1. Introduction
A healthy person may dismiss sunscreen use on a summer’s day but skin protection and skin care more broadly confer essential maintenance to the body’s largest organ beyond our own natural mechanisms. The skin plays a vital role in maintaining homeostasis, offering protection, and containing our organs and fluids [
1]. When the skin is afflicted by disease, these essential functions are disrupted, putting the entire person at risk. One disease that easily comes to mind is skin cancer. Statistics from Center for Disease Control and Prevention reveal that approximately 6 million people in the U.S. receive treatment for various skin cancers annually, with an annual expenditure of nearly
$9 billion on skin cancer treatment [
2]. Cancer, though, is far from the only manifestation of skin disease that people should be aware of. The National Institute of Arthritis and Musculoskeletal and Skin Diseases identifies 13 significant benign skin disorders [
3]. While not inherently cancerous, these major disorders still negatively impact the quality of life of individuals and can even transform into life-threatening conditions if infected or neglected.
Furthermore, social stigma toward irregular skin conditions can place undue psychological stress on the individual through isolation and low self-worth. Even in mild cases, it is still possible for the affected individual to be forcefully exempted from fully participating in otherwise expected society roles due to the sick role phenomenon [
4]. It is also worth noting that all demographics can get skin cancer: age, sex, race, ethnicity show differences in severity and incidence, but no group can claim immunity [
5,
6,
7]. All things considered, it is imperative to emphasize the importance of awareness and advancements in dermatology, especially given the recent surge in diagnoses and evolving treatment options fueled by technological progress.
In exploring this landscape, this topical review aims to cover key topics discussed in the field of dermatology as an introduction. Publications from PubMed and Google Scholar and reports from research centers and governmental organizations focused on skin disease were pulled, evaluated, and used to summarize key features of the pathophysiology of major skin cancers and disorders and explore conventional diagnostic methods and current treatment approaches.
2. Overview of Human Skin Diseases
2.1. Skin Cancers
This subsection aims to provide a brief description of the disease, its risk factors, and its incidence. Basal and squamous cell cancer also have some coverage on diagnosis and conventional treatment options.
2.1.1. Basal Cell Cancer
The most common head and neck skin cancer, it is slow growing with low metastatic potential with locally invasive. The main risk factor is ultraviolet (UV) radiation. Diagnosis relies on clinical examination as well as skin biopsy for confirmation and assessing recurrence risk. Treatment options include surgical excision, Mohs surgery, cryosurgery, electrodesiccation and curettage, imiquimod or fluorouracil, photodynamic therapy, and radiation therapy [
8,
9,
10]. Despite being so common, basal cell cancer and squamous cell skin cancer cases are difficult to estimate because most cancer registries do not require and do not receive records of cases [
7].
2.1.2. Squamous Cell Cancer
The second most prevalent skin cancer in the U.S., it often emerges from precursor lesions and has the potential to metastasize. UV radiation is the primary risk factor. Surgical excision is the primary treatment, with Mohs micrographic surgery preferred for head and neck cases or those with high-risk characteristics. Radiation therapy is an option for older patients or those unable to undergo surgery. Immunosuppression increases lifetime risk and metastasis [
8,
11].
2.1.3. Melanoma Skin Cancer
A metastasizing cancer that originates in melanocytes and can appear in various colors, from brown to white. It often starts on the trunk in men and the legs in women, with darker skin offering some protection but not immunity. Risk factors include strong evidence of exposure to ultraviolet radiation, moles, family history, weight, and fair phenotype. Statistically, American men under 50 years have seen incidence rates decline 1% per year while men over 50 years have stabilized rates. American women, however, see stable rates for those under 50 years but 1% increase per year for those over 50 years [
7]. Trauma and chronic inflammation are associated with non-cutaneous melanoma, particularly acral melanoma. Although melanoma is less common than some skin cancers, it has a higher tendency to metastasize if not diagnosed and treated [
12]. Even so, mortality rates in the U.S. have declined 2% from 2016-2020 [
7].
2.1.4. Merkel Cell Skin Cancer
A rare, metastasizing skin cancer primarily typically affecting individuals over 50 years old. Risk factors include the Merkel cell polyomavirus, UV exposure, and immunosuppression. Diagnosis involves clinical examination, tissue biopsy, and cytokeratin-20 immunohistochemistry [
13]. There is scant data to generate national incidence and prevalence rates, however the largest dataset in Europe of this cancer type reports that men on average had it more than women with incidence rates increasing 3.9% per year during 2004-2018 [
14].
2.1.5. Lymphoma
Originating in the lymphatic system, lymphoma is the result of either T or B lymphocytes becoming cancerous. Risk factors for lymphoma include certain viral infections, exposure to certain chemicals and pesticides, family history, and a weakened immune system. Common methods for diagnosing lymphoma include blood tests, imaging studies like CT scans and PET scans, and lymph node biopsy [
15,
16,
17]. The incidence of non-Hodgkin lymphoma decreased by about 1% per year 2015- 2019 [
7].
2.1.6. Kaposi Sarcoma
Kaposi sarcoma results from Kaposi sarcoma-associated herpesvirus (KSHV) infection in blood and lymphatic vessel cells. Risk factors include KSHV infection, which is more prevalent among those with weakened immune systems. Diagnosis relies on recognizing characteristic skin lesions [
17,
18]. Global declines in incidence in Europe, Latin America, the U.S., and Africa are said to be matched with declines in HIV incidence and prevalence due to safe sex practices, public awareness, and viral therapies [
19,
20].
2.2. Benign Skin Disorders
This subsection aims to provide a brief description of the disorder and its primary risk factors.
2.2.1. Acne
A condition caused when blocked skin follicles from a plug caused by oil from glands, bacteria, and dead cells clump together and swell [
3]. There are many environmental and behavioral contributors to the development of acne including but not limited to air pollution, certain skincare products, medications, hormonal and, more recently, diet and stress. [
21,
22] In addition, genetic inheritance of specific polymorphisms has been observed to yield the acne phenotype more prominently for certain ethnic groups [
23,
24,
25,
26].
2.2.2. Alopecia Areata
A condition causing hair to fall out in small, round patches [
3]. Risk factors include but are not limited to history of atopic dermatitis for the individual and family, inheritance of various single nucleotide polymorphisms, pre-existing autoimmune thyroid diseases, and psychological stress [
27,
28,
29,
30].
2.2.3. Atopic Dermatitis
A skin disease characterized by itchiness, redness, swelling, cracking, and scaling [
3]. It is closely associated with asthma, with several studies observing the comorbidity of the two conditions [
31]. Risk factors include but are not limited to sex of the individual, existing allergies, family history of allergens, air pollution [
31,
32,
33,
34].
2.2.4. Epidermolysis Bullosa
A group of genetic connective tissue diseases causing painful blisters to form on the skin and scar [
3]. Unlike the previous disorders, the consequences of severe epidermolysis bullosa can result in organ damage and failure. The most common form is epidermolysis bullosa simplex, which is restricted to the epidermis of the skin with minimal scarring. Both mutations to modifier genes and to epigenetic mechanisms contribute to the loss of tissue integrity [
35,
36]. Non-genetic risk factors may include expression of Inflammatory Bowel Disease, reported in children, SARS-CoV-2 infection, [
37,
38].
2.2.5. Hidradenitis Suppurativa
A chronic inflammatory condition characterized by pimple-like bumps, boils, and tunnels under the skin [
3]. It is associated with bacterial infection of the apocrine sweat glands. Risk factors include but are not limited to age, sex, stress, and even early squamous cell cancer. It remains unclear whether race is a risk factor with various studies in support of it such as Mokos et al. (2023) and others that remain skeptical such as Bryd et al. (2023) [
39,
40,
41,
42,
43,
44].
2.2.6. Ichthyosis
A rare autosomal recessive congenital disorder causing dry, thickened skin that resembles fish scales [
3]. Reported genetic causes of this disorder are severe mutations in the Adenosine-triphosphate-Binding Cassette A12 gene resulting in an extreme form of ichthyosis and the Vitamin D Receptor gene (polymorphism) resulting in a milder form. [
43,
44]. In the case of the latter, age and raised serum ALP levels have been suggested to be risk factors [
45,
46].
2.2.7. Pachyonychia Congenita
A rare congenital disorder causing thick nails, painful calluses, and other symptoms [
3]. Variations in keratin genes, specially KRT6A and KRT6B [
47].
2.2.8. Pemphigus
A disease where the immune system attacks healthy skin cells, resulting in blisters [
3]. Having pemphigus may lead to osteopenia, osteoporosis, and pathologic fractures although further investigation is needed [
48,
49,
50]. One case report describes an interesting case of induced autoimmunity, preventing pemphigus despite the patient having Hodgkin’s lymphoma, a risk factor for pemphigus [
50]. Other suggested risk factors are sex thymic diseases, and P wave dispersion increases and diversity of diet intake [
51,
52,
53,
54].
2.2.9. Psoriasis
A skin disease that causes red, scaly, painful, swollen skin [
3]. While specific risk factors are still unclear for psoriasis, it is known to be associated with cardiovascular mortality, myocardial infarction and stroke in its moderate to severe forms [
55].
2.2.10. Raynaud’s Phenomenon
A condition shunting blood vessels and causing insufficient blood flow to the hands and feet [
3]. Risk factors include migraine headaches, rheumatologic disease, vaso-occlusive diseases, hematologic disorders, physical injury, viral infection, carpal tunnel syndrome [
56]. In one case report, there is an association of Raynaud’s phenomenon with long-term silica exposure [
57].
2.2.11. Rosacea
A long-term disease leading to reddened skin, pimples, and skin thickening, often affecting the face [
3]. Risk factors include high temperatures,
Demodex mites, overuse of aggressive face cleansers, cardiovascular diseases [
58,
59,
60,
61]. There is a suggestion that alcohol consumption may also be a risk factor, however, follow-up studies of this scenario are required to investigate [
62].
2.2.12. Scleroderma
A condition causing tight, hard skin and potential harm to blood vessels and organs [
3]. In systemic sclerosis patients, ACE inhibitors with concomitant arterial hypertension have been shown to be a risk factor as well as high levels of RNA polymerase III antibodies [
63,
64]. Smoking interestingly has not been shown to be a risk factor [
65].
2.2.13. Vitiligo
A common disorder leading to white patches of skin due to the destruction of melanocytes [
3]. It may manifest in childhood or in adulthood. There is some correlation between Hepatitis C virus presence and adult-onset of vitiligo [
66]. Polymorphisms in tumor necrosis factor-α and -β have been reported to be a risk factor albeit only in one population [
67].
3. Pathophysiology of Human Skin Diseases
3.1. Common Pathophysiological Factors
This subsection aims to describe typically shared traits of skin diseases. Due to the broad scope of this review, it will not go into the details of each skin disease’s complex pathogenesis.
3.1.1. Inflammation
Inflammation is a natural immune response to protect against harmful stimuli, such as pathogens or tissue injury. In the context of skin diseases, when the skin is exposed to irritants, allergens, infections, or autoimmune reactions, the immune system triggers an inflammatory response [
68]. This response can manifest as redness, swelling, heat, and pain. The issue arises with chronic or excessive inflammation contributing to skin diseases. TRPV-ion channels trigger the release of pro-inflammatory mediators such as calcitonin gene-related peptide and substance P, perpetuating conditions like psoriasis, atopic dermatitis, prurigo, and rosacea. Immune cells within the skin, including mononuclear cells, dendritic cells, and mast cells, also express TRPV1, further amplifying inflammation by releasing cytokines and neuropeptides [
69]. This amplification can cause abnormal cell growth in the case of wound repair forming a healing tissue niche resembling the tumor stroma if hijacked, a gateway toward skin cancer [
70].
3.1.2. Immune System Dysregulation
Because the immune system triggers inflammation as an innate response to foreign particles, there is overlap of the consequences of irregularity in the immune system and in the inflammation response. For instance, T cell populations on the skin, even rare ones like γδ T cells, have a specific role in allergic skin inflammation and in maintaining tissue homeostasis [
71,
72]. Effector T cells and memory T cells are accompanied by natural killer cells and T cells expressing MHCR gene on the skin. This impressive ensemble of specific immunity can collapse with interference in signaling or antigen expression. The classic case is with the HIV virus which puts individuals at higher risk for developing non-melanoma skin cancers compared to the general population [
73]. Human papillomaviruses have a similar impact with promoting squamous cell carcinoma [
74].
3.1.4. Environmental Triggers
The top trigger for skin cancer is UV radiation followed by ionizing radiation. Due to the high energy of UV waves, it causes DNA damage and genetic mutations, especially by mutating pyrimidines into cyclobutene pyrimidine dimers that then are “fixed” by natural nucleotide substitution mechanisms. UV radiation (and also ionizing radiation) are impacted by the ozone (or decline of), latitude, altitude, and weather conditions [
75,
76].
3.2. Biomarkers by Skin Disease
Major advances in elucidating gene pathways that may trigger skin diseases are thanks to the fields of computational biology and genomics. For instance, the Cancer Genome Atlas Program characterized over 20,000 primary cancers and matched normal samples to 33 cancer types [
77]. Because of the rising possibilities of which gene pathways could contribute to skin diseases, the following sections are not meant to be taken as comprehensive.
3.2.1. Skin Cancers (Basal Cell Cancer, Squamous Cell Carcinoma, Melanoma)
3.2.1.1. Melanoma
For melanoma, risk factors are primarily genetic over environmental with telomere genes as a common source of mutations. The typical biomarkers of melanoma are BRAF, NRAS, PI3K-AKT/PTEN, p53, CDK4/CDKN2A, c-KIT, MC1R, cadherin [
78]. With the overlap of melanoma with other cancer families, several genes have stood out to be shared between melanoma and at least one other cancer type: CDKN2A/p16, CDK4, BRCA2, POT1, MITF, RB1, P53, BAP1, PTEN, CHEK2. Many melanoma genes are not just shared with other cancers but are also shared with neurological diseases. PLA2G6, BAP1, DCC, ERBB4, KIT, MAPK2, MITF, PTEN, and TP53 have all been implicated in both melanoma and Parkinson’s [
79]. Turning over to non-coding DNA markers, telomeres have been long established to be mutated in melanoma, especially in the TERT promoter, although the exact degree across subtypes of melanoma and the relationship with different mutations is still being investigated [
80].
3.2.1.2. Basal Cell Cancer
For basal cell cancer specifically, the Hedgehog pathway has mutations in multiple genes, promoting this type of cancer directly just as effectively as UV overexposure. While PTCH1 has been reported as the primary driver for basal cell cancer when bound excessively to the Hedgehog ligand, many secondary drivers exist that affect downstream components of the pathway such MYCN, PPPC, SK19, LATS1, ERBB2, PIK23C, N-RAS, K-RAS, H-RAS, PTPN14, RB1, and FBX7 and pathways IGF-PI3K-AKT, EGFR-MEK-ERK, and Hippo pathways [
81,
82].
3.2.1.3. Squamous Cell Carcinoma
For squamous cell carcinoma, which has the highest mutational burden of all cancers, there are many possible mutations that can occur. One, the EGFR-MAPK pathway is overexpressed resulting in downstream effects via RAS/RAS GTPase. Two, RAS adaptor proteins themselves may also be mutated, yielding metastasizing tumors. Three, RAF proteins (serine/threonine kinases activated by RAS) if activated and heterodimerize, then activate kinase MEK which promotes new tumor formation. Four, NOTCH1 coactivated by MAML1 if knocked out no longer can fulfill its tumor suppressor role. Five, TGF beta receptors are somewhat more complicated to target due to the opposite roles the SMAD proteins play downstream and upstream. Droll & Bao (2021) go in depth on the alterations in the aforementioned pathways that contribute to this cancer type. They also describe what effectors are associated with squamous cell carcinoma: p53, p63, p73, cyclin D1, p14, p16, and MYC [
83].
The epigenetics of this cancer are also worth looking at. DNA hypomethylation, histone hypomethylation, and BAF complex loss of function have been reported [
83].
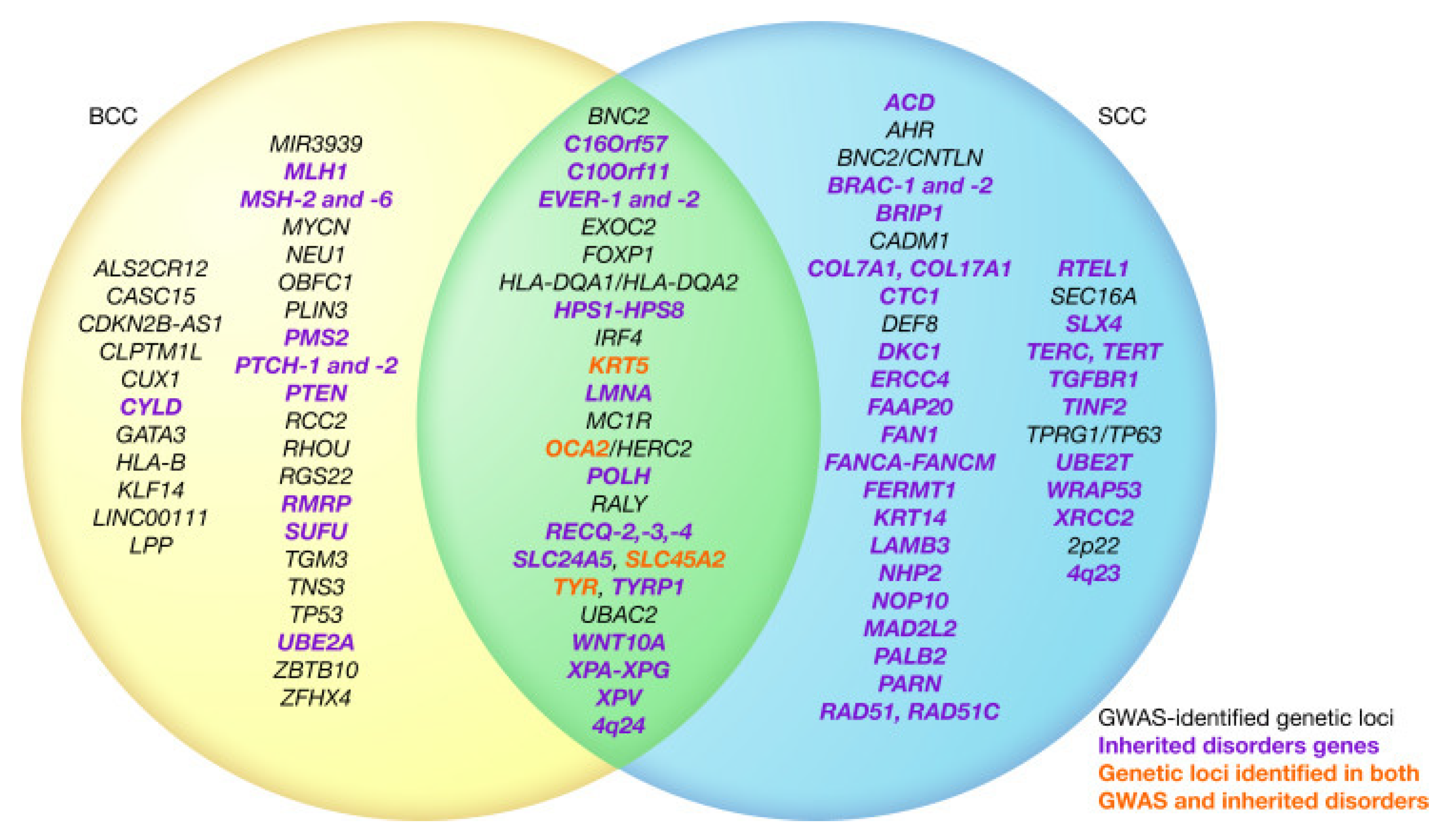
As far as similarities go, both squamous cell carcinoma (right) and basal cell carcinoma (left) share several genetic factors as summarized in Choquet et al. (2020) [
83] (p. 3).
Figure 1.
Venn Diagram of Two Most Common Cancer Type Genes From Choquet et al. (2020).
Figure 1.
Venn Diagram of Two Most Common Cancer Type Genes From Choquet et al. (2020).
3.2.2. Benign Skin Disorders (Atopic Dermatitis, Pemphigus Vulgaris, Psoriasis, Rosacea, Vitiligo)
3.2.2.1. Atopic Dermatitis
While over 30 genetic loci have been linked to atopic dermatitis, loss of function mutation in filaggrin gene has been reported to be the strongest known genetic risk factor. Other genes that confer atopic dermatitis are interleukin-4 and -13 and their respective receptors. GWAS studies and other multi-omics investigations have found more candidate genes [
84]. For elderly individuals, the rise in immunosenescence of the skin can be traced by decrease in T-cell surface markers globally, a decrease in Langerhans cells expressing HBD3, increase in IgG and IgA levels, and a decrease in IgM levels [
85].
3.2.2.2. Pemphigus Vulgaris
Desmoglein-3, a Ca2+-dependent cell adhesion molecule, is considered the primary autoantigen. Additionally, proteomic studies have revealed several other autoantibodies, including desmocollins 1 and 3, muscarinic and nicotinic acetylcholine receptor subtypes, mitochondrial proteins, human leukocyte antigen molecules, thyroid peroxidase, and hSPCA1 [
86,
87,
88]. These autoantibodies play various roles in keratinocyte physiology and cell adhesion.
3.2.2.3. Psoriasis
Psoriasis is characterized by cytokines TNF-α, IFN- γ, IL-10, IL-12, IL17, IL-22, IL-23 and small molecules MAPK Inhibitors, PDE-4 inhibitors, JAK inhibitors [
88]. Cytokines typically have varying structures, modes of detection (surface bound receptor, serum levels), sites of production, specific function, and effects, all of which are succinctly described by Parab & Doshi (2022). In addition, MAPK and p38 are overexpressed in psoriasis and directly contribute to cytokine production. Blocking these proteins, the cAMP pathway via the PDE enzyme, and the JAK-STAT system via JAK1 and STAT3 are ideal approaches for treatment. In the presence of IL-1β and TNF-α, Langerhans cells fail to migrate and so induce CD4+ T cell proliferation during the inflammation response.
On the other hand, the IL-23/IL-17 axis with antimicrobial peptide HBD3 when activated lead to the development of psoriasis [
88,
89]. Another axis of players that can influence psoriasis is the HPA axis. Deregulation of the cross talk between the varying levels of signaling in the HPA axis have been reported to contribute to psoriasis and psoriasis itself can feed into the feed forward mechanism of the HPA axis through expressing pro-inflammatory cytokines and hormones in response to stress [
90].
3.2.2.4. Rosacea
Upregulation of TLR2 promotes the expression of antimicrobial peptide cathelicidin, which is converted to LL-37, a promoter of angiogenesis and skin redness. TLR2 also activates NLRP3, leading to pustule formation and vascular reactivity. PAR2 activation results in inflammation, pruritus, and physical pain. Temperature changes, exercise, UV exposure, spicy food, and alcohol, may also cause TRP ankyrin and vanilloid subfamilies to the release of vasoactive neuropeptides like substance P and calcitonin gene-related peptide [
91,
92]. These genes, when upregulated, collectively contribute to the pathogenesis of rosacea.
3.2.2.5. Vitiligo
The pathogenesis of vitiligo involves several genes and molecular processes, with a focus on the IFN-γ-CXCL9/10-CXCR3 axis and its connection to the JAK/STAT pathway. Melanocytes in vitiligo patients have reduced adhesiveness and heightened susceptibility to oxidative stress [
93].
Polymorphisms in HLA-A confer the most significant genetic risk followed by antigen presentation genes (HLA-DRB1/DQA1, CPVL). Other biomarkers that have been suggested that those that mediate immune target cell lysis (GZMB, FASLG), regulate adaptive immunity (FOXP3, CTLA4, IL2RA, BACH2), drive innate immunity (TICAM1, IFIH1, CD80), and regulate melanocytes (TYR, PMEL, MC1R, OCA2-HERC2, IRF4) [
94].
Various studies have also demonstrated the significance of the IFN-γ-CXCL9/10-CXCR3 axis in vitiligo. This axis inhibits melanogenesis, induces apoptosis of melanocytes, and recruits T cells to the skin. These processes are interconnected with the JAK/STAT pathway. Cytokines such as HSP70i, IL-15, IL-17/23, and TNF, are recruited. The WNT signaling pathway and regulatory T cells are also implicated in vitiligo [
93,
94,
95].
4. Diagnosis of Human Skin Diseases
4.1. Clinical Examination Methods
This subsection aims to describe common examination methods used by clinicians in evaluating skin.
The clinician in interacting with the patient can inquire about any history and recent changes in lifestyle and assess all skin with real-time communication. Even so, there are limitations to superficial assessments, calling for more sophisticated tools.
Typically, additional clinical measures to identify and assess skin disease include taking a biopsy or a scraping for culturing to determine the cell type. For cases of fungi or bacterial infection, a black or wood light is used to shine UV light in a dark room. For viral infection, a Tzanck test is done to obtain fluid from blisters for analysis. When the medical practitioner wishes to observe how the skin responses to a potential allergen, there are four skin tests: use test, patch test, prick test, and intradermal test. The last two may prompt anaphylaxis and are used with caution [
96]. For hard to see features such as scabies burrows, dermatoscopes are used and are useful to distinguish between malignant and benign pigmented lesions [
97,
98].
4.2. Histopathology
This subsection aims to describe common methods used to further characterize skin samples at a microscopic level.
Generally, histology slides of representative tissue are first assessed with the naked eye, then with microscope at low power, and finally with microscope at high power. There are many staining techniques and markers available in dermatopathology with the five below as the most common:
Hematoxylin and Eosin
Immunohistochemistry
Immunofluorescence (Direct and Indirect)
In Situ Hybridization and Fluorescence In Situ Hybridization
Polymerase Chain Reaction
There are also 17 special stains for specific targets such as fungi, bacteria, mast cells, and various molecules. See page 155 from Ladoyanni (2022) for details [
97].
4.3. Imaging Techniques
This subsection lists digital photographic imaging, confocal microscopy, optical coherence tomography (OCT), Raman spectroscopy, and high-frequency ultrasound as established imaging platforms and are used to glean precise and microscopic information that the naked eye cannot perceive.
4.3.1. Digital Photography
Digital photography dates back the longest but is still frequently used for its reflection, back scatter, and polarizing filters to define features such as vascularity and pigmentation. 3D imaging allows for more accurate depictions of lesions compared to 2D photography [
98].
4.3.2. Confocal Microscopy
Confocal microscopy is able to look at a much smaller scale and much higher resolution than digital photography with improved methods for light refraction and excitement. Reflectance confocal microscopy is one type that can visualize skin lesions without a biopsy while remaining accurate, although it is limited in that it requires extensive image analysis to determine cell type and is grayscale [
98]. Here is where the ex vivo confocal microscopy steps in. This method images biopsies both in reflectance and fluorescence modes and then combines them to form a pseudo hematoxylin and eosin stain. Advances have allowed for the adoption of multiple fluorescent direct and indirect antibody stains to be visualized simultaneously with confocal, expanding the versality of this imaging platform [
98,
99]. While confocal is ideal for going to the cellular level of tissue, OCT imaging is strictly useful for tissue level visualizations with a lower resolution than that for histology.
4.3.3. OCT Imaging
OCT imaging is a high resolution, non-invasive, real-time imaging technique that uses safe near-infrared light and accompanying interferometry techniques to compare backscattered light from the specimen to a reference sample and generate a 2D or 3D reconstructed image. In the context of dermatology, OCT imaging can capture the stratum corneum, epidermis, upper dermis, appendages, and blood vessels, even blood flow [
99]. By providing detailed images of tissue microstructure without the need for invasive procedures, OCT imaging has improved medical understanding of organ tissue and their structure.
4.3.4. High-Frequency Ultrasound
Ultrasound can accurately discriminate between skin elements, making it another useful imaging method. Keratin in the epidermis, collagen in the dermis, and fascia in the subcutis are depicted as white from high-intensity echoes from the ultrasound waves while fat globules are depicted as gray from low-intensity echoes [
99]. High-frequency ultrasound trumps OCT imaging in the sense that it has a higher scan depth despite a lower resolution.
4.3.5. Raman Spectroscopy
A non-destructive analytical technique, it is utilized in dermatology to examine the vibrational modes of chemical bonds within skin tissues. It works by shining laser light on the skin, and the scattered light carries information about the molecular composition and structure of the skin’s components [
100]. This method can reveal insights into skin composition, identify specific molecules, and assess various skin conditions and disorders. Its versatility allows for it to be useful for the skin globally considering how heterogeneous and multifunctional it is with differentiated layers. Advances have been made to combine it with confocal microscopy to determine the chemical characterization of skin. Darvin (2023) summarizes 12 non-invasive modern imaging platforms and their advantages, limitations, possibilities, and prospects.
4.3.6. Fluorescence Imaging
It involves fluorescent probes that emit light when excited by specific wavelengths of light. These endogenous and exogenous probes conjugated with antibodies have advanced protein and nucleic acid visualization to be color coded under microscopy in response to controlled light streams. Ultraviolet light is typically used; however, near-infrared lasers can be useful if multiple photon emission fluorescence is of interest [
98]. Another specificity fluorescence imaging has is the use of quenched activity-based probe. It typically consists of a molecular scaffold with a reporter component (often a fluorescent dye or a radioactive tag) and a reactive group designed to bind specifically to an enzyme or protein of interest. Importantly, the probe’s reporter component is initially “quenched” or non-fluorescent due to its proximity to another chemical group within the probe. When the probe binds to its target enzyme or protein and undergoes a chemical reaction, this quenching effect is relieved, resulting in a detectable fluorescent signal.
4.3.7. Machine Learning Algorithms
The tremendous improvement in deep learning algorithms has propelled machine learning in the realm of image detection and analysis. In particular, automating the analysis of reflectance confocal images and OCT images has enabled success in epidermal layer classification, dermo-epidermal junction identification, lesion detection, and cell identification. That said, the consistency of the efficacy of these algorithms varies due to diverse datasets in terms of size, sampled body site, skin features, phototype, and any hidden image processing. Additional challenges include poor image quality, high noise artifacts, low contrast in images; for supervised learning models, there is the added difficulty meeting the need labor-intensive manual labeling of images for supervised models. The most pressing hurdle is the absence of a universally accepted gold standard machine learning models can be held to [
101].
Even with all the promise AI brings with image identification and analysis alone, the adoption of AI algorithms faces several challenges. Firstly, there’s the issue of interpretability, as deep learning algorithms are often seen as black boxes, making it difficult to understand their predictions. Secondly, the need for vast amounts of correctly labeled data to train these models is ever present. Open-access databases like the Human Against Machine (HAM10000) and International Skin Imaging Collaboration are undeniably valuable, yet open-source databases can house data that is poorly labeled, organized, or processed. Additionally, complex data formats for imaging like OCT have been around for years yet the proportion of open-source databases remains much lower than that for images along These challenges are compounded by a lack of standardization in image storage and sharing and potential biases due to over-representation of certain skin conditions and inadequate diversity in skin tones, limiting the applicability of AI models across different patient demographics [
102,
103].
5. Standard Treatment Approaches for Skin Diseases
5.1. Topical and Oral Medications
This subsection aims to cover the various categories of skin disease treatments.
5.1.1. Topical Preparations Overview
Preparations tend to consist of an active drug mixed with an inactive ingredient known as the vehicle. The choice of vehicle affects the consistency of the product and how the drug interacts with the skin (
Table 1) [
104]. Other considerations are any side effects on other systems. For example, corticosteroids reduce inflammation by suppressing the immune response, therefore, they pose a risk for heightened infection and should be used strategically or with antifungal, antibacterial, or antiviral supplementary agents.
Some vehicles are better suited for certain functions than others and have differing sensations when applied. For instance, solutions are ideal cleansing agents because water is a powerful solvent while ointments and powders are better protective agents. Sunscreen is a common example. There are eight categories of impact topical drugs can be described: 1) cleansing, 2) protective, 3) moisturizing, 4) drying, 5) anti-itch, 6) anti-inflammatory, 7) anti-infective, 8) keratolytic.
5.1.3. Oral Preparations Overview
Oral medications for skin diseases offer systemic treatment advantages such consistent dosages, ensuring precise drug delivery to the bloodstream. This convenience is especially beneficial for individuals with extensive affected areas. However, oral medications can affect organs beyond the skin, and usually require monitoring is essential to assess effectiveness and detect potential side effects. Some traditional formulations of oral medications can be in the form of tablets (chewable, effervescent, buccal, sublingual), capsules, liquids, suspensions, powders.
5.1.4. Topical and Oral Antibiotics
Topical antibiotics are available in various forms, such as creams, ointments, gels, lotions, and powders, making them suitable for treating a wide range of skin and mucosal infections. Dallo et al. (2023) summarizes common topical antibiotics used in dermatology (
Table 2) [
105].
Some well-known oral antibiotics are doxycycline, minocycline, cephalexin, erythromycin, sarecycline, azithromycin for many of the skin disorders discussed [
106,
107].
Recent research has uncovered the importance of the skin microbiome in maintaining skin health, calling for more careful prescription of topical and oral antibiotics. Antibiotic resistance generating super strains of bacteria are real concerns of unintended side effects of use of particularly oral antibiotics [
108]. A case study of this danger is with the discontinuation of early tetracycline oral antibiotics to treat acne [
106]. Additionally, the skin has its own natural microbiome for healthy maintenance. Dréno et al. (2020) recognizes a shift in understanding how rather than
Cutibacterium acnes hyperproliferation, it is the loss of balance between the different
C. acnes phylotypes and a collapse of the skin microbiome that results in acne development [
109].
5.1.5. Topical and Oral Retinoids
Topical retinoids are dermatological medications derived from vitamin A that work by promoting skin cell turnover, unclogging pores, and stimulating collagen production. While effective, they can also cause skin irritation, dryness, and increased sensitivity to sunlight. Furthermore, they are contraindicated in pregnancy due to the developmental role of retinoic acid in embryogenesis.
There are four main approved topical retinoids on the U.S. market (FDA) and Canadian market (Health Canada): Tretinoin, Tazarotene, Adapalene, and Trifarotene. All have been used for acne vulgaris and Tazarotene is also applicable to plaque psoriasis [
110].
5.1.4. Topical and Oral Antifungals
Topical antifungals are designed to treat fungal infections of the skin, nails, and mucous membranes. They work by inhibiting the growth and reproduction of fungi. These antifungal creams, ointments, or solutions are used for conditions like athlete’s foot, ringworm, and fungal nail infections. The two dominating causes are tinea pedis and onychomycosis [
111]. For the latter condition, there are oral antifungal alternatives, however, they tend to have stronger side effects and more contraindications than topical antifungals despite faster and quicker recoveries. Efinaconazole, tavaborole, ciclopirox and amorolfine are some common topical antifungals while terbinafine, itraconazole and fluconazole are common oral options with rising alternative therapies posaconazole, fosravuconazole, voriconazole, and oteseconazole [
112,
113,
114].
5.1.5. Topical and Oral Corticosteroids
Corticosteroids help reduce skin inflammation, itching, and redness associated with conditions such as eczema, psoriasis, and dermatitis. They are available in ointments, creams, lotions, gels, solutions, foams, and shampoos. Topical corticosteroids are applied directly to the affected skin, while oral forms may be prescribed for more severe cases. Long-term or improper use of corticosteroids can lead to side effects like skin thinning, adrenal suppression, and even rosacea and dermatitis [
115].
Betamethasone, clobetasol, fluocinonide, flurandrenolide, halobetasol, amcinonide, desoximetsaone, halcinonide, fluticasone, hydrocortisone, triamcinolone, desonide are some established topical corticosteroids that vary by potency are generally treat eczema, psoriasis, dermatitis [
116]. Pemphigus can be treated by corticosteroids, considered the first line of therapy [
117].
5.1.6. Antiviral Medications
5.1.7. Immunosuppressants and Chemotherapy
Medications used to treat cancer tend to address both the corrupted system and the immune system. They also offer an alternative to using corticosteroids which are used as first-line treatment for autoimmune cases such as pemphigus [
118]. Some diseases have treatments that avoid corticosteroids all together such as kaposi sarcoma [
119].
Immunosuppressants used in other therapies such as solid organ transplantation can put patients at risk for skin cancer. Thus, autoimmune diseases can be found as a comorbidity with skin diseases, primarily skin cancer. For example, prednisone and azathioprine suppress the inflammatory response and the formation of DNA in several types of lymphocytes, although alternatives like mycophenolate for azathioprine are viable options [
120]. As far as comparisons go for these two popular immunosuppressants, azathioprine has better affordability, safety, and efficacy; while mycophenolate has fewer side effects and better tolerability for patients. Other high risk immunosuppressants like azathioprine include calcineurin inhibitors and voriconazole. Low risk options mainly consist of mTOR inhibitors. For patients with multiple skin cancers, acitretin or isotretinoin are used instead.
Abatacept, a CTLA-4 inhibitor, has been associated with an increased risk of melanoma, while natalizumab, an integrin inhibitor, has limited evidence suggesting a cancer risk. In contrast, rituximab, a CD20 inhibitor, appears to be protective against malignancy, including skin cancer. Ibrutinib, a BTK inhibitor, has shown a significant increase in reporting of nonmelanoma skin cancer and melanoma cases. JAK inhibitors, such as tofacitinib, baricitinib, filgotinib, and upadacitinib, exhibit mixed results in relation to skin cancer risk, with some studies suggesting an elevated risk, especially for NMSC. Alemtuzumab, a CD52 inhibitor, has preliminary data suggesting no significant cancer association [
121]. More long-term data are needed to provide more definitive guidance.
5.2. Mechanical Therapy
This subsection describes therapies that utilize some physical mechanism in treating skin conditions: phototherapies, laser therapies, Mohs surgery, cryotherapy, and abrasion.
5.2.1. UV Light Therapy
Perhaps surprisingly, light can be turned around as a therapy for skin diseases, even though UV light and other forms of radiation are a common source of skin damage. Phototherapy has been reported to induce apoptosis, T-cell activation in some diseases like psoriasis and vitiligo. Phototherapy is popular because of its wide availability, ease of administration, affordability, and therapeutic efficacy [
122]. Vieyra-Garcia and Wolf (2021) summarize the leading phototherapies into three categories: psoralen plus UV A (PUVA), narrow-band UV B (NB-UVB), and UV A1. They can then be further divided into oral or topical administration.
PUVA utilizes psoralens and UV A light to treat skin diseases such as psoriasis. Psoralens are naturally occurring substances found in plants like figs, celery, and parsley and have been used in medicine for thousands of years. In modern PUVA therapy, psoralens, such as 8-methoxypsoralen (8-MOP) or 5-methoxypsoralen (5-MOP), are administered either orally or topically before exposure to UVA light (320-400 nm). This treatment is particularly effective for conditions like psoriasis and cutaneous T-cell lymphoma. With UVA light, psoralens activate and interacts with cellular components, including DNA, to induce the p53 pathway for cell cycle arrest and eventual apoptosis. While 8-MOP is the standard psoralen in PUVA therapy, 5-MOP is a safe alternative with less erythema but more melanogenic properties, making it suitable for specific skin conditions. It has since the 1970s been expanded to be combined with other treatments like oral retinoids, methotrexate, or vitamin D derivatives. Part of PUVA’s effectiveness comes from how deeply it can reach skin tissue: UVA has a longer wavelength than UV B and so can reach the dermis [
122].
UVB phototherapy follows in popularity with a shorter but more intense impact region. Compared to broad-band UVB that utilizes the entire UVB fraction (280-320 nm), NB-UVB was later found to be more effective than broad-band UVB in most cases because it covers wavelengths 311-312 nm, thus, similarly to PUVA, the longer wavelength aided more in the body’s response to psoriasis to biologics and induce apoptosis in keratinocytes, contributing to rapid plaque resolution. That said, NB-UVB can also induce levels of chemokine associated with acute epidermal injury to rise, a concern for patients with the Koebner response new lesions or patches of the diseased skin condition form at the “injury” site. Transcriptomic studies have revealed that NB-UVB exposure can shift the expression of numerous genes and induce the secretion of antimicrobial peptides and immune-modulating factors. Despite not deeply penetrating the dermis, NB-UVB indirectly influences the local environment, contributing to its therapeutic effects.
UVA1 therapy is the most recent of the three and came about to treat skin conditions like atopic dermatitis and scleroderma. It was designed to reduce the risk of sunburn with relatively longer exposure times than UVB and PUVA therapies require much shorter exposures. These high doses can reduce immune cell and mast cell population by triggering apoptosis , leading to clinical improvement. UVA1 contributes to the generation of superoxide anions, the release of cytochrome c, the activation of apoptosis initiating factor and the cleavage of caspase 3, resulting in prompt apoptosis in lymphocytes and immature mast cells [
122].
5.2.2. Laser Therapy
Since the 1960s, laser therapy for skin diseases has served as a non-invasive treatment that utilizes specialized medical lasers to target and address various skin conditions. Different types of lasers are employed, each tailored to specific skin issues (
Table 4). These lasers emit focused beams of intense light, which are absorbed by targeted pigments or tissues in the skin. Ablative lasers, for example, remove the epidermis and stimulate collagen production by heating up the dermis [
123]. Different wavelengths, durations, and coverage will have varying effects on skin damage and collagen production, depending on the condition being treated. The procedure also includes the application of a numbing cream or local anesthesia for comfort, and protective eyewear is used to shield the eyes from the laser light.
Laser therapy can effectively address acne scars, wrinkles, pigmentation disorders like melasma, vascular conditions such as rosacea, unwanted hair, and even tattoo removal. A related category, laser-assisted drug delivery, has been used in adults and adolescents for squamous cell carcinoma. As for infections, it is not recommended for antibiotic or antifungal cases although antiviral when treating the face and genitalia; and antifungal prophylaxis is not recommended [
129].
5.2.3. Mohs Micrographic Surgery
This technique was developed by Dr. Frederic Mohs in the 1930s as a means to fix and excise cutaneous tumors and has been used to remove basal cell and squamous cell carcinomas [
130,
131]. Since then, it has been refined and expanded to more skin cancers with established practices for post-Mohs surgical wounds. In its current state, the fresh tissue technique and the fixed tissue technique are used with five-year recurrence rates as low as 1% for basal cell carcinomas and 3-5% for squamous cell carcinomas [
131].
There has been extended use of this technique with melanoma despite the prevailing wide excision method. Even though Dr. Mohs established its use with melanoma patients, the American Academy of Dermatology Melanoma Guidelines recommend wide excision but suggest MMS as an alternate for anatomically constrained sites [
132]. The wide margin of normal skin is needed because visual inspection of margins prior to excision is inherently inaccurate and often fails to detect subclinical tumor extension, yet even wide excision does not typically have established safe margins that are agreed upon. The concern with using Mohs’ surgery is that the 10- to 20-mm margins of excision that are recommended for wide excision are rarely achieved and so the narrower margins of Mohs’ surgery stand to miss malignant tumor.
Brodland (2023) argues that this argumentation is not actually supported by clinical trials for wide excision versus Moh’s surgery and present-day use of Mohs’s surgery to remove melanoma have proven effective regardless. Improvements in immunostaining for melanoma antigens have helped in this aspect [
133,
134]. Other studies have reviewed case uses of Mohs’ surgery and noted little to no significant impact on patient survival and/or melanoma recurrence [
135,
136]. Another reason why Mohs’ surgery is not as common as may be expected is due to the lack of local access to a Mohs surgeon. A survey of 402 general dermatologists reported lack of local access to a Mohs surgeon was the most common deterring reason for melanoma in situ and malignant melanoma referral to Mohs’s surgery.
5.2.4. Cryotherapy
Cryotherapy involves the application of extreme cold to the skin’s surface in the management of various skin conditions. Commonly employed for dermatological purposes, cryotherapy is especially effective in warts, actinic keratosis, and certain precancerous lesions. During the procedure, a cryogen, often liquid nitrogen, is used to freeze the targeted skin lesions, causing the affected cells to undergo necrosis and eventual sloughing. The freezing process promotes vasoconstriction, reducing blood flow and minimizing inflammation, thereby facilitating the removal of abnormal skin growths [
138]. Alternatives to cryotherapy include laser therapy and drug regimes, however cryotherapy is a relatively quick and minimally invasive outpatient procedure, often requiring no anesthesia, making it a well-tolerated option for patients. However, it may cause temporary discomfort, redness, or blistering at the treatment site and so is not the best option for pain intolerant patients.
One subtle benefit of cryotherapy compared to traditional excision methods is enhanced immunity. In the case of cryosurgery, the antigens present on the dead malignant cells are retained, allowing for a host immune response to develop [
139,
140].
5.2.5. Dermabrasion
Dermabrasion is utilized to enhance skin appearance by precisely removing its outer layers, similar to laser resurfacing. This non-surgical approach is effective in addressing fine lines, scars, sun damage, and other nuanced skin irregularities. The procedure involves using a high-speed rotating instrument, such as a wire brush or diamond wheel, to gently exfoliate and resurface the skin. Dermabrasion encourages skin regeneration, resulting in a smoother complexion. While generally safe, dermabrasion may cause temporary redness, swelling, and sensitivity.
Dermabrasion has been used in combination with other skincare methods in the treatment of acne and showed to be more effective than when used in isolation [
141]. Pang et al. (2023) suggest that eschar (type of necrotic tissue) dermabrasion can be used to treat deep second-degree wounds with mild scarring and no limitation in joint movement.
6. Conclusions
Dermatology remains an ever-expanding field with the rise of new digital tools like AI and new methods like laser therapies, opening new niches and potential for improved therapies and treatments. While this review is not exhaustive of the diversity of diseases and treatments available in trial, it touches on several pertinent and relevant conditions as anchors to begin understanding the field of dermatology as a whole. Additionally, it highlights gaps in knowledge for some of the discussed skin disorders still pending investigation.
Author Contributions
Conceptualization, H.G.; investigation, N.E.; writing—original draft preparation, N.E.; writing—review and editing, H.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yousef, H.; Alhajj, M.; Sharma, S.; Anatomy, Skin (Integument), Epidermis; StatPearls Publishing: Treasure Island, 2022; https://www.ncbi.nlm.nih.gov/books/NBK470464/.
- National Center for Chronic Disease Prevention and Health Promotion. Health and Economic Benefits of Skin Cancer Interventions. Center for Disease Control and Prevention, 2022. Available online: https://www.cdc.gov/chronicdisease/programs-impact/pop/skin-cancer.htm (accessed on 27 September 2023).
- National Institute of Arthritis and Musculoskeletal and Skin Diseases. Skin Diseases, National Institutes of Health. 2023. Available online: https://www.niams.nih.gov/health-topics/skin-diseases (accessed on 27 September 2023).
- Segall, A. The Sick Role Concept: Understanding Illness Behavior. Journal of Health and Social Behavior, 17(2), 162–169, 1976. [CrossRef]
- US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Skin Cancer. Washington (DC): Office of the Surgeon General (US); 2014. Foreword from the Acting Surgeon General, U.S. Department of Health and Human Services. Available from: https://www.ncbi.nlm.nih.gov/books/NBK247178/.
- Laughter, M.R.; Maymone, M.B.C; Karimkhani, C. The Burden of Skin and Subcutaneous Diseases in the United States From 1990 to 2017. JAMA Dermatol. 2020; 156(8):874–881. [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J Clin. 2023; 73(1): 17-48. [CrossRef]
- Madan, V.; Lear, J.T.; Szeimies, R.M.; Non-melanoma skin cancer. Lancet. 2010; 375(9715):673-685. [CrossRef]
- McDaniel, B; Badri, T; Steele, RB; Basal Cell Carcinoma. In StatPearls. Treasure Island (FL): StatPearls Publishing; September 19, 2022.
- Tanese, K. Diagnosis and Management of Basal Cell Carcinoma. Curr Treat Options Oncol. 2019;20(2):13. [CrossRef]
- Howell, J.Y; Ramsey, M.L. Squamous Cell Skin Cancer. In StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. https://www.ncbi.nlm.nih.gov/books/NBK441939/.
- Guo, W.; Wang, H.; Li, C. Signal pathways of melanoma and targeted therapy. Signal Transduct Target Ther. 6(1):424; 2021. [CrossRef]
- Patel, P.; Hussain, K. Merkel cell carcinoma. Clin Exp Dermatol. 46(5):814-819. 2021. [CrossRef]
- Mistry, K.; Levell, N.J.; Hollestein, L. Trends in incidence, treatment and survival of Merkel cell carcinoma in England 2004-2018: a cohort study. Br J Dermatol. 188(2):228-236. 2023. [CrossRef]
- Dummer, R.; Vermeer, M.H.; Scarisbrick, J.J. Cutaneous T cell lymphoma. Nat Rev Dis Primers. 7(1):61. 2021 Aug 26. [CrossRef]
- Goodlad, J.R.; Cerroni, L.; Swerdlow, S.H. Recent advances in cutaneous lymphoma-implications for current and future classifications. Virchows Arch. 482(1):281-298. 2023. [CrossRef]
- Goyal, A.; LeBlanc, R.E.; Carter, J.B. Cutaneous B-Cell Lymphoma. Hematol Oncol Clin North Am. 33(1):149-161. 2019. [CrossRef]
- Agaimy, A.; Mueller, S.K.; Harrer, T.; Bauer, S.; Thompson, L.D.R. Head and Neck Kaposi Sarcoma: Clinicopathological Analysis of 11 Cases. Head Neck Pathol. 12(4):511-516. 2018. [CrossRef]
- Carrilho, C.; Lunet, N. Global trends in Kaposi sarcoma incidence and mortality: the need for action to reduce inequalities. Lancet Glob Health. 11(10):e1479. 2023. [CrossRef]
- Hussein, H.A.M.; Okafor, I.B.; Walker, L.R.; Abdel-Raouf, U.M.; Akula, S.M. Cellular and viral oncogenes: the key to unlocking unknowns of Kaposi’s sarcoma-associated herpesvirus pathogenesis. Arch Virol. 163(10):2633-2643. 2018. [CrossRef]
- Claudel, J.P.; Auffret, N.; Leccia, M.T.; Poli, F; Dréno, B. Acne and nutrition: hypotheses, myths and facts. J Eur Acad Dermatol Venereol. 32(10):1631-1637. 2018. [CrossRef]
- Karadağ, A.S.; Balta, İ.; Saricaoğlu, H. The effect of personal, familial, and environmental characteristics on acne vulgaris: a prospective, multicenter, case controlled study. G Ital Dermatol Venereol. 154(2):177-185. 2019. [CrossRef]
- Bakry, O.; Shoeib, M.; Soliman, S.; Kamal, L. Neutrophil Cytosolic Factor-1 Genotyping in Acne Vulgaris. Skin Pharmacol Physiol. ;34(1):51-56. 2021. [CrossRef]
- Heng, A.H.S; Say, Y.H.; Sio, Y.Y.; Ng, Y.T.; Chew, FT. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 14(1):103. 2021. [CrossRef]
- Ibrahim, A.A.; Salem, R.M.; El-Shimi, O.S.; Baghdady, S.M.A; Hussein, S. IL1A (-889) gene polymorphism is associated with the effect of diet as a risk factor in Acne Vulgaris. J Cosmet Dermatol. 18(1):333-336. 2019. [CrossRef]
- Teder-Laving, M.; Kals, M.; Reigo, A. Genome-wide meta-analysis identifies novel loci conferring risk of acne vulgaris [published online ahead of print, 2023 Mar 16] [published correction appears in Eur J Hum Genet. 2023 Apr 20. ]. Eur J Hum Genet. 10.1038/s41431-023-01326-8. 2023. [CrossRef]
- Fujii, H.; Endo, Y.; Dainichi, T. Predictive factors of response to pulse methylprednisolone therapy in patients with alopecia areata: A follow-up study of 105 Japanese patients. J Dermatol. 46(6):522-525. 2019. [CrossRef]
- Jacobsen, E.W.; Pedersen, O.B.; Andorsdóttir, G.; Jemec, G.B.E; Bryld, L.E. Family recurrence risk of alopecia areata in the Faroe Islands. Clin Exp Dermatol. 44(7):e224-e229. 2019. [CrossRef]
- Moravvej, H.; Tabatabaei-Panah, P.S.; Abgoon, R. Genetic variant association of PTPN22, CTLA4, IL2RA, as well as HLA frequencies in susceptibility to alopecia areata. Immunol Invest. 47(7):666-679. 2018. [CrossRef]
- Kinoshita-Ise, M.; Martinez-Cabriales, S.A.; Alhusayen, R. Chronological association between alopecia areata and autoimmune thyroid diseases: A systematic review and meta-analysis. J Dermatol. 46(8):702-709. 2019. [CrossRef]
- Tsakok, T.; Woolf, R.; Smith, C.H.; Weidinger, S.; Flohr, C. Atopic dermatitis: the skin barrier and beyond. Br J Dermatol. 180(3):464-474. 2019. [CrossRef]
- Ho, C.L.; Chang, L.I.; Wu, W.F. The prevalence and risk factors of atopic dermatitis in 6-8 year-old first graders in Taipei. Pediatr Neonatol. 60(2):166-171. 2019. [CrossRef]
- Belugina, I.N.; Yagovdik, N.Z.; Belugina, O.S.; Belugin, S.N. Outdoor environment, ozone, radionuclide-associated aerosols and incidences of infantile eczema in Minsk, Belarus. J Eur Acad Dermatol Venereol. 32(11):1977-1985. 2018. [CrossRef]
- Nishijima, H.; Suzuki S.; Kondo, K.; Yamasoba, T.; Yanagimoto, S. Environmental factors associated with allergic rhinitis symptoms in Japanese university students: A cross-sectional study [published correction appears in Auris Nasus Larynx. 2019 Jun;46(3):485]. Auris Nasus Larynx. 45(5):1006-1013. 2018. [CrossRef]
- Prodinger, C.; Bauer, J.W.; Laimer, M. Translational perspectives to treat Epidermolysis bullosa-Where do we stand?. Exp Dermatol. 29(11):1112-1122. 2020. [CrossRef]
- Yenamandra, V.K.; Vellarikkal, S.K.; Chowdhury, M.R. Genotype-Phenotype Correlations of Dystrophic Epidermolysis Bullosa in India: Experience from a Tertiary Care Centre. Acta Derm Venereol. 98(9):873-879. 2018. [CrossRef]
- Diaconescu, S.; Strat, S.; Balan, G.G. Dermatological Manifestations in Pediatric Inflammatory Bowel Disease. Medicina (Kaunas). 2020;56(9):425. 2020. [CrossRef]
- Abdollahimajd, F.; Youssefian, L.; Pourani, M.R.; Vahidnezhad, H.; Uitto, J. Coronavirus disease 2019 and epidermolysis bullosa: Report of three cases. Dermatol Ther. 33(6):e14194. 2020. [CrossRef]
- Byrd, A.S.; Rosenberg, A.Z.; Shipman, W.D. Hidradenitis suppurativa in Black and White patients - a clinical study. Eur Rev Med. Pharmacol Sci. 27(3 Suppl):92-98. 2023. [CrossRef]
- De D.R.; Rick, J.W.; Shih, T.; Hsiao, J.L.; Hamzavi, I.; Shi, V.Y. COVID-19 Infection in Hidradenitis Suppurativa Patients: A Retrospective Study. Skin Appendage Disord. 9(3):203-206. 2023. [CrossRef]
- Gierek, M.; Niemiec, P.; Szyluk, K.; Ochala-Gierek, G.; Bergler-Czop, B. Hidradenitis suppurativa and squamous cell carcinoma: a systematic review of the literature. Postepy Dermatol Alergol. 40(3):350-354. 2023. [CrossRef]
- Mokos, Z.B.; Čagalj, A.M.; Marinović, B. Epidemiology of Hidradenitis Suppurativa [published online ahead of print, 2023 Sep 9]. Clin Dermatol. S0738-081X(23)00099-8. 2023. [CrossRef]
- Lee, J.H.; Kwon, H.S.; Jung, H.M.; Kim, G.M.; Bae, J.M. Prevalence and comorbidities associated with hidradenitis suppurativa in Korea: a nationwide population-based study. J Eur Acad Dermatol Venereol. 32(10):1784-1790. 2018. [CrossRef]
- Garg, A.; Wertenteil, S.; Baltz, R.; Strunk, A.; Finelt, N. Prevalence Estimates for Hidradenitis Suppurativa among Children and Adolescents in the United States: A Gender- and Age-Adjusted Population Analysis. J Invest Dermatol. 138(10):2152-2156. 2018. [CrossRef]
- Shrestha, A.B.; Biswas, P.; Shrestha, S. Harlequin ichthyosis: A case report and literature review. Clin Case Rep. 10(12):e6709. 2022. [CrossRef]
- Kaushik, H.; Mahajan, R.; Dabas, G. A cross-sectional study to find association of VDR gene polymorphism with non-syndromic congenital ichthyosis and with vitamin D deficiency. Arch Dermatol Res. 315(3):551-557. 2023. [CrossRef]
- Smith, F.J.D.; Hansen, C.D.; Hull, P.R. Kaspar, R.L.; McLean, I.; O’Toole, E.; Sprecher, E. Pachyonychia Congenita. 2006 Jan 27 [Updated 2017 Nov 30]. In: Adam MP, Mirzaa GM, Pagon RA. editors. GeneReviews®. Seattle (WA): University of Washington, Seattle; 1993-2023. https://www.ncbi.nlm.nih.gov/books/NBK1280/.
- Chovatiya, R.; Silverberg, J.I. Association of pemphigus and pemphigoid with osteoporosis and pathological fractures. Arch Dermatol Res. 312(4):263-271. 2020. [CrossRef]
- Kang, M.; Bilgic, A. Radjenovic M, Murrell DF. Osteoporosis and bone health in autoimmune blistering skin disease-an evidenced based review. J Eur Acad Dermatol Venereol. 34(12):2745-2756. 2020. [CrossRef]
- De Medeiros, V.L.S.; Monteiro-Neto, A.U.; França, D.D.T.; Castelo Branco, R.; de Miranda Coelho, É.O.; Takano, D.M. Pemphigus Vulgaris After COVID-19: a Case of Induced Autoimmunity. SN Compr Clin Med. 3(8):1768-1772. 2021. [CrossRef]
- Siddig, O.; Mustafa, M.B.; Kordofani, Y.; Gibson, J.; Suleiman, A.M. The epidemiology of autoimmune bullous diseases in Sudan between 2000 and 2016. PLoS One. 16(7):e0254634. 2021. [CrossRef]
- Lin, N.; Li, X.; Lang, Y.; Han, J. Case Report: Pemphigus in Young Patients With Thymic Anomalies. Front Med (Lausanne). 2022;9:844223. 2022. [CrossRef]
- Seifollahi, A.; Fazl, M.R.; Setayesh, L. The Association Between Dietary Diversity Score and Cardiovascular Risk Factors Among Patients With Pemphigus Vulgaris: A Cross Sectional Study. Clin Nutr Res.11(4):289-301. 2022. [CrossRef]
- Namazi, N.; Ariaeenejad, S.; Azad, M.E.; Pishgahi, M. Risk of Atrial Fibrillation in Pemphigus Vulgaris. Indian J Dermatol. 67(6):639-644. 2022. [CrossRef]
- Cozzani, E.; Rosa, G.M.; Burlando, M.; Parodi, A. Psoriasis as a cardiovascular risk factor: updates and algorithmic approach. G Ital Dermatol Venereol. 153(5):659-665. 2018. [CrossRef]
- Kadian-Dodov, D. Cold Hands or Feet: Is It Raynaud’s or Not?. Med Clin North Am. 107(5):829-844. 2023. [CrossRef]
- Lomanta, J.M.J.; Atienza, M.A.; Gonzales, J.R.M. Erasmus Syndrome: A Case Report and Literature Review. Am J Case Rep. 23:e937061. 2022. [CrossRef]
- Nobeyama, Y.; Aihara, Y.; Asahina, A. Characteristics of Rosacea and Similar Diseases in Patients Wearing Face Masks. Skin Appendage Disord. 8(6):462-468. 2022. [CrossRef]
- Li, G.; Wang, B.; Zhao, Z. Excessive cleansing: an underestimating risk factor of rosacea in Chinese population. Arch Dermatol Res.313(4):225-234. 2021. [CrossRef]
- Tsai, T.Y.; Chiang, Y.Y.; Huang, Y.C. Cardiovascular Risk and Comorbidities in Patients with Rosacea: A Systematic Review and Meta-analysis. Acta Derm Venereol. 100(17):adv00300. 2020. [CrossRef]
- Zhang, J.; Yan, Y.; Jiang, P. Association between rosacea and cardiovascular disease: A systematic review and meta-analysis. J Cosmet Dermatol. 20(9):2715-2722. 2021. [CrossRef]
- Liu, L.; Xue, Y.; Chen, Y. Alcohol consumption and the risk of rosacea: A systematic review and meta-analysis. J Cosmet Dermatol. 21(7):2954-2961. 2022. [CrossRef]
- Bütikofer, L.; Varisco, P.A.; Distler, O. ACE inhibitors in SSc patients display a risk factor for scleroderma renal crisis-a EUSTAR analysis. Arthritis Res Ther. 22(1):59. 2020. [CrossRef]
- Hesselstrand, R.; Scheja, A.; Wuttge, D.M. Scleroderma renal crisis in a Swedish systemic sclerosis cohort: survival, renal outcome, and RNA polymerase III antibodies as a risk factor. Scand J Rheumatol. 41(1):39-43. 2012. [CrossRef]
- Chaudhary, P.; Chen, X.; Assassi, S. Cigarette smoking is not a risk factor for systemic sclerosis. Arthritis Rheum. 63(10):3098-3102. 2011. [CrossRef]
- Fawzy, M.M.; Hammad, N.M.; Sharaf, A.L.; Khattab, F. Hepatitis C virus infection could be a risk factor for adult-onset vitiligo in Egyptian patients: A cross-sectional study. J Cosmet Dermatol. 21(10):4983-4989. 2022. [CrossRef]
- Al-Harthi, F.; Zouman, A.; Arfin, M.; Tariq, M.; Al-Asmari, A. Tumor necrosis factor-α and -β genetic polymorphisms as a risk factor in Saudi patients with vitiligo. Genet Mol Res. 12(3):2196-2204. 2013. [CrossRef]
- Naik, S.; Fuchs, E. Inflammatory memory and tissue adaptation in sickness and in health. Nature. 607(7918):249-255. 2022. [CrossRef]
- Marek-Jozefowicz, L.; Nedoszytko, B.; Grochocka, M. Molecular Mechanisms of Neurogenic Inflammation of the Skin. Int J Mol Sci. 24(5):5001. 2023. [CrossRef]
- Jakovija, A; Chtanova, T. Skin immunity in wound healing and cancer. Front Immunol. 2023;14:1060258. 2023. [CrossRef]
- Novak, N.; Tordesillas, L.; Cabanillas, B. Diversity of T cells in the skin: Novel insights. Int Rev Immunol. 42(3):185-198. 2023. [CrossRef]
- Castillo-González, R.; Cibrian, D.; Sánchez-Madrid, F. Dissecting the complexity of γδ T-cell subsets in skin homeostasis, inflammation, and malignancy. J Allergy Clin Immunol. 147(6):2030-2042. 2021. [CrossRef]
- Venanzi Rullo, E.; Maimone, M.G.; Fiorica, F. Non-Melanoma Skin Cancer in People Living With HIV: From Epidemiology to Clinical Management. Front Oncol. 11:689789. 2021. [CrossRef]
- Hasche, D.; Akgül, B. Prevention and Treatment of HPV-Induced Skin Tumors. Cancers (Basel). 15(6):1709. 2023. [CrossRef]
- Choquet, H. Ashrafzadeh, S.; Kim, Y.; Asgari, M.M.; Jorgenson, E. Genetic and environmental factors underlying keratinocyte carcinoma risk. JCI Insight. 5(10):e134783. 2020. [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int J Dermatol. 49(9):978-986. 2010. [CrossRef]
- National Human Genome Research Institute. Available online: https://www.genome.gov/dna-day/15-ways/cancer-genomics (accessed on September 12 2023).
- Liu, Y.; Sheikh, M.S. Melanoma: Molecular Pathogenesis and Therapeutic Management. Mol Cell Pharmacol. 2014;6(3):228. PMID: 2574553.
- Bataille, V. It’s Not All Sunshine: Non-sun-related Melanoma Risk-factors. Acta Derm Venereol. 100(11):adv00137. 2020. [CrossRef]
- Newton-Bishop, J.; Bishop, D.T.; Harland, M. Melanoma Genomics. Acta Derm Venereol. 100(11):adv00138. 2020. [CrossRef]
- Krakowski, A.C.; Hafeez, F.; Westheim, A.; Pan, E.Y.; Wilson, M. Advanced basal cell carcinoma: What dermatologists need to know about diagnosis. J Am Acad Dermatol. 86(6S):S1-S13. 2022. [CrossRef]
- Basset-Seguin, N.; Herms, F. Update in the Management of Basal Cell Carcinoma. Acta Derm Venereol. 100(11):adv00140. 2020. [CrossRef]
- Droll S., Bao X. Oh, the Mutations You’ll Acquire! A Systematic Overview of Cutaneous Squamous Cell Carcinoma. Cell Physiol Biochem. 2021;55(S2):89-119. [CrossRef]
- Løset, M.; Brown, S.J.; Saunes, M.; Hveem, K. Genetics of Atopic Dermatitis: From DNA Sequence to Clinical Relevance. Dermatology.235(5):355-364. 2019. [CrossRef]
- Bocheva, G.S.; Slominski, R.M.; Slominski, A.T. Immunological Aspects of Skin Aging in Atopic Dermatitis. Int J Mol Sci. 22(11):5729. 2021. [CrossRef]
- Malik, A.M.; Tupchong, S.; Huang, S.; Are, A.; Hsu, S.; Motaparthi, K. An Updated Review of Pemphigus Diseases. Medicina (Kaunas). 57(10):1080. 2021. [CrossRef]
- Amber, K.T.; Valdebran, M.; Grando, S.A. Non-Desmoglein Antibodies in Patients With Pemphigus Vulgaris. Front Immunol. 9:1190. 2018. [CrossRef]
- Parab, S.; Doshi, G. An update on emerging immunological targets and their inhibitors in the treatment of psoriasis. Int Immunopharmacol. 113(Pt A):109341. 2022. [CrossRef]
- Yan, B.; Liu, N.; Li, J. The role of Langerhans cells in epidermal homeostasis and pathogenesis of psoriasis. J Cell Mol Med. 24(20):11646-11655. 2020. [CrossRef]
- Marek-Jozefowicz, L.; Czajkowski, R.; Borkowska, A. The Brain-Skin Axis in Psoriasis-Psychological, Psychiatric, Hormonal, and Dermatological Aspects. Int J Mol Sci. 2022;23(2):669. 2022. [CrossRef]
- Daou, H.; Paradiso, M.; Hennessy, K.; Seminario-Vidal, L. Rosacea and the Microbiome: A Systematic Review. Dermatol Ther (Heidelb). 11(1):1-12. 2021. [CrossRef]
- van Zuuren, E.J.; Arents, B.W.M.; van der Linden, M.M.D.; Vermeulen, S.; Fedorowicz, Z.; Tan, J. Rosacea: New Concepts in Classification and Treatment. Am J Clin Dermatol. 22(4):457-465. 2021. [CrossRef]
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology. 236(6):571-592. 2020. [CrossRef]
- Frisoli, M.L.; Essien, K.; Harris, J.E. Vitiligo: Mechanisms of Pathogenesis and Treatment. Annu Rev Immunol. 38:621-648. 2020. [CrossRef]
- Feng, Y.; Lu, Y. Advances in vitiligo: Update on therapeutic targets. Front Immunol. 13:986918. 2022. [CrossRef]
- Benedetti, J. Diagnosis of Skin Disorders. In MSD Manual Consumer Version. 2022. Available on: https://www.msdmanuals.com/home/skin-disorders/biology-of-the-skin/diagnosis-of-skin-disorders.
- Ladoyanni, E. Histopathology of the Skin: General Principles. Atlas of Dermatology, Dermatopathology and Venereology; Smoller, B., Bagherani, N., Eds; Springer Nature: Switzerland; 2022, pp. 145-160.
- Schneider, S.L.; Kohli, I.; Hamzavi, I.H.; Council, M.L.; Rossi, A.M.; Ozog, D.M. Emerging imaging technologies in dermatology: Part I: Basic principles. J Am Acad Dermatol. 80(4):1114-1120. 2019. [CrossRef]
- Atak, M.F.; Farabi, B.; Navarrete-Decshent, C.; Rubinstein, G.; Rajadhyaksha, M.; Jain, M. Confocal Microscopy for Diagnosis and Management of Cutaneous Malignancies: Clinical Impacts and Innovation. Diagnostics (Basel). 13(5):854. 2023. [CrossRef]
- Darvin, M.E. Optical Methods for Non-Invasive Determination of Skin Penetration: Current Trends, Advances, Possibilities, Prospects, and Translation into In Vivo Human Studies. Pharmaceutics. 15(9):2272. 2023. [CrossRef]
- Lboukili, I.; Stamatas, G.; Descombes, X. Automating reflectance confocal microscopy image analysis for dermatological research: a review. J Biomed Opt. 27(7):070902. 2022. [CrossRef]
- Widaatalla, Y.; Wolswijk, T.; Adan, F. The application of artificial intelligence in the detection of basal cell carcinoma: A systematic review. J Eur Acad Dermatol Venereol. 37(6):1160-1167. 2023. [CrossRef]
- Brown-Korsah, J.B.; McKenzie, S.; Omar, D.; Syder, N.C.; Elbuluk, N.; Taylor, S.C. Variations in genetics, biology, and phenotype of cutaneous disorders in skin of color - Part I: Genetic, biologic, and structural differences in skin of color. J Am Acad Dermatol. 87(6):1239-1258. 2022. [CrossRef]
- Keri, J.E. Treatment of Skin Disorders. In Merck Manual Consumer Version. 2022. Available on: https://www.merckmanuals.com/home/skin-disorders/treatment-of-skin-disorders/treatment-of-skin-disorders.
- Dallo, M.; Patel, K.; Hebert, A.A. Topical Antibiotic Treatment in Dermatology. Antibiotics (Basel). 12(2):188. 2023. [CrossRef]
- Jo, J.H.; Harkins, C.P.; Schwardt, N.H. Alterations of human skin microbiome and expansion of antimicrobial resistance after systemic antibiotics. Sci Transl Med. 13(625):eabd8077. 2021. [CrossRef]
- Nagler, A.R.; Del Rosso, J. The Use of Oral Antibiotics in the Management of Rosacea. J Drugs Dermatol. 18(6):506. 2019.
- Baldwin, H. Oral Antibiotic Treatment Options for Acne Vulgaris. J Clin Aesthet Dermatol. 13(9):26-32. 2020.
- Dréno, B.; Dagnelie, M.A.; Khammari, A.; Corvec, S. The Skin Microbiome: A New Actor in Inflammatory Acne. Am J Clin Dermatol. 21(Suppl 1):18-24. 2020. [CrossRef]
- Callender, V.D.; Baldwin, H.; Cook-Bolden, F.E.; Alexis, A.F.; Stein Gold, L.; Guenin, E. Effects of Topical Retinoids on Acne and Post-inflammatory Hyperpigmentation in Patients with Skin of Color: A Clinical Review and Implications for Practice. In Am J Clin Dermatol. 23(1):69-81. 2022. [CrossRef]
- Rotta, I.; Sanchez, A.; Gonçalves, P.R.; Otuki, MF; Correr, CJ. Efficacy and safety of topical antifungals in the treatment of dermatomycosis: a systematic review. Br J Dermatol. 166(5):927-933. 2012. [CrossRef]
- Gupta, A.K.; Stec, N.; Summerbell, R.C. Onychomycosis: a review. J Eur Acad Dermatol Venereol. 34(9):1972-1990. 2020. [CrossRef]
- Kovitwanichkanont, T.; Chong, A.H. Superficial fungal infections. Aust J Gen Pract. 48(10):706-711. 2019. [CrossRef]
- Gupta, A.K.; Talukder, M.; Venkataraman, M. Review of the alternative therapies for onychomycosis and superficial fungal infections: posaconazole, fosravuconazole, voriconazole, oteseconazole. Int J Dermatol. 61(12):1431-1441. 2022. [CrossRef]
- Goa, K.L. Clinical pharmacology and pharmacokinetic properties of topically applied corticosteroids. A review. Drugs. 36 Suppl 5:51-61. 1998. [CrossRef]
- Stacey, SK; McEleney, M. Topical Corticosteroids: Choice and Application. In Am Fam Physician. 103(6):337-343. 2021.
- Zhao, W.; Wang, J.; Zhu, H.; Pan, M. Comparison of Guidelines for Management of Pemphigus: a Review of Systemic Corticosteroids, Rituximab, and Other Immunosuppressive Therapies. Clin Rev Allergy Immunol.;61(3):351-362. 2021. [CrossRef]
- Zhao, W.; Wang, J.; Zhu, H.; Pan, M. Comparison of Guidelines for Management of Pemphigus: a Review of Systemic Corticosteroids, Rituximab, and Other Immunosuppressive Therapies. Clin Rev Allergy Immunol. 61(3):351-362. 2021. [CrossRef]
- Wang, L.L.; Lin, S.K.; Stull, C.M. Cutaneous Oncology in the Immunosuppressed. Dermatol Clin. 2023;41(1):141-162. 2023. [CrossRef]
- Griffith, C.F. Skin cancer in immunosuppressed patients. JAAPA. 35(2):19-27. 2022. [CrossRef]
- Kreher, M.A.; Konda, S.; Noland, M.M.B.; Longo, M.I.; Valdes-Rodriguez, R. Risk of melanoma and nonmelanoma skin cancer with immunosuppressants, part II: Methotrexate, alkylating agents, biologics, and small molecule inhibitors. J Am Acad Dermatol. 88(3):534-542. 2023. [CrossRef]
- Vieyra-Garcia, P.A.; Wolf, P. A deep dive into UV-based phototherapy: Mechanisms of action and emerging molecular targets in inflammation and cancer. In Pharmacol Ther. 222:107784. 2021. [CrossRef]
- Mayo Clinic. Available on: https://www.mayoclinic.org/tests-procedures/laser-resurfacing/about/pac-20385114 (accessed on 20 October 2023).
- Braun, S.A.; Schrumpf, H.; Buhren, B.A.; Homey, B.; Gerber, P.A. Laser-assisted drug delivery: mode of action and use in daily clinical practice. J Dtsch Dermatol Ges. 2016;14(5):480-488. [CrossRef]
- Stanford Healthcare. Available online: https://stanfordhealthcare.org/medical-treatments/p/pulsed-dye-laser-treatment.html (accessed on 15 November 2023).
- Ibrahim, A.M.; Omar, G.A.B.; Hamdino, M. Long-pulsed Nd: YAG laser (1064 nm) versus intralesional botulinum toxin type (A) in acne vulgaris therapy: a split face study. Int J Dermatol. 2023;62(6):822-830. [CrossRef]
- Kao, Y.C.; Lin, D.Z.; Kang, Y.N.; Chang, C.J.; Chiu, W.K.; Chen, C. Efficacy of Laser in Hair Removal: A Network Meta-analysis. J Cosmet Laser Ther. 2023;25(1-4):7-19. [CrossRef]
- Mallat, F.; Chaaya, C.; Aoun, M.; Soutou, B.; Helou, J. Adverse Events of Light-Assisted Hair Removal: An Updated Review. J Cutan Med Surg. 2023;27(4):375-387. [CrossRef]
- Labadie, J.G.; Ibrahim, S.A.; Worley, B. Evidence-Based Clinical Practice Guidelines for Laser-Assisted Drug Delivery. In JAMA Dermatol. 158(10):1193-1201. 2022. [CrossRef]
- Swanson, N.A. Mohs Surgery: Technique, Indications, Applications, and the Future. Arch Dermatol. 1983;119(9):761–773. [CrossRef]
- Robins, P.; Ebede, T.L.; Hale, E.K. The Evolution of Mohs Surgery. Skin Cancer Foundation. https://www.skincancer.org/treatment-resources/mohs-surgery/history-of-mohs/.
- Swetter, S.M.; Tsao, H.; Bichakjian, C.K.; Curiel-Lewandrowski, C.; Elder, D.E.; Gershenwald, J.E.; Guild, V.; Grant-Kels, J.M.; Halpern, A.C.; Johnson, T.M.; Sober, A.J.; Thompson, J.A.; Wisco, O.J.; Wyatt, S.; Hu, S.; Lamina, T. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80(1):208-250. [CrossRef]
- Brodland, D.G. Mohs Micrographic Surgery for Melanoma: Evidence, Controversy, and a Critical Review of Excisional Margin Guidelines. Dermatol Clin. 2023;41(1):79-88. [CrossRef]
- Elgash, M.; Young, J.; White, K.; Leitenberger, J.; Bar A. An Update and Review of Clinical Outcomes Using Immunohistochemical Stains in Mohs Micrographic Surgery for Melanoma [published online ahead of print, 2023 Sep 18]. Dermatol Surg. 2023. [CrossRef]
- Crum, O.M.; Campbell, E.H.; Chelf, C.J.; Demer, A.M.; Brewer, J.D. Disease-specific survival of malignant melanoma after Mohs micrographic surgery is not impacted by initial margins: A systematic review and meta-analysis. JAAD Int. 2023;13:140-149. Published 2023 Jun 28. [CrossRef]
- Beal, B.T.; Udkoff, J.; Aizman, L.; Etzkorn, J.; Zitelli, J.A.; Miller, C.J.; Shin, T.M.; Sobanko, J.F.; Brodland, D.G. Outcomes of invasive melanoma of the head and neck treated with Mohs micrographic surgery - A multicenter study. J Am Acad Dermatol. 2023;89(3):544-550. [CrossRef]
- Neill, B.C.; Siscos, S.M.; Bar, A.A.; Seger, E.W.; Latour, E.; Tolkachjov, S.N. Factors Influencing General Dermatologists When Referring Patients with Head and Neck Melanoma for Mohs Micrographic Surgery: A Nationwide Cross-Sectional Survey. Dermatol Surg. 2023;49(5):451-455. [CrossRef]
- Ashique, K.T.; Kaliyadan, F.; Jayasree, P. Cryotherapy: Tips and Tricks. J Cutan Aesthet Surg. 2021;14(2):244-247. [CrossRef]
- Sabel, M.S. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58(1):1-11. [CrossRef]
- Liao, Y.; Chen, Y.; Liu, S.; Wang, W.; Fu, S.; Wu, J. Low-dose total body irradiation enhances systemic anti-tumor immunity induced by local cryotherapy. J Cancer Res Clin Oncol. 2023;149(12):10053-10063. [CrossRef]
- Goberdhan, L.T.; Schneider, K; Makino, E.T.; Mehta R.C. Combining Diamond-Tip Dermabrasion Treatments and Topical Skincare in Participants with Dry, Hyperpigmented, Photodamaged or Acne-Prone/Oily Facial Skin: A Clinical Usage Study. Clin Cosmet Investig Dermatol. 2023;16:2645-2657. Published 2023 Sep 25. [CrossRef]
Table 1.
Comparison of Topical Vehicle Types.
Table 1.
Comparison of Topical Vehicle Types.
| Vehicle |
Benefits |
Drawbacks |
| Ointments |
Stronger drug delivery, low irritancy |
Greasy feel and difficult to wash off |
| Creams |
Easy to apply, relatively nonirritating |
Evaporate easily resulting in weak moisture and skin barrier formation, water content can allow for microbial growth |
| Lotions |
easy application |
low drug delivery, see cream drawbacks |
| Foams |
absorbed rapidly, can be used in hairy areas |
can leave skin dry due to alcohol and drying agents |
| Solutions |
easy to apply |
requires additional agents to ease irritation caused by solvent. |
| Powders |
effective at drying skin |
tricky application, inhalation danger, limited effectiveness with oily skin types, cannot form protective barrier on skin |
| Gels |
easy application, useful for cleaning debris that cannot be washed with water |
low moisturization capability |
Table 2.
List of Common Topical Antibiotics from Dallo et al. (2023).
Table 2.
List of Common Topical Antibiotics from Dallo et al. (2023).
| Skin Disease |
Common Antibiotics |
| Rosacea |
Azelaic acid, Minocycline, Metronidazole |
| Acne Vulgaris |
Azelaic Acid, Benzoyl Peroxide, Clindamycin, Dapsone, Minocycline |
| Hidradenitis Suppurativa |
Clindamycin |
| Dermatitis |
Fusidic Acid. Mupirocin |
| Skin and Soft-Tissue Infection |
Amikacin, Clindamycin, Fusidic Acid, Mupirocin, Ozenoxacin, Retapamulicin |
Table 3.
Viruses and State of Antiviral Treatments.
Table 3.
Viruses and State of Antiviral Treatments.
| Virus |
Skin Disease State |
Vaccine Availability |
| Herpes Simplex Virus |
Herpes Labialis, Herpetic Whitlow |
No, No |
| Varicella-Zoster Virus |
Chickenpox, Shingles |
Yes, Yes |
| Human Papillomavirus Virus |
Common Warts, Plantar Warts, Genital Warts |
No, No, Yes |
| Measles Virus |
Measles |
Yes |
| Coxsackievirus |
Hand, Foot, and Mouth Disease |
No |
| Human Immunodeficiency Virus |
Induced Rash |
No |
| Molluscum Contagiosum Virus |
Molluscum Contagiosum |
No |
| Dengue Virus |
Dengue Fever |
Yes |
Table 4.
Common Types of Lasers.
Table 4.
Common Types of Lasers.
| Laser |
Target |
Reference |
| CO2 Laser |
Scars, warts, wrinkles, skin outgrowths (benign and cancerous) |
Mayo Clinic [123], Braun et al. (2016) [124] |
| Erbium YAG Laser |
See above |
Braun et al. (2016) [124], Mohammed (2023) [126] |
| Pulsed Dye Laser |
Vascular skin conditions like rosacea, scar tissue, hemangioma |
Stanford Healthcare [125], Mallat et al. (2023) [128] |
| Alexandrite and Diode Laser |
Unwanted hair and pigmentation disorders |
Kao et al. (2023) [127], Mallat et al. (2023) [128] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).