Submitted:
01 December 2023
Posted:
01 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
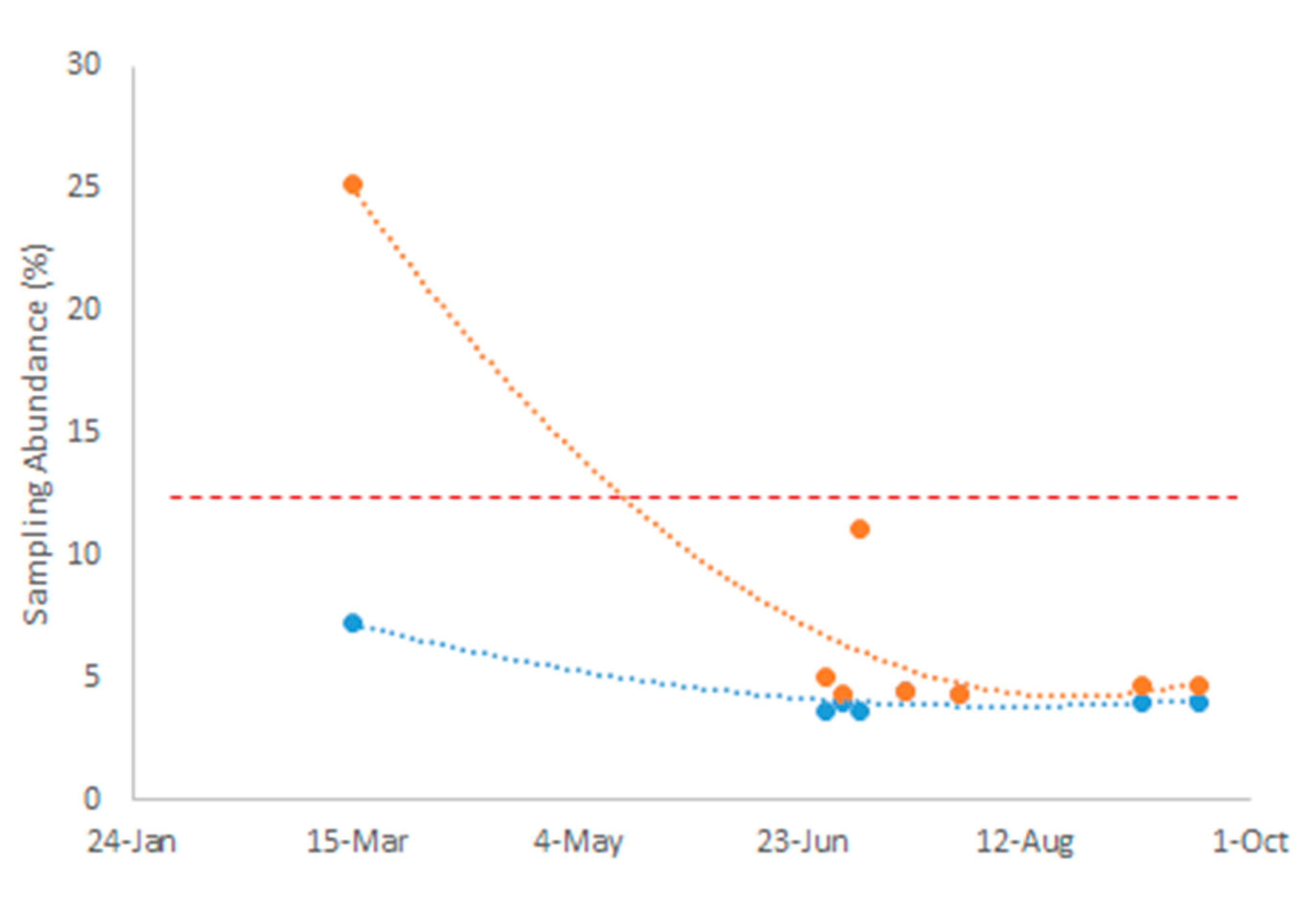
2.1. Mosquito Collection
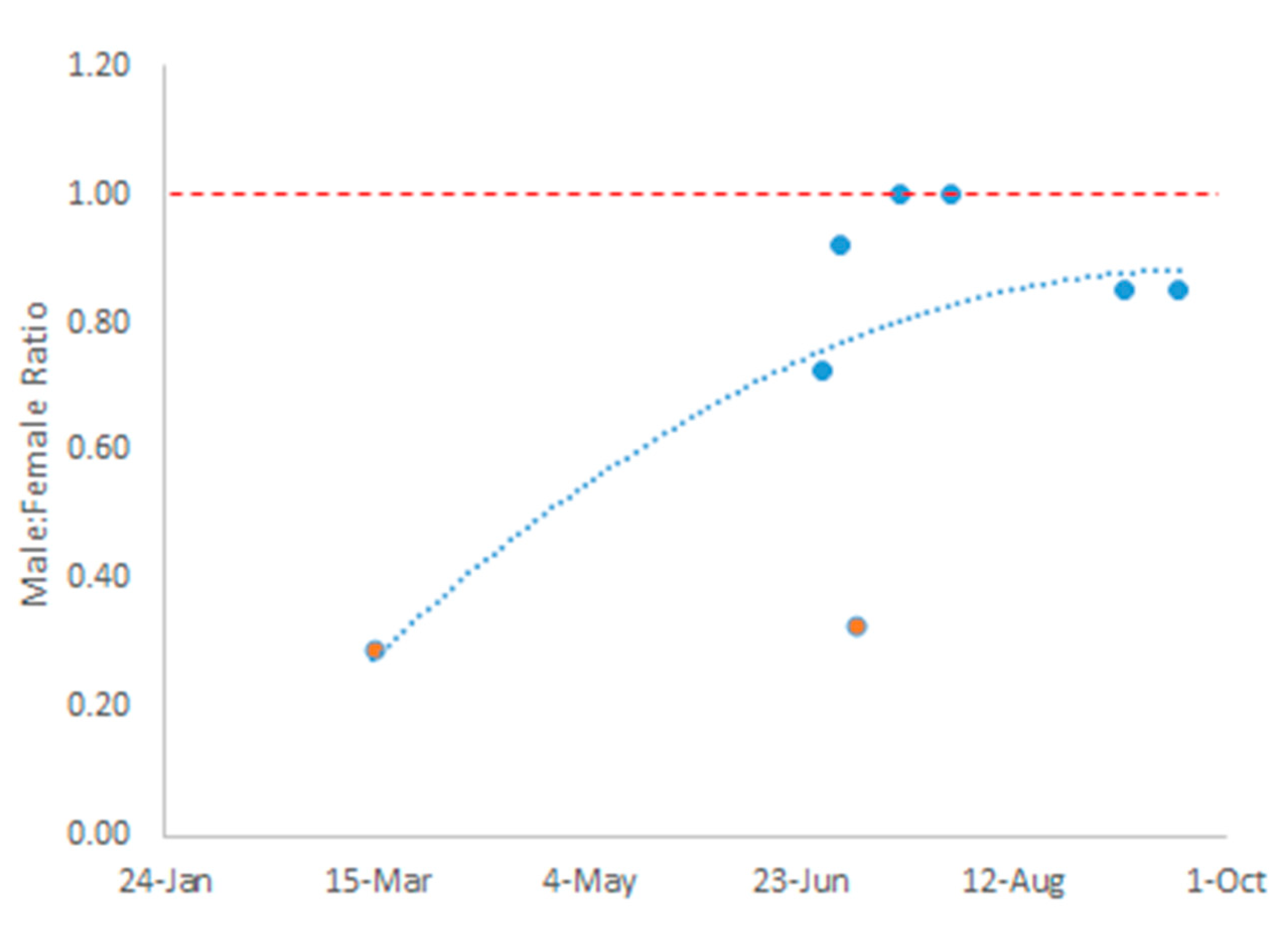
2.2. Sex Ratio Determination
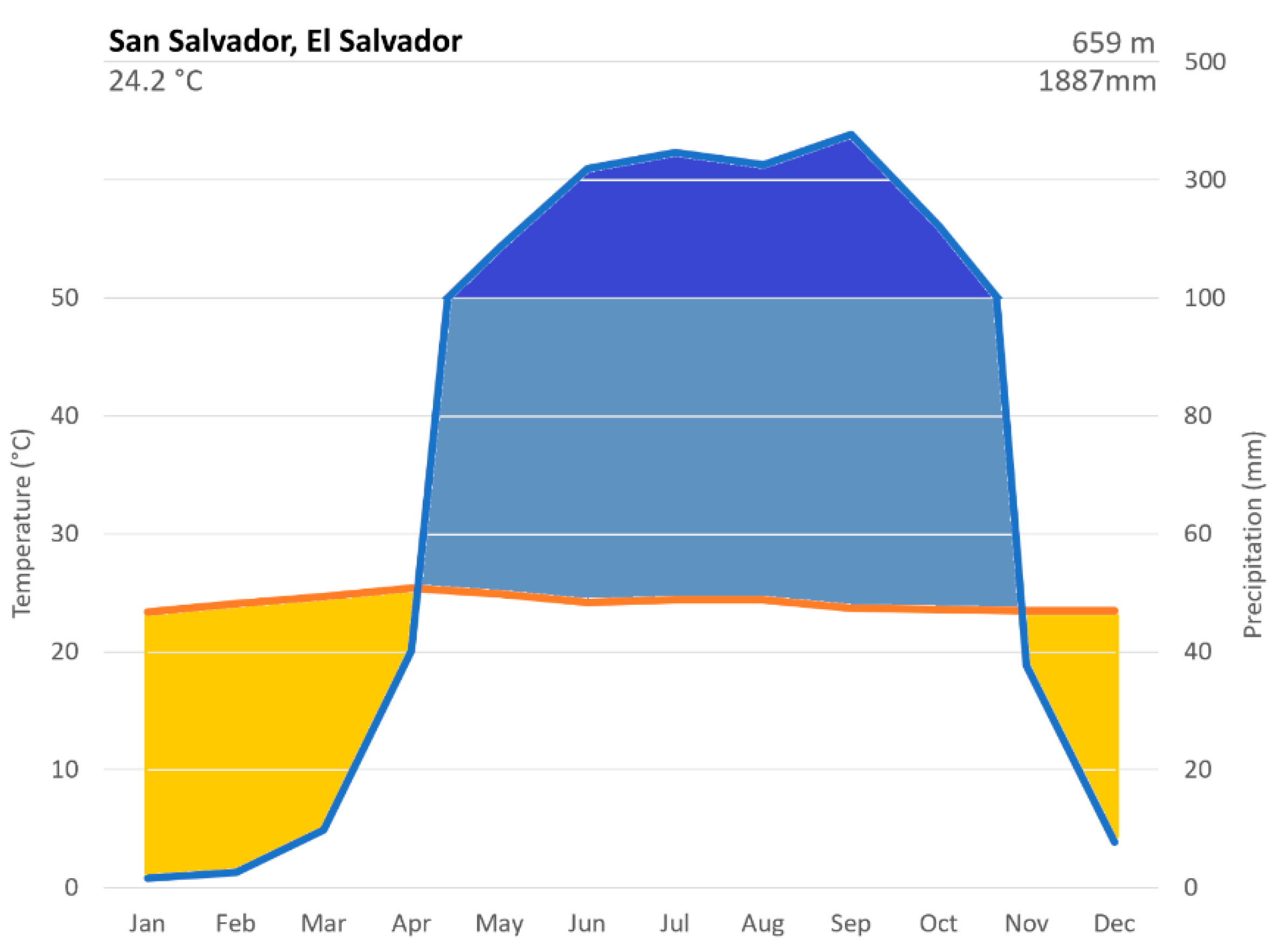
2.3. Climate Diagram
3. Results
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Organization, W.H. Vector-borne diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 28 November 2023).
- Guzman, M.G.; Gubler, D.J.; Izquierdo, A.; Martinez, E.; Halstead, S.B. Dengue infection. Nat Rev Dis Primers 2016, 2, 16055. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.L.; Courtenay, O.; Kelly-Hope, L.A.; Scott, T.W.; Takken, W.; Torr, S.J.; Lindsay, S.W. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis 2020, 14, e0007831. [Google Scholar] [CrossRef]
- Pimenta, P.F.; Touray, M.; Miller, L. The journey of malaria sporozoites in the mosquito salivary gland. J Eukaryot Microbiol 1994, 41, 608–624. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J.; Ooi, E.E.; Vasudevan, S.; Farrar, J. Dengue and Dengue Hemorrhagic Fever, 2nd Edition; CABI: 2014.
- Charnov, E.L. The Theory of Sex Allocation; Princeton University Press: 1982.
- Hamilton, W.D. Extraordinary sex ratios. A sex-ratio theory for sex linkage and inbreeding has new implications in cytogenetics and entomology. Science 1967, 156, 477–488. [Google Scholar] [CrossRef]
- Fisher, R.A. The genetical theory of natural selection: a complete variorum edition; Oxford University Press: 1999.
- A. W. F. Edwards. Natural Selection and the Sex Ratio: Fisher’s Sources. The American Naturalist 1998, 151, 564–569. [CrossRef]
- Andrew Olaf Shelton. The Ecological and Evolutionary Drivers of Female-Biased Sex Ratios: Two-Sex Models of Perennial Seagrasses. The American Naturalist 2010, 175, 302–315. [Google Scholar] [CrossRef] [PubMed]
- Pérez, I.d.L.G.; Carretero, M.A.; Font, E. Intensity of male-male competition predicts morph diversity in a color polymorphic lizard. Evolution 2017, 71, 1832–1840. [Google Scholar] [CrossRef]
- Barrett, S.C.; Yakimowski, S.B.; Field, D.L.; Pickup, M. Ecological genetics of sex ratios in plant populations. Philos Trans R Soc Lond B Biol Sci 2010, 365, 2549–2557. [Google Scholar] [CrossRef] [PubMed]
- Vergés, A.; Paul, N.A.; Steinberg, P.D. Sex and life-history stage alter herbivore responses to a chemically defended red alga. Ecology 2008, 89, 1334–1343. [Google Scholar] [CrossRef]
- Groat-Carmona, A.M.; Orozco, S.; Friebe, P.; Payne, A.; Kramer, L.; Harris, E. A novel coding-region RNA element modulates infectious dengue virus particle production in both mammalian and mosquito cells and regulates viral replication in Aedes aegypti mosquitoes. Virology 2012, 432, 511–526. [Google Scholar] [CrossRef]
- Groat-Carmona, A.M.; Kain, H.; Brownell, J.; Douglass, A.N.; Aly, A.S.I.; Kappe, S.H.I. A Plasmodium α/β-hydrolase modulates the development of invasive stages. Cellular Microbiology 2015, 17, 1848–1867. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Callahan, A.G.; Yang, Q.; Jasper, M.; Arif, M.A.K.; Afizah, A.N.; Nazni, W.A.; Hoffmann, A.A. An elusive endosymbiont: Does Wolbachia occur naturally in Aedes aegypti? Ecology and Evolution 2020, 10, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Xiong, J.; Lu, D.; Wang, G.; Ai, H. Transcriptome Sequencing Analysis and Functional Identification of Sex Differentiation Genes from the Mosquito Parasitic Nematode, Romanomermis wuchangensis. PLOS ONE 2016, 11, e0163127. [Google Scholar] [CrossRef]
- Simberloff, D. Risks of biological control for conservation purposes. BioControl 2012, 57, 263–276. [Google Scholar] [CrossRef]
- Holdridge, L.R. Determination of World Plant Formations From Simple Climatic Data. Science 1947, 105, 367–368. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Scientific Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- Bullock, S.H.; Mooney, H.A.; Medina, E. Seasonally Dry Tropical Forests; Cambridge University Press: Cambridge, 1995. [Google Scholar]
- Dexter, K.G.; Pennington, R.T.; Oliveira-Filho, A.T.; Bueno, M.L.; Silva de Miranda, P.L.; Neves, D.M. Inserting Tropical Dry Forests Into the Discussion on Biome Transitions in the Tropics. Frontiers in Ecology and Evolution 2018, 6. [Google Scholar] [CrossRef]
- Pablo-Cea, J.D.; Velado-Cano, M.A.; Noriega, J.A. A first step to evaluate the impact of ecotourism on biodiversity in El Salvador: a case study using dung beetles in a National Park. Journal of Ecotourism 2021, 20, 51–69. [Google Scholar] [CrossRef]
- Szabó, B.; Korányi, D.; Gallé, R.; Lövei, G.L.; Bakonyi, G.; Batáry, P. Urbanization decreases species richness, and increases abundance in dry climates whereas decreases in wet climates: A global meta-analysis. Science of The Total Environment 2023, 859, 160145. [Google Scholar] [CrossRef]
- Slominski, A.H.; Burkle, L.A. Solitary bee life history traits and sex mediate responses to manipulated seasonal temperatures and season length. Frontiers in Ecology and Evolution 2019, 7, 314. [Google Scholar] [CrossRef]
- Vides-Hernández, G.L.; Velado-Cano, M.A.; Pablo-Cea, J.D.; Carmona-Galindo, V.D. Patrones de riqueza y diversidad de aves en áreas verdes del centro urbano de San Salvador, El Salvador. Huitzil 2017, 18, 272–280. [Google Scholar] [CrossRef]
- de Araújo, H.R.C.; Kojin, B.B.; Capurro, M.L. Sex determination and Aedes population control. Parasites & Vectors 2018, 11, 644. [Google Scholar] [CrossRef] [PubMed]
- Shettima, A.; Joseph, S.; Ishak, I.H.; Abdul Raiz, S.H.; Abu Hasan, H.; Othman, N. Evaluation of Total Female and Male Aedes aegypti Proteomes Reveals Significant Predictive Protein–Protein Interactions, Functional Ontologies, and Differentially Abundant Proteins. Insects 2021, 12, 752. [Google Scholar] [CrossRef] [PubMed]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Scientific Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Robinson, A.S. Sex-ratio manipulation in relation to insect pest control. Annu Rev Genet 1983, 17, 191–214. [Google Scholar] [CrossRef]
- Benelli, G.; Jeffries, C.L.; Walker, T. Biological Control of Mosquito Vectors: Past, Present, and Future. Insects 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Lu, L.; Fu, G.; Zhong, S.; Ding, G.; Xu, R.; Zhu, G.; Shi, N.; Fan, F.; Liu, Q. Epidemiology and vector efficiency during a dengue fever outbreak in Cixi, Zhejiang Province, China. Journal of vector ecology 2009, 34, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, E. The sex structure of the larval populations of the urban mosquito Culex pipiens pipiens f. molestus Forskal (Diptera, Culicidae) in St. Petersburg. Entomological review 2011, 91, 729–734. [Google Scholar] [CrossRef]
- Mwingira, V.; Mboera, L.E.; Dicke, M.; Takken, W. Exploiting the chemical ecology of mosquito oviposition behavior in mosquito surveillance and control: a review. Journal of Vector Ecology 2020, 45, 155–179. [Google Scholar] [CrossRef]
- Gubler, D.J. The partnership for dengue control - a new global alliance for the prevention and control of dengue. Vaccine 2015, 33, 1233. [Google Scholar] [CrossRef]
- Lance, D.; McInnis, D. Biological basis of the sterile insect technique. Sterile Insect Technique 2021, 113–142. [Google Scholar]
- Hendrichs, J.; Robinson, A. Sterile insect technique. In Encyclopedia of insects; Elsevier: 2009; pp. 953–957.
- Benedict, M.Q.; Robinson, A.S. The first releases of transgenic mosquitoes: an argument for the sterile insect technique. Trends in parasitology 2003, 19, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Jayachandran, S.; Prabagaran, S.R. Evidence for the natural occurrence of Wolbachia in Aedes aegypti mosquitoes. FEMS microbiology letters 2019, 366, fnz055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zheng, X.; Xi, Z.; Bourtzis, K.; Gilles, J.R. Combining the sterile insect technique with the incompatible insect technique: I-impact of Wolbachia infection on the fitness of triple-and double-infected strains of Aedes albopictus. PloS one 2015, 10, e0121126. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Moreira, L.A. Can Wolbachia be used to control malaria? Memórias do Instituto Oswaldo Cruz 2011, 106, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, D.; Li, Y.; Yang, C.; Wu, Y.; Liang, X.; Liang, Y.; Pan, X.; Hu, L.; Sun, Q. Incompatible and sterile insect techniques combined eliminate mosquitoes. Nature 2019, 572, 56–61. [Google Scholar] [CrossRef]
- Zhang, D.; Lees, R.S.; Xi, Z.; Bourtzis, K.; Gilles, J.R. Combining the sterile insect technique with the incompatible insect technique: III-robust mating competitiveness of irradiated triple Wolbachia-infected Aedes albopictus males under semi-field conditions. PloS one 2016, 11, e0151864. [Google Scholar] [CrossRef] [PubMed]
- Jawara, M.; Pinder, M.; Drakeley, C.J.; Nwakanma, D.C.; Jallow, E.; Bogh, C.; Lindsay, S.W.; Conway, D.J. Dry season ecology of Anopheles gambiae complex mosquitoes in The Gambia. Malaria Journal 2008, 7, 1–9. [Google Scholar] [CrossRef]
- Minakawa, N.; Githure, J.I.; Beier, J.C.; Yan, G. Anopheline mosquito survival strategies during the dry period in western Kenya. Journal of medical entomology 2001, 38, 388–392. [Google Scholar] [CrossRef]
- Charlwood, J.; Vij, R.; Billingsley, P. Dry season refugia of malaria-transmitting mosquitoes in a dry savannah zone of east Africa. The American journal of tropical medicine and hygiene 2000, 62, 726–732. [Google Scholar] [CrossRef]
- Animut, A.; Negash, Y. Dry season occurrence of Anopheles mosquitoes and implications in Jabi Tehnan District, west Gojjam Zone, Ethiopia. Malaria Journal 2018, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bank, W. Population density in El Salvador from 2011 to 2021 (in number of inhabitants per square kilometer). Available online: https://www-statista-com.laverne.idm.oclc.org/statistics/882993/population-density-el-salvador/ (accessed on 25 November 2023).
- Nazari, B.; Keshavarz, M. Water population density: Global and regional analysis. Theoretical and Applied Climatology 2023, 1–15. [Google Scholar] [CrossRef]
- Fernández, D.M., Alfredo; Sarmanto, Natalia. Diagnóstico de la prestación de los servicios de agua potable y alcantarillado en El Salvador.; 2023; p. 25 p.
- Joyce, A.L.; Torres, M.M.; Torres, R.; Moreno, M. Genetic variability of the Aedes aegypti (Diptera: Culicidae) mosquito in El Salvador, vector of dengue, yellow fever, chikungunya and Zika. Parasites & vectors 2018, 11, 1–14. [Google Scholar]
- Choi, J.; Carmona-Galindo, V.; Paredes, G.R.; Marín Recinos, M.F.; de Abrego, V.C. An exploration of land use and poverty as an integrative model for mitigating Chagas disease in El Salvador. BIOS 2020, 91, 125–132, 128. [Google Scholar] [CrossRef]
- Benelli, G. Managing mosquitoes and ticks in a rapidly changing world–Facts and trends. Saudi Journal of Biological Sciences 2019, 26, 921–929. [Google Scholar] [CrossRef]
- Ferraguti, M.; Martínez-de La Puente, J.; Roiz, D.; Ruiz, S.; Soriguer, R.; Figuerola, J. Effects of landscape anthropization on mosquito community composition and abundance. Scientific reports 2016, 6, 29002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




