Submitted:
25 November 2023
Posted:
01 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and discussion
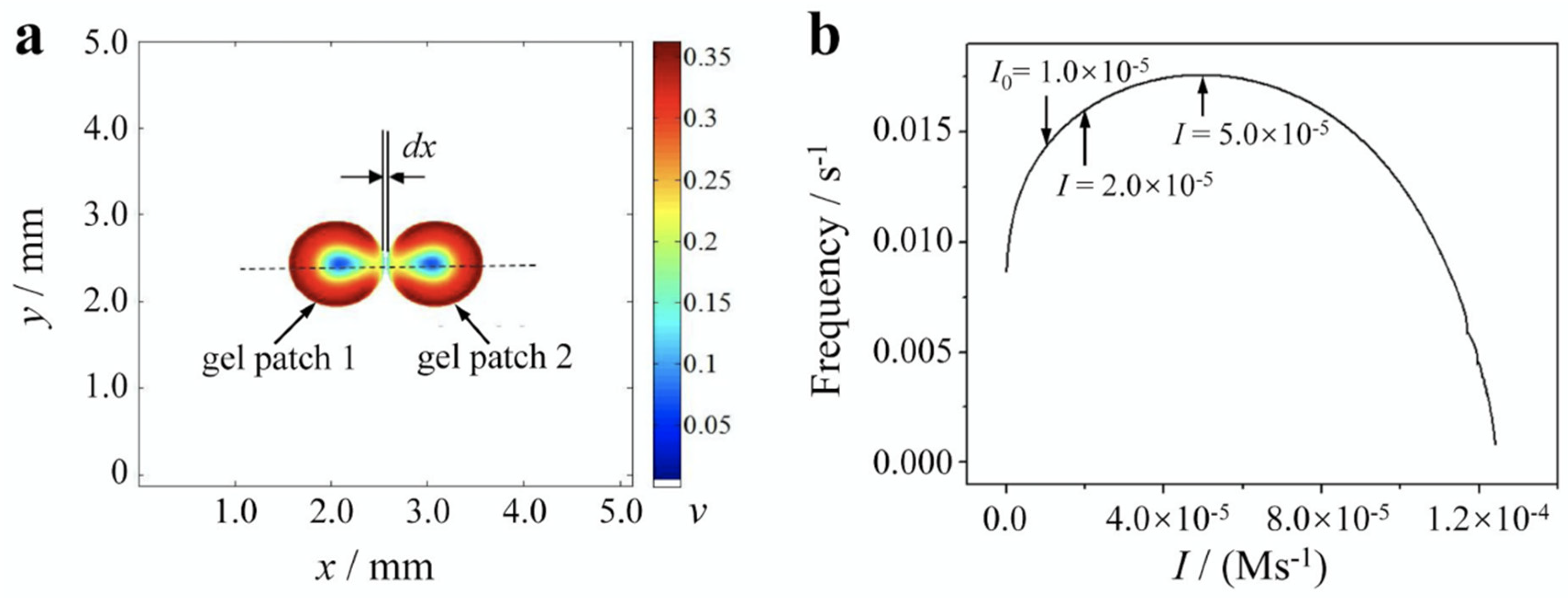
2.1. Model and schematic of the system
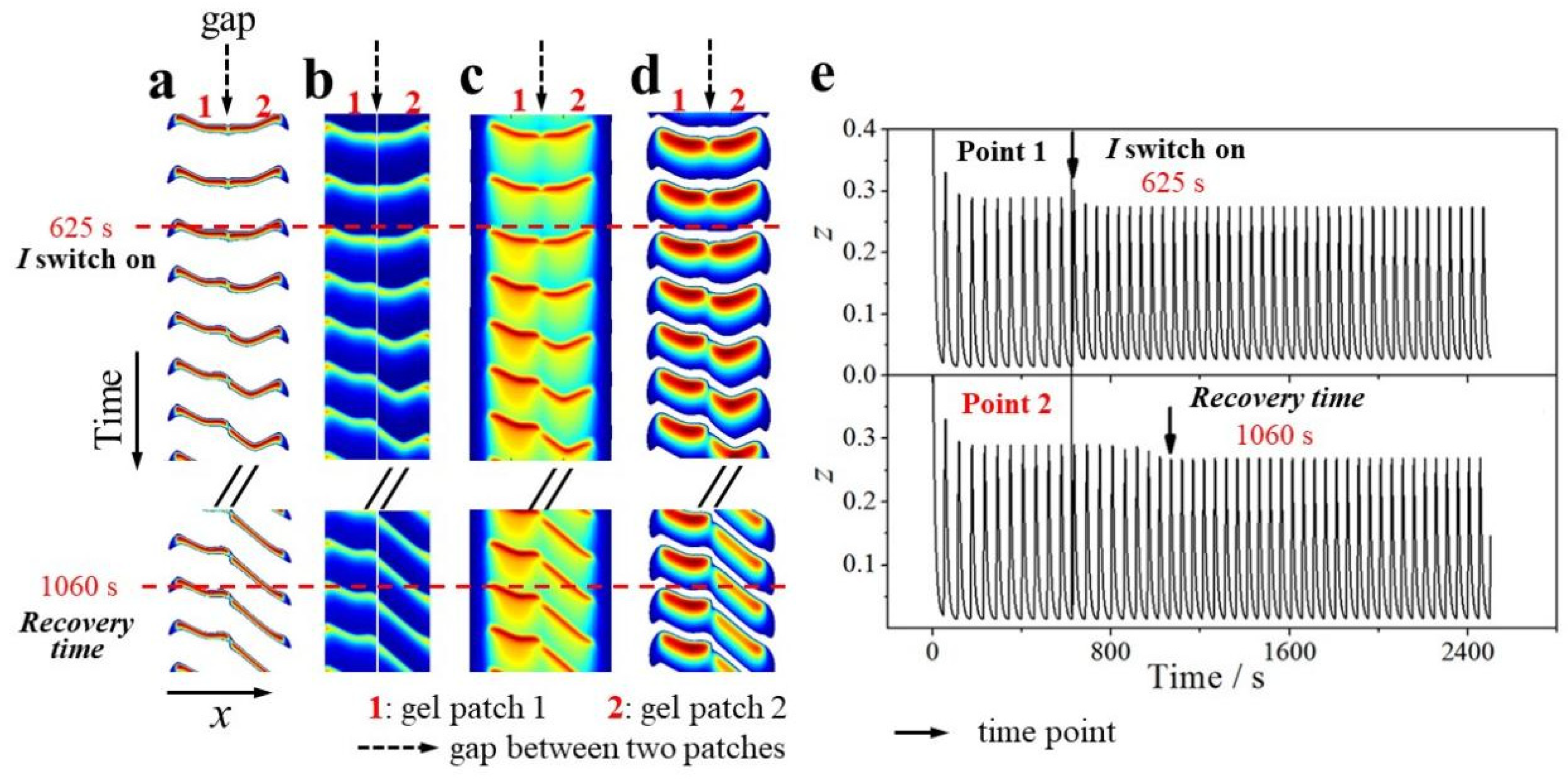
2.2. Recovery of synchrony oscillation between gel patches
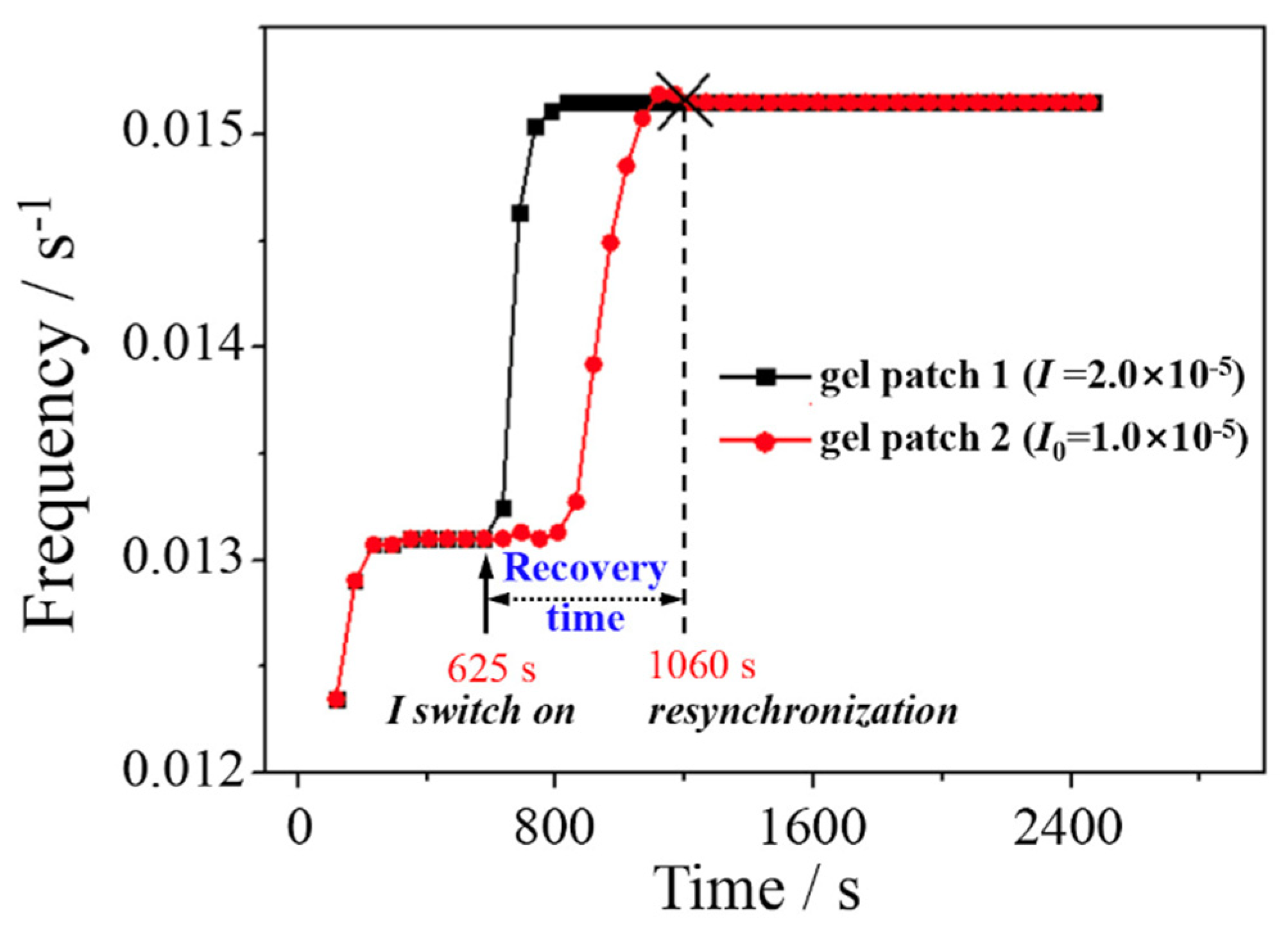
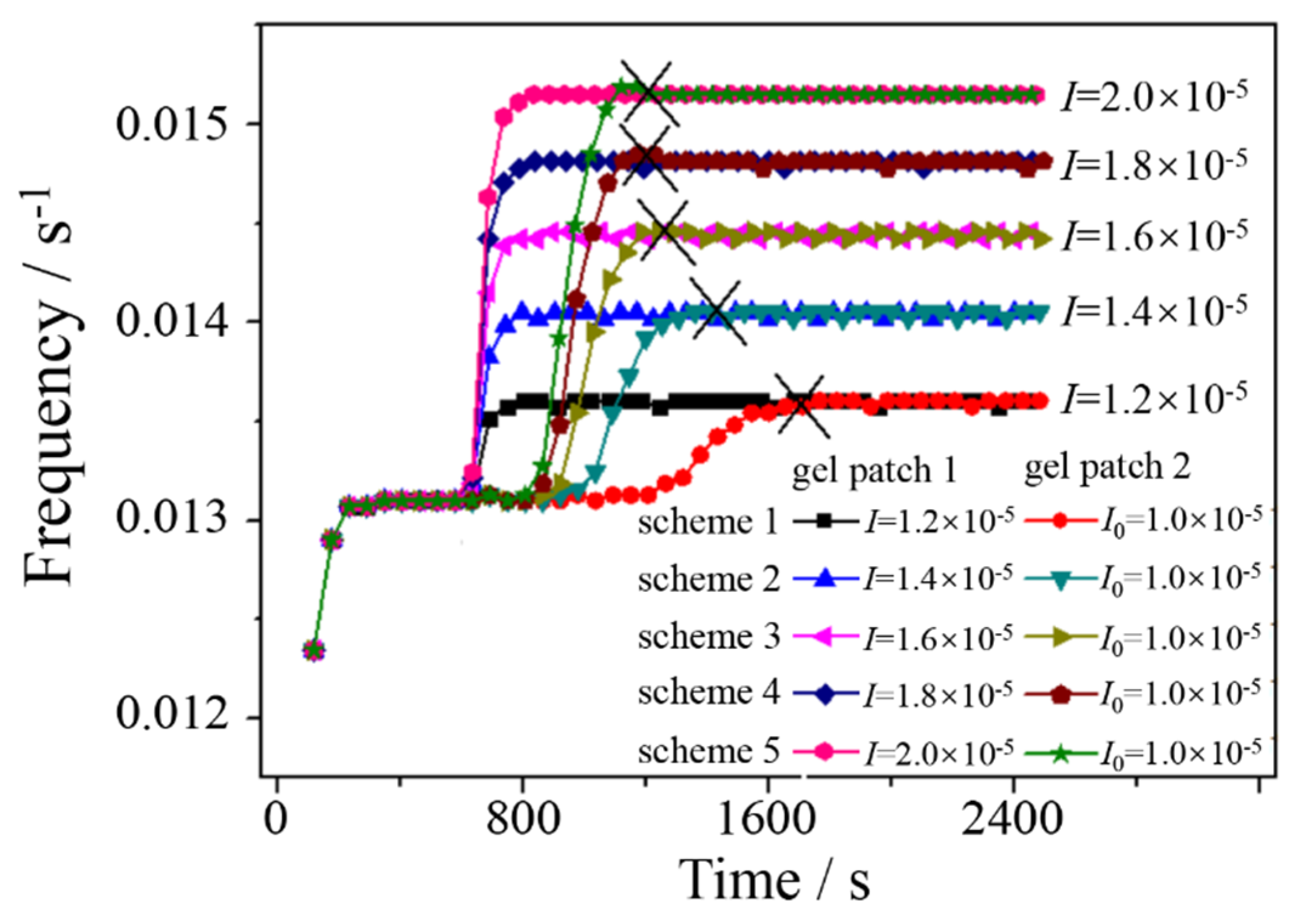
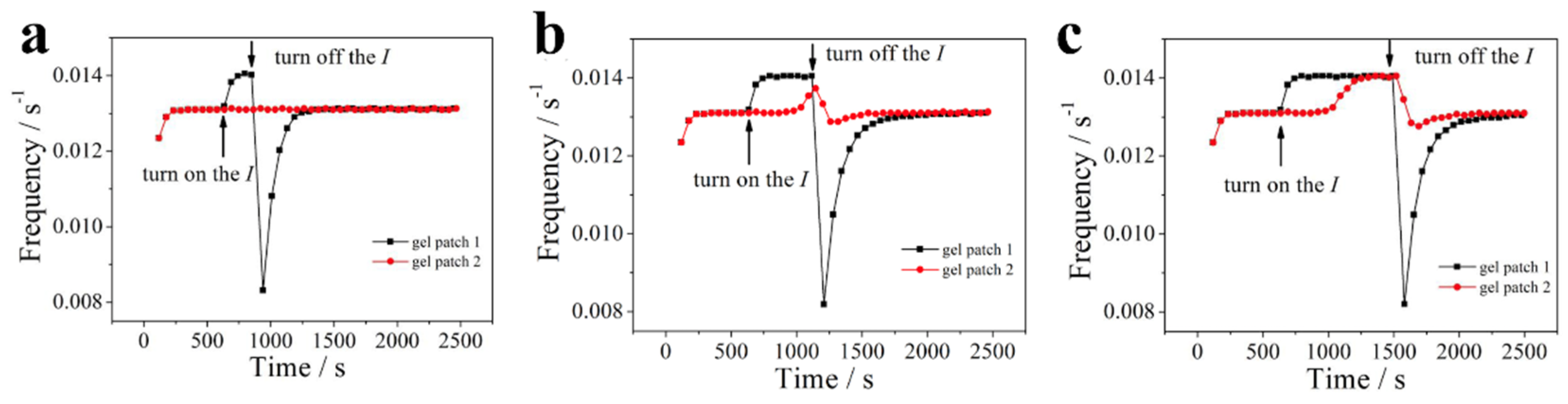
2.3. Recovery time of synchronization modulated by illumination
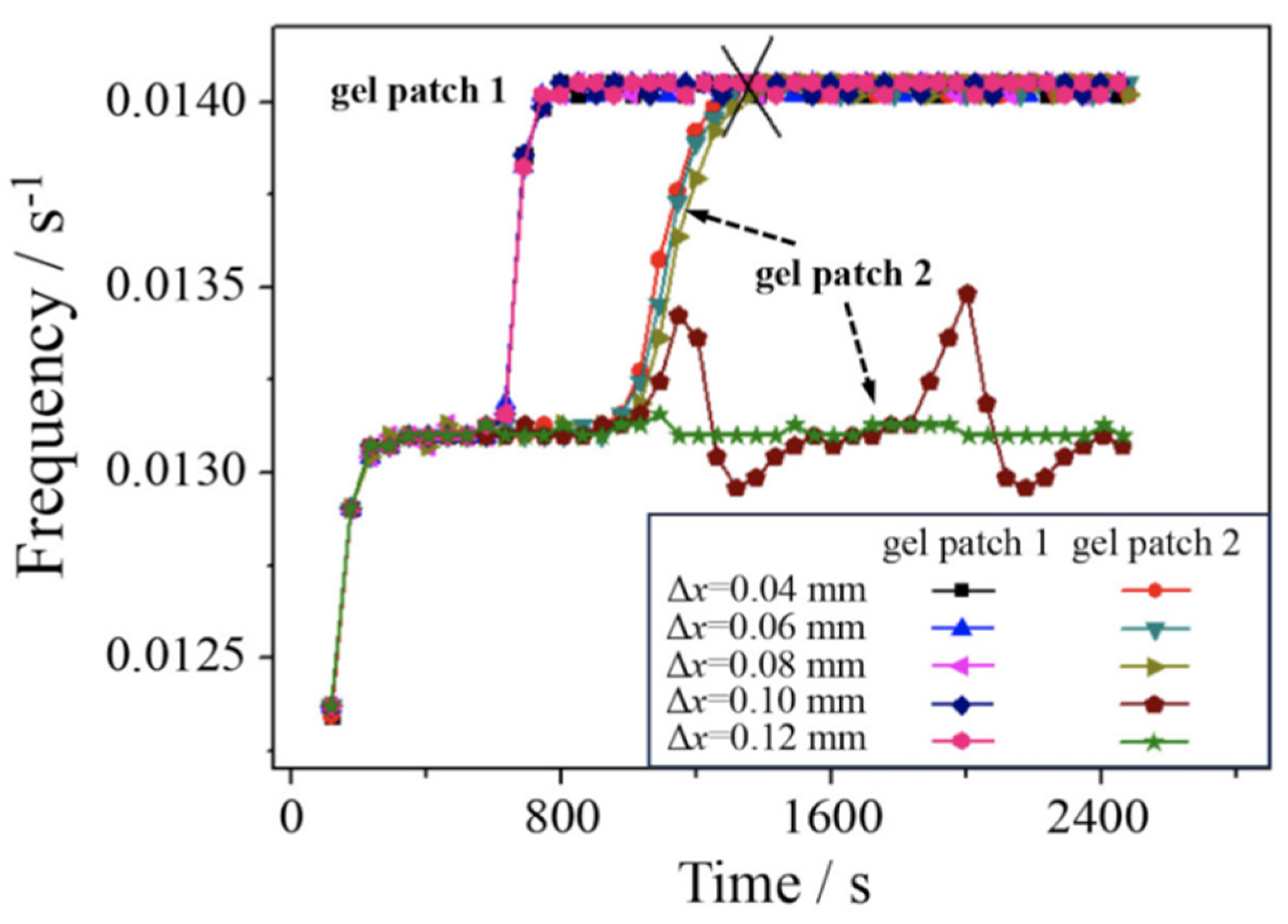
2.4. Recovery time of synchronization modulated by the patches-distant
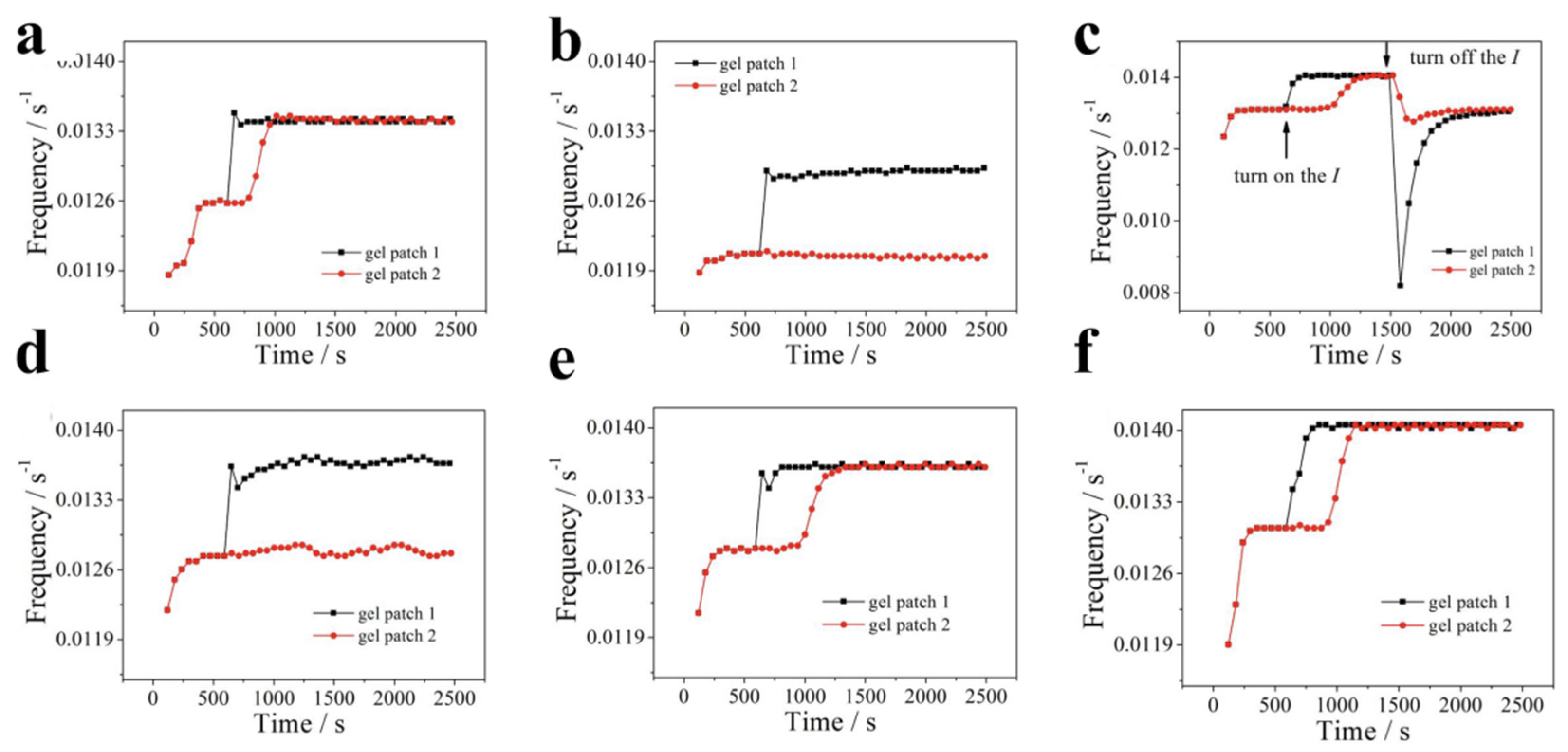
2.5. Effect of illumination-duration on the recovery time
2.6. Signal molecules for the synchrony recovery
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Balanov, A.; Janson, N.; Postnov, D.; Sosanovtseva, O. Synchronization from simple to complex. Springer, 2008, pp. 1-19.
- Winfree, A. T. The Geometry of Biological Time. Springer, 1980, pp. 347-366.
- Bechinger, C.; Leonardo, R. D.; Löwen, H. Active particles in complex and crowded environments. Rev. Mod. Phys. 2016, 88, 045006. [CrossRef]
- Merindol, R.; Walther. A. Materials learning from life: concepts for active adaptive and autonomous molecular systems. Chem. Soc. Rev. 2017, 46, 5588-5619. [CrossRef]
- Soh, S.; Byrska, M.; Kandere-Grzybowska, K.; Grzybowski, B. A. Reaction-Diffusion Systems in Intracellular Molecular Transport and Control. Angew. Chem. Int. Ed. 2010, 49, 4170-4198. [CrossRef]
- Alexander, R. M. Principles of Animal Locomotion. Princeton, 2002, pp. 21-48.
- Pollard, T. D.; Earnshaw, W. C.; Lippincott-Schwartz, J.; Johnson, G. Cell biology. Elsevier Health Sciences, 2016, pp. 24-56.
- Ji, F. T.; Wu, Y. L.; Pumera, M.; Zhang, L. Collective Behaviors of Active Matter Learning from Natural Taxes Across Scales. Adv. Mater. 2022, 35, 202203959. [CrossRef]
- Oates, A. C.; Morelli, L. G.; Ares, S. Patterning embryos with oscillations: structure, function and dynamics of the vertebrate segmentation clock. Development 2012, 139, 625–639. [CrossRef]
- Squire, J. M. In muscle sarcomeres, myosin are threaded in thick-filaments interdigitized with actin fibrils. Curr. Opin. Struct. Biol. 1997, 7, 247–257. [CrossRef] [PubMed]
- Umbach, G.; Tan, R.; Jacobs, J.; Pfeiffer, B. E.; Lega, B. Flexibility of functional neuronal assemblies supports human memory. Nat. Commun. 2022, 13. 6162. [CrossRef]
- He, L.; Wang, X.; Tang, H. L; Montell, D. J. Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat. Cell Biol. 2012, 12, 1133–1142. [CrossRef]
- Chen, C.; Liu, S.; Shi, X. Q.; Chate, H.; Wu, Y. L. Weak synchronization and large-scale collective oscillation in dense bacterial suspensions. Nature 2017, 542, 210-214. [CrossRef] [PubMed]
- Tu, H. Q.; Li, S.; Xu, Y. L.; Zhang, Y. C.; Li, P. Y.; Liang, L. Y. Rhythmic cilia changes support SCN neuron coherence in circadian clock. Science 2023, 380, 972-979. [CrossRef]
- Gilpin, W.; Bull, M. S.; Prakash, M. The multiscale physics of cilia and flagella. Nat. Rev. Phys. 2020, 2, 74–88. [CrossRef]
- Ghosh, A. K.; Chance, B.; Pye, E. K. Metabolic coupling and synchronization of NADH oscillations in yeast cell populations. Arch. Biochem. Biophys. 1971, 145, 319-331. [CrossRef]
- Ellison, D.; Mugler, A.; Brennan, M. D.; Lee, S. H.; Huebner, R. J.; Dhamir, E. R.; Woo, L. A. Cell-cell communication enhances the capacity of cell ensembles to sense shallow gradients during morphogenesis. Proc. Natl. Acad. Sci. USA. 2016, 113, E679-E688. [CrossRef] [PubMed]
- Smith, H. M. Synchronous flashing of fireflies. Science 1935, 82, 151-152. [CrossRef] [PubMed]
- Farkas, I.; Helbing, D.; Vicsek, T. Mexican waves in an excitable medium. Nature 2002, 419, 131–132. [CrossRef]
- Andersan, P.W. More is different. Science 1972, 4, 393-396. [CrossRef] [PubMed]
- Vantomme, G.; Elands, L.; Gelebart, A. H. Coupled liquid crystalline oscillators in Huygens’ synchrony. Nat. Mater. 2021, 20, 1702–1706. [CrossRef]
- Li, S. G.; Batra, R.; Brown, D.; Chang, H. D.; Ranganathan, N.; Hoberman, C.; Rus, D.; Lipson, H. Particle robotics based on statistical mechanics of loosely coupled components. Nature 2019, 567, 361-365. [CrossRef]
- Litschel, T.; Norton, M. M.; Tserunyan, V.; Fraden, S. Engineering reaction–diffusion networks with properties of neural tissue. Lab. Chip 2018, 18, 714-722. [CrossRef]
- Lerch, M. M.; Grinthal, A.; Aizenberg, J. Viewpoint Homeostasis as Inspiration Toward Interactive Materials. Adv. Mater. 2020, 32, 1905554. [CrossRef]
- Totz, J. F. Synchronization and Waves in Active Media. Springer, 2019, pp. 37-52.
- Yoshida, R.; Takahashi, T.; Yamaguchi. T.; Ichijo, H. Self-Oscillating Gel. J. Am. Chem. Soc. 1996, 118, 5134–5135. [CrossRef]
- Kim, Y. S.; Tamate, R.; Akimoto, A. M. Recent developments in self-oscillating polymeric systems as smart materials: from polymers to bulk hydrogels. Mater. Horiz. 2017, 4, 38-54. [CrossRef]
- Kuksenok, O.; Dayal, P.; Bhattacharya, A. Chemo-responsive, self-oscillating gels that undergo biomimetic communication. Chem. Soc. Rev. 2013, 42, 7257-7277. [CrossRef]
- Osypova, A.; Dubner, M.; Panzarasa, G. Oscillating reactions meet polymers at interfaces. Materials 2020, 13, 2957. [CrossRef]
- Yashin, V. V.; Balazs, A. C. Chemomechanical synchronization in heterogeneous self-oscillating gels. Phys. Rev. E 2008, 77, 046210. [CrossRef] [PubMed]
- Yashin, V. V.; Balazs, A. C. Controlling chemical oscillations in heterogeneous Belousov-Zhabotinsky gels via mechanical strain. Phys. Rev. E 2009, 79, 046214. [CrossRef]
- Yashin, V. V.; Suzuki, S.; Yoshida, R.; Balazs, A. C. Controlling the dynamic behavior of heterogeneous self-oscillating gels. J. Mater. Chem. 2012, 22, 13625. [CrossRef]
- Tateyama, S.; Shibuta, Y.; Yoshida, R. Direction Control of Chemical Wave Propagation in Self-Oscillating Gel Array. J. Phys. Chem. B 2008, 112, 1777. [CrossRef] [PubMed]
- Ito, K.; Ezaki, T.; Suzuki. S.; Kobayashi, R.; Hara, Y.; Nakatra, S. Synchronization of Two Self-Oscillating Gels Based on Chemo-Mechanical Coupling. J. Phys. Chem. B 2016, 120, 2977. [CrossRef]
- Fang, Y.; Yashin, V. V.; Levitan, S. P.; Balazs, A. C. Pattern recognition with “materials that compute”. Sci. Adv. 2016, 2, e1601114. [CrossRef] [PubMed]
- Lu, X.; Ren, L.; Gao, Q.; Zhao, Y.; Wang, S.; Yang, J.; Epstein, I. R. Photophobic and phototropic movement of a self-oscillating gel. Chem. Commun. 2013, 49, 7690-7692. [CrossRef] [PubMed]
- Ren, L.; She, W.; Gao, Q.; Pan, C.; Ji, C.; Epstein, I. R. Retrograde and Direct Wave Locomotion in a Photosensitive Self-Oscillating Gel. Angew. Chem. Int. Ed. 2016, 55, 14301-14305. [CrossRef]
- Ren, L.; Wang, M.; Pan, C.; Gao, Q.; Liu, Y.; Epstein, I. R. Autonomous reciprocating migration of an active material. Proc. Nati. Acad. Sci. USA. 2017, 114, 8704-8709. [CrossRef]
- Ren, L.; Wang, L.; Gao, Q.; Teng, R.; Xu, Z.; Wang, J.; Pan, C.; Epstein, I. R. Programmed Locomotion of an Active Gel Driven by Spiral Waves. Angew. Chem. Int. Ed. 2020, 59, 7106 -7112. [CrossRef]
- Ren, L.; Yuan, L.; Gao, Q.; Teng, R.; Wang, J.; Epstein, I. R. Chemomechanical Origin of Directed Locomotion Driven by Internal Chemical Signals. Sci. Adv. 2020, 6, eaaz9125. [CrossRef] [PubMed]
- Wang, J.; Ren, L.; Teng, R.; Epstein, I. R.; Wang, H.; Zhang, M.; Yuan, L.; Gao, Q. Y. Rotational Locomotion of an Active Gel Driven by Internal Chemical Signals. J. Phys. Chem. Lett. 2021. 12. 11987-11991. [CrossRef] [PubMed]
- Teng, R.; Gao, Q. Y; Yuan, L.; Ren, L.; Wang, J.; Wang, Y. J.; Epstein, I. R.; Heterogeneity-driven collective-motion patterns of active gels. Cell Rep. Phys. Sci. 2022, 3, 100933. [CrossRef]
- Teng, R.; Li, Y.; Ren, L.; Epstein, I. R.; Gao, Q. Y. Transitions of Collective Motions Driven by Phase Resetting. Chem. Phys. Chem. 2023, 24, e202300336. [CrossRef] [PubMed]
- Lu, X. J.; Ren, L.; Gao, Q. Y.; Yang, Y. Y.; Zhao, Y. M.; Huang, J.; Lu. X. L.; Epstein, I. R. Multiple Length Scale Instabilities of Unidirectional Pulse propagation in a diffusion-fed gel. J. Phys. Chem. Lett. 2013, 4, 3891–3896. [CrossRef]
- Ren, L.; Fan, B.; Gao, Q.; Zhao, Y.; Luo, H.; Xia, Y.; Lu, X.; Epstein, I. R. Experimental, numerical, and mechanistic analysis of the nonmonotonic relationship between oscillatory frequency and photointensity for the photosensitive Belousov–Zhabotinsky oscillation. Chaos 2015, 25, 064607. [CrossRef]
- Amemiya, T.; Ohmori, T.; Yamaguchi, T. An Oregonator-Class Model for Photoinduced Behavior in the Ru(bpy)32+-Catalyzed Belousov-Zhabotinsky Reaction. J. Phys. Chem. A 2000, 104, 336-344. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




