Submitted:
29 November 2023
Posted:
30 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
- a conservation status of Least Concern: the British Isles is wholly dependent on immigrants from the continent and they arrive in substantial numbers,
- A wide distribution range to increase the prospect the species may require adaptations to deal with different environmental gradients and to increase the chance the study can be replicated globally: V. cardui is a long-distance migrant with a wide distribution range, inhabiting all continents except for Australia and Antarctica [25].
- Larvae are easily attainable in high numbers and ethically sourced, breeding requires minimal demands for teachers and students with the animal being relatively robust: there are retailers that breed butterflies to sell as pets and they are found almost anywhere, from coastal to urban area, with it being one of the few species that can breed intensively in a variety of habitats;
- There is some indication that the species show gradual and measurable changes across the thermal range for which our study was to be conducted: adults are first seen in late March and numbers continue to rise through May and June as further migrants arrive from the continent [26]. A few publications have shown V. cardui vary in developmental timing and wing morphology across different temperatures.
- Cosmopolitan so the final release of the butterflies is unlikely to cause any environmental damage itself. This species is not a conservation concern.
2. Materials and Methods
2.1. Source and Husbandry of V. cardui
2.2. Incubator Design
2.2.1. Experimental Design
2.2.2. Measurements
2.2.3. Data Analysis for Baseline Butterfly Experiment
3. Results
3.1. Survival Rate of V. cardui to Temperature Variation.
3.2. Morphological Variations of V. cardui Larvae in Response to Temperature Variation
3.3. Morphological and Phenological Variations of V. cardui in Relation to Temperature
4. Discussion
4.1. Temperatures Effect on Phenological and Morphological Attributes of V. cardui
4.2. Flexibility of experimental set-up
4.3. Active learning in environmental education
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Physiological observation on 10.05.23 | Physiological observations on 13.05.23 | ||||||
|---|---|---|---|---|---|---|---|
| Group 1: Room Temperature (18.5 ̊C) | |||||||
| No. | Length (mm) | Colour | Instar Stage | No. | Length (mm) | Colour | Instar Stage |
| 1 | 10 | B | 3rd | 1 | 14 | B | 4th |
| 2 | 10 | B | 3rd | 2 | 13 | B | 4th |
| 3 | 10 | B | 3rd | 3 | 14 | B | 4th |
| 4 | 9 | B | 3rd | 4 | 13 | B | 4th |
| 5 | 11 | B | 3rd | 5 | 16 | B | 4th |
| 6 | 10 | B | 3rd | 6 | 15 | B | 4th |
| 7 | 11 | B | 3rd | 7 | 16 | B | 4th |
| 8 | 11 | B | 3rd | 8 | 16 | B | 4th |
| 9 | 9 | B | 3rd | 9 | 13 | B | 4th |
| 10 | 9 | B | 3rd | 10 | 13 | B | 4th |
| 11 | 10 | B | 3rd | 11 | 14 | B | 4th |
| 12 | 9 | B | 3rd | 12 | 13 | B | 4th |
| 13 | 11 | B | 3rd | 13 | 15 | B | 4th |
| 14 | 12 | B | 3rd | 14 | 16 | B | 4th |
| 15 | 10 | B | 3rd | 15 | 14 | B | 4th |
| 16 | 10 | B | 3rd | 16 | 14 | B | 4th |
| 17 | 9 | B | 3rd | 17 | 13 | B | 4th |
| 18 | 11 | B | 3rd | 18 | 15 | B | 4th |
| 19 | 10 | B | 3rd | 19 | 15 | B | 4th |
| 20 | 10 | B | 3rd | 20 | 14 | B | 4th |
| Group 2: 25 ̊C | |||||||
| 21 | 10 | B | 3rd | 21 | 15 | B | 4th |
| 22 | 9 | B | 3rd | 22 | 13 | B | 4th |
| 23 | 11 | B | 3rd | 23 | 16 | B | 4th |
| 24 | 11 | B | 3rd | 24 | 16 | B | 4th |
| 25 | 9 | B | 3rd | 25 | 13 | B | 4th |
| 26 | 11 | B | 3rd | 26 | 14 | B | 4th |
| 27 | 10 | B | 3rd | 27 | 13 | B | 4th |
| 28 | 9 | B | 3rd | 28 | 13 | B | 4th |
| 29 | 10 | B | 3rd | 29 | 14 | B | 4th |
| 30 | 10 | B | 3rd | 30 | 14 | B | 4th |
| 31 | 10 | B | 3rd | 31 | 14 | B | 4th |
| 32 | 10 | B | 3rd | 32 | 13 | B | 4th |
| 33 | 11 | B | 3rd | 33 | 16 | B | 4th |
| 34 | 9 | B | 3rd | 34 | 13 | B | 4th |
| 35 | 10 | B | 3rd | 35 | 14 | B | 4th |
| 36 | 11 | B | 3rd | 36 | 15 | B | 4th |
| 37 | 11 | B | 3rd | 37 | 16 | B | 4th |
| 38 | 11 | B | 3rd | 38 | 15 | B | 4th |
| 39 | 9 | B | 3rd | 39 | 13 | B | 4th |
| 40 | 9 | B | 3rd | 40 | 13 | B | 4th |
| Group 3: 30 ̊C | |||||||
| 41 | 10 | B | 3rd | 41 | 15 | B | 4th |
| 42 | 9 | B | 3rd | 42 | 13 | B | 4th |
| 43 | 9 | B | 3rd | 43 | 13 | B | 4th |
| 44 | 11 | B | 3rd | 44 | 15 | B | 4th |
| 45 | 9 | B | 3rd | 45 | 13 | B | 4th |
| 46 | 12 | B | 3rd | 46 | 16 | B | 4th |
| 47 | 10 | B | 3rd | 47 | 14 | B | 4th |
| 48 | 10 | B | 3rd | 48 | 14 | B | 4th |
| 49 | 11 | B | 3rd | 49 | 15 | B | 4th |
| 50 | 10 | B | 3rd | 50 | 14 | B | 4th |
| 51 | 10 | B | 3rd | 51 | 14 | B | 4th |
| 52 | 11 | B | 3rd | 52 | 15 | B | 4th |
| 53 | 12 | B | 3rd | 53 | 16 | B | 4th |
| 54 | 9 | B | 3rd | 54 | 13 | B | 4th |
| 55 | 10 | B | 3rd | 55 | 14 | B | 4th |
| 56 | 12 | B | 3rd | 56 | 16 | B | 4th |
| 57 | 11 | B | 3rd | 57 | 14 | B | 4th |
| 58 | 11 | B | 3rd | 58 | 15 | B | 4th |
| 59 | 10 | B | 3rd | 59 | 14 | B | 4th |
| 60 | 9 | B | 3rd | 60 | 13 | B | 4th |
| Group 4: 35 ̊C | |||||||
| 61 | 10 | B | 3rd | 61 | 14 | B | 4th |
| 62 | 10 | B | 3rd | 62 | 14 | B | 4th |
| 63 | 11 | B | 3rd | 63 | 15 | B | 4th |
| 64 | 9 | B | 3rd | 64 | 13 | B | 4th |
| 65 | 12 | B | 3rd | 65 | 16 | B | 4th |
| 66 | 10 | B | 3rd | 66 | 14 | B | 4th |
| 67 | 10 | B | 3rd | 67 | 14 | B | 4th |
| 68 | 11 | B | 3rd | 68 | 15 | B | 4th |
| 69 | 11 | B | 3rd | 69 | 15 | B | 4th |
| 70 | 11 | B | 3rd | 70 | 16 | B | 4th |
| 71 | 9 | B | 3rd | 71 | 13 | B | 4th |
| 72 | 10 | B | 3rd | 72 | 14 | B | 4th |
| 73 | 9 | B | 3rd | 73 | 13 | B | 4th |
| 74 | 9 | B | 3rd | 74 | 14 | B | 4th |
| 75 | 11 | B | 3rd | 75 | 15 | B | 4th |
| 76 | 9 | B | 3rd | 76 | 13 | B | 4th |
| 77 | 11 | B | 3rd | 77 | 16 | B | 4th |
| 78 | 10 | B | 3rd | 78 | 13 | B | 4th |
| 79 | 10 | B | 3rd | 79 | 14 | B | 4th |
| 80 | 10 | B | 3rd | 80 | 14 | B | 4th |
| Group 5: 40 ̊C | |||||||
| 81 | 10 | B | 3rd | 81 | 15 | B | 4th |
| 82 | 11 | B | 3rd | 82 | 16 | B | 4th |
| 83 | 12 | B | 3rd | 83 | 16 | B | 4th |
| 84 | 9 | B | 3rd | 84 | 13 | B | 4th |
| 85 | 10 | B | 3rd | 85 | 14 | B | 4th |
| 86 | 11 | B | 3rd | 86 | 15 | B | 4th |
| 87 | 11 | B | 3rd | 87 | 15 | B | 4th |
| 88 | 12 | B | 3rd | 88 | 16 | B | 4th |
| 89 | 11 | B | 3rd | 89 | 15 | B | 4th |
| 90 | 10 | B | 3rd | 90 | 13 | B | 4th |
| 91 | 9 | B | 3rd | 91 | 13 | B | 4th |
| 92 | 9 | B | 3rd | 92 | 13 | B | 4th |
| 93 | 9 | B | 3rd | 93 | 13 | B | 4th |
| 94 | 10 | B | 3rd | 94 | 14 | B | 4th |
| 95 | 11 | B | 3rd | 95 | 15 | B | 4th |
| 96 | 10 | B | 3rd | 96 | 14 | B | 4th |
| 97 | 11 | B | 3rd | 97 | 16 | B | 4th |
| 98 | 9 | B | 3rd | 98 | 13 | B | 4th |
| 99 | 9 | B | 3rd | 99 | 13 | B | 4th |
| 100 | 10 | B | 3rd | 100 | 15 | B | 4th |
| Physiological Changes three days post incubation: Date - 16.06.23 | |||||
|---|---|---|---|---|---|
| Group 1: Room Temp (18.5̊C) | |||||
| Number | Length (mm) | Colour | Number | Length (mm) | Colour |
| 1 | 15 | B | 11 | 20 | B |
| 2 | 17 | B/W | 12 | 16 | B |
| 3 | 17 | B | 13 | 22 | B |
| 4 | 20 | B | 14 | 16 | B/W |
| 5 | 18 | B | 15 | 19 | B |
| 6 | 18 | B | 16 | 17 | B |
| 7 | 20 | B | 17 | 18 | B |
| 8 | 15 | B | 18 | 19 | B |
| 9 | 15 | B | 19 | 17 | B |
| 10 | 20 | B | 20 | 26 | B/W |
| Group 2 : 25̊C | |||||
| 21 | 35 | B/W | 31 | 35 | B/W |
| 22 | 28 | B/W | 32 | 40 | B/W |
| 23 | 40 | B/W | 33 | 37 | B/W |
| 24 | 27 | B/W | 34 | 39 | B/W |
| 25 | 37 | W | 35 | 40 | B/W |
| 26 | 37 | B/W | 36 | 35 | B/W |
| 27 | 33 | B/W | 37 | 37 | B/W |
| 28 | 35 | B/W | 38 | 34 | B/W |
| 29 | 36 | W | 39 | 34 | B/W |
| 30 | 35 | B/W | 40 | 28 | B/W |
| Group 3: 30̊C | |||||
| 41 | 37 | B/W | 51 | 38 | B/W |
| 42 | 36 | B/W | 52 | 40 | B/W |
| 43 | 40 | W | 53 | 37 | W |
| 44 | 40 | W | 54 | 33 | B/W |
| 45 | 37 | B/W | 55 | 40 | W |
| 46 | 33 | W | 56 | 36 | B/W |
| 47 | 33 | W | 57 | 35 | B/W |
| 48 | 35 | B/W | 58 | 37 | B/W |
| 49 | 31 | B/W | 59 | 39 | B/W |
| 50 | 37 | B/W | 60 | 36 | B/W |
| Group 4: 35̊C | |||||
| 61 | - | W | 71 | 41 | W |
| 62 | 35 | W | 72 | 40 | W |
| 63 | 39 | W | 73 | 43 | W |
| 64 | 40 | W | 74 | 42 | B/W |
| 65 | 41 | W | 75 | 37 | W |
| 66 | 32 | B/W | 76 | 37 | W |
| 67 | 38 | W | 77 | 38 | W |
| 68 | 42 | W | 78 | 37 | B/W |
| 69 | 36 | W | 79 | 40 | W |
| 70 | 35 | W | 80 | 41 | W |
| Group 5: 40̊C | |||||
| 81 | - | W | 91 | - | W |
| 82 | - | W | 92 | 30 | W |
| 83 | - | W | 93 | - | W |
| 84 | 30 | W | 94 | 35 | W |
| 85 | - | W | 95 | - | W |
| 86 | - | W | 96 | - | W |
| 87 | - | W | 97 | 34 | W |
| 88 | 39 | W | 98 | - | W |
| 89 | - | W | 99 | - | W |
| 90 | - | W | 100 | 30 | W |
| Phenological Records | |||||||
|---|---|---|---|---|---|---|---|
| Group 1: Room Temperature (18.5̊C) | |||||||
| No. | Pupation Date | Pupation Emergence | Pupation Duration (days) | No. | PupationDate | Pupation Emergence | Pupation Duration (days) |
| 1 | 23.05.23 | 05.06.23 | 13 | 11 | 23.05.23 | 04.06.23 | 12 |
| 2 | 23.05.23 | 05.06.23 | 13 | 12 | 23.05.23 | 05.06.23 | 13 |
| 3 | 23.05.23 | 04.06.23 | 12 | 13 | 22.05.23 | 03.06.23 | 12 |
| 4 | 24.05.23 | 06.06.23 | 13 | 14 | 23.05.23 | 04.06.23 | 12 |
| 5 | 23.05.23 | 04.06.23 | 12 | 15 | 23.05.23 | 04.06.23 | 12 |
| 6 | 23.05.23 | 05.06.23 | 13 | 16 | 23.05.23 | 04.06.23 | 12 |
| 7 | 22.05.23 | 02.06.23 | 11 | 17 | 24.05.23 | 05.06.23 | 12 |
| 8 | 23.05.23 | 04.06.23 | 12 | 18 | 23.05.23 | 03.06.23 | 11 |
| 9 | 24.05.23 | 05.06.23 | 12 | 19 | 23.05.23 | 04.06.23 | 12 |
| 10 | 23.05.23 | 04.06.23 | 12 | 20 | 22.05.23 | 03.06.23 | 12 |
| Group 2: 25̊C | |||||||
| 21 | 18.05.23 | 25.05.23 | 7 | 31 | 17.05.23 | 24.05.23 | 7 |
| 22 | 18.05.23 | 25.05.23 | 7 | 32 | 17.05.23 | - | - |
| 23 | 18.05.23 | 25.05.23 | 7 | 33 | 18.05.23 | 25.05.23 | 7 |
| 24 | 18.05.23 | 25.05.23 | 7 | 34 | 18.05.23 | 25.05.23 | 7 |
| 25 | 18.05.23 | 25.05.23 | 7 | 35 | 19.05.23 | 27.05.23 | 8 |
| 26 | 18.05.23 | - | - | 36 | 18.05.23 | 25.05.23 | 7 |
| 27 | 17.05.23 | 25.05.23 | 8 | 37 | 17.05.23 | 25.05.23 | 8 |
| 28 | 17.05.23 | 25.05.23 | 8 | 38 | 18.05.23 | 25.05.23 | 7 |
| 29 | 18.05.23 | 25.05.23 | 7 | 39 | 18.05.23 | 25.05.23 | 7 |
| 30 | 18.05.23 | 26.05.23 | 8 | 40 | 18.05.23 | 26.05.23 | 8 |
| Group 3: 30̊C | |||||||
| 41 | 17.05.23 | 24.05.23 | 7 | 51 | 17.05.23 | 21.05.23 | 4 |
| 42 | 17.05.23 | 23.05.23 | 6 | 52 | 17.05.23 | 23.05.23 | 6 |
| 43 | 17.05.23 | 23.05.23 | 6 | 53 | 17.05.23 | 23.05.23 | 6 |
| 44 | 17.05.23 | 23.05.23 | 6 | 54 | - | - | - |
| 45 | 17.05.23 | 22.05.23 | 5 | 55 | 17.05.23 | 23.05.23 | 6 |
| 46 | 17.05.23 | 23.05.23 | 6 | 56 | 17.05.23 | 22.05.23 | 5 |
| 47 | 18.05.23 | 23.05.23 | 5 | 57 | 17.05.23 | 23.05.23 | 6 |
| 48 | 18.05.23 | Failed | - | 58 | 18.05.23 | 24.05.23 | 6 |
| 49 | 18.05.23 | 24.05.23 | 6 | 59 | 17.05.23 | 23.05.23 | 6 |
| 50 | 18.05.23 | Failed | - | 60 | 17.05.23 | 23.05.23 | 6 |
| Group 4: 35̊C | |||||||
| 61 | - | - | - | 71 | 18.05.23 | Failed | - |
| 62 | - | - | - | 72 | - | - | - |
| 63 | 17.05.23 | Failed | - | 73 | 18.05.23 | 23.05.23 | 5 |
| 64 | - | - | - | 74 | 18.05.23 | Failed | - |
| 65 | 17.05.23 | Failed | - | 75 | 18.05.23 | 23.05.23 | 5 |
| 66 | 19.05.23 | Failed | - | 76 | 17.05.23 | Failed | - |
| 67 | 17.05.23 | Failed | - | 77 | 18.05.23 | 24.05.23 | 6 |
| 68 | 18.05.23 | Failed | - | 78 | 17.05.23 | 23.05.23 | 6 |
| 69 | 18.05.23 | Failed | - | 79 | 17.05.23 | 23.05.23 | 6 |
| 70 | 17.05.23 | 23.05.23 | 6 | 80 | 18.05.23 | 23.05.23 | 5 |
| Group 5: 40̊C | |||||||
| 81 | - | - | - | 91 | - | - | - |
| 82 | - | - | - | 92 | 18.05.23 | Failed | - |
| 83 | - | - | - | 93 | - | - | - |
| 84 | 17.05.23 | Failed | - | 94 | 18.05.23 | Failed | - |
| 85 | - | - | - | 95 | - | - | - |
| 86 | - | - | - | 96 | - | - | - |
| 87 | - | - | - | 97 | 17.05.23 | Failed | - |
| 88 | - | - | - | 98 | - | - | - |
| 89 | - | - | - | 99 | - | - | - |
| 90 | - | - | - | 100 | - | - | - |
| Morphological Attributes Upon Emergence | |||||||
|---|---|---|---|---|---|---|---|
| Group 1: Room Temperature (18.5̊C) | |||||||
| No. | Pupa Position | Note | Wingspan (mm) | No. | Pupa Position | Note | Wingspan (mm) |
| 1 | Hanging | Perfectly formed | 64 | 11 | Hanging | Perfectly formed | 66 |
| 2 | Hanging | Perfectly formed | 62 | 12 | Hanging | Perfectly formed | 66 |
| 3 | Ground | Perfectly formed | 62 | 13 | Hanging | Perfectly formed | 64 |
| 4 | Hanging | Perfectly formed | 64 | 14 | Hanging | Perfectly formed | 62 |
| 5 | Hanging | Perfectly formed | 60 | 15 | Hanging | Perfectly formed | 62 |
| 6 | Hanging | Perfectly formed | 64 | 16 | Hanging | Perfectly formed | 68 |
| 7 | Hanging | Perfectly formed | 62 | 17 | Hanging | Perfectly formed | 62 |
| 8 | Hanging | Perfectly formed | 64 | 18 | Hanging | Perfectly formed | 62 |
| 9 | Hanging | Perfectly formed | 62 | 19 | Ground | Perfectly formed | 64 |
| 10 | Hanging | Perfectly formed | 66 | 20 | Hanging | Perfectly formed | 62 |
| Group 2: 25̊C | |||||||
| 21 | Hanging | Perfectly formed | 66 | 31 | Hanging | Perfectly formed | 64 |
| 22 | Hanging | Perfectly formed | 64 | 32 | Ground | Failed to emerge | - |
| 23 | Hanging | Perfectly formed | 68 | 33 | Hanging | Perfectly formed | 62 |
| 24 | Ground | Perfectly formed | 64 | 34 | Hanging | Perfectly formed | 68 |
| 25 | Hanging | Perfectly formed | 66 | 35 | Hanging | Perfectly formed | 64 |
| 26 | Ground | Failed to emerge | - | 36 | Hanging | Perfectly formed | 66 |
| 27 | Hanging | Right wings larger than the left | 60 | 37 | Hanging | Perfectly formed | 68 |
| 28 | Ground | Perfectly formed | 68 | 38 | Hanging | Perfectly formed | 64 |
| 29 | Hanging | Perfectly formed | 68 | 39 | Hanging | Perfectly formed | 66 |
| 30 | Hanging | Severely deformed on all wings, stuck in chrysalis | - | 40 | Hanging | Perfectly formed | 62 |
| Group 3: 30̊C | |||||||
| 41 | Hanging | Perfectly formed | 66 | 51 | Ground | Deformity in left forewing | 64 |
| 42 | Hanging | Perfectly formed | 70 | 52 | Hanging | Perfectly formed | 64 |
| 43 | Hanging | Perfectly formed | 70 | 53 | Hanging | Perfectly formed | 66 |
| 44 | Hanging | Perfectly formed | 68 | 54 | - | - | - |
| 45 | Ground | Deformity of right hind wing | 66 | 55 | Hanging | Slight uplift in outer left forewing | 64 |
| 46 | Hanging | Perfectly formed | 64 | 56 | Ground | Perfectly formed | 66 |
| 47 | Hanging | Perfectly formed | 68 | 57 | Hanging | Perfectly formed | 68 |
| 48 | Hanging | Failed to emerge | - | 58 | Hanging | Perfectly formed | 66 |
| 49 | Ground | Small, deformity of both the right hind & forewing | 44 | 59 | Ground | Severely deformed on all wings. Got stuck in chrysalis. | - |
| 50 | Ground | Failed to emerge | - | 60 | Ground | Perfectly formed | 66 |
| Group 4: 35̊C | |||||||
| 61 | - | - | - | 71 | Ground | Failed to emerge | - |
| 62 | - | - | - | 72 | - | - | - |
| 63 | Hanging | Failed to emerge | - | 73 | Ground | Perfectly formed | 66 |
| 64 | - | - | - | 74 | Ground | Failed to emerge | - |
| 65 | Ground | Failed to emerge | - | 75 | Ground | Perfectly formed | 58 |
| 66 | Ground | Failed to emerge | - | 76 | Ground | Failed to emerge | - |
| 67 | Ground | Failed to emerge | - | 77 | Ground | Severely deformed on all wings, got stuck in chrysalis | - |
| 68 | Hanging | Failed to emerge | - | 78 | Hanging | Perfectly formed | 66 |
| 69 | Ground | Failed to emerge | - | 79 | Hanging | Perfectly formed | 64 |
| 70 | Hanging | Severely deformed on all wings, got stuck in chrysalis | - | 80 | Ground | Abdomen stuck in chrysalis | 66 |
| Group 5: 40̊C | |||||||
| 81 | - | - | - | 91 | - | - | - |
| 82 | - | - | - | 92 | Ground | Failed to emerge | - |
| 83 | - | - | - | 93 | - | - | - |
| 84 | Hanging | Failed to emerge | - | 94 | Ground | Failed to emerge | - |
| 85 | - | - | - | 95 | - | - | - |
| 86 | - | - | - | 96 | - | - | - |
| 87 | - | - | - | 97 | Hanging | Failed to emerge | - |
| 88 | - | - | - | 98 | - | - | - |
| 89 | - | - | - | 99 | - | - | - |
| 90 | - | - | - | 100 | - | - | - |
References
- Jones, R; Bursens, P. The effects of active learning environments: how simulations trigger affective learning. Eur. Political Sci. 2015, 14, 254–265. [Google Scholar] [CrossRef]
- Gano-Phillips, S. Affective learning in general education. Special topic: Assessment in University General Education Program 2009, 6, 1–44. [Google Scholar]
- Vaishnav, S.R. Learning Style and Academic Achievement of Secondary School Students. Learning Style and Academic Achievements 2013, 1, 2277–7733. [Google Scholar]
- Marleny, L.; Aloysius, C.; Hadi, S. Emotional Intelligence among Auditory, Reading, and Kinaesthetic Learning Styles of Elementary School Students in Ambon-Indonesia. International Electronic Journal of Elementary Education 2017, 10, 83–91. [Google Scholar]
- Gifford, R.; Nilsson, A. Personal and social factors that influence pro-environmental concern and behaviour: A review. Int. J. Psychol. 2014, 49, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Heinen, R.; Gols, R.; Thakur, M.P. Climate change-mediated temperature extremes and insects: From outbreaks to breakdowns. Glob. Change Biol. 2020, 26, 6685–6701. [Google Scholar] [CrossRef]
- Song, H.; Kemp, D.B.; Tian, L.; Chu, D.; Song, H.; Dai, X. Thresholds of temperature change for mass extinctions. Nat. Comm. 2021, 12, 4694. [Google Scholar] [CrossRef] [PubMed]
- Román-Palacios, C.; Wiens, J.J. Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. USA 2020, 117, 4211–4217. [Google Scholar] [CrossRef]
- Chmura, H.E.; Kharouba, H.M.; Ashander, J.; Ehlman, S.M.; Rivest, E.B.; Yang, L.H. The mechanisms of phenology: the patterns and processes of phenological shifts. Ecol. Monogr. 2019, 89, e01337. [Google Scholar] [CrossRef]
- Gérard, M.; Vanderplanck, M.; Wood, T.; Michez, D. Global warming and plant–pollinator mismatches. Emerg. Top. Life Sci. 2020, 4, 77–86. [Google Scholar]
- Buckley, L.B.; Kingsolver, J.G. Environmental variability shapes evolution, plasticity and biogeographic responses to climate change. Glob. Ecol. Biogeogr. 2019, 28, 1456–1468. [Google Scholar] [CrossRef]
- Tseng, M.; Bevanda, C.; Bhatti, S.S.; Black, E.N.; Chang, E.; Chiang, J.; Dhaliwal, H.; Dimitriou, A.; Gong, S.Y.; Halbe, E.; Harris, N. Effects of temperature on monarch caterpillar pigment variation in nature. Insect Conserv. Divers. 2023, 16, 164–171. [Google Scholar] [CrossRef]
- Cohen, J.M.; Lajeunesse, M.J.; Rohr, J.R. A global synthesis of animal phenological responses to climate change. Nat. Clim. Change 2018, 8, 224–228. [Google Scholar] [CrossRef]
- Miller-Struttmann, N.E.; Geib, J.C.; Franklin, J.D.; Kevan, P.G.; Holdo, R.M.; Ebert-May, D.; Lynn, A.M.; Kettenbach, J.A.; Hedrick, E.; Galen, C. Functional mismatch in a bumble bee pollination mutualism under climate change. Science 2015, 349, 1541–1544. [Google Scholar] [CrossRef] [PubMed]
- Kudo, G.; Cooper, E.J. When spring ephemerals fail to meet pollinators: mechanism of phenological mismatch and its impact on plant reproduction. Proc. Natl. Acad. Sci. USA 2019, 286, 20190573. [Google Scholar] [CrossRef] [PubMed]
- Both, C.; Van Turnhout, C.A.; Bijlsma, R.G.; Siepel, H.; Van Strien, A.J.; Foppen, R.P. Avian population consequences of climate change are most severe for long-distance migrants in seasonal habitats. Proc. R. Soc. Lond., B 2010, 277, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.E.; Holleman, L.J. Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc. R. Soc. Lond., B 2001, 268, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Posledovich, D.; Toftegaard, T.; Wiklund, C.; Ehrlén, J.; Gotthard, K. The developmental race between maturing host plants and their butterfly herbivore–the influence of phenological matching and temperature. J. Anim. Ecol. 2015, 84, 1690–1699. [Google Scholar] [CrossRef]
- Richardson, A.D. PhenoCam: An evolving, open-source tool to study the temporal and spatial variability of ecosystem-scale phenology. Agric. For. Meteorol. 2023, 342, 109751. [Google Scholar] [CrossRef]
- Lawrence, A. The first cuckoo in winter: phenology, recording, credibility and meaning in Britain. Glob. Environ. Change 2019, 19, 173–179. [Google Scholar] [CrossRef]
- van der Kolk, H.J.; WallisDeVries, M.F.; Van Vliet, A.J. Using a phenological network to assess weather influences on first appearance of butterflies in the Netherlands. Ecol. Indic. 2016, 69, 205–212. [Google Scholar] [CrossRef]
- Roy, B.D.; Sparks, H.T. Phenology of British butterflies and climate change. Glob. Change Biol. 2001, 6, 407–416. [Google Scholar] [CrossRef]
- Stefanescu, C.; Penuelas, J.; Filella, I. Effects of climatic change on the phenology of butterflies in the northwest Mediterranean Basin. Glob. Change Biol. 2003, 9, 1494–1506. [Google Scholar] [CrossRef]
- Chowdhury, S.; Fuller, A.R.; Dingle, H.; Chapman, W.J.; Zalucki, M. Migration in butterflies: a global overview. Biol. Rev. 2021, 96, 1462–1483. [Google Scholar] [CrossRef] [PubMed]
- Stefanescu, C.; Paramo, F.; Akesson, S.; Alarcon, M.; Avila, A.; Brereton, T.; Carnicer, J.; Cassar, L.; Fox, R.; Heliola, J.; Hill, J.; Hirneisen, N.; Kjellen, N.; Kuhn, E.; Kuussaari, M.; Leskinen, M.; Liechti, F.; Musche, M.; Regan, E.; Reynolds, D.; Roy, D.; Ryrholm, N.; Schmaljohann, H.; Settele, J.; Thomas, C.; Swaay, C.; Chapman, J. Multi-generational long-distance migration of insects: studying the painted lady butterfly in Western Palaearctic. Echography 2012, 36, 474–486. [Google Scholar] [CrossRef]
- Ubach, A.; Stefanescu, C.; Wiklund, C. Timing of mating, reproductive status and resource availability in relation to migration in the painted lady butterfly. An. Behav. 2021, 172, 145–153. [Google Scholar]
- Kassambara, A.; Kosinski, M.; Biecek, P.; Fabian, S. Survminer: Drawing Survival Curves using ‘ggplot2’. https://cran.rstudio.com/web/packages/survminer/index.html (accessed on 20th November 2023).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. Springer: New York 2016. [CrossRef]
- Brookes, M, E.; Kristensen, K.; Van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. “glmmTMB Balances Speed and Flexibility Among Packages for Zero-inflated Generalized Linear Mixed Modelling”. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hartig, F.; Lohse, L. Residual Diagnostics for Hierarchical (Multi-Level/ Mixed Regression Models). https://cran.r-project.org/web/packages/DHARMa/index.html (accessed on 20th November 2023).
- Lenth, V.R.; Bolker, B.; Buerkner, P.; Gine-Vazquez, I.; Herve, M.; Jung, M.; Love, J.; Miguez, F.; Riebl, H.; Singmann, H. Emmeans: Estimated Marginal Means, aka Least-Squares Means. https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 20th November 2023).
- Babosova, M.; Pereckova, M.; Porhajasova, J. Fixation of the Pupae of Selected Butterfly Species and Factors Affecting their Emerging. Pol. J. Environ. Stud. 2021, 30, 1521–1529. [Google Scholar] [CrossRef]
- Huang, Y.; McPherson, J.; Jiggins, C.; Montejo-Kovacevich, G. Effects of temperature on the development of Heliconius erato butterflies. University of Cambridge: United Kingdom 2022. [CrossRef]
- Koda, K.; Nakamura, H. Effects of temperature on the development and survival of an endangered butterfly, Lycaeides argyrognomon (Lepidoptera: Lycaenidae) with estimation of optimal and threshold temperatures using linear and nonlinear models. Entomol. Sci. 2021, 15, 162–170. [Google Scholar] [CrossRef]
- Stevens, D. Pupal development temperature alters adult phenotype in the speckled wood butterfly, Pararge aegeria. J. Therm. Biol. 2004, 29, 205–210. [Google Scholar] [CrossRef]
- Atkinson, D.; Sibly, R. Why are organisms usually bigger in colder environments? Making sense of a life history puzzle. Trends Ecol. Evol. 1997, 12, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Na, S.; Lee, E.; Kim, H.; Choi, S.; Yi, H. The relationship of mean temperature and 9 collected butterfly species’ wingspan as the response of global warming. J. Ecol. Environ. 2021, 45, 1–8. [Google Scholar] [CrossRef]
- Wilson, R.J.; de Siqueira, A.F.; Brooks, S.J.; Price, B.W.; Simon, L.M.; van der Walt, S.J.; Fenberg, P.B. Applying computer vision to digitised natural history collections for climate change research: Temperature-size responses in British butterflies. Methods Ecol. Evol. 2023, 14, 372–384. [Google Scholar] [CrossRef]
- Sekar, S. A meta-analysis of the traits affecting dispersal ability in butterflies: can wingspan be used as a proxy? J. Anim. Ecol. 2021, 81, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Altizer, S.; Davis, A.K. Populations of monarch butterflies with different migratory behaviors show divergence in wing morphology. Evolution 2010, 64, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Soule, A.; Decker, L.; Hunter, M. Effects of diet and temperature on monarch butterfly wing morphology and flight ability. J. Ins. Conserv. 2020, 24, 961–975. [Google Scholar] [CrossRef]
- Kautz, M.; Imron, A.; Dworschak, K.; Schopf, R. Dispersal variability and associated population-level consequences in tree-killing bark beetles. Mov. Ecol. 2016, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M. Stress-induced color-pattern modifications and evolution of the Painted Lady butterflies Vanessa cardui and Vanessa kershawi. Zool. Sci. 2007, 24, 811–819. [Google Scholar] [CrossRef] [PubMed]
- Solensky, M.J.; Larkin, E. Temperature-induced variation in larval coloration in Danaus plexippus (Lepidoptera: Nymphalidae). Ann. Entomol. Soc. Am. 2003, 96, 211–216. [Google Scholar] [CrossRef]
- Yamanaka, A.; Tanaka, A.; Kitazawa, C. Pupal color polyphenism regulated by temperature and photoperiod in the Asian comma butterfly, Polygonia caureum (Lepidoptera: Nymphalidae). Zool. Stud. 2012, 51, 1432–1437. [Google Scholar]
- Trott, C. Reshaping our world: Collaborating with children for community-based climate change action. Action Res. 2021, 17, 42–62. [Google Scholar] [CrossRef]
- Baker, C.; Clayton, S.; Bragg, E. Educating for resilience: parent and teacher perceptions of children’s emotional needs in response to climate change. Environ. Edu. Res. 2021, 27, 687–705. [Google Scholar] [CrossRef]
- Balestri, M.; Campera, M.; Budiadi, B.; Imron, M.A.; Nekaris, K.A.I. Active learning increases knowledge and understanding of wildlife friendly farming in middle school students in Java, Indonesia. Knowledge 2023, 3, 401–413. [Google Scholar] [CrossRef]
- Cordero, E.; Todd, A.; Abellera, D. Climate Change Education and the Ecological Footprint. Bull. Am. Meteorol. Soc. 2008, 89, 865–872. [Google Scholar] [CrossRef]
- Trott, C. Childrens constructive climate change engagement: Empowering awareness, agency, and action. Environ. Edu. Res. 2019, 26, 532–554. [Google Scholar] [CrossRef]


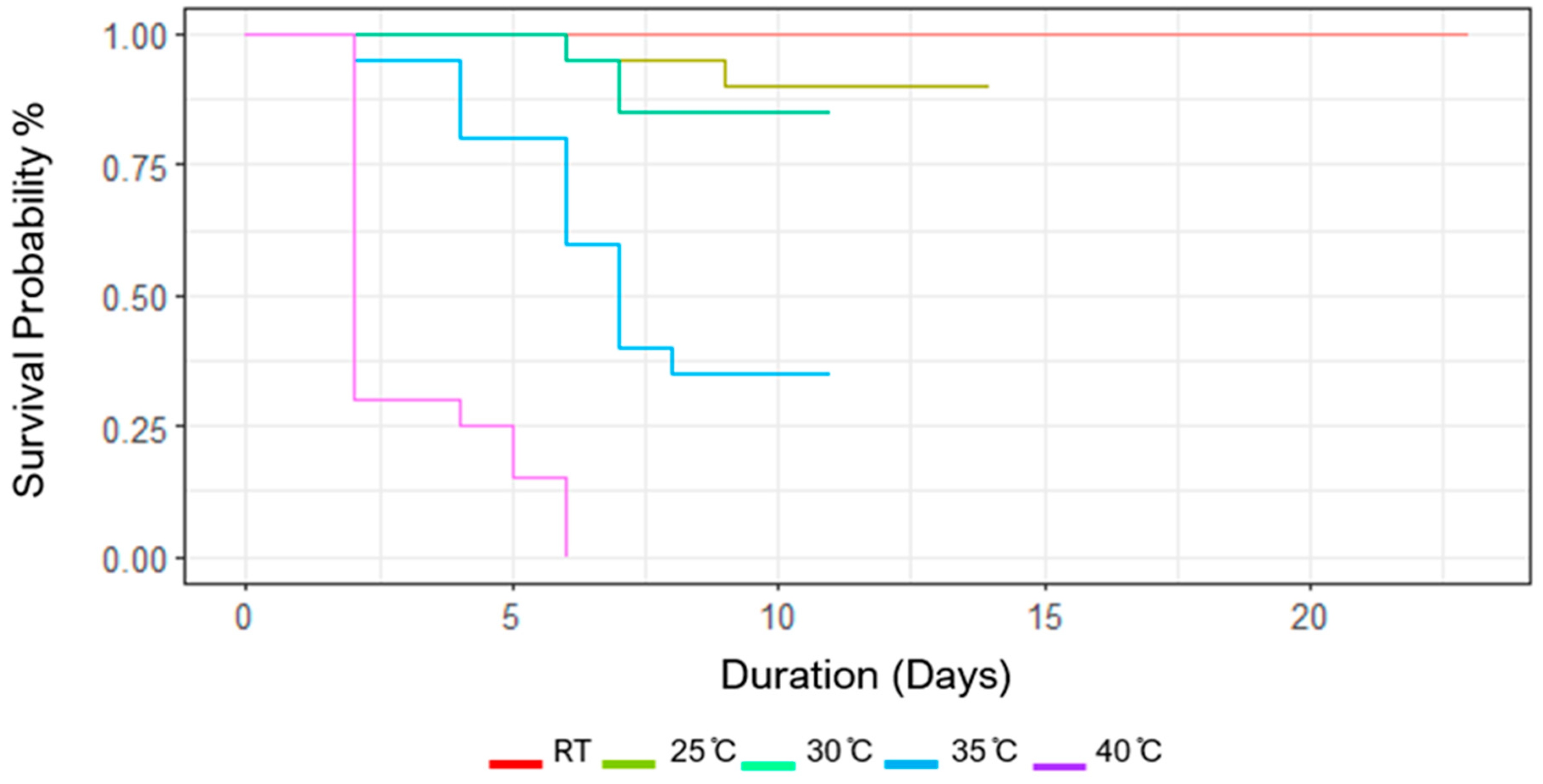
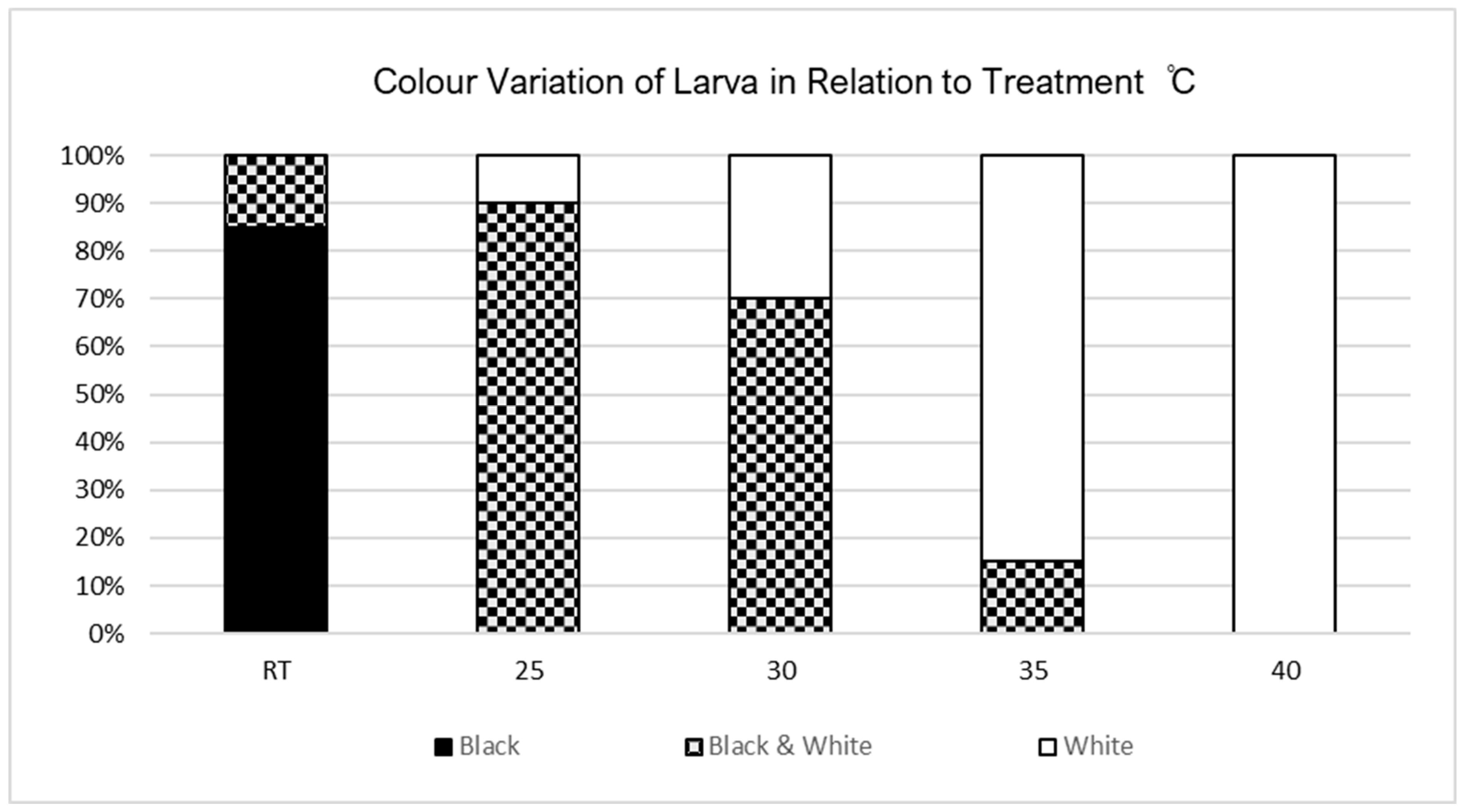
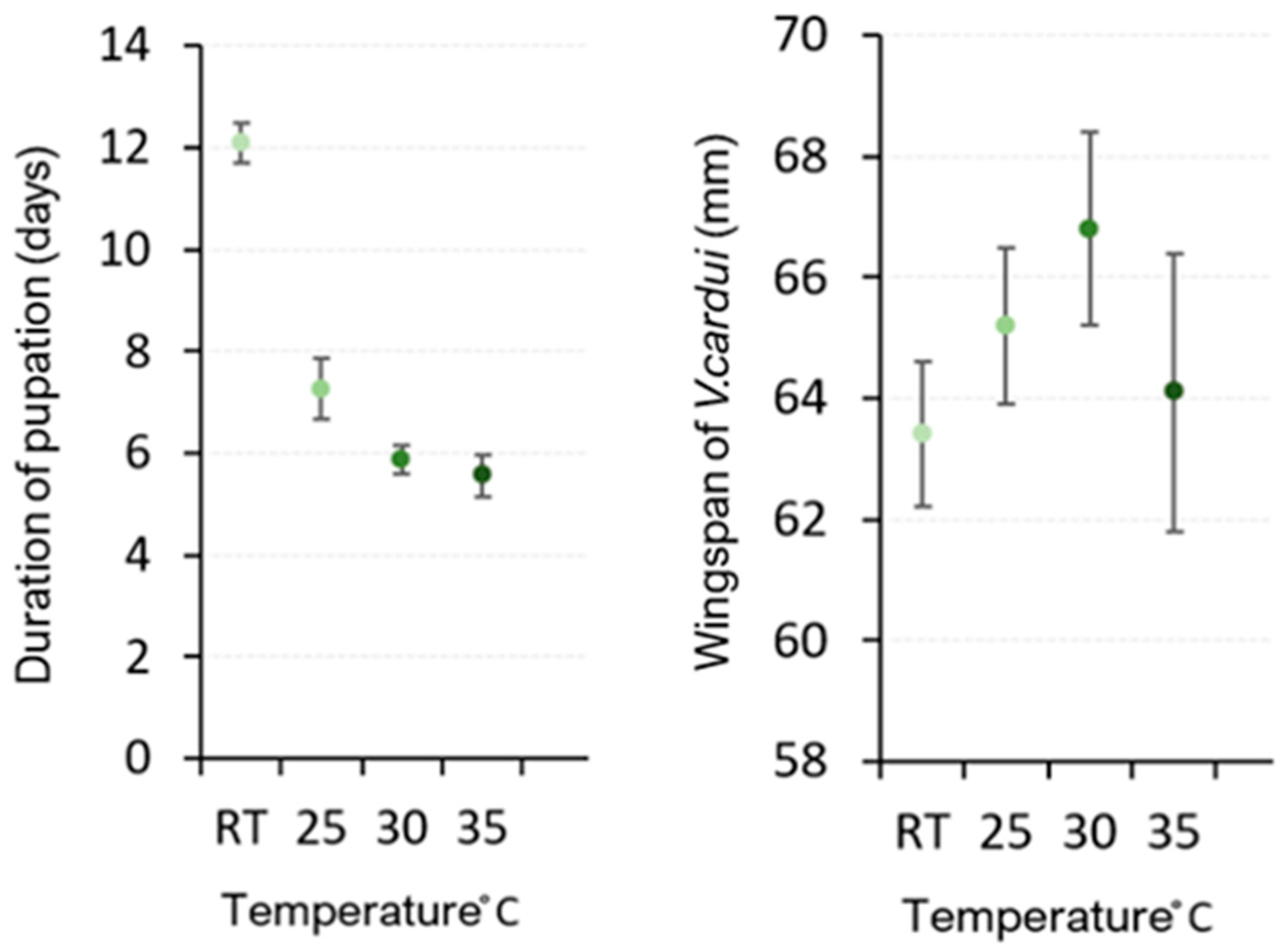
 |
Equipment | Site | Price |
| Basking clip-on spot lamp | Amazon | £19.99 | |
| Basking spotlight 50w* | Reptilush | £4.80 | |
| Habistat Dimming Thermostat | Swell UK | £55.99 | |
| 20 pack A3 white Polystyrene foam boards | Amazon | £18.99 | |
| 30cm mesh habitat net** | Insect Lore | £15.00 | |
| Total | £130.76 | ||
| Response variable | Predictor | Estimate | Std. Error | z-value | p-value |
|---|---|---|---|---|---|
| Duration of pupation | Intercept | 2.492 | 0.013 | 190.88 | <0.0001*** |
| 25 ̊C | -0.509 | 0.021 | -23.56 | <0.0001*** | |
| 30 ̊C | -0.722 | 0.022 | -32.68 | <0.0001*** | |
| 35 ̊C | -0.778 | 0.033 | -23.53 | <0.0001*** | |
| Wingspan of adults | Intercept | 4.148 | 0.007 | 548.4 | <0.0001*** |
| 25 ̊C | 0.028 | 0.011 | 2.6 | 0.0095** | |
| 30 ̊C | 0.525 | 0.121 | 4.3 | 0.0001*** | |
| 35 ̊C | 0.012 | 0.016 | 0.7 | 0.465 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





