1. Introduction:
Cardiovascular disease (CVD) serves as an umbrella term encompassing various heart and blood vessel-related conditions, including coronary artery disease [
1], hypertension, cerebrovascular disease, peripheral vascular disease (PVD), cardiomyopathies, and valvular heart disease [
2]. The predominant cause of CVD is atherosclerosis, a progressive condition marked by the accumulation of lipids and fibrins within artery walls, irregular immune responses, and disrupted cholesterol metabolism. Alarmingly, CVD contributes to nearly one-third of global fatalities and stands as the leading cause of death in developed nations. Currently, more than 30% of Americans are affected by some form of CVD. In European Society of Cardiology (ESC) member countries, ischemic heart disease is responsible for 45% of female and 39% of male CVD-related deaths. Notably, CVD surpasses cancer in causing fatalities when combined for both sexes in ESC member countries [
3].
The proportion of premature deaths (70 years) attributed to CVD varies significantly between high- and low-income countries. This discrepancy is particularly pronounced among women, with approximately 36% of premature deaths due to CVD in middle-income nations and 16% in high-income ones [
4]. For men, the figures stand at 36% in high-income countries and 24% in middle-income ones. Encouragingly, since 1990, age-standardized mortality rates (ASMRs) for CVD have dropped by 47% in men and 42% in women. The reduction in ASMRs has been remarkable in high-income countries, exceeding 50% for both genders. However, the decline in middle-income countries has been less pronounced, with some even witnessing an increase [
5].
Despite advancements in healthcare technology and lower mortality rates, there remains a pressing need for technological innovation in the clinical management of CVD due to the projected rise in CVD-related deaths [
5,
6]. It is widely believed that many of these fatalities could be prevented through simple lifestyle changes. This underscores the necessity for better education, comprehensive prevention initiatives, and accurate early clinical diagnosis. While invasive procedures can provide definitive diagnoses, they often come with significant risks that must be carefully considered. Consequently, there is a growing demand for precise diagnostics and safe, non-invasive imaging techniques to offer detailed insights into the onset and progression of CVD [
7].
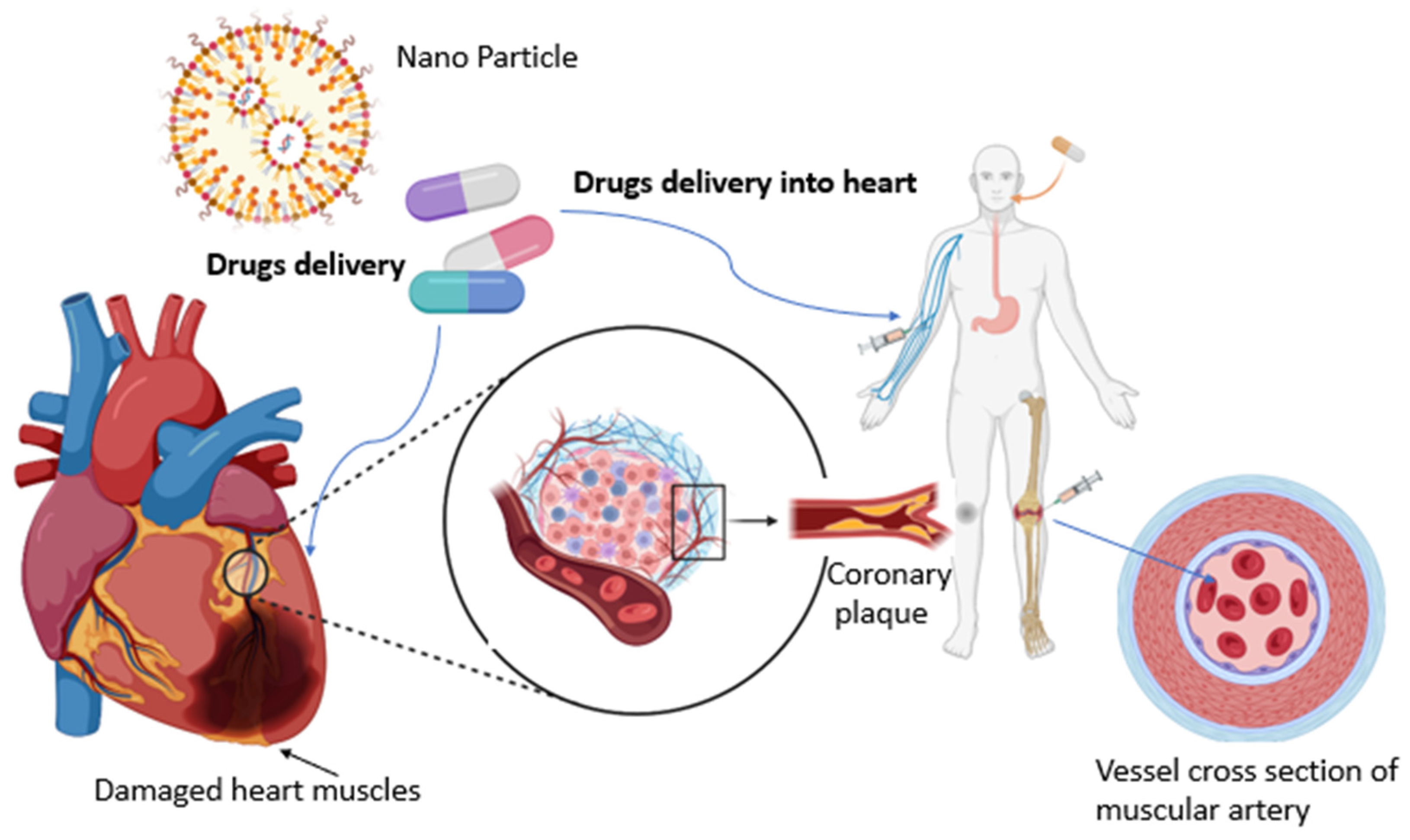
The treatment options for CVD vary significantly based on the severity of the condition, particularly for disorders with a gradual pathogenesis spanning decades. Nanotechnology, a field dedicated to manipulating matter on a nearly atomic scale to create innovative technologies, materials, and structures at nanoscale dimensions, holds promise in advancing various scientific domains, including materials science, engineering, energy, industry, and medicine. In the context of cardiovascular nanomedicine research, the focus has been on developing tailor-made nanomaterials for enhanced targeting. Nanoparticles (NPs) have gained prominence in the biomedical industry, finding applications as diagnostic tools, biosensors, and drug delivery vehicles. Over the past few decades, a plethora of nanomaterials have been engineered, with recent insights suggesting that NPs can serve as effective drug delivery carriers capable of crossing the blood-brain barrier (BBB). To facilitate the transportation of other drugs through the BBB or to function as therapeutic agents for various conditions, NPs with superior biodegradability and biocompatibility can be designed [
8]. This review aims to provide an up-to-date overview of how nanotechnology is currently employed in the diagnosis and treatment of common CVDs.
2. Research Review Methodology
In our comprehensive literature review, we conducted extensive searches across various reputable databases, including but not limited to Google Scholar, Pub_Med, Research_Gate, NCBI, Elsevier, and Wiley_Online Library. We employed a wide range of keywords, encompassing nanotechnologies, nanomedicines, nano diagnosis, as well as specific terms related to cardiovascular health such as CVD, myocardial infarction, coronary and heart disease.
3. Nano-Diagnosis of CVD:
The effectiveness of personalized therapy for cardiovascular disease (CVD) hinges on two crucial factors: early identification and precise diagnosis. However, recent research involving the use of gold nano-biosensors for CVD imaging has captured the attention of the scientific community and is poised to become a major focus in the future. The discoveries presented in this study have the potential to engage medical researchers and encourage further exploration of nano-biosensor applications in the CVD [
1,
9].
Interventional cardiology is an evolving field that has shown promise in harnessing nanotechnology as a cutting-edge, multidisciplinary approach. It is being employed in various multimodal imaging techniques, including the detection of nanoparticle-enhanced gadolinium (Gd) contrast for improved detection of atherosclerotic CVD and optical coherence tomography (OCT)/infrared luminescence [
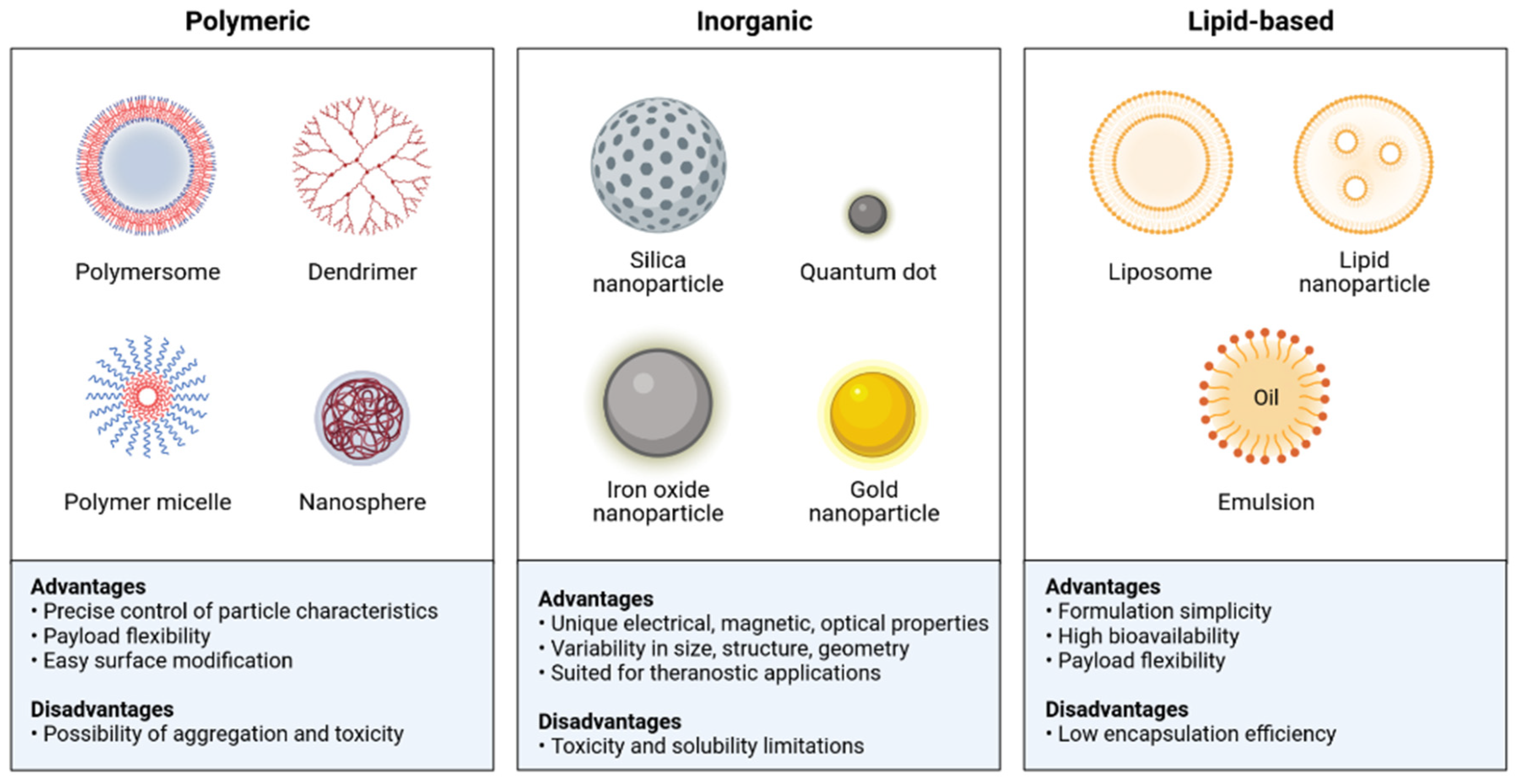
4] for characterizing coronary plaques. Here are summarized the different classes of the nanoparticles used in CVDs (
Figure 1).
In contrast to traditional metal-based contrast agents, 19F-MRI offers numerous advantages for imaging CVD, allowing for direct detection of labeled cells, ensuring clear identification and quantification. Recent advancements in 19F MRI-based in vivo cell quantification, actual clinical applications of 19F drugs, and the growing interest in cellular therapies are bringing 19F imaging technology closer to clinical adoption [
10].
Myeloid cell tracking via 19F-MRI, as demonstrated by Senders et al., is part of a multimodal hot-spot imaging technique that employs a novel high-density lipoprotein-derived nano tracer containing a perfluoro-crown ether payload (19F-HDL) [
11]. This approach enables the rapid visualization of myeloid cell migration from the bone marrow and spleen in atherosclerotic mice with myocardial infarction (MI) after administering the nano tracer. Simultaneously, the research showcased the accumulation of circulating pro-inflammatory myeloid cells in atherosclerotic plaques and at the site of MI using ex vivo methods. These myeloid cells can also be detected in vivo through positron emission tomography imaging or optical modalities when the 19-F HDL nano tracer is labeled with zirconium-89 and fluorophores [
12].
Electrochemical immunosensors play a crucial role in translating biological signals resulting from antigen-antibody interactions into electrical signals. They offer high sensitivity, rapid signal response, the ability to detect low-abundance samples, ease of use, and cost-effectiveness in biomarker detection. Chauhan et al. developed an electrochemical immunosensor for the detection of cardiac troponin I (cTnI), a biomarker for heart disease. Their sensor, based on nanostructured metal chalcogenide (molybdenum tetra selenide) embedded in reduced graphene oxide, holds promise for future cTnI detection and enhancing our understanding of CVD [
13].
Serum biomarker analysis, specifically cardiac troponin I, using on-chip microarrays, yielded a substantial increase in near-infrared fluorescence, allowing for ultra-sensitive and quantitative detection using minimal serum samples. The pGold chip assay outperformed the gold standard chemiluminescence immunoassay used in cardiology clinics, significantly improving MI diagnostic sensitivity to 100% and specificity to 95.54% [
14].
Khushaim et al. developed nano-biosensors based on porous graphite carbon nitride (PCN-AuNPs) for rapid and sensitive point-of-care measurement of cTnI. These sensors offer advantages such as a highly sensitive detection limit, quick analysis, and minimal sample volume requirements [
15].
Arshad et al. employed direct deposition of molybdenum disulfide (MoS2) nanosheets to construct a field-effect transistor (FET) for diagnosing CVD. MoS2 nanosheets conjugated with gold nanoparticles (AuNPs) demonstrated robust electrical signal responses in the detection of CVD, aiming to enhance current imaging agents for early CVD identification and characterization [
16].
The ultimate goal in CVD diagnosis is to elevate existing imaging agents to a level where they enable early identification and characterization of the disease [
17]. Targeted drug delivery systems, utilizing nanoparticles to minimize systemic side effects and improve drug localization and efficacy in thrombotic and atherosclerotic lesions, represent a significant shift from traditional pharmacological therapies that result in complete systemic exposure. Hybrid nanoparticles, known as "theranostics," allow simultaneous imaging and treatment, facilitating real-time assessment of therapeutic effectiveness [
18]. Nanoparticles have diverse potential applications in managing CVD, including imaging inflammation in myocardial infarction, in situ detection of thrombosis, plaque and aneurysm detection and characterization, ultra-sensitive monitoring of cardiovascular markers, and targeted delivery of athero-protective or thrombolytic drugs. Additionally, nanoparticles can assist in endothelialization of stents and myocardial regeneration as part of cell-based treatments [
19]. Summarizes the different categories of nanoparticles used in the diagnosis of cardiovascular disease (
Table 1).
Li et al. used time-resolved (TR) luminescent lanthanide metal-organic framework nanoprobes for detecting creatine kinase (CK) activity. These nanoprobes, known as Eu-QPTCA, offer an improved detection limit of 1.0 U/L, significantly enhancing early identification of acute myocardial infarction [
28] [
29].
Hong et al. developed a cost-effective electrochemiluminescence (ECL) immunosensor for detecting the acute MI biomarker cTnI, utilizing a dual-signal system (Ru(bpy)32+/TPA and SnO2 NFs/K2S2O8). This ECL immunosensor demonstrated stability and selectivity in detecting cTnI in real samples, offering reliable results [
9].
Ardestani et al. created the 99mTc-Dendrimer Glyco Conjugate (99mTc-DGC) for myocardial viability scanning, which proved to be a safe and effective tool for early-stage MI diagnosis. Meanwhile, Sultan et al. demonstrated the influence of a chemokine receptor 2 (CCR2) targeted gold nano-cluster conjugated with extracellular loop 1 invers-peptide (AuNC-ECL1i) on atherosclerosis in murine models [
30], providing valuable insights into atherosclerosis progression and regression, with potential implications for clinical use as a targeted molecular imaging probe [
28].
For early detection of cTnI in acute MI, Mansuriya et al. introduced an ultrasensitive enzyme-free electrochemical nano-immunosensor based on a screen-printed gold electrode (SPGE) enhanced with graphene quantum dots (GQDs) and AuNPs. This sensor exhibited exceptional specificity for cTnI, with minimal interference from non-target biomolecules [
31].
Eom et al. developed an enzyme-based electrochemical nano-biosensor, the platinum nanocluster (Pt-NC), capable of detecting cholesterol in saliva. This sensor provides a quick and reliable means of measuring cholesterol levels, while also demonstrating high specificity in detecting lactate, glucose, dopamine, uric acid, and ascorbic acid [
32].
In summary, ongoing research in the field of nano-biosensors is reshaping the landscape of CVD diagnosis and treatment, with promising applications in early detection, precise diagnosis, and targeted therapy. These advancements hold the potential to significantly impact the field of cardiology and improve patient care.
4. The Nanomedicine:
Nanomedicine development has paved the way for several notable advancements, including the efficient delivery of hydrophobic drugs and biologics, precise targeting of disease sites, and the circumvention of biological barriers. Over the past few decades, nanomedicine has made significant inroads into clinical practice, and ongoing pharmaceutical research continues to yield increasingly sophisticated nanomedical solutions [
33].
Within the European Union's nanomedicine market, a variety of nanoscale technologies have found application, including nanoparticles, liposomes, nanocrystals, nano-emulsions, polymeric-protein conjugates, and nano complexes. Notably, nanomedicine encompasses both biological and non-biological pharmaceuticals. While non-biological complex drugs (NBCD) derive their active components from synthetic structures, biological nanomedicines are sourced from living organisms [
34].
Nanoparticles, in particular, show promise in various therapeutic applications, such as the regeneration of fibrotic cardiomyocytes, the regression of atherosclerotic plaque, and the eradication of bacterial biofilms to enhance and extend the antimicrobial effects in cases of infective endocarditis [
15].
5. MRI Scan in Nanomedicine:
To enhance the quality of magnetic resonance imaging (MRI scans, researchers have identified a collection of dual-targeted nanoparticles (NPs), incorporating enclosed iron oxide NPs referred to as mito-magneto [
35]. These MM-encapsulated NPs have exhibited the potential to augment the contrast in MRI scans. Moreover, their diagnostic capabilities were substantiated through in vivo imaging studies, demonstrating the successful distribution of MM-encapsulated dual-targeted NPs in the heart and aorta of mice [
15].
The therapeutic effectiveness of these dual-targeted NPs was accomplished by the selective recruitment of macrophages to plaque areas, facilitated by the presence of mannose receptor targeting ligands and the optimization of NP composition. Remarkably, despite the inclusion of MM encapsulation, these dual-targeted NPs exhibited therapeutic efficacy without provoking adverse immune responses. In another research study, the achievement of background-free near-infrared X-ray-excited luminescence (NIR-XEL) imaging for in vivo thrombosis was demonstrated. This was achieved using thrombin-activatable scintillating nanoprobes, which were engineered using thrombin-cleavable dye-peptide conjugates and highly luminescent lanthanide-doped scintillator nanocrystals (NCs) [
36].
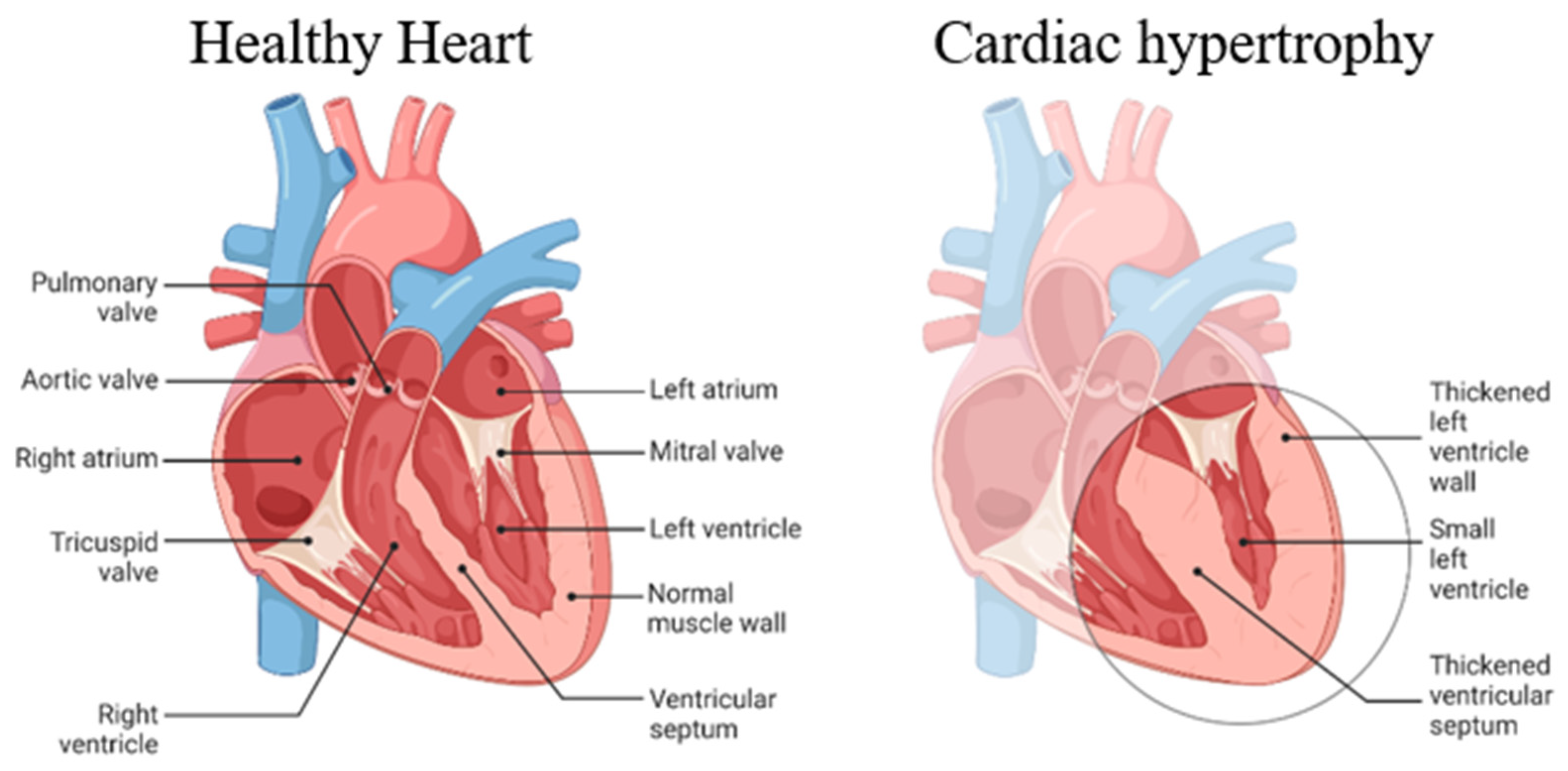
6. Advances in Nanoparticle-Based Therapies for Cardiac Hypertrophy and Myocardial Health:
Cardiac hypertrophy, a condition characterized by the enlargement of the heart muscle, poses a significant threat to cardiovascular health. Recent research has explored the potential of innovative nanoparticle-based therapies to combat cardiac hypertrophy and enhance myocardial health [
36] (
Figure 2).
Here is the basic demonstration of the mechanism of cardiac hypertrophy (
Figure 3).
6.1. Curcumin-Capped Gold-Loaded Poly (lactic-co-glycolic acid) Nanoparticles (CAuPLGA Nps):
Liu et al. demonstrated the remarkable potential of curcumin-capped gold-loaded poly (lactic-co-glycolic acid) nanoparticles (CAuPLGA Nps) in inhibiting cardiac hypertrophy in a Wistar rat model. Their research revealed that these nanoparticles not only effectively reduced heart hypertrophy but also led to improved survival rates, enhanced cardiac diastolic and systolic functions, and maintained stable left ventricle pressure and heart weight. These benefits can be attributed to the anti-inflammatory and antioxidant properties of CAuPLGA Nps, their ability to inhibit cardiomyocyte growth, facilitate enhanced drug delivery, and protect against cholesterol accumulation and myocardial infarction [
37].
6.2. Small Interfering RNA (siRNA) Nanoparticles for Advanced Atherosclerosis:
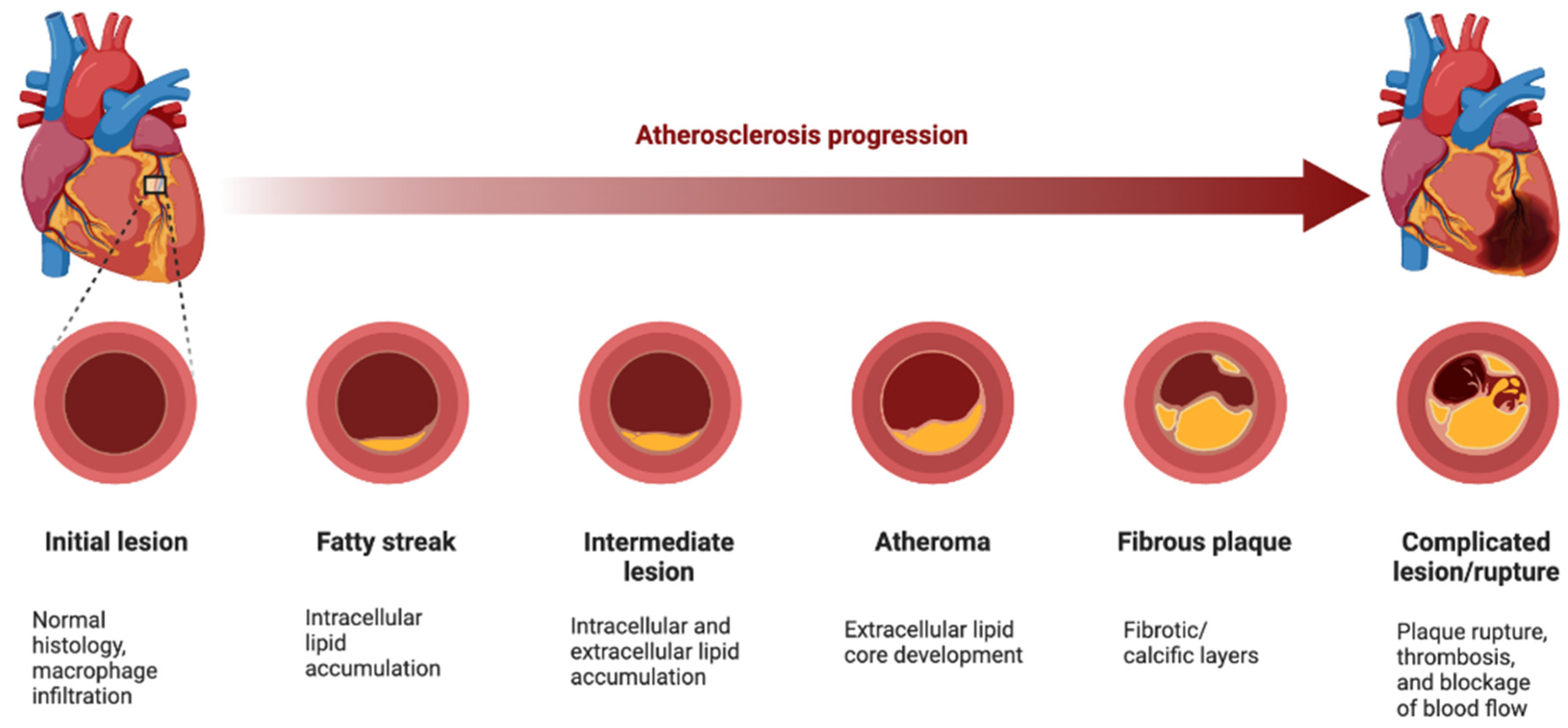
Huang et al. introduced a procedure for manufacturing small interfering RNA (siRNA) nanoparticles (NPs) designed to selectively suppress Ca2+/calmodulin-dependent protein kinase (CaMKII) activity in macrophages. This approach offers promising prospects for treating advanced atherosclerosis and improving plaque stability [
38] and also some discussed the principle mechanism of the atherosclerosis process (
Figure 4).
6.3. Nano Sodium Alginate Bioglass in Myocardial Infarction Treatment:
Guan et al. conducted research involving nano sodium alginate Bio-glass for the treatment of myocardial infarction (MI). Their findings indicated that this innovative approach significantly improved cardiac function, attributed to its capacity to promote cell proliferation [
38].
6.4. Nanoemulsion Curcumin (NEC) in Cardioprotection against PM2.5-Induced Cardiac Injury:
El Tabaa et al. investigated Nanoemulsion curcumin (NEC) as a potential cardioprotective agent against cardiac injuries induced by Particulate Matter 2.5 μm (PM2.5). Their results suggest that NEC might exert its effects via inhibiting the Protein kinase R-like endoplasmic reticulum kinase-Eukaryotic initiation factor-2α pathway [
39].
7. Nanoparticle-Based Approach for Myocardial Fibrosis:
Gao et al. utilized Bellidifolin (BEL) and encapsulated it in HS15 to create BEL nano-micelles with a high encapsulation rate (90%). These nano-micelles exhibited excellent stability and sustained release characteristics. Importantly, this innovative approach showed promise in reducing myocardial fibrosis by lowering the production of collagen III, collagen I, α-SMA, and Smad-2 proteins in myocardial cells [
40].
The studies mentioned above collectively signify the significant progress made in utilizing nanoparticle-based therapies to combat cardiac hypertrophy and enhance myocardial health. These innovative approaches offer potential solutions for a range of cardiac conditions from hypertrophy to myocardial infarction and fibrosis. The findings underscore the evolving landscape of cardiovascular research and the promise that nanoparticle-based treatments hold in improving cardiac health.
8. Nanoparticle- based delivery of RNA-Therapeutics
There is a tremendous burden on health and the economy. While heart failure incidents are increasing world wide, there are few options available for this patient cohort. The need to combat CVDs is therefore urgent.
There is a possibility of using drugs that target proteins in these procedures. Based on the fact that RNA molecule play key roles in many biological processes, they might emerge as promising therapeutic candidates for CVD as well.
Other than that, recent studies dedicate themselves to the issue of using miRNA for the diagnosis of CVD, by investigating sensitive and specific biomarkers for cardiovascular diseases in blood samples. microRNAs (miRNAs/miRs) have in that context been identified as very promising biomarkers to differentiate ST-elevation myocardial infarction (STEMI) patients form TTS patients [
41,
42].
Finally, in a variety of diseases, microRNAs (miRNAs) disrupt post-transcriptional regulation by negatively regulating mRNA expression [
43,
44].
Lastly, in a variety of diseases, microRNAs (miRNAs) disrupt post-transcriptional regulation by negatively regulating mRNA expression. Numerous preclinical studies have evaluated miRNA delivery to the heart via nanoparticle-mediated delivery [
45,
46].
RNA transcript delivery systems have also been employed with metal- and carbon-based nanoparticles [
47].
Of note, cardiovascular applications based on nanoparticles can target hemopoietic cells in addition to cardiomyocytes, cardiac fibroblasts, and endothelial cells [
48,
49].
In fact, nanoparticles can target a variety of innate immune system processes and components, such as neutrophils, macrophage polarization and biology, and transendothelial migration, to reduce inflammation in the heart [
48,
49].
Nevertheless, these RNA modalities must first get past a billion years of evolutionary barriers that have prevented RNAs on the outside of cells from entering cells in order to realize their full potential. The main obstacle to the widespread development of RNA therapeutics has been breaking through the lipid bilayer to deliver RNA into cells, but recent developments in chemistry have started to pierce this evolutionary shield.
More research is required now that RNA chemistries have developed to the point where they allow for improved stability and innate immune system avoidance while also maintaining high on-target activity profiles to develop non-toxic endosomal escape agents and most importantly, target these molecules to particular cell types or tissues [
50].
Thirdly, RNA therapeutics can pharmacoevolve their sequence at the same rate as disease, whether it be pandemic influenza or cancer, in contrast to static small molecules and antibodies. These characteristics offer RNA-based medicines a great deal of promise for treating incurable human diseases (once delivery is figured out).
9. Challenges and Prospects in Nanomedicine Delivery:
In a comprehensive retrospective analysis of various clinical trials utilizing systemically administered nanoparticles, the nanomedicine field faces substantial obstacles. The results of this study cast a sobering light on nano delivery, revealing that a mere 0.7% (median) of the administered nanoparticles successfully reach their intended target areas. Despite this discouraging outlook, this research yields crucial insights for the field of nanomedicine, particularly in the context of treating inflammatory disorders such as cardiovascular diseases (CVDs). We explore the parallel challenges faced in delivering nanomedicines to these complex disease sites and emphasize the significance of the enhanced permeation and retention (EPR) effect, a widely employed strategy for targeting nanoparticles to sites like atherosclerotic plaques and inflamed blood vessels.
The effective delivery of nanomedicines presents multifaceted challenges. These challenges extend to various aspects, from the formulation of nanoparticles to their transport within the body. One of the most glaring issues is the low rate of successful delivery, with a median of only 0.7% of administered nanoparticles reaching their intended destinations. This inefficiency underscores the pressing need for innovative solutions in the field.
9.1. Enhanced Permeation and Retention (EPR) Effect:
To tackle these challenges, the application of the EPR effect has emerged as a promising approach. This strategy leverages the abnormal vasculature and increased permeability of tumor tissues or inflamed regions, allowing nanoparticles to passively accumulate at these sites. The EPR effect is a fundamental mechanism for nanoparticle delivery, as it capitalizes on the unique characteristics of disease locations, such as atherosclerotic plaques and inflamed blood vessels.
The delivery of nanomedicines faces substantial challenges, primarily the low efficiency in reaching target areas. Nevertheless, the study of nanomedicine delivery provides crucial insights for addressing these obstacles. In the context of inflammatory disorders like CVDs and cancer, the EPR effect offers a promising avenue for enhancing the precision of nanoparticle delivery [
51].
10. Conclusion:
In summary, the field of nano therapies is rapidly advancing and holds great promise for the treatment of cardiovascular diseases (CVDs). Soon, nanotechnology-based treatments are poised to replace conventional invasive cardiology procedures, expanding the horizons of microtechnology in the diagnosis and management of coronary artery disease (CAD). Nevertheless, substantial challenges persist, primarily stemming from issues related to the consistent and effective delivery of nanotherapeutics. To address this, innovative approaches are essential to enhance the precise targeting of systemically administered nanoparticles. This can be achieved through the modification of the physical properties of nanoparticles or by leveraging immune cells as carriers, thus optimizing the efficacy of nano-therapies in the context of cardiovascular diseases and other inflammatory disorders.
References
- Senders, M.L., et al., Probing myeloid cell dynamics in ischaemic heart disease by nanotracer hot-spot imaging. 2020. 15(5): p. 398-405. [CrossRef]
- Chandarana, M., A. Curtis, and C.J.A.N. Hoskins, The use of nanotechnology in cardiovascular disease. 2018. 8: p. 1607-1619. [CrossRef]
- Murray, C.J. and A.D.J.N.E.J.o.M. Lopez, Measuring the global burden of disease. 2013. 369(5): p. 448-457.
- Update, A.S.J.C., Heart disease and stroke statistics–2017 update. 2017. 135: p. e146-e603. [CrossRef]
- Timmis, A., et al., European Society of Cardiology: cardiovascular disease statistics 2021. 2022. 43(8): p. 716-799. [CrossRef]
- Tavakol, M., S. Ashraf, and S.J.J.G.j.o.h.s. Brener, Risks and complications of coronary angiography: a comprehensive review. 2012. 4(1): p. 65. [CrossRef]
- Wang, D.K., et al., Nanotechnology applications for cardiovascular disease treatment: Current and future perspectives. 2021. 34: p. 102387. [CrossRef]
- Avasthi, A., et al., Magnetic nanoparticles as MRI contrast agents. 2020: p. 49-91.
- Chen, H., et al., The applications of electrochemical immunosensors in the detection of disease biomarkers: a review. 2023. 28(8): p. 3605. [CrossRef]
- Chauhan, D., et al., Nanostructured transition metal chalcogenide embedded on reduced graphene oxide based highly efficient biosensor for cardiovascular disease detection. 2020. 155: p. 104697. [CrossRef]
- Xu, W., et al., Diagnosis and prognosis of myocardial infarction on a plasmonic chip. 2020. 11(1): p. 1654. [CrossRef]
- Arshad, M.M., et al. Molybdenum Disulfide (MoS 2)/Gold Nanoparticles (AuNPs)-based Field-effect Transistor for C-reactive Protein Detection: Early Diagnosis of Cardiovascular Disease. in 2019 IEEE International Conference on Sensors and Nanotechnology. 2019. IEEE.
- Khushaim, W., et al., Porous graphitic carbon nitrides integrated biosensor for sensitive detection of cardiac troponin I. 2022. 12: p. 100234. [CrossRef]
- Zapp, E., et al., Label-Free Immunosensor Based on Liquid Crystal and Gold Nanoparticles for Cardiac Troponin I Detection. 2022. 12(12): p. 1113. [CrossRef]
- Hussaarts, L., et al., Equivalence of complex drug products: advances in and challenges for current regulatory frameworks. 2017. 1407(1): p. 39-49. [CrossRef]
- Mahan, M.M. and A.L.J.J.o.n. Doiron, Gold nanoparticles as X-ray, CT, and multimodal imaging contrast agents: formulation, targeting, and methodology. 2018. 2018.
- Varna, M., et al., Gold nanoparticles in cardiovascular imaging. 2018. 10(1): p. e1470. [CrossRef]
- Li, X., et al., Lanthanide metal–organic framework nanoprobes for the in vitro detection of cardiac disease markers. 2019. 11(47): p. 43989-43995. [CrossRef]
- Hong, C., et al., A dual-signal electrochemiluminescence immunosensor for high-sensitivity detection of acute myocardial infarction biomarker. 2021. 194: p. 113591. [CrossRef]
- Zhou, Z., et al., Two-stage oxygen delivery for enhanced radiotherapy by perfluorocarbon nanoparticles. 2018. 8(18): p. 4898. [CrossRef]
- Koudrina, A., et al., Exploring the Unique Contrast Properties of Aptamer–Gadolinium Conjugates in Magnetic Resonance Imaging for Targeted Imaging of Thrombi. 2021. 13(8): p. 9412-9424. [CrossRef]
- Lux, J. and A.D.J.C.o.i.c.b. Sherry, Advances in gadolinium-based MRI contrast agent designs for monitoring biological processes in vivo. 2018. 45: p. 121-130. [CrossRef]
- Xu, Q., et al., Quantum dots in cell imaging and their safety issues. 2021. 9(29): p. 5765-5779. [CrossRef]
- Sim, S. and N.K.J.B.r. Wong, Nanotechnology and its use in imaging and drug delivery. 2021. 14(5): p. 1-9.
- Sharifi, S., et al., Superparamagnetic iron oxide nanoparticles for in vivo molecular and cellular imaging. 2015. 10(5): p. 329-355. [CrossRef]
- Cole, J.A., M.A. Tully, and M.E.J.B.r.n. Cupples, “They should stay at their desk until the work’s done”: a qualitative study examining perceptions of sedentary behaviour in a desk-based occupational setting. 2015. 8(1): p. 1-9. [CrossRef]
- Nie, H., Y. Fu, and C.-H.J.B. Wang, Paclitaxel and suramin-loaded core/shell microspheres in the treatment of brain tumors. 2010. 31(33): p. 8732-8740. [CrossRef]
- Ardestani, M.S., et al., Synthesis and characterization of novel 99mTc-DGC nano-complexes for improvement of heart diagnostic. 2020. 96: p. 103572.
- Howard, K.A.J.N., Nanomedicine: Working towards defining the field. 2016: p. 1-12.
- Sultan, D., et al., Assessment of ultrasmall nanocluster for early and accurate detection of atherosclerosis using positron emission tomography/computed tomography. 2021. 36: p. 102416. [CrossRef]
- Mansuriya, B.D. and Z.J.N. Altintas, Enzyme-free electrochemical nano-immunosensor based on graphene quantum dots and gold nanoparticles for cardiac biomarker determination. 2021. 11(3): p. 578. [CrossRef]
- Eom, K.S., et al., Sensitive and non-invasive cholesterol determination in saliva via optimization of enzyme loading and platinum nano-cluster composition. 2020. 145(3): p. 908-916. [CrossRef]
- Wu, L.-P., et al., Grand challenges in nanomedicine. 2020. 106: p. 110302. [CrossRef]
- Mühlebach, S.J.A.d.d.r., Regulatory challenges of nanomedicines and their follow-on versions: a generic or similar approach? 2018. 131: p. 122-131.
- Fujimoto, Z., et al., The tetramer structure of the glycoside hydrolase family 27 α-galactosidase I from Umbelopsis vinacea. 2009. 73(10): p. 2360-2364. [CrossRef]
- Liu, Y., et al., In vivo evaluation of enhanced drug carrier efficiency and cardiac anti-hypertrophy therapeutic potential of nano-curcumin encapsulated photo-plasmonic nanoparticles combined polymerized nano-vesicles: A novel strategy. 2019. 199: p. 111619.
- Huang, X., et al., Synthesis of siRNA nanoparticles to silence plaque-destabilizing gene in atherosclerotic lesional macrophages. 2022. 17(3): p. 748-780. [CrossRef]
- Guan, T., et al., Evaluation of the Effect of Nano-Sodium Alginate-Bioglass on Cardiac Function of Myocardial Infarction Based on Cardiac Ultrasound. 2022. 68(3): p. 67-76. [CrossRef]
- El Tabaa, M.M., et al., SERCA2a directs the cardioprotective role of nano-emulsion curcumin against PM2. 5-induced cardiac injury in rats by prohibiting PERK-eIF2α pathway. 2022. 311: p. 121160. [CrossRef]
- Gao, F., et al., Preparation, characterization and in vitro study of bellidifolin nano-micelles. 2022. 12(34): p. 21982-21989.
- Couch, L.S., et al., Circulating microRNAs predispose to takotsubo syndrome following high-dose adrenaline exposure. Cardiovasc Res, 2022. 118(7): p. 1758-1770. [CrossRef]
- Jaguszewski, M., et al., A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur Heart J, 2014. 35(15): p. 999-1006. [CrossRef]
- Li, C., et al., Mechanism of action of non-coding RNAs and traditional Chinese medicine in myocardial fibrosis: Focus on the TGF-beta/Smad signaling pathway. Front Pharmacol, 2023. 14: p. 1092148.
- Thum, T., Noncoding RNAs and myocardial fibrosis. Nat Rev Cardiol, 2014. 11(11): p. 655-63. [CrossRef]
- Hartmann, D., et al., MicroRNA-Based Therapy of GATA2-Deficient Vascular Disease. Circulation, 2016. 134(24): p. 1973-1990.
- Deng, S., et al., Neonatal Heart-Enriched miR-708 Promotes Proliferation and Stress Resistance of Cardiomyocytes in Rodents. Theranostics, 2017. 7(7): p. 1953-1965. [CrossRef]
- Zeng, Y., et al., A Circular RNA Binds To and Activates AKT Phosphorylation and Nuclear Localization Reducing Apoptosis and Enhancing Cardiac Repair. Theranostics, 2017. 7(16): p. 3842-3855. [CrossRef]
- Duivenvoorden, R., et al., Nanoimmunotherapy to treat ischaemic heart disease. Nat Rev Cardiol, 2019. 16(1): p. 21-32. [CrossRef]
- Leuschner, F., et al., Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol, 2011. 29(11): p. 1005-10. [CrossRef]
- Hache, M., et al., Intrathecal Injections in Children With Spinal Muscular Atrophy: Nusinersen Clinical Trial Experience. J Child Neurol, 2016. 31(7): p. 899-906.
- Dowaidar, M.J.L.A.J.o.P., Nanomedicines for enhanced permeability and retention (EPR)-stratified patients have the potential to improve treatment outcomes. 2023. 42(2): p. 566-596.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).