Submitted:
28 November 2023
Posted:
29 November 2023
You are already at the latest version
Abstract
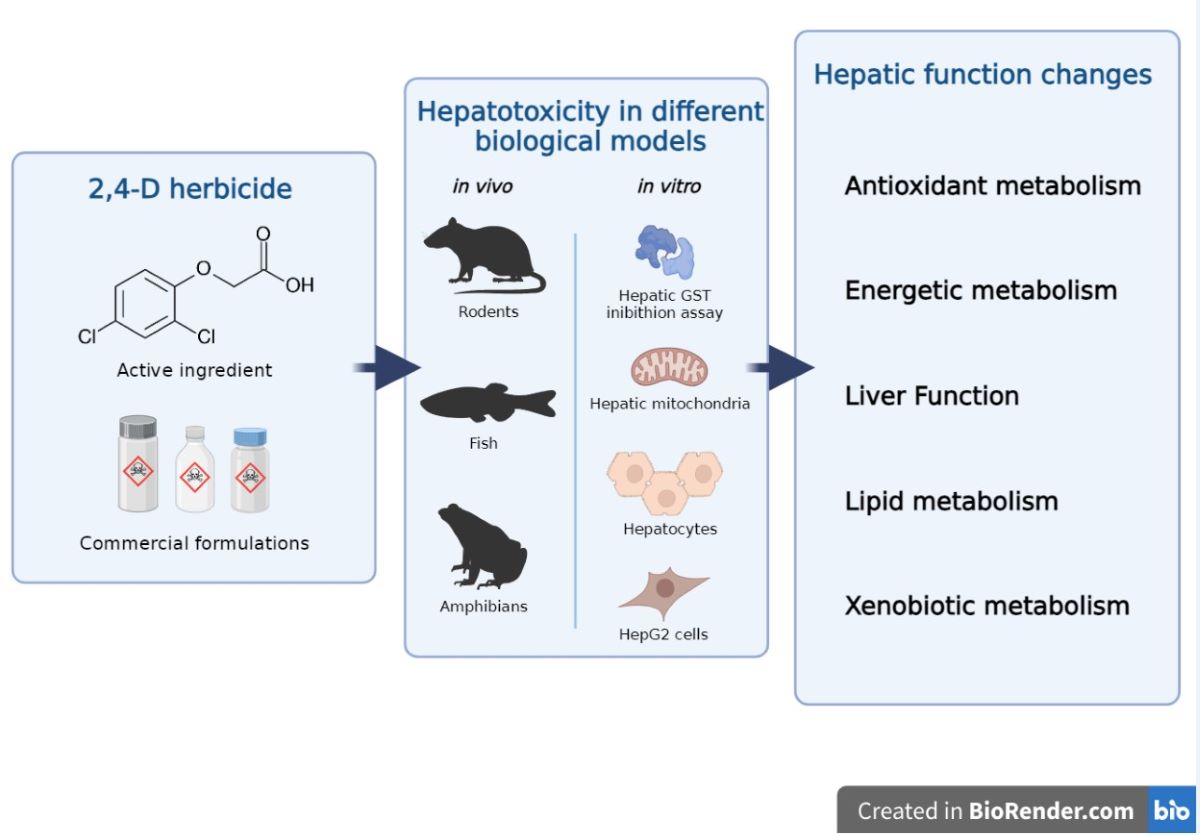
Keywords:
1. Introduction
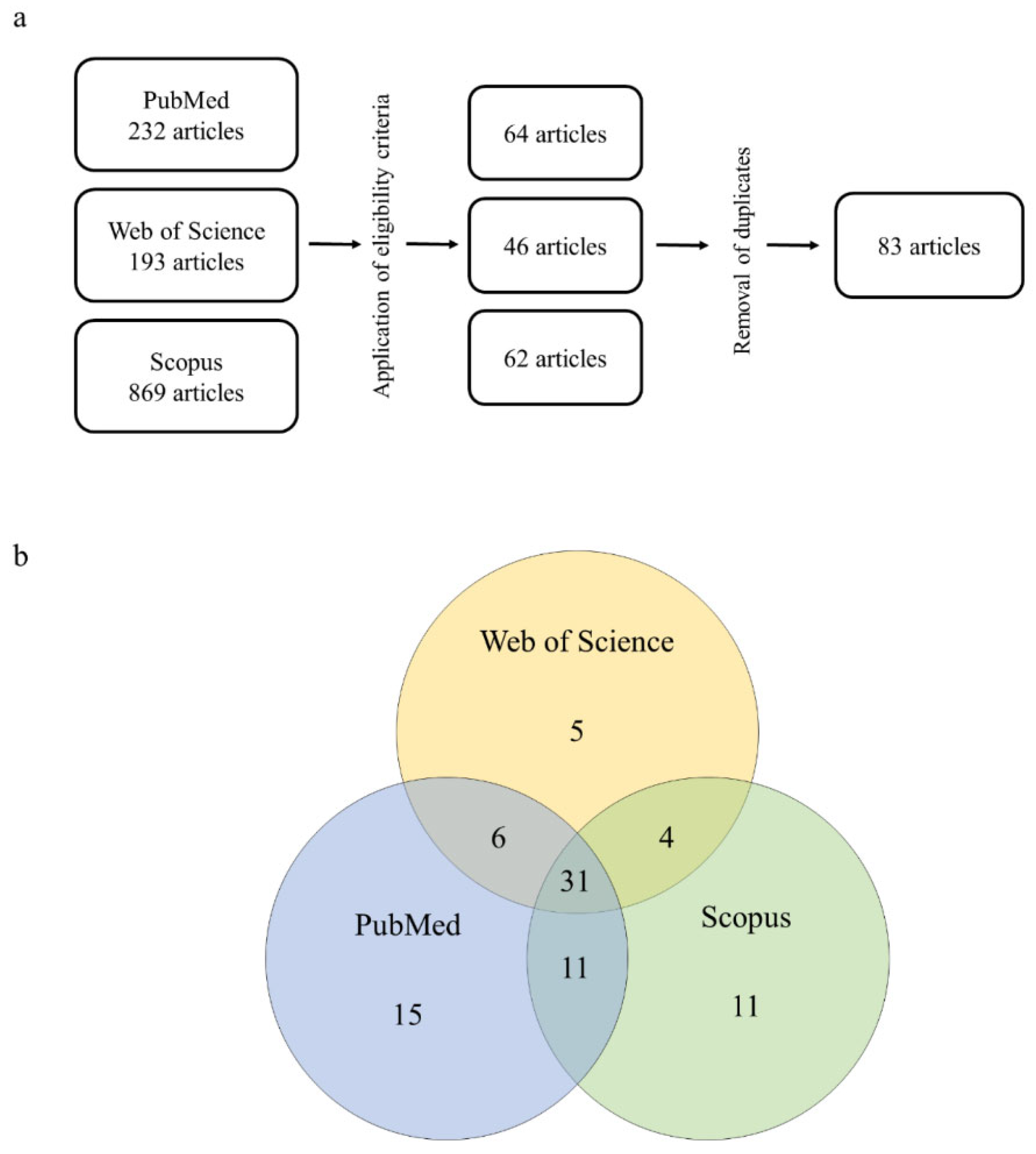
2. Materials and Methods
2.1. Overview
3. Results and discussion
3.1. Historical review and geographical distribution
3.2. Chemical compounds
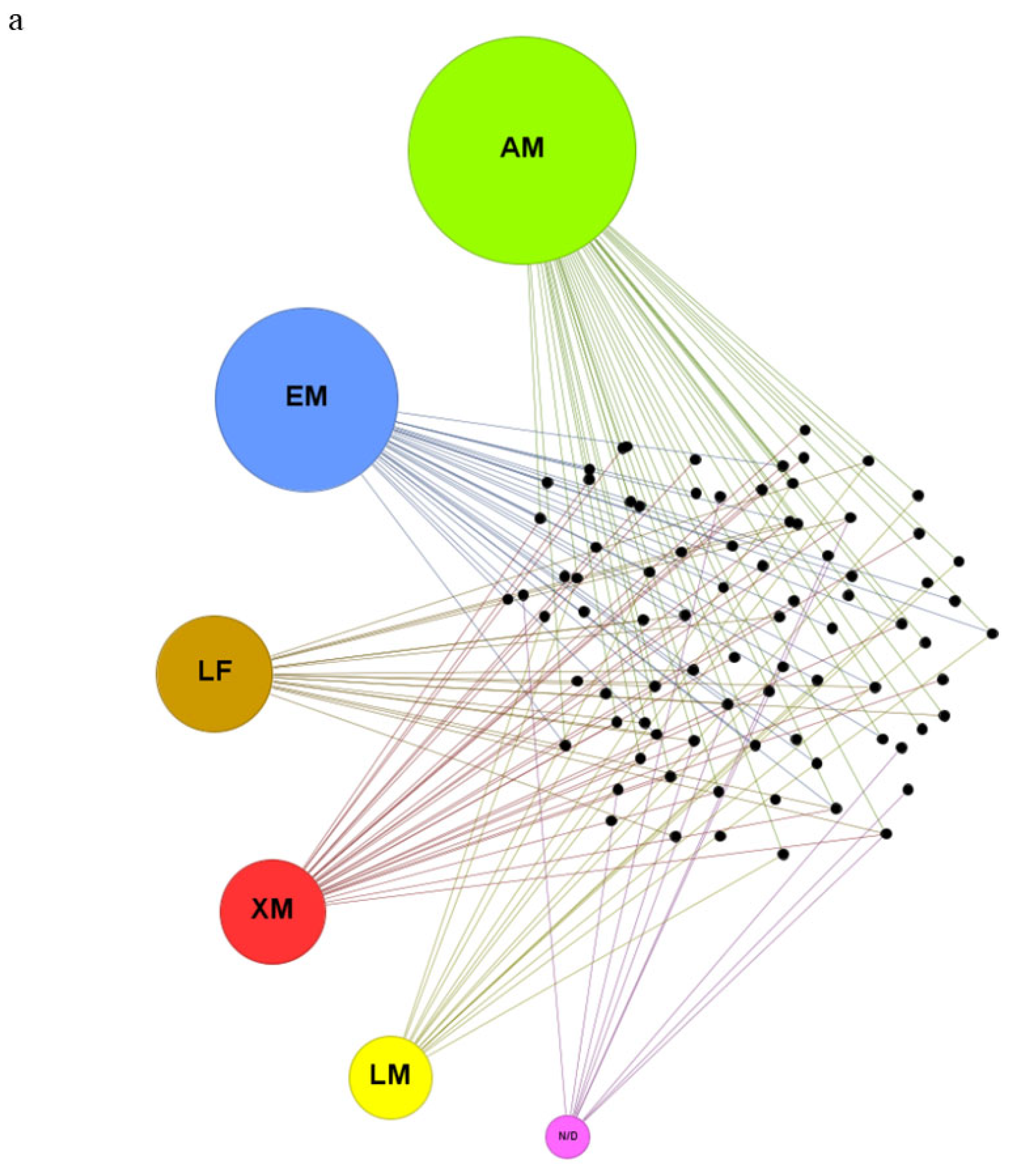
3.3. Biological models
3.4. Morphological markers
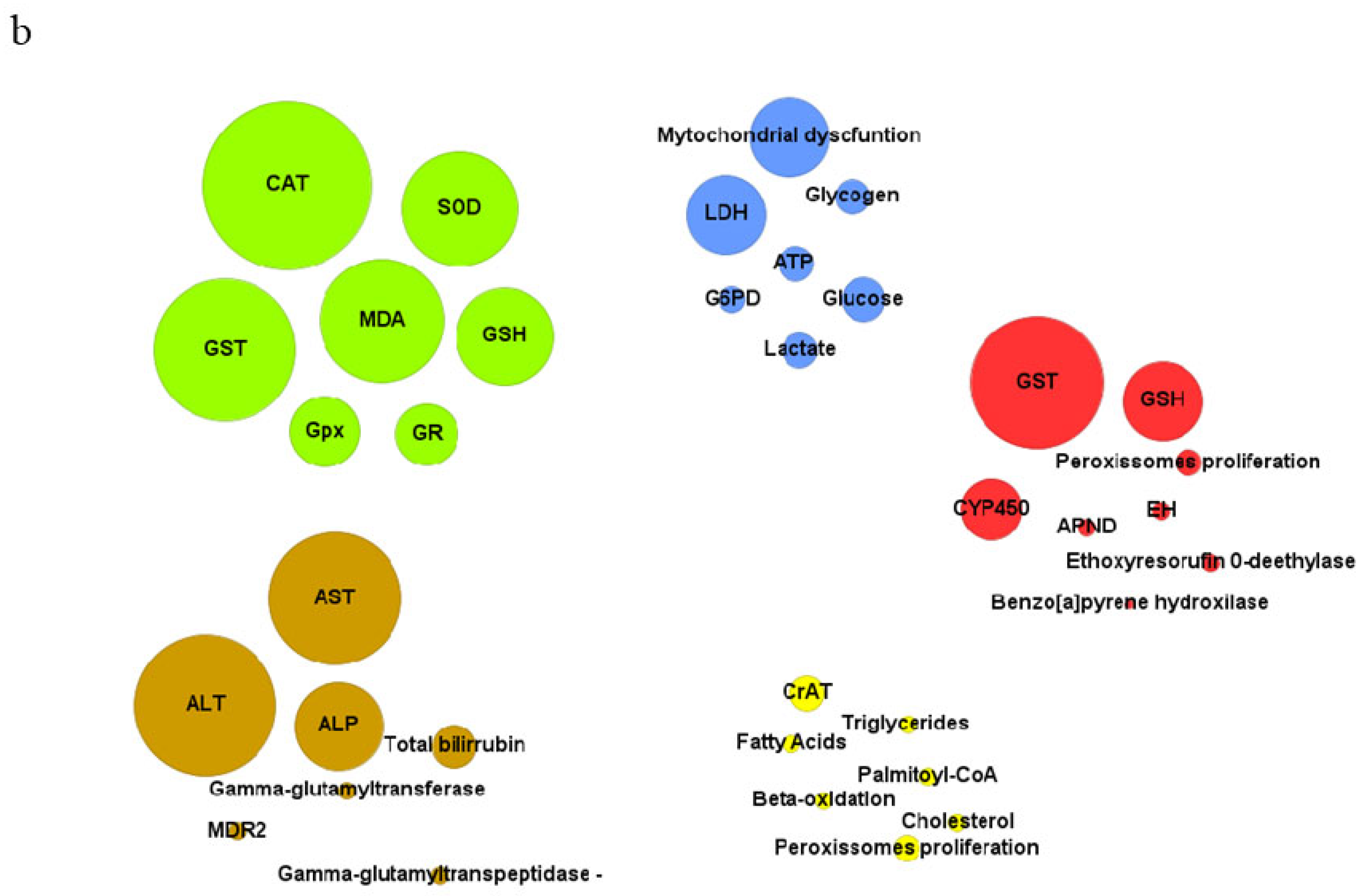
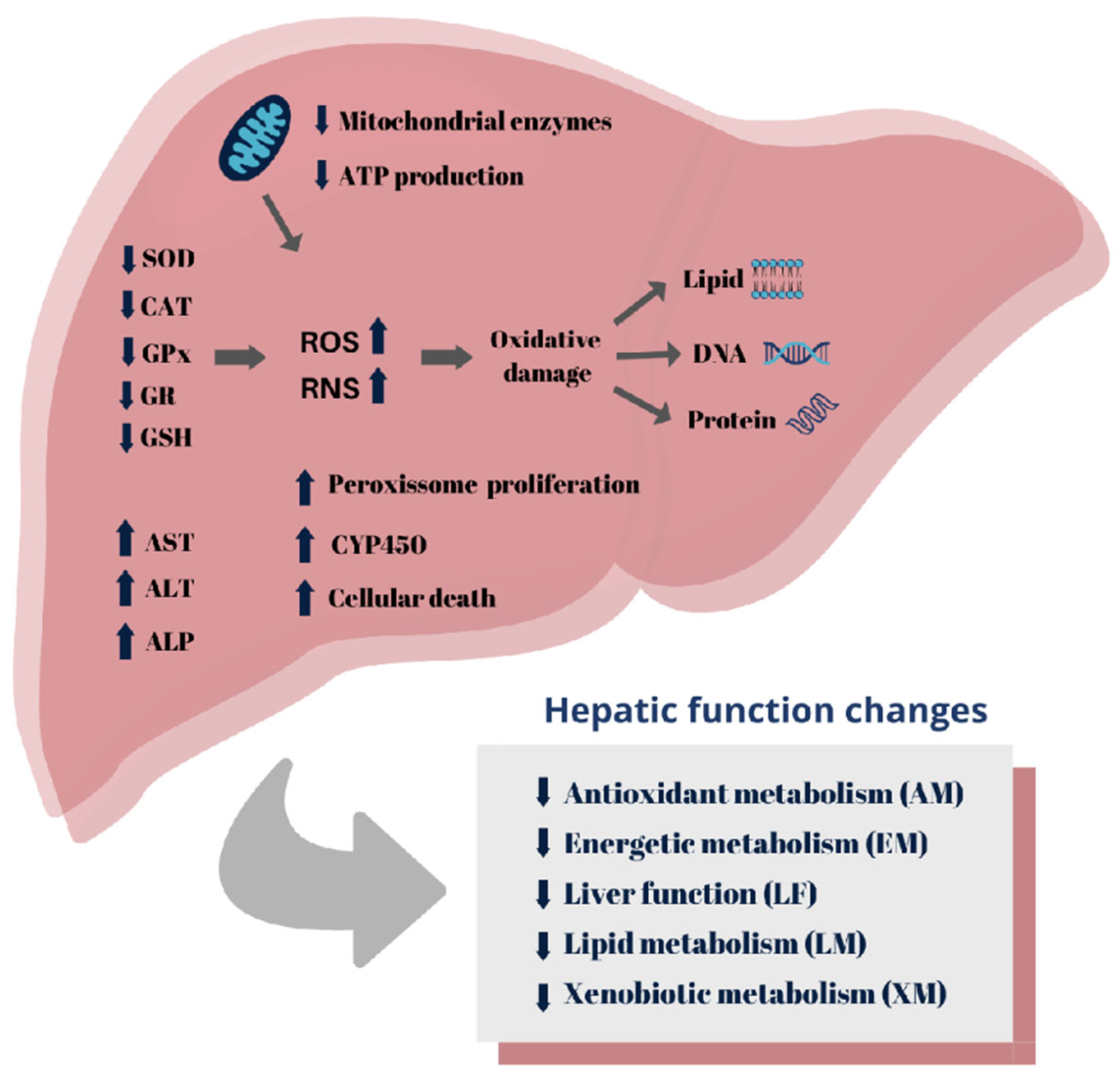
3.5. Toxicity biomarkers
3.5.1. Antioxidant metabolism
3.5.2. Energetic metabolism
3.5.3. Lipid metabolism
3.5.4. Liver Function
3.5.5. Xenobiotic metabolism
3.6. Hepatoprotective assessments
3.7. Pesticide mixtures containing 2,4-D
3.8. Conclusions and perspectives
Author Contributions
Funding
Acknowledgments
Declaration of competing interest
References
- Li K, Wu JQ, Jiang LL, Shen LZ, Li JY, He ZH, et al. Developmental toxicity of 2,4-dichlorophenoxyacetic acid in zebrafish embryos. Chemosphere. 2017 Mar;171:40–8. [CrossRef]
- Martins RX, Vieira L, Souza JACR, Silva MGF, Muniz MS, Souza T, et al. Exposure to 2,4-D herbicide induces hepatotoxicity in zebrafish larvae. Comp Biochem Physiol Part C Toxicol Pharmacol. 2021 Oct;248:109110. [CrossRef]
- Da Silva AP, Morais ER, Oliveira EC, Ghisi N de C. Does exposure to environmental 2,4-dichlorophenoxyacetic acid concentrations increase mortality rate in animals? A meta-analytic review. Environ Pollut. 2022 Jun;303:119179. [CrossRef]
- Freitas L, Valadares L de A, Camozzi M, de Oliveira P, Ferreira Machado M, Lima F. Animal models in the neurotoxicology of 2,4-D. Hum Exp Toxicol. 2019 Oct;38(10):1178–82. [CrossRef]
- Magnoli K, Carranza CS, Aluffi ME, Magnoli CE, Barberis CL. Herbicides based on 2,4-D: its behavior in agricultural environments and microbial biodegradation aspects. A review. Environ Sci Pollut Res. 2020 Nov;27(31):38501–12. [CrossRef]
- Freisthler MS, Robbins CR, Benbrook CM, Young HA, Haas DM, Winchester PD, et al. Association between increasing agricultural use of 2,4-D and population biomarkers of exposure: findings from the National Health and Nutrition Examination Survey, 2001–2014. Environ Health. 2022 Dec;21(1):23.
- IBAMA. Pesticide Commercialization Reports. [Internet]. Ministry of the Environment.; 2022. Available from: https://www.gov.br/ibama/en-us/topics/chemicals-and-biology/pesticides/pesticide-commercialization-report.
- Casimero M, Abit MJ, Ramirez AH, Dimaano NG, Mendoza J. Herbicide use history and weed management in Southeast Asia. Adv Weed Sci. 2022 Dec 22;40(spe1):e020220054. [CrossRef]
- Liu W, Li H, Tao F, Li S, Tian Z, Xie H. Formation and contamination of PCDD/Fs, PCBs, PeCBz, HxCBz and polychlorophenols in the production of 2,4-D products. Chemosphere. 2013 Jul;92(3):304–8. [CrossRef]
- Dehnert GK, Karasov WH, Wolman MA. 2,4-Dichlorophenoxyacetic acid containing herbicide impairs essential visually guided behaviors of larval fish. Aquat Toxicol. 2019 Apr;209:1–12.
- Gaaied S, Oliveira M, Le Bihanic F, Cachot J, Banni M. Gene expression patterns and related enzymatic activities of detoxification and oxidative stress systems in zebrafish larvae exposed to the 2,4-dichlorophenoxyacetic acid herbicide. Chemosphere. 2019 Jun;224:289–97.
- Islam F, Wang J, Farooq MA, Khan MSS, Xu L, Zhu J, et al. Potential impact of the herbicide 2,4-dichlorophenoxyacetic acid on human and ecosystems. Environ Int. 2018 Feb;111:332–51.
- Ensminger MP, Budd R, Kelley KC, Goh KS. Pesticide occurrence and aquatic benchmark exceedances in urban surface waters and sediments in three urban areas of California, USA, 2008–2011. Environ Monit Assess. 2013 May;185(5):3697–710.
- Rodil R, Quintana JB, Concha-Graña E, López-Mahía P, Muniategui-Lorenzo S, Prada-Rodríguez D. Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere. 2012 Mar;86(10):1040–9. [CrossRef]
- Meftaul IM, Venkateswarlu K, Dharmarajan R, Annamalai P, Megharaj M. Movement and Fate of 2,4-D in Urban Soils: A Potential Environmental Health Concern. ACS Omega. 2020 Jun 9;5(22):13287–95. [CrossRef]
- Yamini Y, Saleh A. Ultrasound-assisted emulsification microextraction combined with injection-port derivatization for the determination of some chlorophenoxyacetic acids in water samples: Sample Preparation. J Sep Sci. 2013 Jul;36(14):2330–8.
- Tsaboula A, Papadakis EN, Vryzas Z, Kotopoulou A, Kintzikoglou K, Papadopoulou-Mourkidou E. Environmental and human risk hierarchy of pesticides: A prioritization method, based on monitoring, hazard assessment and environmental fate. Environ Int. 2016 May;91:78–93. [CrossRef]
- Nault ME, Netherland MD, Mikulyuk A, Skogerboe JG, Asplund T, Hauxwell J, et al. Efficacy, selectivity, and herbicide concentrations following a whole-lake 2,4-D application targeting Eurasian watermilfoil in two adjacent northern Wisconsin lakes. Lake Reserv Manag. 2014 Jan 2;30(1):1–10.
- SISAGUA. Detection and concentration of pesticides from 2014 to 2017 in human drinking water. [Internet]. Ministry of Health; 2018. Available from: http://www.vigilanciasanitaria.sc.gov.br/index.php/saude-ambiental/sisagu.
- Zuanazzi NR, Ghisi NDC, Oliveira EC. Analysis of global trends and gaps for studies about 2,4-D herbicide toxicity: A scientometric review. Chemosphere. 2020 Feb;241:125016.
- Tichati L, Trea F, Ouali K. Potential Role of Selenium Against Hepatotoxicity Induced by 2,4-Dichlorophenoxyacetic Acid in Albino Wistar Rats. Biol Trace Elem Res. 2020 Mar;194(1):228–36. [CrossRef]
- Tichati L, Trea F, Ouali K. The antioxidant study proprieties of Thymus munbyanus aqueous extract and its beneficial effect on 2, 4-Dichlorophenoxyacetic acid -induced hepatic oxidative stress in albino Wistar rats. Toxicol Mech Methods. 2021 Mar 24;31(3):212–23.
- Troudi A, Ben Amara I, Samet AM, Zeghal N. Oxidative stress induced by 2,4-phenoxyacetic acid in liver of female rats and their progeny: Biochemical and histopathological studies. Environ Toxicol. 2012 Mar;27(3):137–45.
- Elufioye TO, Habtemariam S. Hepatoprotective effects of rosmarinic acid: Insight into its mechanisms of action. Biomed Pharmacother. 2019 Apr;112:108600. [CrossRef]
- Vainio H, Nickels J, Linnainmaa K. Phenoxy acid herbicides cause peroxisome proliferation in Chinese hamsters. Scand J Work Environ Health. 1982 Mar;8(1):70–3. [CrossRef]
- Vainio H, Linnainmaa K, Kähönen M, Nickels J, Hietanen E, Marniemi J, et al. Hypolipidemia and peroxisome proliferation induced by phenoxyacetic acid herbicides in rats. Biochem Pharmacol. 1983 Sep;32(18):2775–9. [CrossRef]
- Hietanen E, Linnainmaa K, Vainio H. Effects of Phenoxyherbicides and Glyphosate on the Hepatic and Intestinal Biotransformation Activities in the Rat. Acta Pharmacol Toxicol (Copenh). 1983 Aug;53(2):103–12. [CrossRef]
- Kawashima Y, Katoh H, Nakajima S, Kozuka H, Uchiyama M. Effects of 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid on peroxisomal enzymes in rat liver. Biochem Pharmacol. 1984 Jan;33(2):241–5.
- Y Kawashima, N Hanioka, H Kozuka. Induction of microsomal stearoyl-CoA desaturase by the administration of various phenoxyacetic acid derivatives. J Pharmacobiodyn. 1984;7((5)):286–93.
- Katoh H, Nakajima S, Kawashima Y, Kozuka H, Uchiyama M. Induction of rat hepatic long-chain acyl-CoA hydrolases by various peroxisome proliferators. Biochem Pharmacol. 1984 Apr;33(7):1081–5.
- Hietanen, E., Ahotupa, M., Heinonen, T., Hämäläinen, H., Kunnas, T., Linnainmaa, K., Mäntylä, E., & Vainio, H. (1985). Enhanced peroxisomal beta-oxidation of fatty acids and glutathione metabolism in rats exposed to phenoxyacetic acids. Toxicology, 34(2), 103–111. [CrossRef]
- Lundgren B, Meijer J, DePIERRE JW. Examination of the structural requirements for proliferation of peroxisomes and mitochondria in mouse liver by hypolipidemic agents, with special emphasis on structural analogues of 2-ethylhexanoic acid. Eur J Biochem. 1987 Mar;163(2):423–31. [CrossRef]
- Gorzinski S. Acute, pharmacokinetic, and subchronic toxicological studies of 2,4-dichlorophenoxyacetic acid*1, *2. Fundam Appl Toxicol. 1987 Oct;9(3):423–35.
- Lundgren B, Meijer J, DePierre JW. Induction of cytosolic and microsomal epoxide hydrolases and proliferation of peroxisomes and mitochondria in mouse liver after dietary exposure to p-chlorophenoxyacetic acid, 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid. Biochem Pharmacol. 1987 Mar;36(6):815–21.
- Mustonen R, Elovaara E, Zitting A, Linnainmaa K, Vainio H. Effects of commercial chlorophenolate, 2,3,7,8-TCDD, and pure phenoxyacetic acids on hepatic peroxisome proliferation, xenobiotic metabolism and sister chromatid exchange in the rat. Arch Toxicol. 1989 May;63(3):203–8. [CrossRef]
- A G Abdellatif, V Préat, J Vamecq, R Nilsson, M Roberfroid. Peroxisome proliferation and modulation of rat liver carcinogenesis by 2,4-dichlorophenoxyacetic acid, 2,4,5-trichlorophenoxyacetic acid, perfluorooctanoic acid and nafenopin. Carcinogenesis. 1990;1899–902. [CrossRef]
- Kuntz DJ, Rao NGS, Berg IE, Khattree R, Chaturvedi AK. Toxicity of mixtures of parathion, toxaphene and/or 2,4-D in mice. J Appl Toxicol. 1990 Aug;10(4):257–66. [CrossRef]
- Kozuka H, Yamada J, Horie S, Watanabe T. Characteristics of induction of peroxisomal fatty acid oxidation-related enzymes in rat liver by drugs: Relationships between structure and inducing activity. Biochem. Pharmacol. 1991, 41, 617-623. [CrossRef]
- N Inomata, H Yoshida, Y Aoki, M Tsunoda, M Yamamoto. Effects of MCPA and other phenoxyacid compounds on hepatic xenobiotic metabolism in rats. 1991;171–82. [CrossRef]
- Chaturvedi AK, Kuntz DJ, Rao NGS. Metabolic aspects of the toxicology of mixtures of parathion, toxaphene and/or 2,4-D in mice. J Appl Toxicol. 1991 Aug;11(4):245–51. [CrossRef]
- Knopp D, Schiller F. Oral and dermal application of 2,4-dichlorophenoxyacetic acid sodium and dimethylamine salts to male rats: Investigations on absorption and excretion as well as induction of hepatic mixed-function oxidase activities. Arch Toxicol. 1992 Feb;66(3):170–4.
- C A Paulino, J L Guerra, G H Oliveira, J Palermo-Neto. Acute, subchronic and chronic 2,4-dichlorophenoxyacetic acid (2,4-D) intoxication in rats. Vet Hum Toxicol. 1996;38:348–52.
- Miranda S, Vollrath V, Wielandt AM, Loyola’ G, Bronfman’ M, Chianale J. Overexpression of mdr2 gene hy peroxisopmroleiferators inthe mouse liver.
- Badawi AF, Cavalieri EL, Rogan EG. Effect of chlorinated hydrocarbons on expression of cytochrome P450 1A1, 1A2 and 1B1 and 2- and 4-hydroxylation of 17β-estradiol in female Sprague–Dawley rats.
- Di Paolo O, de Duffard AME, Duffard R. In vivo and in vitro binding of 2,4-dichlorophenoxyacetic acid to a rat liver mitochondrial protein. Chem Biol Interact. 2001 Sep;137(3):229–41. [CrossRef]
- Ozaki K, Mahler JF, Haseman JK, Moomaw CR, Nicolette ML, Nyska A. Unique Renal Tubule Changes Induced in Rats and Mice by the Peroxisome Proliferators 2,4-Dichlorophenoxyacetic Acid (2,4-D) and WY-14643. Toxicol Pathol. 2001 Jun;29(4):440–50. [CrossRef]
- Ge R, Tao L, Kramer PM, Cunningham ML, Pereira MA. Effect of peroxisome proliferators on the methylation and protein level of the c- myc protooncogene in B6C3F1 mice liver. J Biochem Mol Toxicol. 2002 Jan;16(1):41–7. [CrossRef]
- Yilmaz HR, Yuksel E. Effect of 2,4-dichlorophenoxyacetic acid on the activities of some metabolic enzymes for generating pyridine nucleotide pool of cells from mouse liver. Toxicol Ind Health. 2005 Aug;21(7–8):231–7. [CrossRef]
- Celik I, Tuluce Y, Isik I. Influence of subacute treatment of some plant growth regulators on serum marker enzymes and erythrocyte and tissue antioxidant defense and lipid peroxidation in rats. J Biochem Mol Toxicol. 2006 Aug;20(4):174–82.
- Aydýn H, Baran A, Demirel G, Yýldýrým M. Effects of 2,4-Dichlorophenoxyacetic acid (2,4-D) treatment on the epididymal spermatozoa, blood serum transaminases and its accumulation in liver of rats. 2006.
- Nakbi A, Tayeb W, Grissa A, Issaoui M, Dabbou S, Chargui I, et al. Effects of olive oil and its fractions on oxidative stress and the liver’s fatty acid composition in 2,4-Dichlorophenoxyacetic acid-treated rats. Nutr Metab. 2010;7(1):80.
- Tayeb W, Nakbi A, Trabelsi M, Attia N, Miled A, Hammami M. Hepatotoxicity induced by sub-acute exposure of rats to 2,4-Dichlorophenoxyacetic acid based herbicide “Désormone lourd.” J Hazard Mater. 2010 Aug 15;180(1–3):225–33.
- Nakbi A, Tayeb W, Dabbou S, Chargui I, Issaoui M, Zakhama A, et al. Hypolipidimic and antioxidant activities of virgin olive oil and its fractions in 2,4-diclorophenoxyacetic acid–treated rats. Nutrition. 2012 Jan;28(1):81–91. [CrossRef]
- Tayeb W, Nakbi A, Cheraief I, Miled A, Hammami M. Alteration of lipid status and lipid metabolism, induction of oxidative stress and lipid peroxidation by 2,4-dichlorophenoxyacetic herbicide in rat liver. Toxicol Mech Methods. 2013 Jul;23(6):449–58. [CrossRef]
- Kalipci E, Ozdemir C, Oztas H. Assessing eco-toxicological effects of industrial 2,4-D acid iso-octylester herbicide on rat pancreas and liver. Biotech Histochem. 2013 May;88(3–4):202–7. [CrossRef]
- Mazhar FM, Moawad KM, El-Dakdoky MH, Amer AS. Fetotoxicity of 2,4-dichlorophenoxyacetic acid in rats and the protective role of vitamin E. Toxicol Ind Health. 2014 Jun;30(5):480–8.
- Al-Baroudi DA, Arafat R, El-kholy T. Hepatoprotective effect of chamomile capitula extract against 2,4-dichlorophenoxyacetic acid-induced hepatotoxicity in rats. Life Science Journal. 2014;11(8):34–40.
- Dakhakhni TH, Raouf GA, Qusti SY. Evaluation of the toxic effect of the herbicide 2, 4-D on rat hepatocytes: an FT-IR spectroscopic study. Eur Biophys J. 2016 May;45(4):311–20. [CrossRef]
- Satapathy A., M. Rao. Protective effect of Curcumin on 2, 4- Dichlorophenoxy acetic acid exerted Hepatotoxicity in Mice. Research Journal of Pharmacy and Technology. 2018;11(2):637–42.
- Shafeeq S, Mahboob T. Magnesium supplementation ameliorates toxic effects of 2,4-dichlorophenoxyacetic acid in rat model. Hum Exp Toxicol. 2020 Jan;39(1):47–58.
- Bonfim DJP, Magalhães LR, Chagas PHN, Serra FDM, Benatti LAT, Nai GA. Hepatic, renal, and pancreatic damage associated with chronic exposure to oral and inhaled 2,4-dichlorophenoxy acetic acid (2,4-d): an environmental exposure model in rats. Comp Clin Pathol. 2020 Oct;29(5):1001–10. [CrossRef]
- Bueno Franco Salla G, Bracht L, Valderrama Parizotto A, Comar JF, Peralta RM, Bracht F, et al. Kinetics of the metabolic effects, distribution spaces and lipid-bilayer affinities of the organo-chlorinated herbicides 2,4-D and picloram in the liver. Toxicol Lett. 2019 Oct;313:137–49. [CrossRef]
- Shafeeq S, Mahboob T. 2,4-Dichlorophenoxyacetic acid induced hepatic and renal toxicological perturbations in rat model: Attenuation by selenium supplementation. Toxicol Ind Health. 2021 Mar;37(3):152–63.
- Ince S, Demirel HH, Zemheri-Navruz F, Arslan-Acaroz D, Kucukkurt I, Acaroz U, et al. Synergistic toxicity of ethanol and 2,4-dichlorophenoxyacetic acid enhances oxidant status, DNA damage, inflammation, and apoptosis in rats. Environ Sci Pollut Res. 2022 Sep 9;30(4):10710–23. [CrossRef]
- Nechalioti PM, Karampatzakis T, Mesnage R, Antoniou MN, Ibragim M, Tsatsakis A, et al. Evaluation of perinatal exposure of glyphosate and its mixture with 2,4-D and dicamba οn liver redox status in Wistar rats. Environ Res. 2023 Jul;228:115906. [CrossRef]
- Gallagher E, Digiulio R. Effects of 2, 4-dichlorophenoxyacetic acid and picloram on biotransformation, peroxisomal and serum enzyme activities in channel catfish (Ictalurus punctatus). Toxicol Lett. 1991 Jun;57(1):65–72.
- Neskovid N, Karan V, Elezovic I, Poleksic V, Budimir M. Toxic effects of 2,4-D herbicide on fish. J Environ Sci Health Part B. 1994;29(2):265–79.
- Oruç EÖ, Üner N. Combined effects of 2,4-D and azinphosmethyl on antioxidant enzymes and lipid peroxidation in liver of Oreochromis niloticus. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 2000 Dec;127(3):291–6. [CrossRef]
- Ackers JT, Johnston MF, Haasch ML. Immunodetection of hepatic peroxisomal PMP70 as an indicator of peroxisomal proliferation in the mummichog, Fundulus heteroclitus. Mar Environ Res. 2000 Jul;50(1–5):361–5. [CrossRef]
- Özcan Oruç E, Üner N. Marker enzyme assesment in the liver of cyprinus carpio (L.) exposed to 2,4-D and azinphosmethyl: Marker Enzymes in Cyprinus Carpio. J Biochem Mol Toxicol. 2002 Aug;16(4):182–8.
- da Fonseca MB, Glusczak L, Silveira Moraes B, de Menezes CC, Pretto A, Tierno MA, et al. The 2,4-D herbicide effects on acetylcholinesterase activity and metabolic parameters of piava freshwater fish (Leporinus obtusidens). Ecotoxicol Environ Saf. 2008 Mar;69(3):416–20.
- Cattaneo R, Loro VL, Spanevello R, Silveira FA, Luz L, Miron DS, et al. Metabolic and histological parameters of silver catfish (Rhamdia quelen) exposed to commercial formulation of 2,4-dichlorophenoxiacetic acid (2,4-D) herbicide. Pestic Biochem Physiol. 2008 Nov;92(3):133–7.
- Matviishyn TM, Kubrak OI, Husak VV, Storey KB, Lushchak VI. Tissue-specific induction of oxidative stress in goldfish by 2,4-dichlorophenoxyacetic acid: Mild in brain and moderate in liver and kidney. Environ Toxicol Pharmacol. 2014 Mar;37(2):861–9.
- Vigário AF, Sabóia-Morais SMT. Effects of the 2,4-D herbicide on gills epithelia and liver of the fish Poecilia vivipara. Pesqui Veterinária Bras. 2014 Jun;34(6):523–8.
- Menezes C, Fonseca MB, Leitemperger J, Pretto A, Moraes BS, Murussi CR, et al. Commercial formulation containing 2,4-D affects biochemical parameters and morphological indices of silver catfish exposed for 90 days. Fish Physiol Biochem. 2015 Apr;41(2):323–30. [CrossRef]
- Yakovenko BV, Tretyak OP, Mekhed OB, Iskevych OV. Effect of herbicides and surfactants on enzymes of energy metabolism in European carp. Ukr J Ecol. 2018 Mar 22;8(1):948–52. [CrossRef]
- Kaya İ, Yılmaz M, Kaya MM, Kükürt bdulsamed, Karapehlivan M. The Effects of Carbaryl and 2,4- Dichlorophenoxyacetic Acid on Oxidative Stress Index in Capoeta capoeta (Guldensteadt 1773). Pak J Zool [Internet]. 2018 [cited 2023 Jul 14];51(1). Available from: http://researcherslinks.com/current-issues/The-Effects-of-Carbaryl-and-Dichlorophenoxyacetic/20/1/1847/htm.
- Zaffaroni NP, Zavanella T, Cattaneo A, Arias E. The toxicity of 2,4-dichlorophenoxyacetic acid to the adult crested newt. Environ Res. 1986 Oct;41(1):79–87. [CrossRef]
- Van Meter RJ, Glinski DA, Purucker ST, Henderson WM. Influence of exposure to pesticide mixtures on the metabolomic profile in post-metamorphic green frogs (Lithobates clamitans). Sci Total Environ. 2018 May;624:1348–59.
- Curi LM, Peltzer PM, Sandoval MT, Lajmanovich RC. Acute Toxicity and Sublethal Effects Caused by a Commercial Herbicide Formulated with 2,4-D on Physalaemus albonotatus Tadpoles. Water Air Soil Pollut. 2019 Jan;230(1):22. [CrossRef]
- Dierickx PJ. Interaction of chlorophenoxyalkyl acid herbicides with rat-liver glutathione S-transferases. Food Chem Toxicol. 1983 Oct;21(5):575–9. [CrossRef]
- Vessey DA, Boyer TD. Differential activation and inhibition of different forms of rat liver glutathione S-transferase by the herbicides 2,4-dichlorophenoxyacetate (2,4-D) and 2,4,5-trichlorophenoxyacetate (2,4,5-T). Toxicol Appl Pharmacol. 1984 May;73(3):492–9. [CrossRef]
- Dierickx PJ. Hepatic glutathione S-transferases in rainbow trout and their interaction with 2,4-dichlorophenoxyacetic acid and 1,4-benzoquinone. Comp Biochem Physiol Part C Comp Pharmacol. 1985 Jan;82(2):495–500. [CrossRef]
- Singh S. Inhibition of human glutathione S-transferases by 2,4-dichlorophenoxyacetate (2,4-D) and 2,4,5-trichlorophenoxyacetate (2,4,5-T). Toxicol Appl Pharmacol. 1985 Nov;81(2):328–36.
- Elia AC, Mantilacci L, Natali M, Principato G. Association of glutathione peroxidase activity with an acidic glutathione S-transferase in carp liver. Ital J Zool. 2000 Jan 1;67(1):39–43. [CrossRef]
- Dierickx PJ. Interaction of 1,4-benzoquinone and 2,4-dichlorophenoxyacetic acid with microsomal glutathione transferase from rat liver. Arch Int Physiol Biochim. 1988 Jan;96(1):1–5.
- Özaslan MS, Demir Y, Aksoy M, Küfrevioğlu ÖI, Beydemir Ş. Inhibition effects of pesticides on glutathione- S -transferase enzyme activity of Van Lake fish liver. J Biochem Mol Toxicol. 2018 Sep;32(9):e22196.
- Dixon A, Osterloh J, Becker C. Inhibition of Palmitoyl Co-enzyme A Hydrolase in Mitochondria and Microsomes by Pharmaceutical Organic Anions. J Pharm Sci. 1990 Feb;79(2):103–5.
- Zychlinski L, Zolnierowicz S. Comparison of uncoupling activities of chlorophenoxy herbicides in rat liver mitochondria. Toxicol Lett. 1990 Jun;52(1):25–34.
- Palmeira C. M., Moreno A. J., Madeira V. M. Interactions of herbicides 2,4-D and dinoseb with liver mitochondrial bioenergetics. Toxicol Appl Pharmacol. 1994;127(1):50–7.
- Pereira LF, Campello AP, Silveira O. Effect of tordon 2,4-D 64/240 triethanolamine BR on the energy metabolism of rat liver mitochondria. J Appl Toxicol. 1994 Jan;14(1):21–6.
- Oakes DJ, Pollak JK. Effects of a herbicide formulation, Tordon 75D®, and its individual components on the oxidative functions of mitochondria. Toxicology. 1999 Aug;136(1):41–52.
- Palmeira C. M., Moreno A. J., Madeira V. M. C. Metabolic alterations in hepatocytes promoted by the herbicides paraquat, dinoseb and 2,4-D. 68:24–31.
- Palmeira CM, Moreno AJ, Madeira VMC. Thiols metabolism is altered by the herbicides paraquat, dinoseb and 2,4-D: A study in isolated hepatocytes. Toxicol Lett. 1995 Nov;81(2–3):115–23. [CrossRef]
- Li C, Grillo MP, Benet LZ. In vitro studies on the chemical reactivity of 2,4-dichlorophenoxyacetyl-S-acyl-CoA thioester. Toxicol Appl Pharmacol. 2003 Mar;187(2):101–9.
- Salvo LM, Malucelli MIC, da Silva JRMC, Alberton GC, Silva De Assis HC. Toxicity assessment of 2,4-D and MCPA herbicides in primary culture of fish hepatic cells. J Environ Sci Health Part B. 2015 Jul 3;50(7):449–55. [CrossRef]
- Tuschl H, Schwab C. Cytotoxic effects of the herbicide 2,4-dichlorophenoxyacetic acid in HepG2 cells. Food Chem Toxicol. 2003 Mar;41(3):385–93. [CrossRef]
- Tuschl H, Schwab CE. Flow cytometric methods used as screening tests for basal toxicity of chemicals. Toxicol In Vitro. 2004 Aug;18(4):483–91. [CrossRef]
- Bharadwaj L, Dhami K, Schneberger D, Stevens M, Renaud C, Ali A. Altered gene expression in human hepatoma HepG2 cells exposed to low-level 2,4-dichlorophenoxyacetic acid and potassium nitrate. Toxicol In Vitro. 2005 Aug;19(5):603–19.
- Barrón Cuenca J, De Oliveira Galvão MF, Ünlü Endirlik B, Tirado N, Dreij K. In vitro cytotoxicity and genotoxicity of single and combined pesticides used by Bolivian farmers. Environ Mol Mutagen. 2022 Jan;63(1):4–17.
- Olson RJ, Trumble TE, Gamble W. Alterations in cholesterol and fatty acid biosynthesis in rat liver homogenates by aryloxy acids. Biochem J. 1974 Aug 15;142(2):445–8.
- Santagostino A, Leone MP, Maci R, Casale A, Marabini L. Effects of Phenoxyacetic Acid Herbicides on Chicken Embryo Liver Drug Metabolizing Enzymes. Pharmacol Toxicol. 1991 Feb;68(2):110–4. [CrossRef]
- Evangelista de Duffard A, Fabra de Peretti A, Castro de Cantarini S, Duffard R. Effects of 2,4-dichlorophenoxyacetic acid butyl ester on chick liver. Arch Environ Contam Toxicol [Internet]. 1993 Aug [cited 2023 Jul 14];25(2). Available from: http://link.springer.com/10.1007/BF0021213.
- Adeva-Andany MM, Pérez-Felpete N, Fernández-Fernández C, Donapetry-García C, Pazos-García C. Liver glucose metabolism in humans. Biosci Rep. 2016 Dec 1;36(6):e00416. [CrossRef]
- Almazroo OA, Miah MK, Venkataramanan R. Drug Metabolism in the Liver. Clin Liver Dis. 2017 Feb;21(1):1–20.
- Ore A, Akinloye O. Oxidative Stress and Antioxidant Biomarkers in Clinical and Experimental Models of Non-Alcoholic Fatty Liver Disease. Medicina (Mex). 2019 Jan 24;55(2):26. [CrossRef]
- Nagy K, Duca RC, Lovas S, Creta M, Scheepers PTJ, Godderis L, et al. Systematic review of comparative studies assessing the toxicity of pesticide active ingredients and their product formulations. Environ Res. 2020 Feb;181:108926. [CrossRef]
- Mesnage R, Antoniou MN. Ignoring Adjuvant Toxicity Falsifies the Safety Profile of Commercial Pesticides. Front Public Health. 2018 Jan 22;5:361. [CrossRef]
- Bambino K, Morrison J, Chu J. Hepatotoxicity in Zebrafish Larvae. In: Hansen JM, Winn LM, editors. Developmental Toxicology [Internet]. New York, NY: Springer New York; 2019 [cited 2023 Nov 24]. p. 129–38. (Methods in Molecular Biology; vol. 1965). Available from: http://link.springer.com/10.1007/978-1-4939-9182-2_.
- Ingber DE. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat Rev Genet. 2022 Aug;23(8):467–91.
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007 May;8(5):353–67.
- Ruiz de Arcaute C, Soloneski S, Larramendy ML. Toxic and genotoxic effects of the 2,4-dichlorophenoxyacetic acid (2,4-D)-based herbicide on the Neotropical fish Cnesterodon decemmaculatus. Ecotoxicol Environ Saf. 2016 Jun;128:222–9.
- Wilkins BJ, Pack M. Zebrafish Models of Human Liver Development and Disease. In: Terjung R, editor. Comprehensive Physiology [Internet]. 1st ed. Wiley; 2013 [cited 2022 Jun 17]. p. 1213–30. Available from: https://onlinelibrary.wiley.com/doi/10.1002/cphy.c12002.
- Trefts E, Gannon M, Wasserman DH. The liver. Curr Biol. 2017 Nov;27(21):R1147–51.
- Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020 May;152:116–41.
- Cichoż-Lach H. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 2014;20(25):8082. [CrossRef]
- Han KH. Relationships among alcoholic liver disease, antioxidants, and antioxidant enzymes. World J Gastroenterol. 2016;22(1):37. [CrossRef]
- Massarsky A, Kozal JS, Di Giulio RT. Glutathione and zebrafish: Old assays to address a current issue. Chemosphere. 2017 Feb;168:707–15. [CrossRef]
- Ramanathan R, Ali AH, Ibdah JA. Mitochondrial Dysfunction Plays Central Role in Nonalcoholic Fatty Liver Disease. Int J Mol Sci. 2022 Jun 30;23(13):7280. [CrossRef]
- Datta S, Sahdeo S, Gray JA, Morriseau C, Hammock BD, Cortopassi G. A high-throughput screen for mitochondrial function reveals known and novel mitochondrial toxicants in a library of environmental agents. Mitochondrion. 2016 Nov;31:79–83.
- Igbinosa EO, Odjadjare EE, Chigor VN, Igbinosa IH, Emoghene AO, Ekhaise FO, et al. Toxicological Profile of Chlorophenols and Their Derivatives in the Environment: The Public Health Perspective. Sci World J. 2013;2013:1–11.
- Shannon RD, Boardman GD, Dietrich AM, Bevan DR. Mitochondrial response to chlorophenols as a short-term toxicity assay. Environ Toxicol Chem. 1991 Jan;10(1):57–66.
- Mansouri A, Gattolliat CH, Asselah T. Mitochondrial Dysfunction and Signaling in Chronic Liver Diseases. Gastroenterology. 2018 Sep;155(3):629–47. [CrossRef]
- Paradies G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J Gastroenterol. 2014;20(39):14205. [CrossRef]
- Arya DS, Arora S, Malik S, Nepal S, Kumari S, Ojha S. Effect of Piper betle on cardiac function, marker enzymes, and oxidative stress in isoproterenol-induced cardiotoxicity in rats. Toxicol Mech Methods. 2010 Nov;20(9):564–71.
- Maharajan K, Muthulakshmi S, Nataraj B, Ramesh M, Kadirvelu K. Toxicity assessment of pyriproxyfen in vertebrate model zebrafish embryos ( Danio rerio ): A multi biomarker study. Aquat Toxicol. 2018 Mar;196:132–45.
- Song MJ, Park C, Kim H, Han S, Lee SH, Lee DH, et al. Carnitine acetyltransferase deficiency mediates mitochondrial dysfunction-induced cellular senescence in dermal fibroblasts. Aging Cell. 2023 Nov;22(11):e14000.
- Okumoto K, Tamura S, Honsho M, Fujiki Y. Peroxisome: Metabolic Functions and Biogenesis. In: Lizard G, editor. Peroxisome Biology: Experimental Models, Peroxisomal Disorders and Neurological Diseases [Internet]. Cham: Springer International Publishing; 2020. p. 3–17. [CrossRef]
- Fransen M, Lismont C, Walton P. The Peroxisome-Mitochondria Connection: How and Why? Int J Mol Sci. 2017 May 24;18(6):1126.
- Kleiboeker B, Lodhi IJ. Peroxisomal regulation of energy homeostasis: Effect on obesity and related metabolic disorders. Mol Metab. 2022;65:101577. [CrossRef]
- Giannini EG. Liver enzyme alteration: a guide for clinicians. Can Med Assoc J. 2005 Feb 1;172(3):367–79. [CrossRef]
- Sookoian S, Pirola CJ. Liver enzymes, metabolomics and genome-wide association studies: From systems biology to the personalized medicine. World J Gastroenterol. 2015;21(3):711. [CrossRef]
- Fernandes M da S, Iano FG, Rocia V, Yanai MM, Leite A de L, Furlani TA, et al. Alkaline phosphatase activity in plasma and liver of rats submitted to chronic exposure to fluoride. Braz Arch Biol Technol. 2011 Dec;54(6):1187–92.
- Veith A, Moorthy B. Role of cytochrome P450s in the generation and metabolism of reactive oxygen species. Curr Opin Toxicol. 2018 Feb;7:44–51.
- Warner J, Hardesty J, Zirnheld K, McClain C, Warner D, Kirpich I. Soluble Epoxide Hydrolase Inhibition in Liver Diseases: A Review of Current Research and Knowledge Gaps. Biology. 2020 Jun 12;9(6):124.
- Monticelli Barizon RR, Kummrow F, Fernandes De Albuquerque A, Assalin MR, Rosa MA, Cassoli De Souza Dutra DR, et al. Surface water contamination from pesticide mixtures and risks to aquatic life in a high-input agricultural region of Brazil. Chemosphere. 2022 Dec;308:136400.
- Mansano AS, Moreira RA, Dornfeld HC, Freitas EC, Vieira EM, Daam MA, et al. Individual and mixture toxicity of carbofuran and diuron to the protozoan Paramecium caudatum and the cladoceran Ceriodaphnia silvestrii. Ecotoxicol Environ Saf. 2020 Sep;201:110829. [CrossRef]
- Aparecida M, Campos Ventura- Camargo BD, Miyuki M. Toxicity of Herbicides: Impact on Aquatic and Soil Biota and Human Health. In: Price A, editor. Herbicides - Current Research and Case Studies in Use [Internet]. InTech; 2013 [cited 2023 Jul 18]. Available from: http://www.intechopen.com/books/herbicides-current-research-and-case-studies-in-use/toxicity-of-herbicides-impact-on-aquatic-and-soil-biota-and-human-healt.
- Lushchak VI, Matviishyn TM, Husak VV, Storey JM, Storey KB. Pesticide toxicity: a mechanistic approach. EXCLI J 17Doc1101 ISSN 1611-2156 [Internet]. 2018 [cited 2023 Jul 18]; Available from: https://www.excli.de/vol17/Lushchak_08112018_proof.pd.
- Zhang C, Wang N, Xu Y, Tan HY, Li S, Feng Y. Molecular Mechanisms Involved in Oxidative Stress-Associated Liver Injury Induced by Chinese Herbal Medicine: An Experimental Evidence-Based Literature Review and Network Pharmacology Study. Int J Mol Sci. 2018 Sep 13;19(9):2745. [CrossRef]
- Zhao S, Iyengar R. Systems Pharmacology: Network Analysis to Identify Multiscale Mechanisms of Drug Action. Annu Rev Pharmacol Toxicol. 2012 Feb 10;52(1):505–21. [CrossRef]
- Cotterill JV, Palazzolo L, Ridgway C, Price N, Rorije E, Moretto A, et al. Predicting estrogen receptor binding of chemicals using a suite of in silico methods – Complementary approaches of (Q)SAR, molecular docking and molecular dynamics. Toxicol Appl Pharmacol. 2019 Sep;378:114630.
- Liebsch M, Grune B, Seiler A, Butzke D, Oelgeschläger M, Pirow R, et al. Alternatives to animal testing: current status and future perspectives. Arch Toxicol. 2011 Aug;85(8):841–58. [CrossRef]
- Bastian M., Heymann S., Jacomy M. (2009). Gephi: an open source software for exploring and manipulating networks. International AAAI Conference on Weblogs and Social Media. [CrossRef]


| Biological model | Exposure compounds | Exposure conditions | Cellular and tissues damage | Impaired biochemical markers | References |
| Chinese Hamsters |
Commercial formulation (550 g/L) |
AR: oral gavage T: 9 days C: 100 mg/kg of body weight |
NA | ND: Peroxissomes plorifaration | Vainio et al. (1982) [25] |
| Rattus novergicus |
Commercial formulation (550 mg/kg) |
AR: oral gavage T: 2 weeks C: 100-200 mg/kg of body weight |
NA |
LM: peroxissome proliferation, CrAT, protein lipases AM: CAT |
Vainio et al. (1983) [26] |
| Rattus novergicus |
Commercial formulation ( 550 g/L) |
AR.: intragastrically gavage T: 2 weeks C: 100, 150 and 200 mg/kg of body weight |
NA |
XM: EH, UDPglucuronosyltransferase , GST AM: GST |
Hietanen et al. (1983) [27] |
| Rattus novergicus | Active ingredient |
AR: feeding T: 14 h C: 0.25% w/w |
NA |
LM: CrAT, palmitoyl-CoA, triglycerides AM: CAT |
Kawashima et al. (1984) [28] |
| Rattus novergicus | not specified |
AR.: feeding T: 14 days C: 0.5 % of diet |
NA | LM: stearoyl-CoA | Kawashima et al. (1984) [29] |
| Biological model | Exposure compounds | Exposure conditions | Cellular and tissues damage | Impaired biochemical markers | References |
| Rattus novergicus | Active ingredient |
AR: feeding and subcutaneously T: 1 or 2 weeks C: 0.25% of diet or 0.93 mmole or 1.86 mmole per kg of body weight |
NA | LM: acyl-CoA hydrolase II; β oxidation | Katoh et al. (1984) [30] |
| Rattus novergicus |
Commercial formulation ( 550 g/L ) |
AR: intragastrically ET: 14 days CT: 1 mmol/kg of body weight |
NA |
LM: peroxissome proliferation, β-oxidation AM: GR |
Hietanen et al. (1985) [31] |
| Mus musculus | not specified |
AR: diet T: 4 days C: --- |
Increase liver somatic index |
LM: palmitoil-CoA, CrAT EM: cytochrome oxidase |
Lundgren et al. (1987) [32] |
| Rattus novergicus | Active ingredient |
AR: gavage and feeding T: single dose and 13 days C: 553 mg/kg and 1090 mg/kg (single dose); 0, 15, 60, 100, or 150 mg/kg/day (13 days) |
Dose levels of 100 or 150 mg/kg/day produced minimal swelling and increased staining homogeneity in the liver cells and were associated with a slight elevation of liver weight |
LF: ALT, ALP EM: glucose |
Gorzinskj et al. (1987) [33] |
| Biological model | Exposure compounds | Exposure conditions | Cellular and tissues damage | Impaired biochemical markers | References |
| Mus musculus | not specified |
AR: feeding T: 4 days C: 100 mg/kg/bw |
NA |
XM: EH, CYP450, GST, peroxissome proliferation AM: GST |
Lundgren et al. (1987) [34] |
| Rattus novergicus |
Active ingredient (>99%) |
AR.: intragastrically gavage T: 2 weeks C: 100 mg/kg of body weight |
NA | XM: peroxisome proliferation, CYP450, UDP-glucunorosyl transferase, NADPH diaphorase | Mustonen et al. (1989) [35] |
| Rattus novergicus | Active ingredient |
AR.: feeding T: 7 months C: 0.05% of diet |
NA | LM: peroxissome proliferation, acyl Coa oxidase, dicarboxylyl CoA oxidase | Abdellatif et al.. (1990) [36] |
| Mus musculus |
Active ingredient (97- 99%) |
AR.: oral intubathion T: 14 days exposure + 7 days recovery C: 50 mg/kg |
Increase liver/ body weight ratio | LF: ALT | Kuntz et al. (1990) [37] |
| Rattus novergicus | Active ingredient |
AR: feeding T: 6 days C: 1.680 ppm |
NA |
LM: CrAT; carnitine palmitoyltransferase fatty acyl-CoA dehydrogenase cyanide-insensitive fatty acyl-CoA, peroxissome proliferation AM: CAT |
Kozuka (1991) [38] |
| Biological model | Exposure compounds | Exposure conditions | Cellular and tissues damage | Impaired biochemical markers | References |
| Rattus novergicus | not specified |
AR.: oral T: 2 weeks C: 200 mg/kg/day |
NA | XM: NADPH cytocrome C reductase, aniline hydroxylase, Cytocrome B, NADPH ferricicyanide reductase, aminopyrine N-demethylase | N Inomata et al. (1991) [39] |
| Mus musculus |
Active ingredient (>97%) |
AR: oral intubation T: 7 days C: 50 mg/kg of body weight |
NA | XM: amidopyrine N-demethylas, Benzo [a]pyrene hydroxilase | Chaturvedi et al. (1991) [40] |
| Rattus novergicus | Commercial formulation |
AR: oral and middorsal skin applications T: single dose C: 1.9 and 2.6 mg/kg of body weight |
NA | XM: CYP450, ethylmorphine N-demethylase, ethoxyresorufin O-deethylase | Knopp and Schiller (1992) [41] |
| Rattus novergicus | not specified |
AR: oral T: single dose; 30 days and 180 days C: 600 mg/kg (single dose) and 200 ppm (30 and 180 days) |
NA |
LF: AST, ALT, ALP EM: LDH, amylase, glucose ND: Creatinine |
Paulino et al. (1996) [42] |
| Mus musculus | Active ingredient |
AR.: feeding T: 4 days C: 0.125% of diet |
NA | LF: mdr2 gene | Miranda et al. (1997) [43] |
| Biological model | Exposure compounds | Exposure conditions | Cellular and tissues damage | Impaired biochemical markers | References |
| Rattus novergicus | Active ingredient |
AR: oral gavage T: single dose C: 375 mg/L |
NA | XM: CYP1A1, CYP1A2, CYP1B1 | Badawi et al. (2000) [44] |
| Rattus novergicus |
Active ingredient (>98%) |
AR: injections T: 30 days C: 70 mg/kg of body weight |
NA | EM: mitochondrial dysfunction | Di Paolo et al. (2001) [45] |
| Rattus novergicus ; Mus musculus and Syrian hamsters |
Active ingredient |
AR: feeding T: 3 months C: 0, 12, 28, 83, 250, 700, and 1,680 ppm (M. musculus); 0, 17, 83, 250, 750, 1,250, and 2,500 ppm (R. novergicus); 0, 12, 100, 500, 1,000, and 5,000 ppm (Syrian hamsters) |
Increase of mice liver weith |
XM: CYP450; peroxissome proliferation AM: CAT |
Ozaki et al. (2001) [46] |
| Mus musculus | Active ingredient |
AR: feeding T: 6 days C: 1.680 ppm |
NA | ND: c-myc gene | Ge et al. (2002) [47] |
| Mus musculus | Active ingredient |
AR: Intraperitoneally T: 55 days C: 3.8 mg/kg bw |
NA | EM: LDH, MDH | Yilmaz and Yuksel (2005) [48] |
| Biological model | Exposure compounds | Exposure conditions | Cellular and tissues damage | Impaired biochemical markers | References |
| Rattus novergicus | not specified |
AR: drink water T: 25 day C: 50 and 100 ppm |
NA |
AM: SOD, GSH, GR, MDA EM: LDH, creatine kinase LF: AST XM: GSH |
Celik et al. (2006) [49] |
| Rattus novergicus | Active ingredient |
AR: Feed and drink water T: 30 days C: 25 ppm and 50 ppm (water) and 50 ppm and 100 ppm (food) |
No hepatic damage was observed, but the level of 2,4-D in the liver was found to be significantly higher in both the feed and water groups compared to the control group. | NA | Aydin et al. (2006) [50] |
| Rattus novergicus | Active ingredient |
AR: drink water T: 21 days C: 600 ppm or 126 mg/kg |
Vascular congestion, cytoplasmic vacuolization, and mononuclear cells’ infiltration |
AM: SOD, CAT, GPx, MDA LF: AST, ALT, ALP, γ-glutamyl transpeptidase EM: LDH |
Troudi et al. (2012) [23] |
| Rattus novergicus |
Commercial formulation (600 g/L) |
AR: oral gavage T: 4 weeks C: 5 mg/ kg/ bw |
NA |
AM: SOD, CAT, GPx, GR, MDA LF: AST, ALT, ALP, γ-GGT, total bilirubin. LM: change of fatty acid composition |
Nakbi. et al (2010) [51] |
| Biological model | Exposure compounds | Exposure conditions | Cellular and tissues damage | Impaired biochemical markers | References |
| Rattus novergicus |
Commercial formulation (600 g/L) |
AR: oral gavage T: 4 weeks C: 15, 75 and 150 mg/kg of body weight |
Body weight decreased and the liver weight increased significantly .2,4-D induced hepatic cord disruption, focal necrosis, vessel dilation and pycnotic nucleus. |
LF: AST, ALT, ALP, γ-GGT AM: CAT, GR |
Tayeb et al. (2010) [52] |
| Rattus novergicus |
Commercial formulation (600 g/L) |
AR: oral gavage T: 4 weeks C: 5 mg/kg of body weight/ day |
Vascular congestion and wide sinusoidal spaces and a necrotic |
AM: SOD, CAT, GPx, MDA LF: AST, ALT LM: low density lipoprotein, cholesterol |
Nakbi et al. (2012) [53] |
| Rattus novergicus |
Commercial formulation (600 g/L) |
AR: oral gavage T: 28 days C: 15, 75 and 150 mg/kg/bw/day |
NA |
AM: SOD, CAT, GPx, GR, MDA LM: change of fatty acid composition |
Tayeb et al. (2013) [54] |
| Rattus novergicus | not specified |
AR: feeding T: 16 weeks C: 200 mg/kg/day |
2,4-D acid iso-octylester caused the formation of atypical cell foci (ACF) in the pancreata and livers of rats. | NA | Kalipici et al. (2013) [55] |
| Biological model | Exposure compounds | Exposure conditions | Cellular and tissues damage | Impaired biochemical markers | References |
| Rattus novergicus |
Active ingredient (≥ 90%) |
AR: oral gavage T: 19 days C: 100 mg/kg of body weight |
NA | AM: CAT, MDA, total antioxidant capacity | Mazhar. et al. (2014) [56] |
| Rattus novergicus | Commercial formulation |
AR: oral T: 28 days C: 75 or 150 mg/kg of body weight |
2,4-D. increased liver weight and induced nuclear changes in liver cells, including alterations in size and shape, irregularity, and slight distention of nuclear envelope, Hepatic nuclei exhibited varying degrees of pyknosis, disaggregation and apoptosis. |
LF: AST, ALT, ALP, total bilirubin AM: GR, SOD EM: LDH |
Al-Baroudi et al. (2014) [57] |
| Rattus novergicus | Commercial formulation |
AR: oral gavage T: 24 h (single dose) C: 639 mg/kg of body weight |
NA |
AM: hydroperoxyl and carbonyl lipids EM: glycogen |
Dakhakhni et al. (2016) [58] |
| Mus musculus | Active ingredient |
AR: oral T: 45 days C: 30, 60, 90 mg/kg/day |
Vascular and hepatocellular lesions with necrotic changes and focal areas of necrosis in the liver |
AM: GSH, SOD, CAT, GPx, GR, GST and total –SH EM: ATP and SDH XM: GSH and GST |
Satapathy and Rao (2018) [59] |
| Biological model | Exposure compounds | Exposure conditions | Cellular and tissues damage | Impaired biochemical markers | References |
| Rattus novergicus | Active ingredient |
AR: oral gavage T: 4 weeks C: 150 mg/Kg/day |
NA |
AM: SOD, CAT, GSH, MDA LF: AST, ALT XM: GSH ND: Urea and creatinine |
Shafeeq and Mahboob (2020) [60] |
| Rattus novergicus | Commercial formulation (806 g/L) |
AR: inhalation and feed T: 6 months C: 3.71/6.19 and 9.28×10−3 g a.i./ha |
The groups exposed to oral 2,4-D had a higher incidence of steatosis, and exposed to high doses had increased liver inflammation. | LF: ALT | Bonfim et al (2020) [61] |
| Rattus novergicus |
Commercial formulation (600 g/L) |
AR: oral gavage T: 4 weeks C: 5 mg/kg/b.w./day |
Rat livers shown perivascular inflammatory infiltration around the vessel, sinusoidal dilatation and vacuolization of hepatocytes |
AM: SOD, CAT, GSH, GPx, GST, MDA LF: AST, ALT, ALP, total bilirubin EM: LDH XM: GST, GSH |
Tichati et al. (2020) [21] |
| Rattus novergicus |
Active ingredient (> 98%) |
AR: cannulation of portal and cava veins liver T: 20 min. C: 10 – 400 µM |
Membrane lipid bilayer deformity | EM: NADH, NAD+ , lactate, glycolisis, gluconeogenesis | Salla et al. (2019) [62] |
| Biological model | Exposure compounds | Exposure conditions | Cellular and tissues damage | Impaired biochemical markers | References |
| Rattus novergicus | Active ingredient |
AR: oral gavage T: 4 weeks C: 150 mg/Kg/day |
NA |
AM: SOD, CAT, GSH, MDA LF: AST, ALT, ALP XM: GSH ND: urea and creatinine |
Shafeeq and Mahboob (2021) [63] |
| Rattus novergicus |
Commercial formulation (600 g/L) |
AR: oral gavage T: 30 days C: 5 mg/kg/b.w |
2,4-D increases relative and absolute liver weights. Furthermore, 2,4-D induces severe infiltration of mononuclear inflammatory cells with vacuolar degeneration around a dilated central lobular vein, congestion of the hepatic sinusoids, and degenerative hepatocytes with largely vacuolated cytoplasm and a large number of lipid droplets. | AM: SOD, CAT, GPx, GST, MDA, carbonyl proteins LF: AST, ALT, ALP, γ-GGT EM: LDH XM: GST, GSH |
Tichati et al. (2021) [22] |
| Biological model | Exposure compounds | Exposure conditions | Cellular and tissues damage | Impaired biochemical markers | References |
| Rattus novergicus |
Commercial formulation (480 g/L) |
AR: oral T: 60 days C: 5 mg/kg of body weight |
In the liver tissue of rats, focal areas of mononuclear cell infiltration in the pericentral and periacinal region, sinusoidal dilatation, and hyperemia in the vessels and areas of pyknosis and parenchymal degeneration in the nuclei of hepatocytes were determined |
LF: AST, ALT, ALP AM: SOD, GSH, CAT, MDA XM: GSH ND: NF-κB, COX-2, TNF-α, MCP-I, TGFβI, and CYP2E P53, Bax/Bcl-2, caspase-3, caspase-8, caspase-9, and PARP |
Sinan Ince et al. (2022) [64] |
| Rattus novergicus | 2,4-D, gliphosate and dicamba (not specified) |
AR: drink water T: 90 days C: gliphosate (0.5 mg/kg bw/day) + 2,4-D (0.3 mg/kg bw/day) + dicamba (0.02 mg/kg bw/ day) |
NA | AM: GSH and MDA | Nechalioti et al. (2023) [65] |
| Ictalurus punctatus |
Active ingredient 2,4-D (>99%) Picloram (>99%) |
AR: water expossure T: 10 days C: 22.5, 7.5, and 2.25 mg/L |
NA | XM: ethoxyresorufin 0-deethylase | Gallagher and Digiulio (1991) [66] |
| Biological model | Exposure compounds | Exposure conditions | Cellular and tissues damage | Impaired biochemical markers | References |
| Cyprinus carpio |
Active ingredient (>98%) |
AR: water exposure T: 96 h and 14 days C: 310, 295 and 270 mg/L (96 h ) 150, 200, and 250 mg/L (14 days) |
Hepatocycites shown slight vacuolar degeneration and pycnotic nuclei (some of them displaced) | LF: AST, ALT | Neskovic et al. (1994) [67] |
| Oreochromis niloticus |
Commercial formulation (500 g/L) |
AR: water exposure T: 96 h C: 27 ppm |
NA |
AM: SOD, GPx, GR EM: glucose-6-phosphate dehydrogenase |
Oruç. and Uner (2000) [68] |
| Fundulus heteroclitus | not specified |
AR: water expossure T: 21 days C: 0.04, 0.41, and 4.1 µM |
NA | ND: peroxissome proliferation | Ackers et al. (2000) [69] |
| Cyprinus carpio |
Commercial formulation (500 g/L) |
AR: water exposure T: 96 h C: 87 ppm |
NA | AM: GST, SOD EM: G6PD XM: GST |
Oruç and Uner (2002) [70] |
| Leporinus obtusidens |
Commercial formulation (868 g/L) |
AR: water exposure T: 96 h C: 1 and 10 mg/L |
NA | EM: glycogen, lactate, glucose | Fonseca et al. (2008) [71] |
| Rhamdia quelen |
Commercial formulation (720 g/L ) |
AR: water exposure T: 96 h C: 0, 400, 600 and 700 mg/L |
Hepatocytes vacualization and changes in its arrangement cords | EM: glycogen,lactate, glucose | Cattaneo et al. (2008) [72] |
| Carassius auratus | Active ingredient |
AR: water exposure T: 90 h C: 1, 10 and 100 mg/L |
NA | AM: carbonyl proteins, lipid peroxidases LM: lipid peroxidases |
Matviishyn et al. (2014) [73] |
| Poecilia vivipara | Commercial formulation (868g/L) |
AR: water exposure T: 48 h C: 10,20 and 40μl |
Swollen nuclei and cytoplasmic vacuolization. Finally, the 40 μl/L group presented blood vessel alterations indicating vasodilatation, hepatocytes with swollen nuclei, Ito cells, and micronuclei. | NA | Vigário and Sabóia-Morais (2014) [74] |
| Rhamdia quelen |
Commercial formulation (720 g/L) |
AR: water exposure T: 90 days C: 0.5 and 2 mg/L |
NA |
AM: CAT, MDA EM: glycogen; lactate, glucose |
Menezes. et al. (2015) [75] |
| Cyprinus carpio L | not specified |
A.R.: water exposure T: --- C: 0.2 mg/dm3 |
NA | EM: ICDH, LDH, G6PD | Yakovenko et al. (2018) [76] |
| Biological model | Exposure compounds | Exposure conditions | Cellular and tissues damage | Impaired biochemical markers | References |
| Capoeta capoeta | not specified |
AR: water expossure T: 7 days C: 10 and 20 mg/L |
NA |
AM: plasma oxidative status index LF: AST |
Kaya et al. (2018) [77] |
| Danio rerio |
Active ingredient (> 97%) |
AR: water exposure T: 48 h C: 2.5, 5 and 10 mg/L |
Hepatocytes had heterogeneous eosiniphilic, cytosol vacualization and cell nucleus were eccentric, Loss of cell boundaries and liver with necrotic appearence, Release of cytosolic content among adjacent cells |
LF: AST, ALT, ALP AM: CAT, GST XM: GST EM: LDH |
Martins et al. (2021) [2] |
| Triturus cristatus carnifex | Commercial formulation (37% of 2,4-D as iso-octylic ester) | AR: water exposure T: 3 months C: 25, 50, 75, 100, 125, and 150 ppm |
Vacuolar degeneration of liver parenchyma and necrosis of kidney tubules | NA | Zaffaroni et al. (1986) [78] |
| Lithobates clamitans |
Active ingredient (>98%) |
AR: soil exposition T: 2 days C: 14.3 µg/cm² |
NA | NA | Van Meter et al. (2018) [79] |
| Biological model | Exposure compounds | Exposure conditions | Cellular and tissues damage | Impaired biochemical markers | References |
| Physalaemus albonotatus | Commercial formulation) (48.5% w/v of active ingredient) | AR: water exposure T: 96 h (acute) and 49 days (chronic) C: 350, 700, 1400, and 2400 mg/L(acute); 43.7, 87.5, 175 or 262.5 mg/L (chronic) |
The liver of treated tadpoles showed enlargement of hepatic sinu- soids , hypervascularization, dilation of blood vessels, and vacuolization of hepatocytes | NA | Curi et al. (2019) [80] |
| Biological model | Exposure compounds | Exposure conditions | Impaired biochemical markers | References |
| Liver GST of Rattus novergicus | Active ingredient |
AR.: enzyme kinetics T: --- C:--- |
AM: GST XM: GST |
Dierickx (1983) [81] |
| Liver GST of Rattus novergicus |
Active ingredient (>99%) |
AR: enzyme kinetics T: --- C: 2 -12 mM |
XM: GST AM: GST |
Vessey and Boyer (1984) [82] |
| Liver GST of Salmo gairdneri | Active ingredient |
AR: enzyme kinetics T: --- C: 2 mM |
AM: GST XM: GST |
Dierick (1985) [83] |
| Liver GST of Homo sapiens (autopsy) |
Active ingredient (>97%) |
AR: --- T: --- C: --- |
AM: GST XM: GST |
Singh (1985) [84] |
| Liver GST of Cyprinus carpio | not specified |
AR: cell culture T: --- C: --- |
AM: GST XM: GST |
Elia et al. (2000) [85] |
| Biological model | Exposure compounds | Exposure conditions | Impaired biochemical markers | References |
| Liver GST of Rattus novergicus | not specified |
AR: --- T: --- C: --- |
AM: GST XM: GST |
Dierickx (1988) [86] |
| Liver GST of Chalcalburnus tarichii Pallas |
Active ingredient |
AR.: --- T: --- C: 0.6, 0.23 and 0.57 mM |
AM: GST XM: GST |
Özaslan et al. (2018) [87] |
| Liver mitochondria of Rattus novergicus | Active ingredient |
AR.: cell culture T: --- C: 0, 0.2, 0.5, 1.0, and 2 mM. |
LM: palmitoyl CoA hydrolase , fatty acyl CoA EM: mitochondrial dysfunction |
Dixon et al. (1990) [88] |
| Liver mitochondria of Rattus novergicus | not specified |
AR: cell culture T: --- C: 0.1 - 4.0 mM |
EM: mitochondrial dysfunction | Zychlinski and Zolnierowicz (1990) [89] |
| Liver mitochondria of Rattus novergicus | Active ingredient |
AR: cell culture T: --- C: 100, 200, 300, 400, 500, 600, 700 and 800 µM |
EM: SDH, cytochrome c reductase, mitochondrial dysfunction | Palmeira et al. (1994) [90] |
| Biological model | Exposure compounds | Exposure conditions | Impaired biochemical markers | References |
| Liver mitochondria of Rattus novergicus | Commercial formulation (2,4-D 1.08 M + Picloram 0.265 M) |
AR: cell culture T: --- C: 66.2 nmol picloram + 270 nmol 2,4-D mg-1 protein |
EM: NADH oxidase, NADH cytochrome c reductase, ATP, mitochondrial dysfunction | Pereira et al. (1994) [91] |
| Liver mitochondria of Rattus novergicus | Commercial formulation. Tordon (2,4-D 300 g/L + picloram 75 g/L) |
AR.: cell culture T: --- C: --- |
EM: mitochondrial dysfunction | Oakes and Pollak (1999) [92] |
| Liver Rattus novergicus mitochondria |
Active ingredient (>98%) |
AR: injections T: 30 days C: 70 mg/kg of body weight |
EM: mitochondrial dysfunction | Di Paolo et al. (2001) [45] |
| Hepatocytes of Rattus novergicus | Active ingredient |
AR: cell culture T: --- C: 1-10 mM |
EM: LDH, ATP, ADP, AMP, NADH, NAD+ AM: GSH, GSSG XM: GSH, GSSG |
Palmeira et al. (1994) [93] |
| Biological model | Exposure compounds | Exposure conditions | Impaired biochemical markers | References |
| Hepatocytes of Rattus novergicus |
Active ingredient (> 98%) |
AR: cell culture T: 200 min C: 1, 5 and 10 mM |
AM: MDA, proteins thiol, GSH XM: GSH |
Palmeira et al. (1995) [94] |
| Hepatocytes of Rattus novergicus | Active ingredient |
AR: cell culture ET: 3 months C.T: 1 mM |
NA | Li et al. (2003) [95] |
| Hepatocytes of Metynnis roosevelti | Active ingredient |
AR: cell culture T: --- C: 0.275, 2.75 and 27.5 mg/L |
EM: mitochondrial dysfunction | Salvo et al. (2015) [96] |
| HepG2 cells | Active ingredient |
AR: cell culture T: 48 h C: 4, 8 and 16 mM |
EM: mitochondrial dysfunction ND: Cell cicle alterations, apoptose, DNA damage |
Tuschl and Schwab (2003) [97] |
| HepG2 cells | Active ingredient |
AR: cell culture T: 48 h C: 8, 14 and 16 mM |
ND: Cell cycle alterations, apoptosis, DNA damage | Tuschl and Schwab (2004) [98] |
| Biological model | Exposure compounds | Exposure conditions | Impaired biochemical markers | References |
| HepG2 cells | Commercial formulation |
AR: cell culture T: --- C: 0.1 nM to 4 mM |
ND: Genes involved to stress response, cell cycle control, immunological and DNA repair genes. (FTH1, FTL, PCNA, DCLRE1C, TCLK1, JM11, VEGF, USP19, DDB2, IL1RL1, PTGER3 and GTF2A. | Bharadwaj et al. (2005) [99] |
| HepG2 cell |
Active ingredient (>90%) |
AR.: cell culture T: --- C: 0.001 - 0.1 mM |
NA | Barrón Cuenca et al. (2022) [100] |
| Liver homogenates of Rattus novergicus | Active ingredient |
AR: cell culture T: --- C: --- |
LM: cholesterol | Olson et al. (1974) [101] |
| Biological model | Exposure compounds | Exposure conditions | Impaired biochemical markers | References |
| Chicken embryo |
Commercial formulation (37%) |
AR.: injected into the air cell of the eggs T: 19 days C: 1, 2 and 4 mg/egg |
XM: ethoxycoumarin O-deethylase, GST AM: GST |
Santagostino et al. (1991) [102] |
| Chicken Liver |
Commercial formulation (31.6% w/v) |
AR: Fertilized eggs were externally treated T: 21 days C: 3.1 mg |
EM: G6Pase LM: total lipids AM: CAT |
Duffard et al. (1993) [103] |
| Biological model | Hepatoprotective agent | Concentrations and time of exposure | Hepatoprotective effects | References |
| Rattus novergicus | Extra virgin olive oil (EVOO) and its hydrophilic fraction (OOHF) | C: 2,4-D (5 mg/kg body weight) + EVOO (300 μl/day) or OOHF (1 mL/day) T: 4 weeks |
EVOO and OOHF suplementation induced a significant increase in the antioxidant enzyme activities (SOD, CAT, GPx and GR), liver marker (AST, ALT and total bilirubin) and a decrease in the conjugated dienes (CD) and thiobarbituric acid-reactive substances (TBARs) levels in the liver. | Nakbi, A. et al. (2010) [51] |
| Rattus novergicus | Extra virgin olive oil (EVOO) and its hydrophilic fraction (OOHF) | C: 2,4-D (5 mg/kg body weight) + EVOO (300 μl/day) or OOHF (1 mL/day) T: 4 weeks |
EVOO and OOHF suplementation induced a significant increase in the antioxidant enzyme activities (SOD, CAT, GPx), liver marker (AST, ALT and total bilirubin), and decreased MDA levels in the liver. | Nakbi, A. et al. (2012) [53] |
| Biological model | Hepatoprotective agent | Concentrations and time of exposure | Hepatoprotective effects | References |
| Rattus novergicus | Chamomile capitula extract | C: 2,4-D (75 or 150 mg/kg body weight) + Chamomile capitula extract- (500 mg/kg body weight) T: 28 days |
Chamomile capitula extract presented antioxidant effects, improving the levels of SOD and GR. The levels of hepatic enzymes AST, ALT, ALP, and LDH decreased, as well as total bilirubin. Additionally, the degenerative damages in the hepatic tissue caused by 2,4-D were also alleviated. | Al-Baroudi et al. (2014) [57] |
| Mus musculus | Curcumin | C: 2,4-D (30, 60, 90 mg/kg/day) + Curcumin (10 mg/kg/day) T: 45 days |
Curcumin supplementation exhibited antioxidant effects, mainly normalizing the levels of GSH, GR, and lipid peroxidation. Furthermore, curcumin supplementation reduced hepatic tissue damage. | Satapathy and Rao (2018) [59] |
| Biological model | Hepatoprotective agent | Concentrations and time of exposure | Hepatoprotective effects | References |
| Rattus novergicus | Magnesium (Mg) | C: 2,4-D (150 mg/kg body weight/day) + Mg supplement (50 mg/kg body weight/day) T: 4 weeks |
Mg supplementation exhibited its antioxidant properties by significantly improving urea, creatinine SOD, MDA, CAT, GSH and MDA levels and antioxidant enzyme activities. Hepatic markers were also improved: AST, ALT and ALP and absolute liver weight. | Shafeeq and Mahboob (2020) [60] |
| Rattus novergicus | Selenium (Se) | C: 2,4-D (5 mg/kg body weight/day) + Se supplement (1 mg/kg body weight/day) T: 4 weeks |
Se supplementation in 2,4- D-treated rats elicited a reduction in the toxic effects of the pesticide by improving the studied parameters (absolute liver weight, total bilirubin, AST, ALP, LDH, MDA and carbonyl proteins) which was confirmed by the histological study of the liver. | Tichati, L. et al. (2020) [21] |
| Rattus novergicus | Selenium (Se) | C: 2,4-D (150 mg/kg body weight/day) + Se supplement (1 mg/kg body weight/day) T: 4 weeks |
Se supplementation exhibited its antioxidant properties by significantly improving urea, creatinine, ALP, AST, and ALT, and MDA levels and antioxidant enzyme activities. Hepatic and renal toxicities were attenuated by Se supplementation. | Shafeeq and Mahboob (2021) [63] |
| Biological model | Hepatoprotective agent | Concentrations and time of exposure | Hepatoprotective effects | References |
| Rattus novergicus | Thymus munbyanus extract (AETM) | C: 2,4-D (5 mg/kg body weight) + AETM (10 ml/kg body weight) T: 30 days |
AETM supplementation showed a marked enhancement in the above altered hepatic functional and antioxidant parameters (CAT, GST, total bilirubin, AST, ALP, MDA, carbonyl proteins) and liver histopathology. | Tichati, L. et al. (2021) [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





