1. Introduction
Breast cancer stands out as the most prevalent malignancy among women worldwide. The increasing number of breast cancer survivors can be attributed to advancements in early diagnosis and improvements in treatment efficacy [
1,
2]. Consequently, an expanding cohort of women is exposed to the risk of developing contralateral breast cancer (CBC). Within the domain of breast cancer research and treatment, CBC emerges as a significant focal point. This terminology denotes the manifestation of a novel and distinct breast cancer occurrence in the contralateral breast of a patient previously diagnosed with breast cancer of the other breast. CBC carries an adverse prognosis, characterized by an augmented incidence of distant metastases and elevated mortality rates linked to breast cancer [
3].
While surgical intervention remains the primary therapeutic approach for breast cancer, complementary strategies such as chemotherapy, radiation therapy, hormone therapy, and immunotherapy have clinical applications. Patients typically receive surgical recommendations for either mastectomy or breast-conserving surgery. Nevertheless, both options entail the potential for post-operative breast asymmetry. Consequently, a substantial number of women opt for symmetrizing reduction procedures, which result in a more harmonious aesthetic outcome and contribute to an enhanced quality of life [
4].
For most patients with primary breast cancer, the risk of developing distant metastases surpasses the risk of developing contralateral breast cancer. Approximately 10-12% of women treated for primary breast cancer experience distant recurrence during a mean follow-up of slightly over 5 years [
5]. However, the risk of contralateral breast cancer is 1.3 to 1.9 times higher than the risk of primary breast cancer in the general population [
3,
6] and reaches up to a 10,5% cumulative incidence in 20 years (3).
Consequently, the tissue excised during symmetrizing surgery undergoes pathological examination to discern any potential signs of cancer. This specimen was previously resected in multiple fragments and not marked in any specific way. On several occasions, CBC or in situ lesions within this specimen were incidentally discovered in our institution. This serendipitous discovery prompted us to reconsider the entire protocol from surgical removal to examination at the pathology department. The absence of marking and inking, and removal in pieces made it challenging to precisely identify the tumor's location and ascertain whether it had been completely excised. Secondly, it was sometimes challenging to define tumor size, which is important for staging and defining the need for adjuvant (systemic) therapy Depending on the tumor's characteristics, many patients were required to undergo either mastectomy or whole-breast radiation therapy. By introducing a new protocol on July 20, 2022, our objective was to enhance our ability to provide detailed information regarding the potential CBC, such as tumor size and the margin status. This improved precision in diagnosis may allow for the adjustment of treatment plans accordingly, potentially involving re-excision, chemotherapy, targeted therapy, or a boost of radiation therapy.
1.1. Breast cancer treatment guidelines
The treatment approach for breast cancer depends on the tumor type and is discussed within a Multidisciplinary Oncology Board (MOB).
The importance of adjuvant therapy varies depending on individual patients and tumor characteristics, with certain factors, like tumor size and margin status, being more challenging to ascertain in cases of non-oncologic resection without protocolized removal and specimen handling.
Tumor size primarily influences the determination of chemotherapy, targeted therapy, and hormonal therapy. The assessment of tumor grade or receptor status is generally not significantly affected by the mode of resection. Determining surgical margins is crucial for considering additional surgery and radiotherapy, possibly with a boost.
Follow-up is indicated for other diagnoses such as lobular carcinoma in situ (LCIS) For atypical ductal hyperplasia (ADH), no intervention is recommended.
2. Materials and Methods
A retrospective cohort study was performed examining patients who received a symmetrizing breast reduction, due to previous breast cancer treatment in the contralateral breast, between January 2018 and December 2022 at the Maastricht University Medical Center+ (MUMC+) in the Netherlands.
The inclusion criteria for this analysis involved patients in whom (pre)malignancies were identified within the breast reduction tissue.
2.1. Data management
Data was recorded from the patient’s medical records concerning oncological, radiological, and pathological parameters, with baseline characteristics: age, age at the time of first and second diagnoses, body mass index (BMI), history of intoxication, and medication history. These variables were deemed essential for a comprehensive understanding of breast cancer dynamics and their potential influence on outcomes.
The study also scrutinized initial cancer diagnosis details, pathology reports, and treatment modalities, serving as crucial context for comprehending the patients' breast cancer journeys. Concerning the pathology we collected information on histological subtype, tumor diameter, grade, lymph vascular space invasion, hormonal and Her2 receptors, and margin status. Concerning radiology, we collected information on BI-RADS classification, tumor localization, and diameter. The BI-RADS classification, which stands for "Breast Imaging Reporting and Data System," is a standardized system used in radiology and mammography to describe and report findings related to breast imaging. [
7]. Data were collected for the primary and secondary diagnosis.
2.2. Surgical technique
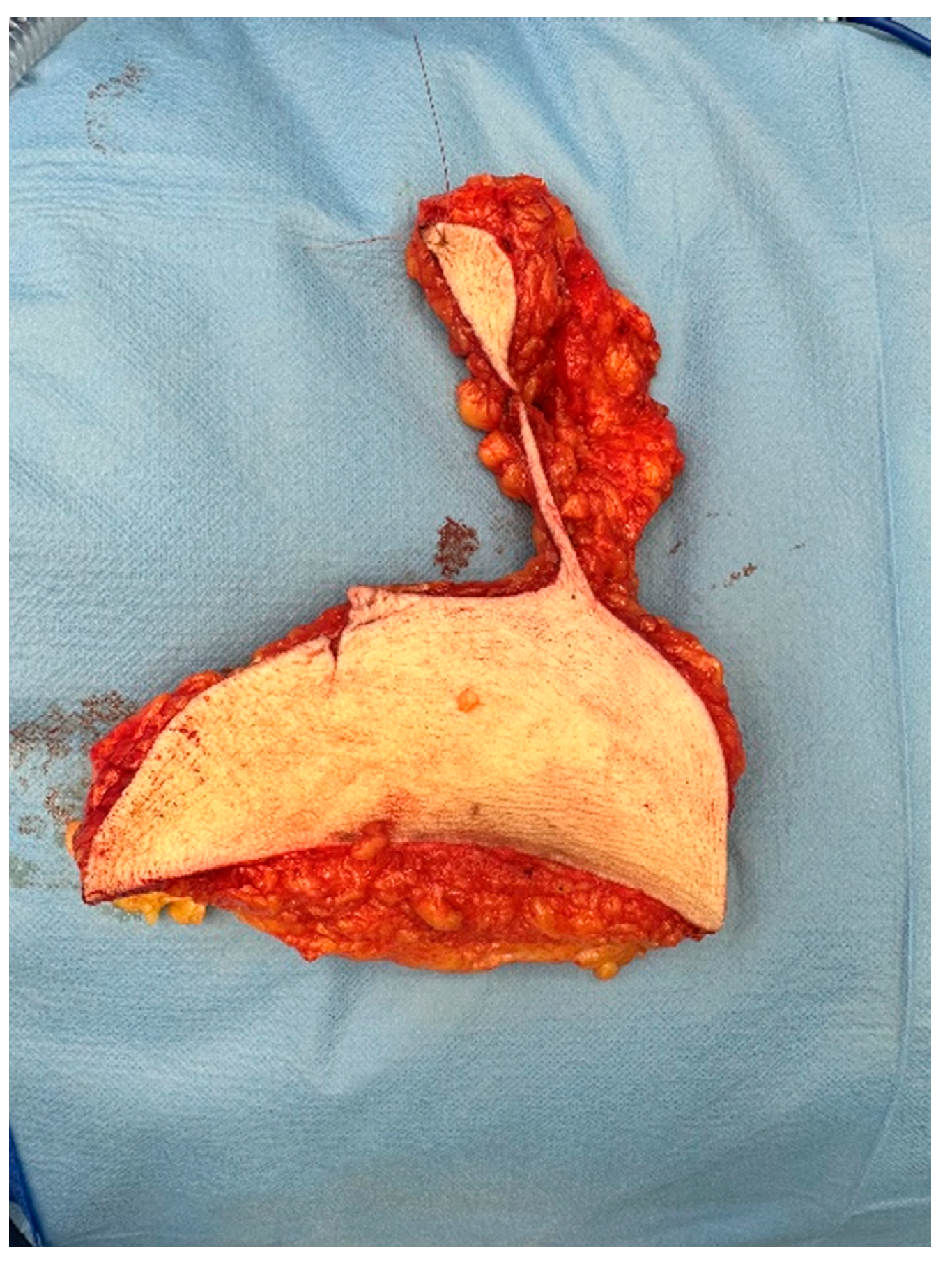
All patients underwent a contralateral breast reduction surgery for symmetry using the Wise pattern technique. This is the most common technique used in breast reduction surgery [
8]. The surgical protocol was changed on 20 July 2022 as resections were made in toto and marking of the excised tissue was done cranially (
Figure 1).
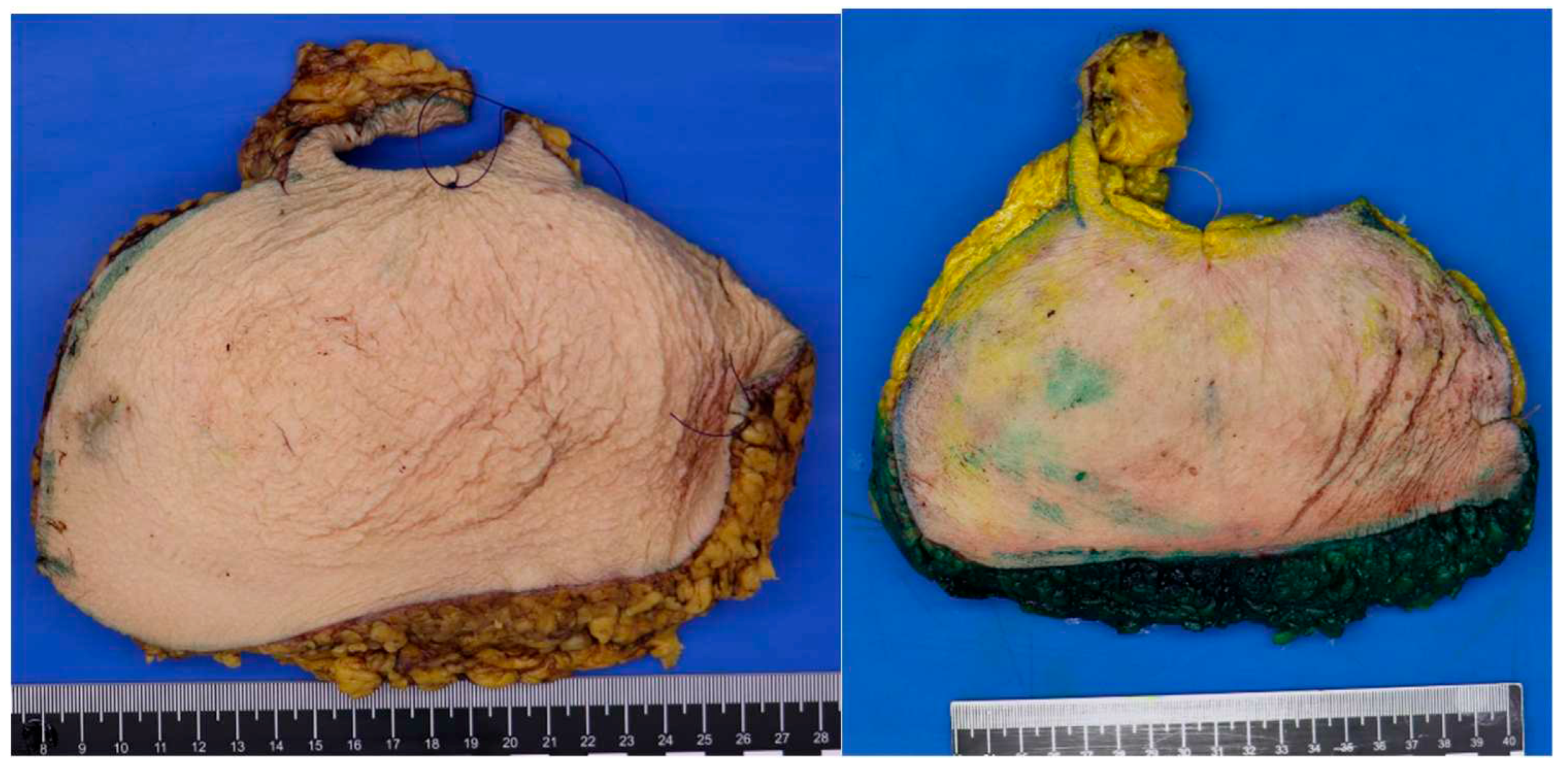
2.3. Pathology: protocol and methods
The pathology protocol changes involved inking the reduction preparations of patients with a previous breast malignancy and in patients above the age of 50 (
Figure 2). Additionally, the transected slices at grossing are saved in their original order. In case of an accidental finding at microscopy, it is possible to identify the selection site of the original piece obtained for microscopy. Additional sampling can be done to reconstruct tumor size and margin status. (
Figure 2).
3. Results
3.1. Participants
Between January 2018 and December 2022, 648 patients had a symmetrizing contralateral breast reduction. In 10 of them a (pre-)malignancy was found in the breast reduction tissue. These 10 patients were included for further investigation. The incidence rate was 1,5% in 5 years.
3.2. Patient demographics and clinical characteristics
Baseline patient characteristics. See
Table 1.
3.3. Main results
3.3.1. Pathological information
Table 2.
Pathological information of the primary and secondary tumor.
Table 2.
Pathological information of the primary and secondary tumor.
| |
Primary tumor N = 10 |
Secondary tumor N = 10 |
|
|
| |
Type |
Grade |
Receptor |
Size (mm) |
Type |
Grade |
Receptor |
Size (mm) |
Margin |
| 1 |
DCIS |
3 |
ER/PR+, HER2- |
25 |
DCIS |
2 |
N/A |
18 |
Positive |
| 2 |
DCIS |
3 |
ER/PR+, HER2- |
13 |
DCIS |
2 |
N/A |
3 |
Negative |
| 3 |
DCIS |
2 |
ER/PR+, HER2- |
41 |
LCIS |
N/A |
N/A |
N/A |
Negative |
| 4 |
DCIS |
2 |
ER/PR+ |
12 |
LCIS |
N/A |
N/A |
N/A |
Negative |
| 5 |
DCIS |
2 |
ER/PR+ |
15 |
NST |
N/A |
ER/PR+ |
1,5 |
Negative |
| 6 |
NST |
3 |
Triple negative |
37 |
LCIS |
N/A |
N/A |
N/A |
Negative |
| 7 |
NST |
3 |
Triple negative |
53 |
LCIS |
N/A |
N/A |
N/A |
Negative |
| 8 |
NST |
2 |
ER/PR+, HER2- |
11 |
NST |
2 |
ER/PR+, HER2- |
6 |
Negative |
| 9 |
NST |
2 |
ER/PR+, HER2 amplified |
24 |
ADH |
N/A |
N/A |
N/A |
Negative |
| 10 |
NST |
2 |
ER- |
42 |
ADH |
N/A |
N/A |
N/A |
Negative |
3.3.2. Radiological and surgical information
Regarding the primary tumor, a BI-RADS classification of 5 was assigned to all patients. Of these, eight opted for a lumpectomy, and two chose to undergo mastectomy. Among these patients, three underwent axillary clearance, while six opted for a sentinel node procedure. Furthermore, five patients received neoadjuvant chemotherapy. Additionally, two patients received adjuvant chemotherapy, and six received adjuvant radiotherapy. The median diameter of the primary tumor, as ascertained from mammographic imaging, was measured at 24 mm. (
Table 3)
As for the secondary tumor, a BI-RADS classification of 2 was assigned to nine out of ten patients, and one patient received a classification of 1. Based on the pathology, two patients elected to undergo mastectomy upon diagnosis, and one of them received adjuvant radiotherapy. It is noteworthy that none of these secondary tumors were diagnosed in the pre-operative imaging screening. (
Table 3)
3.3.3. Follow up
In this study, all patients underwent yearly follow-up examinations for 5 years, which included mammograms of the contralateral breast following their initial tumor diagnosis. Four patients stood out in this regard, as they completed their 5-year follow-up but were subsequently diagnosed with CBC. Notably, their mammograms consistently showed benign findings (BI-RADS 2) over the entire 5-year follow-up period. One of these patients developed CBC 29 years after concluding the 5-year follow-up, while the other patients received the CBC diagnosis 12, 3, and 1 year(s) after completing their 5-year follow-up.
Furthermore, two additional patients completed their 4-year follow-up, maintaining a BI-RADS 2 classification throughout this time before eventually receiving a CBC diagnosis. Similarly, four patients completed their 3-year follow-up, with BI-RADS 2 findings consistently reported during each examination before eventually being diagnosed with a second case of breast cancer.
However, it's worth noting that for one patient, the radiological data was unavailable for the primary tumor. The first diagnosis was in 1992, giving us no BI-RADS classification or other radiological information.
Before every breast reduction surgery, a mammogram of the breast that gets the symmetrical reduction is mandatory and it should be no older than 6 months to rule out any malignancies. The pre-operative breast reduction protocol changed on 1st October 2023, and since that date year one-year-old mammogram has been sufficient. This means that patients from our study could have had a mammogram older than 6 months before surgery [
9].
3.3.4. Treatment plan after the secondary diagnosis
For six out of ten patients, there was no alteration in their treatment plan, nor was it recommended by the MOB. These patients were diagnosed with either LCIS or ADH. The clinical management in these cases aligned with established guidelines, which advocate for radiological follow-up over a period of five years.
Conversely, the 3 patients who received a diagnosis of DCIS, invasive lobular carcinoma, or invasive carcinoma NST underwent comprehensive discussions within the MOB. In each of these cases, the recommendations encompassed either mastectomy or lumpectomy, an increased dosage of radiotherapy, or diligent follow-up, all depending on margins. One of the patients, diagnosed with invasive carcinoma NST, had clear tumor margins and was either advised to have adjuvant radiotherapy or a wait-and-see policy. She opted for the latter. The second patient, diagnosed with invasive carcinoma NST, was advised to have a mastectomy or radiotherapy, based on the unclear margins. She underwent a mastectomy. Both diagnoses were made before the protocol changed, leaving the second patient with no options.
Worth noting is that one patient with DCIS received this diagnosis after the protocol modification, affording them the option of choosing a lumpectomy instead of a mastectomy. The margins of the tumor were not radical, but because of the changed protocol, localization of the tumor was made and a lumpectomy was possible. Nevertheless, due to concerns about the potential recurrence of breast cancer and the breast reconstruction possibilities, the patient opted for a mastectomy with autologous tissue reconstruction.
4. Discussion
In the context of this study, CBC was observed with an incidence of 1.5% in the population under investigation at our institution. This incidence also includes cases of pre-malignancies. However, when considering only malignancies, excluding cases of LCIS, the incidence rate stood at 0.77%. This is slightly higher than the 0,44% of the general CBC incidence [
3]. The occurrence of invasive carcinoma in general reduction mammoplasties can differ between 0,06-5%, the larger cohorts show an average incidence ranging from 0.06% to 0.38%. The incidence of CBC is twice as high [
10]. Interestingly, out of the 10 patients who had (pre)malignant conditions, they underwent regular follow-up examinations with imaging for 5 years after the primary diagnosis. During these follow-ups, their BIRADS stages consistently indicated 1 or 2. This suggests that, without the symmetrizing breast reduction surgery, the diagnosis of CBC might have been delayed or never been made.
The introduction of the revised protocol has significantly broadened the range of available treatment options for patients who have received a breast cancer diagnosis, all while maintaining oncologic safety. The incorporation of complete specimen excision and marking has notably improved the precision of tumor localization. This, in turn, has opened the possibility of considering radiotherapy and surveillance as alternatives to immediate surgical intervention. The utilization of specimen marking simplifies the tumor localization process, providing a more comprehensive assessment of the tumor's size. This information is crucial for guiding adjuvant systemic therapy. Without specimen marking, accurately pinpointing the tumor's exact location becomes challenging, leaving questions about margin status. In such cases, mastectomy or comprehensive chest radiation may become the only viable treatment option. When we reviewed our patient cohort, it is worth mentioning that only one individual had DCIS identified in the resected specimen following the protocol amendment. She chose to undergo a mastectomy, primarily due to concerns about potential disease recurrence. This patient represents the only case in our study that truly benefited from the protocol change. However, in the meantime we have had referral cases from centers without a specific protocol and encountered similar problems, stressing the need for a wider implementation.
It is important to note that the financial impact of specimen marking and inking is minimal, effectively eliminating any potential financial burden. Both marking and inking procedures are brief, requiring only a minimal amount of time during the surgical procedure and pathology specimen handling, respectively. Moreover, the removal of the specimen in toto does not alter the aesthetic outcome.
Upon changing the breast cancer treatment protocol, it becomes evident that there are relatively few consequences for specific categories including LCIS. LCIS is not classified as cancer but as a pre-malignancy. The cumulative incidence of breast cancer following a diagnosis of LCIS is approximately 1-2% per year, resulting in a relative risk that is 8-10 times higher than the general population. Because it is challenging to delineate the region with classic LCIS, and the cumulative risk of progression to invasive lobular carcinoma is low. According to Dutch guidelines, a 5-year follow-up is sufficient in these instances [
11,
12]. Since the diagnosis of LCIS does not involve any physical treatment options, psychological support might be a topic to discuss after the diagnosis. Currently, in North America, the standard of care includes the screening of distress symptoms in cancer patients at all stages of their illness. The high prevalence of distress symptoms specific to the diagnosis of breast cancer indicates that healthcare providers should give increased attention to these symptoms. Although LCIS is not a malignancy, it does lead to uncertainty and fear of breast cancer. Early screening and the provision of interventions for distress can enhance adherence to improve quality of life and be a form of treatment. [
13,
14]
Concerns have arisen regarding the potential impact of pre-operative mammograms on the accuracy of predicting malignancies in the reduction specimen. This has led to discussions about the necessity of conducting a more comprehensive pre-operative mammogram assessment. However, upon closer scrutiny, it becomes apparent that smaller abnormalities, such as LCIS and ADH, often go unnoticed on mammograms. Consequently, the outcomes of these specific diagnoses remain unaffected by the pre-operative mammogram.
In general, one of the most relevant benefits of this protocol change is the gain of shared decision-making. Patients have more treatment options available, giving them a higher level of autonomy. [
15]
5. Conclusions
The revised breast cancer contralateral reduction protocol has broadened therapeutic choices by better tumor assessment through resection in toto, excision specimen marking, and inking. This enhances oncological safety. The financial impact is minimal. Overall, the revised protocol improves treatment flexibility and gains shared decision-making. Thereby, this article creates more awareness regarding the risk of contralateral breast cancer in reduction specimens. We highly suggest every plastic surgery and pathology department introduce this new protocol as standard care.
Author Contributions
Conceptualization, Shan Shan Qiu. and Loes Kooreman; methodology, Shan Shan Qiu, Zoë Kuijlaars, Carmen Rijvers, Nadine Hillberg, and Loes Kooreman; validation, Shan Shan Qiu, Carmen Rijvers, Nadine Hillberg, Zoë Kuijlaars, and Loes Kooreman; formal analysis, Zoë Kuijlaars.; investigation, Zoë Kuijlaars; resources, Zoë Kuijlaars, Loes Kooreman, and Carmen Rijvers; data curation, Zoë Kuijlaars, Loes Kooreman, Carmen Rijvers; writing—original draft preparation, Zoë Kuijlaars.; writing—review and editing, Zoë Kuijlaars, Nadine Hillberg, Loes Kooreman, Carmen Rijvers, and Shan Shan Qiu; visualization, Zoë Kuijlaars.; supervision, Shan Shan Qiu; project administration, Shan Shan Qiu. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Maastricht University Medical Center (METC 2023-3751, 5th May 2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nederland, N.B.O. Borstkanker. Available online: https://richtlijnendatabase.nl/richtlijn/borstkanker/algemeen.html (accessed on 7 February 2020).
- Nederland, I.k. NKR cijfers. Available online: https://iknl.nl/nkr.
- Xiong, Z.; Yang, L.; Deng, G.; Huang, X.; Li, X.; Xie, X.; Wang, J.; Shuang, Z.; Wang, X. Patterns of Occurrence and Outcomes of Contralateral Breast Cancer: Analysis of SEER Data. J Clin Med 2018, 7, 133. [Google Scholar] [CrossRef] [PubMed]
- McGaughey, A. Body image after bilateral prophylactic mastectomy: an integrative literature review. J Midwifery Womens Health 2006, 51, e45–49. [Google Scholar] [CrossRef] [PubMed]
- Colzani, E.; Johansson, A.L.; Liljegren, A.; Foukakis, T.; Clements, M.; Adolfsson, J.; Hall, P.; Czene, K. Time-dependent risk of developing distant metastasis in breast cancer patients according to treatment, age and tumour characteristics. Br J Cancer 2014, 110, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Scheepens, J.C.C.; Veer, L.V.; Esserman, L.; Belkora, J.; Mukhtar, R.A. Contralateral prophylactic mastectomy: A narrative review of the evidence and acceptability. Breast 2021, 56, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Magny, S.J.; Shikhman, R.; Keppke, A.L. Breast Imaging Reporting and Data System. In StatPearls; Treasure Island (FL); 2023. [Google Scholar]
- Becker, D.B. The Paisley Pattern Breast Reduction. Plast Surg (Oakv) 2019, 27, 189–194. [Google Scholar] [CrossRef] [PubMed]
- NVPC. Mammareductie. Available online: https://richtlijnendatabase.nl/richtlijn/mammareductie/preoperatieve_beeldvorming.html.
- Uson Junior, P.L.S.; Callegaro Filho, D.; Bugano, D.D.G.; Geyer, F.C.; de Nigro Corpa, M.V.; Goncalves, P.D.S.; Simon, S.D.; Kaliks, R.A. Incidental Findings in Reduction Mammoplasty Specimens in Patients with No Prior History of Breast Cancer. An Analysis of 783 Specimens. Pathol Oncol Res 2018, 24, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Nederland, N.B.O. Borstkanker. Available online: https://richtlijnendatabase.nl/richtlijn/borstkanker/algemeen.html.
- Dinapoli, L.; Colloca, G.; Di Capua, B.; Valentini, V. Psychological Aspects to Consider in Breast Cancer Diagnosis and Treatment. Curr Oncol Rep 2021, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Fortin, J.; Leblanc, M.; Elgbeili, G.; Cordova, M.J.; Marin, M.F.; Brunet, A. The mental health impacts of receiving a breast cancer diagnosis: A meta-analysis. Br J Cancer 2021, 125, 1582–1592. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Liu, X.; Wu, Q.; Shi, Y. The Effects of Cognitive-Behavioral Stress Management for Breast Cancer Patients: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Cancer Nurs 2020, 43, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Faiman, B.; Tariman, J.D. Shared Decision Making: Improving Patient Outcomes by Understanding the Benefits of and Barriers to Effective Communication. Clin J Oncol Nurs 2019, 23, 540–542. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).