Submitted:
28 November 2023
Posted:
29 November 2023
You are already at the latest version
Abstract
Keywords:
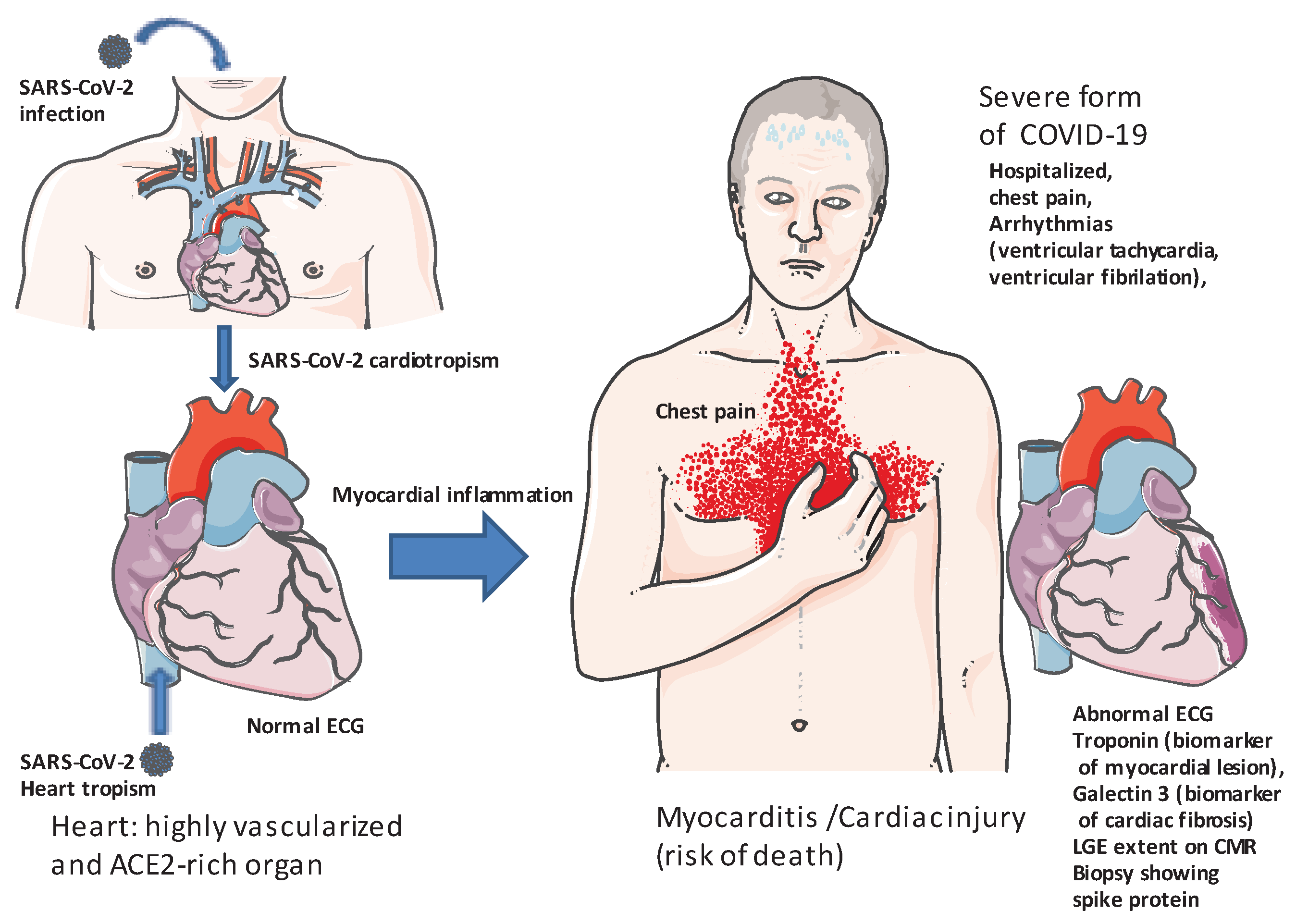
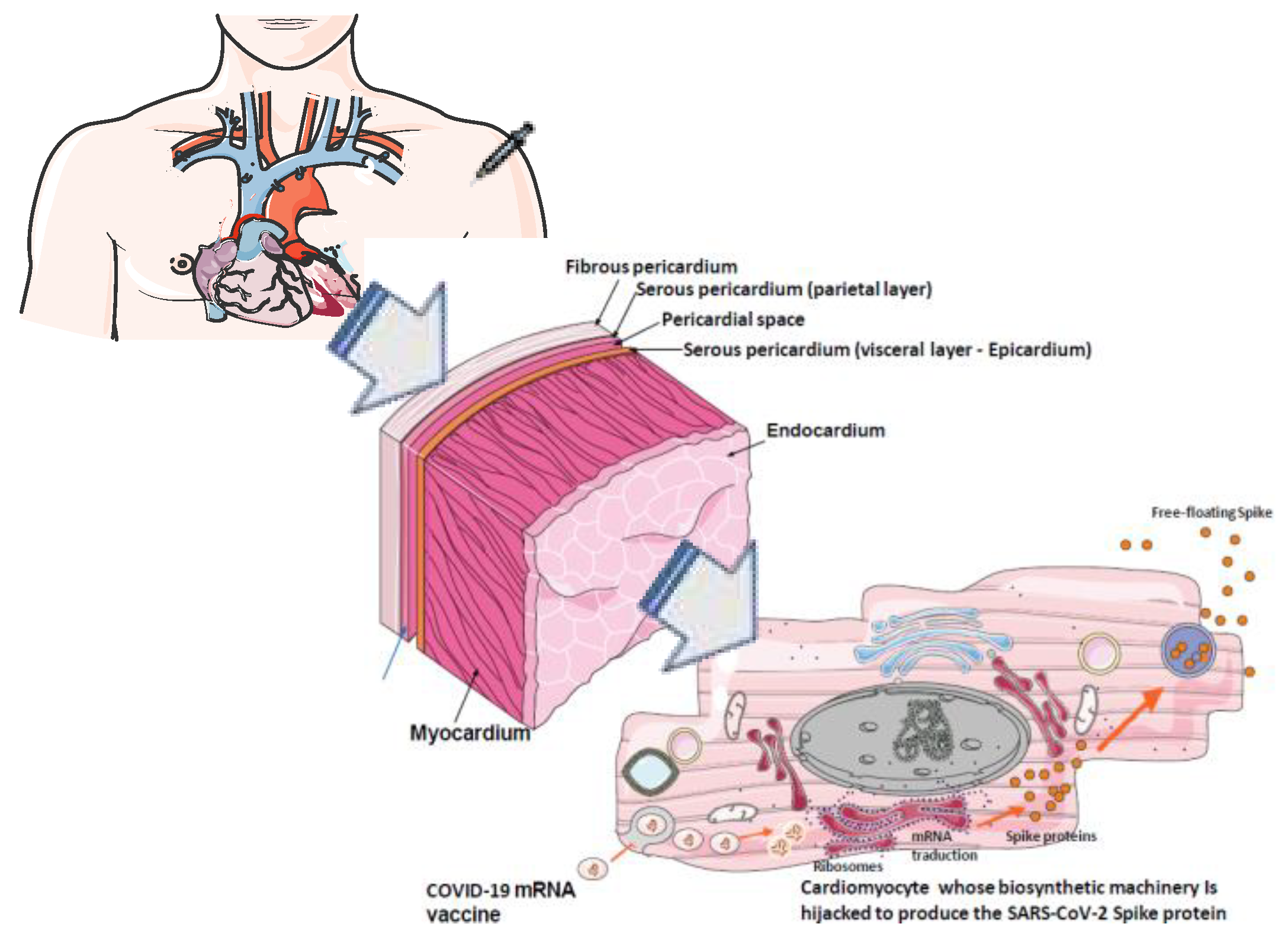
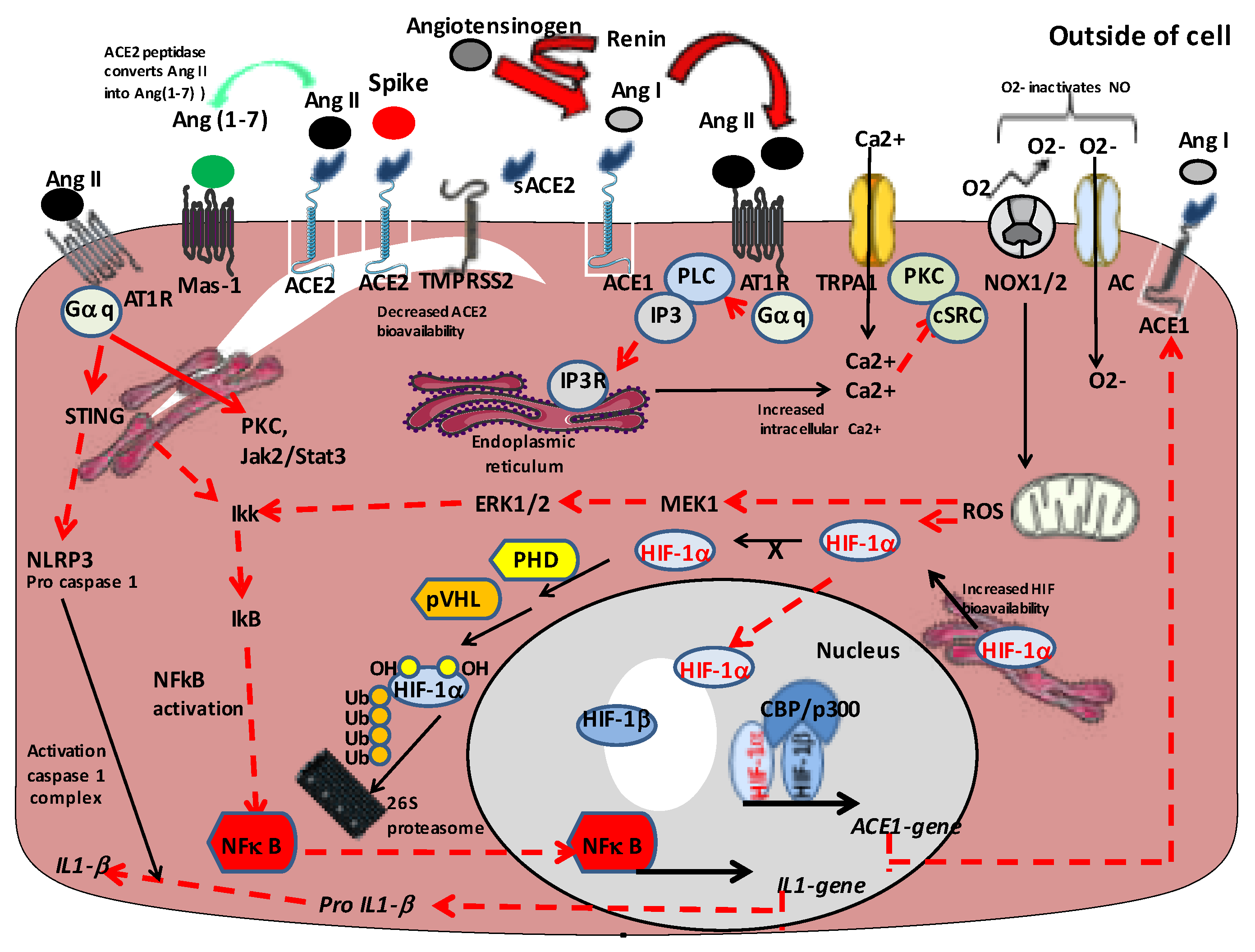
1. Introduction
| Diagnostic criteria for myocarditis | CDC* criteria |
Brighton Collaboration criteria |
|---|---|---|
|
Level 1 (confirmed) Symptoms consistent with myocarditis and at least one of: Abnormal histopathology OR Elevated troponin AND abnormal CMR |
Level 1 (definitive) Symptoms consistent with myocarditis and at least one of: Abnormal histopathology OR Elevated troponin AND abnormal CMR OR Elevated troponin AND abnormal TTE |
|
| Level 2 (probable) | Level 2 (probable) | |
| Symptoms consistent with myocarditis and at least one of: Elevated troponin OR Abnormal ECG OR Abnormal TTE OR Abnormal CMR |
Symptoms consistent with myocarditis and at least one of: Elevated troponin OR CKMB OR Abnormal ECG OR Abnormal TTE |
|
| Level 3 (possible case) | ||
| Symptoms consistent with myocarditis AND Enlarged heart on CXR OR non-specific ECG abnormalities |
||
|
CMR diagnostic criteria for myocarditis |
Diagnostic target Myocardial edema Myocardial injury Hyperemia Myocardial necrosis |
Lake Louise criteria (LLC) T2-weighted imaging, increased Bright signal intensity Increased global early gadolinium enhancement ratio between myocardium and skeletal muscle. At least one focal lesion with non-ischemic regional distribution on late gadolinium enhancement Pericardial effusion; Systolic left ventricular wall motion abnormality |
2.1. Toward the generation of COVID-19 mRNA vaccines
2.2. The Pfizer/BioNTech’s BNT162b2 and Moderna’s mRNA-1273 vaccines and post-vaccine myocarditis
2.3. Shedding light on the manufacture of mRNA-based COVID-19 vaccines
- Isolation of SARS-CoV-2 and the extraction of its RNA genome. Synthesis by reverse transcription (RT) of a double stranded (ds) DNA template of the gene coding for the spike protein. A synthetic DNA sequence encoding the viral spike protein is inserted into a bacterial plasmid (7,824 base pairs for the Pfizer BNT162b2 mRNA vaccine and 6,777 base pairs for the Moderna mRNA 1273 vaccine) that contains a bacterial origin (ori) of replication and a kanamycin resistant (aminoglycoside phosphotransferase Neo/Kan) gene [50]. Notably, the wild type spike sequence (NCBI accession: NC_045512) is found to be 45.3% identical to the BNT162b2 vaccine and the GC content is 37.3% for the wild type and 56.9% for the BNT162b2 vaccine. Eight small ORFs were found to overlap the Pfizer BNT162b2 mRNA vaccines compared to eleven overlapping ORFs in the wild type [51].
- The recombinant bacterial plasmid containing a double stranded DNA copy of the gene coding for the spike protein as well as a DNA-dependent RNA-polymerase promoter and a kanamycin-resistance selection gene are stored at -150°C until use. The, plasmid is then transfected into an Escherichia coli (E. coli) bacteria that has been made competent for DNA uptake (the construct includes missense codons leading to two major changes in the S2 spike protein sequence with K986P and V987P substitution aimed at stabilizing the protein, the proline is a very rigid amino acid forming a bend aimed at improving the stability of the spike protein by preventing the conformational change of the pre-fusion into the post-fusion structure) [52,53].
- E.coli colonies are grown at 37°C for 24 hours on Petri dishes filled with solid medium. During this process the plasmid is transmitted to daughter bacteria of the E.coli colonies when the bacteria divide (bacteria multiply every 20 minutes). To avoid event of plasmid loss its maintenance is enforced by selection with a kanamycin antibiotic added to the growth medium. Bacteria are then grown into flasks filled with medium and then moved into a large fermenter that contains up to 300 liters of a nutrient broth where they are grown for four days. After amplification in bacteria serving as a master cell bank [54], bacteria are chemically broken down and the plasmid DNA is purified from bacterial debris. The products were tested for purity and gene sequence control. Each one liter batch of plasmid DNA is intended to finally produce about one million doses of the vaccine.
- The ring-shaped plasmid is linearized through the action of a restriction enzyme releasing the sequence encoding the synthetic spike. The cell-free in vitro transcription of DNA into RNA is achieved using a T7 RNA polymerase to generate a synthetic mRNA with a 5' cap. The sequence of mRNA is 4100-4300 nucleotides long with a 5'cap [55]. At this stage, the synthetic nucleoside N-methyl-pseudouridine (mψU) is incorporated into the artificial RNA instead of the natural uridine nucleoside to further increase RNA stability, to enhance translation efficiency in host cells, and to remove alternative start codons - avoid overlapping ORFs - and internal ribosome entry sites, thus preventing non-specific recognition by ribosomal complexes [56]. The addition of a 5′ cap structure is a critical part of this production step that has been improved by new technology suitable for large-scale production [57,58]. A poly(A) tail is needed for efficient translation of mRNA vaccines and is also a critical part during manufacture [59].
- In vitro transcription is followed by several steps of mRNA purification, including the removal of DNA and dsRNA, which could lead to an excessive innate immune response by dsRNA sensing [60] and mRNA is filtered and frozen. Analysis of mRNA requires diverse techniques such as RT-qPCR, capillary and gel electrophoresis, high-pressure liquid chromatography (HPLC) and immunoblotting. The Food and Drug Administration (FDA, USA), recommends manufacturers to limit amount of residual DNA in the final product to be below 10 ng/dose and the size of DNA to be below the size of a functional gene [61].
- The thawed mRNA is mixed with water. In a separate process, the oily lipids are mixed with ethanol, and mRNA and lipids (including phospholipids, cholesterol, cationic lipids and polyethylene glycol lipids that are mixed together) are mixed to create lipid nanoparticles [62]. When the lipids come into contact with the mRNA, electric charge pulls them together in a nanosecond. The mRNA is enveloped in several layers of clinically translatable lipid nanoparticles (multilayer liposomes), forming an oily protective vaccine particle. Liposomes or lipid nanoparticles (LNPs) which facilitate the mRNA cytosolic transport, are known to function as adjuvants can also modulate the immune response [63,64]. The newly made vaccine is filtered to remove the ethanol, concentrated and filtered again to remove any impurities, and finally sterilized. Machines inject 0.45 ml of a concentrated vaccine solution into vials, enough for six doses after dilution. The vials are sealed with foil and capped with purple lids and stored at -70°C. After further quality testing, the vials from the same batch are ready to ship.
- Packaged vaccine doses (preserved at low temperature), are shipped and processed for the market. The mRNA-based COVID-19 vaccine is administered by injection into the deltoid muscle leading to capture of mRNA by muscle cells. The lipid nanoparticles protect RNA (a fragile molecule) from RNAse-dependent degradation and facilitate cellular uptake by lipid fusion with lipids of cell membrane. Spike coding RNA is released into target cell cytoplasm.
2.4. Biodistribution and persistence of COVID-19 mRNA vaccines
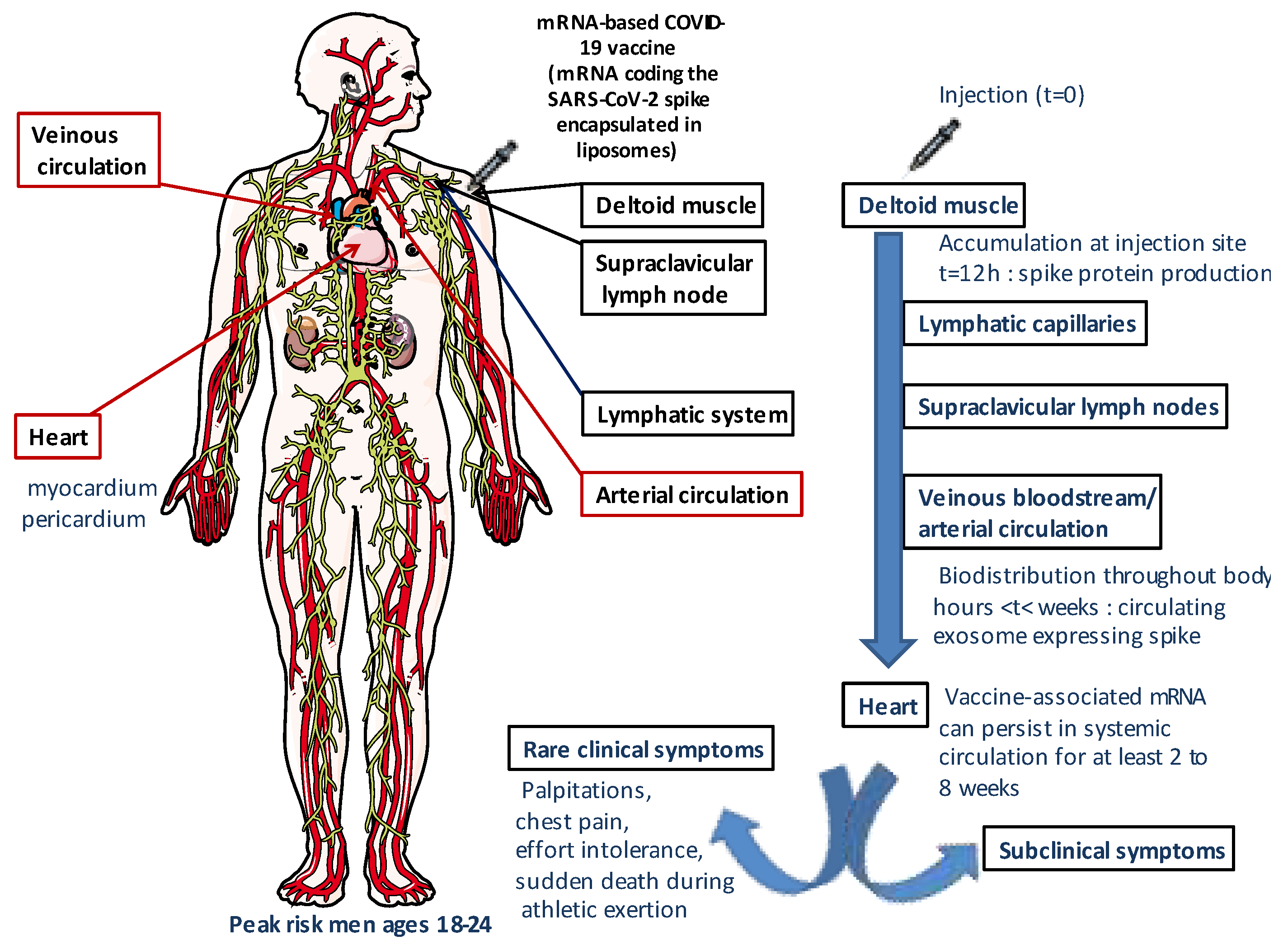
2.5. Previous hypothesis proposed in an attempt to explain the increased risk of post mRNA-based COVID-19 vaccines myocarditis
2.6. Possible role of circulating spike protein in post-mRNA-based COVID-19 vaccines associated myocarditis
3. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang C, et al. A Pneumonia Outbreak Associated With a New Coronavirus of Probable Bat Origin. Nature (2020) 579: 270–273. [CrossRef]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A Novel Coronavirus From Patients With Pneumonia in China, 2019. N. Engl. J. Med. (2020) 382:727–733. [CrossRef]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet (2020) 395(10223): 497–506. [CrossRef]
- Devaux CA, Lagier JC. Unraveling the Underlying Molecular Mechanism of ‘Silent Hypoxia’ in COVID-19 Patients Suggests a Central Role for Angiotensin II Modulation of the AT1R-Hypoxia-Inducible Factor Signaling Pathway. J. Clin. Med. (2023) 12: 2445. [CrossRef]
- Han L, Zhao S, Li S, Gu S, Deng X, Yang L, Ran J. Excess cardiovascular mortality across multiple COVID-19 waves in the United States from March 2020 to March 2022. Nature Cardiovasc. Res. (2023) 2: 322-333. [CrossRef]
- Pollack A, Kontorovich AR, Fuster V, Dec GW. Viral myocarditis—diagnosis, treatment options, and current controversies. Nature Rev. Cardiol. (2015)12: 670–680. [CrossRef]
- Sozzi FB, Gherbesi E, Faggiano A, Gnan E, Maruccio A, Schiavonne M, Lacuzio L, Carugo S. Viral Myocarditis: Classification, Diagnosis, and Clinical Implications. Front. Cardiovasc. Med. (2022) 9: 908663. [CrossRef]
- Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, et al. Cardiovascular magnetic resonance in myocarditis: a JACC White paper. J. Am. Coll. Cardiol. (2009) 53(17): 1475-1487. [CrossRef]
- Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R. et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on myocardial and pericardial diseases. Eur. Heart J. (2013) 34(33): 2636-2648. [CrossRef]
- Luetkens JA, Faron A, Isaak A, Dabir D, Kuetting D, Feisst A, Schmeel FC, Sprinkart AMThomas D. Comparison of Original and 2018 Lake Louise Criteria for diagnosis of acute myocarditis: results of a validation cohort. Radiol. Cardiothor. Imag. (2019) 1(3): e190010. [CrossRef]
- Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, Shah RV, Sims DB, Thiene G, Vardeny O. Recognition and initial management of fulminant myocarditis: a scientific statement from the American heart association. Circulation (2020) 141(6): e69-92. [CrossRef]
- Marshall TR, Schrader S, Voss L, Buttery JP, Crawford NW, Cheng DR. A comparison of post-COVID vaccine myocarditis classification using the Brighton Collaboration criteria versus Centre for Dieasise Control criteria. Australian Gov. Dept Health and Aged Care, Com. Dis. Intell. (2023) 47: electronic publication 19/01/2023. [CrossRef]
- Hamadeh A, Aldujeli A, Briedis K, Tecson KM, Sanz-Sanchez J, Al Dujeili M, Al-Obeidi A, Diez JL, Zaliunas R, Stoler R, McCullough PA. Characteristics and Outcomes in Patients Presenting With COVID-19 and ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. (2020) 131: 1-6. [CrossRef]
- Mele D, Flamigni F, Rapezzi C, Ferrari R. Myocarditis in COVID-19 patients: current problems. Intern. Emerg. Med. (2021) 16: 1123–1129. [CrossRef]
- Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nature Med. (2022) 28: 583-590. [CrossRef]
- Castiello T, Georgiopoulos G, Finocchiaro G, Claudia M, Gianatti A, Delialis D, Aimo A, Prasad S. COVID-19 and myocarditis: a systematic review and overview of current challenges. Heart Failure Rev. (2022) 27: 251–261. [CrossRef]
- Kornowski R, Witberg G. Acute myocarditis caused by COVID-19 disease and following COVID-19 vaccination. Openheart (2022) 9: e001957. [CrossRef]
- Pillay J, Gaudet L, Wingert A, Bialy L, Mackie AS, Paterson DI, Hartling L. Incidence, risk factors, natural history, and hypothesised mechanisms of myocarditis and pericarditis following covid-19 vaccination: living evidence syntheses and review. Brit. Med. J. (2022) 378: e069445. [CrossRef]
- Heidecker B, Dagan N, Balicer R, Eriksson U, Rosano G, Coats A, Tschöpe C, Kelle S, Poland GA, Frustaci A, et al. Myocarditis following COVID-19 vaccine: incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. (2022) 24(11): 10.1002/ejhf.2669. [CrossRef]
- Devaux CA, Camoin-Jau L An update on angiotensin-converting enzyme 2 structure/functions, polymorphism, and duplicitous nature in the pathophysiology of coronavirus disease 2019: Implications for vascular and coagulation disease associated with severe acute respiratory syndrome coronavirus infection. Front. Microbiol. (2022) 13: 1042200. [CrossRef]
- Katoto PDMC, Byamungu LN, Brand AS, Tamuzi JL, Kakubu MAM, Wiysonge CS, Gray G. Systematic review and meta-analysis of myocarditis and pericarditis in adolescents following COVID-19 BNT162b2 vaccination. npj Vaccines (2023) 8:89. [CrossRef]
- Eberhardt N, Noval MG, Kaur R, Amadori L, Gildea M, Sajja S, Amadori L, Das D, Cihoroz B, Stewart OJ, et al. SARS-CoV-2 infection triggers pro-atherogenic inflammatory responses in human coronary vessels. Nature Cardiovasc. Res. (2023) published online 28 September 2023. [CrossRef]
- Oleszak F, Maryniak A., Botti E, Abrahim C, Salifu MO,Youssef M, Henglein VL, McFariane SJ. Myocarditis associated with COVID-19. Am. J. Med. Case Rep. (2020) 8: 498–502. [CrossRef]
- Wu S, Zou G, Lin K, Zhang D. Effects of COVID-19 on the cardiovascular system and implications for management. J. Xiangya Med. (2021) 6: 7. [CrossRef]
- Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, Olsha-Castell S, Arad D, Hasin T, Levi N, et al. Myocarditis after BNT162b2 mRNA Vaccine against COVID-19 in Israel. N. Engl. J. Med. (2021) 385: 2140–2149. [CrossRef]
- Husby A, Vinslov Hansen J, Fosbol E, Myrup Thiesson E, Madsen M, Thomsen RW, Sorensen HT, Andersen M, Wohlfahrt J, Gislason G, et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: Population based cohort study. Brit. Med. J. (2021) 375: e068665. [CrossRef]
- Patone, M. Patone M., Mei XW, Handunnetthi L, Dixon S, Zaccardi F, Shankar-Hari M, Watkinson P, Khunti K, Harnden A, Coupland CAC, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nature Med. (2022) 28(2), 410–422. [CrossRef]
- Heymans S, Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nature Rev. Cardiol. (2022) 19: 75-77. [CrossRef]
- Heymans S, Cooper LT. Author Correction: Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nature Rev. Cardiol. (2023) 20: 575. [CrossRef]
- Pastor Pueyo P, Gambo-Ruberte E, Gayan Ordas J, Blanco LM, Figal DP, Larranaga Moreira JM, Gomez Barrado JJ, Gonzalez Clla D, Almenar Bonet L, Corbi Pascual MJ, et al. Vaccine–carditis study: Spanish multicenter registry of inflammatory heart disease after COVID-19 vaccination. Clin. Res. Cardiol. (2023) Jun 27. [CrossRef]
- Jiang J, Chan L, Kauffman J, Narula J, Charney AW, Oh W, Nadkami G, N3C Consortium. Impact of vaccination on major adverse cardiovascular events in patients with COVID-19 infection. J. Am. Coll. Cardiol. (2023) 81(9): 928-930. [CrossRef]
- Wassif M, Lo P, Satouris P, Swan L, Tardo D, Kovacic JC, Muller D, Muthiah K, Kotlyar E, Bart NK. Acute Myocarditis and Pericarditis after mRNA COVID-19 vaccinations - A single-centre retrospective analysis. Heart Lung Circul. (2023) 32: 467-479. [CrossRef]
- Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman P, Mascherbauer J, Nezafat R, Salerno M, Schelbert EB, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. (2017) 19: 75. [CrossRef]
- Buchan SA, Seo CY, Johnson C, Alley S, Kwong JC, Nasrren S, Calzavara A, Lu D, Harris TM, Yu K, Wilson SE. Epidemiology of Myocarditis and Pericarditis Following mRNA Vaccination by Vaccine Product, Schedule, and Interdose Interval Among Adolescents and Adults in Ontario, Canada. JAMA Network Open. (2022) 5(6):e2218505. [CrossRef]
- Bramwell VW, Perrie Y. The rational design of vaccines. Drug Discov. Today (2005) 10: 1527–1534. [CrossRef]
- Schijns V, Majhen D, van der Ley P, Thakur A, Summerfield A, Berisio R, Nativi C, Fernandez-Tejada A, Alvarez-Dominguez C, Gizurarson S, Zamyatina A, et al. Rational vaccine design in times of emerging diseases: the critical choices of immunological correlates of protection, vaccine antigen and immunomodulation. Pharmaceutics (2021) 13(4): 501. [CrossRef]
- Walsh EE, Frenck RW Jr., Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N. Engl. J. Med. (2020) 383(25): 2439-2450 . [CrossRef]
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. COVE Study Group. Efficacy and safety of the mRNA-1273 Sars-Cov-2 vaccine. N. Engl. J. Med. (2021) 384(5): 403-416. [CrossRef]
- Teo, SP. Teo SP. Review of COVID-19 mRNA vaccines: BNT162b2 and mRNA-1273. J. Pharm. Pract. (2021) 35(6):947-951. [CrossRef]
- Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, Hernán MA, Lipsitch M, Kohane I, Netzer D, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. (2021) 385: 1078– 1090. [CrossRef]
- Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation (2021) 144: 471–484. [CrossRef]
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. (2020) 383(27): 2603-2615. [CrossRef]
- Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy. Thromb. Res.( 2020) 191: 9–14. [CrossRef]
- Salah HM, Mehta JL. COVID-19 vaccine and myocarditis. Am. J. Cardiol. (2021) 157: 146–148. [CrossRef]
- Shiravi AA, Ardekani A, Sheikhbahaei E, Heshmat-Ghahdarijani K. Cardiovascular complications of SARS-CoV-2 vaccines: An overview. Cardiol. Ther. (2022) 11: 13–21. [CrossRef]
- Lai FTT, Li X, Peng K, Huang L, Ip P, Tong X, Ling Chui CS, Fai Wan EY, Ho Wong CK, Yin Chan EW, et al. Carditis After COVID-19 Vaccination with a Messenger RNA Vaccine and an Inactivated Virus Vaccine: A Case–Control Study. Ann. Intern. Med.( 2022) 175: 362–370. [CrossRef]
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science (2020), 367(6483): 1260-1263. [CrossRef]
- Beasley, DW. Beasley DW. New international guidance on quality, safety and efficacy of DNA vaccines. npj Vaccines (2020) 5: 53. [CrossRef]
- Tinari, S. Tinari S. The EMA covid-19 data leak, and what it tells us about mRNA instability. Brit. Med. J. (2021) 372: n627. [CrossRef]
- Speicher DJ, Rose J, Gutschi LM, Wiseman D, McKernan K. DNA fragments detected in monovalent and bivalent Pfizer/BioNTech and Moderna modRNA COVID-19 vaccines from Ontario, Canada: Exploratory dose response relationship with serious adverse events. Open Sci. Framework (2023). Preprint not peer reviewed. https://osf.io/xv3nz.
- Beaudoin CA, Bartas M, Volna´ A, Pecinka P, Blundell TL Are there hidden genes in DNA/RNA vaccines? Front. Immunol. (2022) 13: 801915. [CrossRef]
- Nance KD, Meier JL. Modifications in an emergency: the role of N1-Methylpseudouridine in COVID-19 vaccines. ACS Cent Sci. (2021) 7:748–756. [CrossRef]
- Schoenmaker L, Witzigmann D, Kulkarni JA, Verbeke R, Kersten G, Jiskoot W, Crommelin DJA. mRNA-lipid nanoparticle COVID-19 vaccines: structure and stability. Int. J. Pharm. (2021) 601: 120586. [CrossRef]
- Cott E, deBruyn E, Corum J April 28, 2021 (The New York Times). How Pfizer Make its Covid-19 Vaccine. https://www.nytimes.com/interactive/2021/health/pfizer-coronavirus-vaccine.html (Accessed on 20 june 2023).
- Abu Abed, O.S. Abu Abed, O.S. Gene therapy avenues and COVID-19 vaccines. Genes Immun. (2021) 22: 120–124. [CrossRef]
- Xia, X. Xia X. Detailed dissection and critical evaluation of the Pfizer/BioNTech and Moderna mRNA vaccines. Vaccines (2021) 9(7): 734. [CrossRef]
- Kyriakopoulos AM, Mc Cullough PA. Synthetic mRNAs; Their Analogue Caps and Contribution to Disease. Diseases (2021) 9(3): 57. [CrossRef]
- Kim SC, Sekhon SS, Shin WR, Ahn G, Cho BK, Ahn JY, Kim YH. Modifications of mRNA vaccine structural elements for improving mRNA stability and translation efficiency. Mol. Cell. Toxicol. (2022) 18(1): 1-8. [CrossRef]
- Jackson NAC, Kester KE, Casimiro D, Gurunathan S, DeRosa F. The promise of mRNA vaccines: a biotech and industrial perspective. npj Vaccines (2020) 5: 11. [CrossRef]
- Nelson J, Sorensen EW, Mintri S, Rabideau AE, Zheng W, Besin G, Khatwani N, Su SV, Miracco EJ, Issa WJ, et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci. Adv. (2020) 6(26): eaaz6893. [CrossRef]
- Shin J, Wood D, Robertson J, Minor P, Penden K, WHO Informal Consultation Group. WHO informal consultation on the application of molecular methods to assure the quality, safety and efficacy of vaccines, Geneva, Switzerland, 7-8 April 2005. Biologicals (2007) 35(1): 63-71. [CrossRef]
- Han X, Zhang H, Butowska K, Swingle KL, Alameh MG, Weissman D, Mitchell MJ. An ionizable lipid toolbox for RNA delivery. Nature Com. (2021) 12: 7233. [CrossRef]
- Pulendran B, Arunachalam PS, O'Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nature Rev. Drug Discov. (2021) 20: 454-475. [CrossRef]
- Kobiyama K, Ishii KJ. Making innate sense of mRNA vaccine adjuvanticity. Nature Immunol. (2022) 23: 472-482. [CrossRef]
- Yan B, Chakravorty S, Mirabelli C, Wang L, Trujillo-Ochoa JL, Chauss D, Kumar D, Lionakis MS, Olson MR, Wobus CE, et al. Host-virus chimeric events in SARS-CoV-2-infected cells are infrequent and artifactual. J. Virol. (2021) 95: e00294-21. [CrossRef]
- Grigoriev A, Kelley JJ, Guan L. Sequences of SARS-CoV-2 "hybrids" with the human genome: signs of non-coding RNA ?. J. Virol. (2022) 96(2): e01462-21. [CrossRef]
- McKernan K, Helbert Y, Kane LT, Mc Laughlin S. Sequencing of bivalent Moderna and Pfizer mRNA vaccines reveals nanogram to microgram quantities of expression vector dsDNA per dose. Scienceopen (2023) preprint not peer reviewed. [CrossRef]
- Zhang L, Richards A, Barrasa MI, Hughes SH, Young RA, Jaenisch RA. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc. Natl. Acad. Sci., USA (2021) 118(21): e2105968118. [CrossRef]
- Smits N, Rasmussen J, Bodea GO, Amarilla AA, Gerdes P, Sanchez-Luque FJ, Ajjikuttira P, Modhiran N, Liang B, Faivre J, et al. No evidence of human genome integration of SARS-CoV-2 found by long-read DNA sequencing. Cell Rep. (2021) 36(7): 109530. [CrossRef]
- Gunter HM, Idrisoglu S, Singh S, Han DJ, Ariens E, Peters JR, Wong T, Cheetham SW, Xu J, Rai SK, et al. mRNA vaccine quality analysis using RNA sequencing. Nature Com. (2023) 14: 5663. [CrossRef]
- Aldén M, Olofsson Falla, F, Yang D, Barghouth M, Luan C, Rasmussen M, De Marinis Y. Intracellular Reverse Transcription of Pfizer BioNTech COVID-19 mRNA Vaccine BNT162b2 In Vitro in Human Liver Cell Line. Curr. Issues Mol. Biol. (2022) 44: 1115–1126. [CrossRef]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. From RNA to Protein. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA (2002) Available online: https://www.ncbi.nlm.nih.gov/books/NBK26829/ (accessed on 6 April 2023).
- Shamir M, Baron Y, Phillips R, Milo R. SnapShot: Timescales in cell biology. Cell (2016) 164: 1302. [CrossRef]
- Amanat F, Thapa M, Lei T, Ahmed SMS, Adelsberg DC, Carreño JM, Strohmeier S, Schmitz AJ, Zafar S, Zhou JQ, et al. SARS-CoV-2 mRNA vaccination induces functionally diverse antibodies to NTD, RBD, and S2. Cell (2021) 184: 3936–3948.e10. [CrossRef]
- Angyal A, Longet S, Moore SC, Payne RP, Harding A, Tipton T, Rongkard P, Ali M, Hering LM, Meardon N, et al. T-Cell and antibody responses to first BNT162b2 vaccine dose in previously infected and SARS-CoV-2-naive UK health-care workers: A multicentre prospective cohort study. Lancet Microbe (2022) 3: e21–e31. [CrossRef]
- Cantoni D, Siracusano G, Mayora-Neto M, Pastori C, Fantoni T, Lytras S. Analysis of Antibody Neutralisation Activity against SARS-CoV-2 Variants and Seasonal Human Coronaviruses NL63, HKU1, and 229E Induced by Three Different COVID-19 Vaccine Platforms. Vaccines (2023) 11: 58. [CrossRef]
- Fertig TE, Chitoiu L, Marta DS, Ionescu VS, Cismasiu VB, Radu E, Angheluta G, Dobre M, Serbanescu A, Hinescu ME, Gherghiceanu M. Vaccine mRNA Can Be Detected in Blood at 15 Days Post-Vaccination. Biomedicines (2022) 10: 1538. [CrossRef]
- Cognetti JS, Miller BL Monitoring serum spike protein with disposable photonic biosensors following SARS-CoV-2 vaccination. Sensors (2021). 21(17): 17. [CrossRef]
- Liang F, Lindgren G, Lin A, Thompson EA, Ols S, Röhss J, John S, Hassett K, Yuzhakov O, Bahl K, et al. Efficient Targeting and Activation of Antigen-Presenting Cells In Vivo after Modified MRNA Vaccine Administration in Rhesus Macaques. Mol. Ther. (2017) 25: 2635–2647. [CrossRef]
- Chaudhary N, Weissman D, Whitehead KA. MRNA Vaccines for Infectious Diseases: Principles, Delivery and Clinical Translation. Nat. Rev. Drug Discov.( 2021) 20: 817–838. [CrossRef]
- Krauson AJ, Casimero FVC, Siddiquee Z, Stone JR. Duration of SARS-CoV-2 mRNA vaccine persistence and factors associated with cardiac involvement in recently vaccinated patients. npj Vaccines (2023) 8: 141. [CrossRef]
- Li C, Lee A, Grigoryan L, Arunachalam PS, Scott MKD, Trisal M, Wimmers F, Sanyal M, Weidenbacher PA, Feng Y, et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nature Immunol. (2022) 23: 543–555. [CrossRef]
- Tahtinen S, Tong AJ, Himmels P, Oh J, Paler-Martinez A, Kim L, Wichner S, Oei Y, McCarron MJ, Freund EC, et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nature Immunol. (2022) 23: 532–542. [CrossRef]
- Bansal S, Perincheri S, Fleming T, Poulson C, Tiffany B, Bremner RM, Mohanakumar T. Cutting Edge: Circulating exosomes with COVID spike protein are induced by BNT162b2 (Pfizer-BioNTech) vaccination prior to development of antibodies: A novel mechanism for immune activation by mRNA vaccines. J. Immunol. (2021) 207: 2405-2410. [CrossRef]
- Seneff S, Nigh G, Kyriakopoulos, Mc Cullough PA. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem. Toxicol. (2022) 164: 113008. [CrossRef]
- Meo SA, Bukhari AI, Akram J, Meo AS, Klonoff DC. COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. (2021) 25: 1663-1669. [CrossRef]
- Li B, Jiang AY, Raji I, Atyeo C, Raimondo TM, Gordon AGR, Rhym LH, Samad T, MacIsaac C, Witten J., et al. Enhancing the immunogenicity of lipid-nanoparticle mRNA vaccines by adjuvanting the ionizable lipid and the mRNA. Nature Biomed. Eng. (2023) Sep 7. Online ahead of print. [CrossRef]
- Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, Madden TD, Hope MJ, Weissman D. Expression kinetics of nucleosidemodified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control Release. (2015) 217: 345–351. [CrossRef]
- Wayment-Steele HK, Kim DS, Choe CA, Nicol JJ, Wellington-Oguri R, Watkins AM, Parra Sperberg RA, Huang PS, Participants E, Das R. Theoretical basis for stabilizing messenger RNA through secondary structure design. Nucleic Acids Res. (2021) 49 (18): 10604–10617. [CrossRef]
- Castruita JAS, Schneider UV, Mollerup S, Leineweber TD, Weis N, Bukh,J, Pedersen MS, Westh H. SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination. Apmis (2023) 131(3): 128–132. [CrossRef]
- Röltgen K, Nielsen SC, Silva O, Younes SF, Zaslavsky M, Costales C, Yang F, Wirz OF, Solis D, Hoh RA, Wang A, et al. Immune imprinting, breadth of variant recognition, and germinal center response in human SARS-CoV-2 infection and vaccination. Cell (2022) 185, 1025–1040. [CrossRef]
- Ogata AF, Cheng CA, Desjardins M, Senussi Y, Sherman AC, Powell M, Novack L, Von S, Li X, Baden LR, Walt DR. Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) vaccine antigen detected in the plasma of mRNA-1273 vaccine recipients. Clin. Infect. Dis. (2022) 74(4): 715–718. [CrossRef]
- Brogna C, Cristoni S, ,Marino G, Montano L, Viduto V, Fabrowski M, Lettieri G, Piscopo M. Detection of recombinant Spike protein in the blood of individuals vaccinated against SARS-CoV-2: Possible molecular mechanisms. Proteomics Clin Applications (2023): 2300048. [CrossRef]
- King WW, Petersen MR, Matar RM, Budweg JB, Cuervo Pardo L, Petersen JW. Myocarditis following mRNA vaccination against SARS-CoV-2, a case series. Am. Heart. J. Plus Cardiol. Res. Pract. (2021) 8: 100042. [CrossRef]
- Williams CB, Choi JI, Hosseini F, Roberts J, Ramanathan K, Ong K. Acute myocarditis following mRNA-1273 SARS-CoV-2 vaccination. CJC Open (2021) 3: 1410–1412. [CrossRef]
- Torjsesen, I. Torjsesen I. COVID-19: Pfizer-BioNTech vaccine is “likely” responsible for deaths of some elderly patients, Norwegian review finds. Brit. Med. J. (2021) 373: n1372. [CrossRef]
- Sun CLF, Jaffre E, Levi R. Increased emergency cardiovascular events among under-40 population in Israel during vaccine rollout and third COVID-19 wave. Nat. Sci. Rep. (2022) 12: 6978. [CrossRef]
- Husby A, Lovdal Gulseth H, Hovi P, Vinslov Hansen J, Pihlström N, Gunnes N, Härkänen T, Dahl J, Karistad O, Heliö T, et al. Clinical outcomes of myocarditis after SARS-CoV-2 mRNAvaccination in four Nordic countries: Population based cohort study. Brit. Med. J. Med. (2023) 2: e000373. [CrossRef]
- Tsilingiris D, Vallianou NG, Karampela I, Liu J, Dalamaga M. Potential implications of lipid nanoparticles in the pathogenesis of myocarditis associated with the use of mRNA vaccines against SARS-CoV-2. Metab. Open (2022) 13: 100159. [CrossRef]
- Tang H, Tanaka G, Unterman T, Bursztajn H. Detoxifying the fear of epigenetic changes due to COVID vaccination. Am. J. Med. (2022) 135(6): 665-666. [CrossRef]
- Yamaguchi Y, Kato Y, Edahiro R, Sondergaard JN, Murakami T, Amiya S, Nameki S, Yoshimine Y, Morita T, Takesshima Y, et al. Consecutive BNT162b2 mRNA vaccination induces short-term epigenetic memory in innate immune cells. JCI Insight (2022) 7(22): e163347. [CrossRef]
- Heymans S, Eriksson U, Lehtonen J, Cooper LT Jr. The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J. Am. Coll. Cardiol. (2016) 68: 2348–2364. [CrossRef]
- Khani E, Entezari-Maleki T. Hypertensive crisis following COVID-19 vaccination. J. Clin. Pharmacol. (2022) 62: 1047–1048. [CrossRef]
- Bouhanick B, Brusq C, Bongard V, Tessier S, Montastruc JL, Senard JM, Montastruc F, Herin F. Blood pressure measurements after mRNA-SARS-CoV-2 tozinameran vaccination: A retrospective analysis in a university hospital in France. J. Hum. Hypertens. (2022) 36: 580–581. [CrossRef]
- Simonini M, Scarale MG, Tunesi F, Manunta P, Lanzani C. COVID-19 vaccines effect on blood pressure. Eur. J. Intern. Med. (2022) 105: 109–110. [CrossRef]
- Angeli F, Reboldi G, Trapasso M, Santilli G, Zappa M, Verdecchia P. Blood pressure increase following COVID-19 vaccination: A systematic overview and meta- analysis. J. Cardiovasc. Dev. Dis. (2022) 9: 150. [CrossRef]
- Gundry, SF. Gundry SF. mRNA COVID Vaccines Dramatically Increase Endothelial Inflammatory Markers and ACS Risk as Measured by PULS Cardiac Test: A Warning. Circulation (2021) 144, A10712. correction to: Abstract 10712. [CrossRef]
- Salzman MB, Huang CW, O’Brien CM, Castillo RD. Multisystem Inflammatory Syndrome after SARS-CoV-2 Infection and COVID-19 Vaccination. Emerg. Infect. Dis. (2021) 27: 1944–1948. [CrossRef]
- Buchhorn R, Meyer C, Schulze-Forster K, Junker J, Heidecke H. Autoantibody Release in Children after Corona Virus mRNA Vaccination: A risk factor of Multisystem Inflammatory Syndrome? Vaccines (2021) 9: 1353. [CrossRef]
- Thurner L, Kessel C, Fadle N, Seidel F, Kindermann I, Tschöpe C, Kheiroddin P, Kiblboeck D, Hoffmann MC, Bette B, et al. IL-1RA antibodies in myocarditis after SARS-CoV-2 vaccination. N. Engl. J. Med. (2022) 387(16): 1524-1527. [CrossRef]
- Arthur JM, Forrest JC, Boehme KW, Kennedy JL, Owens S, Herzog C, Liu J, Harville TO. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PLos One (2021) 16(9): e0257016. [CrossRef]
- Hallmann E, Sikora D, Poniedzialek B, Szymanski K, Kondratiuk K, Zurawski J, Brydak L, Rzymski P. IgG autoantibodies against ACE2 in SARS-CoV-2 infected patients. J. Med. Virol. (2023) 95(1): e28273. [CrossRef]
- Lebedin M, Vazquez Garcia C, Spatt L, Ratswohl C, Thibeault C, Ostendorf L, Alexander T, Paul F, Sander LE, Kurth F, de la Rosa K. Discriminating promiscuous from target-specific autoantibodies in COVID-19. Eur J Immunol.(2023) 53(5):e2250210. [CrossRef]
- Qiu Y, Zhao YB, Wang Q, Li JY, Zhou ZJ, Liao CH, Ge XY. Predicting the Angiotensin Converting Enzyme 2 (ACE2) Utilizing Capability as the Receptor of SARS-Cov-2. Microbes Infect. (2020) 22(4-5): 221–225. [CrossRef]
- Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Guo L, Guo R, Chen T, Hu J, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nature Com. (2020) 11: 1620. [CrossRef]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell (2020) 181: 271–280.e8 : . [CrossRef]
- Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, Zhang Q, Shi X, Wang Q, Zhang L, Wang X. Structure of the SARSCoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature (2020). 581: 215–220. [CrossRef]
- Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara H, Geng Q, Auerbach A, Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature (2020) 581: 221–224. [CrossRef]
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science (2020) 367: 1444–1448. [CrossRef]
- Hendren NS, Drazner MH, Bozkurt B, Cooper LT. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation (2020). 141: 1903–1914. [CrossRef]
- Tu WJ, McCuaing RD, Melino M, Rawle DJ, Le TT, Yan K, Suhrbier A, Johnston RL, Koufariotis LT, Waddell N, et al. Targeting novel LSD1-dependent ACE2 demethylation domains inhibits SARS-CoV-2 replication. Cell Discov. (2021) 7: 37. [CrossRef]
- Tu WJ, Melino M, Dunn J, McCuaig RD, Bielefeldt-Ohmann H, Tsimbalyuk S, Forwood JK, Ahuja T, Vandermeide J, Tan X, et al. In vivo inhibition of nuclear ACE2 translocation protects against SARS-CoV-2 replication and lung damage through epigenetic imprinting. Nature Com. (2023) 14: 3680. [CrossRef]
- Devaux CA, Rolain JM, Raoult D. ACE2 Receptor Polymorphism: Susceptibility to SARS-Cov-2, Hypertension, Multi-Organ Failure, and COVID-19 Disease Outcome. J. Microbiol. Immunol. Infect. (2020) 53(3): 425–435. [CrossRef]
- Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nature Rev. Cardiol. (2020) 17: 259-260. [CrossRef]
- Silhol F, Sarlon G, Deharo JC, Vaïsse B. Downregulation of ACE2 induces overstimulation of the renin–angiotensin system in COVID-19: should we block the renin–angiotensin system? Hypertens. Res. (2020) 43: 854–856. [CrossRef]
- Miesbach, W. Miesbach W. Pathological role of angiotensin II in severe COVID-19. TH Open (2020) 4(2): e138-e144. [CrossRef]
- Jahani, M. Jahani, M., Dokaneheifard, S., Mansouri, K. Hypoxia: A key feature of COVID-19 launching activation of HIF-1 and cytokine storm. J. Inflamm. (2020). 17: 33. [CrossRef]
- Costela-Ruiz VJ, Illescas6Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodriguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev .(2020) 54: 62-75. [CrossRef]
- Osman IO, Melenotte C, Brouqui P, Million M, Lagier JC, Parola P, Stein A, La Scola B, Meddeb L, Mege JL, Raoult D, Devaux CA. Expression of ACE2, Soluble ACE2, Angiotensin I, Angiotensin II and Angiotensin-(1-7) Is Modulated in COVID-19 Patients. Front. Immunol. (2021) ,12: 625732. [CrossRef]
- Zoufaly A, Poglitsch M, Aberle JH, Hoepler W, Seitz T, Traugott M, Grieb A, Pawelka E, Laferl H, Wenisch C, et al. Human Recombinant Soluble ACE2 in Severe COVID-19. Lancet Resp. Med. (2020) 8: 115–158. [CrossRef]
- Reindl-Schwaighofer R, Hödlmoser S, Domenig O, Krenn K, Eskandary F, Krenn S, Schörgenhofer C, Rumpf B, Karolyi M, Traugott MT, et al. The systemic renin-angiotensin system in COVID-19. Sci. Rep. (2022). 12: 20117. [CrossRef]
- Devaux CA, Raoult D The impact of COVID-19 on populations living at high altitude: Role of hypoxiainducible factors (HIFs) signaling pathway in SARS-CoV-2 infection and replication. Front. Physiol. (2022) 13: 960308. [CrossRef]
- Devaux CA, Camoin-Jau L. Molecular Mimicry of the Viral Spike in the SARS-CoV-2 Vaccine Possibly Triggers Transient Dysregulation of ACE2, Leading to Vascular and Coagulation Dysfunction Similar to SARS-CoV-2 Infection. Viruses (2023) 15: 1045. [CrossRef]
- Meylan S, Livio F, Foerster M, Genoud PJ, Marguet F, Wuerzner G. CHUV COVID Vaccination Center. Stage III hypertension in patients after mRNA-based SARS-CoV-2 vaccination. Hypertension (2021) 77: e56–e57. [CrossRef]
- Zappa M, Verdecchia P, Spanevello A, Visca D, Angeli F. Blood pressure increase after Pfizer/BioNTech SARS-CoV-2 vaccine. Eur. J. Intern. Med. (2021) 90: 111–113. [CrossRef]
- Rhea EM, Logsdon AF, Hansen KM, Williams LM, Reed MJ, Baumann KK, Holden SJ, Raber J, Banks WA, Erickson MA. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nature Neurosci. (2021) 24: 368-378. [CrossRef]
- Yonker, L. Yonker L.M, Swank Z, Bartsch YC, Burns MD, Kane A, Boribong BP, Davis JP, Loiselle M, Novak T, Senussi Y, et al. Circulating spike protein detected in post–COVID-19 mRNA vaccine myocarditis. Circulation (2023) 147: 867-876. [CrossRef]
- Bozkurt B, Shedding Light on Mechanisms of Myocarditis With COVID-19 mRNA Vaccines. Circulation (2023) 147: 877–880. [CrossRef]
- Forte, E. Forte E. Circulating spike protein may contribute to myocarditis after COVID-19 vaccination. Nat Cardiovasc. Res. (2023). 2: 100 . [CrossRef]
- Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA (2020) 324: 799-801 . [CrossRef]
- Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, Ibrahim H, Friedman GH, Thompson C, Alviar CL, et al. ST-segment elevation in patients with covid-19 — a case series. N. Engl. J. Med. (2020) 382: 2478-2480. [CrossRef]
- Toidokoro D, Hiroi Y. Cardiovascular implications of the COVID-19 pandemic. J. Cardiol. (2022) 79(4): 460-467. [CrossRef]
- Modin D, Claggett B, Sindet-Pedersen C, Lassen MCH, Skaarup KG,Jensen JUS, Fralick M, Schou M, Lamberts M, Gerds T, et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation (2020) 142: 2080-2082. [CrossRef]
- Mohkhedkar M, Krishna Venigalia SS, Janakiraman V. Untangling COVID-19 and autoimmunity: Identification of plausible targets suggests multi organ involvement. Mol. Immunol. (2021) 137: 105–113. [CrossRef]
- Moody R, Sonda S, Johnston FH, Smith KJ, Stephens N, McPherson M, Flanagan KL, Poebanski M. Antibodies against Spike protein correlate with broad autoantigen recognition 8 months post SARS-CoV-2 exposure, and anti-calprotectin autoantibodies associated with better clinical outcomes. Front. Immunol. (2022) 13: 945021. [CrossRef]
- Schreckenberg R, Woitasky N, Itani N, Czech L, Ferdinandy P, Schulz R. Cardiac side effects of RNA-based SARS-CoV-2 vaccines: Hidden cardiotoxic effects of mRNA-1273 and BNT162b2 on ventricular myocyte function and structure. Brit. J. Pharmacol. (2023) Oct 12. Online ahead of print. [CrossRef]
- Gaitzsch E, Czermak T, Ribeiro A, Heun Y, Bohmer M, Merkle M, Merkle M, Mannell H, Schulz C, Wörnle M, Pircher J. Double-stranded DNA induces a prothrombotic phenotype in the vascular endothelium. Sci. Rep. (2017) 7(1):1112. [CrossRef]
- Ma X, Xin D, She R, Liu D, Ge J, Mei Z. Novel insight into cGAS-STING pathway in ischemic stroke: from pre- to post-disease. Front Immunol. (2023) 14: e1275408. [CrossRef]
- Nakahara T, Iwabuchi Y, Miyazawa R, Tonda K, Shiga T, Strauss HW, Antoniades C, Narula J, Jinzaki M. Assessment of Myocardial 18F-18F-FDG Uptake at PET/CT in Asymptomatic SARS-CoV-2-vaccinated and Nonvaccinated individuals. Radiology (2023) 308 (3), Published Online Sep 19 2023. https://pubs.rsna.org/doi/10.1148/radiol.23074.
- Barmada A, Klien J, Ramaswamy A, Brodsky NN, Jaycox JR, Sheilkha H, Jones KM, Habet V, Campbell M, Sumida TS, et al. Cytokinopathy with aberrant cytotoxic lymphocytes and profibrotic myeloid response in SARS-CoV-2 mRNA vaccine–associated myocarditis. Sci. Immunol. (2023) 8: eadh3455. [CrossRef]
- Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of Sex and Gender on COVID-19 Outcomes in Europe. Biol Sex Differ (2020) 11: 29. [CrossRef]
- AlGhatrif M, Tanaka T, Moore AZ, Bandinelli S, Lakatta EG, Ferrucci L. Age-associated difference in circulating ACE2, the gateway for SARS-COV-2, in humans: results from the InCHIANTI study. GeroScience (2021) 43: 619–627. [CrossRef]
- Lanjanian H, Moazzam-Jazi M, Hedayati M, Akbarzadeh M, Guity K, Sedaghati-Khayat B, Azizi F, Daneshpour MS. SARS-CoV-2 infection susceptibility infuenced by ACE2 genetic polymorphisms: insights from Tehran cardio-metabolic genetic study. Sci. Rep. (2021) 11: 1529. [CrossRef]
- Cao Y, Li L, Feng Z, Wan S, Huang P, Sun X, Wen F, Huang X, Ning G, Wang W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. (2020) 6: 4–7. [CrossRef]
- Benetti E, Tita R, Spiga O, Ciolfi A, Birolo G, Bruselles A, Doddato G, Giliberti A, Marconi C, Musacchia F, et al. ACE2 gene variants may underlie interindividual variability and susceptibility to COVID-19 in the Italian population. Eur. J. Hum. Genet. (2020) 28: 1602–1614. [CrossRef]
- Othman H, Bouslama Z, Brandenburg JT, da Rocha J, Hamdi Y, Ghedira K, Srairi-Abid N, Hazelhurst S. Interaction of the spike protein RBD from SARS-CoV-2 with ACE2: similarity with SARS-CoV, hot-spot analysis and effect of the receptor polymorphism. Biochem. Biophys. Res. Commun. (2020) 527: 702–708. [CrossRef]
- Suryamohan K, Diwanji D, Stawiski EW, Gupta R, Miersch S, Liu J, Chen C, Jiang YP, Fellouse FA, Sathirapongsasuti JF, et al. Human ACE2 receptor polymorphisms and altered susceptibility to SARSCoV-2. Comm. Biol. (2021). 4: 475. [CrossRef]
- Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. (2020) 116: 1097–1100. [CrossRef]
- Hikmet F, Méar L, Edvinsson A, Micke P, Uhlén M, Lindskog, C. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. (2020) 16: e9610. [CrossRef]
- Nassar M, Nso N, Gonzalez C, Lakhdar S, Alshamam M, Elshafey M, Abdalazeem Y, Nyein A, Punzalan B, Durrance RJ, et al. COVID-19 vaccine-induced myocarditis: Case report with literature review. Diab. Metabolism Synd. : Clin. Res. Rev (2021) 15: 102205. [CrossRef]
- Yu CKM, Tsao S, Ng CWK, Chua GT, Chan KL, Shi J, Chan YYT, lp P, Kwan MYW, Cheung YF. Cardiovascular assessment up to one year after COVID-19 vaccine-associated myocarditis. Circulation (2023) 148: 436-439. [CrossRef]
- Stowe J, Miller E, Andrews N, Whitaker HJ Risk of myocarditis and pericarditis after a COVID-19 mRNA vaccine booster and after COVID-19 in those with and without prior SARSCoV-2 infection: A self-controlled case series analysis in England. PLoS Med (2023) 20(6): e1004245. [CrossRef]
- Abd El-Aziz TM, Al-Sabi A, Stockand JD. Human recombinant soluble ACE2 (hrsACE2) shows promise for treating severe COVID19. Sign. Transduct. Target Ther. (2020) 5: 258 . [CrossRef]
- Higuchi Y, Suzuki T, Arimori T, Ikemura N, Mihara E, Kirita Y, Ohgitani E, Mazda O, Motooka D, Nakamura S, et al. Engineered ACE2 receptor therapy overcomes mutational escape of SARS-CoV-2. Nature Com. (2021) 12: 3802 . [CrossRef]
- Krishnamurthy S, Lockey RF, Kolliputi N. Soluble ACE2 as a potential therapy for COVID-19. Am. J. Physiol. Cell Physiol. (2021) 320: C279–C281. [CrossRef]
- Focosi D, McConnell S, Casadevall A, Cappello E, Valdiserra G, Tuccori M. Monoclonal antibody therapies against SARS-CoV-2. Lancet Inf. Dis. (2022) 22(11): E311-E326. [CrossRef]
- Onodera Y, Liang J, Li Y, Griffin B, Thanabalasingam T, Lu C, Zhu JY, Liu M, Moraes T, Zheng W, et al. Inhalation of ACE2 as a therapeutic target on sex-bias differences in SARS-CoV-2 infection and variant of concern. iScience (2023) 26: 107470. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





