Submitted:
28 November 2023
Posted:
29 November 2023
You are already at the latest version
Abstract
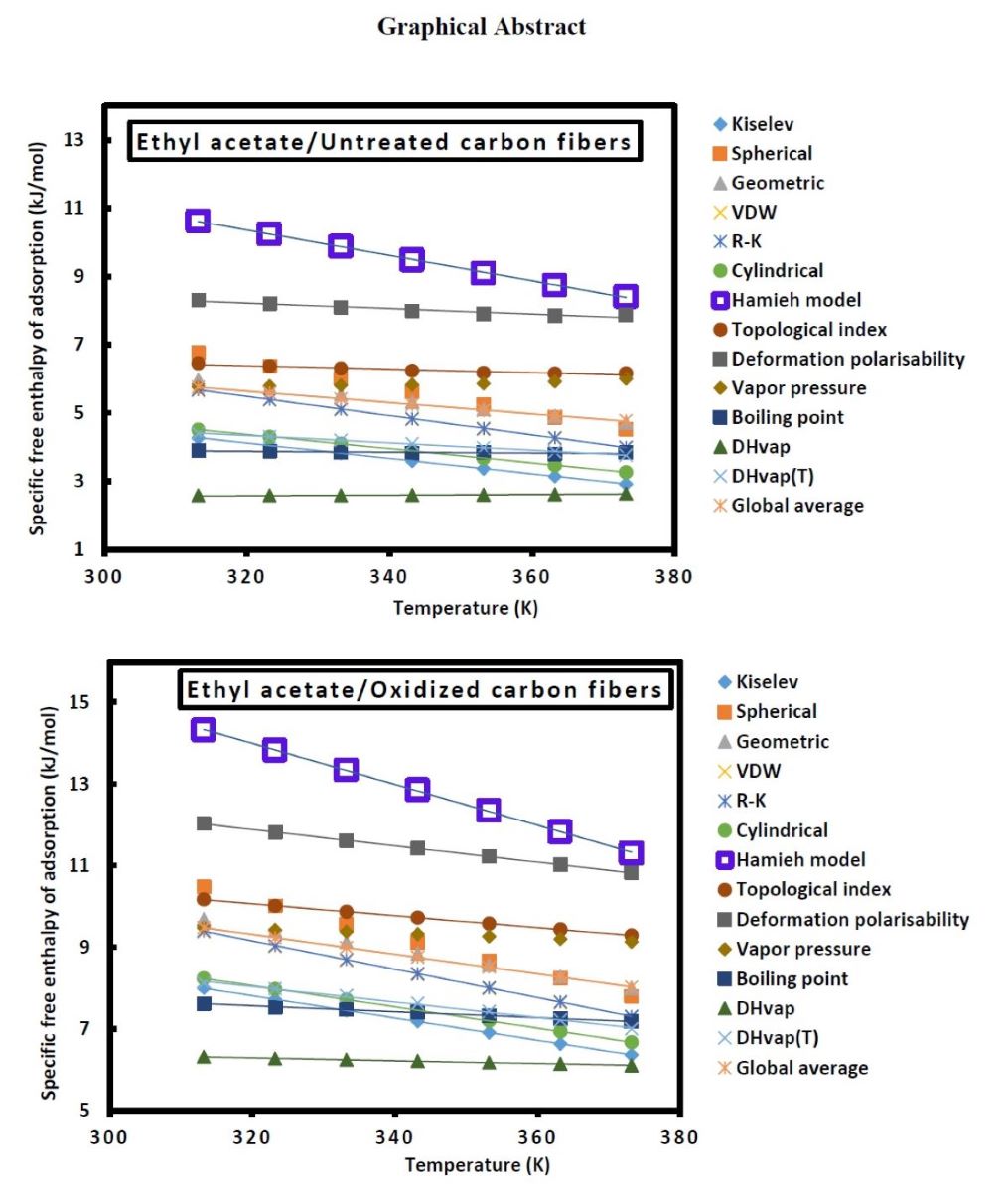
Keywords:
1. Introduction
2. IGC methods and models
2.1. Classical methods
- -
- the boiling point of the solvents [24]
- -
- the vapor pressure of the probes at a fixed temperature [25,26]
- -
- the dispersive component of the surface energy of the solvent [14]
- -
- the deformation polarizability [27]
- -
- the standard enthalpy of vaporization (supposed constant) of organic molecules [28,29]
- -
- the topological index of the solvents [30,31].
2.2. Recent progress
Molecular models
- -
- Kiselev results
- -
- Two-dimensional Van der Waals (VDW) equation
- -
- Two-dimensional Redlich-Kwong (R-K) equation
- -
- geometric model based on the real form of molecules
- -
- cylindrical model based on cylindrical form of molecules
- -
- spherical model based on spherical form of molecules.
Hamieh thermal model
3. Experimental
3.1. Materials and solvents
3.2. GC Conditions
3.3. Results
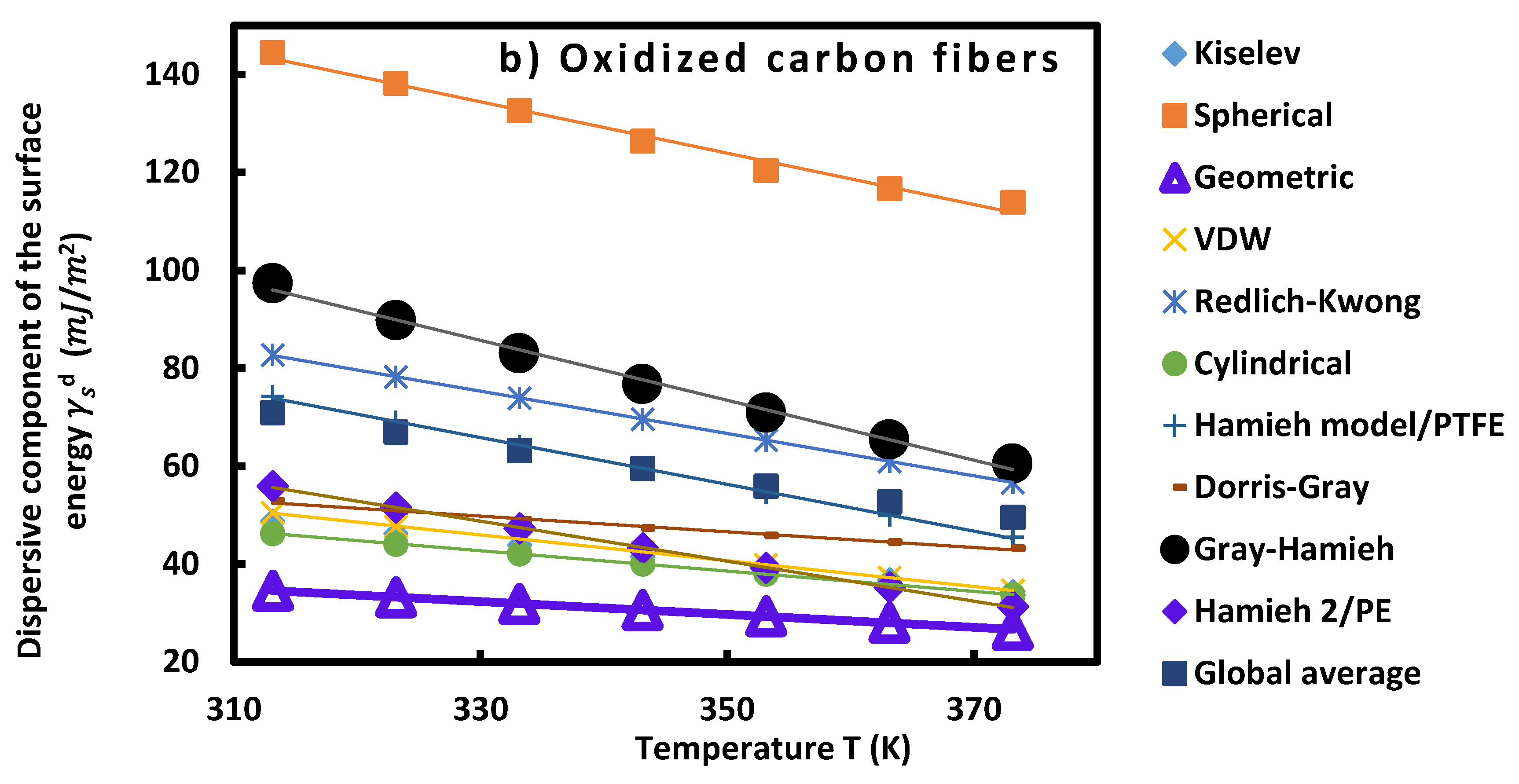
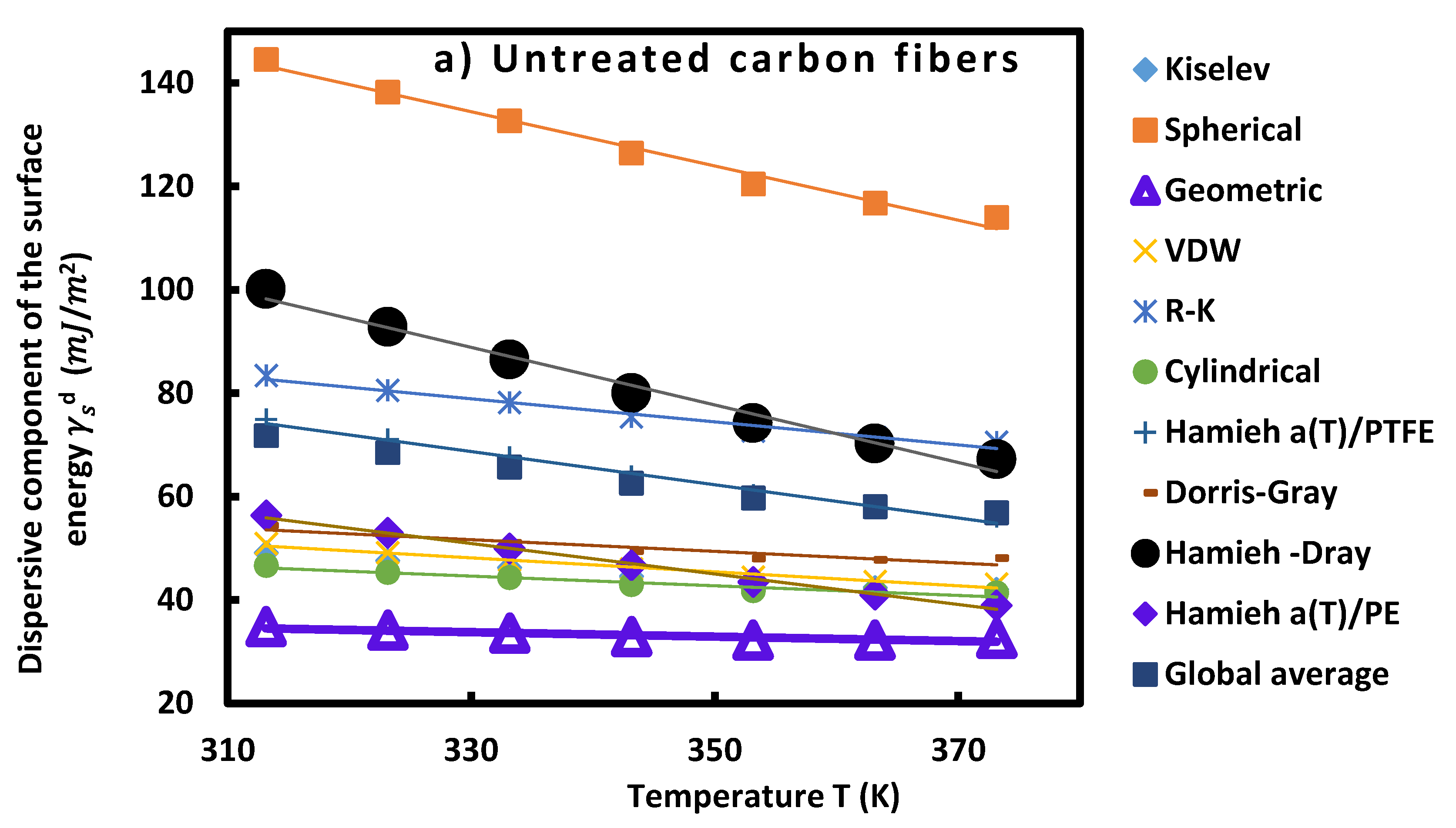
3.3.1. Dispersive component of surface energy of carbon fibers
- -
- the dispersive surface entropy , given by
- -
- the extrapolated values at 0 K
- -
- and the maximum temperature defined by: .
| Untreated carbon fibers (a) | ||||
| Molecular model |
(mJ/m2) |
(mJ m-2 K-1) |
(mJ/m2) |
(K) |
| Kiselev | = -0.13T + 88.0 | -0.13 | 88.0 | 702 |
| Cylindrical | = -0.53T + 307.5 | -0.52 | 307.5 | 586 |
| VDW | = -0.07T + 55.3 | -0.07 | 55.3 | 848 |
| Geometric | = -0.14T + 92.7 | -0.14 | 92.7 | 686 |
| Redlich-Kwong | = -0.22T + 152.5 | -0.22 | 152.5 | 684 |
| Spherical | = -0.11T + 80.6 | -0.11 | 80.6 | 739 |
| Hamieh a(T)/PTFE | = -0.32T + 174.7 | -0.32 | 174.7 | 544 |
| Dorris-Gray | = -0.16T + 104.0 | -0.16 | 104.0 | 655 |
| Hamieh-Gray | = -0.56T + 272.5 | -0.56 | 272.5 | 490 |
| Hamieh a(T)/PE | = -0.29T + 148.2 | -0.29 | 148.2 | 503 |
| Global average | = -0.26T + 151.2 | -0.26 | 151.2 | 590 |
| Oxidized carbon fibers (b) | ||||
| Molecular model | (mJ/m2) | (mJ m-2 K-1) | (mJ/m2) | (K) |
| Kiselev | = -0.24T + 123.7 | -0.24 | 123.7 | 517 |
| Cylindrical | = -0.52T + 307.5 | -0.52 | 307.5 | 586 |
| VDW | = -0.13T + 75.8 | -0.13 | 75.8 | 576 |
| Geometric | = -0.26T + 132.9 | -0.26 | 132.9 | 505 |
| Redlich-Kwong | = -0.43T + 218.0 | -0.43 | 218.0 | 504 |
| Spherical | = -0.21T + 111.3 | -0.21 | 111.3 | 536 |
| Hamieh a(T)/PTFE | = -0.48T + 223.5 | -0.48 | 223.5 | 468 |
| Dorris-Gray | = -0.16T + 102.7 | -0.16 | 102.7 | 641 |
| Hamieh-Gray | = -0.61T + 287.6 | -0.61 | 287.6 | 470 |
| Hamieh a(T)/PE | = -0.41T + 183.6 | -0.41 | 183.6 | 449 |
| Global average | = -0.36T + 182.5 | -0.36 | 182.5 | 511 |
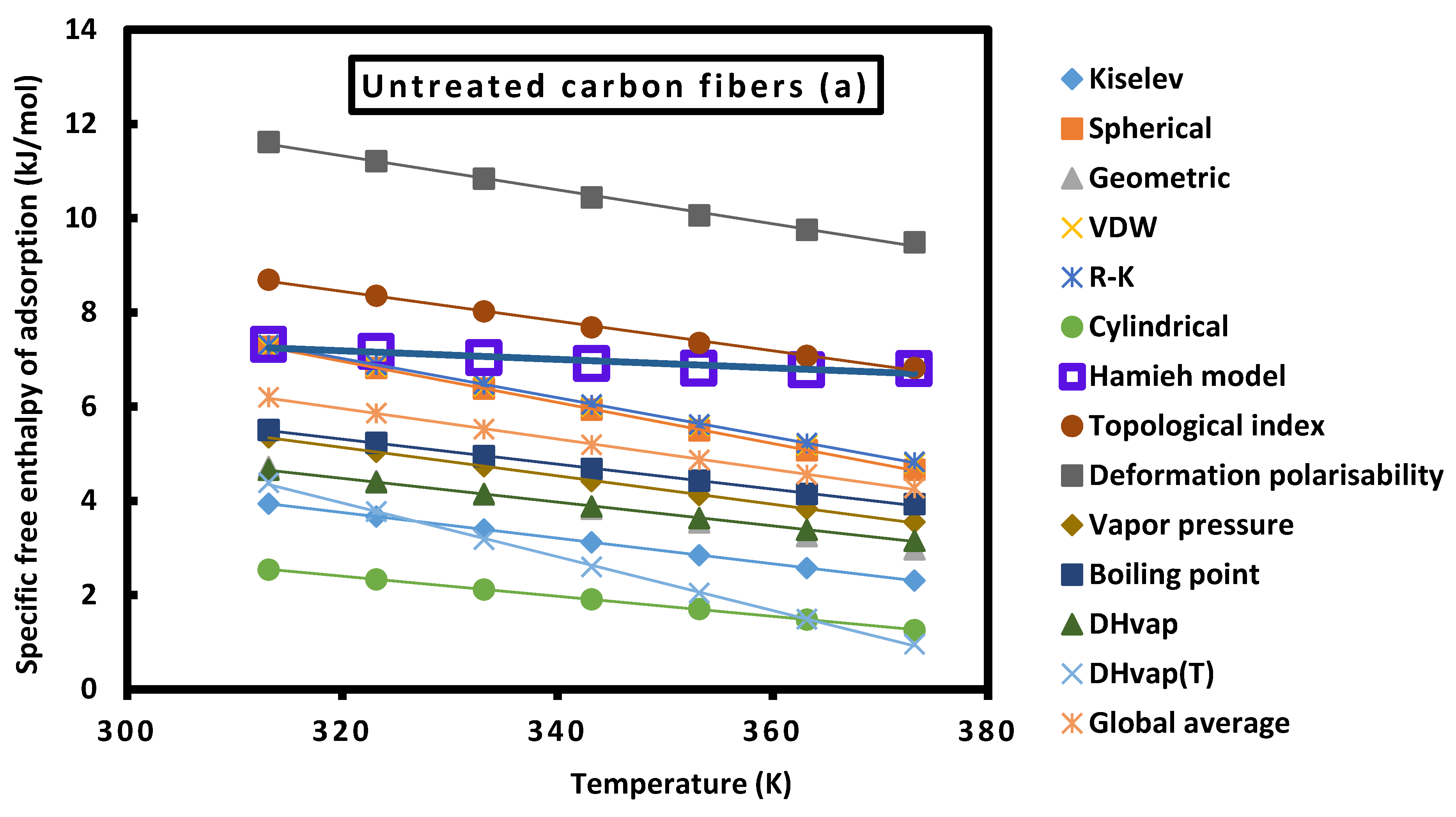
3.3.2. Specific variables of adsorption and Lewis’s acid-base constants
Enthalpic and entropic acid base constants
| Untreated carbon fibers (a) | ||||||||
| Probes | CCl4 | CH2Cl2 | CHCl3 | Benzene | Ether | THF | EA | Acetone |
| Kiselev | 1.075 | 1.200 | 6.011 | 0.765 | 12.135 | 12.456 | 11.321 | 16.459 |
| Spherical | 4.020 | 6.751 | 11.991 | 12.195 | 9.371 | 20.915 | 18.464 | 22.925 |
| Geometric | 9.512 | 14.044 | 41.587 | 6.642 | 5.827 | 13.830 | 12.562 | 10.470 |
| VDW | 2.523 | 4.995 | 19.769 | 12.685 | 8.460 | 20.658 | 14.704 | 18.342 |
| R-K | 2.601 | 5.081 | 19.691 | 12.494 | 8.370 | 20.331 | 14.483 | 18.015 |
| Cylindrical | 1.541 | 16.596 | 38.802 | -3.287 | 4.682 | 9.464 | 11.266 | 9.969 |
| Hamieh model | 1.475 | 1.900 | 6.011 | 1.100 | 13.852 | 13.093 | 18.923 | 13.540 |
| Topological index | 7.292 | 17.167 | 54.714 | 5.528 | 9.132 | 18.524 | 9.042 | 13.164 |
| Deformation polarizability | 9.504 | 0.707 | 47.722 | 9.133 | 11.308 | 22.853 | 10.797 | 15.845 |
| Vapor pressure | -3.789 | 2.297 | 44.039 | 4.700 | 6.777 | 14.770 | 4.576 | 2.082 |
| Boiling point | -4.167 | 0.110 | 41.913 | 4.990 | 9.162 | 13.796 | 4.262 | 4.487 |
| DHvap | -3.839 | 2.382 | 42.347 | 4.584 | 7.495 | 12.540 | 2.302 | 2.438 |
| DHvap(T) | 4.069 | 7.408 | 53.671 | 9.034 | 20.773 | 22.216 | 7.813 | -4.978 |
| Average values | 2.447 | 6.203 | 32.944 | 6.197 | 9.796 | 16.573 | 10.809 | 10.981 |
| Oxidized carbon fibers (b) | ||||||||
| Probes | CCl4 | CH2Cl2 | CHCl3 | Benzene | Ether | THF | EA | Acetone |
| Kiselev | 4.200 | 6.958 | -18.895 | 3.170 | 16.203 | 20.300 | 16.512 | 28.623 |
| Spherical | 7.350 | 13.845 | -11.197 | 16.232 | 13.477 | 29.914 | 24.441 | 35.723 |
| Geometric | 14.586 | 20.524 | 15.981 | 10.199 | 8.598 | 21.950 | 18.548 | 22.065 |
| VDW | 6.045 | 11.728 | -5.159 | 17.185 | 12.309 | 29.983 | 20.583 | 31.148 |
| R-K | 6.053 | 11.843 | -4.140 | 16.892 | 12.292 | 29.521 | 20.294 | 30.633 |
| Cylindrical | 4.982 | 23.658 | 12.922 | -1.537 | 7.551 | 16.915 | 16.682 | 21.332 |
| Hamieh model | 4.782 | 2.937 | 11.416 | 2.931 | 17.063 | 20.099 | 30.039 | 24.811 |
| Topological index | 13.884 | 25.326 | 29.357 | 9.583 | 12.066 | 28.413 | 14.729 | 27.207 |
| Deformation polarizability | 16.481 | 4.283 | 20.425 | 14.201 | 14.861 | 33.962 | 18.262 | 31.156 |
| Vapor pressure | -0.393 | 6.459 | 16.558 | 8.635 | 9.739 | 23.202 | 11.251 | 13.724 |
| Boiling point | -0.789 | 11.799 | 12.989 | 8.864 | 12.220 | 22.385 | 9.874 | 16.157 |
| DHvap | -0.338 | 6.433 | 13.555 | 8.384 | 9.992 | 20.772 | 7.396 | 13.503 |
| DHvap(T) | 7.717 | 11.939 | 24.057 | 13.722 | 22.735 | 29.753 | 14.091 | 6.858 |
| Average values | 6.505 | 12.133 | 9.067 | 9.882 | 13.008 | 25.167 | 17.131 | 23.303 |
| Models or methods | KA | KD | KD/KA | R² | 10-3ωA | 10-3ωD | ωD/ωA | R² |
|---|---|---|---|---|---|---|---|---|
| Kiselev | 0.14 | 0.29 | 2.2 | 0.9705 | 0.31 | 0.41 | 1.3 | 0.9876 |
| Spherical | 0.10 | 2.53 | 25.3 | 0.0475 | 0.26 | 4.40 | 17.0 | 0.0893 |
| Geometric | 0.05 | 1.99 | 43.7 | 0.0366 | 0.17 | 2.94 | 17.7 | 0.1959 |
| Van der Waals | 0.09 | 2.49 | 26.7 | 0.0375 | 0.27 | 4.36 | 16.4 | 0.0847 |
| Redlich-Kwong | 0.09 | 2.46 | 27.0 | 0.0371 | 0.24 | 4.04 | 16.5 | 0.0844 |
| Cylindrical | 0.12 | -0.16 | -1.4 | 0.3736 | 0.30 | -0.47 | -1.6 | 0.4863 |
| Hamieh model | 0.14 | 0.44 | 3.1 | 0.9252 | 0.17 | 0.75 | 4.5 | 0.9309 |
| Topological index | 0.11 | 1.66 | 15.1 | 0.2264 | 0.28 | 1.09 | 3.8 | 0.6131 |
| Deformation polarizability | 0.13 | 2.21 | 17.3 | 0.1226 | 0.30 | 1.69 | 5.6 | 0.4612 |
| Vapor pressure | 0.12 | 0.69 | 5.9 | 0.2498 | 0.24 | 1.15 | 4.7 | 0.4934 |
| Boiling point | 0.11 | 0.76 | 7.1 | 0.2089 | 0.29 | 0.06 | 0.2 | 0.5289 |
| DHvap | 0.09 | 0.70 | 7.5 | 0.1873 | 0.27 | 0.09 | 0.3 | 0.5091 |
| DHvap(T) | 0.14 | 1.89 | 13.4 | 0.1419 | 0.50 | 2.43 | 4.8 | 0.4308 |
| Average values | 0.14 | 1.28 | 8.9 | 0.3193 | 0.30 | 1.64 | 5.5 | 0.5303 |
| Models or methods | KA | KD | KD/KA | R² | 10-3ωA | 10-3ωD | ωD/ωA | R² |
|---|---|---|---|---|---|---|---|---|
| Kiselev | 0.20 | 0.78 | 3.9 | 0.7242 | 0.34 | 1.40 | 4.1 | 0.8568 |
| Spherical | 0.16 | 3.34 | 21.2 | 0.0643 | 0.30 | 5.97 | 20.1 | 0.0689 |
| Geometric | 0.09 | 2.78 | 29.7 | 0.06 | 0.20 | 4.01 | 20.1 | 0.1061 |
| Van der Waals | 0.15 | 3.37 | 22.5 | 0.0515 | 0.33 | 5.77 | 17.5 | 0.0671 |
| Redlich-Kwong | 0.15 | 0.15 | 1.0 | 0.0516 | 0.30 | 5.33 | 17.6 | 0.0673 |
| Cylindrical | 0.18 | 0.22 | 1.2 | 0.7422 | 0.38 | -0.73 | -1.9 | 0.5582 |
| Hamieh model | 0.19 | 1.06 | 5.4 | 0.98 | 0.20 | 2.52 | 12.6 | 0.9244 |
| Topological index | 0.17 | 2.60 | 15.5 | 0.1805 | 0.35 | 2.68 | 7.8 | 0.3816 |
| Deformation polarizability | 0.19 | 3.31 | 17.4 | 0.1127 | 0.38 | 3.75 | 9.9 | 0.2279 |
| Vapor pressure | 0.17 | 1.43 | 8.3 | 0.2066 | 0.37 | 0.69 | 1.9 | 0.4483 |
| Boiling point | 0.16 | 1.54 | 9.5 | 0.1829 | 0.34 | 1.06 | 3.2 | 0.3909 |
| DHvap | 0.15 | 1.40 | 9.5 | 0.1691 | 0.31 | 0.86 | 2.7 | 0.3832 |
| DHvap(T) | 0.18 | 2.79 | 15.8 | 0.106 | 0.46 | 4.52 | 9.8 | 0.2163 |
| Average values | 0.20 | 2.00 | 10.2 | 0.2506 | 0.35 | 2.70 | 7.8 | 0.3182 |
| Solid surface | KA | KD | KD/KA | 10-3ωA | 10-3ωD | ωD/ωA |
|---|---|---|---|---|---|---|
| Untreated carbon fibers (a) | 0.14 | 0.44 | 3.1 | 0.17 | 0.75 | 4.5 |
| Oxidized carbon fibers (b) | 0.19 | 1.06 | 5.4 | 0.20 | 2.52 | 12.6 |
| Ratio fibers (b)/fibers (a) | 1.36 | 2.41 | 1.74 | 1.18 | 3.36 | 2.80 |
Specific and total surface energies of carbon fibers
Conclusion
Funding
Conflicts of Interest
References
- Collins, M.N.; Culebras, M.; Ren, G. Chapter 8 - The use of lignin as a precursor for carbon fiber–reinforced composites, Editor(s): D. Puglia, C. Santulli, F. Sarasini, Micro and Nanolignin in Aqueous Dispersions and Polymers, Elsevier, 2022, Pages 237-250, ISBN 9780128237021. [CrossRef]
- Morgan, P. Carbon Fibers and Their Composites, CRC Press, 2005 May 20. 1200 pages, eBook ISBN: 9780429116827. [CrossRef]
- Park, S.J. Carbon Fibers, Springer, Dordrecht, The Netherlands, 2015. Publisher: Springer Dordrecht, Springer Series in Materials Science (SSMATERIALS, volume 210), 330 pages. [CrossRef]
- Frank, E.; Steudle, L.M.; Ingildeev, D.; Spörl, J.M.; Buchmeiser, M.R. Carbon fibers: Precursor systems, processing, structure, and properties. Angew Chem Int Ed Engl. 2014, 53, 5262–5298. [Google Scholar] [CrossRef]
- Peijs, T.; Kirschbaum, R.; Jan Lemstra, P. Chapter 5: A critical review of carbon fiber and related products from an industrial perspective. Advanced Industrial and Engineering Polymer Research 2022, 5, 90–106. [Google Scholar] [CrossRef]
- Roberts, T. The Carbon Fiber Industry: Global Strategic Market Evaluation 2006–2010; Materials Technology Publications: Watford, UK, 2006. [Google Scholar]
- Red, C. Aerospace will continue to lead advanced composites market in 2006. Composites Manuf. 2006, 7, 24–33. [Google Scholar]
- Papirer, E.; Brendlé, E.; Ozil, F.; Balard, H. Comparison of the surface properties of graphite, carbon black and fullerene samples, measured by inverse gas chromatography. Carbon 1999, 37, 1265–1274. [Google Scholar] [CrossRef]
- Chung, D.L. Carbon Fiber Composites; Butterworth-Heinemann: Boston, MA, USA, 1994; pp. 3–65. [Google Scholar]
- Donnet, J.B.; Bansal, R.C. Carbon Fibers, 2nd ed.; Marcel Dekker: New York, NY, USA, 1990; pp. 1–145. [Google Scholar]
- Friedlander, H.N.; Peebles, L.H. Jr.; Brandrup, J.; Kirby, J.R. On the chromophore of polyacrylonitrile. VI. Mechanism of color formation in polyacrylonitrile. Macromolecules. 1968, 1, 79–86. [Google Scholar] [CrossRef]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials (Basel). 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Qiao, Z.; Ding, C. Recent progress in carbon fibers for boosting electrocatalytic energy conversion. Ionics 2022, 28, 5259–5273. [Google Scholar] [CrossRef]
- Schultz, J.; Lavielle, L.; Martin, C. The role of the interface in carbon fibre-epoxy composites. J. Adhes. 1987, 23, 45–60. [Google Scholar] [CrossRef]
- Schultz, J.; Lavielle, L.; Martin, C. Propriétés de surface des fibres de carbone déterminées par chromatographie gazeuse inverse. J. Chim. Phys. 1987, 84, 231. [Google Scholar] [CrossRef]
- Hamieh, T. Surface acid-base properties of carbon fibres. Advanced powder Technol. 1997, 8, 279–289. [Google Scholar] [CrossRef]
- Hamieh, T. Study of the temperature effect on the surface area of model organic molecules, the dispersive surface energy and the surface properties of solids by inverse gas chromatography. J. Chromatogr. A 2020, 1627, 461372. [Google Scholar] [CrossRef] [PubMed]
- Hamieh, T.; Ahmad, A.A.; Roques-Carmes, T.; Toufaily, J. New approach to determine the surface and interface thermodynamic properties of H-β-zeolite/rhodium catalysts by inverse gas chromatography at infinite dilution. Scientific Reports 2020, 10, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Hamieh, T. New methodology to study the dispersive component of the surface energy and acid–base properties of silica particles by inverse gas chromatography at infinite dilution. Journal of Chromatographic Science 2022, 60, 126–142. [Google Scholar] [CrossRef]
- Hamieh, T. New Physicochemical Methodology for the Determination of the Surface Thermodynamic Properties of Solid Particles. AppliedChem 2023, 3, 229–255. [Google Scholar] [CrossRef]
- Hamieh, T.; Schultz, J. New approach to characterise physicochemical properties of solid substrates by inverse gas chromatography at infinite dilution. I. Some new methods to determine the surface areas of some molecules adsorbed on solid surfaces, J. Chromatogr. A 2002, 969, 17–26. [Google Scholar] [CrossRef]
- Hamieh, T.; Schultz, J. «New approach to characterise physicochemical properties of solid substrates by inverse gas chromatography at infinite dilution. II. Study of the transition temperatures of poly(methyl methacrylate) at various tacticities and of poly(methyl methacrylate) adsorbed on alumina and silica». Journal of Chromatography A 2002, 969, 27–36. [Google Scholar]
- Hamieh, T.; Fadlallah, M.-B.; Schultz, J. New approach to characterise physicochemical properties of solid substrates by inverse gas chromatography at infinite dilution. III. Determination of the acid-base properties of some solid substrates (polymers, oxides and carbon fibres): A new model. J. Chromatogr. A. 2002, 969, 37–47. [Google Scholar] [CrossRef]
- Sawyer, D.T.; Brookman, D.J. Thermodynamically based gas chromatographic retention index for organic molecules using salt-modified aluminas and porous silica beads. Anal. Chem. 1968, 40, 1847–1850. [Google Scholar] [CrossRef]
- Saint-Flour, C.; Papirer, E. Gas-solid chromatography. A method of measuring surface free energy characteristics of short carbon fibers. 1. Through adsorption isotherms. Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 337–341. [Google Scholar] [CrossRef]
- Saint-Flour, C.; Papirer, E. Gas-solid chromatography: Method of measuring surface free energy characteristics of short fibers. 2. Through retention volumes measured near zero surface coverage, Ind. Eng. Chem. Prod. Res. Dev. 1982, 21, 666–669. [Google Scholar] [CrossRef]
- Donnet, J.-B.; Park, S.; Balard, H. Evaluation of specific interactions of solid surfaces by inverse gas chromatography. Chromatographia 1991, 31, 434–440. [Google Scholar] [CrossRef]
- Chehimi, M.M.; Abel, M.-L.; Perruchot, C.; Delamar, M.; Lascelles, S.F.; Armes, S.P. The determination of the surface energy of conducting polymers by inverse gas chromatography at infinite dilution. Synth Met 1999, 104, 51–59. [Google Scholar] [CrossRef]
- Chehimi, M.M.; Pigois-Landureau, E. Determination of acid–base properties of solid materials by inverse gas chromatography at infinite dilution. A novel empirical method based on the dispersive contribution to the heat of vaporization of probes. J. Mater. Chem. 1994, 4, 741–745. [Google Scholar] [CrossRef]
- Brendlé, E.; Papirer, E. A new topological index for molecular probes used in inverse gas chromatography for the surface nanorugosity evaluation, 2. Application for the Evaluation of the Solid Surface Specific Interaction Potential. J. Colloid Interface Sci. 1997, 194, 217–2224. [Google Scholar] [CrossRef] [PubMed]
- Brendlé, E.; Papirer, E. A new topological index for molecular probes used in inverse gas chromatography for the surface nanorugosity evaluation, 1. Method of Evaluation. J. Colloid Interface Sci. 1997, 194, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, V. The Donor-acceptor Approach to Molecular Interactions, Plenum. New York, 1978.
- Dorris, G.M.; Gray, D.G. Adsorption of n-alkanes at zero surface coverage on cellulose paper and wood fibers. J. Colloid Interface Sci. 1980, 77, 353–362. [Google Scholar] [CrossRef]
- Fowkes, F.M. in: Surface and interfacial aspects of biomedical polymers, Vol. I, pp. 337–372, Ed: J. D. Andrade, Plenum Press, New York (1985).
- Hamieh, T.; Schultz, J. Etude par chromatographie gazeuse inverse de l’influence de la température sur l’aire de molécules adsorbées. J. Chim. Phys. 1996, 93, 1292–1331. [Google Scholar] [CrossRef]
- Hamieh, T.; Schultz, J. Study of the adsorption of n-alkanes on polyethylene surface - State equations, molecule areas and covered surface fraction. Comptes Rendus de l’Académie des Sciences, Série IIb 1996, 323, 281–289. [Google Scholar]
- Hamieh, T.; Schultz, J. A new method of calculation of polar molecule area adsorbed on MgO and ZnO by inverse gas chromatography. Comptes Rendus de l’Académie des Sciences, Série IIb 1996, 322, 627–633. [Google Scholar]
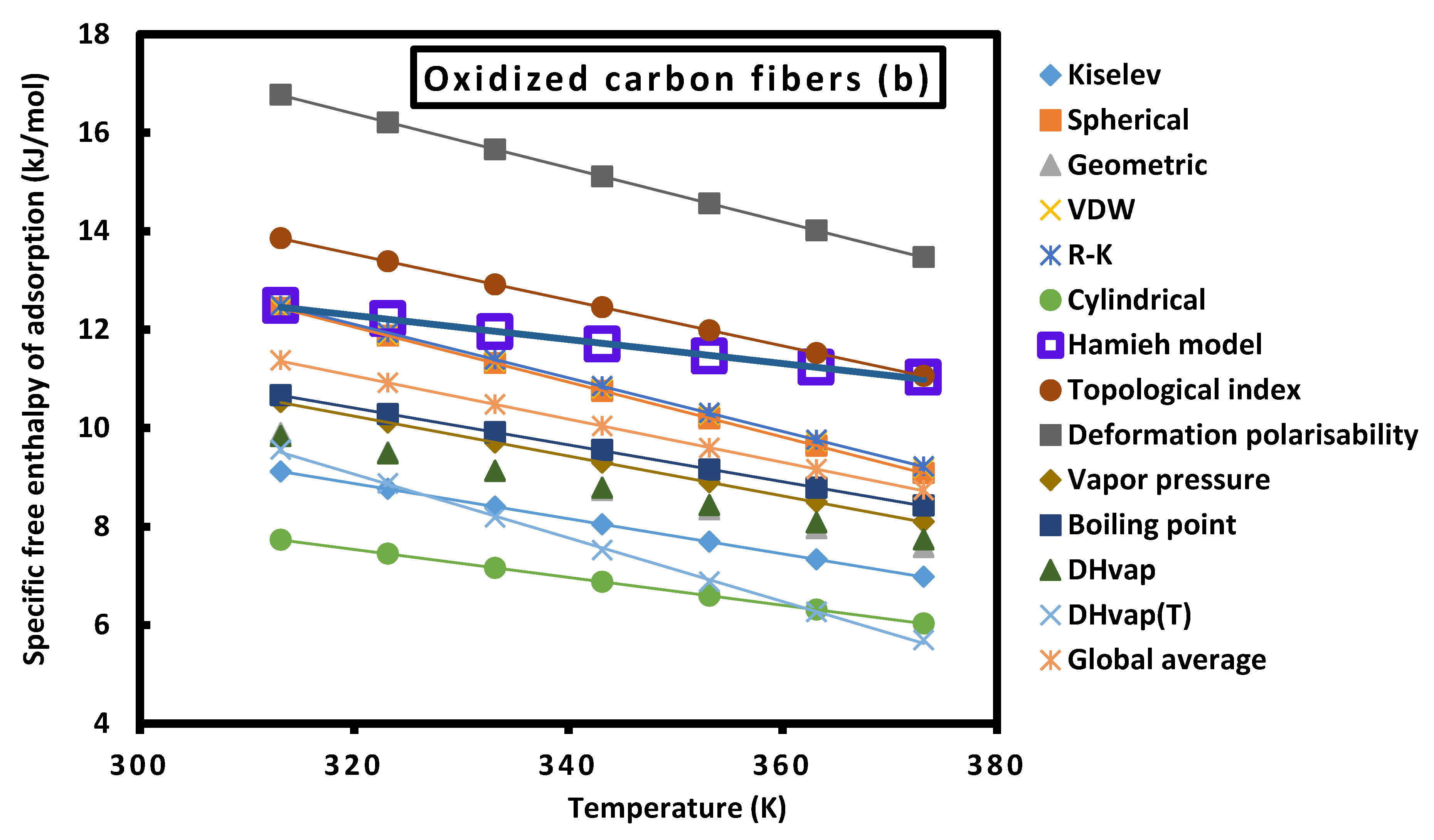
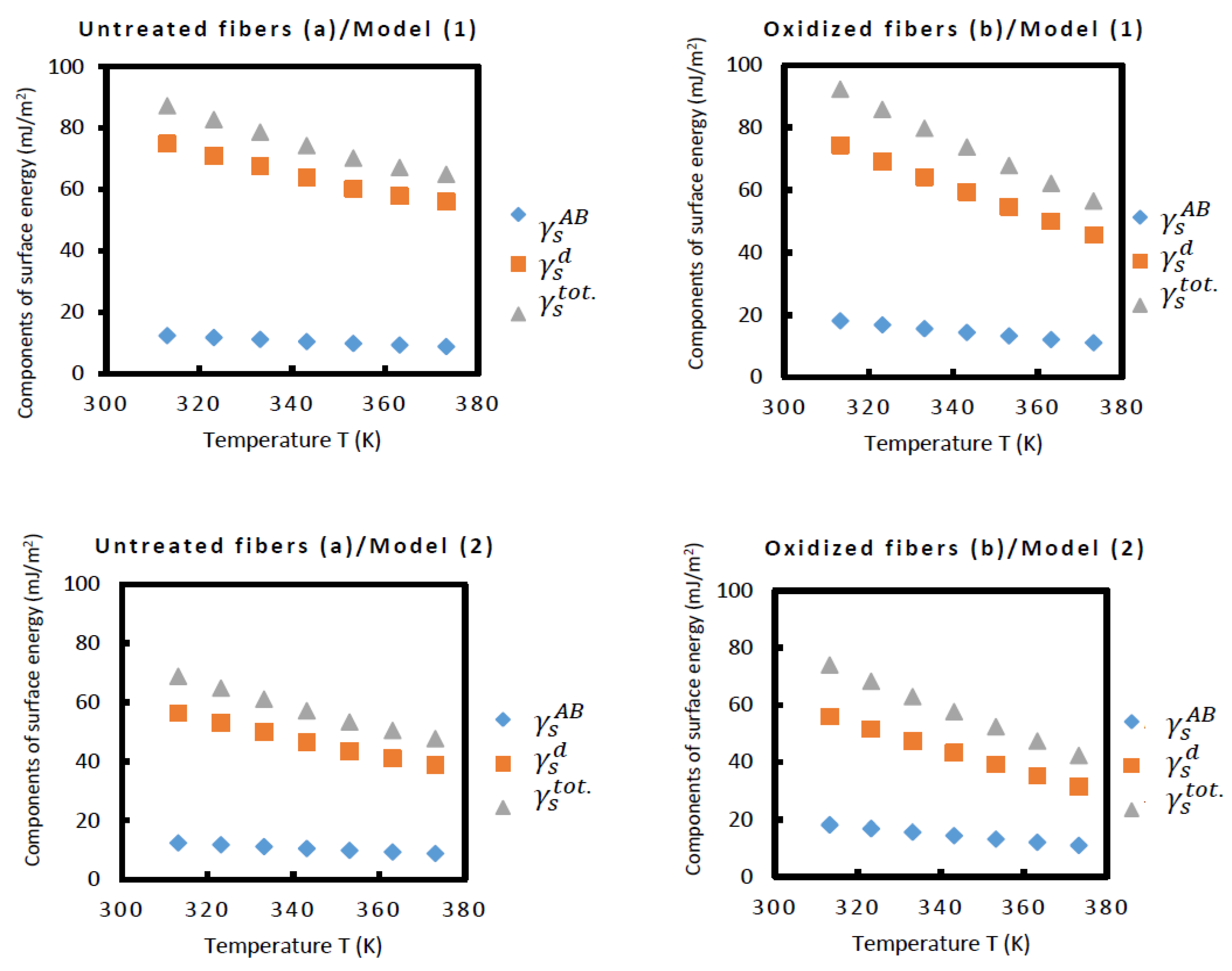
| () | Untreated carbon fibers | Oxidized carbon fibers | ||
| T(K) | CH2Cl2 | Ethyl acetate | CH2Cl2 | Ethyl acetate |
| 313.15 | 1.274 | 10.622 | 1.274 | 10.622 |
| 323.15 | 1.254 | 10.246 | 1.254 | 10.246 |
| 333.15 | 1.234 | 9.881 | 1.234 | 9.881 |
| 343.15 | 1.214 | 9.489 | 1.214 | 9.489 |
| 353.15 | 1.194 | 9.094 | 1.194 | 9.094 |
| 363.15 | 1.174 | 8.746 | 1.174 | 8.746 |
| 373.15 | 1.154 | 8.411 | 1.154 | 8.411 |
| In mJ/m2 | Untreated carbon fibers | Oxidized carbon fibers | ||||
| T(K) | ||||||
| 313.15 | 0.88 | 43.88 | 12.45 | 1.02 | 79.70 | 18.06 |
| 323.15 | 0.85 | 40.75 | 11.79 | 0.95 | 74.19 | 16.77 |
| 333.15 | 0.82 | 37.82 | 11.17 | 0.88 | 68.96 | 15.54 |
| 343.15 | 0.80 | 34.81 | 10.53 | 0.81 | 63.83 | 14.35 |
| 353.15 | 0.77 | 31.91 | 9.91 | 0.74 | 58.79 | 13.20 |
| 363.15 | 0.74 | 29.45 | 9.35 | 0.68 | 53.86 | 12.08 |
| 373.15 | 0.72 | 27.19 | 8.82 | 0.62 | 49.06 | 11.00 |
| In mJ/m2 | Thermal model 1 | ||||
| T(K) | |||||
| 313.15 | 74.9 | 87.3 | 74.3 | 92.3 | |
| 323.15 | 71.0 | 82.8 | 69.1 | 85.8 | |
| 333.15 | 67.6 | 78.7 | 64.1 | 79.7 | |
| 343.15 | 63.8 | 74.3 | 59.3 | 73.7 | |
| 353.15 | 60.2 | 70.2 | 54.6 | 67.8 | |
| 363.15 | 57.9 | 67.2 | 50.0 | 62.1 | |
| 373.15 | 56.1 | 64.9 | 45.5 | 56.5 | |
| In mJ/m2 | Thermal model 2 | ||||
| T(K) | |||||
| 313.15 | 56.3 | 68.8 | 55.9 | 74.0 | |
| 323.15 | 53.0 | 64.8 | 51.5 | 68.3 | |
| 333.15 | 49.9 | 61.1 | 47.4 | 62.9 | |
| 343.15 | 46.6 | 57.2 | 43.3 | 57.6 | |
| 353.15 | 43.5 | 53.4 | 39.2 | 52.4 | |
| 363.15 | 41.1 | 50.4 | 35.2 | 47.3 | |
| 373.15 | 38.9 | 47.7 | 31.3 | 42.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





