Guzelturk, B.; Po, H.; Dabard, C.; Fu, N.; Makke, L.; Lhuillier, E.; Ithurria, S. 2D II–VI Semiconductor Nanoplatelets: From Material Synthesis to Optoelectronic Integration. Chem. Rev. 2023, 123 (7), 3543-3624. [CrossRef]
- Chen, Z.; Nadal, B.; Mahler, B.; Aubin, H.; Dubertret B. Quasi-2D Colloidal Semiconductor Nanoplatelets for Narrow Electroluminescence. Adv. Funct. Mater. 2014, 24, 295-302. [CrossRef]
- Vashchenko, A. A.; Vitukhnovskii, A. G.; Lebedev, V. S.; Selyukov, A. S.; Vasiliev, R. B.; Sokolikova, M. S. Organic Light-Emitting Diode with an Emitter Based on a Planar Layer of CdSe Semiconductor Nanoplatelets. JETP Lett. 2014, 100, 86−90. [CrossRef]
- Yang, Z.; Pelton, M.; Fedin, I.; Talapin, D.V.; Waks, E. A room temperature continuous-wave nanolaser using colloidal quantum wells. Nat. Commun. 2017, 8, 143. [CrossRef]
- Wu, M.; Ha, S. T.; Shendre, S.; Durmusoglu, E. G.; Koh, W.-K.; Abujetas, D. R.; Sánchez-Gil, J. A.; Paniagua-Domínguez, R.; Demir, H. V.; Kuznetsov, A. I. Room-temperature lasing in colloidal nanoplatelets via Mie-resonant bound states in the continuum. Nano Lett. 2020, 20, 8, 6005–6011. [CrossRef]
- Saidzhonov, B. M.; Zaytsev, V. B.; Berekchiian, M. V.; Vasiliev, R. B. Highly Luminescent Copper-Doped Ultrathin CdSe Nanoplatelets for White-Light Generation. J. Lumin. 2020, 222, 117134. [CrossRef]
- Saidzhonov, B. M.; Zaytsev, V. B.; Eliseev, A. A.; Grishko, A. Y.; Vasiliev, R. B. Highly Luminescent Gradient Alloy CdSe1-XSx Nanoplatelets with Reduced Reabsorption for White-Light Generation. ACS Photonics 2020, 7, 3188−3198. [CrossRef]
- Yang, G.; Kazes, M.; Oron, D. Chiral 2D Colloidal Semiconductor Quantum Wells. Adv. Funct. Mater. 2018, 28(28), 1802012. [CrossRef]
- Gao, X.; Zhang, X.; Zhao, L.; Huang, P.; Han, B.; Lv, J.; Qiu, X.; Wei, S. H.; Tang, Z. Distinct Excitonic Circular Dichroism between Wurtzite and Zincblende CdSe Nanoplatelets. Nano Lett. 2018, 18 (11), 6665– 6671. [CrossRef]
- Kurtina, D.A.; Garshev, A.V.; Vasil’eva, I.S.; Shubin, V.V.; Gaskov, A.M.; Vasiliev, R.B. Atomically-Thin Population of Colloidal CdSe Nanoplatelets: Growth of Rolled-up Nanosheets and Strong Circular Dichroism Induced by Ligand Exchange. Chem. Mater. 2019, 31, 9652. [CrossRef]
- Kuznetsova, V. A.; Mates-Torres, E.; Prochukhan, N.; Marcastel, M.; Purcell-Milton, F.; O’Brien, J.; Visheratina, A. K.; Martinez-Carmona, M.; Gromova, Y.; Garcia-Melchor, M.; Gun’ko, Y. K. Effect of Chiral Ligand Concentration and Binding Mode on Chiroptical Activity of CdSe/CdS Quantum Dots. ACS Nano 2019, 13 (11), 13560– 13572. [CrossRef]
- Choi, J.K.; Haynie, B.E.; Tohgha, U.; Pap, L.; Elliott, K.W.; Leonard, B.M.; Dzyuba, S.V.; Varga, K.; Kubelka, J.; Balaz, M. Chirality Inversion of CdSe and CdS Quantum Dots without Changing the Stereochemistry of the Capping Ligand. ACS Nano 2016, 10 (3), 3809-3815. [CrossRef]
- Kurtina, D.A.; Grafova, V.P.; Vasil’eva, I.S.; Maksimov, S.V.; Zaytsev, V.B.; Vasiliev, R.B. Induction of Chirality in Atomically Thin ZnSe and CdSe Nanoplatelets: Strengthening of Circular Dichroism via Different Coordination of Cysteine-Based Ligands on an Ultimate Thin Semiconductor Core. Materials 2023, 16, 1073. [CrossRef]
- Puri, M.; Ferry, V.E. Circular Dichroism of CdSe Nanocrystals Bound by Chiral Carboxylic Acids. ACS Nano 2017, 11 (12), 12240–12246. [CrossRef]
- Vasiliev, R.B.; Lazareva, E.P.; Karlova, D.A.; Garshev, A.V.; Yao, Y.; Kuroda, T.; Gaskov, A.M.; Sakoda, K. Spontaneous folding of CdTe nanosheets induced by ligand exchange. Chem. Mater. 2018, 30, 1710–1717. [CrossRef]
- Mahler, B.; Nadal, B.; Bouet, C.; Patriarche, G.; Dubertret, B. Core/Shell Colloidal Semiconductor Nanoplatelets. J. Am. Chem. Soc. 2012, 134, 18591−18598. [CrossRef]
- Ithurria, S.; Talapin, D. V. Colloidal Atomic Layer Deposition (c-ALD) using Self-Limiting Reactions at Nanocrystal Surface Coupled to Phase Transfer between Polar and Nonpolar Media. J. Am. Chem. Soc. 2012, 134, 18585−18590. [CrossRef]
- Garcia, A. R.; de Barros, R. B.; Lourenco, J. P.; Ilharco, L. C. The Infrared Spectrum of Solid L-Alanine: Influence of pH-Induced Structural Changes. J.Phys. Chem. A 2008, 112, 8280−8287. [CrossRef]
- Berezhinsky, L. I.; Dovbeshko, G. I.; Lisitsa, M. P.; Litvinov, G. S. Vibrational spectra of crystalline 𝛽-alanine. Spectrochim. Acta, Part A 1998, 54, 349. [CrossRef]
- Griffith, E. C.; Vaida, V. Ionization state of L-Phenylalanine at the Air−Water Interface. J. Am. Chem. Soc. 2013, 135, 710–716. [CrossRef]
- Iwai, H.; Egawa, C. Molecular Orientation and Intermolecular Interaction in Alanine on Cu(001). Langmuir 2010, 26 (4), 2294–2300. [CrossRef]
- Zheng, X.; Xu, K.; Wang, Y.; Shenb, R.; Wang, Q. Study of hydrogen explosion control measures by using L-phenylalanine for aluminum wet dust removal systems. RSC Adv. 2018, 8, 41308–41316. [CrossRef]
- Delikanli, S.; Yu, G.; Yeltik, A.; Bose, S.; Erdem, T.; Yu, J.; Erdem, O.; Sharma, M.; Sharma, V.K.; Quliyeva, U.; Shendre, S.; Dang, C.; Zhang, D.H.; Sum, T.C.; Fan, W.; Demir, H.V. Ultrathin Highly Luminescent Two-Monolayer Colloidal CdSe Nanoplatelets. Adv. Funct. Mater. 2019, 29 (35), 1901028. [CrossRef]
- Vasiliev, R.B.; Lebedev, A.I.; Lazareva, E. P.; Shlenskaya, N.N.; Zaytsev, V.B.; Vitukhnovsky, A.G.; Yao, Y.; Sakoda, K. High-energy exciton transitions in quasi-two-dimensional cadmium chalcogenide nanoplatelets. Phys. Rev. B. 2017, 95, 165414. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).