1. Introduction
Important neglected arboviruses of public health concern such as dengue, Zika, chikungunya and Yellow Fever viruses, are transmitted by Aedes aegypti , especially in the tropics (Mayer et al., 2017). The impact of these arboviruses has increased in the last 50 years with the spreading of the mosquito vector into new geographic regions with naïve human populations, increasing the incidence of these diseases (Weaver SC., 2014). The distribution of Aedes aegypti is concentrated in tropical and subtropical regions of the world (Huang et al., 2019), where it is considered anthropophilic and is found indoors and outdoors of houses in the urban areas (Badolo et al., 2022). Several factors like the tendency of this mosquito species to oviposit in man-made breeding sites, its close association with human habitations and environmental factors may explain its distribution (Lounibos LP., 2002). Aedes aegypti originated in Africa and exists in two subspecies named Aedes aegypti and Aedes formosus which have morphological, ecological and genetic differences (Brown et al., 2011; Gloria-Soria et al., 2016). Aedes aegypti formosus is the feral form, more zoophilic, and breeds in tree holes, while the urban form Aedes aegypti aegypti is more anthropophilic and breeds in artificial containers (Ouattara et al., 2019). Urbanisation, international exchanges and adaptation to human environment are the main driving factors of Aedes aegypti spread (Rose et al., 2020).
Life history traits of mosquitoes are influenced by certain extrinsic factors specific to the region of origin ( Arevalo-Cortes et al., 2022). For example, it has been shown that insecticides affect the life history traits of Aedes aegypti mosquitoes: on natural populations the effect on life history traits were proportional to resistance level; however, several viability parameters were strongly affected in the laboratory selected populations compared to its unselected control (Martins et al., 2012). Other important extrinsic factors influencing the life history traits are the pH of the breeding site water and the availability of food and biological. Indeed, the development time, adult size and survival of Aedes aegypti was influenced when the larvae were reared under different diets (Sasmita et al., 2019; Yan et al., 2021). In addition, these changes in the growth rate and the development time can result in significant size-associated differences in the vectorial capacity due to modifications in lipid metabolism (Arrese et Soulages, 2010). Similarly, variation in Aedes aegypti body size is associated with significantly different expression patterns of genes and metabolites that are associated with immunity, and reproduction, directly impacting vector competence (Price et al., 2015). Ae. aegypti is the primary vector of dengue virus in the urban areas of Burkina Faso (Robert et al., 1990). The proximity to human beings and the wide distribution of Ae. aegypti across the country make it an important vector to study the extent of its adaptation to local environmental conditions.
In mosquito vectors, female body size affects the fecundity. Female mosquitoes with larger body sizes lay more eggs than females with smaller bodies during their first gonotrophic cycle (Breigel, 1990). In addition, body size and teneral reserves acquired during larval development affect blood feeding and blood utilization for egg production with an impact on the production of viral particles and vector dispersion. Survival is a key component of vectorial capacity, an epidemiological standpoint. Increased survival allows the vector to produce more offspring, to increase the dispersion over greater distances, and to survive long enough to become infectious, and then to deliver more infectious bites during the remainder of its lifetime (Brady et al., 2013). Indeed, it has been shown that larger Aedes albopictus (Skuse) females have higher human host attack rates and obtain multiple blood meals (from multiple hosts) more frequently than smaller females (Xue et al., 1995). However, the relationship between body size and survival or fecundity is not positive and predictable, for example in Aedes aegypti, a negative association between body size and longevity was reported (Joy et al., 2010).
In this study, we evaluated the life history traits of the Aedes aegypti mosquito from different geographic areas in Burkina Faso. Briefly, the objectives of this study were to evaluate the development time of Aedes aegypti in three ecological zones, to assess the fecundity and survival and finally to measure the wings length of these mosquitoes to see whether the body size impacts the longevity and fecundity.
2. Methodology
2.1. Study Sites
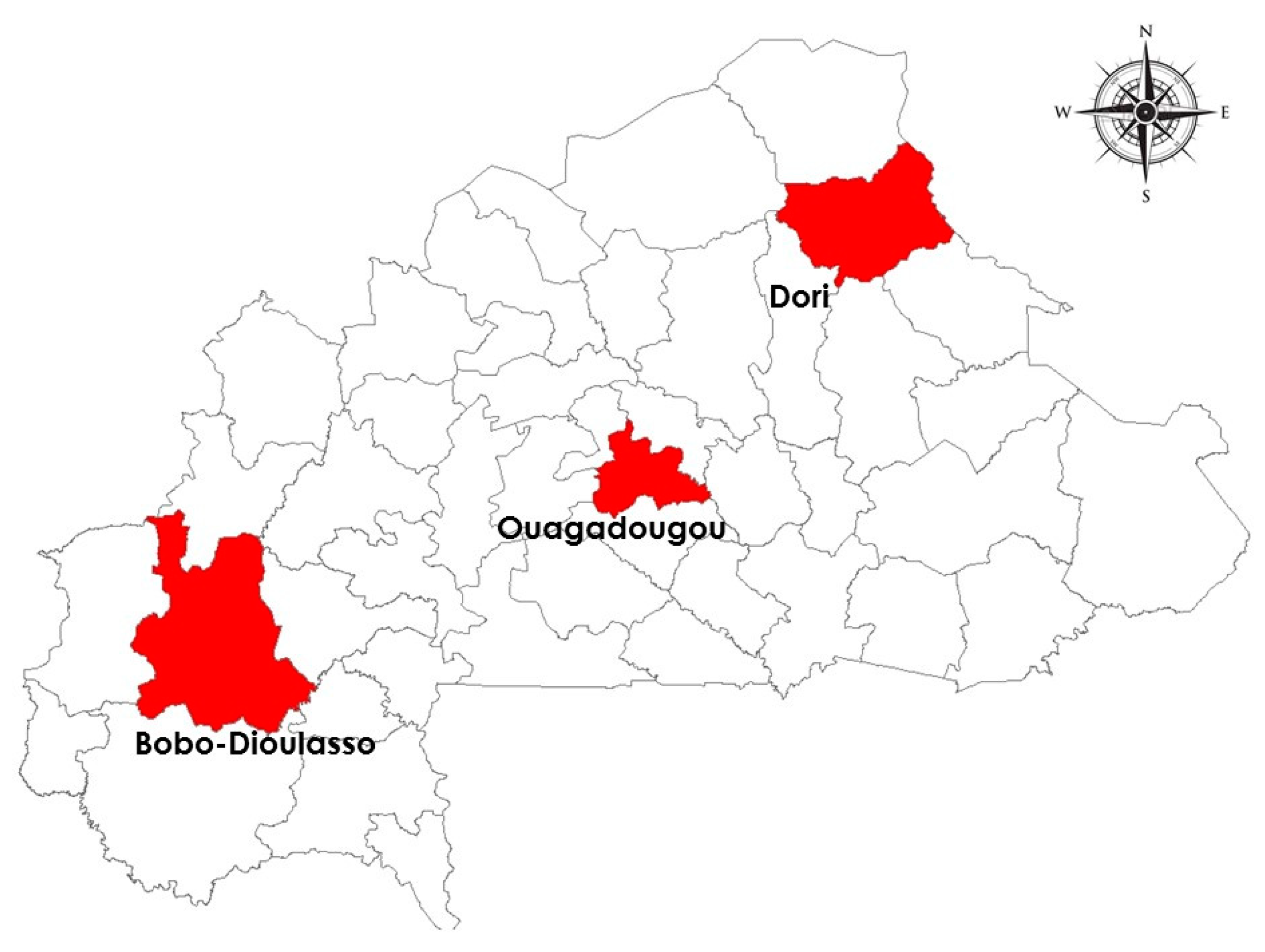
Burkina Faso is divided into three ecological zones with decreasing annual rainfall in quantity, duration of the rainy season and increasing temperatures along the North-South gradient. The study was conducted in four different sites between August and September 2021 during the rainy season. The sites were selected to be representative of the three ecological zones of the country (Figure 1).
Figure 1.
Mosquito collection sites in the three ecological zone of Burkina Faso. Source: Base Nationale des Données Topographiques (BNDT).
Figure 1.
Mosquito collection sites in the three ecological zone of Burkina Faso. Source: Base Nationale des Données Topographiques (BNDT).
Bobo-Dioulasso (Bobo) (11°09’N; 004°16’W), located in Western Burkina Faso, is the second biggest city of the country distant from Ouagadougou by about 365 km. The climate is of the Sudanian type, characterized by a relatively long rainy season from June to October with a yearly rainfall > 900 mm and a dry season from November to May. The annual average temperature is about 31°C (Zoure et al., 2020). The vegetation is dominated by Savannah-type plants with woodland, tree savannah, gallery forests and trees planted by local residents.
Ouagadougou (12° 21’58’’N; 1°31’5’’W) is the capital city of the country, located in the central part. The climate is Sudano-Sahelian with an average annual rainfall of 600–900 mm. The annual average is 33°C (Zoure et al., 2020) and the rain lasts from July to September. Two sites were chosen based on the urbanisation degree. 1200 logements (1200 logts) (12°22’N; 1°29’W) is an urban site located 2 km from the International airport. The roads of this site are paved and limited by a channel that allows polluted water evacuation. Toudweogo (Toud) (12°26’N; 001°30’W') is a peri-urban area located in the North of the capital city at 6 km from 1200 logts. The vegetation of this site is sparse with unpaved roads and a poor waste management system.
Dori (14°01’N; 001°02’W) is located in the northern part of the country about 265 km from the capital city. This part of the country experiences a dry season of 6-8 months with an annual mean rainfall of 500 mm. The climate is Sahelian, characterized by a night/day variation of temperature with an annual average temperature of 35°C (Zoure et al., 2020).
2.2. Mosquito Collection
Aedes mosquito larvae were sampled in the study sites between August and September 2021. Larvae were collected in natural and artificial breeding sites, like used tires, drums, flowerpots, and plastic and metal containers. After collection, samples were brought back to the insectary of Laboratoire d’Entomologie Fondamentale et Appliquée to separate Aedes larvae from other species and reared to adults in the insectary under 27±2°C and 75±5% relative humidity. These adults were allowed to feed on mice and the resulting eggs (F1) were used in all the experiments, such as development time, the fecundity and the longevity assays.
2.3. Larval Development Time
The development time from the first instar larvae to adults was assessed by rearing mosquito larvae in a plastic cup in five replicates. Briefly, twenty-four hours after hatching, larvae were placed in a small plastic cup at a density of 20 per cup, filled with 100 ml of distilled water and 0.04 mg of diet (crushed cat’s biscuit). Water and food were renewed daily to avoid water pollution due to food rests and pupae were removed and placed in a cup for emergence within the cage. Time to pupation, number and sex of emerging adults and their wings length were measured. All the experiments were performed at laboratory conditions of 25±2°C temperature and 75±5% relative humidity.
2.4. Mosquito Fecundity and Longevity
Mosquito adults aged 3-5 days were held in the laboratory at 25±2°C and blood-fed on anesthetised mice for one hour. After feeding, 20 blood-fed mosquitoes were individually put in a plastic cup with 10% sugar-soaked cotton on top, to allow them to lay eggs. After 5 days, these mosquitoes were removed, their eggs were counted and their abdomen was dissected to count the retained eggs. The eggs were dried in insectary conditions for 3 days and put in hatching conditions for 5 days to assess their fertility. The fecundity was assessed as the total number of eggs collected divided by the total number of ovipositing mosquitoes (P. T. LEISNHAM, L. M. SALA, 2008).
The longevity is the time between emergence and mosquito death. Mosquito longevity was measured as the time between blood-feeding and death, which was assessed daily under laboratory conditions. We assessed the longevity post-blood meal, because taking a blood meal is realistic and one factor of the vectorial capacity is how a vector can survive after an infectious blood meal (Barreaux et al., 2018). To this end, approximately 50 fed and unfed mosquitoes were kept separately in paper cups and fed with 10% sugar solution. Every day, dead mosquitoes were removed and the length of both wings was measured to assess the relationship between longevity and wing length.
2.5. Morphology and Morphometry
All mosquitoes used for the morphology and morphometry measurements were provided from the different experiments of the development time, the fecundity and the longevity assays.
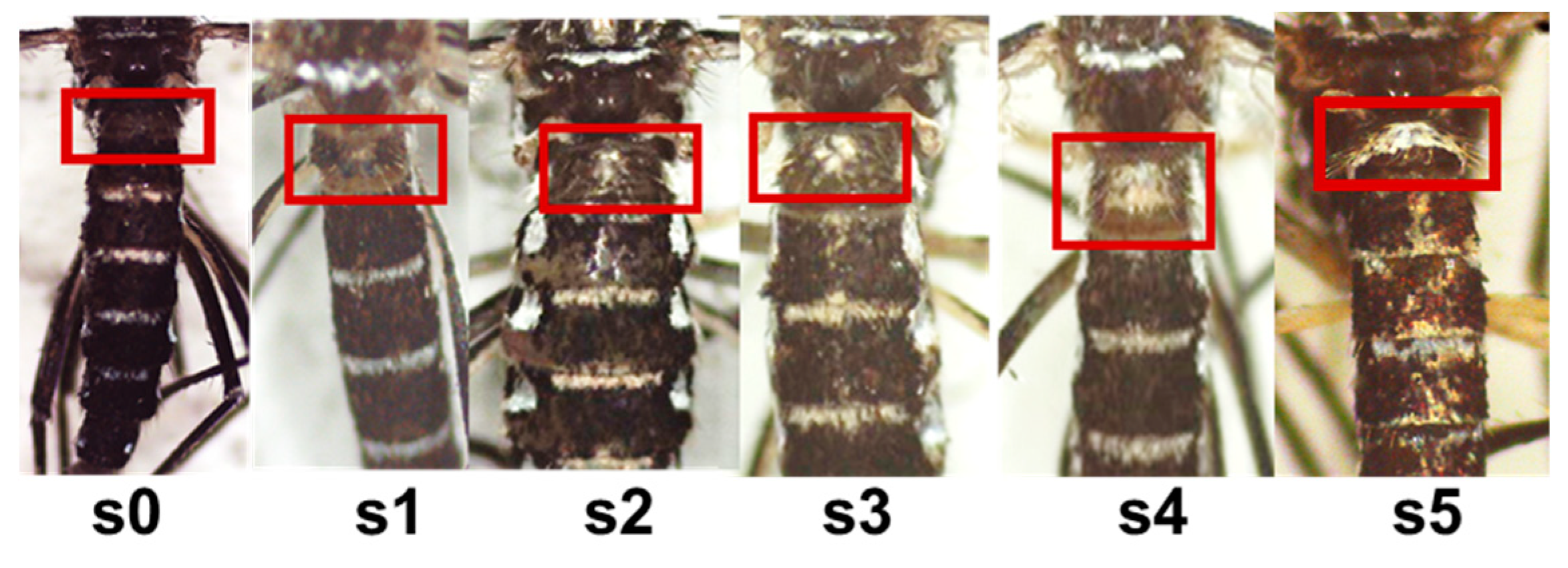
Daily emerging mosquitoes from the immature development assay were kept in the freezer before processing. Each mosquito was identified according to McBride et al., 2015 which attributes a score from 1 to 5 with regard to white scales abundance on the first abdominal tergite (figure 2). Mosquitoes without any white scale were identified as Aedes formosus. After identification, mosquito wings were removed under a stereomicroscope equipped with a photography system. The wings were placed on microscope slides and photographed using the LEICA application. Wing length was used as a proxy for body size. Both wings of each individual were measured from the tip to the distal end of the alula with the Image J software (Barreaux et al., 2016). The mean length of the two wings was used in the analyses or only one when the other one was damaged. The sample was removed when both wings were damaged.
Figure 2.
Morphotype of Aedes aegypti mosquitoes (Sanon 2021).
Figure 2.
Morphotype of Aedes aegypti mosquitoes (Sanon 2021).
The morphotype was assessed according the extent of white scaling on the first abdominal tergite using an ordinal scale from 1 to 5 according to McBride et al., (2015). The S0 morphotype lacking of white scales on the first abdominal tergite is recorded as formosus-like; S1, up to a few scattered white scales; S2, small patch of white scales at midline; S3, contiguous patch of white scales at midline stretching from top to bottom of tergite and covering up to 60% of visible area; S4, contiguous patch of white scales covering 60 - 90% of visible area; S5, contiguous patch of white scales covering >90% of visible area. Pictures in the figure were taken from mosquito samples collected in different locations from Burkina Faso as part of this study.
3. Statistical Analysis
All data were analysed with R version 4.0.4. The normality of the datasets was checked using Shapiro-Wilk. The dataset of the development time to adult emergence was not normally distributed (P-value>0.05), a simple linear model was used to evaluate the effect of the site on the emergence time. To estimate the effect of the different site on the longevity of the mosquitoes, we performed a Cox proportional hazards regression model using the R package ‘survival’. The number of mosquitoes that died per day was used as a response variable and an interaction between physiological status (fed and unfed) mosquitoes and the study sites (Bobo, Dori, 1200 logements and Toudweogo) was included as an explanatory variable. All analyses were performed with 95% confidence.
A general linear model was used to assess the relationship between the fecundity, the wing length, the survival and the sites. For the development of the immature stages, we analysed the effect of wing length regarding the different emergence day (days), the sex and the sites without interactions and this is expressed in the following equation:
To access the influence body size and fecundity (longevity), we analysed fecundity (longevity) regarding the interactions between sites and wing length. Sites were considered as factors and wing length was a covariate. Below are the following equations:
4. Results
4.1. Larvae to adult development time
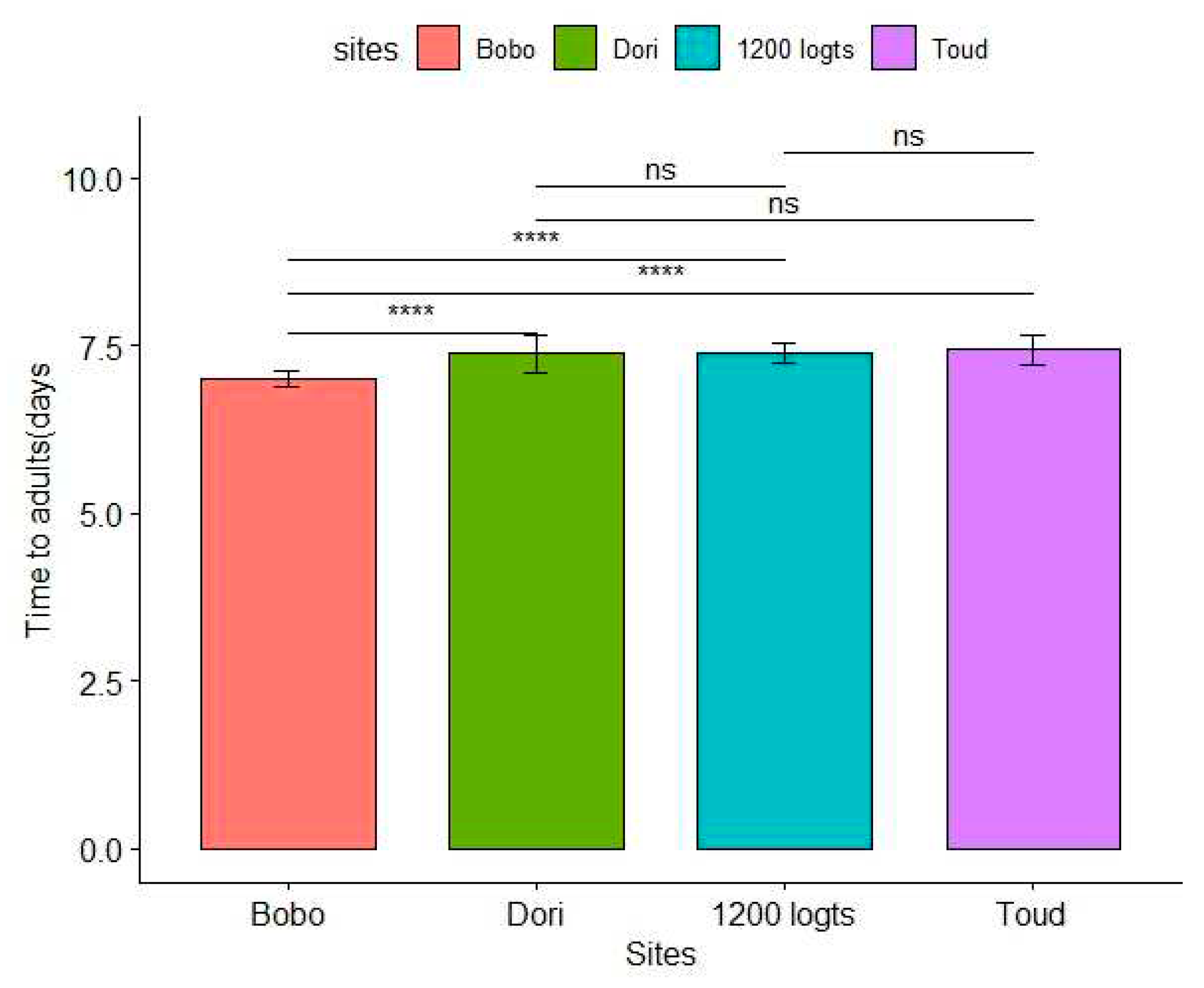
The mean development time from the first instar larvae to adults varied significantly in the different sites with the longest time in the sites of 1200 logts and Toud (7.39±0.14 and 7.43 ± 0.21 days for both sites) and the lowest time at Bobo (7.0 ± 0.12 days) (
Figure 3). The development time of mosquitoes from Bobo were significantly different from the three others sites (P<0.05). The structural equation confirmed a significant effect of the sites on the adult emergence time, indicating that mosquitoes from Bobo had a faster adult emergence time compared to the other sites (supplemental table 1; P<0.05).
4.2. Adult’s Body Size and Aedes aegypti Morphotypes
Mosquitoes that emerged during immature development time were removed and sexed on daily basis and their wing length was measured. The mean adult’s body size was 2.53±0.33; 2.57±0.36; 2.56± 0.35 and 2.48± 0.34 mm respectively for Bobo, Dori, 1200 logts and Toud. Mosquitoes from Dori had the biggest size, while those from Toud had smaller bodies. There was a significant difference between the body size of mosquitoes from Dori and Toud (P=0.03), while mosquitoes from the three urban sites across the different ecological zones did not show any difference in their body size (P>0.05).
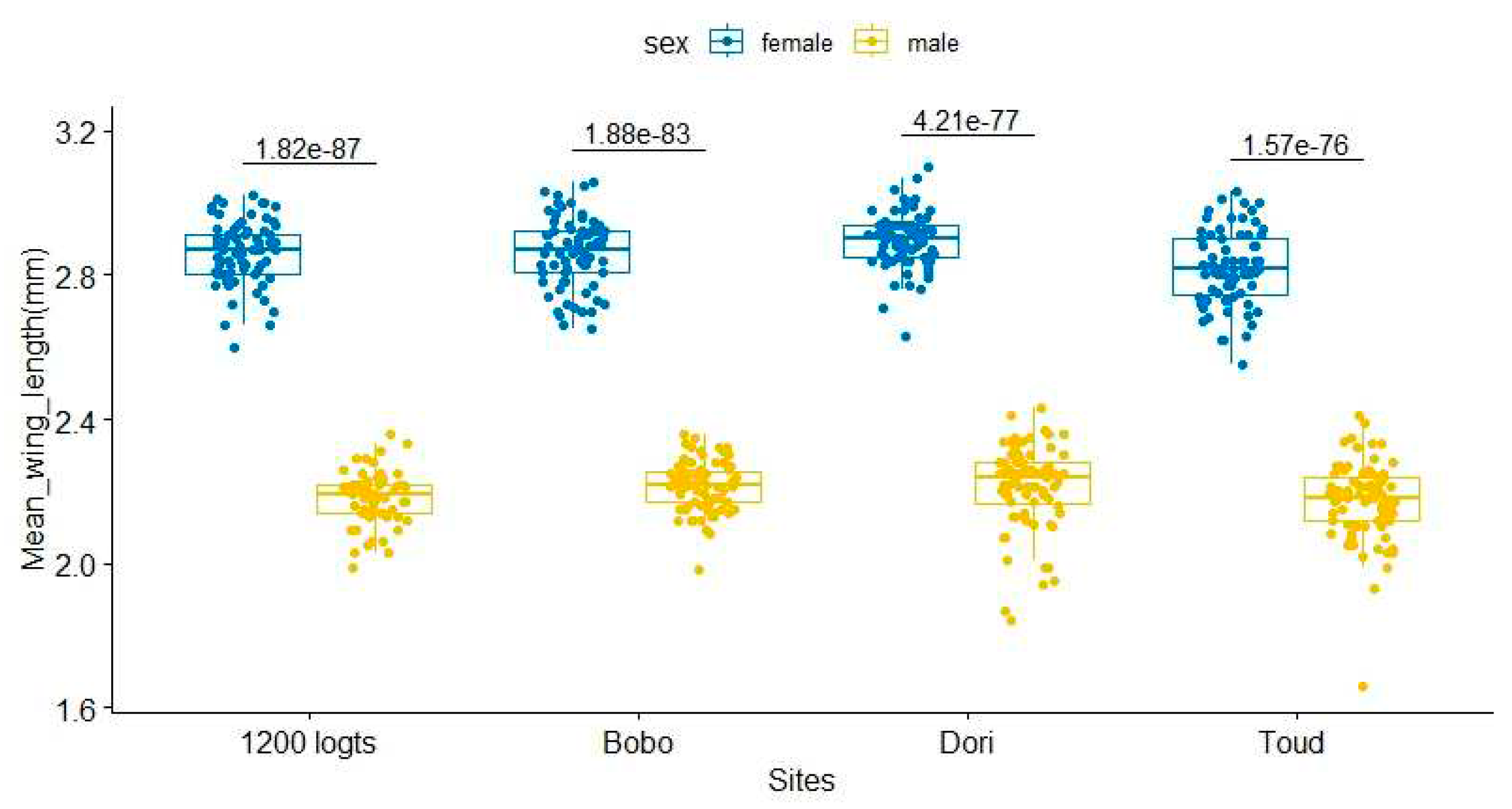
In all the study sites, we found a sexual dimorphism with a significant difference in body size between males and females. The mean body size of the females was higher (2.87±0.09; 2.90±0.07; 2.82±0.106; 2.86±0.08 mm) compared to that of the males respectively in Bobo, Dori, 1200 logts and Toud (2.22± 0.008; 2.22± 0.13; 2.18± 0.108; 2.19± 0.07 mm), (Figure 5). We observed that most of the adult mosquitoes emerged in three days intervals, therefore we decided to compare the wings of these mosquitoes by sex and days. When compared per emergence day within the site, no significant difference was found in body size during the different emergence days in the sites of Bobo and Dori. However, in 1200 logts and Toud, male and female body size was significantly different according to days post-emergence (
Table 1). Indeed, with a generalized linear model (
Table 2), the regression coefficient of wing length on emergence days, sex and sites showed an interaction between the body size, the sex and the emergence days, thus the body size was strongly influenced by the date of the mosquito emergence and the site (P<0.05). Mosquitoes emerging later had a bigger body size compared to those that emerged earlier, and males had smaller bodies than females (P<0.05).
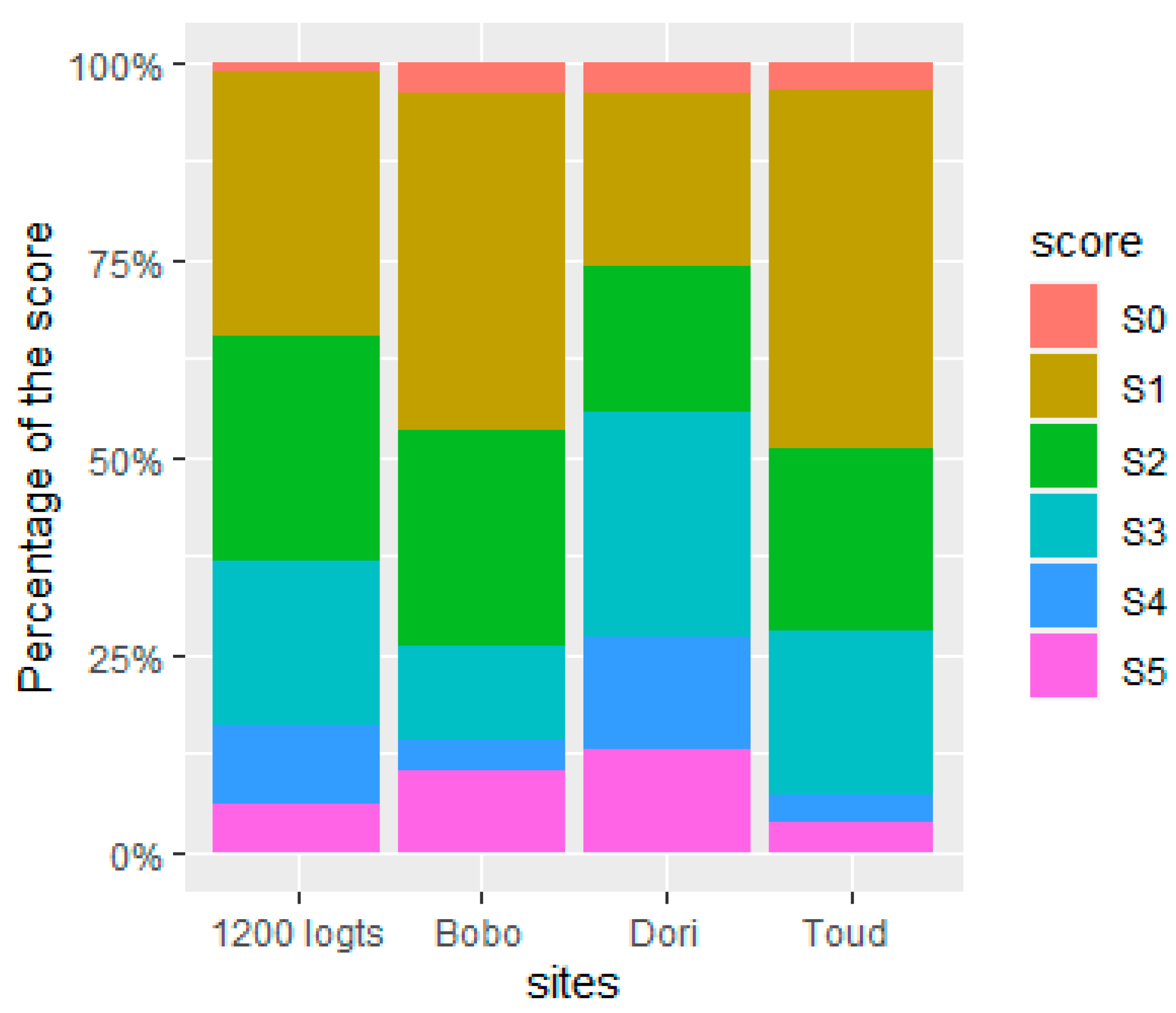
At their emergence, 317 mosquitoes (male and female) were identified to the according Ae. aegypti and Ae. formosus forms (presence or absence of the pale scale in the first abdominal tergite respectively). Out of the 317 mosquitoes, 3.2% (10/317) were identified as Ae. formosus and 96.8% (307/317) were Ae. aegypti. In the Aedes aegypti mosquitoes, score 1 was predominant with 37.1% (114/307), while mosquitoes with score 4 and 5 were less represented with 8.1% (25/307) and 8.5% (26/307), respectively. Between study sites, mosquitoes with score 1 were more predominant in three study sites, except in Dori where score 3 was most abundant. Mosquitoes with score 5 were less represented in the three sites (score 3 in Bobo).
Figure 4.
Wing length of male and female mosquitoes in the different study sites. Dots present the values of individual mosquitoes, while boxes show the position of the minimum, maximum and median values along with the position of the lower and the upper quartiles. The number on top of the boxplot indicates the p-value.
Figure 4.
Wing length of male and female mosquitoes in the different study sites. Dots present the values of individual mosquitoes, while boxes show the position of the minimum, maximum and median values along with the position of the lower and the upper quartiles. The number on top of the boxplot indicates the p-value.
Table 1.
Standardized regression coefficients of the structural equation model of effect on the wing length of the emergence days, the sex and the study sites.
Table 1.
Standardized regression coefficients of the structural equation model of effect on the wing length of the emergence days, the sex and the study sites.
| Predictors |
Estimate |
Std. error |
z-value |
Pr(>|t|) |
| Intercept |
2.861 |
0.011 |
242.22 |
< 2e-16 |
| Day [d1] |
|
|
|
|
| Day d2 |
0.014 |
0.010 |
1.39 |
0.16 |
| Day d3 |
0.03 |
0.016 |
2.25 |
0.02
|
| Sex[female] |
|
|
|
|
| Sex male |
-0.65 |
0.008 |
-74.84 |
< 2e-16 |
| Site [1200 logts] |
|
|
|
|
| Site Bobo |
0.02 |
0.01 |
1.94 |
0.05 |
| Site Dori |
-0.03 |
0.01 |
3.21 |
0.001
|
| Site Toud |
-0.01 |
0.01 |
-1.38 |
0.16 |
Table 2.
Standardized regression coefficients of the structural equation model of the fecundity on the wing length and study sites with and without interactions effect.
Table 2.
Standardized regression coefficients of the structural equation model of the fecundity on the wing length and study sites with and without interactions effect.
| Predictors |
Estimate |
Std. error
|
z-value |
Pr(>|t|) |
| Intercept |
-149.406 |
71.948 |
-2.077 |
0.039 |
| Site [1200 logts] |
|
|
|
|
| Bobo |
-20.90 |
101.16 |
-0.20 |
0.83 |
| Dori |
163.97 |
85.93 |
1.90 |
0.05 |
| Toud |
41.69 |
92.86 |
0.44 |
0.65 |
| Wing length |
88.71 |
28.89 |
3.07 |
0.02 |
| Wing length: site[1200 logts] |
|
|
|
|
| Wing length: site Bobo |
8.56 |
40.16 |
0.21 |
0.83 |
| Wing length: site Dori |
-62.81 |
34.28 |
-1.83 |
0.06 |
| Wing length: site Toud |
-13.08 |
37.17 |
-0.35 |
0.72 |
Figure 5.
Mosquito repartition according to the extent of the white scale in the first abdominal tergite. The different scores were described above.
Figure 5.
Mosquito repartition according to the extent of the white scale in the first abdominal tergite. The different scores were described above.
4.3. Relationship between Fecundity, Longevity and Body Size
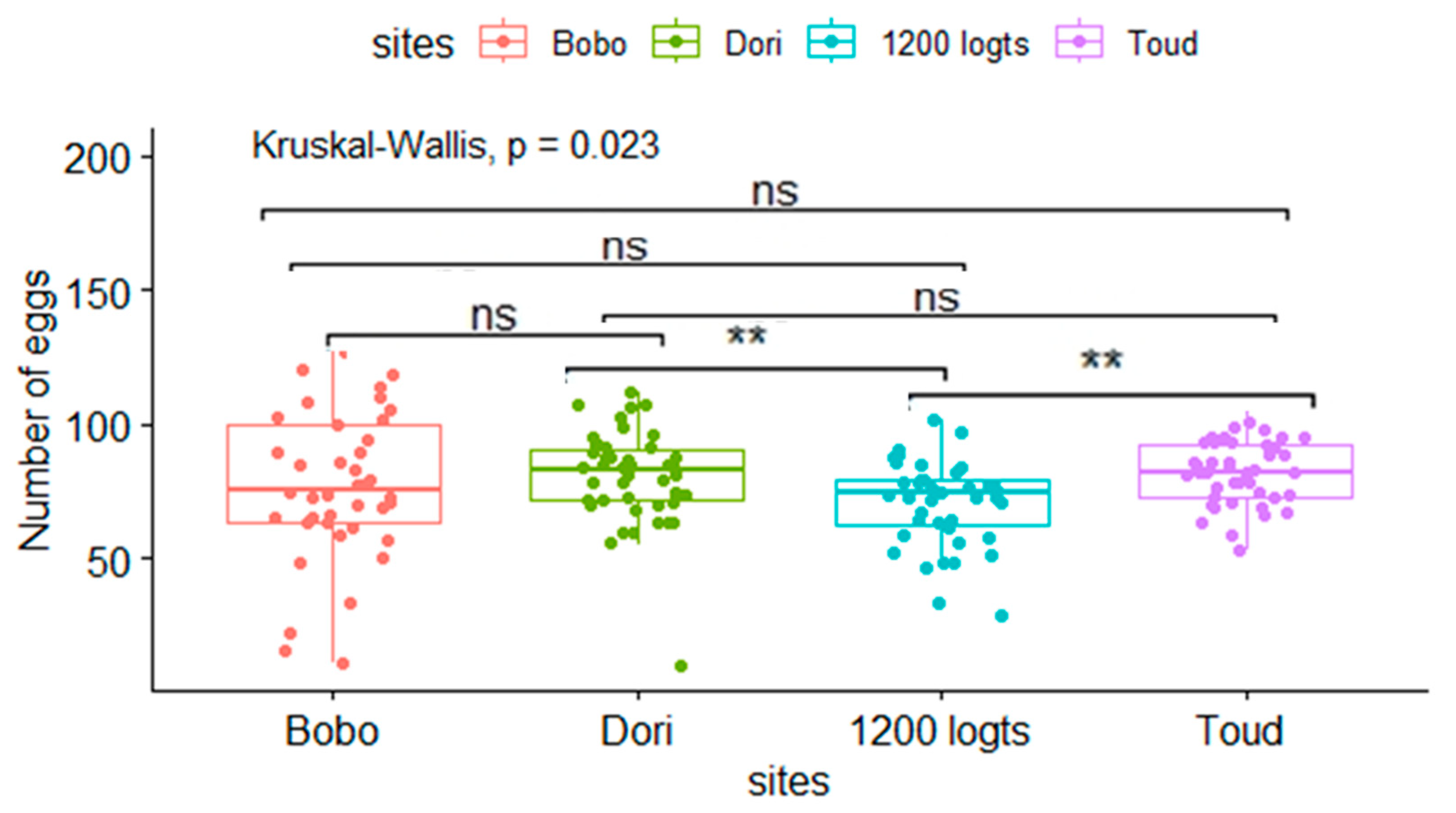
The mean fecundity of the mosquitoes was respectively 77.6 ± 28.5; 80.4 ± 17.7; 71.3 ± 17.1 and 82 ± 12 in Bobo, Dori, 1200 logts and Toud (
Figure 6). The highest fecundity was found for mosquitoes from Toud, while the lowest was found with mosquitoes from 1200 logts. There was a significant difference between 1200 logts and Toud, whereas these two sites did not show a significant difference when compared to the sites of Bobo and Dori. Fecundity was associated with wing length. There were, however, indirect effects, which differed among study sites. Nevertheless, overall no significant interaction was observed between fecundity and wing length in the different sites (P>0.05;
Table 3).
A generalized linear model showed that there was a significant two-way interaction between wing length and longevity, in the semi-urban site of Toud (
Table 3). However, in the three other sites, no significant interaction was apparent between longevity and wing length. The physiological status (fed/unfed) was a determinant with a variation in the fed and unfed ration between study sites shown by the significant interaction (P<0.05; table 5). Overall, blood-fed mosquitoes survived longer than non-blood fed (Figure 7A, additional files). Among these populations, mosquitoes from Dori had a shorter survival time, while mosquitoes from Bobo and 1200 logts had a longer survival time. The mean survival time for the unfed mosquitoes varied from 28 days (Bobo) to 32 days (Toud), while for the fed mosquitoes the mean survival varied from 30 days (Bobo) to 35 days (Toud). The sites of Bobo and Toud had the shortest and longest mean survival time for fed and unfed mosquitoes (supplemental figure 1).
Table 3.
Standardized regression coefficients of the structural equation model on the longevity on the wing length and the study sites with and without interactions effect.
Table 3.
Standardized regression coefficients of the structural equation model on the longevity on the wing length and the study sites with and without interactions effect.
| Predictors |
Estimate |
Std. error |
z-value |
Pr(>|t|)
|
| Intercept |
-19.747 |
28.74 |
-0.68 |
0.49 |
| Site [1200 logts] |
|
|
|
|
| Site Bobo |
-13.13 |
43.44 |
-0.30 |
0.76 |
| Site Dori |
19.67 |
50.14 |
0.39 |
0.69 |
| Site Toud |
107.08 |
46.04 |
2.33 |
0.02 |
| Wing length |
17.08 |
11.43 |
1.49 |
0.13 |
| Status[fed] |
|
|
|
|
| Status[unfed] |
-3.26 |
1.36 |
-2.38 |
0.01 |
| Site [1200 logts]:wing length |
|
|
|
|
| Site Bobo:winglength |
3.60 |
16.84 |
0.21 |
0.83 |
| Site Dori: wing length |
-8.20 |
20.30 |
-0.40 |
0.68 |
| Site Toud: wing length |
-43.34 |
18.61 |
-2.32 |
0.02 |
5. Discussion
This study investigated the life history traits of Aedes aegypti mosquito populations from different ecological zones of Burkina Faso. Some biological traits for populations of the three ecological zones under laboratory conditions were found to be significantly different. In the site of Bobo, the mean adult emergence time was significantly different compared to the three other sites. The difference in the adult development time in our study may be the result of an adaption to the local conditions of these populations in response to the laboratory conditions. Indeed a local adaptation to temperature and other factors has been reported in Aedes aegypti mosquitoes from different geographic origin that differed from five days in development time when reared at the same temperature (Couret et al., 2014). In accordance to this, our results showed that the effect of climatic zone and the distance between the study sites influenced the development time from larvae to adults. The development time increased from Bobo to Dori according a transect of West to North which corresponds to the tree ecological zone of the country. It has been reported that delay in larval development can result into bigger adults (Ezeakacha & Yee, 2019; Bong et al., 2021). This can be explained by the hypothesis that during the development time, male mosquitoes emerge earlier than females that have a bigger body (Ezeakacha & Yee, 2019). However, when we considered only the female Aedes aegypti mosquito population, we did not find a significant difference in their body size at different emergence days post-emergence (Bong et al., 2021). In the different study sites, the body size of mosquitoes presented significant differences with bigger mosquitoes in the site of the north. This can be the result of the adaption ability of Ae. aegypti to extreme conditions in the environment (Mohammed & Chadee, 2011).
The average of the fecundity of Aedes aegypti from the different sites in Burkina Faso reared under laboratory conditions varied from 75 to 80 eggs laid per female. Similar results were previously reported by Carrington et al., 2013 who showed that the mean fecundity of Aedes aegypti mosquitoes reared at under laboratory temperature of 26ºC, was nearly 80 eggs per female. In addition, it was shown that the bigger the body size of the mosquito, the higher the number of eggs it lays (Zirbel et al., 2018), and this may lead to an increase in mosquito density. Our results showed a positive correlation between mosquito fecundity and body size (Yan et al., 2021); however, this relation was not strong enough to conclude that bigger mosquitoes lay higher number of eggs.
It has been reported that mosquitoes with bigger bodies live longer (Lima et al., 2003; Alto et al., 2015), take a larger blood meal and have higher total fecundity than a smaller mosquito (Zirbel et al., 2018; Yan et al., 2021), because larger mosquitoes accumulate higher teneral reserves during their larval development. However, after an initial blood meal survival may no longer be associated with size (Barreaux et al., 2018). Alto et al., 2008 found that body size can alter the susceptibility of Aedes aegypti and Aedes albopictus mosquitoes to dengue virus infection and dissemination. Smaller-size female mosquitoes were significantly more likely to become infected and disseminate virus than larger individuals. According to Alto et al., 2008 this result may be a body mass phenomenon, because in large mosquitoes more tissue was available for virus than small mosquitoes. However, in Ae triseriatus infected with La Crosse virus, larger mosquitoes showed a lower rate of disseminated infection than smaller ones. This phenomenon could be linked to the dynamics of the virus to infect mosquito midgut and its dissemination (Paulson et Hawley, 1991).
Mosquitoes from the different study sites did not show a significant difference in their longevity when reared at a constant temperature. Our results showed that mosquitoes that fed on 10% sugar solution survived nearly as well as those given a single blood meal (Yan et al., 2021). Other studies reported a similar longevity in Aedes aegypti mosquitoes fed on 10% sugar solution and single blood-fed individuals (Joy et al., 2010). Taken together, Aedes aegypti survived at least 30 days (mean survival time), which is long enough for parasite or virus incubation, transmission and the production of more eggs. Indeed, it has been reported that increased survival allows the vector to produce more offspring, to increase the chance of them becoming infected, to survive long enough to become infectious and to deliver more infective bites during the remainder of its lifetime (Brady et al., 2013).
Mosquito size and longevity are important factors in vectorial capacity, as vectors must survive long enough to transmit pathogens. The relationship between body size and longevity suggests that larger mosquitoes survive longer, as they accumulated teneral reserves during immature development. Our results showed a positive and negative relationship between body size and longevity in sugar-fed and single blood-fed mosquitoes, respectively. The positive relationship between body size and longevity could be the result of several biotic and abiotic factors, like food or temperature (Barreaux et al., 2018). Indeed a positive correlation between body size and longevity has been reported in several studies (Alto et al., 2015; Gutiérrez et al., 2020). On the other hand, a negative correlation between body size and longevity was reported as well in Aedes aegypti mosquitoes (Joy et al., 2010). This negative relation might be the effect of a physiological trade-off.
6. Conclusions
This study reports for the first time on the life history traits of Aedes aegypti mosquito populations from different ecological zones of Burkina Faso. The life history traits of mosquitoes are important to understand the epidemiology of mosquito-borne diseases. The development time, fecundity, survival and body size have an important impact on vector dynamics, disease transmission, vector density and the transmission of pathogens. Our study found that the pupation rate did not differ significantly between populations from different ecological zones when reared in laboratory conditions. Similarly, the fecundity under laboratory conditions was not affected by local effects. The relationship between mosquito fecundity, survival and body size was not strong enough to conclude that larger mosquitoes lay a higher number of eggs and survive longer. However, given the complex interactions between biotic and abiotic factors in mosquito life history traits, it would be better to understand the effect of factors like food and different temperature regimens, as mosquito populations are suggested to daily fluctuating temperature and competition for food availability in their different breeding sites. Given the variability of dengue virus infections in Burkina Faso, studying the life history traits of Aedes aegypti mosquitoes in different ecological zones of Burkina Faso could help to understand the epidemiology of dengue virus and of other mosquito-borne viruses that may (re-) emerge in the country.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
A.S contributed to the mosquito collection, the analysis, interpretation of data and prepared the original draft; L.J reviewed the manuscript; A.A.M contributed to the mosquito collection; L.D reviewed and edited the manuscript and A.B reviewed, edited and supervised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a global Minds Scholarship (VLIR-UOS).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alto, B. W., Bettinardi, D. J., & Ortiz, S. (2015). Interspecific larval competition differentially impacts adult survival in dengue vectors. Journal of Medical Entomology, 52(2), 163–170. [CrossRef]
- Alto, B. W., Reiskind, M. H., & Lounibos, L. P. (2008). Size alters susceptibility of vectors to dengue virus infection and dissemination. American Journal of Tropical Medicine and Hygiene, 79(5), 688–695. [CrossRef]
- Andrea Arevalo-Cortes, Granada, Y., Torres, D., & Triana-chavez, O. (2022). Differential Hatching, Development, Oviposition, and Longevity Patterns among Colombian Aedes aegypti Populations. Insects. [CrossRef]
- Arrese, E. L., & Soulages, J. L. (2010). Insect fat body: Energy, metabolism, and regulation. Annual Review of Entomology, 55, 207–225. [CrossRef]
- Badolo, A., Sombie, A., Pignatelli, P., Yaméogo, F., Sanon, A., Wangrawa, W. D., Kanuka, H., Weetman, D., & McCall, P. J. (2019). Baseline data on the bionomics of Aedes aegypti to support dengue control strategies in Burkina Faso. International Journal of Infectious Diseases, 79(2022), 14. [CrossRef]
- Barreaux, A. M. G., Barreaux, P., & Koella, J. C. (2016). Overloading the immunity of the mosquito Anopheles gambiae with multiple immune challenges. Parasites and Vectors, 9(1), 1–4. [CrossRef]
- Barreaux, A. M. G., Stone, C. M., Barreaux, P., & Koella, J. C. (2018). The relationship between size and longevity of the malaria vector Anopheles gambiae (s.s.) depends on the larval environment. Parasites and Vectors, 11(1), 1–9. [CrossRef]
- Bong, L. J., Tu, W. C., & Neoh, K. B. (2021). Interpopulation variations in life history traits and reproductive tactics in Aedes aegypti: A test on populations 50 km apart. Acta Tropica, 213(November 2020), 105750. [CrossRef]
- Brady, O. J., Johansson, M. a, Guerra, C. a, Bhatt, S., Golding, N., Pigott, D. M., Delatte, H., Grech, M. G., Leisnham, P. T., Maciel-de-Freitas, R., Styer, L. M., Smith, D. L., Scott, T. W., Gething, P. W., & Hay, S. I. (2013). Modelling adult Aedes aegypti and Aedes albBrady, O. J., Johansson, M. a, Guerra, C. a, Bhatt, S., Golding, N., Pigott, D. M., … Hay, S. I. (2013). Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and fie. Parasites & Vectors, 6, 351. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3867219&tool=pmcentrez&rendertype=abstract.
- Breigel, H. (1990). Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. Journal of Insect Physiology, 36(3), 165–172.
- Brown, J. E., Mcbride, C. S., Johnson, P., Ritchie, S., Paupy, C., Bossin, H., Lutomiah, J., Fernandez-Salas, I., Ponlawat, A., Cornel, A. J., Black IV, W. C., Gorrochotegui-Escalante, N., Urdaneta-Marquez, L., Sylla, M., Slotman, M., Murray, K. O., Walker, C., & Powell, J. R. (2011). Worldwide patterns of genetic differentiation imply multiple ‘domestications’ of Aedes aegypti, a major vector of human diseases. Proceedings of the Royal Society B: Biological Sciences, 278(1717), 2446–2454. [CrossRef]
- Carrington, L. B., Armijos, M. V., Lambrechts, L., Barker, C. M., & Scott, T. W. (2013). Effects of Fluctuating Daily Temperatures at Critical Thermal Extremes on Aedes aegypti Life-History Traits. PLoS ONE, 8(3). [CrossRef]
- Couret, J., Dotson, E., & Benedict, M. Q. (2014). Temperature, larval diet, and density effects on development rate and survival of Aedes aegypti (Diptera: Culicidae). PLoS ONE, 9(2). [CrossRef]
- Ezeakacha, N. F., & Yee, D. A. (2019). The role of temperature in affecting carry-over effects and larval competition in the globally invasive mosquito Aedes albopictus. Parasites and Vectors, 12(1), 1–11. [CrossRef]
- Gloria-Soria, A., Ayala, D., Bheecarry, A., Calderon-Arguedas, O., Chadee, D. D., Chiappero, M., Coetzee, M., Elahee, K. Bin, Fernandez-Salas, I., Kamal, H. A., Kamgang, B., Khater, E. I. M., Kramer, L. D., Kramer, V., Lopez-Solis, A., Lutomiah, J., Martins, A., Micieli, M. V., Paupy, C., … Powell, J. R. (2016). Global genetic diversity of Aedes aegypti. Molecular Ecology, 25(21), 5377–5395. [CrossRef]
- Gutiérrez, E. H. J., Walker, K. R., Ernst, K. C., Riehle, M. A., & Davidowitz, G. (2020). Size as a proxy for survival in Aedes aegypti (Diptera: Culicidae) mosquitoes. Journal of Medical Entomology, 57(4), 1228–1238. [CrossRef]
- Huang, Y. J. S., Higgs, S., & Vanlandingham, D. L. (2019). Arbovirus. Frontiers in Microbiology, 10(JAN), 1–14. [CrossRef]
- Joy, T. K., A. J. Arik, V. Corby-Harris, A. A. Johnson, and M. A. R. (2010). The impact of larval and adult dietary restriction on lifespan, re_production and growth in the mosquito Aedes aegypti. NIH Public Access. Exp. Gerontol., 45, 685–690. [CrossRef]
- Lima, C. A., Almeida, W. R., Hurd, H., & Albuquerque, C. M. R. (2003). Reproductive aspects of the mosquito Culex quinquefasciatus (Diptera:Culicidae) infected with Wuchereria bancrofti (Spirurida: Onchocercidae). Memorias Do Instituto Oswaldo Cruz, 98(2), 217–222. [CrossRef]
- Lounibos LP. (2002). Invasions by insect vectors of human disease. Annu Rev Entomol, 47:, 233–266. [CrossRef]
- Martins, A. J., Dutra, C., Bellinato, D. F., & Lima, B. P. (2012). Effect of Insecticide Resistance on Development , Longevity and Reproduction of Field or Laboratory Selected Aedes aegypti Populations. PLoS One, 7(3), 1–9. [CrossRef]
- Mayer, S.V., Tesh, R.B., Vasilakis, N. (2017). The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Tropica, 166, 155–163. [CrossRef]
- Mohammed, A., & Chadee, D. D. (2011). Effects of different temperature regimens on the development of Aedes aegypti (L.) (Diptera: Culicidae) mosquitoes. Acta Tropica, 119(1), 38–43. [CrossRef]
- Ouattara, Lissy, Parfait, E., Namountougou, M., Hien, A., Ouari, A., Bonnet, E., & Fournet, F. (2019). Surveys of Arboviruses Vectors in Four Cities Stretching Along a Railway Transect of Burkina Faso : Risk Transmission and Insecticide Susceptibility Status of Potential Vectors. Frontiers in Veterinary Science, 6(May), 1–9. [CrossRef]
- P. T. LEISNHAM, L. M. SALA, and S. . A. J. (2008). Geographic Variation in Adult Survival and Reproductive Tactics of the Mosquito Aedes albopictus. J Med Entomol. 2008 March ; 45(2): 210–221, 23(1), 1–7. [CrossRef]
- Paulson, S. L., & Hawley, W. A. (1991). Effect of body size on the vector competence of field and laboratory populations of Aedes triseriatus for La Crosse virus. Journal of the American Mosquito Control Association, 7(2), 170–175.
- Price, D. P., Schilkey, F. D., Ulanov, A., & Hansen, I. A. (2015). Small mosquitoes, large implications: Crowding and starvation affects gene expression and nutrient accumulation in Aedes aegypti. Parasites and Vectors, 8(1), 1–14. [CrossRef]
- Robert V, Lhuillier M, Meunier D, Sarthou JL, Monteny N, Digoutte JP, C., & M, Germain M, C. R. (1990). [Yellow fever virus, dengue 2 and other arboviruses isolated from mosquitos, in Burkina Faso, from 1983 to 1986. Entomological and epidemiological considerations]. Bull Société Pathol Exotss, 1993;86 (2, 90–100.
- Rui De Xue, John D. Edman, T. W. S. (1995). Age and Body Size Effects on Blood Meal Size and Multiple Blood Feeding by Aedes aegypti (Diptera: Culicidae),. Journal of Medical Entomology, Volume 32,(Issue 4), Pages 471–474,. [CrossRef]
- Sasmita, H. I., Tu, W. C., Bong, L. J., & Neoh, K. B. (2019). Effects of larval diets and temperature regimes on life history traits , energy reserves and temperature tolerance of male Aedes aegypti ( Diptera : Culicidae ): optimizing rearing techniques for the sterile insect programmes. Parasites & Vectors, 1–16. [CrossRef]
- Scott C. Weaver. (2014). Arrival of chikungunya virus in the new world: prospects for spread and impact on public health. PLOS Neglected Tropical Diseases 8:E2921. [CrossRef]
- Yan, J., Kibech, R., & Stone, C. M. (2021). Differential effects of larval and adult nutrition on female survival, fecundity, and size of the yellow fever mosquito, Aedes aegypti. Frontiers in Zoology, 18(1), 1–9. [CrossRef]
- Zirbel, K., Eastmond, B., & Alto, B. W. (2018). Parental and offspring larval diets interact to influence life-history traits and infection with dengue virus in Aedes aegypti. Royal Society Open Science, 5(7). [CrossRef]
- Zoure, A. A., Sare, A. R., Yameogo, F., Somda, Z., Massart, S., Badolo, A., & Francis, F. (2020). Bacterial communities associated with the midgut microbiota of wild Anopheles gambiae complex in Burkina Faso. Molecular Biology Reports, 47(1), 211–224. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).