Submitted:
29 November 2023
Posted:
30 November 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Raw materials and testing techniques
3. Results and discussions
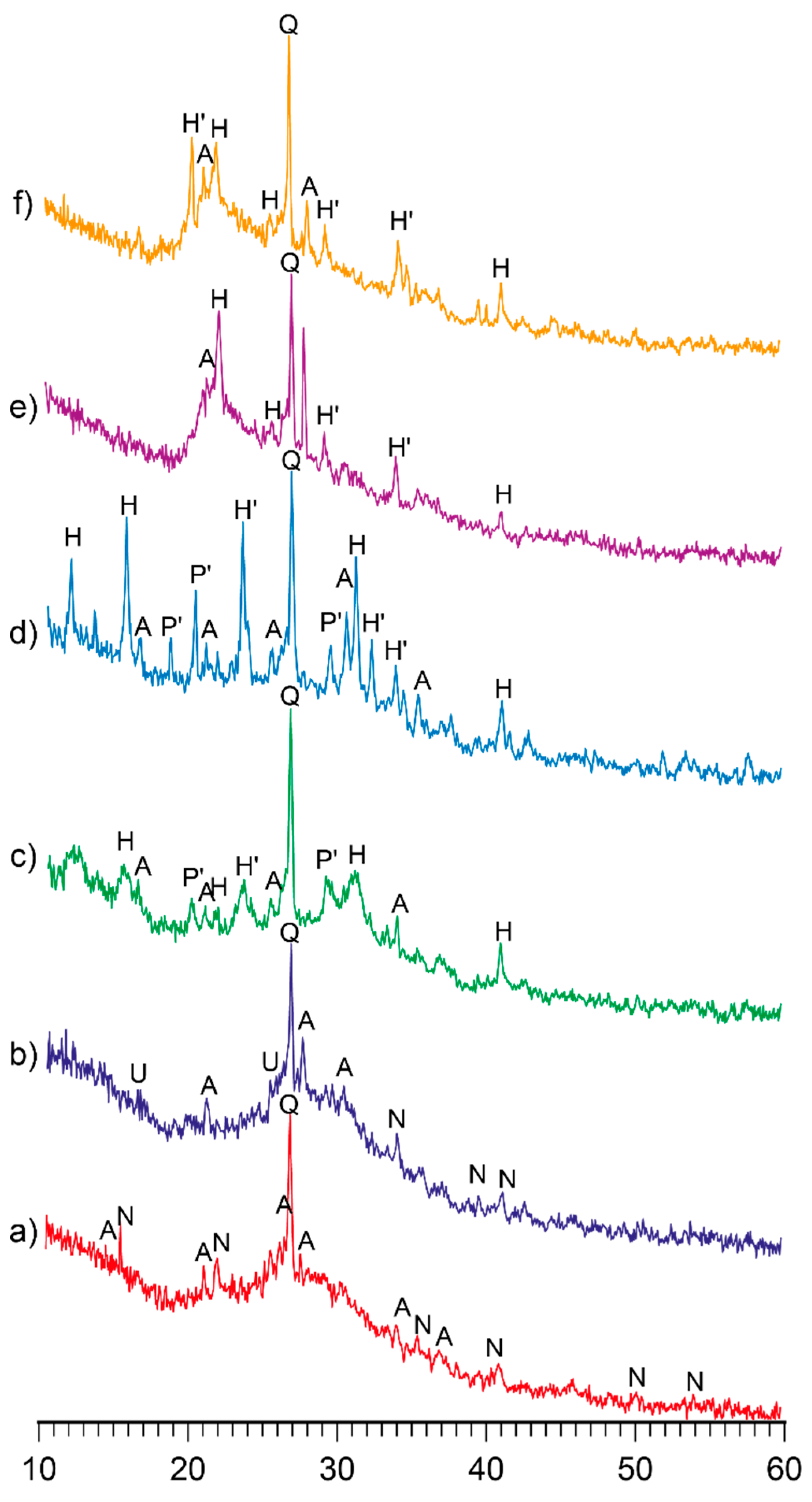
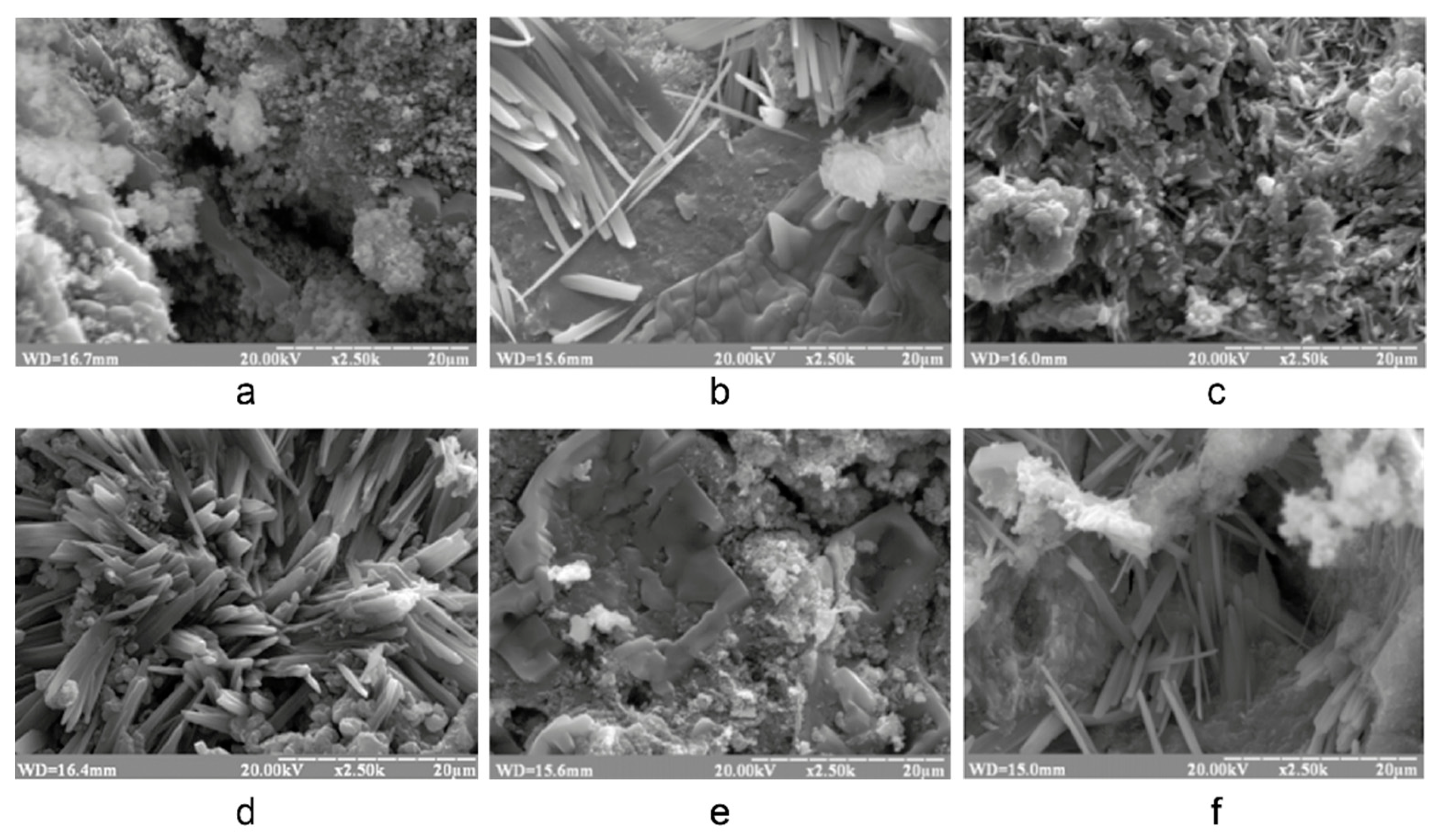
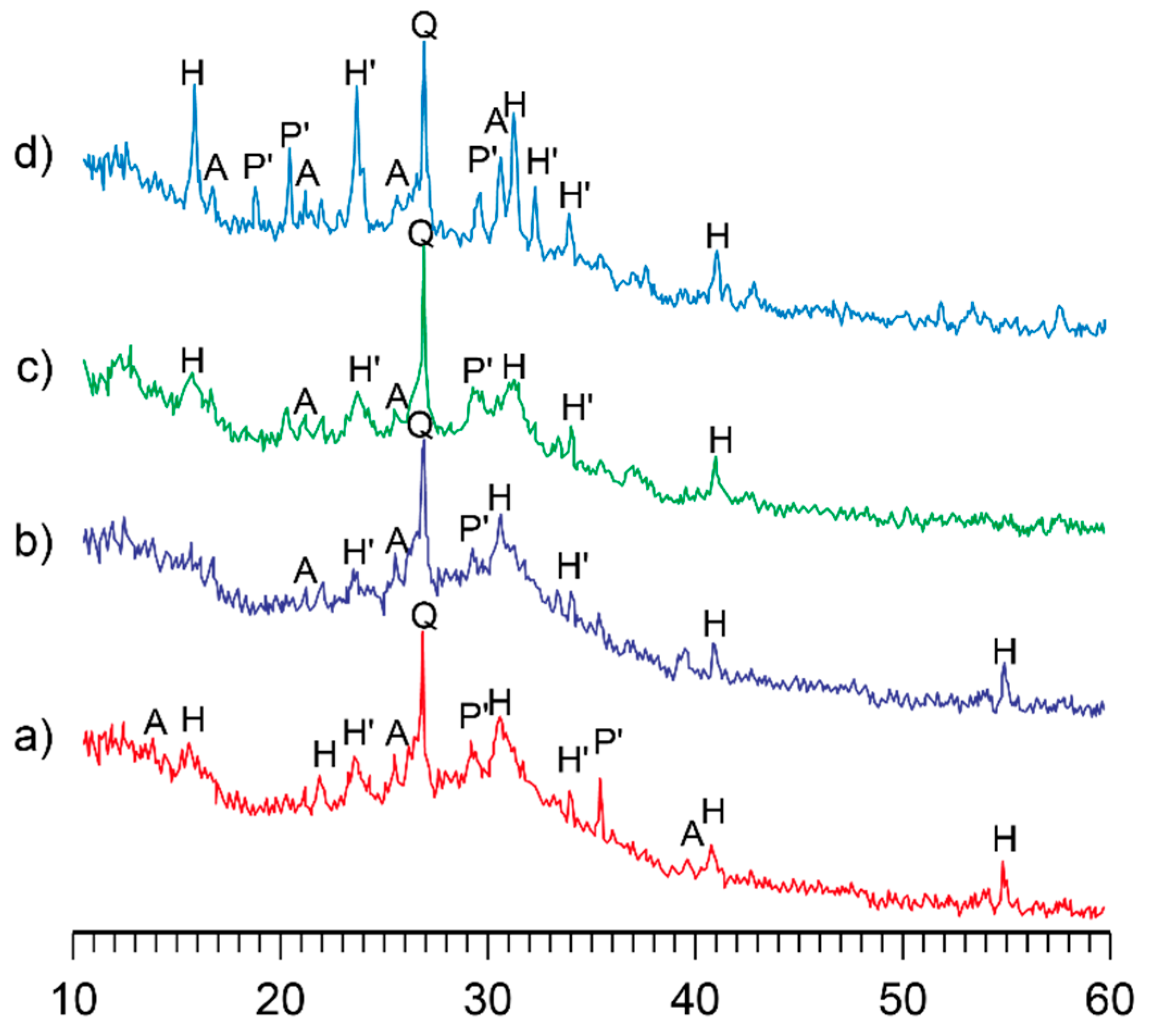
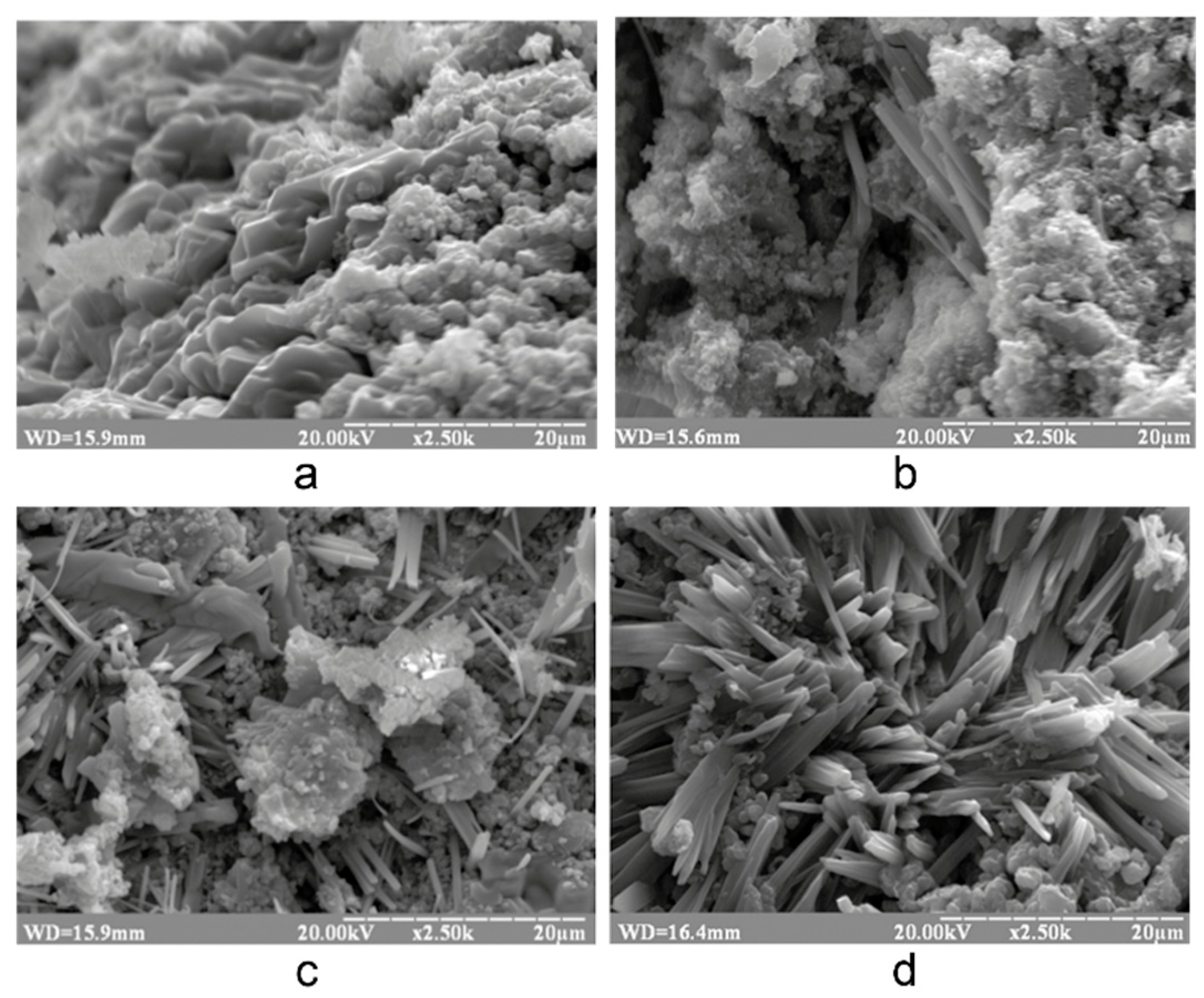
3.1. Research in influence of initial composition and type of curing on structure formation of the alkaline alumino-silicate binder
3.1.1. Influence of mix design
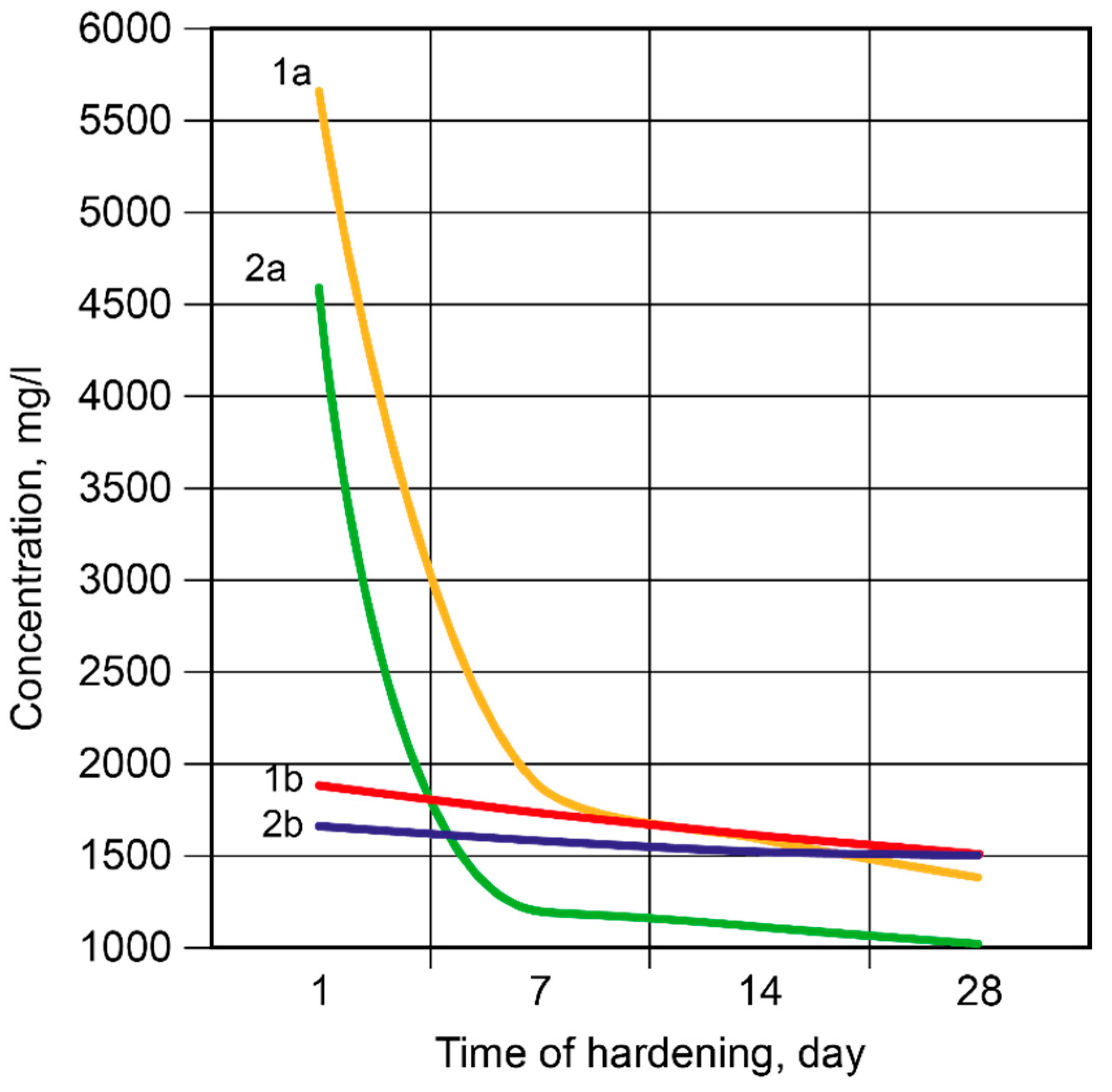
3.1.2. Influence of the curing conditions
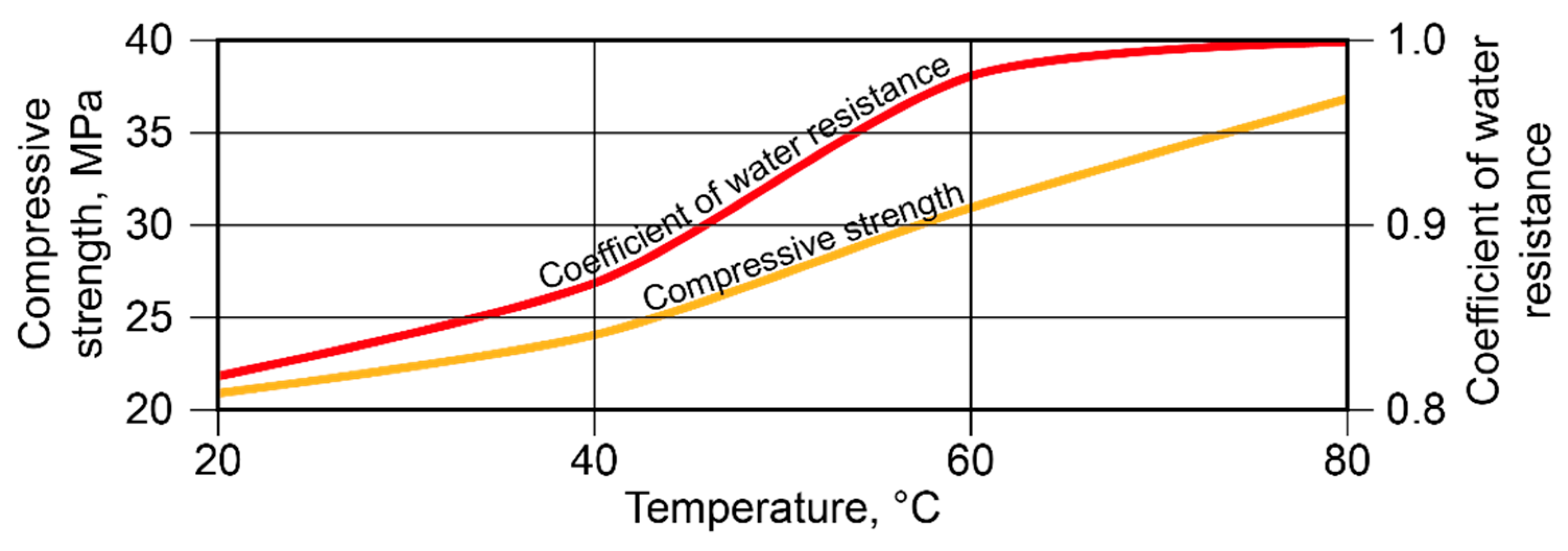
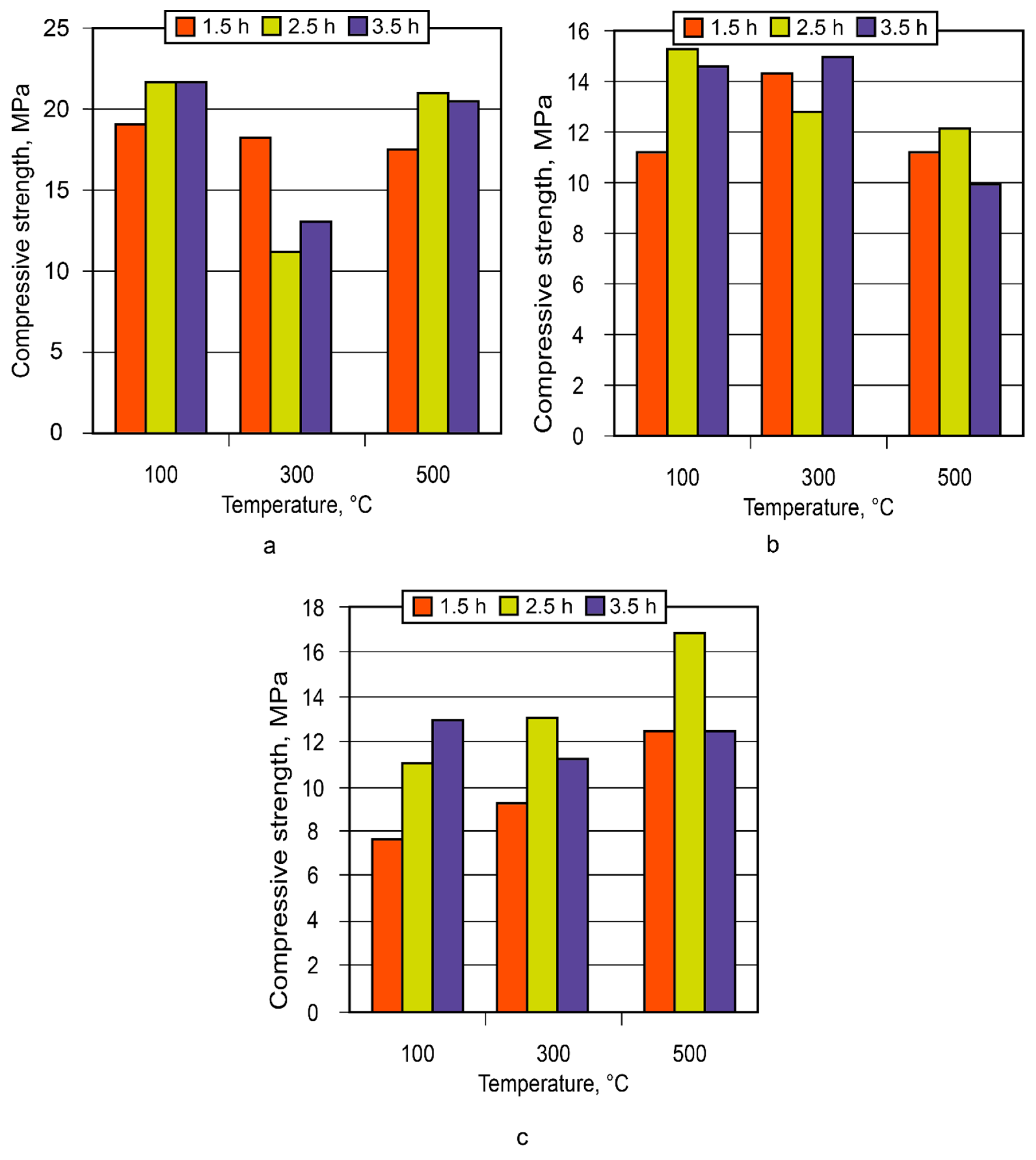
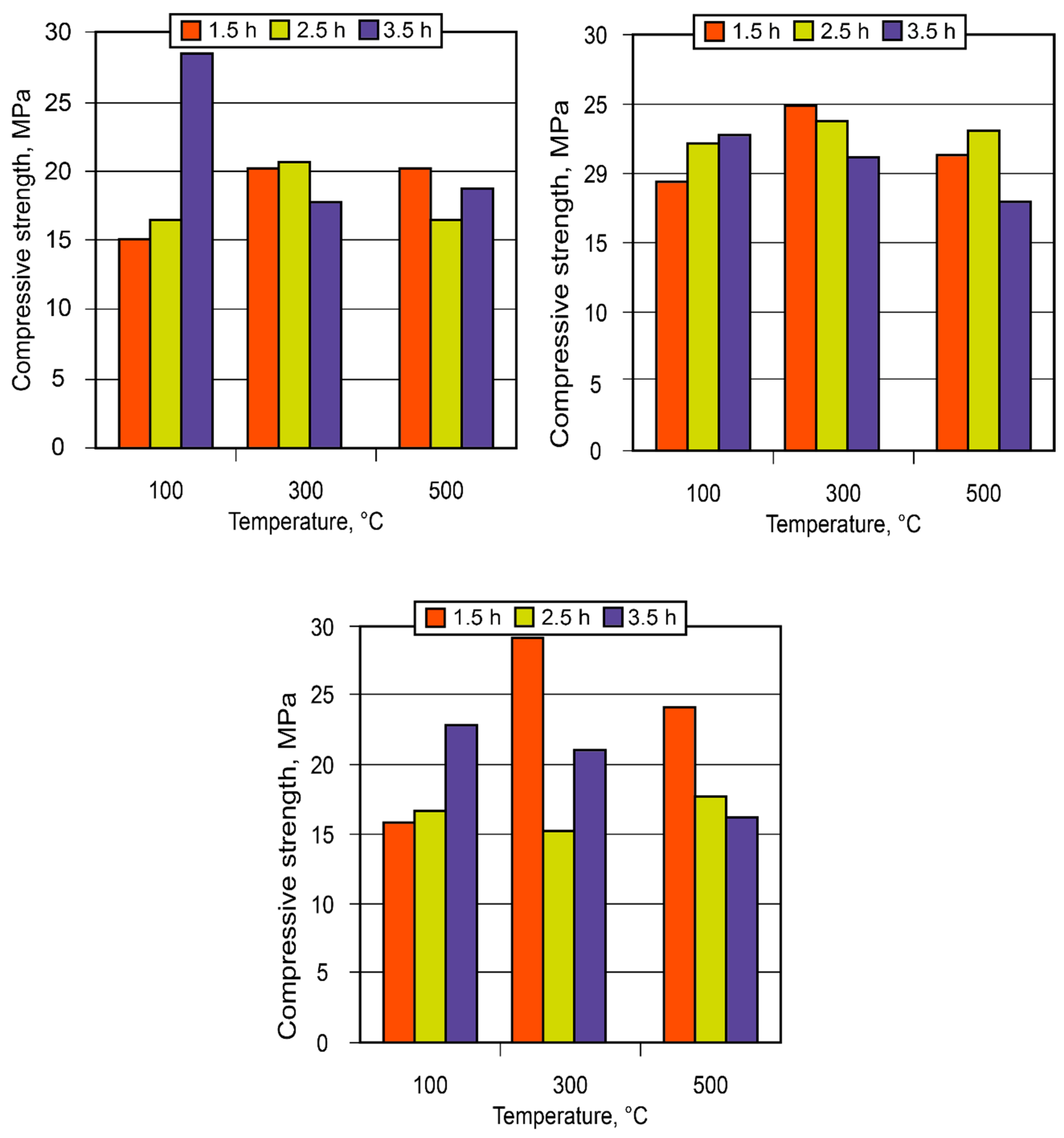
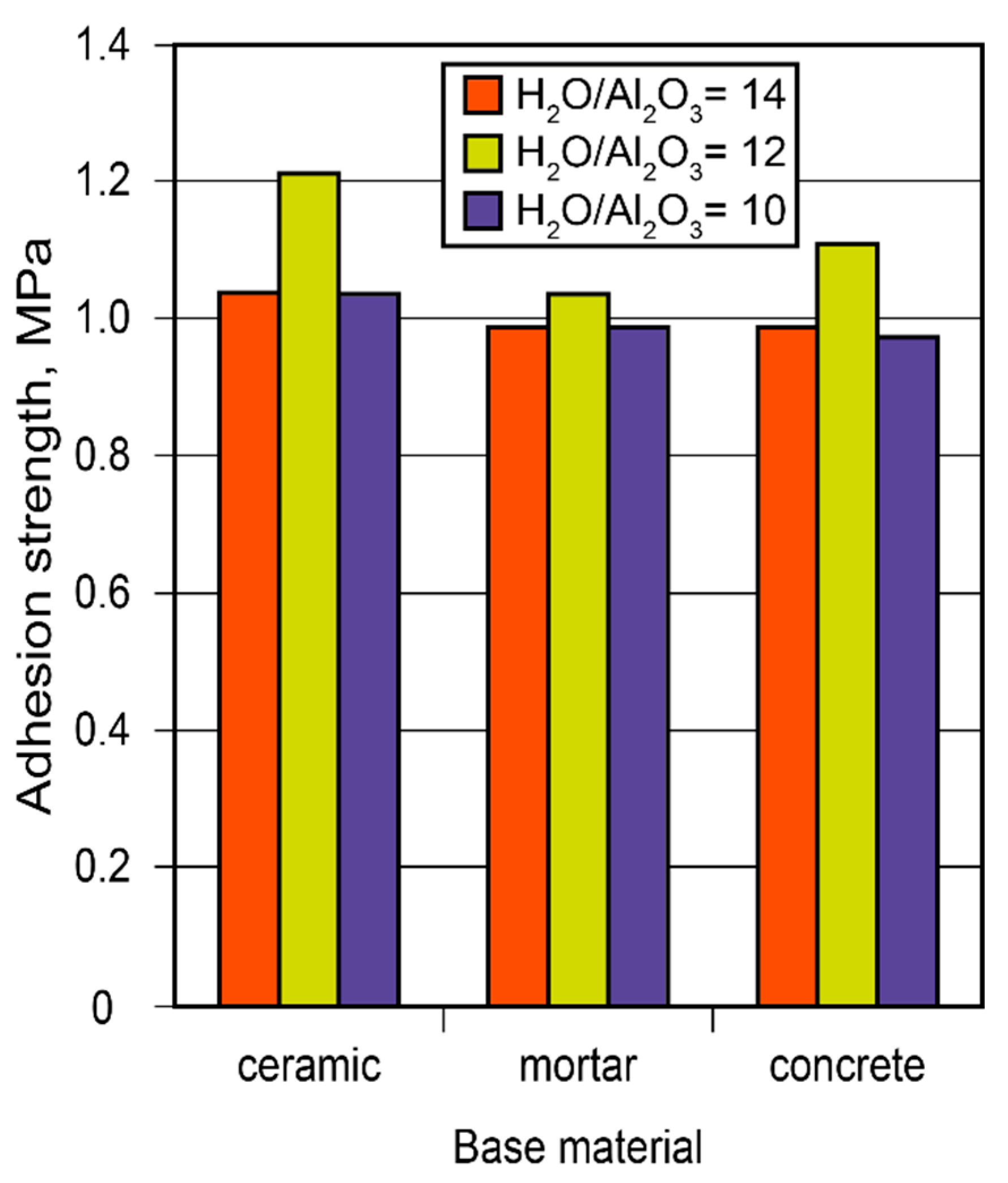
3.2. Manufacture and using of the alkaline alumino-silicate binder
4. Conclusions
- The structure formation of alkaline alumino-silicate binder, represented by the ratios (Na,K)2O/Al2O3= 1, SiO2/Al2O3= 2…7, H2O/Al2O3= 10…15, is due to reaction products, mainly composed of hydro-alumino-silicates like analcime, zeolite P, and garronite. The introduction of calcium to a reactionary mixture causes the formation of zeolite P and analcime as well as calcium hydro-silicates with structure of ksonotlite and girolite types. The additional quantity of SiO2 in the binder determines the dominance zeolite-like formations with increased contents of SiO2, i.e. minerals of Na-shabasite-gmelenite, fogasite and mordenite types.
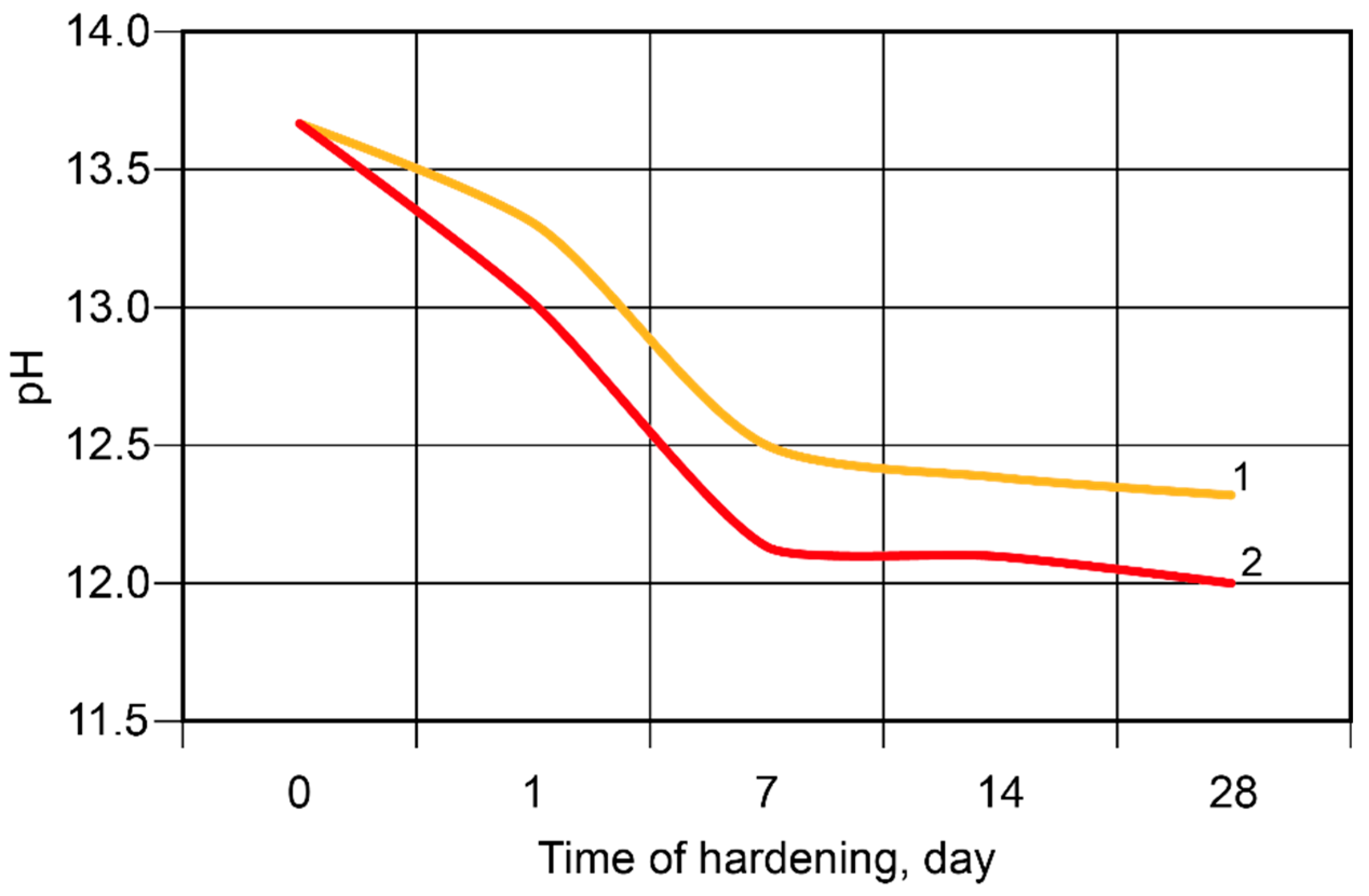
- It was revealed that in the binder, described by H2O/Al2O3 ratio lower than 10, the hardening process passes through the formation of hydro-alumino-silicates at the following stages: amorphous, submicrocrystalline, and crystalline. In the case of the binder with H2O/Al2O3 >10 the formation of submicrocrystalline structure is very poorly expressed. Thus, the nucleation of large crystals happens immediately in the amorphous phase, which results in significant retardation of hardening and crystallization processes, and also deterioration of the structure and properties of artificial stone.
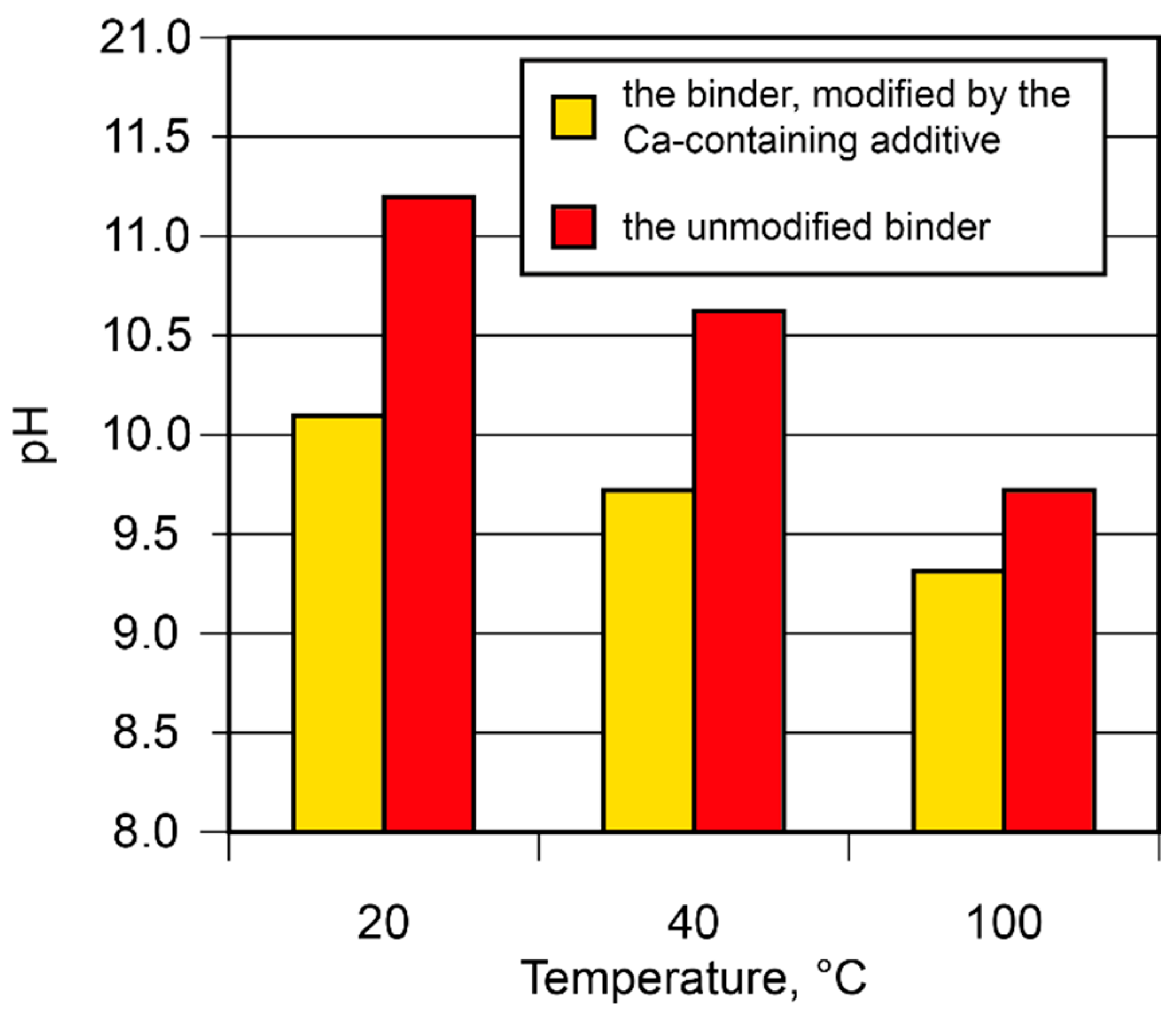
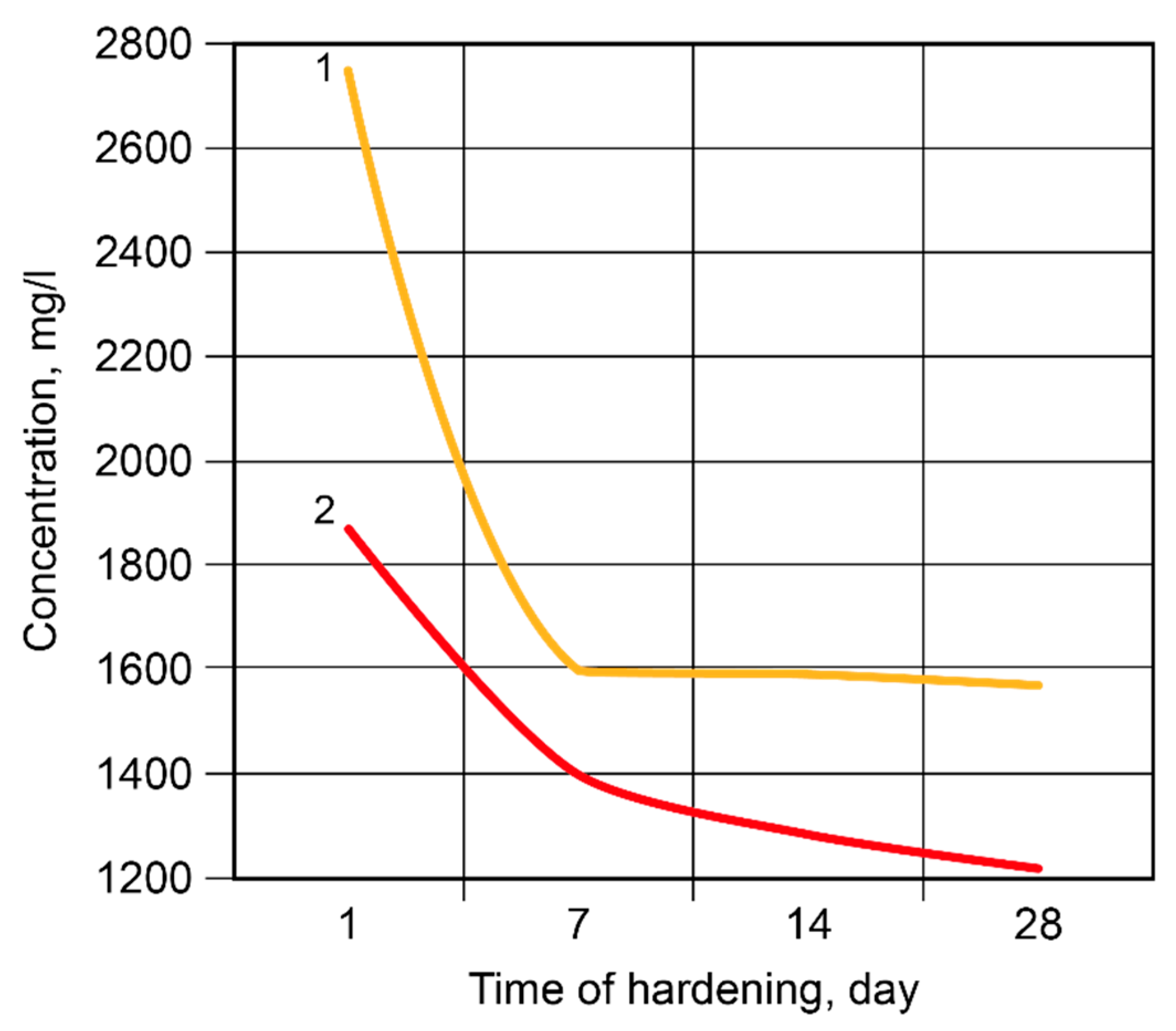
- The hardening of the binder within 28 days is accompanied by a linkage of 93…99 % Na+ and K+-ions. The introduction of the Са-containing additives allows to speed up the linkage of these ions due to the formation of zeolites like amicite, garronite, and gismondine.
- The experience gained for the small- and large-scale industrial use of the binder showed high technical advantages of the insulating composites based on it, especially to protect critical infrastructure facilities from the action of high temperatures and fire.
Acknowledgments
References
- Glukhovsky, V. D. Soilsilicates; Gosstroyizdat: Кyiv, 1959.
- Alkaline and Alkali-Alkaliearth Hydraulic Binders and Concretes; Glukhovsky, V. D., Ed.; High school: Кyiv, 1979.
- Davidovits, J. Geopolymeric Reaction in Archaeological Cements and in Modern Blended Cement; 1988; Vol. l.1, pp 93–105.
- Kryvenko, P.; Rudenko, I.; Kovalchuk, O.; Gelevera, O.; Konstantynovskyi, O. Influence of Dosage and Modulus on Soluble Sodium Silicate for Early Strength Development of Alkali-Activated Slag Cements. Minerals 2023, 13 (9), 1164. [CrossRef]
- Fernández-Jiménez, A.; Pastor, J. Y.; Martín, A.; Palomo, A. High-Temperature Resistance in Alkali-Activated Cement: High Temperature Resistance in Alkali-Activated Cement. Journal of the American Ceramic Society 2010, 93 (10), 3411–3417. [CrossRef]
- Krivenko, P.; Rudenko, I.; Konstantynovskyi, O.; Vaičiukynienė, D. Mitigation of Corrosion Initiated by Cl− and SO42−-Ions in Blast Furnace Cement Concrete Mixed with Sea Water. Materials 2022, 15 (9), 3003. [CrossRef]
- Krivenko, P.; Rudenko, I.; Konstantynovskyi, O. Effect of Technological Factors on Freeze-Thaw Resistance of Alkali-Activated Slag Cement Concrete in NaCl Solution; Kharkiv, Ukraine, 2023; p 040011. [CrossRef]
- Skurchinskaya, Zh. Progress in Alkaline Cements. In Alkaline Cements and Concretes; VIPOL Stock Company: Kyiv, 1994; Vol. 1, pp 271–298.
- Krivenko, P.; Kyrychok, V. Genesis of Structure and Properties of the Zeolite-Like Cement Matrices of the System Na(K)-Al 2 O 3 -SiO 2 -H 2 O within a Temperature Range of 20–1200°C. In Advances in Geopolymer-Zeolite Composites - Synthesis and Characterization; Vizureanu, P., Krivenko, P., Eds.; IntechOpen, 2021. [CrossRef]
- He, P.; Wang, M.; Fu, S.; Jia, D.; Yan, S.; Yuan, J.; Xu, J.; Wang, P.; Zhou, Y. Effects of Si/Al Ratio on the Structure and Properties of Metakaolin Based Geopolymer. Ceramics International 2016, 42 (13), 14416–14422. [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D. E. Compatibility Studies between N-A-S-H and C-A-S-H Gels. Study in the Ternary Diagram Na2O–CaO–Al2O3–SiO2–H2O. Cement and Concrete Research 2011, 41 (9), 923–931. [CrossRef]
- Longhi, M. A.; Rodríguez, E. D.; Walkley, B.; Zhang, Z.; Kirchheim, A. P. Metakaolin-Based Geopolymers: Relation between Formulation, Physicochemical Properties and Efflorescence Formation. Composites Part B: Engineering 2020, 182, 107671. [CrossRef]
- Shi, C.; Krivenko, P. V.; Roy, D. Alkali-Activated Cements and Concretes; Taylor & Francis: London, 2006.
- Wang, J.; Li, F.; Zhou, Z.; Du, P.; Xu, D.; Xie, N.; Cheng, X.; Liu, Y. Effect of Zeolite on Waste Based Alkali-Activated Inorganic Binder Efflorescence. Construction and Building Materials 2018, 158, 683–690. [CrossRef]
- Miranda, T.; Leitão, D.; Oliveira, J.; Corrêa-Silva, M.; Araújo, N.; Coelho, J.; Fernández-Jiménez, A.; Cristelo, N. Application of Alkali-Activated Industrial Wastes for the Stabilisation of a Full-Scale (Sub)Base Layer. Journal of Cleaner Production 2020, 242, 118427. [CrossRef]
- Kang, S.-P.; Kwon, S.-J. Effects of Red Mud and Alkali-Activated Slag Cement on Efflorescence in Cement Mortar. Construction and Building Materials 2017, 133, 459–467. [CrossRef]
- Karrech, A.; Dong, M.; Elchalakani, M.; Shahin, M. A. Sustainable Geopolymer Using Lithium Concentrate Residues. Construction and Building Materials 2019, 228, 116740. [CrossRef]
- Baenla, J.; Bike Mbah, J. B.; Djon Li Ndjock, I. B.; Elimbi, A. Partial Replacement of Low Reactive Volcanic Ash by Cassava Peel Ash in the Synthesis of Volcanic Ash Based Geopolymer. Construction and Building Materials 2019, 227, 116689. [CrossRef]
- Kryvenko, P.; Rudenko, I.; Konstantynovskyi, O. Design of Slag Cement, Activated by Na (K) Salts of Strong Acids, for Concrete Reinforced with Steel Fittings. EEJET 2020, 6 (6 (108)), 26–40. [CrossRef]
- P. Krivenko. Why Alkaline Activation – 60 Years of the Theory and Practice of Alkali-Activated Materials. J. Ceram. Sci. Technol. 2017, No. 03. [CrossRef]
- Alkali Activated Materials: State-of-the-Art Report, RILEM TC 224-AAM; Provis, J. L., Van Deventer, J. S. J., Eds.; RILEM State-of-the-Art Reports; Springer Netherlands: Dordrecht, 2014; Vol. 13. [CrossRef]
- Palomo, A.; Maltseva, O.; Garcia-Lodeiro, I.; Fernández-Jiménez, A. Portland Versus Alkaline Cement: Continuity or Clean Break: “A Key Decision for Global Sustainability.” Front. Chem. 2021, 9, 705475. [CrossRef]
- Mokhort, M. A. Formation of Structure and Properties of Alkaline Geocements. In Tagunsbericht 14 Internationale Baustofftagung “Ibausil”; Bauhaus Universitat Weimar: Weimar; Vol. 1, pp 553–560.
- Krivenko, P.; Mokhort, M.; Petropavlovskyi, O.; Vozniuk, G. Geocement Glues and Composite Materials: Practical Application. In 2007 - International Conference Alkali Activated Materials - Research, Production and Utilization; Praha, 2007; pp 397-412.
- Pavlasova, S.; Skvara, F. High-Temperature Properties of Geopolymer Materials. In 2007 - International Conference Alkali Activated Materials - Research, Production and Utilization; Praha, 2007; pp 523–524.
- Mokhort, M. A.; Popel, G. N. The Studies on Structure-Formation Processes and Microstructure of an Artifical Stone on Alkaline Aluminosilicate Bindersßgeocements. In Alkaline Cements and Concretes; Oranta Ltd: Kyiv, 1999; pp 291–302.
- Davidovits, J. Geopolymers of the First Generation: Siliface-Process, Geopolymer; U.T.C. Université Technologique Compiègne: France, 1988; Vol. 1, pp 49–67.
- J. Davidovits. European patent ЕР 0026687В1 (08.04.1981).
- Mokhort, M. A. Fienfluß von Ausgangyustand Und Erhärtungsbedingungen Bei Der Verfestingung von Geozementen; Bauhaus Universitat Weimar: Weimar, 2003; Vol. 1, pp 137–143.
- Mokhort, M.; Tsibulya, Y. Experience of Applikation of Geocements for Manufacturing of Inorganic Basalt and Organic-Mineral Jute Compisites. In 2007 - International Conference Alkali Activated Materials - Research, Production and Utilization; Praha, 2007; pp 483–492.
- Mokhort, M. A.; Tsibulya, Yu. L. Geocement Composites Based on Basalt Fabric and Alkaline Aluminosilicate Binder. In Cement Combination for Durable Concrete; Thomas Telford Publish: London, 2005; pp 287–292.
- Gontchar, V. P. The Studies on Thermo-Mechanical Characteristics of the Alkaline Aluminosilicate Binders Modified by a Silicon Carbide; Oranta Ltd. Publish: Kyiv, 1999; pp 303–312.
- Baranovskii, A. V. The Use of Alkaline Aluminosilicate Adhesive in the Production of Wood- Based Materials. The News of the Academy of Construction of Ukraine 1997, No. 3, 44–48.
- Baranovskii, A. V. Wood-Based Materials from Alkaline Aluminosilicate Adhesive. PhD degree, 1997.
- Mokhort, M. A.; Dhir, R. K.; Hewlett, P. C.; Csetenyi, L. J. Slag Alkaline and Geocement Concretes on Basis of Organic Filler With High Durability. In Innovations and Developments in Concrete Materials And Construction; Thomas Telford Publishing: London, 2002; pp 597–605.
- Voznyuk, G. V. Environmentally Friendly Composites Based on Geocements for Foundry; Oranta Ltd. Publish: Kyiv, 1999; pp 237–247.
- Torgal, F. P.; Labrincha, J.; Leonelli, C.; Palomo Sánchez, A.; Chindaprasirt, P. Handbook of Alkali-Activated Cements, Mortars and Concretes; Woodhead Publishing Series in Civil and Structural Engineering; Elsevier Woodhead Publishing: Cambridge, England, 2015.
- Krivenko, P.; Guzii, S.; Rudenko, I.; Konstantynovskyi, O. Intumescent Fireproof Coatings Based on Zeolite-Like Cement Matrices. ce papers 2023, 6 (5), 923–929. [CrossRef]
- Gorshkov, B.; Timashev, V.; Savel’ev, V. Methods of Physical-Chemical Analysis of Binders; High School: Moscow, 1984.
- Barrer, B. Hydrothermal Chemistry of Zeolites; World: Moscow, 1985.
- Krivenko, P.; Mokhort, M. Process of Phusico-Chemical Structure Formation in Modified Geocements. In 2007 - International Conference Alkali Activated Materials - Research, Production and Utilization; Praha, 2007; pp 379–396.
- Brek, D. Zeolite Molecular Sieves; World: Moscow, 1976.
- A Practical Guide to Microstructural Analysis of Cementitious Materials, First issued in paperback.; Scrivener, K., Snellings, R., Lothenbach, B., Eds.; CRC Press: Boca Raton London New York, 2017.
- Krivenko, P.; Rudenko, I.; Konstantynovskyi, O.; Vaičiukynienė, D. Feasibility of Incorporating SO42--Ions in Zeolite-like Matrices Based on Alkaline Aluminosilicate Binders. Construction and Building Materials 2023, 391, 131878. [CrossRef]





| Characteristic | Class by density, kg/m3 | |||
|---|---|---|---|---|
| 150 | 200 | 250 | 300 | |
| Alkaline alumino-silicate binder | ||||
| Binder content [% by volume] | 3.0 | 6.0 | 8.0 | 10.0 |
| Compressive strength [MPa] | 0.25 | 0.30 | 0.35 | 0.45 |
| Flexural strength [MPa] | 0.15 | 0.15 | 0.20 | 0.30 |
| Heat conductivity at 25 °С [W/(m⋅°С)] | 0.062 | 0.068 | 0.076 | 0.082 |
| Ordinary portland cement (OPC) | ||||
| Binder content [kg/m3] | − | − | 80 | 110 |
| Compressive strength [MPa] | − | − | 0.15 | 0.25 |
| Flexural strength [MPa] | − | − | 0.16 | 0.20 |
| Heat conductivity at 25 °С [W/(m⋅°С)] | − | − | 0.075 | 0.085 |
| Glued Materials |
Correlation of the total areas at a uniform tearing, % | ||
|---|---|---|---|
| Cohesive destruction (in glue), no more than |
Adhesive destruction (contact zone material-glue), no more than |
Destruction in fibrous layer, not less than | |
| Plate – cardboard | 5 | 5 | 90 |
| Cardboard – steel | 20 | 30 | 50 |
| Plate – steel | 20 | 30 | 50 |
| Plate – aluminium foil | 20 | 30 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





