Submitted:
27 November 2023
Posted:
29 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Understanding Autoimmunity and Its Mechanisms
3. The Impact of COVID-19 Vaccination on Autoimmune Diseases: An Exploration of Possible Triggers and Exacerbations
4. Autoimmune Diseases Associated with COVID19 Vaccine
The pathophysiology of ITP and COVID-19
The association between COVID-19 and Guillain-Barré Syndrome (GBS)
Miller Fisher Syndrome (MFS) and COVID-19
Antiphospholipid Antibodies and Thrombosis in COVID-19 Patients
Kawasakilike Disease and its Association with COVID-19
Impact of Preexisting Autoimmune Rheumatic Diseases on COVID19
The Relationship between Systemic Lupus Erythematosus and COVID-19
The Relationship between Multiple Sclerosis and COVID-19
5. The use of AntiIL-6 receptor antibodies Tocilizumab and Sarilumab for COVID-19 treatment
6. The Use of IL1 Inhibitors in COVID-19 Treatment
7. The Role of Molecular Mimicry in Covid-19 and Autoimmunity
8. Histopathological Signs of Autoimmune Reactions in COVID-19
9. The Role of Autoimmunity in PASC (PostAcute Sequelae of SARS-CoV-2 Infection)
Conclusions and Future Research
References
- Reynolds, J., Putterman, C. Progress and unmet needs in understanding fundamental mechanisms of autoimmunity. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S0896841123000082.
- RamaKrishnan, A., Sankaranarayanan, K. Understanding autoimmunity: The ion channel perspective. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S1568997216300301.
- Gudjonsson, J., Kabashima, K., Eyerich, K. Mechanisms of skin autoimmunity: cellular and soluble immune components of the skin. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S0091674920306862.
- Theofilopoulos, A., Kono, D., Baccala, R. The multiple pathways to autoimmunity. (n.d.) Retrieved November 25, 2023, from www.nature.com/articles/ni.3731.
- Jones, D., Diamond, A. The basis of autoimmunity: an overview. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S0950351X9580787X.
- Sudres, M., Verdier, J., Truffault, F. Pathophysiological mechanisms of autoimmunity. (n.d.) Retrieved November 25, 2023, from nyaspubs.onlinelibrary.wiley.com/doi/abs/10.1111/nyas.13560.
- Simmonds, M., Gough, S. Genetic insights into disease mechanisms of autoimmunity. (n.d.) Retrieved November 25, 2023, from academic.oup.com/bmb/article-abstract/71/1/93/275997.
- Rose, N. Mechanisms of autoimmunity. (n.d.) Retrieved November 25, 2023, from www.thieme-connect.com.
- Correa-Rodríguez, M., Rueda-Medina, B. [HTML][HTML] COVID-19 vaccine literacy in patients with systemic autoimmune diseases. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1007/s12144-022-02713-y.
- Li, X., Gao, L., Tong, X., Chan, V., Chui, C., Lai, F. Autoimmune conditions following mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccination: a descriptive cohort study among 1.1 million vaccinated …. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S0896841122000385.
- Sen, P., Ravichandran, N., Nune, A., Lilleker, J. COVID-19 vaccination-related adverse events among autoimmune disease patients: results from the COVAD study. (n.d.) Retrieved November 25, 2023, from academic.oup.com.
- Boekel, L., Steenhuis, M., Hooijberg, F. Antibody development after COVID-19 vaccination in patients with autoimmune diseases in the Netherlands: a substudy of data from two prospective cohort studies. (n.d.) Retrieved November 25, 2023, from www.thelancet.com.
- Sen, P., Gupta, L., Lilleker, J., Aggarwal, V. [HTML][HTML] COVID-19 vaccination in autoimmune disease (COVAD) survey protocol. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1007/s00296-021-05046-4.
- Boekel, L., Kummer, L., van Dam, K. Adverse events after first COVID-19 vaccination in patients with autoimmune diseases. (n.d.) Retrieved November 25, 2023, from www.thelancet.com.
- Velikova, T., Georgiev, T. [HTML][HTML] SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. (n.d.) Retrieved November 25, 2023, from link.springer.com.
- Chen, Y., Xu, Z., Wang, P., Li, X., Shuai, Z., Ye, D. New-onset autoimmune phenomena post-COVID-19 vaccination. (n.d.) Retrieved November 25, 2023, from onlinelibrary.wiley.com/doi/abs/10.1111/imm.13443.
- Gil-Vila, A., Ravichandran, N. COVID-19 vaccination in autoimmune diseases (COVAD) study: vaccine safety in idiopathic inflammatory myopathies. (n.d.) Retrieved November 25, 2023, from onlinelibrary.wiley.com/doi/abs/10.1002/mus.27681.
- ITP and COVID-19: Risks, Complications, Considerations. (n.d.) Retrieved November 25, 2023, from www.verywellhealth.com/itp-and-covid-19-5190834.
- COVID-19 associated with immune thrombocytopenia. (n.d.) Retrieved November 25, 2023, from www.ncbi.nlm.nih.gov/pmc/articles/PMC8862167/.
- A Case of COVID-19-Induced Immune Thrombocytopenia .... (n.d.) Retrieved November 25, 2023, from www.cureus.com.
- Immune Thrombocytopenic Purpura (ITP) Following .... (n.d.) Retrieved November 25, 2023, from www.cureus.com.
- COVID-19 and ITP: Frequently Asked Questions. (n.d.) Retrieved November 25, 2023, from www.hematology.org/covid-19/covid-19-and-itp.
- Secondary immune thrombocytopenia supposedly .... (n.d.) Retrieved November 25, 2023, from casereports.bmj.com/content/14/5/e242220.
- SARS-CoV-2 Infection Inducing Immune .... (n.d.) Retrieved November 25, 2023, from www.ochsnerjournal.org/content/21/2/187.
- Immune thrombocytopenia and COVID-19: Case report and .... (n.d.) Retrieved November 25, 2023, from journals.sagepub.com/doi/full/10.1177/09612033211021161.
- Immune Thrombocytopenic Purpura Following COVID-19 .... (n.d.) Retrieved November 25, 2023, from www.ncbi.nlm.nih.gov/pmc/articles/PMC10284310/.
- Idiopathic Thrombocytopenic Purpura Related to COVID-19. (n.d.) Retrieved November 25, 2023, from www.journalmc.org/index.php/JMC/article/view/3518/2819.
- Abu-Rumeileh, S., Abdelhak, A., Foschi, M., Tumani, H. [HTML][HTML] Guillain–Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1007/s00415-020-10124-x.
- Gittermann, L., Feris, S. [HTML][HTML] Relation between COVID-19 and Guillain-Barré syndrome in adults: a systematic review. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S2173580820302145.
- El Otmani, H., El Moutawakil, B., Rafai, M. Covid-19 and Guillain-Barré syndrome: more than a coincidence!. (n.d.) Retrieved November 25, 2023, from www.ncbi.nlm.nih.gov/pmc/articles/PMC7180370/.
- Sheikh, A., Chourasia, P., Javed, N. Association of Guillain-Barre syndrome with COVID-19 infection: An updated systematic review. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S0165572821001041.
- Rahimi, K. [HTML][HTML] Guillain-Barre syndrome during COVID-19 pandemic: an overview of the reports. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1007/s10072-020-04693-y.
- Caress, J., Castoro, R., Simmons, Z., Scelsa, S. COVID-19–associated Guillain-Barré syndrome: The early pandemic experience. (n.d.) Retrieved November 25, 2023, from onlinelibrary.wiley.com/doi/abs/10.1002/mus.27024.
- McKean, N., Chircop, C. Guillain-Barré syndrome after COVID-19 vaccination. (n.d.) Retrieved November 25, 2023, from casereports.bmj.com/content/14/7/e244125.abstract.
- Keddie, S., Pakpoor, J., Mousele, C., Pipis, M., Machado, P. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. (n.d.) Retrieved November 25, 2023, from academic.oup.com/brain/article-abstract/144/2/682/6031905.
- Yaqoob, A., Dar, W., Khuja, Z., Bukhari, I. [HTML][HTML] Miller Fisher syndrome associated with COVID 19. (n.d.) Retrieved November 25, 2023, from www.ncbi.nlm.nih.gov/pmc/articles/PMC9648327/.
- Nishiguchi, Y., Matsuyama, H., Maeda, K., Shindo, A. [HTML][HTML] Miller Fisher syndrome following BNT162b2 mRNA coronavirus 2019 vaccination. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1186/s12883-021-02489-x.
- Manganotti, P., Pesavento, V., Buoite Stella, A. [HTML][HTML] Miller Fisher syndrome diagnosis and treatment in a patient with SARS-CoV-2. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1007/s13365-020-00858-9.
- Lantos, J., Strauss, S., Lin, E. COVID-19–associated miller fisher syndrome: MRI findings. (n.d.) Retrieved November 25, 2023, from http://www.ajnr.org/content/41/7/1184.abstract.
- Li, Z., Li, X., Shen, J., Chan, M., Wu, W. [HTML][HTML] Miller Fisher syndrome associated with COVID-19: an up-to-date systematic review. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1007/s11356-021-13233-w.
- Kim, J., Yoon, B., Kim, Y., Kim, J. Miller Fisher syndrome following COVID-19 vaccines: A scoping review. (n.d.) Retrieved November 25, 2023, from onlinelibrary.wiley.com/doi/abs/10.1111/ane.13687.
- Biswas, S., Ghosh, R., Mandal, A., Pandit, A. COVID-19 induced miller fisher syndrome presenting with autonomic dysfunction: a unique case report and review of literature. (n.d.) Retrieved November 25, 2023, from journals.sagepub.com/doi/abs/10.1177/19418744211016709.
- Senel, M., Abu-Rumeileh, S., Michel, D. Miller-Fisher syndrome after COVID-19: neurochemical markers as an early sign of nervous system involvement. (n.d.) Retrieved November 25, 2023, from onlinelibrary.wiley.com/doi/abs/10.1111/ene.14473.
- Siddiqi, A., Khan, T., Tahir, M., Asghar, M., Islam, M. [HTML][HTML] Miller Fisher syndrome after COVID-19 vaccination: Case report and review of literature. (n.d.) Retrieved November 25, 2023, from www.ncbi.nlm.nih.gov/pmc/articles/PMC9276158/.
- COVID-19 and aPL Antibodies: Frequently Asked Questions. (n.d.) Retrieved November 25, 2023, from www.hematology.org/covid-19/covid-19-and-apl-ab.
- Coronavirus-disease-2019-induced antiphospholipid-like .... (n.d.) Retrieved November 25, 2023, from jmedicalcasereports.biomedcentral.com.
- Antiphospholipid antibodies and vitamin D deficiency in .... (n.d.) Retrieved November 25, 2023, from journals.plos.org.
- Antiphospholipid Syndrome & COVID-19: What to Know. (n.d.) Retrieved November 25, 2023, from www.hss.edu.
- COVID-19 and the antiphospholipid syndrome - PMC. (n.d.) Retrieved November 25, 2023, from www.ncbi.nlm.nih.gov/pmc/articles/PMC9527199/.
- Antiphospholipid antibodies and COVID-19 thrombotic .... (n.d.) Retrieved November 25, 2023, from ard.bmj.com/content/80/9/1105.
- Antiphospholipid antibodies in COVID-19: a meta-analysis .... (n.d.) Retrieved November 25, 2023, from rmdopen.bmj.com/content/7/2/e001580.
- Antiphospholipid antibodies and neurological ... - The Lancet. (n.d.) Retrieved November 25, 2023, from www.thelancet.com.
- COVID-19 and antiphospholipid antibodies - Sage Journals. (n.d.) Retrieved November 25, 2023, from http://journals.sagepub.com/doi/10.1177/09612033211062523.
- Lidder, A., Pandit, S., Lazzaro, D. [HTML][HTML] An adult with COVID-19 kawasaki-like syndrome and ocular manifestations. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S2451993620301900.
- Viner, R., Whittaker, E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. (n.d.) Retrieved November 25, 2023, from www.thelancet.com.
- Fang, Y., Aravamudan, V., Sridharan, G. Kawasaki like illness due to COVID-19: a review of the literature. (n.d.) Retrieved November 25, 2023, from jidc.org/index.php/journal/article/view/14185.
- Akca, U., Kesici, S., Ozsurekci, Y., Aykan, H. [HTML][HTML] Kawasaki-like disease in children with COVID-19. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1007/s00296-020-04701-6.
- Toubiana, J., Poirault, C., Corsia, A., Bajolle, F. [HTML][HTML] Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. (n.d.) Retrieved November 25, 2023, from www.bmj.com/content/369/bmj.m2094.long.
- Amirfakhryan, H. Kawasaki-like disease in children with COVID-19: A hypothesis. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S0306987720317278.
- Shaigany, S., Gnirke, M., Guttmann, A., Chong, H. An adult with Kawasaki-like multisystem inflammatory syndrome associated with COVID-19. (n.d.) Retrieved November 25, 2023, from www.thelancet.com.
- Koné-Paut, I., Cimaz, R. Is it Kawasaki shock syndrome, Kawasaki-like disease or pediatric inflammatory multisystem disease? The importance of semantic in the era of COVID-19 pandemic. (n.d.) Retrieved November 25, 2023, from rmdopen.bmj.com/content/6/2/e001333.abstract.
- Berardicurti, O., Conforti, A., Ruscitti, P. The wide spectrum of Kawasaki-like disease associated with SARS-CoV-2 infection. (n.d.) Retrieved November 25, 2023, from www.tandfonline.com/doi/abs/10.1080/1744666X.2021.1847643.
- Pang, R., Zhao, J., Gan, Z., Hu, Z., Xue, X., Wu, Y. Evolution of COVID-19 in patients with autoimmune rheumatic diseases. (n.d.) Retrieved November 25, 2023, from www.ncbi.nlm.nih.gov/pmc/articles/PMC7762508/.
- Antonelli, A. COVID-19 and rheumatic autoimmune systemic diseases: report of a large Italian patients series. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1007/s10067-020-05334-7.
- Montero, F., Martínez-Barrio, J. Coronavirus disease 2019 (COVID-19) in autoimmune and inflammatory conditions: clinical characteristics of poor outcomes. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1007/s00296-020-04676-4.
- Gupta, S., Nakabo, S., Chu, J., Hasni, S., Kaplan, M. Association between anti-interferon-alpha autoantibodies and COVID-19 in systemic lupus erythematosus. (n.d.) Retrieved November 25, 2023, from www.ncbi.nlm.nih.gov/pmc/articles/PMC7658959/.
- Mantovani Cardoso, E., Hundal, J., Feterman, D. Concomitant new diagnosis of systemic lupus erythematosus and COVID-19 with possible antiphospholipid syndrome. Just a coincidence? A case report and review of intertwining pathophysiology. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1007/s10067-020-05310-1.
- Cordtz, R., Kristensen, S., Dalgaard, L. JCM | Free Full-Text | Incidence of COVID-19 Hospitalisation in Patients with Systemic Lupus Erythematosus: A Nationwide Cohort Study from Denmark. (n.d.) Retrieved November 25, 2023, from www.mdpi.com/2077-0383/10/17/3842.
- Barzegar, M., Mirmosayyeb, O. COVID-19 among patients with multiple sclerosis: a systematic review. (n.d.) Retrieved November 25, 2023, from nn.neurology.org/content/8/4/e1001.abstract.
- Sormani, M. An Italian programme for COVID-19 infection in multiple sclerosis. (n.d.) Retrieved November 25, 2023, from www.thelancet.com/lancet/article/S1474-4422(20)30147-2.
- Achiron, A., Dolev, M., Menascu, S. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. (n.d.) Retrieved November 25, 2023, from journals.sagepub.com/doi/abs/10.1177/13524585211003476.
- Loonstra, F., Hoitsma, E. COVID-19 in multiple sclerosis: the Dutch experience. (n.d.) Retrieved November 25, 2023, from journals.sagepub.com/doi/abs/10.1177/1352458520942198.
- Bsteh, G., Bitschnau, C., Hegen, H., Auer, M. Multiple sclerosis and COVID-19: how many are at risk?. (n.d.) Retrieved November 25, 2023, from onlinelibrary.wiley.com/doi/abs/10.1111/ene.14555.
- Simpson-Yap, S., De Brouwer, E., Kalincik, T., Rijke, N. [HTML][HTML] Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. (n.d.) Retrieved November 25, 2023, from n.neurology.org/content/97/19/e1870?ct=.
- Sormani, M., Schiavetti, I., Carmisciano, L. [HTML][HTML] COVID-19 severity in multiple sclerosis: putting data into context. (n.d.) Retrieved November 25, 2023, from nn.neurology.org/content/9/1/e1105.abstract.
- Tallantyre, E., Vickaryous, N., Anderson, V. COVID-19 vaccine response in people with multiple sclerosis. (n.d.) Retrieved November 25, 2023, from onlinelibrary.wiley.com/doi/abs/10.1002/ana.26251.
- Maresa, J., Hartung, H. [PDF][PDF] Multiple sclerosis and COVID-19.. (n.d.) Retrieved November 25, 2023, from biomed.papers.upol.cz.
- Moss, B., Mahajan, K., Bermel, R. Multiple sclerosis management during the COVID-19 pandemic. (n.d.) Retrieved November 25, 2023, from journals.sagepub.com/doi/abs/10.1177/1352458520948231.
- Interleukin-6 Inhibitors. (n.d.) Retrieved November 25, 2023, from www.covid19treatmentguidelines.nih.gov.
- A New Approach to the Management of COVID-19. Antagonists of IL-6: Siltuximab. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1007/s12325-022-02042-3.
- WHO recommends life-saving interleukin-6 receptor blockers for COVID-19 and urges producers to join efforts to rapidly increase access. (n.d.) Retrieved November 25, 2023, from www.who.int.
- Van de Veerdonk, F., Netea, M. Blocking IL-1 to prevent respiratory failure in COVID-19. (n.d.) Retrieved November 25, 2023, from ccforum.biomedcentral.com.
- Kim, J., Lee, J., Yang, J., Lee, K., Effenberger, M. Immunopathogenesis and treatment of cytokine storm in COVID-19. (n.d.) Retrieved November 25, 2023, from www.ncbi.nlm.nih.gov/pmc/articles/PMC7681075/.
- Salle, V. Coronavirus-induced autoimmunity. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S1521661621000310.
- Marino Gammazza, A., Légaré, S., Lo Bosco, G. [HTML][HTML] … with SARS-CoV-2 antigenic epitopes potentially capable of eliciting autoimmunity against endothelial cells: possible role of molecular mimicry in COVID-19. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1007/s12192-020-01148-3.
- Rojas, M., Herrán, M., Ramírez-Santana, C. Molecular mimicry and autoimmunity in the time of COVID-19. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S0896841123000793.
- Vahabi, M., Ghazanfari, T., Sepehrnia, S. Molecular mimicry, hyperactive immune system, and SARS-COV-2 are three prerequisites of the autoimmune disease triangle following COVID-19 infection. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S1567576922006671.
- Cappello, F., Marino Gammazza, A., Dieli, F. Does SARS-CoV-2 trigger stress-induced autoimmunity by molecular mimicry? A hypothesis. (n.d.) Retrieved November 25, 2023, from www.mdpi.com/2077-0383/9/7/2038.
- Ehrenfeld, M., Tincani, A., Andreoli, L., Cattalini, M. Covid-19 and autoimmunity. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S1568997220301610.
- Nunez-Castilla, J., Stebliankin, V., Baral, P., Balbin, C. [HTML][HTML] Potential autoimmunity resulting from molecular mimicry between SARS-CoV-2 spike and human proteins. (n.d.) Retrieved November 25, 2023, from www.mdpi.com/1999-4915/14/7/1415.
- Albert, L., Inman, R. Molecular mimicry and autoimmunity. (n.d.) Retrieved November 25, 2023, from www.nejm.org/doi/full/10.1056/NEJM199912303412707.
- Mehandru, S., Merad, M. [HTML][HTML] Pathological sequelae of long-haul COVID. (n.d.) Retrieved November 25, 2023, from www.nature.com/articles/s41590-021-01104-y.
- Kowalik, M., Trzonkowski, P. COVID-19—Toward a comprehensive understanding of the disease. (n.d.) Retrieved November 25, 2023, from journals.viamedica.pl/cardiology_journal/article/view/68719.
- Rodríguez, Y., Novelli, L., Rojas, M., De Santis, M. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S0896841120301281.
- Yao, X., Li, T., He, Z., Ping, Y., Liu, H. A pathological report of three COVID-19 cases by minimal invasive autopsies. (n.d.) Retrieved November 25, 2023, from europepmc.org/article/med/32172546.
- Su, H., Yang, M., Wan, C., Yi, L., Tang, F., Zhu, H., Yi, F. [HTML][HTML] Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S0085253820303690.
- El Hachem, M., Diociaiuti, A., Concato, C. … , histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: lights and shadows on the relationship with COVID-19 …. (n.d.) Retrieved November 25, 2023, from onlinelibrary.wiley.com/doi/abs/10.1111/jdv.16682.
- Song, Z., Bao, L., Deng, W., Liu, J., Ren, E., Lv, Q. [HTML][HTML] Integrated histopathological, lipidomic, and metabolomic profiles reveal mink is a useful animal model to mimic the pathogenicity of severe COVID-19 patients. (n.d.) Retrieved November 25, 2023, from www.nature.com/articles/s41392-022-00891-6.
- Cooper, S., Tobar, A., Konen, O., Orenstein, N. Long COVID-19 liver manifestation in children. (n.d.) Retrieved November 25, 2023, from journals.lww.com.
- Yong, S. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. (n.d.) Retrieved November 25, 2023, from www.tandfonline.com/doi/abs/10.1080/23744235.2021.1924397.
- Gralinski, L., Baric, R. Molecular pathology of emerging coronavirus infections. (n.d.) Retrieved November 25, 2023, from pathsocjournals.onlinelibrary.wiley.com.
- Pannone, G., Caponio, V. [HTML][HTML] Lung histopathological findings in COVID-19 disease–a systematic review. (n.d.) Retrieved November 25, 2023, from infectagentscancer.biomedcentral.com.
- Li, H., Zhao, Y., Zhou, L., Hu, J. Cutaneous, skin histopathological manifestations and relationship to COVID-19 infection patients. (n.d.) Retrieved November 25, 2023, from onlinelibrary.wiley.com/doi/abs/10.1111/dth.14157.
- Rubio-Muniz, C., Puerta-Peña, M. [HTML][HTML] The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. (n.d.) Retrieved November 25, 2023, from www.ncbi.nlm.nih.gov/pmc/articles/PMC7307079/.
- Proal, A., VanElzakker, M., Aleman, S., Bach, K. SARS-CoV-2 reservoir in post-acute sequelae of COVID-19 (PASC). (n.d.) Retrieved November 25, 2023, from www.nature.com/articles/s41590-023-01601-2.
- Proal, A., VanElzakker, M. [HTML][HTML] Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. (n.d.) Retrieved November 25, 2023, from www.frontiersin.org.
- Reznik, S., Tiwari, A., Ashby Jr, C. [HTML][HTML] Intravenous immunoglobulin: A potential treatment for the post-acute sequelae of SARS-Cov-2 infection?. (n.d.) Retrieved November 25, 2023, from www.ncbi.nlm.nih.gov/pmc/articles/PMC9392972/.
- Peluso, M., Lu, S., Tang, A., Durstenfeld, M., Ho, H. Markers of immune activation and inflammation in individuals with post-acute sequelae of SARS-CoV-2 infection. (n.d.) Retrieved November 25, 2023, from www.medrxiv.org/content/10.1101/2021.07.09.21260287.abstract.
- Moghimi, N., Di Napoli, M., Biller, J., Siegler, J. [HTML][HTML] The neurological manifestations of post-acute sequelae of SARS-CoV-2 infection. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1007/s11910-021-01130-1.
- Dreyer, N., Petruski-Ivleva, N., Albert, L. Identification of a vulnerable group for post-acute sequelae of SARS-CoV-2 (PASC): people with autoimmune diseases recover more slowly from COVID-19. (n.d.) Retrieved November 25, 2023, from www.tandfonline.com/doi/abs/10.2147/IJGM.S313486.
- Newell, K., Waickman, A. Inflammation, immunity, and antigen persistence in post-acute sequelae of SARS-CoV-2 infection. (n.d.) Retrieved November 25, 2023, from www.sciencedirect.com/science/article/pii/S0952791522000759.
- Younger, D. [HTML][HTML] Post-acute sequelae of SARS-CoV-2 infection (PASC): peripheral, autonomic, and central nervous system features in a child. (n.d.) Retrieved November 25, 2023, from link.springer.com/article/10.1007/s10072-021-05345-5.
- Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020; 370(6515): eabd4585.
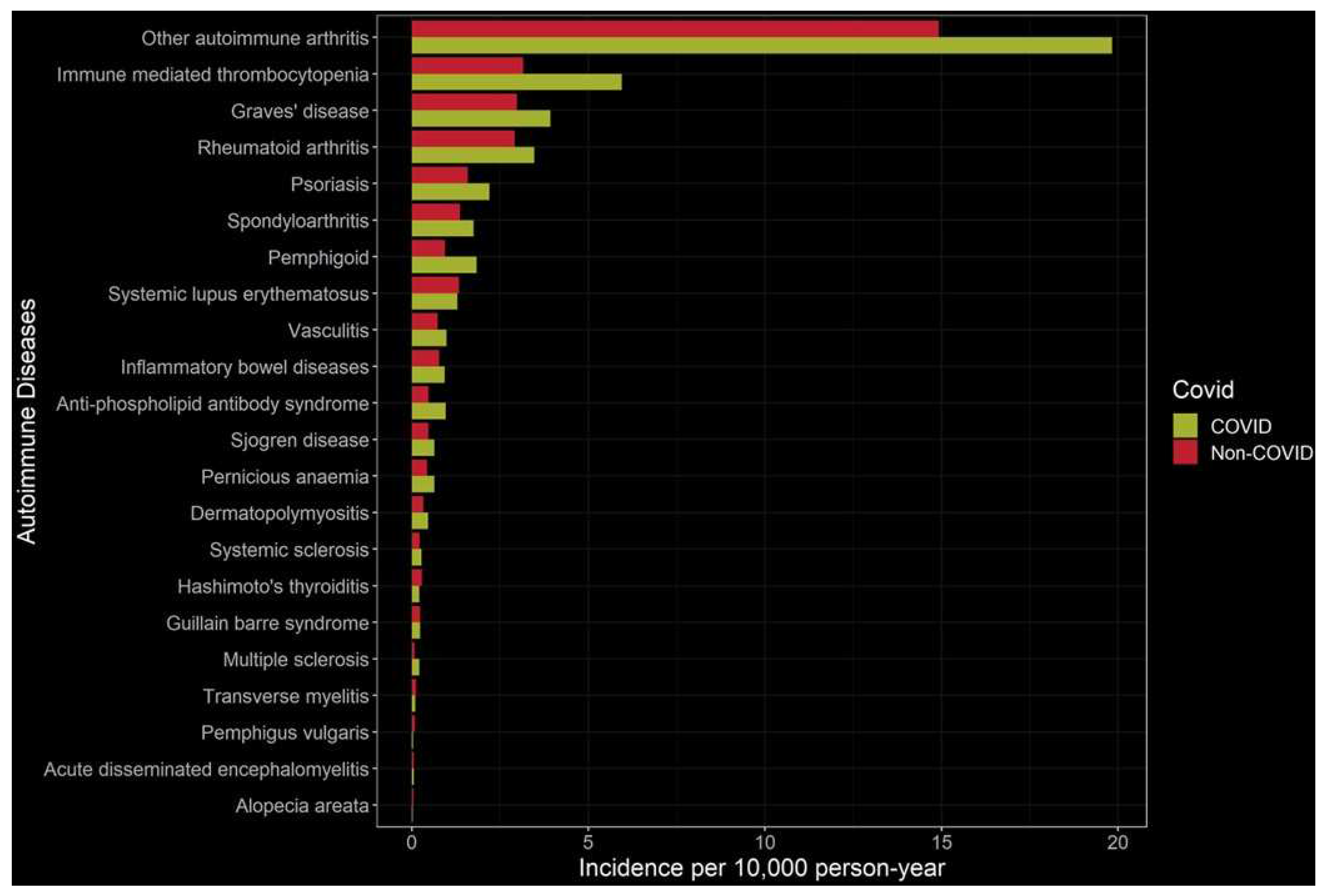
- K. Peng et al., “Risk of autoimmune diseases following COVID-19 and the potential protective effect from vaccination: a population-based cohort study,” EClinicalMedicine, vol. 63, p. 102154, Sep. 2023. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




