1. Introduction
Athletes experience regular cycles of physiological stress, inflammation, oxidative stress, and transient immune dysfunction. Various nutrition-based approaches including increased intake of fruits and phytochemicals are being explored by our research group as countermeasures to these exercise-induced indicators of metabolic stress [
1,
2,
3,
4,
5,
6,
7,
8]. We have focused on the use of multi-omics methods (i.e., lipidomics, metabolomics, proteomics, genomics, epigenetics) to capture the complex biochemical interactions that occur in these types of sports nutrition investigations [
1,
2,
3,
4,
5,
6,
7,
8].
Oxylipins are bioactive lipids that are produced via the oxygenation of polyunsaturated fatty acids (PUFAs) and were designated as the primary outcome of this study. Oxylipins are synthesized from cell membrane PUFAs as they are released under tight regulation by phospholipase A2 (PLA2) in response to cell activation from various stress-related stimuli including exercise [
9,
10,
11,
12,
13]. Cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP) enzyme systems metabolize the released PUFAs into oxylipins that act as autocrine and paracrine lipid mediators in numerous physiological processes [
9,
10,
11]. Depending on the metabolic context, oxylipins can function as beneficial signaling agents or mediators of inflammation, immune dysfunction, and disease [
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31]. A significant proportion of the physiological and immune system effects from n-6 and n-3 PUFAs are mediated through these oxidized metabolites (i.e., lipid mediators) and have emerged as sensitive indicators of metabolic change in nutrition-based interventions [
9,
11,
15,
18,
28,
29,
30] or in various disease states [
10,
20,
21,
22,
23,
24,
25,
26,
27]. Exercise-induced muscle tissue injury, inflammation, and metabolic stress prompt an innate immune response, and lipid mediators are involved in initiating, mediating, and resolving this process [
16,
17,
18,
29,
30,
31].
Our research group showed that exercise-induced increases in pro-inflammatory oxylipins can be countered through carbohydrate ingestion (both bananas and sports drinks), with the largest effects seen for CYP-derived lipid mediators [
5,
29]. We also showed that 2-weeks ingestion of 1 cup/d blueberries increased plasma levels of 24 gut-derived phenolics and strongly countered post-exercise increases in plasma levels of 10 proinflammatory oxylipins derived from arachidonic acid (ARA) and CYP (ARA-CYP) [
29]. In another study with untrained adults, 18 days intake of 1 cup/day blueberries was linked to a reduction in pro-inflammatory dihydroxy-9Z-octadecenoic acids (diHOMES) and sustained elevations in docosahexaenoic acid (DHA)- and eicosapentaenoic acid (EPA)-derived anti-inflammatory oxylipins in response to a 90-minute bout of unaccustomed exercise [
30]. These data indicate that oxylipins are sensitive to both exercise and nutritional influences, and that inflammation resolution from vigorous or muscle-damaging exercise can be improved by fruit carbohydrates and polyphenols.
Mangoes are high in carotenoids (26 mg/100 g), polyphenols (145 mg/100 g), carbohydrates (15 g/100 g), vitamin C (36 mg/100 g), vitamin A (54 µg RAE/100 g), and folate (43 µg/100 g) [
https://fdc.nal.usda.gov/fdc-app.html#/food-details/169910/nutrients; http://phenol-explorer.eu/contents/food/156]. The total antioxidant content of mangoes is high, and this popular fruit has been extensively investigated for health-related effects [
32,
33,
34,
35,
36,
37,
38]. There are many types of polyphenols in mangoes with a predominance of 3,4,5-trihydroxybenzoic acids (gallic acid) and other galloyl-derivatives [
38]. Like other plant sources, mango polyphenols are poorly absorbed in the human small intestine. After biotransformation by colon bacteria, changes in circulating and urine levels of at least 94 polyphenol-related metabolites have been measured [
38]. These gut-derived metabolites from mango ingestion include sulfated, methylated, and glucuronide conjugates of 1,2,3-trihydroxybenzene (pyrogallol) that may exert anti-inflammatory effects as indicated in a limited number of cell culture, animal, and human studies [
32,
33,
34,
36]. Mango polyphenols and gut-derived phenolics may influence the gut microbiome and interact with both gut and liver enzyme systems including CYP and LOX to inhibit inflammatory oxylipins [
32,
39,
40,
41,
42]. Although untested within an exercise context, these data support our hypothesis that intake of mangoes (2 cups per day for two weeks) has the potential for quelling post-exercise increases in pro-inflammatory oxylipins in trained cyclists.
2. Materials and Methods
2.1. Study Participants
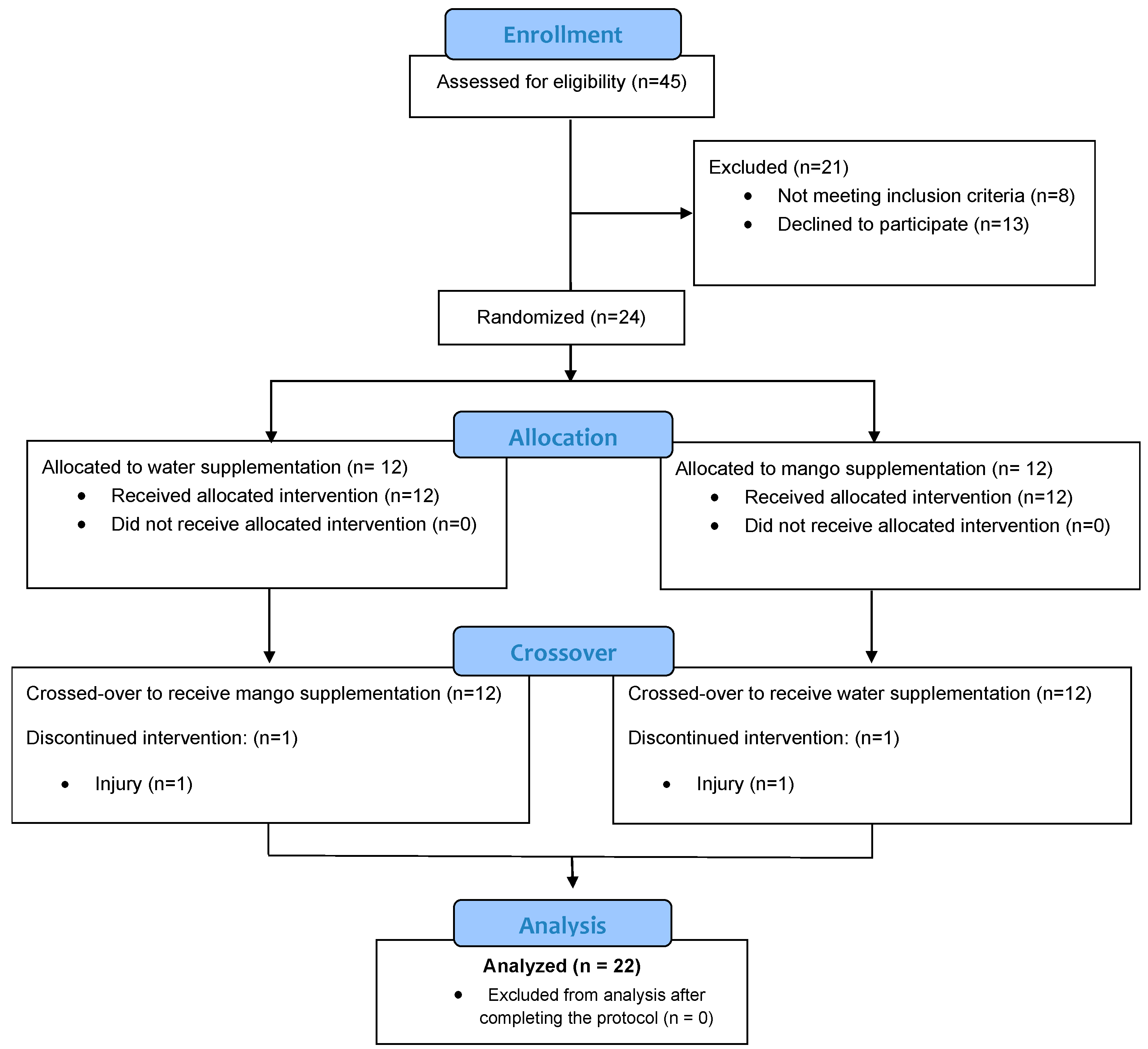
Healthy male and female cyclists between the ages of 18 and 60 years were recruited who were capable of cycling at race pace for 2.25 h in a laboratory setting. Of 45 adults assessed for eligibility, 24 were recruited into the study, and 22 (n=13 males, n=9 females) completed all study requirements (
Figure 1). The two subjects dropping out of the study experienced training-induced injuries. During the 6-week period when data were being collected, participants maintained their typical training regimen, did not ingest mangoes except what was provided during the study, and avoided the use of vitamin and mineral supplements, herbs, and medications that could influence inflammation. Participants signed informed consent forms and study procedures were approved by the Institutional Review Board at Appalachian State University. Trial Registration: ClinicalTrials.gov, U.S. National Institutes of Health, identifier: NCT05409105. The research procedures were conducted at the Human Performance Laboratory operated by Appalachian State University at the North Carolina Research Campus (NCRC) in Kannapolis, NC.
2.2. Research Design
This study examined the effects of mango ingestion in moderating exercise-induced inflammation in a randomized crossover trial with 22 cyclists. In random order with trials separated by a 2-week washout period, the cyclists ingested 330 g/day mangoes with 0.5 liters water or 0.5 liters water alone for 2 weeks, followed by a 2.25-h cycling bout challenge. Blood and urine samples were collected pre- and post-2 weeks supplementation, with additional blood samples collected immediately post-exercise, and then 1.5-h, 3-h, and 24-h post-exercise.
2.2.1. Pre-Study Baseline Testing
After voluntarily signed IRB-approved consent forms, study participants were tested for maximal aerobic capacity (VO2max) during a graded, cycling test with continuous metabolic monitoring with the Cosmed CPET metabolic cart (Cosmed, Rome, Italy). Body composition was measured with the BodPod body composition analyzer (Cosmed, Rome, Italy). Three-day food records and 24-h urine collection kits were supplied with thorough instructions. Participants were given a food list restricting high fat foods with instructions to record all food and beverage intake (only from what was listed) during the 3-day period before the second lab visit. Macro-and micro-nutrient intake was assessed from the 3-day food records using the Food Processor dietary analysis software system (Version 11.11, ESHA Research, Salem, OR, United States).
2.2.2. Pre-Supplementation Lab Visits
Study participants reported to the lab in an overnight fasted state and turned in their 3-day food records and 24-hour urine samples. A blood sample was collected. Study participants randomized to the mango trial were given a 2-weeks supply of frozen Tommy Atkins mangoes (pulp only in plastic freezer bags), with instructions to store the fruit in their freezers. Mangoes from Mexico were supplied, processed, and cubed by the National Mango Board (Orlando, FL). The mango pulp was portioned into daily serving sizes and frozen. Participants were instructed to eat 165 grams each day of mangoes with the first meal, and then another 165 grams with the last meal of the day during a 2-week period. Participants were allowed to eat the mango pulp as provided, mixed with yogurt, or in smoothies. Bottled water was supplied to all study participants with instructions to drink 0.5 liters water with the mangoes during the first meal and then another 0.5 liters during the last meal. Participants were told to save the freezer bags and then bring them back to the next lab visit after the 2-week supplementation period. Participants randomized to the water-only trial were instructed to drink 0.5 liters of water with the first meal and then another 0.5 liters of water with the last meal. Participants crossed over to the other supplement after a 2-week washout interval during the second 2-week supplementation period. Participants were instructed to taper their exercise training during the 3-day period before the next lab visit to get rested for the 2.25 hour cycling session. Participants were also given another 3-day food record (with the food list), with instructions to record all food and beverage intake (only from what is listed) during the 3-day period before the next lab visit. A 24-hour urine collection kit was given to participants with instructions to collect all urine during the entire day before the next lab visit.
2.2.3. Cycling Sessions
Study participants reported to the lab in an overnight fasted state and turned in their 3-day food records, the empty mango freezer bags (to monitor compliance), and the 24-hour urine sample. A blood sample was collected. All participants drank 0.5 liters of water, and those in the mango trial consumed 165 grams of mangoes. No other foods or beverages were allowed.
After warming up, participants cycled 2.25 hours at an intensity close to a race of this duration (about 60% maximal watts power). Participants cycled on their own bicycles fitted to Saris H3 direct drive smart trainers (Madison, WI, USA) with monitoring by Zwift online training platform (Long Beach, CA). Heart rate, cycling speed, cadence, distance, and power, breathing rate, and oxygen intake were measured after 15 minutes and then every 30 minutes during the cycling session using the cycling trainer, platform, and Cosmed metabolic cart. Participants ingested 250 ml water every 15 minutes with no other beverage or food allowed.
After completing the cycling session, additional blood samples were collected immediately post-exercise, and then again after 1.5 and 3 hours. During the first 1.5 hours after the cycling session, participants drank water (7 ml/kg) and rested. After the blood draw 1.5 hours after exercise, participants in the mango trial consumed 165 grams of mangoes. Participants in the water-only trial drank 0.45 liters of a 6% carbohydrate sports beverage. Three hours after finishing the cycling session, participants were allowed to leave the performance lab. Participants were told to eat and drink beverages as desired from the assigned food list until the next morning and to avoid any additional vigorous exercise.
Participants returned the next morning in an overnight fasted state and provided a blood sample. For the next two weeks, participants were told to follow their normal eating and training routines. Participants then crossed over to the opposite trial arm and repeated all study procedures.
2.3. Sample Analysis
Plasma and urine aliquots were prepared and stored in a − 80°C freezer until analysis for oxylipins and mango phenolic metabolites after the study was completed.
2.3.1. Plasma Oxylipins
Plasma arachidonic acid (ARA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and oxylipins were analyzed using a liquid chromatography-multiple reaction monitoring mass spectrometry (LC-MRM-MS) method as fully described elsewhere [
43]. Resultant data files were processed with Skyline software (version 22.2.0.351) and the auto-integrated peaks were inspected manually. Concentrations of each oxylipin were determined from calibration curves of each analyte, which were constructed by normalizing to the selected deuterated internal standards followed by linear regression with 1/x weighting (
Supplementary Table S1). Analytes with coefficients of variation relative to the quality control standards of <30% were included in the statistical analysis.
A summary composite variable was calculated for 49 of 67 oxylipins that had significant exercise-induced time effects. Based on prior studies from our research group [
29,
30,
31], two other composite variables were calculated. Eight oxylipins generated from arachidonic acid and cytochrome P-450 (ARA-CYP) were grouped and these included 5,6-, 8,9-, 11,12-, and 14,15-dihydroxy-eicosatetraenoic acid (diHETrEs), 16-, 17,- 18-hydroxy-eicosatetraenoic acids (HETEs), and the 20-HETE metabolite 20-carboxy-arachidonic acid (20-coohAA). Four abundant oxylipins generated from linoleic acid with CYP and lipoxygenease (LOX) were also grouped including 9,10- dihydroxy-9Z-octadecenoic acid (DiHOME), 12,13-DiHOME, 9- hydroxy-octadecadienoic acid (HODE), and 13-HODE (LA-DiHOMES+HODES).
2.3.2. Urine Mango Metabolites
Creatinine concentrations in urine samples were quantified using an optical method that relies on the Jaffe reaction in a 96-well format (modified from reference [
44]). Prior to mass spectrometry analysis, urine samples were centrifuged (15,000 rpm for 10 minutes) and the supernatants diluted to a standard creatinine concentration of 2.5 mM.
UPLC–ESI–TOF-MRM analysis was performed using a Waters Xevo G2-XS QTOF mass spectrometer (Waters Corporation) coupled to an ACQUITY I-Class UPLC (Waters Corporation). A sample volume of 2 µL was separated on a Kinetex 2.6 µm PFP 100 Å 100 x 2.1 mm LC column (Phenomenex) maintained at 37°C. A binary gradient using 0.1% formic acid in water (Mobile phase A) and 0.1% formic acid in acetonitrile (mobile phase B), from 2% B to 90% B over 15 min and flow rate gradient ranging from 0.55 ml/min to 0.75 ml/min was utilized for separation (adapted from reference [
45]).
The 12 mango metabolites analyzed were methylated, glucuronidated or sulfated conjugates of pyrogallol, catechol, and gallic acid. These putative metabolites are not commercially available as chemical standards. Therefore, identification was based on accurate mass, fragmentation pattern, and literature precedence. Three structurally related standards having a relevant MS signal range (protocatechuic acid, gallic acid, syringic acid) were utilized for quantitation, based on three previous recent publications [
46,
47,
48] which documented that these metabolites were significantly elevated following mango consumption.
2.3.3. Plasma Glucose
Plasma aliquots (pre-exercise, immediately post-exercise, and 1.5 h post-exercise) were analyzed for glucose concentrations using the protocol described in the glucose colorimetric assay kit (item no. 10009582) (Cayman Chemical, Ann Arbor, MI, USA).
2.4. Statistical Analysis
The data are expressed as mean ± SE. The plasma data were analyzed using the generalized linear model (GLM), repeated measures ANOVA module in SPSS (IBM SPSS Statistics, Version 28.0, IBM Corp, Armonk, NY, United States). The statistical model utilized the within-subjects approach: 2 (trials) × 6 (time points) repeated measures ANOVA and provided time (i.e., the collective effect of the cycling bouts) and interaction effects (i.e., whether the data pattern over time differed between trials). If the interaction effect was significant (p ≤ 0.05), then post hoc analyses were conducted using paired t-tests comparing time point contrasts between trials. An alpha level of p ≤ 0.01 was used after Bonferroni correction for five multiple tests. The urine data were analyzed using the same approach but with a 2 (trials) x 2 (time points) repeated measured ANOVA. The positive false discovery rate (FDR or “q value”) was calculated for multiple testing correction of the plasma oxylipin data.
3. Results
Characteristics for the
n = 22 study participants completing all aspects of the study protocol are summarized in
Table 1. Male and female cyclists had similar ages, percent body fats, and maximal oxygen consumption rates (VO
2max). Male and female cyclists did not differ in exercise-induced changes in the primary outcomes for this study (total plasma oxylipins, supplement × time × sex interaction effect,
p = 0.520). Thus, data from this crossover study are presented for all participants combined.
Three-day food records collected at the beginning and end of each 2-week supplementation period revealed no differences in macro- and micro-nutrient intake both within and between trials (data not shown). The four 3-day food records were averaged and for all 22 cyclists energy intake of the background diet averaged 1,984 ± 106 kcal/day (8.3 ± 0.44 MJ/day), with carbohydrate, protein, fat, and alcohol representing 43.8 ± 1.3, 19.1 ± 0.9, 36.0 ± 0.8, and 2.0 ± 0.6%, respectively of total energy.
Performance data for the water only and mango trials are summarized in
Table 2. As designed, the two trials were similar in all performance measures including cycling distance and speed, watts power output, oxygen consumption, and heart rate.
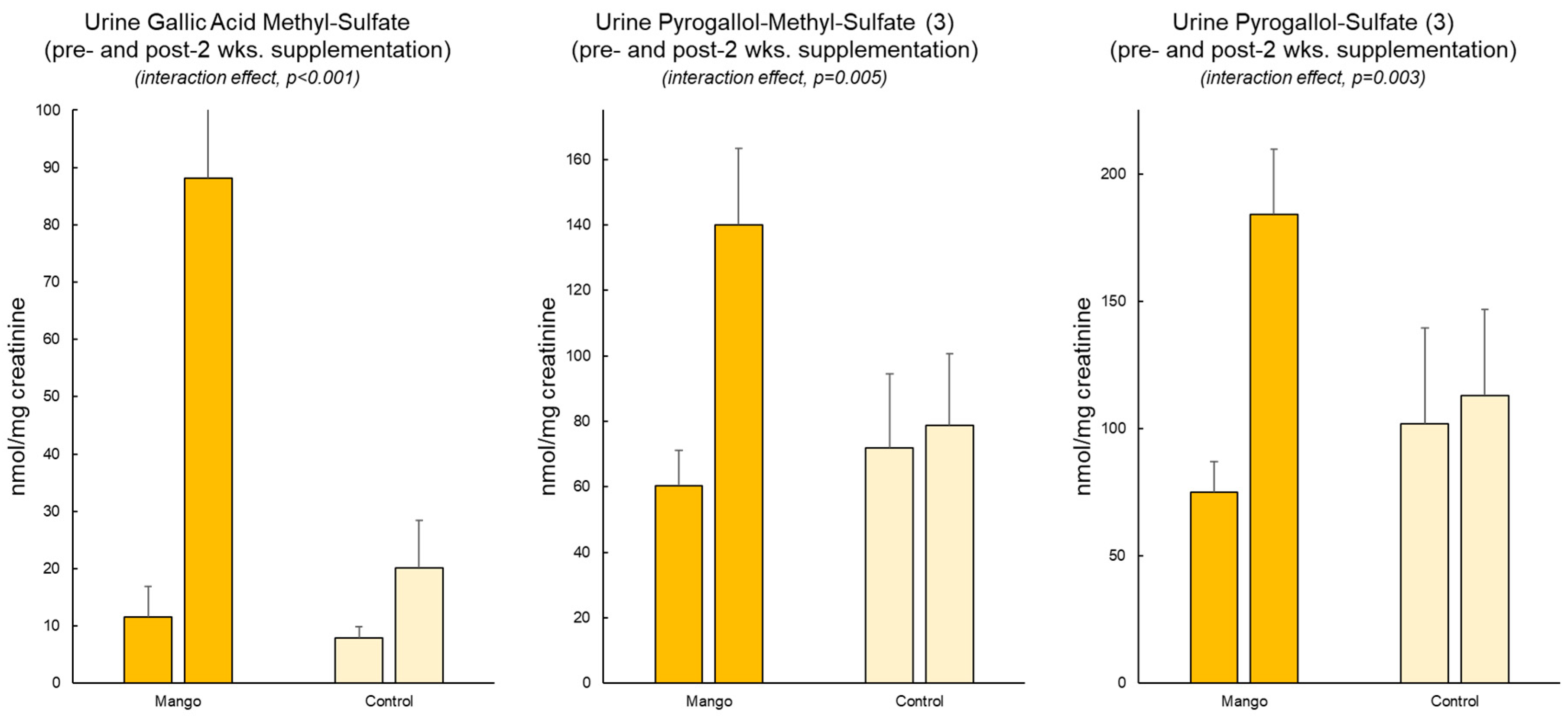
Twelve putative gallotannin-derived metabolites were identified in the urine of subjects who consumed mango (
Table 3). Three of these metabolites differed significantly between the mango and water-only (control) trials (
Figure 2). These included
O-methylgallic acid-
O-sulfate (quantified against syringic acid standard, interaction effect, p<0.001),
O-methylpyrogallol-
O-sulfate (quantified against syringic acid standard, p=0.005), and pyrogallol-
O-sulfate (quantified against protocatechuic acid standard, p=0.003).
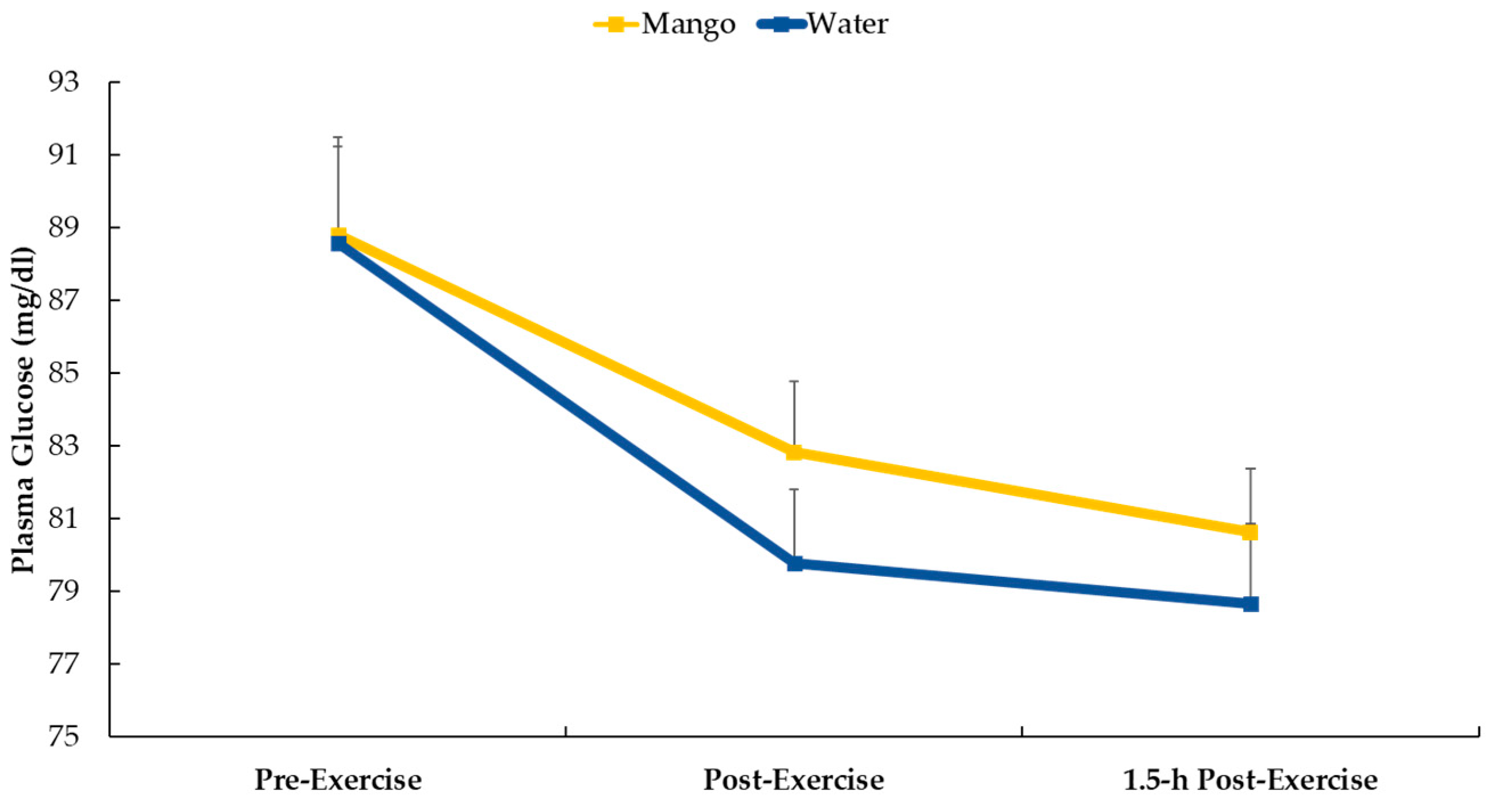
Study participants that were randomized to mango ingestion consumed 165 grams (~25 grams carbohydrate) with water just before the 2.25 h cycling bout.
Figure 3 shows that plasma glucose decreased following the cycling bouts for both supplements (mango and water-only) (p<0.001) with no difference in the pattern of change (interaction effect, p=0.543).
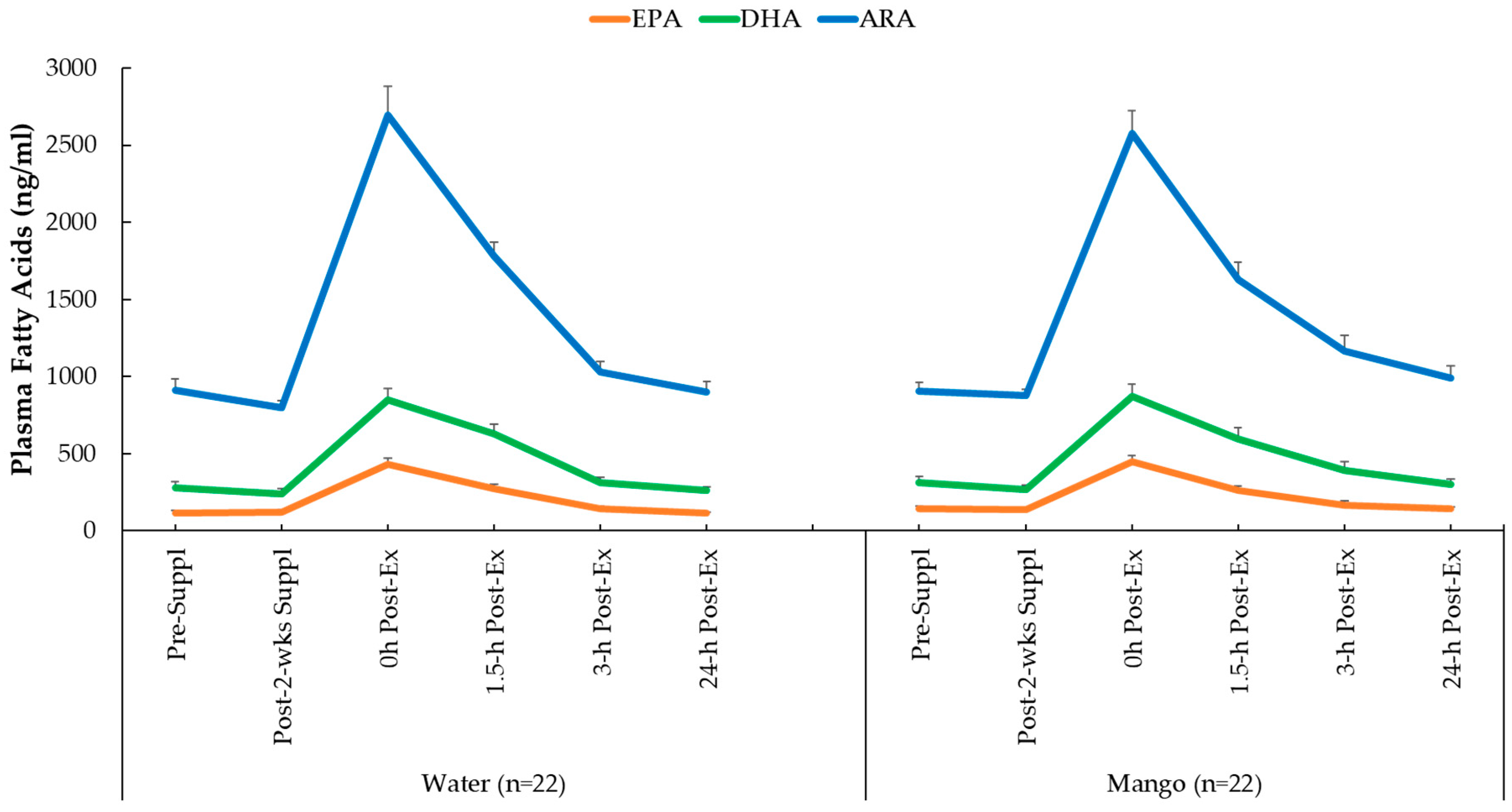
Plasma concentrations for ARA, EPA, and DHA increased significantly in response to the 2.25-h exercise bouts (all time effects, p<0.001), but the pattern of change over time (interaction effects) were not significant (p values=0.412, 0.380, and 0.740, respectively) (
Figure 4).
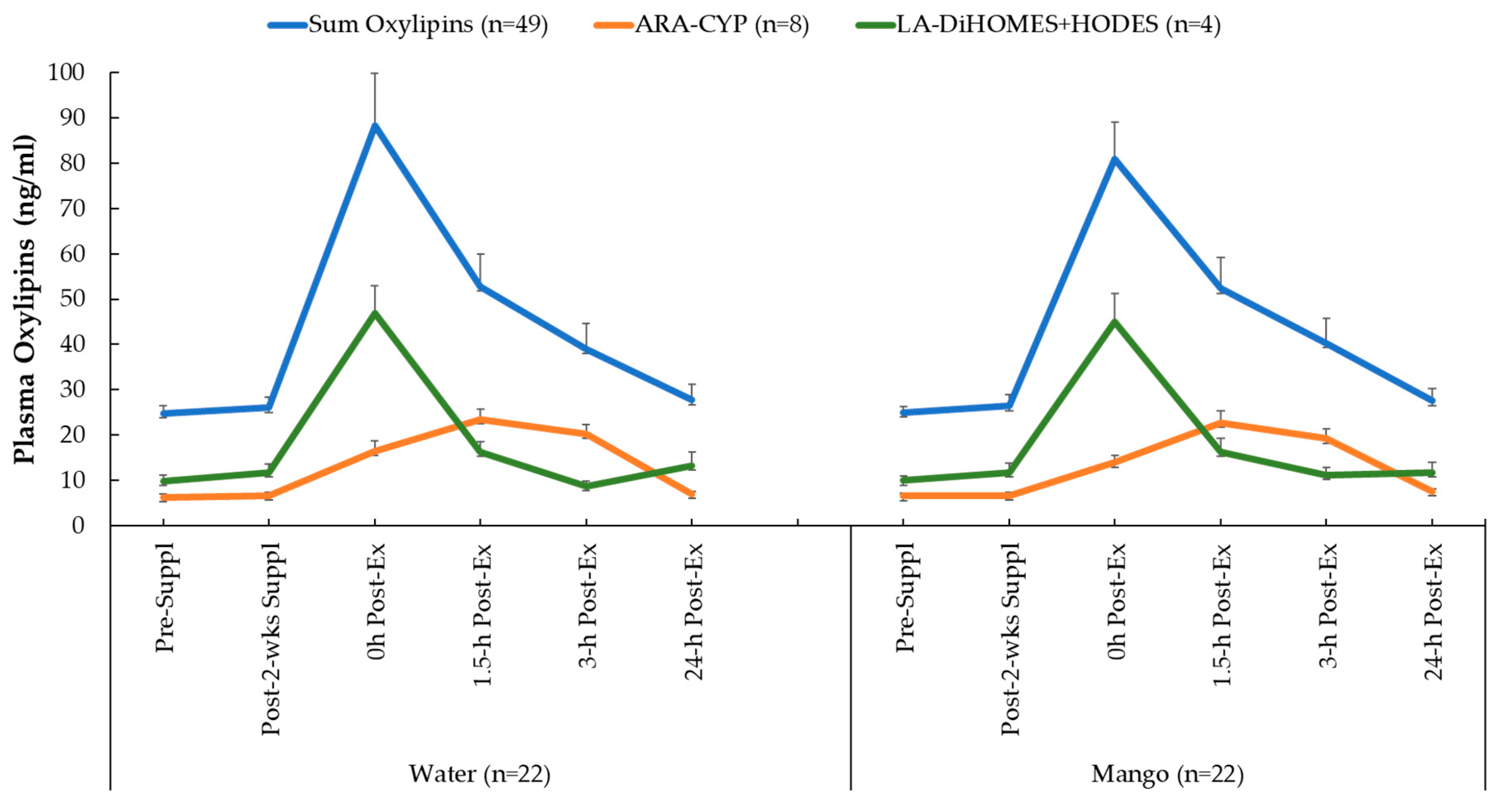
A total of 67 oxylipins were detected in study samples. Of these, 49 exhibited significant time effects during GLM statistical analysis (
Supplementary Table S1). GLM 2 x 6 repeated measures analysis on each of these 49 oxylipins revealed no significant interaction effects. These 49 oxylipins were summed for a composite variable (
Figure 5). GLM analysis showed a significant time effect (
p < 0.001) of 2.25 h cycling on this composite variable of 49 oxylipins, but without trial differences (interaction effect,
p = 0.886). As explained in the methods section of this paper, two other composite variables were calculated that included eight pro-inflammatory oxylipins generated from arachidonic acid and cytochrome P-450 (ARA-CYP) and four abundant and pro-inflammatory oxylipins generated from linoleic acid (LA-DiHOMES+HODES) (
Figure 5). Significant time effects were shown for ARA-CYP and LA-DiHOMES+HODES (
p = 0.003 and
p < 0.001, respectively), but the pattern of change over time did not differ between the mango and water-only trials (
p = 0.610 and
p = 0.168, respectively).
4. Discussion
In this randomized crossover clinical trial with 22 trained male and female cyclists, 2-weeks intake of 330 grams per day of mangoes increased urine concentrations of three targeted gallotannin-related gut-derived phenolics. Substantial increases in plasma concentrations of pro-inflammatory oxylipins were measured following 2.25-h of intensive cycling. Contrary to our hypothesis, however, the pattern of change in these plasma oxylipins did not differ between the mango and water-only trials.
Gallotannins undergo catabolism by the gut microbiota, including hydrolysis and decarboxylation to produce gallic acid, pyrogallol, and catechol. These colonic metabolite products are then absorbed and become subject to phase-II metabolism to produce methylated, glucuronidated, and sulfated conjugates. Previous studies investigating polyphenol metabolism after mango consumption found several of these metabolites were increased in the plasma or urine of subjects after acute, short-term (10 days) and long-term (6 weeks) consumption of mango [
46,
47,
48]. Our study involved intake of a comparable mango dose for 2 weeks, and we expected to see similar results. Indeed, the three metabolites showing significant increases in our study, O-methylgallic acid-O-sulfate, O-methylpyrogallol-O-sulfate, and pyrogallol-O-sulfate (
Figure 2), showed similar increases in the plasma and urine of subjects who consumed mango in the other trials [
46,
47,
48].
Oxylipins are upstream regulators of many physiological processes including inflammation. These oxidized lipids are typically produced from the actions of CYP, COX, and LOX enzyme systems on PUFAs such as LA, ARA, EPA, and DHA. Oxylipins are not stored but are generated during a variety of physiological stressors including prolonged and vigorous exercise. Post-exercise plasma oxylipin concentrations are elevated for several hours, and the magnitude and duration of these responses shown in this study were similar to several other studies from our research group [
5,
7,
29,
30,
31,
49].
Oxylipin generation is a highly controlled process that is sensitive to both nutrition- and exercise-based interventions [
49,
50]. More than 50 enzymes are involved in producing oxylipins, and signaling is conducted by binding to a variety of receptors or by interacting with intracellular pathways [
50,
51,
52]. The enzymes that regulate oxylipin production and the receptors to which they bind are potential targets for metabolites derived from dietary macronutrients and polyphenols [
9,
10,
11,
12,
13,
14,
52]. Underlying mechanisms, however, are poorly understood with large scientific gaps regarding the linkage between dietary change and oxylipin generation. In previous studies, our research group has discovered that the increase of ARA-CYP- and LA-DiHOMES+HODES-derived oxylipins was strongly mitigated when cyclists consumed carbohydrate (both 6% carbohydrate sports beverages and bananas) before, during, and after prolonged and intensive cycling bouts [
5,
29]. Carbohydrate supplementation may influence CYP activity within an exercise stress context by mitigating changes in plasma concentrations of glucose, insulin, and IL-6 [
5,
29]. In the current study, pre-cycling mango-carbohydrate intake (about 25 g) was insufficient to significantly alter plasma glucose levels (
Figure 3).
More recently in two studies, we reported that 2-weeks intake of 1 cup/day blueberries mitigated plasma levels of ARA-CYP oxylipins in cyclists following 75-km cycling bouts, and increased intermediate EPA- and DHA-derived specialized pro-resolving mediators (SPMs) during a 4-day recovery period from eccentric exercise in untrained adults [
29,
30]. Emerging evidence supports a regulatory effect of dietary polyphenols on the enzyme systems that are involved in oxylipin production, but many of these studies are based on cell culture methods with the parent molecules instead of the biotransformed metabolities [
52]. Blueberries are rich in anthocyanins, and limited evidence indicates some linkage between gut-derived metabolites from these flavonoids and enzymatic activity related to selected oxylipin generation [
29,
30]. For example, we showed a modest but significant negative relationship between 1.5 h post-exercise plasma levels of blueberry metabolites (group of 24) and plasma levels of 10 ARA-CYP oxylipins [
29]. One in vitro study showed a mild effect of pyrogallol (a metabolite from gallotannins as found in mangoes) on specific CYPs [
53]. However, the actual blood and tissue levels of pyrogallol metabolites after mango ingestion may fall below the threshold needed to influence COX, LOX, and CYP-generated oxylipins, or the affected enzyme systems may not pertain to those activated in response to exercise stress.
5. Conclusions
This study showed that a large daily dose of mangoes over a 2-week supplementation period failed to alter exercise-induced increases in plasma oxylipins. These results differed from a study using a similar design but with blueberry fruit or bananas [
29]. Thus, mitigation of post-exercise plasma levels of oxylipins may depend on the specific macronutrient and polyphenol content of the fruit used during the 2-week pre-exercise intervention period.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Plasma oxylipins.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, D.C.N., M.A.L.; methodology, C.A.S., D.C.N., A.M.O., R.C.S., J.C.W., M.A.L., Q.Z.; formal analysis, C.A.S., D.C.N., A.M.O., R.C.S., Q.Z..; investigation, C.A.S., D.C.N.; resources, D.C.N., M.A.L., Q.Z.; data curation, C.A.S., D.C.N., A.M.O., R.C.S., Q.Z.; writing—original draft preparation, C.A.S., D.C.N.; writing—review and editing, C.A.S., D.C.N., A.M.O., R.C.S., J.C.W., M.A.L., Q.Z.; visualization, D.C.N., J.C.W., Q.Z.; supervision, C.A.S., D.C.N., M.A.L., Q.Z.; project administration, C.A.S., D.C.N.; funding acquisition, D.C.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Mango Board (Orlando, FL); grant number NMB COA: 603034.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Appalachian State University (protocol code 22-0205, approval date 28 March 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The primary outcome data (plasma oxylipins) are available as a supplementary
Table S1 at Preprints.org.
Acknowledgments
We acknowledge the support by the National Mango Board in providing the mangoes used in this research project.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Nieman, D.C.; Gillitt, N.D.; Henson, D.A.; Sha, W.; Shanely, R.A.; Knab, A.M.; Cialdella-Kam, L.; Jin, F. Bananas as an energy source during exercise: a metabolomics approach. PLoS One 2012, 7, e37479. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Sha, W.; Meaney, M.P.; John, C.; Pappan, K.L.; Kinchen, J.M. Metabolomics-based analysis of banana and pear ingestion on exercise performance and recovery. J Proteome Res 2015, 14, 5367–5377. [Google Scholar] [CrossRef]
- Nieman, D.C.; Davis, J.M.; Henson, D.A.; Walberg-Rankin, J.; Shute, M.; Dumke, C.L.; Utter, A.C.; Vinci, D.M.; Carson, J.A.; Brown, A.; et al. Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol (1985) 2003, 94, 1917–1925. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Sha, W.; Esposito, D.; Ramamoorthy, S. Metabolic recovery from heavy exertion following banana compared to sugar beverage or water only ingestion: a randomized, crossover trial. PLoS One 2018, 13, e0194843. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Chen, G.-Y.; Zhang, Q.; Sakaguchi, C.A.; Stephan, E.H. Carbohydrate intake attenuates post-exercise plasma levels of cytochrome P450-generated oxylipins. PLoS One 2019, 14, e0213676. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Knab, A.M.; Shanely, R.A.; Pappan, K.L.; Jin, F.; Lila, M.A. Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: a randomized trial using a metabolomics approach. PLoS One 2013, 8, e72215. [Google Scholar] [CrossRef]
- Nieman, D.C.; Omar, A.M.; Kay, C.D.; Kasote, D.M.; Sakaguchi, C.A.; Lkhagva, A.; Weldemariam, M.M.; Zhang, Q. Almond intake alters the acute plasma dihydroxy-octadecenoic acid (DiHOME) response to eccentric exercise. Front Nutr 2022, 9, 1042719. [Google Scholar] [CrossRef]
- Shanely, R.A.; Nieman, D.C.; Perkins-Veazie, P.; Henson, D.A.; Meaney, M.P.; Knab, A.M.; Cialdell-Kam, L. Comparison of watermelon and carbohydrate beverage on exercise-induced alterations in systemic inflammation, immune dysfunction, and plasma antioxidant capacity. Nutrients 2016, 8, 518. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in our understanding of oxylipins derived from dietary PUFAs. Adv Nutr 2015, 6, 513–540. [Google Scholar] [CrossRef]
- Caligiuri, S.P.B.; Parikh, M.; Stamenkovic, A.; Pierce, G.N.; Aukema, H.M. Dietary modulation of oxylipins in cardiovascular disease and aging. Am J Physiol Heart Circ Physiol 2017, 313, H903–H918. [Google Scholar] [CrossRef]
- Ostermann, A.I.; Schebb, N.H. Effects of omega-3 fatty acid supplementation on the pattern of oxylipins: a short review about the modulation of hydroxy-, dihydroxy-, and epoxy-fatty acids. Food Funct 2017, 8, 2355–2367. [Google Scholar] [CrossRef]
- Guijas, C.; Rodríguez, J.P.; Rubio, J.M.; Balboa, M.A.; Balsinde, J. Phospholipase A2 regulation of lipid droplet formation. Biochim Biophys Acta 2014, 1841, 1661–1671. [Google Scholar] [CrossRef]
- Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Selectivity of phospholipid hydrolysis by phospholipase A2 enzymes in activated cells leading to polyunsaturated fatty acid mobilization. Biochim Biophys Acta Mol Cell Biol Lipids 2019, 1864, 772–783. [Google Scholar] [CrossRef]
- Shearer, G.C.; Walker, R.E. An overview of the biologic effects of omega-6 oxylipins in humans. Prostaglandins Leukot Essent Fatty Acids 2018, 137, 26–38. [Google Scholar] [CrossRef]
- García-Flores, L.A.; Medina, S.; Gómez, C.; Wheelock, C.E.; Cejuela, R.; Martínez-Sanz, J.M.; Oger, C.; Galano, J.-M.; Durand, T.; Hernández-Sáez, Á.; et al. Aronia-citrus juice (polyphenol-rich juice) intake and elite triathlon training: a lipidomic approach using representative oxylipins in urine. Food Funct 2018, 9, 463–475. [Google Scholar] [CrossRef]
- Vella, L.; Markworth, J.F.; Farnfield, M.M.; Maddipati, K.R.; Russell, A.P.; Cameron-Smith, D. Intramuscular inflammatory and resolving lipid profile responses to an acute bout of resistance exercise in men. Physiol Rep 2019, 7, e14108. [Google Scholar] [CrossRef]
- Markworth, J.F.; Vella, L.; Lingard, B.S.; Tull, D.L.; Rupasinghe, T.W.; Sinclair, A.J.; Maddipati, K.R.; Cameron-Smith, D. Human inflammatory and resolving lipid mediator responses to resistance exercise and ibuprofen treatment. Am J Physiol Regul Integr Comp Physiol 2013, 305, R1281–1296. [Google Scholar] [CrossRef]
- Markworth, J.F.; D’Souza, R.F.; Aasen, K.M.M.; Mitchell, S.M.; Durainayagam, B.R.; Sinclair, A.J.; Peake, J.M.; Egner, I.M.; Raastad, T.; Cameron-Smith, D.; et al. Arachidonic acid supplementation transiently augments the acute inflammatory response to resistance exercise in trained men. J Appl Physiol (1985) 2018, 125, 271–286. [Google Scholar] [CrossRef]
- Rocic, P.; Schwartzman, M.L. 20-HETE in the Regulation of vascular and cardiac function. Pharmacol Ther 2018, 192, 74–87. [Google Scholar] [CrossRef]
- Waldman, M.; Peterson, S.J.; Arad, M.; Hochhauser, E. The role of 20-HETE in cardiovascular diseases and its risk factors. Prostaglandins Other Lipid Mediat 2016, 125, 108–117. [Google Scholar] [CrossRef]
- Hoxha, M.; Zappacosta, B. CYP-derived eicosanoids: Implications for rheumatoid arthritis. Prostaglandins Other Lipid Mediat 2020, 146, 106405. [Google Scholar] [CrossRef]
- Shoieb, S.M.; El-Sherbeni, A.A.; El-Kadi, A.O.S. Subterminal Hydroxyeicosatetraenoic Acids: Crucial Lipid Mediators in Normal Physiology and Disease States. Chem Biol Interact 2019, 299, 140–150. [Google Scholar] [CrossRef]
- Xu, X.; Li, R.; Chen, G.; Hoopes, S.L.; Zeldin, D.C.; Wang, D.W. The role of cytochrome P450 epoxygenases, soluble epoxide hydrolase, and epoxyeicosatrienoic acids in metabolic diseases. Adv Nutr 2016, 7, 1122–1128. [Google Scholar] [CrossRef]
- Dos Santos, L.R.B.; Fleming, I. Role of cytochrome P450-derived, polyunsaturated fatty acid mediators in diabetes and the metabolic syndrome. Prostaglandins Other Lipid Mediat 2020, 148, 106407. [Google Scholar] [CrossRef]
- Valdes, A.M.; Ravipati, S.; Pousinis, P.; Menni, C.; Mangino, M.; Abhishek, A.; Chapman, V.; Barrett, D.A.; Doherty, M. Omega-6 oxylipins generated by soluble epoxide hydrolase are associated with knee osteoarthritis. J Lipid Res 2018, 59, 1763–1770. [Google Scholar] [CrossRef]
- Morisseau, C.; Hammock, B.D. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol 2013, 53, 37–58. [Google Scholar] [CrossRef]
- Zhang, H.; Falck, J.R.; Roman, R.J.; Harder, D.R.; Koehler, R.C.; Yang, Z.-J. Upregulation of 20-HETE synthetic cytochrome P450 isoforms by oxygen-glucose deprivation in cortical neurons. Cell Mol Neurobiol 2017, 37, 1279–1286. [Google Scholar] [CrossRef]
- Knudsen, J.G.; Bertholdt, L.; Gudiksen, A.; Gerbal-Chaloin, S.; Rasmussen, M.K. Skeletal muscle interleukin-6 regulates hepatic cytochrome P450 expression: effects of 16-week high-fat diet and exercise. Toxicol Sci 2018, 162, 309–317. [Google Scholar] [CrossRef]
- Nieman, D.C.; Gillitt, N.D.; Chen, G.-Y.; Zhang, Q.; Sha, W.; Kay, C.D.; Chandra, P.; Kay, K.L.; Lila, M.A. Blueberry and/or banana consumption mitigate arachidonic, cytochrome P450 oxylipin generation during recovery from 75-Km cycling: a randomized trial. Front Nutr 2020, 7, 121. [Google Scholar] [CrossRef]
- Nieman, D.C.; Sakaguchi, C.A.; Omar, A.M.; Davis, K.L.; Shaffner, C.E.; Strauch, R.C.; Lila, M.A.; Zhang, Q. Blueberry intake elevates post-exercise anti-inflammatory oxylipins: a randomized trial. Sci Rep 2023, 13, 11976. [Google Scholar] [CrossRef]
- Nieman, D.C.; Woo, J.; Sakaguchi, C.A.; Omar, A.M.; Tang, Y.; Davis, K.; Pecorelli, A.; Valacchi, G.; Zhang, Q. Astaxanthin supplementation counters exercise-induced decreases in immune-related plasma proteins. Front Nutr 2023, 10, 1143385. [Google Scholar] [CrossRef]
- Kim, H.; Castellon-Chicas, M.J.; Arbizu, S.; Talcott, S.T.; Drury, N.L.; Smith, S.; Mertens-Talcott, S.U. Mango (Mangifera Indica L.) Polyphenols: anti-inflammatory intestinal microbial health benefits, and associated mechanisms of actions. Molecules 2021, 26, 2732. [Google Scholar] [CrossRef]
- Kim, H.; Venancio, V.P.; Fang, C.; Dupont, A.W.; Talcott, S.T.; Mertens-Talcott, S.U. Mango (Mangifera indica L.) polyphenols reduce IL-8, GRO, and GM-SCF plasma levels and increase Lactobacillus species in a pilot study in patients with inflammatory bowel disease. Nutr Res 2020, 75, 85–94. [Google Scholar] [CrossRef]
- Kim, H.; Banerjee, N.; Ivanov, I.; Pfent, C.M.; Prudhomme, K.R.; Bisson, W.H.; Dashwood, R.H.; Talcott, S.T.; Mertens-Talcott, S.U. Comparison of anti-inflammatory mechanisms of mango (Mangifera Indica L.) and pomegranate (Punica Granatum L.) in a preclinical model of colitis. Mol Nutr Food Res 2016, 60, 1912–1923. [Google Scholar] [CrossRef]
- Asuncion, P.; Liu, C.; Castro, R.; Yon, V.; Rosas, M.; Hooshmand, S.; Kern, M.; Hong, M.Y. The effects of fresh mango consumption on gut health and microbiome - randomized controlled trial. Food Sci Nutr 2023, 11, 2069–2078. [Google Scholar] [CrossRef]
- Rosas, M.; Pinneo, S.; O’Mealy, C.; Tsang, M.; Liu, C.; Kern, M.; Hooshmand, S.; Hong, M.Y. Effects of fresh mango consumption on cardiometabolic risk factors in overweight and obese adults. Nutr Metab Cardiovasc Dis 2022, 32, 494–503. [Google Scholar] [CrossRef]
- Pinneo, S.; O'Mealy, C; Rosas, M. Jr; Tsang, M.; Liu, C.; Kern, M.; Hooshmand, S.; Hong, M.Y. Fresh mango consumption promotes greater satiety and improves postprandial glucose and insulin responses in healthy overweight and obese adults. J Med Food 2022, 25, 381–388. [Google Scholar] [CrossRef]
- Cáceres-Jiménez, S.; Rodríguez-Solana, R.; Dobani, S.; Pourshahidi, K.; Gill, C.; Moreno-Rojas, J.M.; Almutairi, T.M.; Crozier, A.; Pereira-Caro, G. UHPLC-HRMS spectrometric analysis: method validation and plasma and urinary metabolite identification after mango pulp intake. J Agric Food Chem 2023, 71, 11520–11533. [Google Scholar] [CrossRef]
- Hartung, N.M.; Fischer, J.; Ostermann, A.I.; Willenberg, I.; Rund, K.M.; Schebb, N.H.; Garscha, U. Impact of food polyphenols on oxylipin biosynthesis in human neutrophils. Biochim Biophys Acta Mol Cell Biol Lipids 2019, 1864, 1536–1544. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Rasmussen, M.K.; Škrlep, M.; Batorek Lukač, N.; Škorjanc, D.; Čandek-Potokar, M. Tissue-specific regulation of CYP3A by hydrolysable tannins in male pigs. Xenobiotica 2016, 46, 591–596. [Google Scholar] [CrossRef]
- Basheer, L.; Kerem, Z. Interactions between CYP3A4 and dietary polyphenols. Oxid Med Cell Longev 2015, 2015, 854015. [Google Scholar] [CrossRef]
- Kampschulte, N.; Alasmer, A.; Empl, M.T.; Krohn, M.; Steinberg, P.; Schebb, N.H. Dietary polyphenols inhibit the cytochrome P450 monooxygenase branch of the arachidonic acid cascade with remarkable structure-dependent selectivity and potency. J Agric Food Chem 2020, 68, 9235–9244. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Zhang, Q. Comprehensive analysis of oxylipins in human plasma using reversed-phase liquid chromatography-triple quadrupole mass spectrometry with heatmap-assisted selection of transitions. Anal Bioanal Chem 2019, 411, 367–385. [Google Scholar] [CrossRef]
- Toora, B.D.; Rajagopal, G. Measurement of creatinine by Jaffe’s reaction--determination of concentration of sodium hydroxide required for maximum color development in standard, urine and protein free filtrate of serum. Indian J Exp Biol 2002, 40, 352–354. [Google Scholar]
- Nieman, D.C.; Kay, C.D.; Rathore, A.S.; Grace, M.H.; Strauch, R.C.; Stephan, E.H.; Sakaguchi, C.A.; Lila, M.A. Increased plasma levels of gut-derived phenolics linked to walking and running following two weeks of flavonoid supplementation. Nutrients 2018, 10. [Google Scholar] [CrossRef]
- Barnes, R.C.; Krenek, K.A.; Meibohm, B.; Mertens-Talcott, S.U.; Talcott, S.T. Urinary metabolites from mango (Mangifera indica L. cv. Keitt) galloyl derivatives and in vitro hydrolysis of gallotannins in physiological conditions. Mol Nutr Food Res 2016, 60, 542–550. [Google Scholar] [CrossRef]
- Barnes, R.C.; Kim, H.; Fang, C.; Bennett, W.; Nemec, M.; Sirven, M.A.; Suchodolski, J.S.; Deutz, N.; Britton, R.A.; Mertens-Talcott, S.U.; et al. Body mass index as a determinant of systemic exposure to gallotannin metabolites during 6-week consumption of mango (Mangifera Indica L.) and modulation of intestinal microbiota in lean and obese individuals. Mol Nutr Food Res 2019, 63, e1800512. [Google Scholar] [CrossRef]
- Fan, J.; Xiao, D.; Zhang, L.; Edirisinghe, I.; Burton-Freeman, B.; Sandhu, A.K. pharmacokinetic characterization of (poly)phenolic metabolites in human plasma and urine after acute and short-term daily consumption of mango pulp. Molecules 2020, 25, 5522. [Google Scholar] [CrossRef]
- Signini, É.F.; Nieman, D.C.; Silva, C.D.; Sakaguchi, C.A.; Catai, A.M. Oxylipin response to acute and chronic exercise: a systematic review. Metabolites 2020, 10. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.-M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: recent advances in the understanding of their biosynthesis, structures, and functions. Prog Lipid Res 2022, 86, 101165. [Google Scholar] [CrossRef]
- Gladine, C.; Fedorova, M. The clinical translation of eicosanoids and other oxylipins, although challenging, should be actively pursued. J Mass Spectrom Adv Clin Lab 2021, 21, 27–30. [Google Scholar] [CrossRef]
- Eccles, J.A.; Baldwin, W.S. Detoxification cytochrome P450s (CYPs) in families 1-3 produce functional oxylipins from polyunsaturated fatty acids. Cells 2022, 12, 82. [Google Scholar] [CrossRef]
- Mohos, V.; Fliszár-Nyúl, E.; Lemli, B.; Zsidó, B.Z.; Hetényi, C.; Mladěnka, P.; Horký, P.; Pour, M.; Poór, M. Testing the pharmacokinetic interactions of 24 colonic flavonoid metabolites with human serum albumin and cytochrome P450 enzymes. Biomolecules 2020, 10, 409. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).