1. Introduction
Autoimmune hepatitis (AIH) is a chronic relapsing and remitting, immune mediated, inflammatory disorder of the liver. AIH presents in young females more frequently, although it can manifest in any age group ranging from infancy to 8th decade. The presentation can be extremely heterogenous from being asymptomatic, to acute on chronic hepatitis, chronic hepatitis and rarely acute fulminant hepatitis. Around one third of patients present with cirrhosis due to undetectable subclinical disease. The diagnosis of AIH is essentially a multidisciplinary diagnosis involving clinical, biochemical, serologic and histologic criteria. AIH is associated with tissue-directed autoantibodies and hypergammaglobulinemia. Timely diagnosis and appropriate therapy can be life saving, as it usually responds well to immunosuppressive therapy. As there is no single pathognomonic or diagnostic feature, the criteria for diagnosis of AIH has seen many revisions over the last few decades. This article aims to describe the evolution in the diagnostic criteria of AIH with special emphasis on the histologic criteria.
1.1. Epidemiology
AIH has a global annual incidence of 1.37 per 100,000 and prevalence of 17.44 per 100,000 with a significant female preponderance (prevalence 12.77 for female and 2.91 for male) [
1]. The pooled prevalence rate in Asian (12.99/100,000) is lower than that in European (19.44/100,000) and American population (22.80/100,000) [
2].
Epidemiological data of AIH in India is scant. In 2015 Amarapurkar et al reported a prevalence of 1.3% amongst all liver disease patients and 8.74% in chronic liver disease [
3].
1.2. Diagnosis
Diagnosis of autoimmune hepatitis relies on clinical history, biochemical findings in the presence of raised IgG and positive serum autoantibodies and favorable histology. Documenting the absence of other chronic liver disease such as viral hepatitis, alcoholic or non-alcoholic steatohepatitis and Wilson’s disease is required. Occasionally, only close observation of the response to steroid therapy or a relapse of disease upon dose reduction or discontinuation of therapy will make the final diagnosis.
1.2.1. Hypergammaglobulinema
Selective elevation of IgG with normal levels of IgA and IgM is a characteristic feature of AIH and is seen in around 90% of chronic AIH patients. Few patients have IgG levels with upper range of normal (relative increase), which fall upon immunosuppressive therapy.
1.2.2. Serum autoantibodies
Presence of autoantibodies remains the hallmark of AIH diagnosis in all scoring systems. Based on serology, AIH can be classified into (1) type I AIH with ANA and/or ASMA and (2) type II AIH with anti-LKM-1 and/ or anti-liver cytosol type-1 antibodies [
4].
Table 1.
Common Autoantibodies and their association with Autoimmune liver disease.
Table 1.
Common Autoantibodies and their association with Autoimmune liver disease.
| Antibody |
Common association |
Other associations |
Method of detection |
| ANA |
AIH type1 |
AIC, PSC, PBC# |
IIF |
| ASMA |
AIH type1 |
AIC, PSC, PBC |
IIF, IB, ELISA |
| LKM-1 |
AIH type2 |
HCV |
IIF, IB, ELISA |
| AMA |
PBC |
Rare- AIH type 1 (low titres) |
IIF, IB, ELISA |
| SLA/LP |
AIH |
- |
IB, ELISA |
| PANCA |
PSC |
AIH, PSC |
IIF, ELISA |
| LKM3 |
AIH type2 |
- |
IIF, IB, ELISA |
| LC1 |
AIH type2 |
HCV |
IIF, IB, ELISA |
1.2.3. Absence of Viral Hepatitis
It is nearly impossible to make histologic distinction of viral hepatitis from AIH. Raised levels of IgG and serum autoantibodies are quite frequently observed in viral hepatitis. Thus, on their own they are insufficient for establishing the diagnosis of AIH. This is especially relevant in view of high prevalence (10-40%) of Hepatitis B and C in Southeast Asia and Africa.
1.3. Early descriptions
One of the earliest publications describe what we now know as AIH, as a persistent, relapsing jaundice with a preponderance for young females, less than 30 years of age, and raised serum globulins [
5]. It was recognised as an “anomaly of immune tolerance”, with viral hepatitis as a trigger. The resemblance to lupoid hepatitis and progression to cirrhosis was described [
6].
Histologically numerous lymphoid cells, dense fibrosis and “remarkable degree of plasma cell infiltration of the liver” was described [
7]. The immune nature of the disease was noted by several subsequent publications and the term lupoid hepatitis and autoimmune hepatitis were used to describe this condition [
8,
9].
1.4. Histology of Autoimmune Hepatitis
Histologic evidence of hepatitis remains the cornerstone of AIH diagnosis along with clinical and serologic parameters. Recent guidelines recommend liver biopsy at presentation to establish the diagnosis of AIH, to exclude other differentials, highlight any concurrent comorbidities, to grade the necroinflammatory activity to take treatment decisions and to evaluate the extent of fibrosis (staging). It is noteworthy that liver histology may be of prognostic significance in terms of progression to cirrhosis or malignancy. Autoimmune hepatitis is histologically a form of chronic hepatitis, thus having portal inflammation, interface activity and lobular inflammation. However, a few features have been described as more commonly seen in AIH, which have been used in the development of the scoring systems (described later in this article).
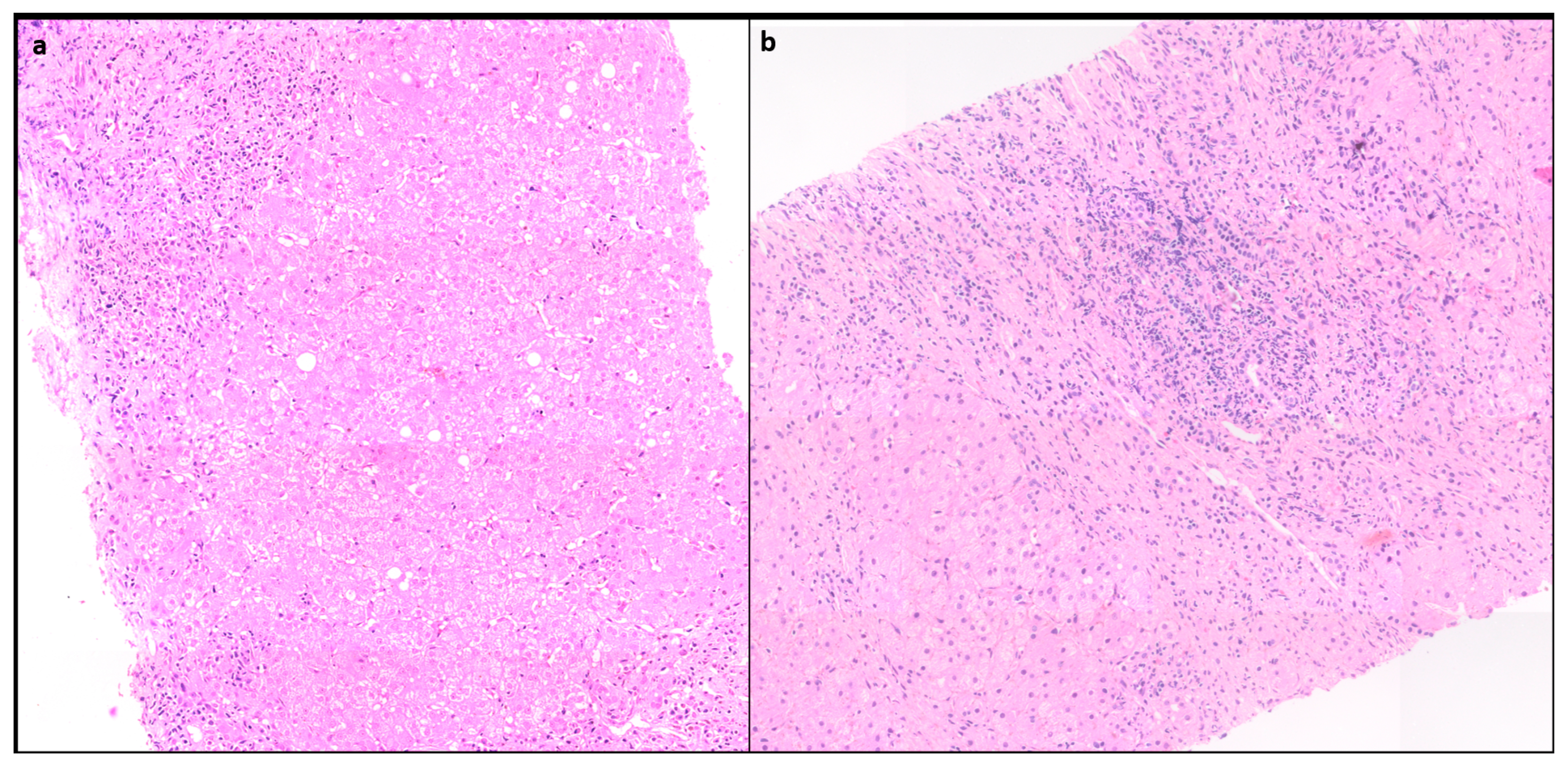
1.4.1. Portal inflammation
Portal inflammation (PI) is usually mononuclear consisting of lymphocytes, plasma cells and few histiocytes. Few neutrophils and eosinophils can be seen. PI can range from mild to severe but is often moderate to severe grade [
10]. (
Figure 1)
1.4.2. Interface Hepatitis
Interface hepatitis with hepatocyte necrosis of the periportal parenchyma is a classic histological finding seen in more than 85% cases and can range from mild to severe [
11]. (
Figure 1) In AIH with severe activity, the PI and interface activity can be associated with bridging necrosis. It is often more severe in AIH than in chronic hepatitis of other etiology.
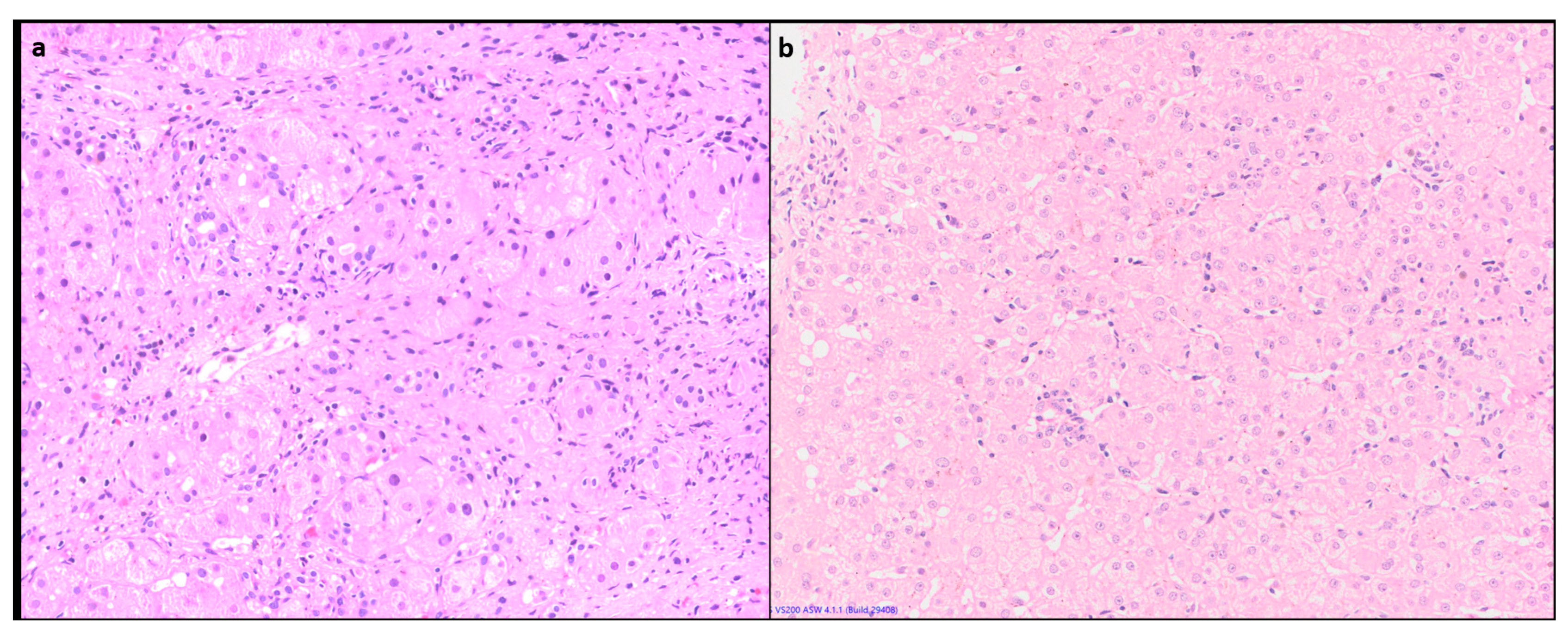
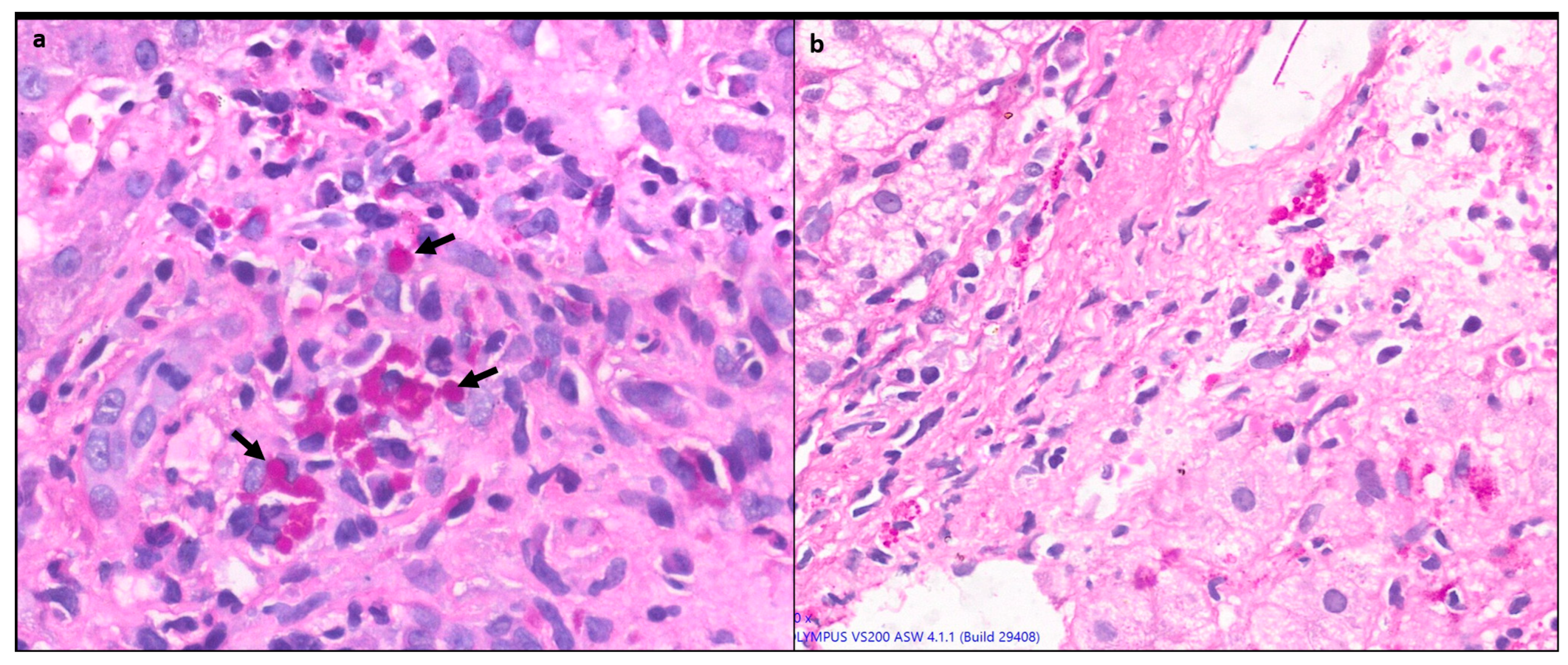
1.4.3. Lobular inflammation (LI) or hepatocyte necrosis
Lobular inflammation of variable severity is usually present ranging from scattered necroinflammatory foci (spotty necrosis) to confluent and/or bridging necrosis. (
Figure 2) This may be associated with lobular disarray, ballooning degeneration and hepatocyte regeneration. Large areas of confluent necrosis can be seen especially in acute fulminant presentation or during disease flares [
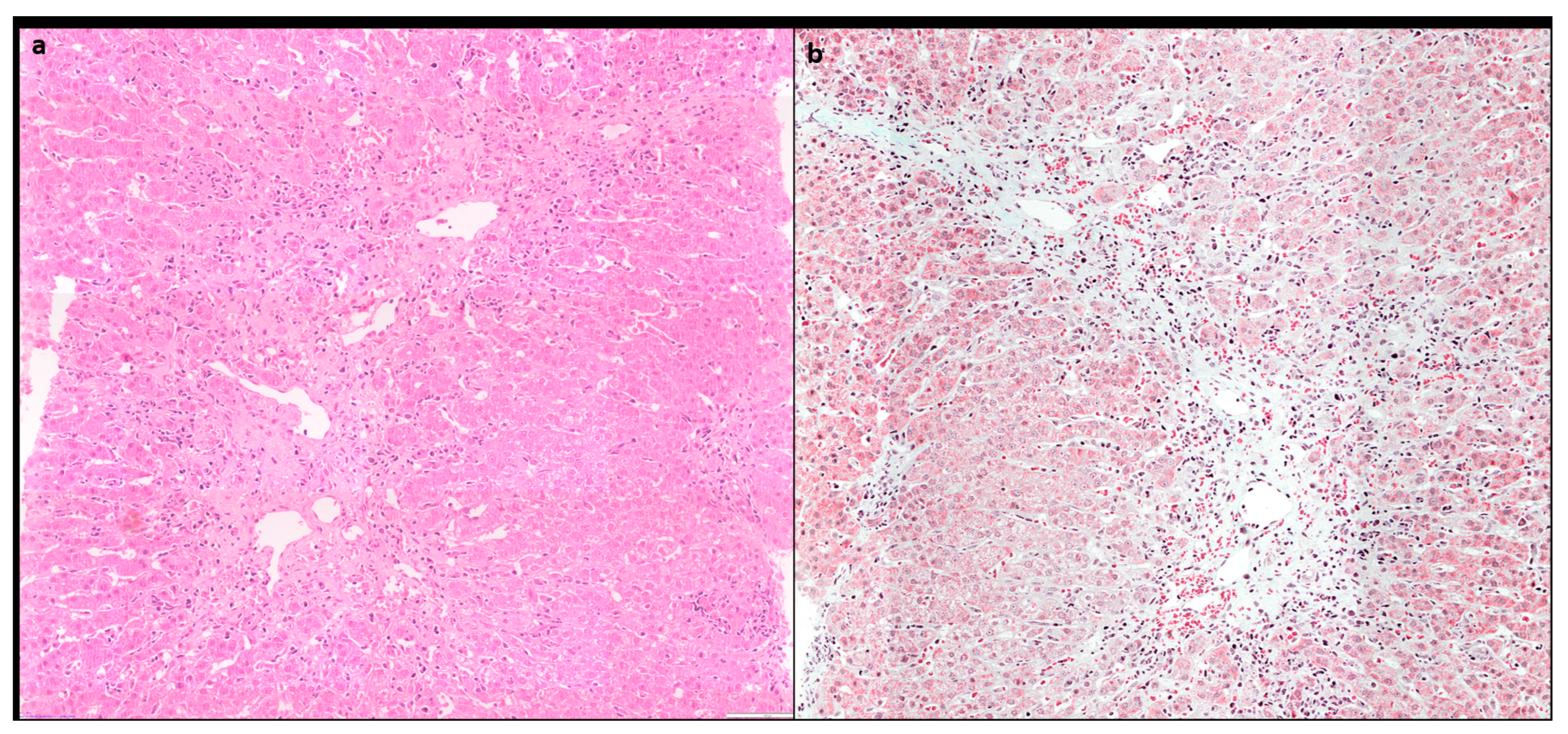
10]. Centrilobular inflammation and necrosis may be prominent in about one third of cases [
12]. (
Figure 3) Rarely it may be the predominant form of injury in acute AIH.
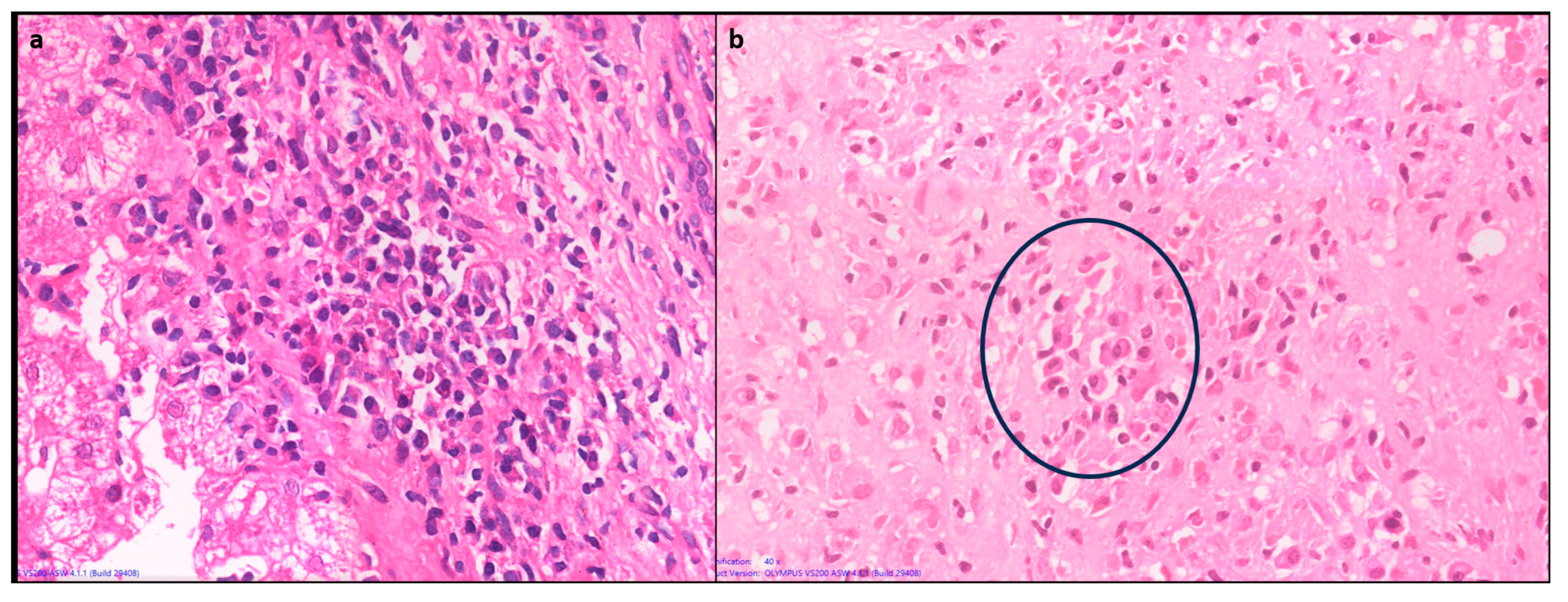
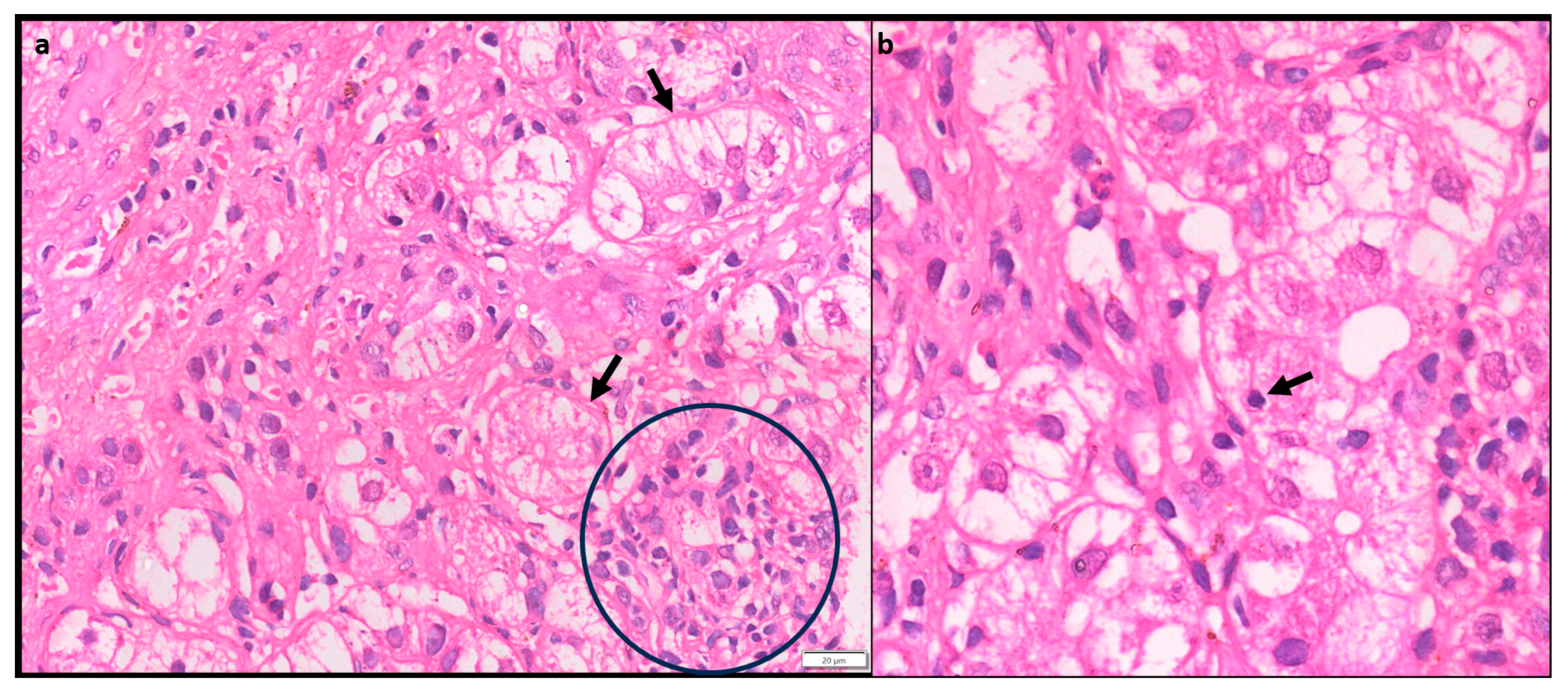
1.4.4. Plasma cell predominance
Plasma cells are prominent in about two thirds of cases of AIH. Plasma cell clusters defined as a collection of 5 or more plasma cells have been noted in AIH [
10,
13]. (
Figure 4)
1.4.5. Hepatocyte rosettes
Rosettes are a regenerative feature where the hepatocytes are arranged around a small, sometimes not visible lumen. (
Figure 5) It can be seen following hepatocellular injury of any cause and may indicate severe injury. Thus, rosettes and emperipolesis may be seen more frequently in AIH (49%) as compared to chronic viral hepatitis [
13].
1.4.6. Emperipolesis
Emperipolesis is a lymphocyte or rarely a plasma cell within the cytoplasm of a hepatocyte. (
Figure 5) This finding has been reported in 65-78% of AIH biopsies [
13]. The lymphocytes are usually CD8+ T lymphocytes [
14]. Emperipolesis may induce hepatocyte apoptosis and thus may be associated with higher serum transaminase levels, and more severe disease activity. Emperipolesis has also been described in Drug induced liver injury, Primary Biliary Cirrhosis and chronic viral hepatitis, therefore it is not pathognomonic for AIH [
10,
13].
1.4.7. Giant cell hepatitis
Around 40% cases of adult post infantile giant cell hepatitis may be associated with autoimmune hepatitis, where there is prominent giant cell transformation of hepatocytes [
15]. It is a progressive disease with survival rate of 50% without transplantation [
16].
1.4.8. Cirrhosis
Around one-third of AIH patients have cirrhosis at the time of presentation, which is usually of the macronodular type [
17]. AIH-related cirrhosis does not have any pathognomonic histological features. Hepatocellular carcinoma is rarely documented in the context of cirrhosis only with an incidence of 0.3-1% per year only [
18].
1.5. Development of scoring systems
The diagnostic scores help the clinicians in making the primary clinical diagnosis, by weighing the different laboratory, serological and histological results. In addition, diagnostic scores are important in stratifying patients by defined unified criteria and help in making scientific studies comparable.
1.5.1. Original Scoring system by the International Autoimmune Hepatitis Group (IAIHG), 1993
This led to development of a diagnostic scoring system of the International Autoimmune Hepatitis Group (IAIHG), created by an international panel in 1993 [
19]. In addition to liver histology (Box1), they defined minimum required parameters related to serum biochemistry, serum immunoglobulins, serum autoantibodies, viral markers and absence of other etiological factors in order to arrive at a “definite” or “probable” diagnosis of AIH. A Scoring system for diagnosis of autoimmune hepatitis was defined, with positive and negative weighted scores assigned to various parameters. Features of liver histology were assigned specific scores (
Table 2), as additional features and not minimum required parameters. The system was designed to be applied to treatment naïve and post treatment liver biopsies.
Liver histology was described as a chronic active hepatitis. In addition they acknowledged that intense activity, presence of numerous plasma cells and liver cell rosettes although suggestive, were not pathognomonic of AIH [
19].
1.5.2. Revised IAIHG modified scoring system,1999
Over time, it became evident that using 1993 criteria, patients with other autoimmune biliary conditions such as primary sclerosing cholangitis (PSC) and primary biliary cirrhosis (PBC) could be ascribed a false positive diagnosis of AIH. In 1999 the 40 member IAIHG revised the criteria in order to obtain greater sensitivity for the diagnosis of AIH and to be able to exclude non-AIH disorders especially biliary disease such as PBC and PSC [
20,
21]. The principal changes in the assigned scores were in the ALP:AST (or ALT) ratio, drug history, liver histology and response to therapy. It was also emphasized that the system was intended mainly for research purposes but may be useful in the diagnosis of difficult cases.
Both the 1993 and 1999 revised criteria clearly imply: 1. Liver histology is an important component in the diagnosis of AIH [
22] 2. While features typical of AIH such as female gender, raised AST, ALT and presence of autoantibodies help in making a diagnosis, this scoring system helps rule out other differentials by assigning negative scores to features such as positive viral serology, use of drugs or alcohol etc. It is important to remember that autoantibodies can be positive in several other chronic liver diseases, including Hepatitis C and NASH [
23]. One of the challenges that remains with these scoring systems, is the diagnosis of AIH when an associated comorbidity is present (such as NASH) or in the diagnosis of AIH overlap syndrome [
24]. In such cases liver histology plays an important role. The IAIHG reiterated the importance of a liver biopsy, preferably seen by a hepatopathologist, to arrive at a definite diagnosis of AIH [
10].
However, these criteria were complex, included a variety of parameters of questionable value and their practical utility for daily clinical use remains challenging, including 13 categories, some of them impractical in children.
1.5.3. Simplified scoring system (SSS) for AIH, 2000
To overcome these difficulties of revised scoring system (1999), IAIHG decided to devise a simplified scoring system for wider applicability in routine clinical practice based on the data of patients with well-established diagnoses. They studied various clinical, biochemical and serologic data, based on the opinion of the experts in the IAIHG. A training set consisting of the data from 250 patients with proven AIH and 193 controls was included [
25]. The validation set included 109 patients with AIH and 284 controls. The controls included PBC, PSC, NASH, viral hepatitis, Wilson’s disease, hemochromatosis and drug/toxin induced liver injury. From this data they developed a simple scoring system which included the autoantibodies (ANA, ASMA, LKM and/or SLA), the total IgG level, absence of viral hepatitis and liver histology. (
Table 3) While in the 1999 revised criteria for autoimmune hepatitis, cholestatic features on histology excluded the diagnosis of autoimmune hepatitis, however the SSS deliberately included these patients, to ensure that these patients get immunosuppressive treatment if the clinical suspicion of AIH is high. The simplified diagnostic criteria scoring system reported a sensitivity and specificity of 88% and 97% respectively for a score of 6 points, defined as ‘‘probable AIH’’; and 81% and 99% respectively, for a score of 7 or more points, defined as ‘‘definite AIH’’[
25]. Subsequent studies revealed a sensitivity for score of >6 from 65% to 95%, and for a score of 7 or more as low as 15 up to 87%; with a positive predictive value of 83-100% and a negative predictive value of 74 to 97% [
26].
Histology was included as essential for the diagnosis of AIH, even though it may not show lesions typical for AIH. Demonstration of hepatitis on histology is considered a prerequisite. Professors Dienes and Lohse defined the liver histology under three categories: ‘typical’, ‘compatible’ and ‘atypical’. (
Table 4) The importance of liver histology lay not just in establishing the diagnosis and the severity of AIH, but also to exclude other disease entities like biliary pathology and identify comorbidities like NASH which is being increasingly recognized nowadays. Main advantage of SSS was its simplicity for use in everyday practice.
Soon after these criteria were published, Yeoman et al evaluated the diagnostic accuracy, utility and comparative evaluation of the simplified score (SSS) in 549 patients with chronic liver disease of which 221 were AIH and 26 were variant syndromes. They found a concordance rate of 90% with 1999 criteria for probable and 61% for definite AIH. The simplified criteria had high specificity (98% for score >/= 6 and 100% for scores >/=7), but exhibit lower sensitivity for scores of >/=7 (70%). The authors felt that the low sensitivity of SSS may be due to omittance of discriminating information as response to corticosteroids. Further, they found a lower frequency (24%) of an overall diagnosis of AIH (probable or definite AIH) among the 70 patients with fulminant liver failure for SSS and 40% for 1999 criteria [
26]. In addition, as the diagnosis of ‘probable’ AIH is based on clinical and laboratory evaluations, and not on treatment response, these patients should not be denied treatment or inclusion in clinical studies if the clinician’s index of suspicion is strong [
27].
De Boer et al. found emperipolesis and rosette formation to be superior histological predictors of AIH than interface hepatitis and plasma cells. Also, they found moderate to severe lymphocytic cholangitis in 28% of AIH patients [
13]. However, others have argued that cases of autoimmune hepatitis, in the absence of rosettes and emperipolesis, would only get a histologic score of 1 point which might result in lower sensitivity [
28]. In addition, these features showed marked interobserver variability and inconsistency in histologic interpretation.
The drawbacks of the simplified criteria, that were gradually recognized, include lack of sensitivity for diagnosis of acute AIH, or AIH with comorbidities [
10,
29,
30]
In a recent study in patients with acute onset AIH, the diagnostic performance of the two AIH scoring systems was assessed. The researchers found that revised version of the original criteria (1999) achieved the diagnosis of AIH in about 30% of patients as compared to the simplified score [
31]. This was predominantly due to normal serum IgG levels and lower frequency of ANA and SMA positivity seen in these patients. Further Joshita et al also reported that SSS is unreliable in diagnosis of acute AIH. More than half AIH patients with acute presentation had a normal IgG level, and the prevalence of seropositivity for ANA and SMA has been reported to be 73% and 28% respectively [
32]. Though more investigative and time consuming, revised version of the original criteria (1999) has higher sensitivity in diagnosing atypical AIH patients.
Furthermore, emperipolesis and resetting are features that indicate hepatic injury and regeneration in the background of hepatitis, therefore, are not specific to AIH. When comparing AIH with grade matched HCV as controls, Gurung et al did not find any significance of these two histologic features in diagnosis of AIH [
33].
1.5.4. Histologic scoring of autoimmune hepatitis by Balitzer et al [28]
Addressing the drawbacks of the SSS, Balitzer et al compared the histologic criteria of the SSS to a revised set of histologic criteria in 88 cases of autoimmune hepatitis, 20 cases of primary biliary cholangitis, and 13 cases of non-autoimmune acute hepatitis. They used histologic score as given in
Table 5. Their study showed an increase in number of cases having a score of >6 using their proposed histologic criteria from 69% to 86% in cases of AIH. The histologic criteria proposed highlight the degree of hepatitic activity (interface and/or lobular) and prominence of plasma cells. Furthermore, a histologic score of 1 required absence of cholestatic features on copper (positive copper or copper associated protein) and CK7 stains, (CK7 positive periportal hepatocytes) thus differentiating from chronic biliary disease [
28].
Using the histologic criteria of SSS, the diagnosis of acute hepatitis has poor sensitivity. Using the revised criteria, the authors demonstrate an improved sensitivity for probable/definite auto- immune hepatitis from 62 to 90% [
28]. This would facilitate timely immunosuppressive therapy which could be lifesaving or prevent rapid progression of fibrosis.
1.5.5. Modified Scoring Criteria proposed by Gurung et al.[33]
Gurung et al conducted a critical appraisal on the histologic features of AIH and found plasma-lymphocytic inflammation, defined as plasma cell predominance and plasma cell clusters (>/=5 plasma cells) were the most reliable distinguishing features in AIH. Another histologic feature evaluated is Kupffer cell hyaline globules (KcHG) [
33,
34]. KcHG defined as round, glassy, well defined/sharply circumscribed intracytoplasmic structures, best seen on Periodic acid Schiff with diastase (PASD) stain, are to be differentiated from ill-defined PAS-D granules in Kupffer cells [
34]. (
Figure 6) Using these criteria (
Table 6) they found a high positive predictive value (70-88%) [
34].
1.5.6. Consensus recommendations for histological criteria of AIH from IAIHG [35]
The proposed histologic criteria for making a diagnosis of AIH over the years were largely based on old studies, and had neither been prospectively validated, nor agreed upon by international consensus. These criteria were focused predominantly on portal based inflammation seen in chronic AIH and cases of acute AIH with lobular based inflammation were often misclassified as drug induced or toxic acute liver injury [
35].
Considering these concerns with the histologic criteria of the SSS and the subsequent studies and recommendations, the European Reference Network on Hepatological Diseases and the European Society of Pathology hosted a workshop on AIH histology in Brussels, Belgium in January 2020 [
25].
The participants were 17 liver pathologists and two hepatologists with expertise in AIH. They proposed in this consensus statement criteria for the histological diagnosis of AIH in the native liver, which would apply to patients with an acute as well as a chronic presentation [
35]. (
Table 7)
Guidelines in this recommendation include the standards of a liver biopsy, which should be done with a
18/16G needle, of 1.5 cm length, with at least 6-8, preferably 10 portal tracts. A connective tissue stain is a must to identify the extent and distribution of fibrosis and helps differentiate from areas of necrosis/collapse. Trichrome, orcein and Sirius red are stains that can be used. They conducted a Delphi round and agreed upon the following statements [
35]:
AIH has no pathognomonic histological features
It is desirable to have the knowledge of the duration of the liver disease is before assessing a liver biopsy for AIH
Emperipolesis and Rosettes should be discarded as diagnostic features for AIH owing to limited specificity
Evaluating a biopsy for AIH involves assessment of the dominant pattern of inflammation, that is, portal (chronic ) hepatitis or lobular (acute ) hepatitis,
The histological spectrum of AIH especially acute presentation now recognizes and includes centrilobular injury with prominent hepatocellular necrosis
Lymphoplasmacytic inflammatory infiltrate refers to predominance of Plasma cells (including plasma cell clusters), in addition to lymphocytes
A plasma cell cluster is defined as more than 5 plasma cells in one focus
A comment on the presence and severity of fibrosis should be included in the pathology report
The likelihood of AIH should be defined as unlikely, possible or likely.
They recommend semi-quantitative assessment of the severity of inflammatory activity of AIH based on the Ishak's modified Histological Activity Index (mHAI) [
36]. Grading of necro-inflammatory activity and staging of fibrosis in AIH can be performed using the systems developed for chronic viral hepatitis. Simple 4-tier grading systems like the Batts and Ludwig [
37], Scheuer [
38] and Metavir [
39] can be used too but they preferred Ishak's mHAI as it allows more detailed assessment of centrilobular necroinflammatory changes and is more appropriate for grading acute lobular damage [
35]. The main highlight of this consensus scoring was inclusion of acute AIH cases as centrilobular injury with hepatocyte necrosis is recognized as a part of the histologic spectrum of AIH, which till now, was conventionally associated with DILI [
35].
The SSS required the exclusion of viral hepatitis, however the consensus recommendation additionally suggests exclusion of viral hepatitis by PCR in the setting of acute hepatitis, and drug history of the last 6 months. History of previous transaminitis supports the chronic relapsing course of AIH [
35].
1.6. Autoantibody negative AIH
‘Autoantibody-negative’ AIH occurs in 10–20% of AIH cases and is defined by the absence of ANA, ASMA, anti-LKM and AMA antibodies at the time of presentation. It clinically. resembles type 1 AIH and has good response rate to steroids [
40]. Liver histology is mandatory for diagnosis in such cases.
1.7. Anti Mitochondrial Antibody-Positive AIH
O’Brien et al. studied 15 patients of AIH who had classical features of AIH on biopsy but were found to be anti-mitochondrial antibody-M2 (AMA) positive. None of these patients had histological evidence of PBC, nor any of them developed PBC on follow-up [
41]. It is controversial whether these patients represent a subtype of AIH or whether treatment with corticosteroids can prevent the subsequent development of PBC or an ‘overlap’ syndrome.
1.8. Overlap syndrome
The majority of cases of so called ‘overlap syndromes’ involve AIH with PBC or PSC. Histologically focal lymphocytic cholangitis and bile duct injury can be seen in 12-20% of AIH cases, therefore their presence does not preclude the diagnosis of AIH [
10,
13]. As per the recent consensus statement, if features of PBC, PSC or NAFLD are present along with typical features of AIH, a liver biopsy can still be classified as possible AIH. However, if a biopsy from a clinically suspected patient of AIH has unusually prominent biliary features such as bile duct loss or features of chronic cholestasis (eg periportal deposits of copper, copper-associated protein or periportal keratin 7-positive cells with an intermediate hepatobiliary phenotype), possibility of PBC or PSC with AIH like features may be considered [
35].
1.9. NAFLD (Nonalcoholic fatty liver disease)
Positive autoantibody serology, usually comprising of low titres of ANA, ASMA and/or AMA, has been documented in 20–48% of NAFLD patients [
42,
43]. Further hypergammaglobulinemia may be seen in some elderly female patients with nonalcoholic steatohepatitis (NASH)-related cirrhosis and the viral markers are negative. Therefore, patients with NASH may be misdiagnosed as probable AIH using the simplified scoring system, underscoring the importance of liver biopsy in such situations [
25,
43]
1.10. Concurrence of AIH with other liver diseases
Concurrence of AIH with viral hepatitis is a very challenging situation for the clinicians as well as the pathologists, since presence of chronic viral hepatitis is an exclusion criteria for AIH in diagnostic scoring systems. This problem is not uncommon in countries where there is a high prevalence of hepatitis virus B (HBV) or hepatitis virus C (HCV) infection. In addition, increased serum IgG and autoantibodies (ASMA up to 66%, ANA up to 41% in chronic hepatitis C) and overlapping histological features of chronic active hepatitis with periportal piecemeal necrosis and lymphoid follicles in chronic HCV can lead to misdiagnosis [
10,
43,
44]. Muratori et al rightly pointed out the issue of LKM1-positivity seen in 38 patients amongst 143 hepatitis C patients. Whether LKM1 antibody titres should only be used in SSS in the absence of HCV infection, remains a topic of debate and further study [
45].
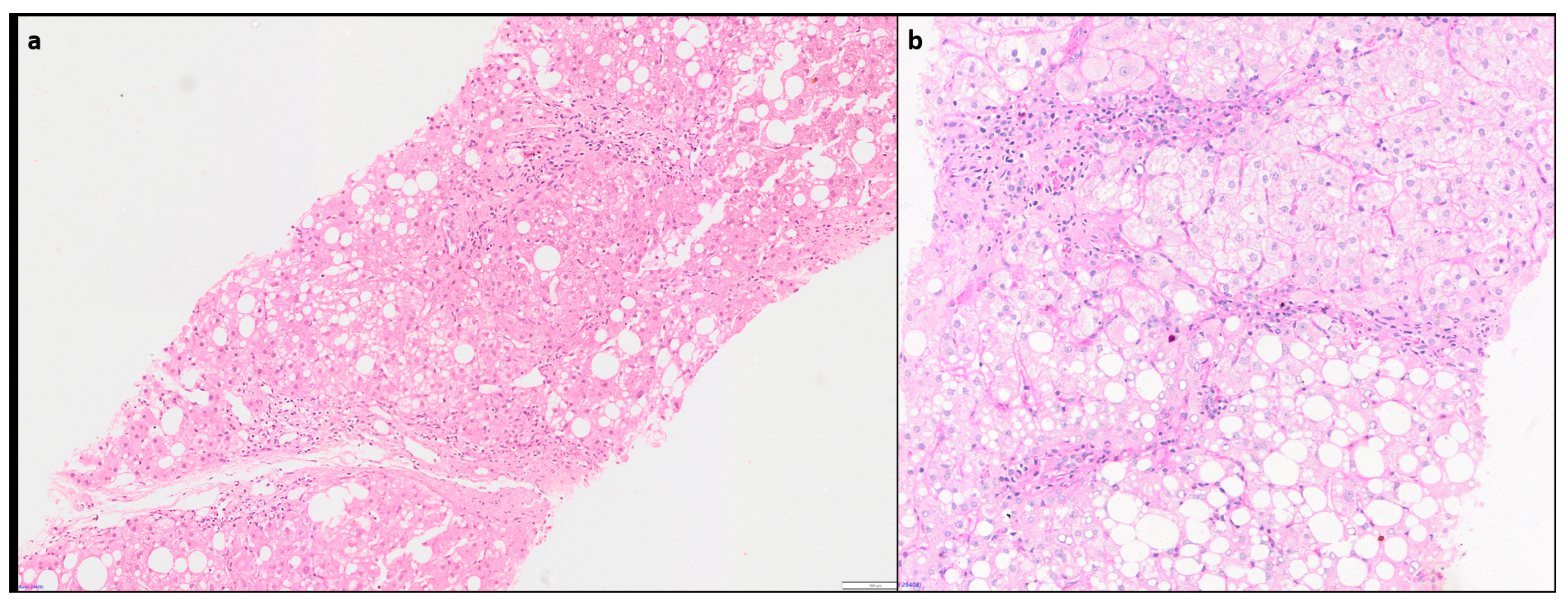
Hepatic comorbidities such as NAFLD and NASH are increasingly seen in 15-30% of AIH cases [
43,
46]. As missing the diagnosis of AIH with its therapeutic and prognostic consequences can be detrimental to the patient, it is important that clinicians are notified of these comorbidities [
35]. Diagnosis of AIH with concurrent Steatohepatitis (SH) of alcoholic or non-alcoholic aetiology can be challenging. Hepatocyte ballooning with rarefaction, Mallory-Denk body and centrilobular ‘chicken- wire’ sinusoidal fibrosis, are characteristic features of SH, and usually not seen in AIH. (
Figure 7) Presence of these findings may guide in diagnosing concurrent SH in an AIH patient having otherwise typical histological and serological features [
10].
1.11. Pediatric Autoimmune hepatitis
The paediatric age group may have certain limitations in diagnosis as over half of the children have no autoantibodies and do not show hypergammaglobulinemia, which lead to false negative results [
47,
48,
49]. Thus, the simplified scoring system has a much lower sensitivity in this age group [
48]. Machancoses et al proposed a simplified diagnostic scoring system after studying 100 cases of pediatric autoimmune hepatitis which included 5 criteria: autoantibodies (0–2points), hypergammaglobulinemia, exclusion of viral hepatitis, exclusion of Wilson’s disease (1 point each) and interface hepatitis or multilobular collapse on liver histology (3 points). Also, a normal cholangiogram is mandatory. They found a sensitivity and specificity of 95.8% and 100% at a score of ≥6. This new proposal was helpful in diagnosing seronegative AIH if the other criteria are met [
46].
1.12. DILI versus AIH
Distinguishing DILI from AIH on histology can be an ardous task, since there are no pathognomonic histologic feature of either DILI or AIH. None of the proposed scoring systems can differentiate hepatitic pattern of DILI from acute AIH as there is significant clinical and histologic overlap [
10,
35]. As described by Dina Tiniakos, patients with DILI often present with centrilobular necrosis with little or no interface hepatitis, which can be the feature of acute AIH as well [
10,
35]. These patients need to be subjected to a trial of steroid monotherapy. Upon response, steroids should be quickly tapered off. Patients with DILI will remain in complete remission unless reexposed to the offending drug. In contrast, patients of AIH will almost always relapse on steroid reduction or withdrawl. It is important to keep such patients under regular follow up for about 5 years with measurements of liver enzymes and IgG levels to recognize a possible delayed relapse of AIH [
43].
1.13. Future way forward and Research
The recent consensus recommendations have proposed the criteria for diagnosis of AIH which could be applied to both acute and chronic disease presentation. But there are still several issues which need to be addressed by future prospective studies and trials. Distinction of acute presentation of AIH and DILI is pivotal, since AIH deserves long-term immunosuppression while DILI does not. The histological criteria most useful to differentiate between DILI and AIH in the context of an acute lobular hepatitis still remain elusive and need to be evaluated by further studies. Also, the performance of various activity scoring systems especially mHAI score need to be endorsed in the setting of acute hepatitis, as conventionally all these were designed for evaluation of chronic viral hepatitis. Detailed histopathologic and clinical prospective studies evaluating the specific features in distinguishing co-morbidity from severe NASH as well as from AIH with just NAFLD, as well as the cogency of scoring systems in case of concurrent comorbidities are required for better understanding. Validation of these consensus criteria across the different populations, encompassing cases with severe acute and chronic manifestations of AIH and evaluation of the optimal diagnostic cut off for defining a plasma cell cluster are topics of future research.
Contributorship statement:
PS and SG have contributed equally, wrote the manuscript and did the final editing. Both authors approved the final manuscript.
Conflicts of Interest
None to declare.
Supplementary Materials
None.
References
- Lv T, Li M, Zeng N, et al. Systematic review and meta-analysis on the incidence and prevalence of autoimmune hepatitis in Asian, European, and American population. J Gastroenterol Hepatol. 2019, 34, 1676–1684. [CrossRef] [PubMed]
- Tunio NA, Mansoor E, Sheriff MZ, Cooper GS, Sclair SN, Cohen SM. Epidemiology of Autoimmune Hepatitis (AIH) in the United States Between 2014 and 2019: A Population-based National Study. J Clin Gastroenterol. 2021, 55, 903–910. [CrossRef] [PubMed]
- Amarapurkar D, Dharod M, Amarapurkar A. Autoimmune hepatitis in India: single tertiary referral centre experience. Trop Gastroenterol. 2015, 36, 36–45. [CrossRef] [PubMed]
- Sebode M, Weiler-Normann C, Liwinski T, Schramm C. Autoantibodies in Autoimmune Liver Disease-Clinical and Diagnostic Relevance. Front Immunol. 2018, 9, 609. [CrossRef]
- Wood IJ, King WE, Parsons PJ, Perry JW, Freeman M, Lim-Brick L. Non-suppurative hepatitis: a study of acute and chronic forms with special reference to biochemical and histological changes. Med J Austr. 1948, 1, 249–261. [CrossRef]
- Mackay, IR. Historical reflections on autoimmune hepatitis. World J Gastroenterol. 2008, 14, 3292–3300. [Google Scholar] [CrossRef] [PubMed]
- Kunkel HG, Ahrens EH, Eisenmenger WJ, Bongiovanni AM, Slater RJ. Extreme hypergammaglobulinemia in young women with liver disease. J Clin Invest. 1951, 30, 654.
- Joske RA, King WE. The L.E.-cell phenomenon in active chronic viral hepatitis. Lancet. 1955, 269, 477–480.
- Bearn AG, Kunkel HG, Slater RJ. The problem of chronic liver disease in young women. Am J Med. 1956, 21, 3–15.
- Tiniakos DG, Brain JG, Bury YA. Role of Histopathology in Autoimmune Hepatitis. Dig Dis. 2015, 33 (Suppl 2), 53–64. [CrossRef]
- Washington MK, Manns MP: Autoimmune hepatitis; in Burt AD, Portmann B, Ferrel L (eds): MacSween’s Pathology of the Liver. Edinburgh, Churchill Livingstone, 2012, pp 467–490.
- Hofer H, Oesterreicher C, Wrba F, Ferenci P, Penner E: Centrilobular necrosis in autoimmune hepatitis: a histological feature associated with acute clinical presentation. J Clin Pathol 2006, 59, 246–249. [CrossRef] [PubMed]
- de Boer YS, van Nieuwkerk CM, Witte BI, Mulder CJ, Bouma G, Bloemena E. Assessment of the histopathological key features in autoimmune hepatitis. Histopathology 2015, 66, 351–362. [CrossRef] [PubMed]
- Miao Q, Bian Z, Tang R, et al. Emperipolesis medi- ated by CD8 T cells is a characteristic histo- pathologic feature of autoimmune hepatitis. Clin Rev Allergy Immunol 2015, 48, 226–235. [CrossRef] [PubMed]
- Estradas J, Pascual-Ramos V, Martínez B, Uribe M, Torre A. Autoimmune hepatitis with giant-cell transformation. Ann Hepatol. 2009, 8, 68–70. [CrossRef]
- Matta B, Cabello R, Rabinovitz M, Minervini M, Malik S. Post-infantile giant cell hepatitis: A single center's experience over 25 years. World J Hepatol. 2019, 11, 752–760. [CrossRef] [PubMed]
- Guindi M: Histology of autoimmune hepatitis and its variants. Clin Liver Dis 2010, 14, 577–590. [CrossRef]
- Ngu JH, Gearry RB, Frampton CM, Stedman CA: Predictors of poor outcome in patients with autoimmune hepatitis: a population based study. Hepatology 2013, 57, 2399–2406. [CrossRef] [PubMed]
- Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology 1993, 18, 998–1005. [CrossRef]
- Alvarez F, Berg PA, Bianchi FB, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999, 31, 929–938. [CrossRef]
- Omagari K, Masuda J, Kato Y, et al. Re-analysis of clinical features of 89 patients with autoimmune hepatitis using the revised scoring system proposed by the International Auto- immune Hepatitis Group. Intern Med 2000, 39, 1008–1012. [CrossRef]
- YatsujiS, HashimotoE, KanedaH, TaniaiM, TokushigeK, Shiratori K. Diagnosing autoimmune hepatitis in non- alcoholic fatty liver disease: is the International Autoimmune Hepatitis Group scoring system useful? J Gastroenterol 2005, 40, 1130–1138. [CrossRef] [PubMed]
- Vergani D, Mieli-Vergani G: Autoimmune manifestations in viral hepatitis. Semin Immunopathol 2013, 35, 73–85. [CrossRef] [PubMed]
- Papamichalis PA, Zachou K, Koukoulis GK, et al. The revised international autoimmune hepatitis score in chronic liver diseases including autoimmune hepatitis/overlap syndromes and autoimmune hepatitis with concurrent other liver disorders. J Autoimmune Dis 2007, 4, 3. [CrossRef]
- Hennes EM, Zeniya M, Czaja AJ, et al. International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008, 48, 169–176.
- Yeoman AD, Westbrook RH, Al-Chalabi T, et al. Diagnostic value and utility of the simplified International Autoimmune Hepatitis Group (IAIHG) criteria in acute and chronic liver disease. Hepatology. 2009, 50, 538–545. [CrossRef]
- Czaja, AJ. Comparability of probable and definite autoimmune hepatitis by international diagnostic scoring criteria. Gastroenterology. 2011, 140, 1472–1480. [Google Scholar] [CrossRef]
- Balitzer D, Shafizadeh N, Peters MG, Ferrell LD, Alshak N, Kakar S. Autoimmune hepatitis: review of histologic features included in the simplified criteria proposed by the international autoimmune hepatitis group and proposal for new histologic criteria. Mod Pathol. 2017, 30, 773–783. [CrossRef]
- Fujiwara K, Yasui S, Tawada A, Fukuda Y, Nakano M, Yokosuka O. Diagnostic value and utility of the simplified International Autoimmune Hepatitis Group criteria in acute-onset autoimmune hepatitis. Liver Int. 2011, 31, 1013–1020. [CrossRef] [PubMed]
- Weiler-Normann C, Lohse AW: Acute auto- immune hepatitis: many open questions. J Hepatol 2014, 61, 727–729. [CrossRef]
- Muratori P, Granito A, Lenzi M, Muratori L. Limitation of the simplified scoring system for the diagnosis of autoimmune Hepatitis with acute onset. Liver Int. 2021, 41, 529–534. [CrossRef]
- Joshita S, Yoshizawa K, Umemura T, et al. Japan Autoimmune Hepatitis Study Group (JAIHSG). Clinical features of autoimmune hepatitis with acute presentation: a Japanese nationwide survey. J Gastroenterol. 2018, 53, 1079–1088. [CrossRef] [PubMed]
- Gurung A, Assis DN, McCarty TR, Mitchell KA, Boyer JL, Jain D. Histologic features of autoimmune hepatitis: a critical appraisal. Hum Pathol. 2018, 82, 51–60. [CrossRef] [PubMed]
- Tucker SM, Jonas MM, Perez-Atayde AR. Hyaline droplets in Kupffer cells: a novel diagnostic clue for autoimmune hepatitis. Am J Surg Pathol 2015, 39, 772–778. [CrossRef] [PubMed]
- Lohse AW, Sebode M, Bhathal PS, et al. Consensus recommendations for histological criteria of autoimmune hepatitis from the International AIH Pathology Group: Results of a workshop on AIH histology hosted by the European Reference Network on Hepatological Diseases and the European Society of Pathology: Results of a workshop on AIH histology hosted by the European Reference Network on Hepatological Diseases and the European Society of Pathology. Liver Int. 2022, 42, 1058–1069.
- Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995, 22, 696–699. [CrossRef] [PubMed]
- Batts KP, Ludwig J: Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 1995, 19, 1409–1417. [CrossRef] [PubMed]
- Scheuer PJ: Classification of chronic viral hepatitis: a need for reassessment. J Hepatol 1991, 13, 372–374. [CrossRef] [PubMed]
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR cooperative study group. Hepatology 1994, 20, 15–20. [CrossRef]
- Czaja AJ: Autoantibody-negative autoimmune hepatitis. Dig Dis Sci 2012, 57, 610–624. [CrossRef]
- O’Brien C, Joshi S, Feld JJ, Guindi M, Dienes HP, Heathcote EJ: Long-term follow-up of antimitochondrial antibody-positive autoimmune hepatitis. Hepatology 2008, 48, 550–556. [CrossRef]
- Tsuneyama K, Baba H, Kikuchi K, et al. Autoimmune features in metabolic liver disease: a single-center experience and review of the literature. Clin Rev Allergy Immunol 2013, 45, 143–148. [CrossRef] [PubMed]
- Lohse, AW. Diagnostic Criteria for Autoimmune Hepatitis: Scores and More. Dig Dis. 2015, 33 (Suppl 2), 47–52. [Google Scholar] [CrossRef] [PubMed]
- Bach N, Thung SN, Schaffner F. The histological features of chronic hepatitis C and autoimmune chronic hepatitis: a comparative analysis. Hepatology. 1992, 15, 572–577. [CrossRef] [PubMed]
- Muratori P, Granito A, Pappas G, Muratori L. Validation of simplified diagnostic criteria for autoimmune hepatitis in Italian patients. Hepatology 2009, 49, 1782–1783, author reply 1783. [CrossRef] [PubMed]
- De Luca-Johnson J, Wangensteen KJ, Hanson J, Krawitt E, Wilcox R. Natural history of patients presenting with autoimmune hepatitis and coincident nonalcoholic fatty liver disease. Dig Dis Sci. 2016, 61, 2710–2720. [CrossRef] [PubMed]
- Ferri PM, Ferreira AR, Miranda DM, Simões E Silva AC. Diagnostic criteria for autoimmune hepatitis in children: a challenge for pediatric hepatologists. World J Gastroenterol. 2012, 7, 4470–4473.
- Mileti E, Rosenthal P, Peters MG. Validation and modification of simplified diagnostic criteria for autoimmune hepatitis in children. Clin Gastroenterol Hepatol. 2012, 10, 417. [CrossRef] [PubMed]
- Arcos-Machancoses JV, Molera Busoms C, Julio Tatis E, et al. Development and validation of a new simplified diagnostic scoring system for pediatric autoimmune hepatitis. Dig Liver Dis. 2019, 51, 1308–1313. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).