Submitted:
27 November 2023
Posted:
30 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Stage 1
2.2. Stage 2
2.3. Stage 3
3. HIV-TB Coinfection
4. Treatment Outcomes
5. Validity and Reliability
6. Definition of Operational Concepts
- Gene mutation is a change to a gene's DNA sequence to produce something different. It creates a permanent change to that gene's sequence. Genetic variations are important for humans to evolve, which is the process of change over generations [35]
- TB mutations are resistance originates in the course of treatment due to genomic mutations in M. tuberculosis. An increase in new cases of drug-resistant TB could be an indicator of high levels of circulating resistant strains [38]
- TB lineages are groups of related M. tuberculosis strains described variously as lineages, families, or clade [35]
- Model is an ideal guidance or imitation. The term "model" refers to something that is accepted or suggested as being admirable [40].
- Collaboration healthcare is defined as the sharing of responsibilities for problem-solving and making decisions to formulate and carry out plans for patient care [38].
- Health stakeholders are individuals or groups who have an interest in a sector, such as the health system. They can influence decisions, shape policy, and create an environment that best serves the needs of those involved in the sector [38].
- Spoligotype is a PCR-based method allowing analysis of strain-dependent polymorphisms observed in spacer sequences present within the direct repeat genomic region of M. tuberculosis complex strains [13].
3. Results
3.1. Gene mutation and Regions of Mutations.
3.2. Heteroresistance
3.3. Type of Lineage
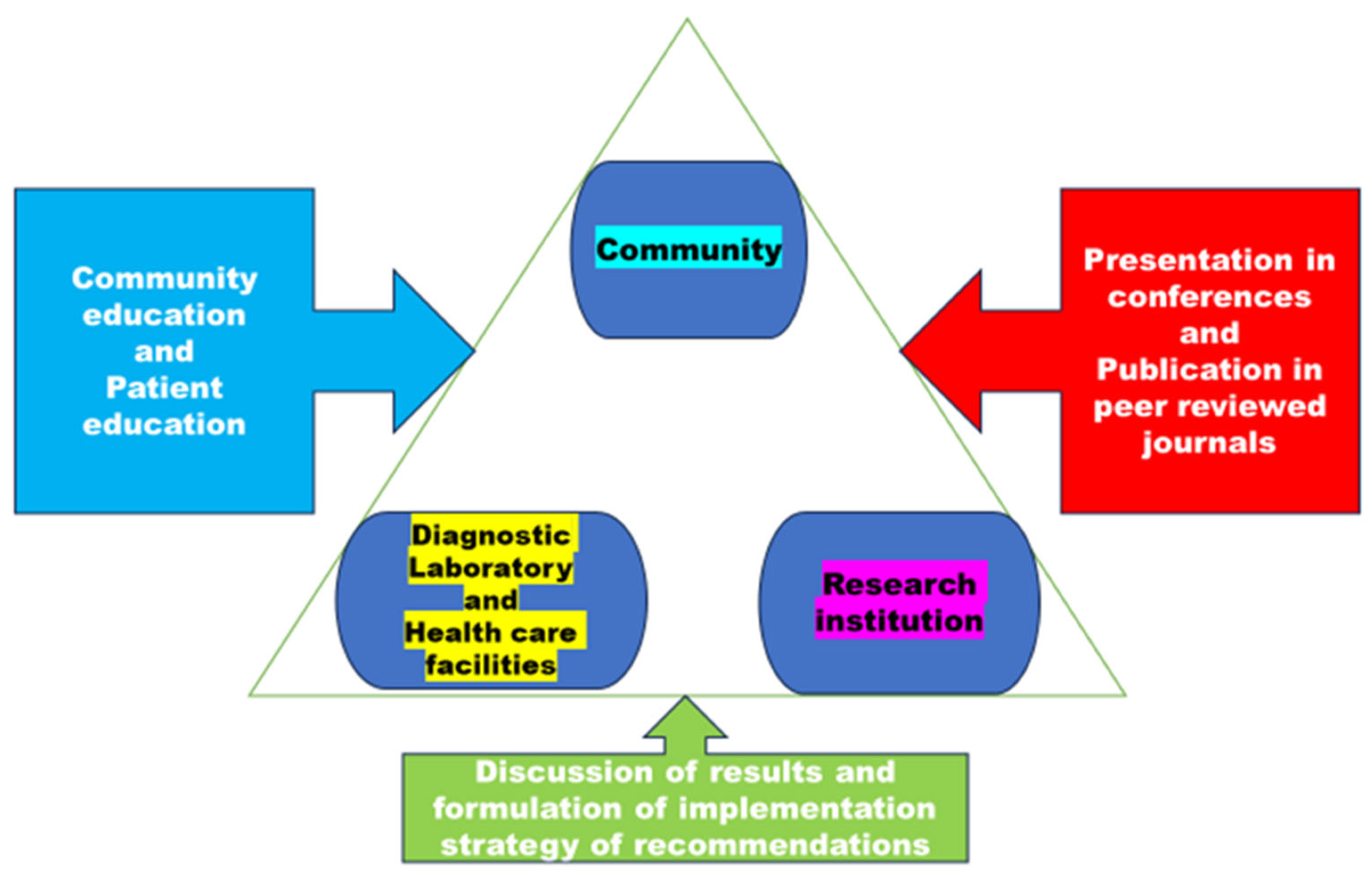
3.4. Significance of the Model
3.5. The Model's Aim to Enhance Capacity, Provide Improved Implementation Guidance, and Ensure Effective Monitoring and Evaluation.
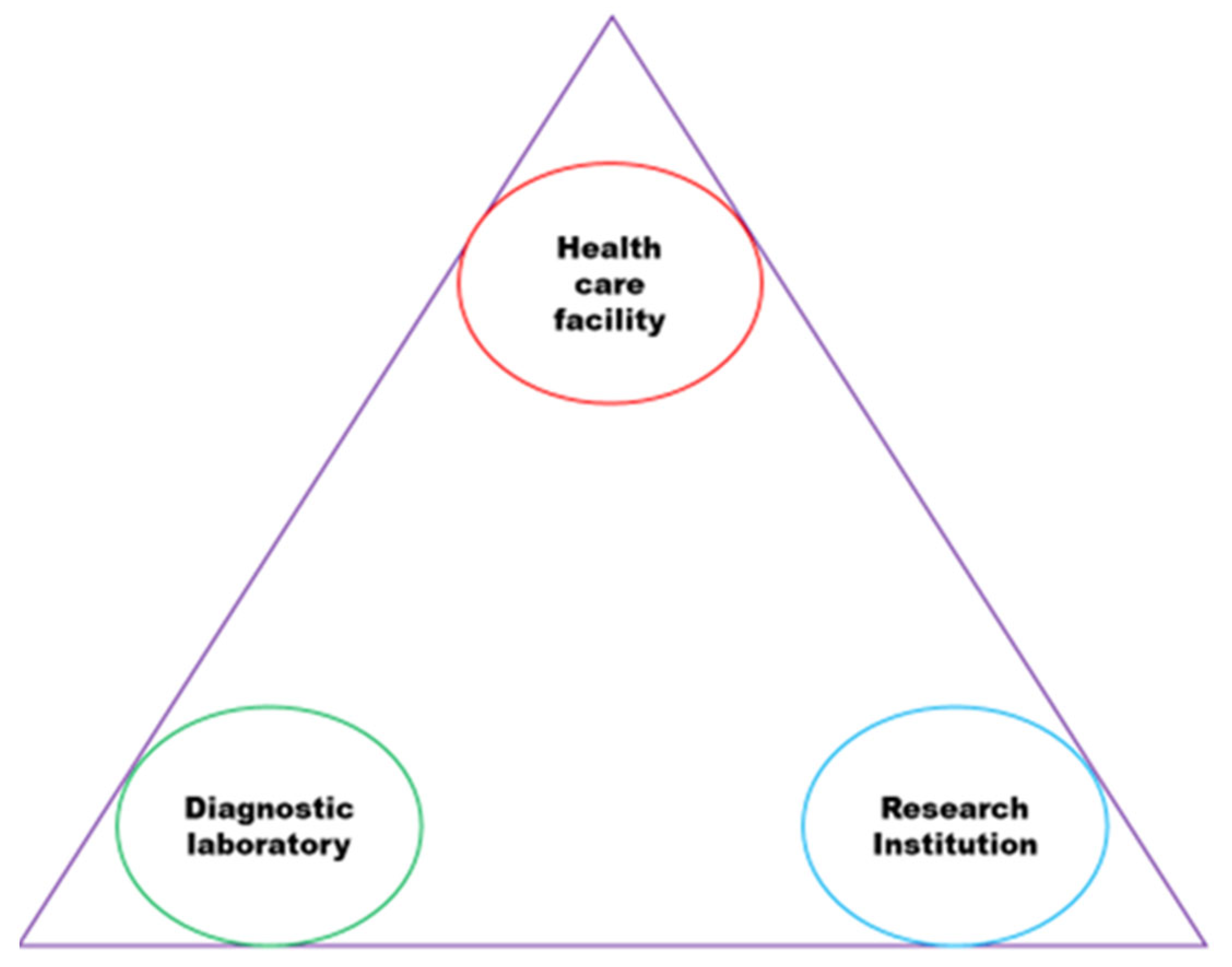
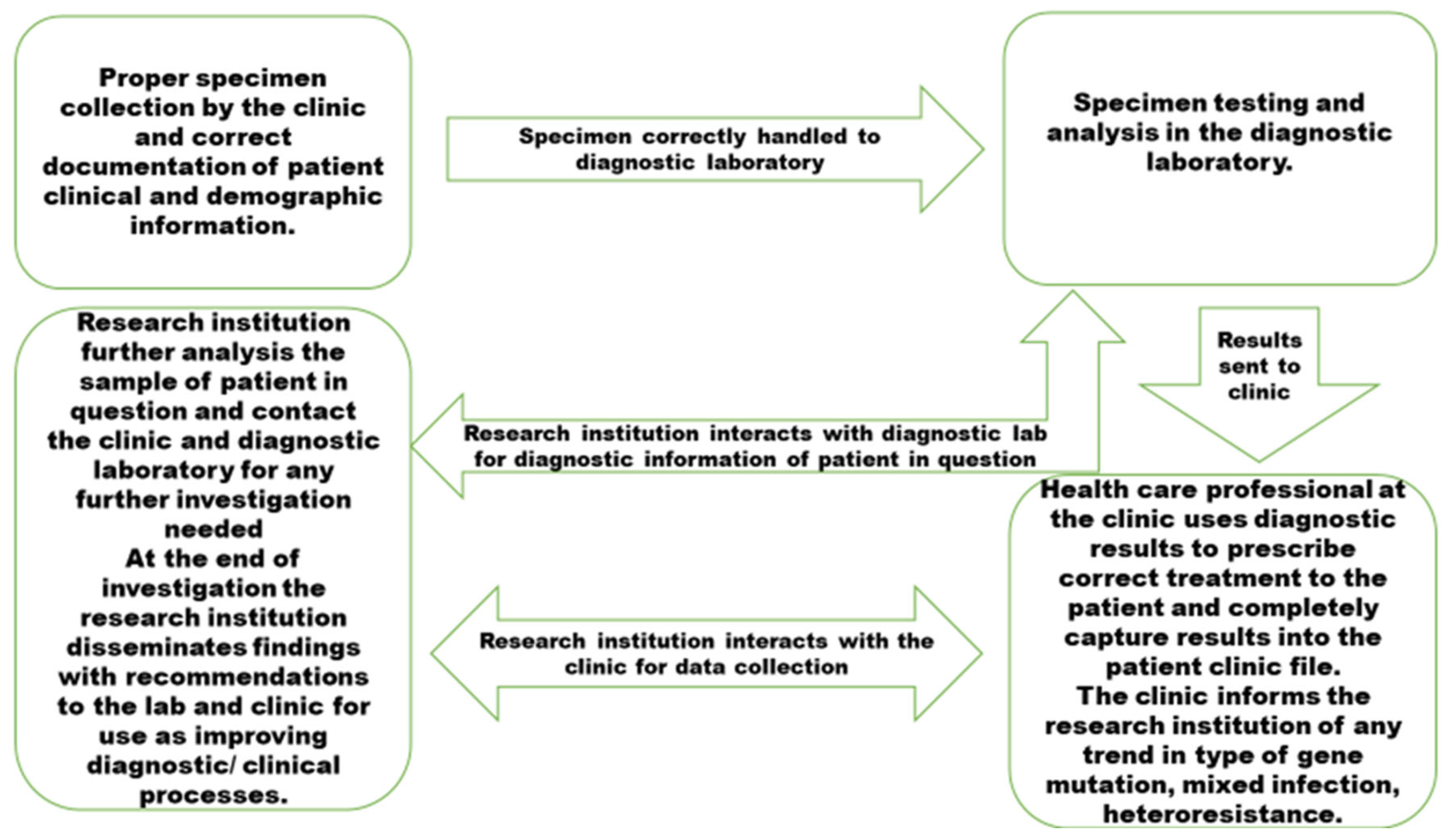
3.6. The Synthesised Model
3.7. The Model's Origin
3.8. Validation of the Model Value
4. Discussion
5. Study Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Notoatmodjo, S. Promosi Kesehatan dan Ilmu Perilaku; Rineka Cipta: Jakarta, Indonesia, 2010. [Google Scholar]
- Bam, T.S.; Aditama, T.Y.; Chiang, C.Y.; Rubaeah, R.; Suhaemi, A. Smoking cessation and smoke-free environments for tuberculosis patients in Indonesia A cohort study. BMC Public Health 2015, 15, 604. [Google Scholar] [CrossRef] [PubMed]
- Vera Rahardjo, S.S.; Murti, B. Health Belief Model and Precede Proceed on the Risk Factors of Multidrug-Resistant Tuberculosis in Surakarta, Central Java. J. Epidemiol. Public Health 2017, 2, 241–254. [Google Scholar]
- Louwagie, G.M.; Ayo-Yusuf, O.A. Tobacco use patterns in tuberculosis patients with high rates of human immunodeficiency virus co-infection in South Africa. BMC Public Health 2013, 13, 1031. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Mahajan, S.; Lal, M.; Toor, A.K.; Deepti, S.S.; Chawla, N. Prevalence of tobacco consumption and smoking and its effect on outcome among microbiologically confirmed new pulmonary tuberculosis patients on daily regimen of DOTS in Amritsar city. J Family Med Prim Care 2022, 11, 2150–2154. [Google Scholar] [PubMed]
- Kant, S.; Maurya, A.K.; Kushwaha, R.A.S.; Nag, V.L. Multi Drug-resistant tuberculosis; an i6trogenic problem. Biosciences Trends 2010, 4, 48–53. [Google Scholar]
- Santha, T.; Garg, R.; Frieden, T.; Chandrasekaran, V.; Subramani, R.; Gopi, P.; Selvakumar, N.; Ganapathy, S.; Charles, N.; Rajamma, J. Risk Factors associated with default, failure, and death among tuberculosis patients treated in a DOTS program in Tiruvallur District, South India, 2000. Int J Tuberc Lung Dis. 2002, 6, 780–788. [Google Scholar] [PubMed]
- Lavigne, M.; Rocher, I.; Steensma, C.; Brassard, P. The impact of smoking on adherence to treatment for latent tuberculosis infection. BMC Public Health 2006, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- WHO. Technical paper on regional strategy for knowledge management to support public health. WHO Regional Office Publisher, 2006; Available online: http://www.who.int/kms/about/en/.
- Veronique, L. Integrated knowledge translation for globally oriented public health practitioners and scientists: framing together sustainable transferontier knowledge translation vision. J Multidisciplinary Health care 2010, 3, 33–47. [Google Scholar]
- Ipe, M. Knowledge sharing in organizations: a conceptual framework. Human Resource Dev Rev 2003, 2, 337–359. [Google Scholar] [CrossRef]
- Pan, S.; Scarborough, H. A sociotechnical view of knowledge sharing at Buckman Laboratories’. J Knowl Manag 1998, 2, 55–66. [Google Scholar] [CrossRef]
- Faye, L.M.; Hosu, M.C.; Oostvogels, S.; Dippenaar, A.; Warren, R.M.; Sineke, N.; Vasaikar, S.; Apalata, T. The Detection of Mutations and Genotyping of Drug-Resistant Mycobacterium Tuberculosis Strains Isolated from Patients in the Rural Eastern Cape Province. Infect. Dis. Rep. 2023, 15, 403–416. [Google Scholar] [CrossRef]
- Faye, L.M.; Hosu, M.C.; Vasaikar, S.; Dippenaar, A.; Oostvogels, S.; Warren, R.M.; Apalata, T. Spatial Distribution of Drug-Resistant Mycobacterium tuberculosis Infections in Rural Eastern Cape Province of South Africa. Pathogens 2023, 12, 475. [Google Scholar] [CrossRef]
- Faye, L.M.; Hosu, M.C.; Iruedo, J.; Vasaikar, S.; Nokoyo, K.A.; Tsuro, U.; Apalata, T. Treatment Outcomes and Associated Factors among Tuberculosis Patients from Selected Rural Eastern Cape Hospitals: An Ambidirectional Study. Trop. Med. Infect. Dis. 2023, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Lipin, M.Y.; Stepanshina, V.N.; Shemyakin, I.G.; Shinnick, T.M. Association of specific mutations in katG, rpoB, rpsL, and rrs genes with spoligotypes of multidrug-resistant Mycobacterium tuberculosis isolates in Russia. Clin. Microbiol. Infect. 2007, 13, 620–626. [Google Scholar] [CrossRef]
- Andersson, D.I.; Nicoloff, H.; Hjort, K. Mechanisms and clinical relevance of bacterial heteroresistance. Nat Rev Microbiol. 2019, 17, 479–496. [Google Scholar] [CrossRef]
- Zheng, Y.; Xia, H.; Bao, X.; Zhao, B.; He, P.; Zhao, Y. Highly Sensitive Detection of Isoniazid Heteroresistance in Mycobacterium Tuberculosis by Droplet Digital PCR. Infection Drug Resistance. 2022, 6245. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Sulaiman, S.A.S.; Hassali, M.A.; Khan, K.U.; Ming, L.C.; Mateen, O.; Ullah, M.O. Effect of smoking on treatment outcome among tuberculosis patients in Malaysia; a multicenter study. BMC Public Health 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hazbon, M.H. Population genetics study of isoniazid resistance mutations and evolution of multidrug-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother 2006, 50, 2640–264. [Google Scholar] [CrossRef] [PubMed]
- van Doorn, H.R.; de Haas, P.E.W.; Kremer, K.; Vandenbroucke-Grauls, C.M.J.E.; Borgdorff, M.W.; van Soolingen, D. Public health impact of isoniazid-resistant Mycobacterium tuberculosis strains with a mutation at amino acid position 315 of katG: a decade of experience in The Netherlands. Clin Microbiol Infect 2006, 12, 769–775. [Google Scholar] [CrossRef]
- Karmakar, M.; Trauer, J.M.; Ascher, D.B.; Denholm, J.T. Hyper transmission of Beijing lineage Mycobacterium tuberculosis: Systematic review and meta-analysis. J. Infect. 2019, 79, 572–581. [Google Scholar] [CrossRef]
- Said, H.; Ratabane, J.; Erasmus, L.; Gardee, Y.; Omar, S.; Dreyer, A.; Ismail, F.; Bhyat, Z.; Lebaka, T.; van der Meulen, M.; et al. Distribution and Clonality of drug-resistant tuberculosis in South Africa. BMC Microbiol. 2021, 21, 157. [Google Scholar] [CrossRef] [PubMed]
- Shangase, Z.; Tsoka-Gwegweni, J.M.; Okem, A. Smoking prevalence among inpatients with drug-resistant tuberculosis in KwaZulu-Natal, South Africa. Tobacco-Induced Diseases. 2018, 16, 276. [Google Scholar] [CrossRef]
- Ano, H.; et al. Relationship between the isoniazid-resistant mutation katGS315T and the prevalence of MDR-/XDR-TB in Osaka, Japan. Int J Tuberc Lung Dis 2008, 12, 1300–1305.
- Swaminathan, S.; Narendran, G. HIV and tuberculosis in India. Journal of Biosciences. 2008, 33, 527. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Mathema, B.; Zhao, Q.; Zheng, X.; Li, D.; Jiang, W.; Wang, W.; Xu, B. Comparison of the sociodemographic and clinical features of pulmonary TB patients infected with sub-lineages within the W-Beijing and non-Beijing Mycobacterium tuberculosis. Tuberculosis 2016, 97, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Kementerian Kesehatan, Republik Indonesia. Information Tuberculosis; Ministry of Health: Jakarta, Indonesia, 2018; Available online: https://pusdatin.kemkes.go.id/article/view/18101500001/infodatin-tuberkulosis-2018.html (accessed on 27 July 2023).
- Holmes, E.A.F.; Hughes, D.A.; Morrison, V.L. Predicting adherence to medications using health psychology theories: A systematic review of 20 years of empirical research. Value Health 2014, 17, 863–876. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global tuberculosis report. WHO/HTM/TB/2017.23, Geneva, 2017.
- van Doorn, H.R.; de Haas, P.E.W.; Kremer, K.; Vandenbroucke-Grauls, C.M.J.E.; Borgdorff, M.W.; van Soolingen, D. Public health impact of isoniazid-resistant Mycobacterium tuberculosis strains with a mutation at amino acid position 315 of katG: a decade of experience in The Netherlands. Clin Microbiol Infect 2006, 12, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Pillay, S.; Magula, N.P. Treatment outcomes of Gene Xpert positive tuberculosis patients in KwaMashu Community Health Centre, KwaZulu-Natal, South Africa: A retrospective review. S. Afr. J. Infect. Dis. 2019, 36, a217. [Google Scholar] [CrossRef] [PubMed]
- Gagneux, S. Impact of bacterial genetics on the transmission of isoniazid-resistant Mycobacterium tuberculosis. PLoSPathog 2006, 2, e61. [Google Scholar] [CrossRef]
- Ameeruddin, N.U.; Luke Elizabeth, H. Impact of isoniazid resistance on the virulence of global and south Indian clinical isolates of Mycobacterium tuberculosis. Tuberculosis (Edinb) 2014, 94, 557–563. [Google Scholar] [CrossRef]
- Ahmad, S.; Mokaddas, E.; Al-Mutairi, N.; Eldeen, H.S.; Mohammadi, S. Discordance across Phenotypic and Molecular Methods.
- for Drug Susceptibility Testing of Drug-Resistant Mycobacterium tuberculosis Isolates in a Low TB Incidence Country. PLoS ONE 2016, 11, e0153563. [CrossRef]
- Tolani, et al. BMC Infectious Diseases 2012, 12, 9. Available online: http://www.biomedcentral.com/1471-2334/12/9.
- Richardson, E.T.; Lin, S.Y.G.; Pinsky, B.A.; Desmond, E.; Banaei, N. The first documentation of isoniazid reversion in Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2009, 13, 1347–1313. [Google Scholar] [PubMed]
- Global Tuberculosis Report 2022 (World Health Organization, 27 January 2023). Available online: https://go.nature.com/3FW2RVs.
- Pai, M.; Kasaeva, T.; Swaminathan, S.N. Eng. J. Med. 2022, 386, 1490–1493. [CrossRef] [PubMed]
- Dlatu, N.; Longo-Mbenza, B.; Oladimeji, K.E.; Apalata, T. Developing a Model for Integrating of Tuberculosis, Human Immunodeficiency Virus and Primary Healthcare Services in Oliver Reginald (O.R) Tambo District, Eastern Cape, South Africa. Int J Environ Res Public Health. 2023, 20, 5977. [Google Scholar] [CrossRef]
- World Health Organization. The power of partnership. Geneva, WHO, 2000 (WHO/HTM/STB/2003.24).
- Biermann, O.; Tran, P.B.; Forse, R.J.; Vo, L.N.Q.; Codlin, A.J.; Viney, K.; Caws, M.; Lönnroth, K. Capitalizing on facilitators and addressing barriers when implementing active tuberculosis case-finding in six districts of Ho Chi Minh City, Vietnam: a qualitative study with key stakeholders. Implement Sci. 2021, 16, 54. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




