Submitted:
20 November 2023
Posted:
01 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials & Methods
2.1. Patient Consent & Tissue Collection
2.2. Cell Lines and Reagents
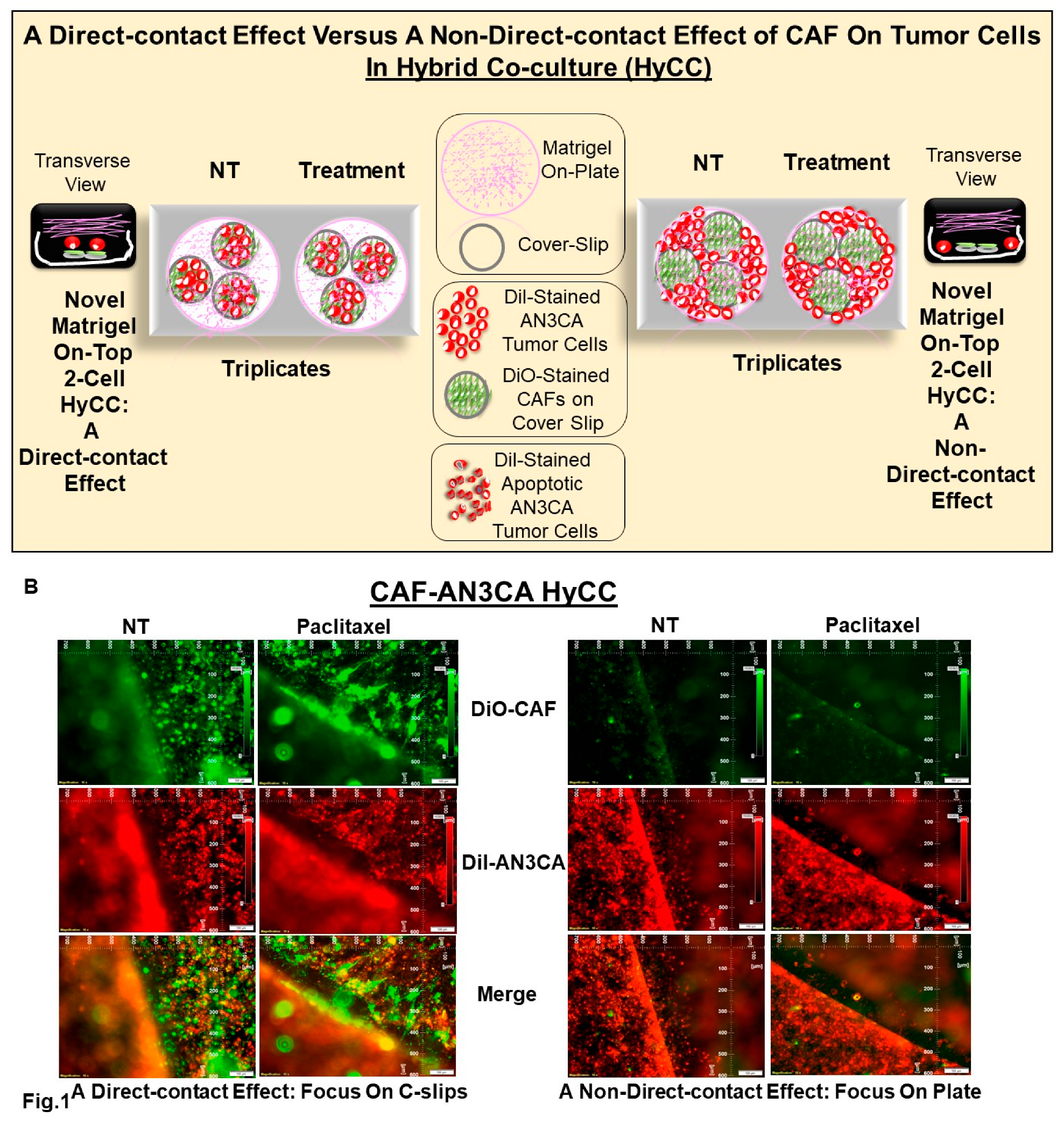
2.3. Establishment of Patient-specific Ex Vivo Tumor-Endometrial CAF-2-Cell Model of Hybrid Co-Culture (HyCC)
2.4. Effect of CAFs On Paclitaxel Alone and In Combination with Targeted Antitumor Drugs Using 2-Cell Hybrid Co-culture Model
3. Results
3.1. Patient Information
3.2. Ex Vivo Primary Culture Of CAFs and Marker-based Verification of CAF
3.3. Patient-specific Ex Vivo Model of Hybrid Co-Culture (HyCC)
3.4. Effect of CAFs in Resisting Paclitaxel in Combination with Pathway-Targeted Drugs
4. Discussion
Funding
Authors’ Contribution
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Patent Status
References
- Brooks, R.A.; Fleming, G.F.; Lastra, R.R.; Lee, N.K.; Moroney, J.W.; Son, C.H.; Tatebe, K.; Veneris, J.L. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin 2019, 69, 258–279. [Google Scholar] [CrossRef] [PubMed]
- Connor, E.V.; Rose, P.G. Management Strategies for Recurrent Endometrial Cancer. Expert Rev Anticancer Ther 2018, 18, 873–885. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, Y.; Weeraratna, A.T. Fibroblasts in cancer: Unity in heterogeneity. Cell 2023, 186, 1580–1609. [Google Scholar] [CrossRef] [PubMed]
- Winterhoff, B.; Konecny, G.E. Targeting fibroblast growth factor pathways in endometrial cancer. Curr Probl Cancer 2017, 41, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Dandara, C. Broadening Drug Design and Targets to Tumor Microenvironment? Cancer-Associated Fibroblast Marker Expression in Cancers and Relevance for Survival Outcomes. OMICS 2020, 24, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Berral-Gonzalez, A.; Lopez-Cade, I.; Galindo-Pumarino, C.; Bueno-Fortes, S.; Martin-Merino, M.; Carrato, A.; Ocana, A.; De La Pinta, C.; Lopez-Alfonso, A.; et al. Cancer-associated fibroblast-derived gene signatures determine prognosis in colon cancer patients. Mol Cancer 2021, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, R.; De, P.; Aske, J.C.; Lin, X.; Dale, A.; Gaster, K.; Espaillat, L.R.; Starks, D.; Dey, N. A CAF-Based Two-Cell Hybrid Co-Culture Model to Test Drug Resistance in Endometrial Cancers. Biomedicines 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, R.; De, P.; Aske, J.C.; Lin, X.; Dale, A.; Gaster, K.; Espaillat, L.R.; Starks, D.; Dey, N. Characterization and Clinical Relevance of Endometrial CAFs: Correlation between Post-Surgery Event and Resistance to Drugs. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Karpel, H.C.; Slomovitz, B.; Coleman, R.L.; Pothuri, B. Treatment options for molecular subtypes of endometrial cancer in 2023. Curr Opin Obstet Gynecol 2023, 35, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.; Shen, S.; Monk, B.J.; Tan, D.S.P.; Nogueira-Rodrigues, A.; Aoki, D.; Sehouli, J.; Makker, V. Looking beyond carboplatin and paclitaxel for the treatment of advanced/recurrent endometrial cancer. Gynecol Oncol 2022, 167, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Markman, M.; Fowler, J. Activity of weekly paclitaxel in patients with advanced endometrial cancer previously treated with both a platinum agent and paclitaxel. Gynecol Oncol 2004, 92, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Kaznatcheev, A.; Peacock, J.; Basanta, D.; Marusyk, A.; Scott, J.G. Fibroblasts and alectinib switch the evolutionary games played by non-small cell lung cancer. Nat Ecol Evol 2019, 3, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Teixeira, A.F.; Zhu, H.J.; Ten Dijke, P. Cancer associated-fibroblast-derived exosomes in cancer progression. Mol Cancer 2021, 20, 154. [Google Scholar] [CrossRef] [PubMed]
- Richards, K.E.; Zeleniak, A.E.; Fishel, M.L.; Wu, J.; Littlepage, L.E.; Hill, R. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene 2017, 36, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: new findings and future perspectives. Mol Cancer 2021, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat Rev Clin Oncol 2021, 18, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, W.; Lin, S.; Liu, B.; Wu, P.; Li, L. Fibroblast diversity and plasticity in the tumor microenvironment: roles in immunity and relevant therapies. Cell Commun Signal 2023, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Ravichandra, A.; Bhattacharjee, S.; Affo, S. Cancer-associated fibroblasts in intrahepatic cholangiocarcinoma progression and therapeutic resistance. Adv Cancer Res 2022, 156, 201–226. [Google Scholar] [CrossRef] [PubMed]
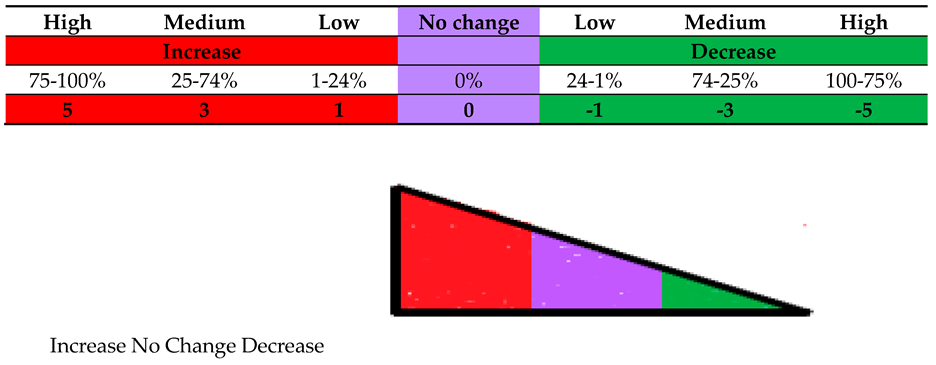
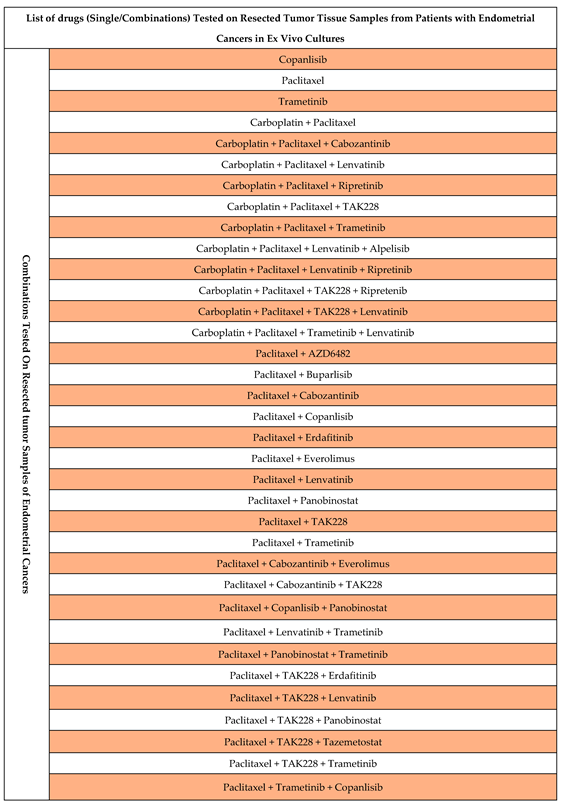
| Combination* # | Drugs used for the Combination |
| A | Paclitaxel plus Copanlisib |
| B | Paclitaxel plus TAK228 |
| C | Paclitaxel plus Lenvatinib |
| D | Paclitaxel plus Trametinib |
| E | Copanlisib |
| Cell Type(s) Involved in the Experi-ment | Treat-ment Type (s) | Culture Platform Type(s) | End- Time of the Experi-ment | Comparison Type(s) | Photo-micrographs Presented in Figure # | Grades* (Arbitrary Scale Based on the % of Fluorescence Intensity of DiI-AN3CA) | Grades* (Arbitrary Scale Based on the % of Fluorescence Intensity of DiO-CAF) |
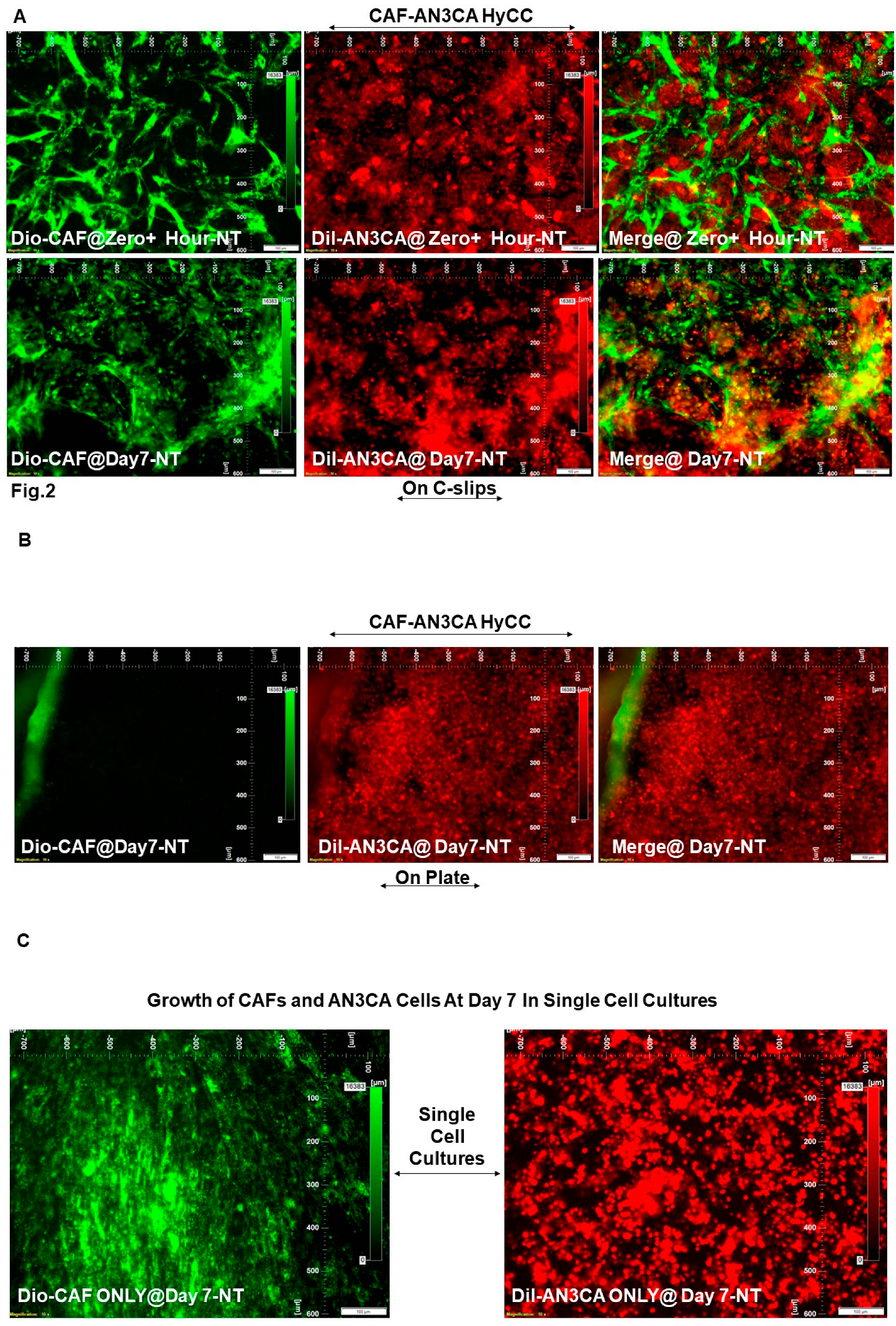
| DiO-CAF & DiI-AN3CA | Vehicle Control (NT) | HyCC On Cover slips (DiO-CAF) | Day 7 | Day Zero+ vs Day7 | Figure 2A | 5 | 3 |
| DiI-AN3CA | HyCC On Plates | Day7 as compared to Day Zero+ | Figure 2B | 5 | Not Applicable (Focused On-Plate) | ||
| DiI-AN3CA | Single Cell Cultures | Day7 as compared to Day Zero+ | Figure 2C (Right Panel) | 5 | Not Applicable | ||
| DiO-CAF | Day7 as compared to Day Zero+ | Figure 2C (Left Panel) | Not Applicable | 5 | |||
| Cell Type(s) Involed in the Experi-ment | Treat-ment Type (s) | Culture Platform Type(s) | End- Time of the Experi-ment | Comparison Type(s) | Photo-micrographs Presented in Figure # | Grades* (Arbitrary Scale Based on the % of Fluorescence Intensity of DiI-AN3CA) | Grades* (Arbitrary Scale Based on the % of Fluorescence Intensity of DiO-CAF) |
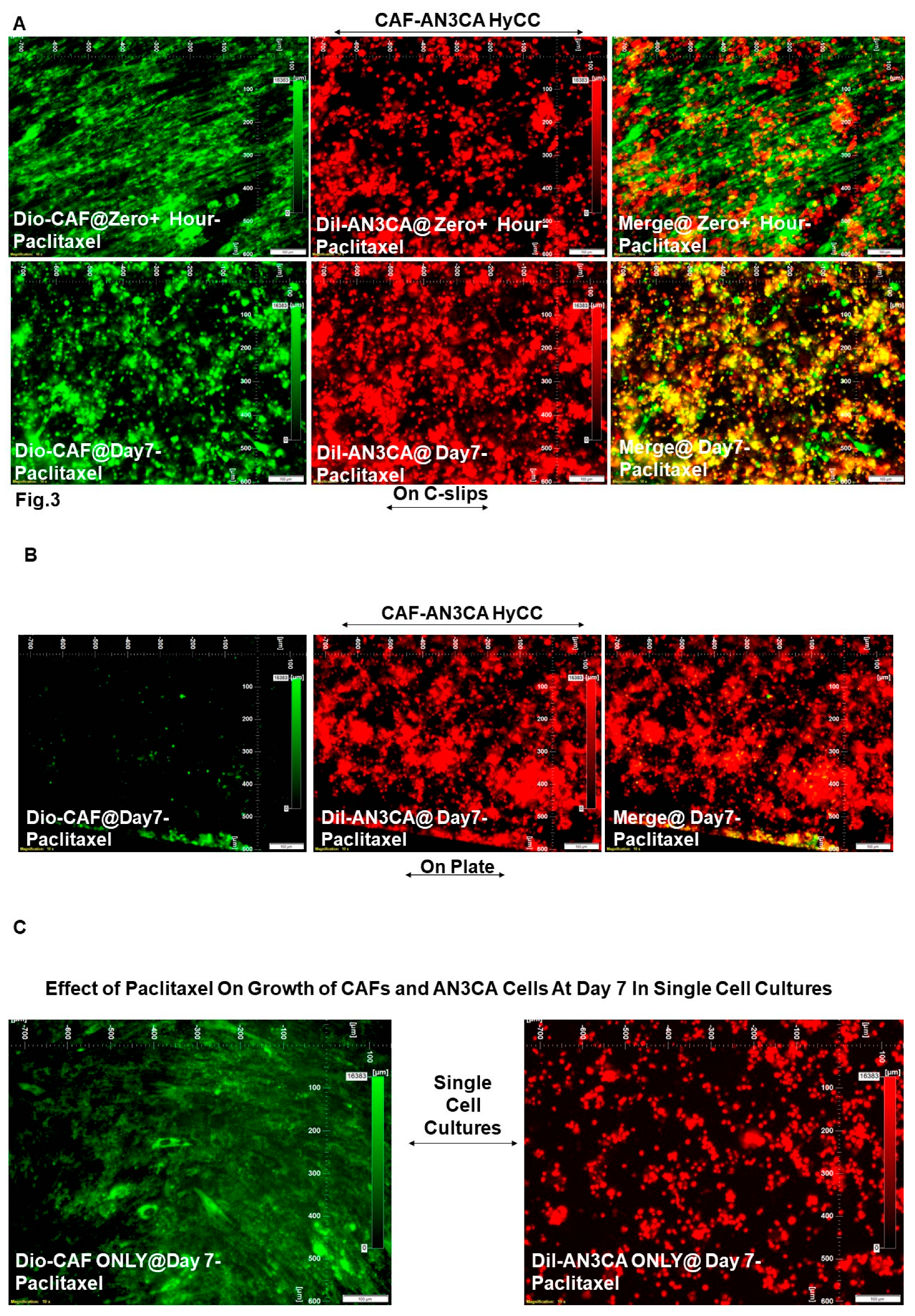
| DiO-CAF & DiI-AN3CA | Pacli-taxel | HyCC On Cover slips (DiO-CAF) | Day 7 | Drug Treatment Vs. No Treatment |
Figure 3A vs. Figure 2A |
0 | 0 |
| DiI-AN3CA | HyCC On Plates | Figure 3B vs. Figure 2B | 0 | Not Applicable (Focused On-Plate) | |||
| DiI-AN3CA | Single Cell Cultures |
Figure 3C (Right Panel) vs. Figure 2C (Right Panel) |
-5 | Not Applicable | |||
| DiO-CAF |
Figure 3C (Left Panel) vs Figure 2C (Left Panel) |
Not Applicable | 0 | ||||
| Cell Type(s) Involed in the Experi-ment | Treat-ment Type (s) | Culture Platform Type(s) | End- Time of the Experi-ment | Comparison Type(s) | Photo-micrographs Presented in Figure # | Grades* (Arbitrary Scale Based on the % of Fluorescence Intensity of DiI-AN3CA) | Grades* (Arbitrary Scale Based on the % of Fluorescence Intensity of DiO-CAF) |
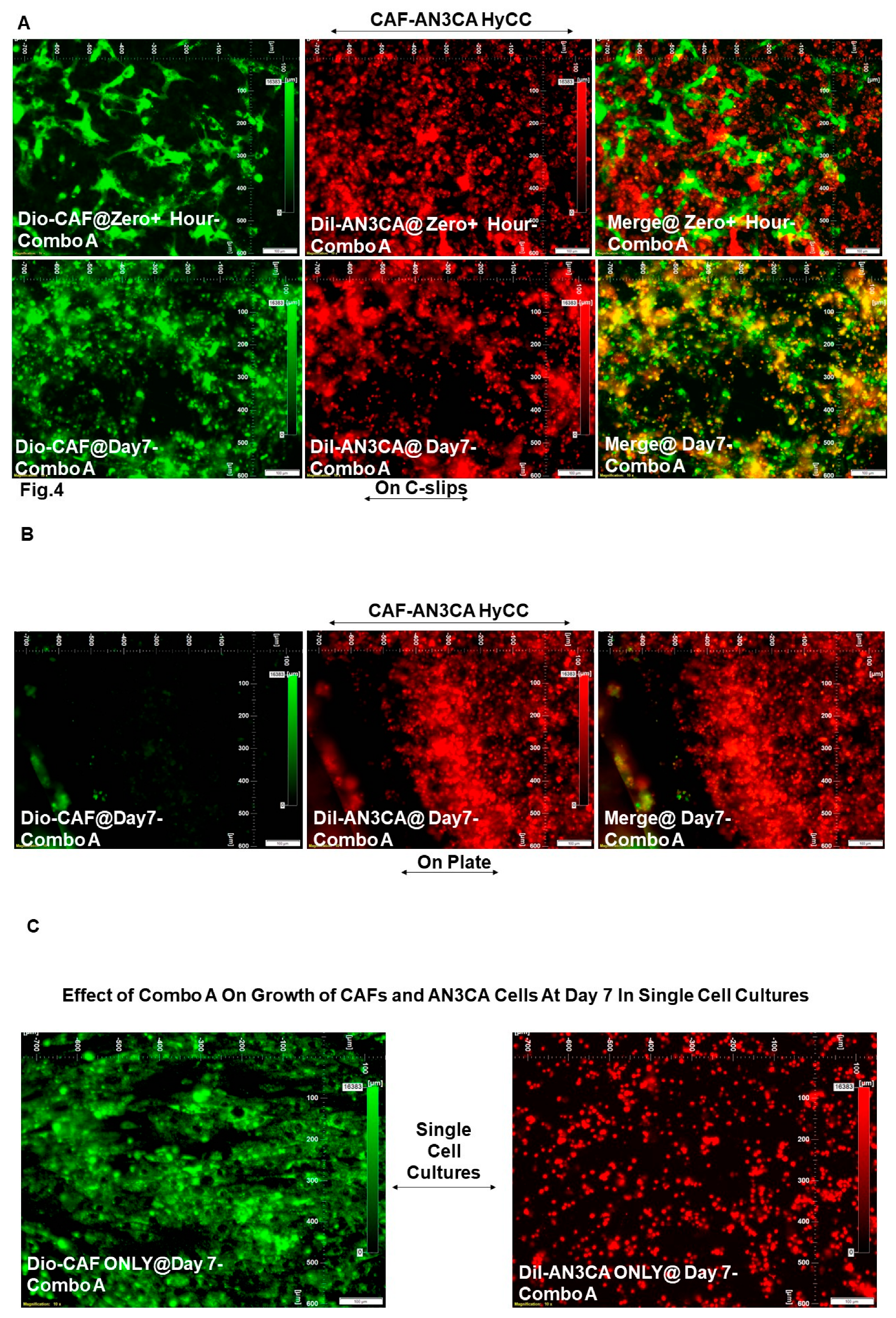
| DiO-CAF & DiI-AN3CA | Pacli-taxel + Copan-lisib (Combo A) |
HyCC On Cover slips (DiO-CAF) | Day 7 | Drug Treatment Vs. No Treatment |
Figure 4A vs. Figure 2A |
0 | 0 |
| DiI-AN3CA | HyCC On Plates | Figure 4B vs. Figure 2B | 0 | Not Applicable (Focused On-Plate) | |||
| DiI-AN3CA | Single Cell Cultures |
Figure 4C (Right Panel) vs. Figure 2C (Right Panel) |
-5 | Not Applicable | |||
| DiO-CAF |
Figure 4C (Left Panel) vs Figure 2C (Left Panel) |
Not Applicable | 0 | ||||
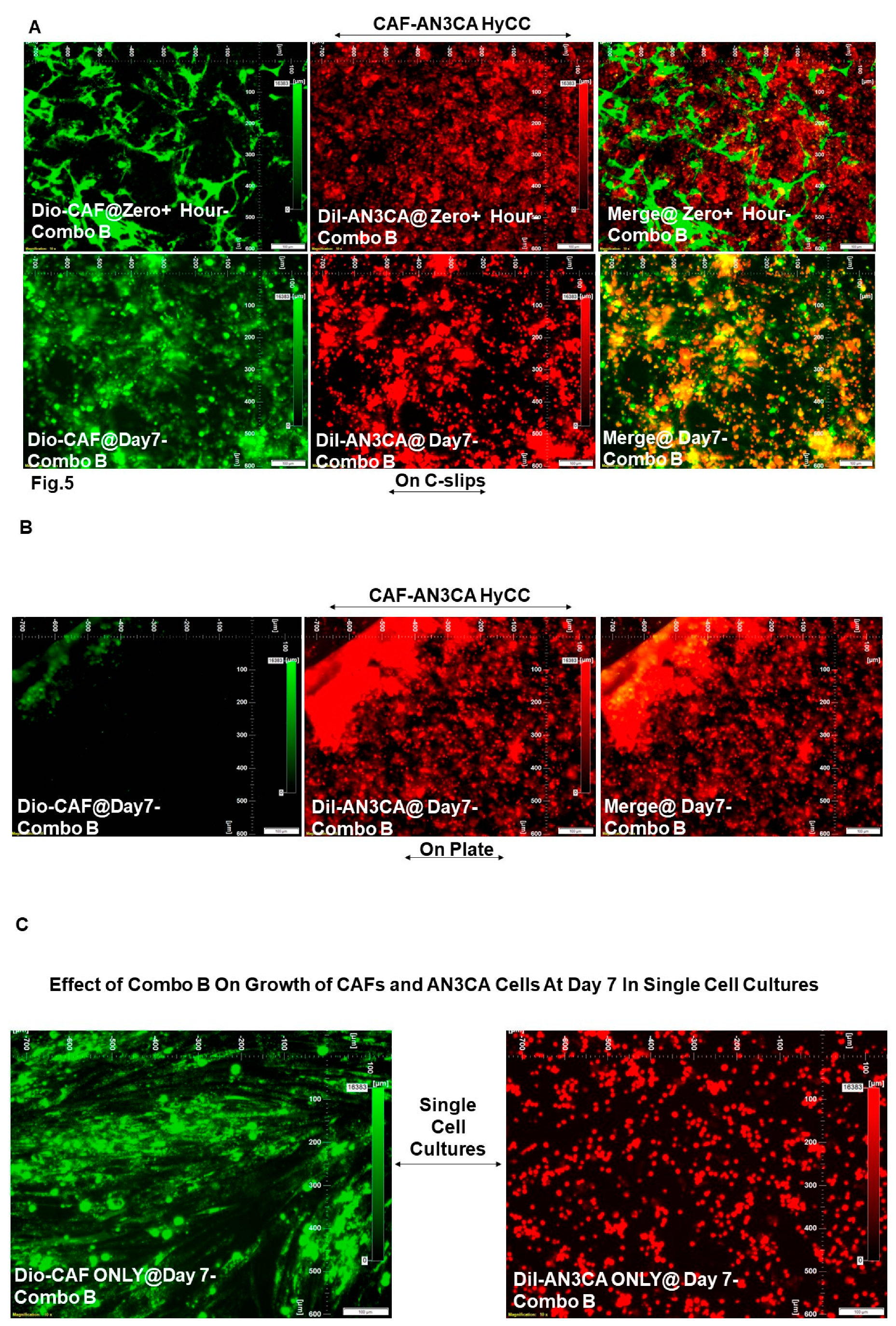
| Cell Type(s) Involed in the Experi-ment | Treat-ment Type (s) | Culture Platform Type(s) | End- Time of the Experi-ment | Comparison Type(s) | Photo-micrographs Presented in Figure # | Grades* (Arbitrary Scale Based on the % of Fluorescence Intensity of DiI-AN3CA) | Grades* (Arbitrary Scale Based on the % of Fluorescence Intensity of DiO-CAF) |
| DiO-CAF & DiI-AN3CA | Pacli-taxel + TAK 228 (Combo B) | HyCC On Cover slips (DiO-CAF) | Day 7 | Drug Treatment Vs. No Treatment |
Figure 5A vs. Figure 2A |
0 | 0 |
| DiI-AN3CA | HyCC On Plates | Figure 5B vs. Figure 2B | 0 | Not Applicable (Focused On-Plate) | |||
| DiI-AN3CA | Single Cell Cultures |
Figure 5C (Right Panel) vs. Figure 2C (Right Panel) |
-5 | Not Applicable | |||
| DiO-CAF |
Figure 5C (Left Panel) vs Figure 2C (Left Panel) |
Not Applicable | 0 | ||||
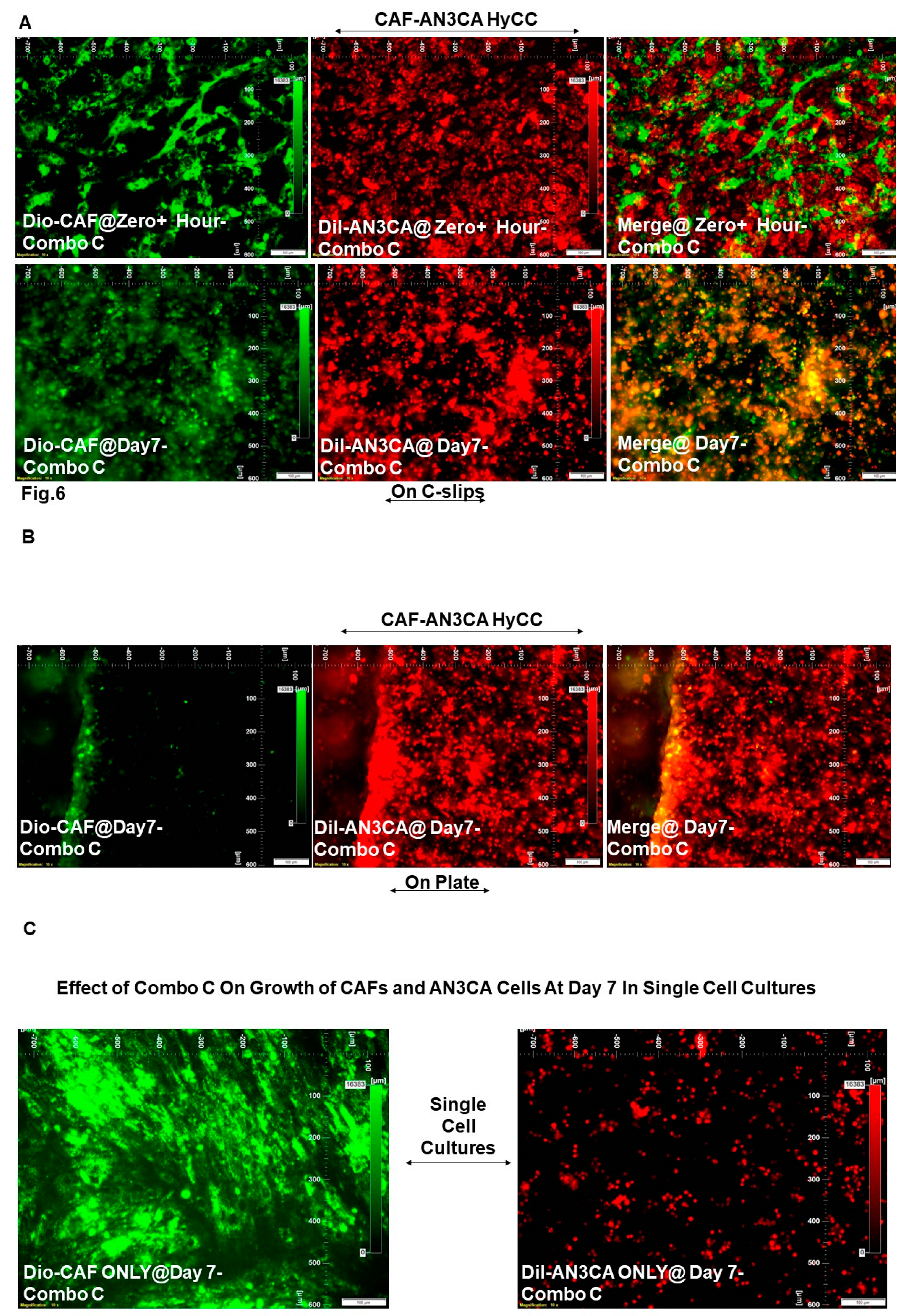
| Cell Type(s) Involed in the Experi-ment | Treat-ment Type (s) | Culture Platform Type(s) | End- Time of the Experi-ment | Comparison Type(s) | Photo-micrographs Presented in Figure # | Grades* (Arbitrary Scale Based on the % of Fluorescence Intensity of DiI-AN3CA) | Grades* (Arbitrary Scale Based on the % of Fluorescence Intensity of DiO-CAF) |
| DiO-CAF & DiI-AN3CA | Pacli-taxel + Lenva-tinib (Combo C) |
HyCC On Cover slips (DiO-CAF) | Day 7 | Drug Treatment Vs. No Treatment |
Figure 6A vs. Figure 2A |
0 | 0 |
| DiI-AN3CA | HyCC On Plates | Figure 6B vs. Figure 2B | 0 | Not Applicable (Focused On-Plate) | |||
| DiI-AN3CA | Single Cell Cultures |
Figure 6C (Right Panel) vs. Figure 2C (Right Panel) |
-5 | Not Applicable | |||
| DiO-CAF |
Figure 6C (Left Panel) vs Figure 2C (Left Panel) |
Not Applicable | 0 | ||||
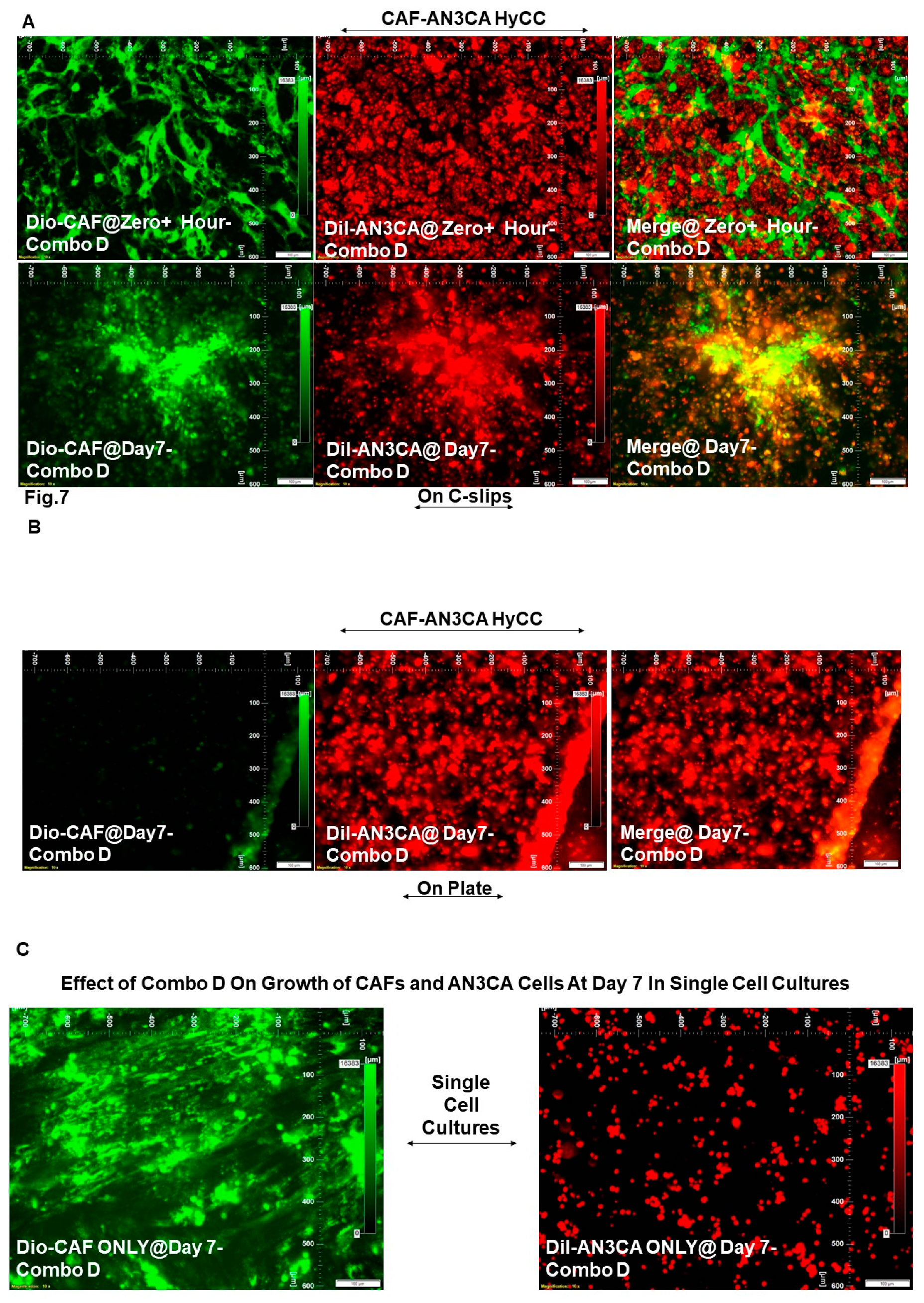
| Cell Type(s) Involed in the Experi-ment | Treat-ment Type (s) | Culture Platform Type(s) | End- Time of the Experi-ment | Comparison Type(s) | Photo-micrographs Presented in Figure # | Grades* (Arbitrary Scale Based on the % of Fluorescence Intensity of DiI-AN3CA) | Grades* (Arbitrary Scale Based on the % of Fluorescence Intensity of DiO-CAF) |
| DiO-CAF & DiI-AN3CA | Pacli-taxel + Trame-tinib (Combo D) |
HyCC On Cover slips (DiO-CAF) | Day 7 | Drug Treatment Vs. No Treatment |
Figure 7A vs. Figure 2A |
0 | 0 |
| DiI-AN3CA | HyCC On Plates | Figure 7B vs. Figure 2B | 0 | Not Applicable (Focused On-Plate) | |||
| DiI-AN3CA | Single Cell Cultures |
Figure 7C (Right Panel) vs. Figure 2C (Right Panel) |
-5 | Not Applicable | |||
| DiO-CAF |
Figure 7C (Left Panel) vs Figure 2C (Left Panel) |
Not Applicable | 0 | ||||
| Cell Type(s) Involed in the Experi-ment | Treat-ment Type (s) | Culture Platform Type(s) | End- Time of the Experi-ment | Comparison Type(s) | Photo-micrographs Presented in Figure # | Grades* (Arbitrary Scale Based on the % of Fluorescence Intensity of DiI-AN3CA) | Grades* (Arbitrary Scale Based on the % of Fluorescence Intensity of DiO-CAF) |
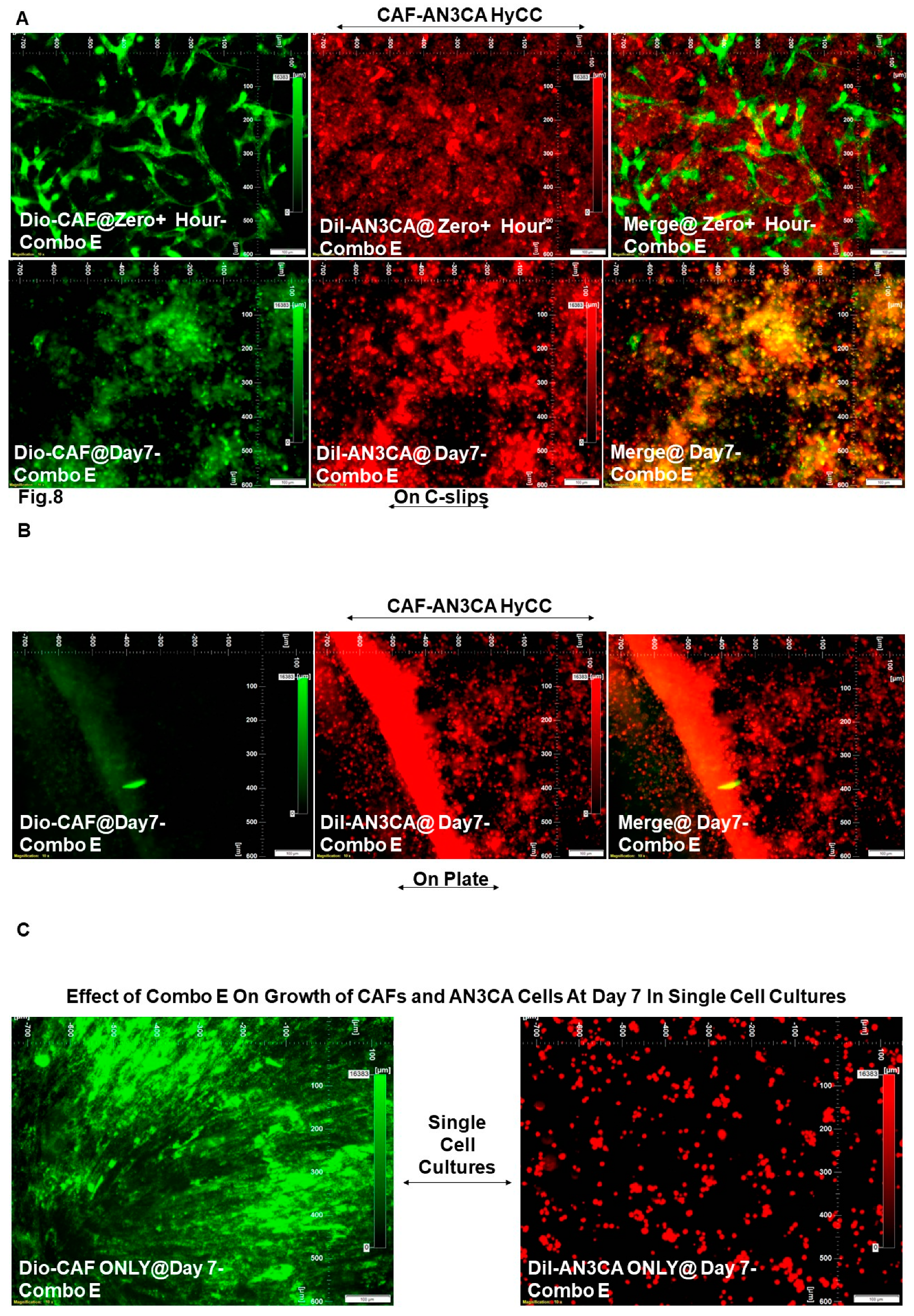
| DiO-CAF & DiI-AN3CA | Copan-lisib (Combo E) | HyCC On Cover slips (DiO-CAF) | Day 7 | Drug Treatment Vs. No Treatment |
Figure 8A vs. Figure 2A |
0 | 0 |
| DiI-AN3CA | HyCC On Plates | Day 7 | Figure 8B vs. Figure 2B | 0 | Not Applicable (Focused On-Plate) | ||
| DiI-AN3CA | Single Cell Cultures | Day 7 |
Figure 8C (Right Panel) vs. Figure 2C (Right Panel) |
-5 | Not Applicable | ||
| DiO-CAF | Day 7 |
Figure 8C (Left Panel) vs Figure 2C (Left Panel) |
Not Applicable | 0 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





