Submitted:
23 November 2023
Posted:
29 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
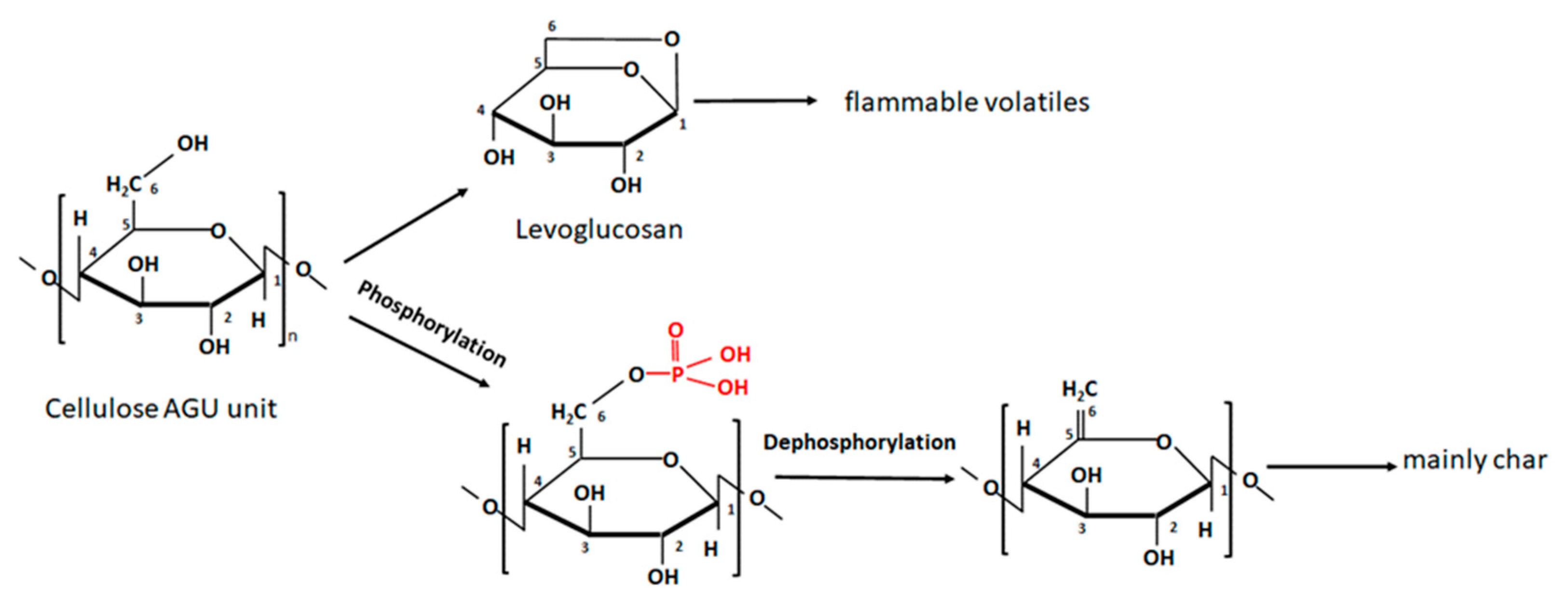
Methods of Nanocellulose Phosphorylation
2. Combination of Conventional Fire Retardants with Nanosized Additives
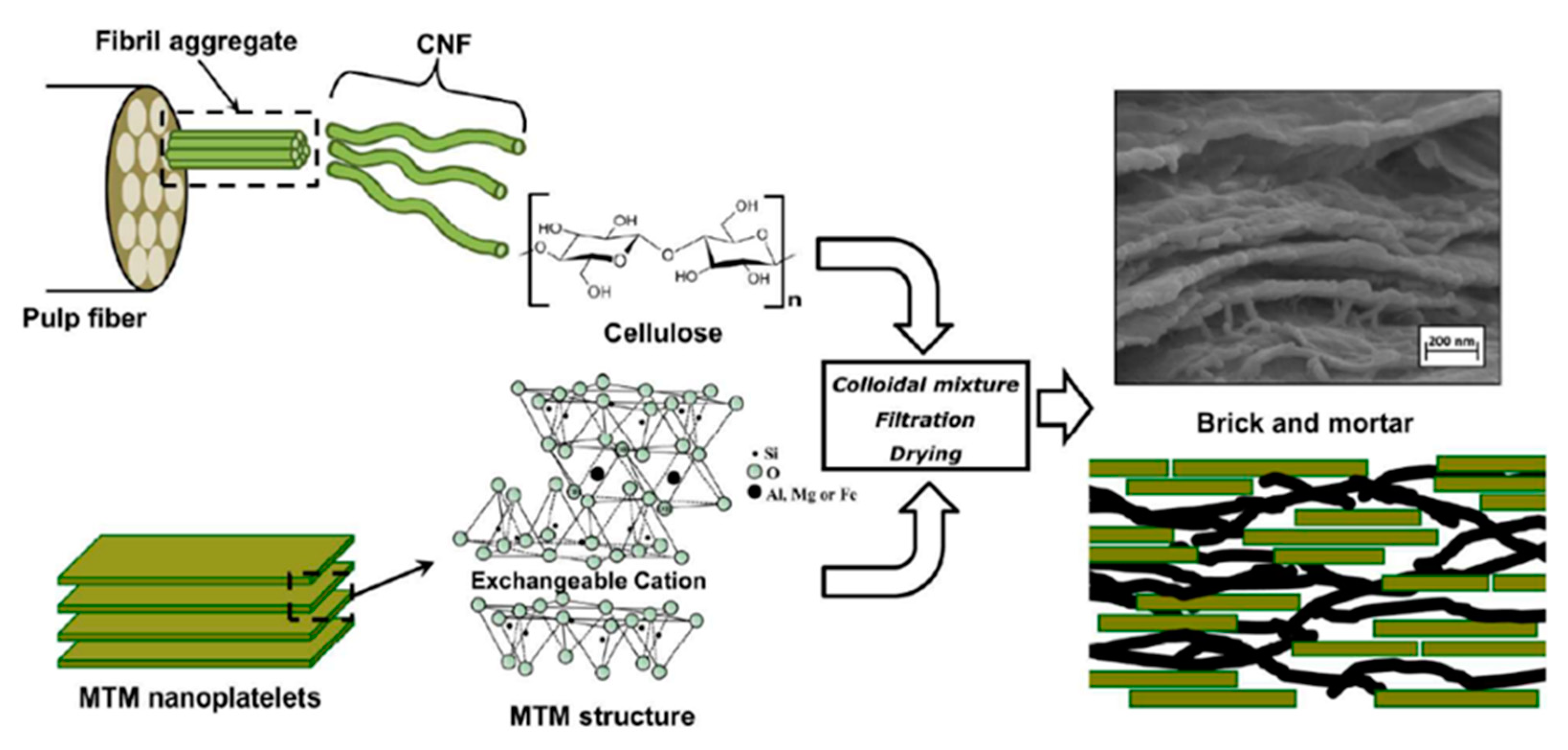
2.1. Nanoclays Case Study
2.2. 2D Carbon-, Black Phosphorus- and MXene—Based Nanomaterials
3. Potential of Lignin and Chitosan in Cellulose-Based FR Coatings
4. Aerogels and Foams with Improved Fire Resistance
4.1. Effect of Cellulose Grade/Modification
4.2. Effect of Nanoclays, Metal-Containing, Graphene Oxide and Conventional FR
4.3. Layer-by-Layer Deposition of FR Coatings—Effect of Chitosan
4.4. Alginate- and Tannin-Based FR Foams
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Ferrer, A.; Tyagi, P.; Yin, Y.; Salas, L.P.; Rojas, O.J. Nanocellulose in thin films, coatings, and plies for packaging applications: A Review. BioResources 2017, 12, 2143–2233. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Oksman, K.; Aitomäki, Y.; Mathew, A.; Siqueira, G.; Zhow, Q.; Butylina, S.; Tanpichai, S.; Zhou, X.; Hooshmand, S. Review of the recent developments in cellulose nanocomposite processing. Compos Part A Appl Sci Manuf 2016, 83, 2–18. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem Soc Rev 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Vanderfleet, O.M.; Cranston, E.D. Production routes to tailor the performance of cellulose nanocrystals. Nat. Rev. Mater. 2021, 6, 124–144. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, W.; Ciesielski, P.N.; Fang, Z.; Zhu, J.Y.; Henriksson, G.; Himmel, M.E.; Hu, L. Wood-Derived Materials for Green Electronics, Biological Devices, and Energy Applications. Chem. Rev. 2016, 116, 9305–9374. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. —Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Sirviö, J.A.; Hasa, T.; Ahola, J.; Liimatainen, H.; Niinimäki, J.; Hormi, O. Phosphonated nanocelluloses from sequential oxidative-reductive treatment—Physicochemical characteristics and thermal properties. Carbohydr Polym 2015, 133, 524–532. [Google Scholar] [CrossRef]
- Ghanadpour, M.; Carosio, F.; Larsson, P.T.; Wågberg, L. Phosphorylated Cellulose Nanofibrils: A Renewable Nanomaterial for the Preparation of Intrinsically Flame-Retardant Materials. Biomacromolecules 2015, 16, 3399–3410. [Google Scholar] [CrossRef]
- Hou, G.; Zhao, S.; Li, Y.; Fang, Z.; Isogai, A. Mechanically robust, flame-retardant phosphorylated cellulose films with tunable optical properties for light management in LEDs. Carbohydr Polym 2022, 298, 120129. [Google Scholar] [CrossRef] [PubMed]
- Qing, Y.; Sabo, R.; Zhu, J.Y.; Agarwal U., Z. Cai Z., Wu Y. A comparative study of cellulose nanofibrils disintegrated via multiple processing approaches. Carbohydr Polym 2013, 97, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Rol, F.; Belgacem, N.M.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Progress in Polymer Science 2019, 88. [Google Scholar] [CrossRef]
- Zhang, S.; Li, S.N.; Wu, Q.; Li, Q.; Huang, J.; Li, W.; Zhang, W.; Wang, S. Phosphorus containing group and lignin toward intrinsically flame retardant cellulose nanofibril-based film with enhanced mechanical properties. Compos B Eng 2021, 212, 108699. [Google Scholar] [CrossRef]
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar] [CrossRef]
- Wågberg, L.; Decher, G.; Norgren, M.; Lindström, T.; Ankerfors, M.; Axnäs, K. The build-up of polyelectrolyte multilayers of microfibrillated cellulose and cationic polyelectrolytes. Langmuir 2008, 24, 784–795. [Google Scholar] [CrossRef]
- Henriksson M.; L. A. Berglund, P. Isaksson, T. Lindström, and T. Nishino. Cellulose nanopaper structures of high toughness. Biomacromolecules 2008, 9, 1579–1585. [CrossRef]
- Noguchi, Y.; Homma, I.; Matsubara, Y. Complete nanofibrillation of cellulose prepared by phosphorylation. Cellulose 2017, 24, 1295–1305. [Google Scholar] [CrossRef]
- Rol, F.; Belgacem, N.; Meyer V., Petit-Conil M.; Bras, J. Production of fire-retardant phosphorylated cellulose fibrils by twin-screw extrusion with low energy consumption. Cellulose 2019, 26, 5635–5651. [Google Scholar] [CrossRef]
- Šturcová, A.; Davies, G.R.; Eichhorn, S.J. Elastic modulus and stress-transfer properties of tunicate cellulose whiskers. Biomacromolecules 2005, 6, 1055–1061. [Google Scholar] [CrossRef]
- Lazar, S.T.; Kolibaba, T.J.; Grunlan, J.C. Flame-retardant surface treatments. Nat. Rev. Mater. 2020, 5, 259–275. [Google Scholar] [CrossRef]
- Donius A.E., Liu A., Berglund L.A., Wegst U.G.K. Superior mechanical performance of highly porous, anisotropic nanocellulose-montmorillonite aerogels prepared by freeze casting. J Mech Behav Biomed Mater 2014, 37, 88–99. [CrossRef] [PubMed]
- Candidate list of substances of very high concern for authorisation. European Chemical agency ECHA.
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- www.flameretardants-online.com/flame-retardants/market.
- Hull, T.R.; Witkowski, A.; Hollingbery, L. Fire retardant action of mineral fillers. Polym Degrad Stab 2011, 96, 1462–1469. [Google Scholar] [CrossRef]
- Scharte, B. Phosphorus-based flame retardancy mechanisms-old hat or a starting point for future development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [PubMed]
- Özer, M.S.; Gaan, S. Recent developments in phosphorus based flame retardant coatings for textiles: Synthesis, applications and performance. Prog Org Coat 2022, 171, 107027. [Google Scholar] [CrossRef]
- Weil, E.D. Fire-protective and flame-retardant coatings—A state-of-the-art review. J. Fire Sci. 2011, 29, 259–296. [Google Scholar] [CrossRef]
- Schartel, B.; Braun, U.; Schwarz, U.; Reinemann, S. Fire retardancy of polypropylene/flax blends. Polymer 2003, 44, 6241–6250. [Google Scholar] [CrossRef]
- Seefeldt, H.; Braun, U.; Wagner, M.H. Residue stabilization in the fire retardancy of wood-plastic composites: Combination of ammonium polyphosphate, expandable graphite, and red phosphorus. Macromol Chem Phys 2012, 213, 2370–2377. [Google Scholar] [CrossRef]
- Ishikawa, T.; Mizuno, K.; Kajiya, T.; Maki, I.; Koshizuka, T.; Takeda, K. Structural decay and flame retardancy of wood as a natural polymer. Combust. Sci. Technol. 2005, 177, 819–842. [Google Scholar] [CrossRef]
- Kashiwagi T.; Harris R.H.; Zhang X., Briber R.M., Cipriano B.H., Raghavan SR.; et al. Flame retardant mechanism of polyamide 6-clay nanocomposites. Polymer 2004, 45, 881–891. [CrossRef]
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Harris, R.; Manias, E.; Giannelis, E.P.; et al. Flammability properties of polymer—Layered-silicate nanocomposites. Polypropylene and polystyrene nanocomposites. Chem Mater 2000, 12, 1866–1873. [Google Scholar] [CrossRef]
- Fu, Q.; Medina, L.; Li, Y.; Carosio, F.; Hajian, A.; Berglund, L.A. Nanostructured Wood Hybrids for Fire-Retardancy Prepared by Clay Impregnation into the Cell Wall. ACS Appl Mater Interfaces 2017, 9, 36154–36163. [Google Scholar] [CrossRef]
- Chen, G.; Chen, C.; Pei, Y.; He, S.; Liu, Y.; Jiang, B.; et al. A strong, flame-retardant, and thermally insulating wood laminate. Chem. Eng. J. 2020, 383, 123109. [Google Scholar] [CrossRef]
- Guo, G.; Park, C.B.; Lee, Y.H.; Kim, Y.S.; Sain, M. Flame retarding effects of nanoclay on wood-fiber composites. Polym Eng Sci 2007, 47, 330–336. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kuboki, T.; Park, C.B.; Sain, M.; Kontopoulou, M. The effects of clay dispersion on the mechanical, physical, and flame-retarding properties of wood fiber/polyethylene/clay nanocomposites. J Appl Polym Sci 2010, 118, 452–461. [Google Scholar] [CrossRef]
- Kashiwagi T., Du F., Winey K.I., Groth K.M., Shields J.R., Bellayer S.P.; et al. Flammability properties of polymer nanocomposites with single-walled carbon nanotubes: Effects of nanotube dispersion and concentration. Polymer 2005, 46, 471–481. [CrossRef]
- Cabello-Alvarado, C.; Reyes-Rodríguez, P.; Andrade-Guel, M.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Cruz-Delgado, V.J.; et al. Melt-mixed thermoplastic nanocomposite containing carbon nanotubes and titanium dioxide for flame retardancy applications. Polymers 2019, 11, 1204. [Google Scholar] [CrossRef] [PubMed]
- Grexa, O.; Poutch, F.; Manikova, D.; Martvonova, H.; Bartekova, A. Intumescence in fire retardancy of lignocellulosic panels. Polym Degrad Stab 2003, 82, 373–377. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Adelnia, H.; Gudarzi, M.M. Intumescent flame retardant polyurethane/reduced graphene oxide composites with improved mechanical, thermal, and barrier properties. J Mater Sci 2014, 49, 243–254. [Google Scholar] [CrossRef]
- Esmailpour, A.; Majidi, R.; Taghiyari, H.R.; Ganjkhani, M.; Armaki, S.M.M.; Papadopoulos, A.N. Improving fire retardancy of beechwood by graphene. Polymers 2020, 12, 303. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, D.S.; Rehovsky, C.; Shojaeiarani, J.; Stark, N.; Bajwa, S.; Dietenberger, M.A. Functionalized cellulose nanocrystals: A potential fire retardant for polymer composites. Polymers 2019, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Bueno, A.B.F.; Bañón, M.V.N.; De Morentín, L.M.; García, J.M. Treatment of natural wood veneers with nano-oxides to improve their fire behaviour. IOP Conf. Ser. Mater. Sci. Eng. 2014. [Google Scholar] [CrossRef]
- Ren, D.; Li, J.; Xu J., Wu Z.; Bao, Y.; Li, N.; et al. Efficient antifungal and flame-retardant properties of ZnO-TiO2-layered double-nanostructures coated on bamboo substrate. Coatings 2018, 8, 341. [Google Scholar] [CrossRef]
- Deraman, A.F.; Chandren, S. Fire-retardancy of wood coated by titania nanoparticles. AIP Conf. Proceedings. Am. Inst. Phys. Inc. 2019, 2155, 020022. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Gilman, J.W.; Butler, K.M.; Harris, R.H.; Shields, J.R.; Asano, A. Flame retardant mechanism of silica gel/silica. Fire Mater 2000, 24, 277–289. [Google Scholar] [CrossRef]
- Alongi, J.; Carletto, R.A.; Di Blasio, A.; Cuttica, F.; Carosio, F.; Bosco, F.; et al. Intrinsic intumescent-like flame retardant properties of DNA-treated cotton fabrics. Carbohydr Polym 2013, 96, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Bosco, F.; Carletto, R.A.; Alongi, J.; Marmo, L.; Di Blasio, A.; Malucelli, G. Thermal stability and flame resistance of cotton fabrics treated with whey proteins. Carbohydr Polym 2013, 94, 372–377. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xuan, S.; Xing, W.; Bai, Z.; et al. Flame retardancy and thermal degradation of intumescent flame retardant poly(lactic acid)/starch biocomposites. Ind Eng Chem Res 2011, 50, 713–720. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Malucelli, G. Flame-retardant systems based on chitosan and its derivatives: State of the art and perspectives. Molecules 2020, 25, 4046. [Google Scholar] [CrossRef]
- Réti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability properties of intumescent PLA starch and lignin. Polym Adv Technol 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Sykam, K.; Försth, M.; Sas, G.; Restás Á., Das O. Phytic acid: A bio-based flame retardant for cotton and wool fabrics. Ind Crops Prod 2021, 164, 113349. [Google Scholar] [CrossRef]
- Božič, M.; Liu, P.; Mathew, A.P.; Kokol, V. Enzymatic phosphorylation of cellulose nanofibers to new highly-ions adsorbing, flame-retardant and hydroxyapatite-growth induced natural nanoparticles. Cellulose 2014, 21, 2713–2726. [Google Scholar] [CrossRef]
- Wu, M.; Huang, Y.; Zhang, T.; Kuga, S.; Ewulonu, C.M. Cellulose nanofibril-based flame retardant and its application to paper. ACS Sustain Chem Eng 2020, 8, 10222–10229. [Google Scholar]
- Fiss, B.G.; Hatherly, L.; Stein, R.S.; Friščić, T.; Moores, A. Mechanochemical Phosphorylation of Polymers and Synthesis of Flame-Retardant Cellulose Nanocrystals. ACS Sustain Chem Eng 2019, 7, 7951–7959. [Google Scholar] [CrossRef]
- Khakalo, A.; Jaiswal, A.K.; Kumar, V.; Gestranius, M.; Kangas, H.; Tammelin, T. Production of High-Solid-Content Fire-Retardant Phosphorylated Cellulose Microfibrils. ACS Sustain Chem Eng 2021, 9, 12365–12375. [Google Scholar] [CrossRef]
- Kokol, V.; Božič, M.; Vogrinčič, R.; Mathew, A.P. Characterisation and properties of homo- and heterogenously phosphorylated nanocellulose. Carbohydr Polym 2015, 125, 301–313. [Google Scholar] [CrossRef]
- Kang, K.Y.; Kim, D.Y. Influence of sulfuric acid impregnation on the carbonization of cellulose. J. Korean Phys. Soc. 2012, 60, 1818–1822. [Google Scholar] [CrossRef]
- Mishra, P.; Pavelek, O.; Rasticova, M.; Mishra, H.; Ekielski, A. Nanocellulose-Based Biomedical Scaffolds in Future Bioeconomy: A Techno-Legal Assessment of the State-of-the-Art. Front. Bioeng. Biotechnol. 2022, 9. [Google Scholar] [CrossRef]
- Lecoeur, E.; Vroman, I.; Bourbigot, S.; Lam T.M., Delobel R. Flame retardant formulations for cotton. Polym Degrad Stab 2001, 74, 487–492. [Google Scholar] [CrossRef]
- Ghanadpour M.; Carosio F., Ruda M.C., Wågberg L. Tuning the Nanoscale Properties of Phosphorylated Cellulose Nanofibril-Based Thin Films to Achieve Highly Fire-Protecting Coatings for Flammable Solid Materials. ACS Appl Mater Interfaces 2018, 10, 32543–32555. [CrossRef]
- Inagaki N.; Nakamura S.; Asai H.; Katsuura K. Phosphorylation of Cellulose with Phosphorous Acid and Thermal Degradation of the Product. J Appl Polym Sci 1976, 20. [CrossRef]
- Suflet, D.M.; Chitanu, G.C.; Popa, VI. Phosphorylation of polysaccharides: New results on synthesis and characterisation of phosphorylated cellulose. React Funct Polym 2006, 66, 1240–1249. [Google Scholar] [CrossRef]
- Ablouh, E.H.; Brouillette, F.; Taourirte, M.; Sehaqui, H.; El Achaby, M.; Belfkira, A. A highly efficient chemical approach to producing green phosphorylated cellulosic macromolecules. RSC Adv 2021, 11, 24206–24216. [Google Scholar] [CrossRef]
- Rol F., Sillard C., Bardet M., Yarava J.R., Emsley L., Gablin C.; et al. Cellulose phosphorylation comparison and analysis of phosphorate position on cellulose fibers. Carbohydr Polym 2020, 229. [CrossRef] [PubMed]
- Shi, Y.; Belosinschi, D.; Brouillette, F.; Belfkira, A.; Chabot, B. Phosphorylation of Kraft fibers with phosphate esters. Carbohydr Polym 2014, 106, 121–127. [Google Scholar] [CrossRef]
- Antoun, K.; Ayadi, M.; El Hage, R.; Nakhl, M.; Sonnier, R.; Gardiennet , C.; et al. Renewable phosphorous-based flame retardant for lignocellulosic fibers. Ind Crops Prod 2022, 186, 115265. [Google Scholar] [CrossRef]
- Yuan, H.B.; Tang, R.C; Yu, C.B. Flame retardant functionalization of microcrystalline cellulose by phosphorylation reaction with phytic acid. Int J Mol Sci 2021, 22, 9631. [Google Scholar] [CrossRef]
- Gospodinova, N.; Grelard, A.; Jeannin, M.; Chitanu, G.C.; Carpov, A.; Thiéry, V.; et al. Efficient solvent-free microwave phosphorylation of microcrystalline cellulose. Green Chem. 2002, 4, 220–222. [Google Scholar] [CrossRef]
- Ghanadpour M., Carosio F., Wågberg L. Ultrastrong and flame-resistant freestanding films from nanocelluloses, self-assembled using a layer-by-layer approach. Appl Mater Today 2017, 9, 229–239. [CrossRef]
- Rol F., Karakashov B., Nechyporchuk O., Terrien M., Meyer V., Dufresne A.; et al. Pilot-Scale Twin Screw Extrusion and Chemical Pretreatment as an Energy-Efficient Method for the Production of Nanofibrillated Cellulose at High Solid Content. ACS Sustain Chem Eng 2017, 5, 6524–6531. [CrossRef]
- Inc. U.I., Standard UL-94: Test for flammability of plastic materials for parts in devices and appliances, 2006, Northbrook, II.
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molekulare Brandbekämpfung—Wie moderne Phosphorchemie zur Lösung der Flammschutzaufgabe beitragen kann. Angew. Chem. 2018, 130, 10608–106026. [Google Scholar] [CrossRef]
- Carosio, F.; Kochumalayil, J.; Cuttica, F.; Camino, G.; Berglund, L. Oriented Clay Nanopaper from Biobased Components—Mechanisms for Superior Fire Protection Properties. ACS Appl Mater Interfaces 2015, 7, 5847–5856. [Google Scholar] [CrossRef] [PubMed]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym Sci 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Liu A., Walther A., Ikkala O., Belova L., Berglund L.A. Clay nanopaper with tough cellulose nanofiber matrix for fire retardancy and gas barrier functions. Biomacromolecules 2011, 12, 633–641. [CrossRef]
- Carosio F, Cuttica F, Medina L, Berglund LA. Clay nanopaper as multifunctional brick and mortar fire protection coating-Wood case study. Mater Des 2016, 93, 357–363. [CrossRef]
- Carosio F., Kochumalayil J., Fina A., Berglund L.A. Extreme thermal shielding effects in nanopaper based on multilayers of aligned clay nanoplatelets in cellulose nanofiber matrix. Adv Mater Interfaces 2016, 3, 1600551. [CrossRef]
- Ming S., Chen G., He J., Kuang Y., Liu Y., Tao R.; et al. Highly transparent and self-extinguishing nanofibrillated cellulose-monolayer clay nanoplatelet hybrid films. Langmuir 2017, 33, 8455–8462. [CrossRef]
- Qin, S.; Pour, M.G.; Lazar, S.; Köklükaya, O.; Gerringer, J.; Song, Y.; et al. Super gas barrier and fire resistance of nanoplatelet/nanofibril multilayer thin films. Adv Mater Interfaces 2019, 6, 1801424. [Google Scholar] [CrossRef]
- Santos, L.P; Da Silva, D.S; Morari, T.H.; Galembeck, F. Environmentally friendly, high-performance fire retardant made from cellulose and graphite. Polymers 2021, 13, 2400. [Google Scholar] [CrossRef] [PubMed]
- ISO 1182:2020 Reaction to fire tests for products—Non-combustibility test.
- Miao, Y.; Wang, X.; Liu, Y.; Liu, Z.; Chen, W. Preparation of graphene oxide/cellulose composites with microcrystalline cellulose acid hydrolysis using the waste acids generated by the hummers method of graphene oxide synthesis. Polymers 2021, 13, 4453. [Google Scholar] [CrossRef] [PubMed]
- Higginbotham, A.L.; Lomeda, J.R.; Morgan A.B., Tour J.M. Graphite oxide flame-retardant polymer nanocomposites. ACS Appl Mater Interfaces 2009, 1, 2256–2261. [Google Scholar] [CrossRef] [PubMed]
- Zhang Z., Yang D., Yang H., Li Y., Lu S., Cai R.; et al. A Hydrophobic Sisal Cellulose Microcrystal Film for Fire Alarm Sensors. Nano Lett 2021, 21, 2104–2110. [CrossRef] [PubMed]
- Cao, C.F.; Yu, B.; Guo, B.F.; Hu, W.J.; Sun, F.N.; Zhang, Z.H.; et al. Bio-inspired, sustainable and mechanically robust graphene oxide-based hybrid networks for efficient fire protection and warning. Chem. Eng. J. 2022, 439, 134516. [Google Scholar] [CrossRef]
- Qiu, S.; Ren, X.; Zhou, X.; Zhang, T.; Song, L.; Hu, Y. Nacre-Inspired Black Phosphorus/Nanofibrillar Cellulose Composite Film with Enhanced Mechanical Properties and Superior Fire Resistance. ACS Appl Mater Interfaces 2020, 12, 36639–36651. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Lai, X.; Li H., Gao J.; Zeng, X.; Huang, X.; et al. A highly efficient flame retardant nacre-inspired nanocoating with ultrasensitive fire-warning and self-healing capabilities. Chem. Eng. J. 2019, 369, 8–17. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhao, Y.; Lai, X.; Jiang, C.; Wang B., Li H.; et al. Skin-inspired multifunctional MXene/cellulose nanocoating for smart and efficient fire protection. Chem. Eng. J 2022, 446, 136899. [Google Scholar] [CrossRef]
- Chollet B., Lopez-Cuesta J.M.; Laoutid, F.; Ferry, L. Lignin nanoparticles as a promising way for enhancing lignin flame retardant effect in polylactide. Materials 2019, 12, 2132. [Google Scholar] [CrossRef]
- Wu, Q.; Ran, F.; Dai, L.; Li, C.; Li, R.; Si, C. A functional lignin-based nanofiller for flame-retardant blend. Int J Biol Macromol 2021, 190, 390–395. [Google Scholar] [CrossRef]
- Dai, P.; Liang, M.; Ma, X.; Luo, Y.; He, M.; Gu, X.; et al. Highly Efficient, Environmentally Friendly Lignin-Based Flame Retardant Used in Epoxy Resin. ACS Omega 2020, 5, 32084–32093. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Li, D.; Ek, M. Improving fire retardancy of cellulosic thermal insulating materials by coating with bio-based fire retardants. Nord Pulp Pap. Res J 2019, 34, 96–106. [Google Scholar] [CrossRef]
- Han, T.; Sophonrat, N.; Evangelopoulos, P.; Persson, H.; Yang W., Jönsson P. Evolution of sulfur during fast pyrolysis of sulfonated Kraft lignin. J Anal Appl Pyrolysis 2018, 133, 162–168. [Google Scholar] [CrossRef]
- Tong, C.; Zhang, S.; Zhong, T.; Fang, Z.; Liu, H. Highly fibrillated and intrinsically flame-retardant nanofibrillated cellulose for transparent mineral filler-free fire-protective coatings. Chem. Eng. J. 2021, 419, 129440. [Google Scholar] [CrossRef]
- Soares Bilhalva Dos Santos, P.; Fuentes Da Silva, S.; Labidi, J.; Gatto, D. Fire resistance of wood treated by emulsion from kraft lignin. Drewno 2016, 59, 199–204. [Google Scholar] [CrossRef]
- Uddin, K.M.A.; Ago, M.; Rojas, O.J. Hybrid films of chitosan, cellulose nanofibrils and boric acid: Flame retardancy, optical and thermo-mechanical properties. Carbohydr Polym 2017, 177, 13–21. [Google Scholar] [CrossRef]
- Pan, H.; Song, L.; Ma, L.; Pan, Y.; Liew, K.M.; Hu, Y. Layer-by-layer assembled thin films based on fully biobased polysaccharides: Chitosan and phosphorylated cellulose for flame-retardant cotton fabric. Cellulose 2014, 21, 2995–3006. [Google Scholar] [CrossRef]
- Carosio, F.; Ghanadpour M., Alongi J.; Wågberg, L. Layer-by-layer-assembled chitosan/phosphorylated cellulose nanofibrils as a bio-based and flame protecting nano-exoskeleton on PU foams. Carbohydr Polym 2018, 202, 479–487. [Google Scholar] [CrossRef]
- Köklükaya, O.; Carosio, F.; Wågberg, L. Superior Flame-Resistant Cellulose Nanofibril Aerogels Modified with Hybrid Layer-by-Layer Coatings. ACS Appl Mater Interfaces 2017, 9, 29082–29092. [Google Scholar] [CrossRef]
- Dorez, G.; Ferry L., Sonnier R.; Taguet, A.; Lopez-Cuesta, J.M. Effect of cellulose, hemicellulose and lignin contents on pyrolysis and combustion of natural fibers. J Anal Appl Pyrolysis 2014, 107, 323–331. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, H.; Shen, L.; Fang, Z.; Zhang, X.; Wang, J.; et al. Chitosan/phytic acid polyelectrolyte complex: A green and renewable intumescent flame retardant system for ethylene-vinyl acetate copolymer. Ind Eng Chem Res 2014, 53, 19199–191207. [Google Scholar] [CrossRef]
- Moussout H, Ahlafi H, Aazza M, Bourakhouadar M. Kinetics and mechanism of the thermal degradation of biopolymers chitin and chitosan using thermogravimetric analysis. Polym Degrad Stab 2016, 130, 1–9. [CrossRef]
- Cogollo-Herrera, K.; Bonfante-Álvarez, H.; De Ávila-Montiel, G.; Barros, A.H.; González-Delgado, Á.D. Techno-economic sensitivity analysis of large scale chitosan production process from shrimp shell wastes. Chem Eng Trans 2018, 70, 2179–2184. [Google Scholar]
- Niu, F.; Wu N., Yu J.; Ma, X. Gelation, flame retardancy, and physical properties of phosphorylated microcrystalline cellulose aerogels. Carbohydr Polym 2020, 242, 116422. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Yu, Z.; Zhu, J.; Zhang, Y.; Ren, X.; Jiang, F. Developing flame-retardant lignocellulosic nanofibrils through reactive deep eutectic solvent treatment for thermal insulation. Chem. Eng. J. 2022, 445, 136748. [Google Scholar] [CrossRef]
- Kim, H.; Park J., Minn K.S.; Pak, S.Y.; Lee, D.; Youn, J.R.; et al. Flame retardant composite foam modified by silylated nanocellulose and tris(2-chloropropyl) phosphate. Fibers Polym. 2019, 20, 2280–2288. [Google Scholar] [CrossRef]
- Carosio, F.; Medina, L.; Kochumalayil, J.; Berglund, L.A. Green and fire resistant nanocellulose/hemicellulose/clay foams. Adv Mater Interfaces 2021, 8, 2101111. [Google Scholar] [CrossRef]
- Wang, L.; Sánchez-Soto, M. Green bio-based aerogels prepared from recycled cellulose fiber suspensions. RSC Adv 2015, 5, 31384–31391. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, X.; Wu, X.; Lu, C. Flame Retardant, Heat Insulating Cellulose Aerogels from Waste Cotton Fabrics by in Situ Formation of Magnesium Hydroxide Nanoparticles in Cellulose Gel Nanostructures. ACS Sustain Chem Eng 2015, 3, 1853–1859. [Google Scholar] [CrossRef]
- He, C.; Huang, J.; Li, S.; Meng, K.; Zhang, L.; Chen, Z.; et al. Mechanically Resistant and Sustainable Cellulose-Based Composite Aerogels with Excellent Flame Retardant, Sound-Absorption, and Superantiwetting Ability for Advanced Engineering Materials. ACS Sustain Chem Eng 2018, 6, 927–936. [Google Scholar] [CrossRef]
- Farooq, M.; Sipponen, M.H.; Seppälä, A.; Österberg, M. Eco-friendly Flame-Retardant Cellulose Nanofibril Aerogels by Incorporating Sodium Bicarbonate. ACS Appl Mater Interfaces 2018, 10, 27407–27415. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, X.; Zhang, P.; Liu, J.; Song, L.; Hu, Y. Nano-fibrillated cellulose-hydroxyapatite based composite foams with excellent fire resistance. Carbohydr Polym 2018, 195, 71–78. [Google Scholar] [CrossRef]
- Huang, C.; Bhagia, S.; Hao, N.; Meng, X.; Liang, L.; Yong, Q.; et al. Biomimetic composite scaffold from an in situ hydroxyapatite coating on cellulose nanocrystals. RSC Adv 2019, 9, 5786–5793. [Google Scholar] [CrossRef]
- Yang, L.; Mukhopadhyay, A.; Jiao, Y.; Yong, Q.; Chen, L.; Xing, Y.; et al. Ultralight, highly thermally insulating and fire resistant aerogel by encapsulating cellulose nanofibers with two-dimensional MoS2. Nanoscale 2017, 9, 11452–11462. [Google Scholar] [CrossRef] [PubMed]
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; et al. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat Nanotechnol 2015, 10, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Wicklein, B.; Kocjan, D.; Carosio, F.; Camino, G.; Bergström, L. Tuning the nanocellulose-borate interaction to achieve highly flame retardant hybrid materials. Chem. Mater. 2016, 28, 1985–1989. [Google Scholar] [CrossRef]
- Cheng, X.; Zhu, S.; Pan, Y.; Deng, Y.; Shi, L.; Gong, L. Fire retardancy and thermal behaviors of Cellulose nanofiber/zinc borate aerogel. Cellulose 2020, 27, 7463–7474. [Google Scholar] [CrossRef]
- Guo, W.; Hu, Y.; Wang, X.; Zhang, P.; Song L., Xing W. Exceptional flame-retardant cellulosic foams modified with phosphorus-hybridized graphene nanosheets. Cellulose 2019, 26, 1247–1260. [Google Scholar] [CrossRef]
- Ghanadpour M., Wicklein B., Carosio F., Wågberg L. All-natural and highly flame-resistant freeze-cast foams based on phosphorylated cellulose nanofibrils. Nanoscale 2018, 10, 4085–4095. [CrossRef]
- Fan, B.; Chen, S.; Yao, Q.; Sun, Q.; Jin, C. Fabrication of cellulose nanofiber/AlOOH aerogel for flame retardant and thermal insulation. Materials 2017, 10, 311. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, J.; Yu, J.; Song, R.; Mi, Q.; He, J.; et al. Transparent and flame retardant cellulose/aluminum hydroxide nanocomposite aerogels. Sci China Chem 2016, 59, 1335–1341. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, J.; Mi, Q.; Yu, J.; Song, R.; Zhang, J. Transparent cellulose-silica composite aerogels with excellent flame retardancy via an in situ sol-gel process. ACS Sustain Chem Eng 2017, 5, 11117–11123. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, Q.; Shen, X.; Xia, Y.; Tan, L.; Kong, Q. Pyrolysis products and thermal degradation mechanism of intrinsically flame-retardant calcium alginate fibre. Polym Degrad Stab 2011, 96, 936–942. [Google Scholar] [CrossRef]
- Berglund L, Nissilä T, Sivaraman D, Komulainen S, Telkki VV, Oksman K. Seaweed-derived alginate-cellulose nanofiber aerogel for insulation applications. ACS Appl Mater Interfaces 2021, 13, 34899–34909. [CrossRef] [PubMed]
- Missio, A.L.; Otoni, C.G.; Zhao, B.; Beaumont, M.; Khakalo, A.; Kämäräinen, T.; et al. Nanocellulose Removes the Need for Chemical Crosslinking in Tannin-Based Rigid Foams and Enhances Their Strength and Fire Retardancy. ACS Sustain Chem Eng 2022, 10, 10303–10310. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Anoshkin, I.V.; Nasibulin, A.G.; Korhonen, J.T.; Seistonen, J.; Pere, J.; Kauppinen, E.I.; Ras, R.H.; Ikkala, O. Modifying native nanocellulose aerogels with carbon nanotubes for mechanoresponsive conductivity and pressure sensing. Adv. Mater 2013, 25, 2428–2432. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, T.; Cai, C.; Liu, K.; Liu, W.; Zhang, M.; Du, H.; Si, C.; Zhang, K. Multifunctional superelastic, superhydrophilic, and ultralight nanocellulose-based composite carbon aerogels for compressive supercapacitor and strain sensor. Adv Funct Mater 2022, 32, 2113080. [Google Scholar] [CrossRef]
- Pääkkö, M.; Vapaavuori, J.; Silvennoinen, R.; Kosonen, H.; Ankerfors, M.; Lindström, T.; Berglund, L.A.; Ikkala, O. Long and entangled native cellulose nanofibers allow flexible aerogels and hierarchically porous templates for functionalities. Soft Matter 2008, 412, 2492–2499. [Google Scholar] [CrossRef]
- Mendez, J.D.; Weder, C. Synthesis, electrical properties, and nanocomposites of poly(3,4-ethylenedioxythiophene) nanorods. Polym Chem 2010, 18, 1237–1244. [Google Scholar] [CrossRef]
- Sehaqui, H.; Salajková, M.; Zhou, Q.; Berglund, L.A. Mechanical performance tailoring of tough ultra-high porosity foams prepared from cellulose nanofiber suspensions. Soft Matter 2010, 6, 1824–1832. [Google Scholar] [CrossRef]
- Isogai, A. Emerging Nanocellulose Technologies: Recent Developments. Adv. Mater. 2021, 33, 2000630. [Google Scholar] [CrossRef] [PubMed]
| Cellulose grade | Reagent | Charge, μmol/g/ DS (0-1) |
UL-94 | TGA/air residue, wt% | TGA/air Tmax2 °C |
Source |
|---|---|---|---|---|---|---|
| CNF | Control/unmodified AGU/(NH4)2HPO4/urea= 1/1.2/4.9a AGU/(NH4)2HPO4/urea= 2.5/10a |
- 912/ 0.15 1840/ 0.41 |
- self-exting.c - |
~0 (800 °C) 9 - |
422 539 - |
[10] |
| CNF (post-P) |
Control/unmodified Hexokinase (EC2.7.1.1.)/ ATP/MgCl2 |
- DS: 0.43 |
- - |
15 (600 °C) 57 |
- - |
[56] |
| CNF | Control/unmodified AGU/(NH4)2HPO4/urea=1/1.2/4.9a |
- 2930 |
- V-0 |
0 ~ 25 (800 °C) |
[19] | |
| NFC | Control P2O5/cellulose=1:1 and 2:1b; (+ melamine) |
- DS:0.15-0.16 |
- - |
0.6 (800 °C) 9.2 |
- - |
[57] |
| NCC (post-P) |
Control/unmodified P2O5/urea P2O5 |
- 3300/ 0.26 950/ 0.08 |
- - - |
4 (500 °C) 30 - |
- - - |
[58] |
| NFC/MFC | AGU/(NH4)2HPO4/urea=1/1.5/10a | 4500/ 0.39 | self-exting.c | - | - | [11] |
| NFC | Control/unmodified Periodate oxidation |
0 320 |
- self-exting.c |
0 (700 °C) 27 |
379 389 |
[9] |
| NFC/MFC | Control/unmodified AGU/(NH4)2HPO4/urea=1/0.5/2a |
- 1540 |
- self-exting.c |
0.6 (800 °C) 20 |
513 625 |
[59] |
| NCC/NFC (post-P) | Control/unmodified NC/H3PO4/water NC/H3PO4/molten urea |
- NFC: 19 NCC: 435 NFC: 1173 NCC: 1038 |
- - - - |
~ 14 (600 °C) ~ 30 ~ 45 ~45 ~ 40 |
- - - - - |
[60] |
| Sample | HRC, J/gK | pHRR, W/g | TpHRR, °C | THR, kJ/g |
|---|---|---|---|---|
| BCNF | 168.9 | 166.2 | 356.1 | 9.7 |
| P-BCNF | 43.2 | 42.7 | 293 | 1.8 |
| BCNF-L | 135.6 | 134.2 | 339.3 | 8.3 |
| P-BCNF-L | 22.8 | 21.1 | 281.2 | 1.3 |
| Sample | T10%, °C | T50%, °C | Tmax1, °C | Tmax2, °C | Char, wt% |
|---|---|---|---|---|---|
| HefCel | 285.2 | 347.2 | 346.8 | 512.5 | 0.6 |
| P-HefCel_0.125* | 226.4 | 330.4 | 268.2 | 529.2 | 2.2 |
| P-HefCel_0.25* | 232.4 | 361.2 | 284.5 | 552.2 | 9.2 |
| P-HefCel_0.5* | 210.6 | 401.4 | 280.4 | 624.8 | 20.0 |
| Sample | IT, s | pHRR, kW/m2 | THR, MJ/m2 | MARHE, kW/m2 | Residue, % |
|---|---|---|---|---|---|
| Wood | 89 ± 5 | 248 ± 9 | 61 ± 2 | 138 ± 10 | 15 ± 1 |
| Coated | 358 ± 58 | 285 ± 50 | 41 ± 5 | 74 ± 9 | 20 ± 2 |
| Sample | IT, s | pHRR, kW/m2 | THR, MJ/m2 | TGA, wt%/800 °C |
|---|---|---|---|---|
| Reference, no coating | 12 ± 4 | 120 ± 6 | 10 ± 1 | 15 ±1 |
| MFC, 100% | 18 ± 1 | 127 ± 9 | 11 ± 1 | 22 ± 1 |
| Sulf. kraft lignin (10%) + MFC | 12 ± 1 | 85 ± 4 | 8 ± 2 | 52 ±0 |
| Kraft lignin (10%) +MFC | 14 ± 2 | 127 ± 8 | 11 ± 1 | 40 ± 2 |
| Nanoclay (10%) + MFC | no ign. | 5 ± 1 | 0.4 ± 0.1 | 87 ± 0 |
| Synergistic FR (10%) + MFC | 34 ± 2 | 47 ± 6 | 4.2 ± 0.2 | 45 ± 3 |
| EG (10%) + MFC | 26 ± 8 | 47 ± 1 | 7 ± 0.4 | 74 ± 2 |
| Sample | THR (kJ/g) | pHRR (W/g) | TpHRR (°C) | R,% |
|---|---|---|---|---|
| CNF | 11.3 | 294 | 351 | 14 |
| CNF/Al(OH)3(56wt%) | 2.0 | 51 | 240 | 50 |
| CNF/Al(OH)3(67 wt%) | 1.7 | 24 | 247 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).