Submitted:
30 November 2023
Posted:
01 December 2023
You are already at the latest version
Abstract
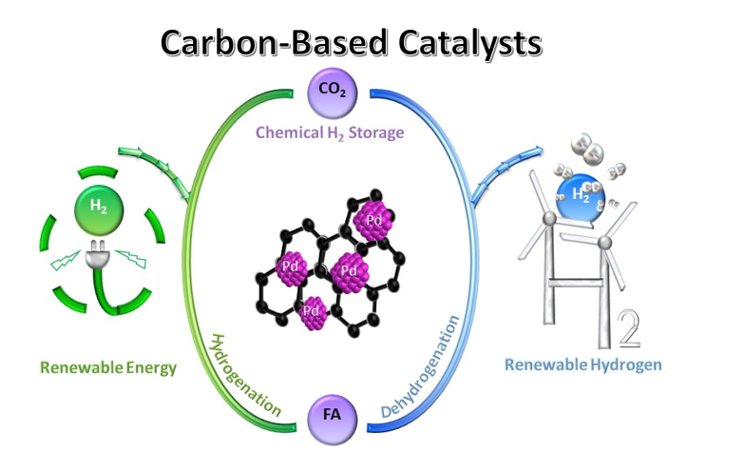
Keywords:
1. Introduction
2. Dehydrogenation of formic acid
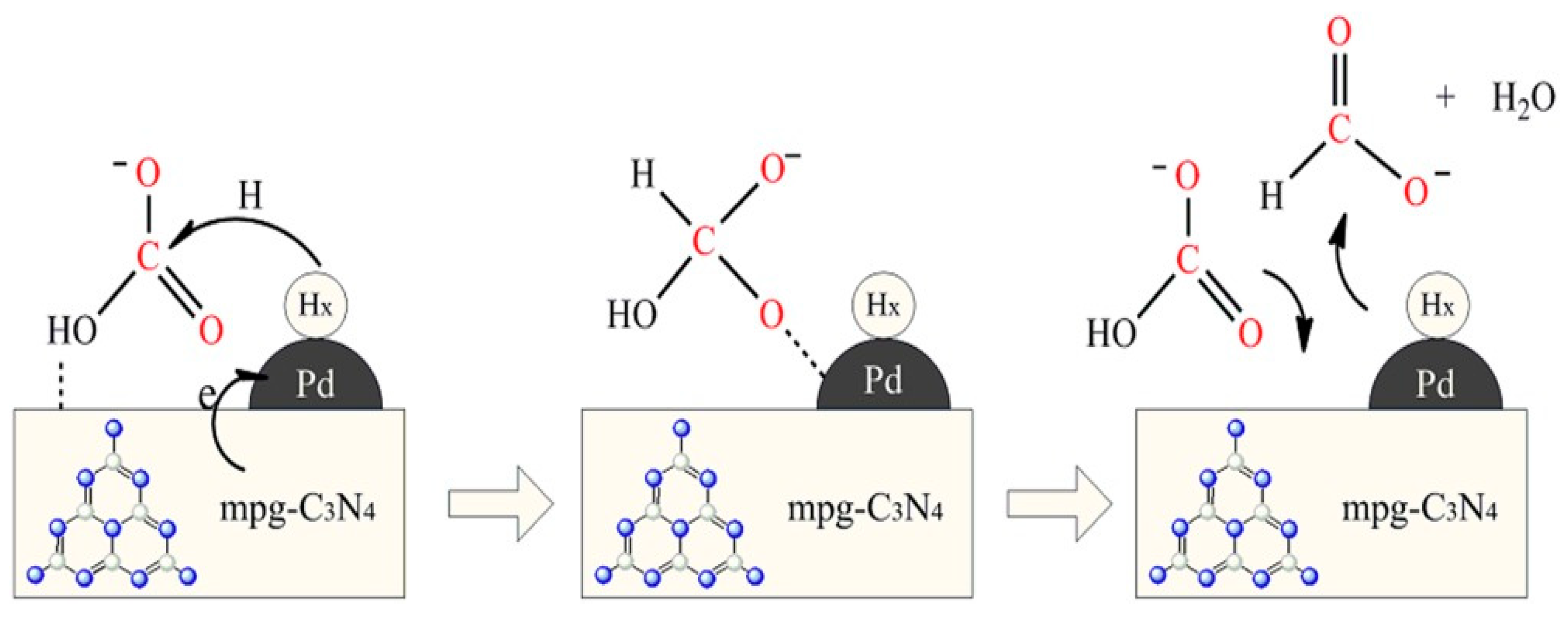
2.1. Carbon-supported monometallic Pd catalysts.
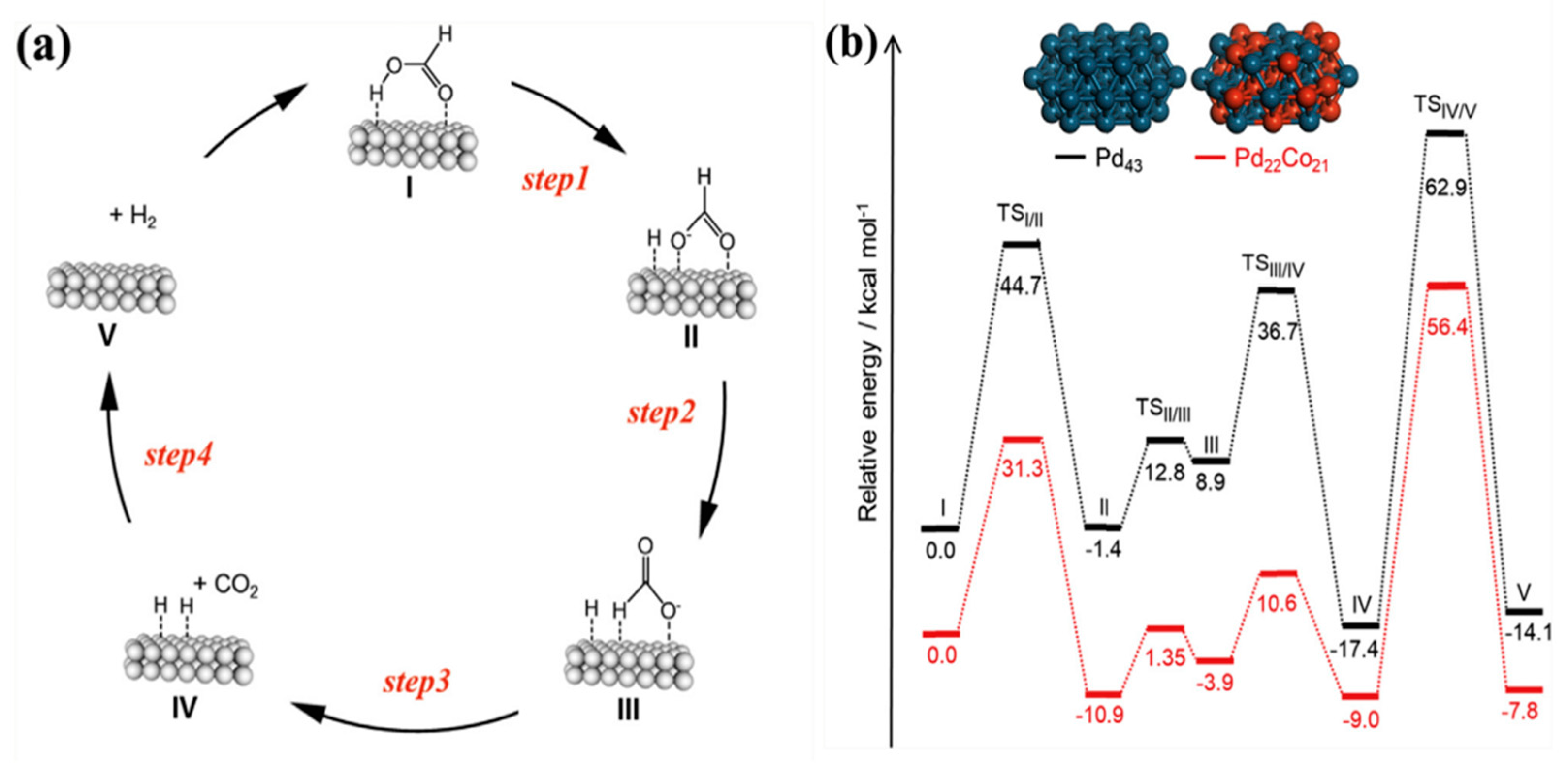
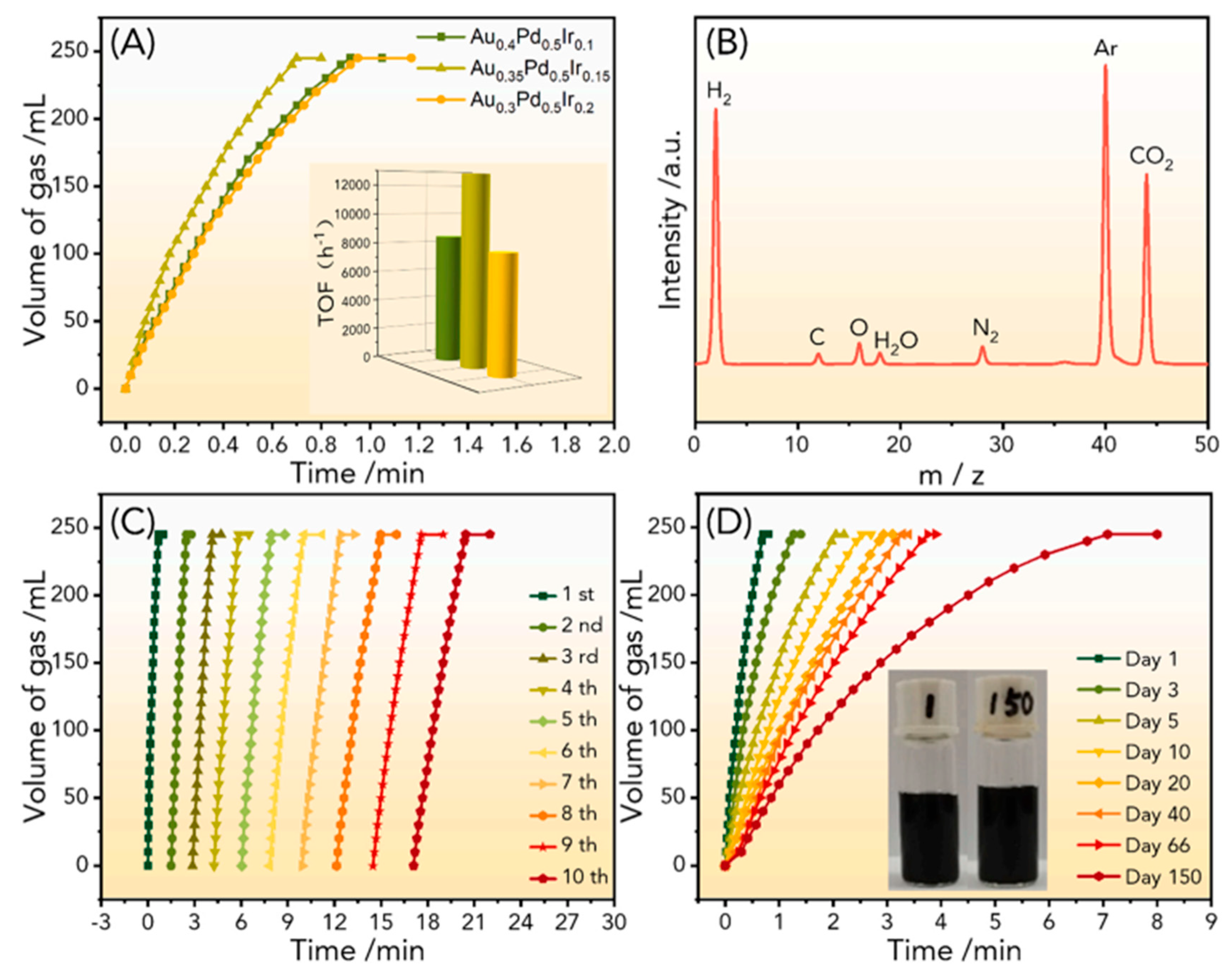
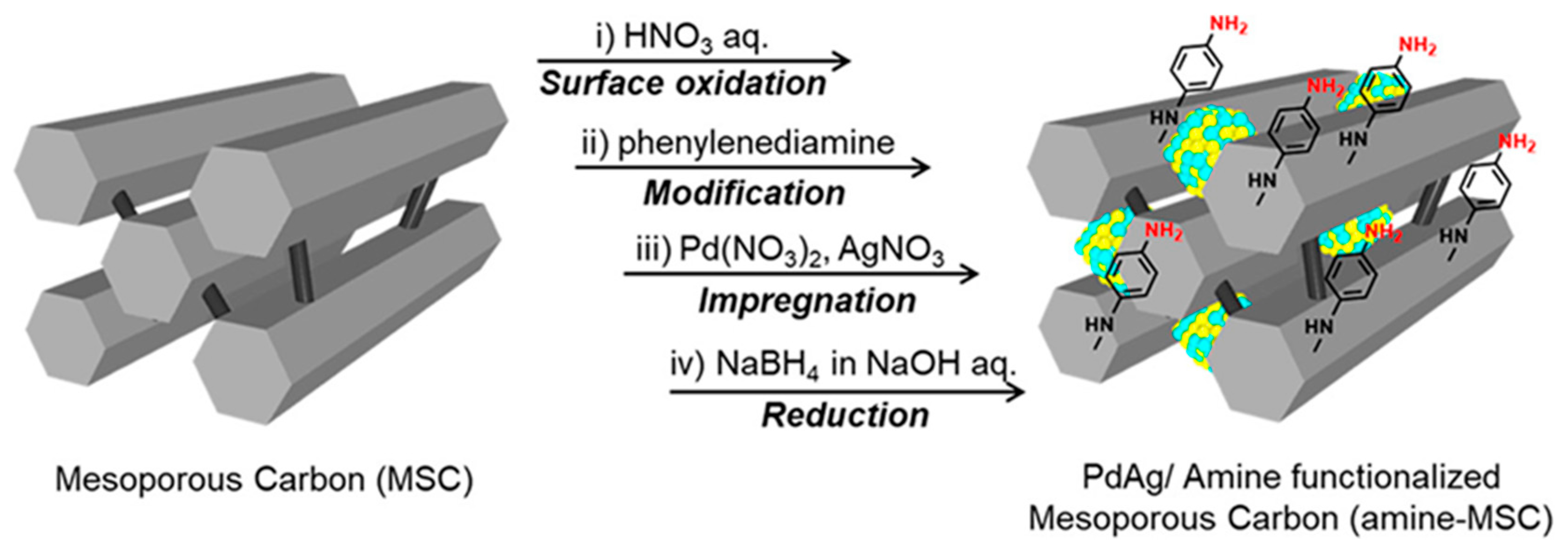
2.2. Carbon-supported multimetallic Pd-based catalysts.
3. Hydrogenation of CO2 to FA
3.1. Carbon-supported monometallic Pd catalysts.
3.2. Carbon-supported multimetallic Pd-based catalysts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Armaroli, N. and Balzani , V., The future of energy supply: Challenges and opportunities, Angew Chem Int Ed Engl, 2007, 46, 52–66. [CrossRef]
- Chow, J., Kopp, R. J., Portney, P. R., Energy Resources and Global Development, Science , 2023, 302, 1528–1531. [CrossRef]
- Papadis E., Tsatsaronis G., Challenges in the decarbonization of the energy sector, Energy, 2020, 205, 118025. [CrossRef]
- Proost, J., Critical assessment of the production scale required for fossil parity of green electrolytic hydrogen, Int J Hydrogen Energy, 2020, 45, 17067–17075. [CrossRef]
- Muthukumar, P., Kumar, A., Afzal, M., Bhogilla, S., Sharma, P., Parida, A., Jana, S., Kumar, E., Pai, R.K., Jain, I.P, Review on large-scale hydrogen storage systems for better sustainability, Int J Hydrogen Energy, 2023, 48, 33223–33259. [CrossRef]
- Aakko-Saksa, P. T., Cook, C., Kiviaho, J., Repo, T., Liquid organic hydrogen carriers for transportation and storing of renewable energy – Review and discussion, J Power Sources, 2018, 396, 803–823. [CrossRef]
- Rao, P. C. Yoon, M., Potential Liquid-Organic Hydrogen Carrier (LOHC) Systems: A Review on Recent Progress, Energies, 2020, 13, 6040. [CrossRef]
- Bulushev, D. A., Ross, J. R. H., Towards Sustainable Production of Formic Acid, ChemSusChem, 2018, 11, 821–836. [CrossRef]
- Enthaler, S., Von Langermann, J., Schmidt, T., Carbon dioxide and formic acid —the couple for environmental-friendly hydrogen storage ?, Energy Environ Sci, 2020, 3, 1207–1217.
- Navlani-García, M., Martis, M., Lozano-Castelló, D., Cazorla-Amorós, D., Mori, K., and Yamashita, H., Investigation of Pd nanoparticles supported on zeolites for hydrogen production from formic acid dehydrogenation, Catal Sci Technol, 2014, 5, 364–371. [CrossRef]
- Wu, Y., Wen, M., Navlani-García, M., Kuwahara, Y., Mori, K. and Yamashita, H., Palladium Nanoparticles Supported on Titanium-Doped Graphitic Carbon Nitride for Formic Acid Dehydrogenation, Chem Asian J, 2017, 12, 860–867. [CrossRef]
- Navlani-García, M., Salinas-Torres, D., Vázquez-Álvarez, F. D., Cazorla-Amorós, D., Formic acid dehydrogenation attained by Pd nanoparticles-based catalysts supported on MWCNT-C3N4 composites, Catal Today, 2022, 397–399, 428–435. [CrossRef]
- Ortega-Murcia, A., Navlani-García, M., Morallón, E., Cazorla-Amorós, D., MWCNT-Supported PVP-Capped Pd Nanoparticles as Efficient Catalysts for the Dehydrogenation of Formic Acid, Front Chem, 2020, 8, 534710. [CrossRef]
- Sun, Q., Chen, B., Wang, N, He, Q., Chang, A., Yang, C.M,. Asakura, H., Tanaka, T., Hülsey, M.J, Yu, J, Yan, N., Zeolite-Encaged Pd–Mn Nanocatalysts for CO2 Hydrogenation and Formic Acid Dehydrogenation, Angewandte Chemie International Edition, 2020, 59, 20183–20191.
- Jin-Jeon H., Chung, Y. M., Hydrogen production from formic acid dehydrogenation over Pd/C catalysts: Effect of metal and support properties on the catalytic performance, Appl Catal B, 2017, 210, 212–222.
- Navlani-García, M., Salinas-Torres, D., Cazorla-Amorós, D., Hydrogen Production from Formic Acid Attained by Bimetallic Heterogeneous PdAg Catalytic Systems, Energies 2019, 12, 4027. [CrossRef]
- Yadav, M., Xu Q., Liquid-phase chemical hydrogen storage materials, Energy Environ Sci, 2012, 5, 9698–9725.
- Jiang, H. L., Singh, S. K., Yan, J. M., Zhang, X. B., Xu, Q., Liquid-Phase Chemical Hydrogen Storage: Catalytic Hydrogen Generation under Ambient Conditions, ChemSusChem, 2010, 3, 541–549. [CrossRef]
- Navlani-García, M., Mori, K., Kuwahara, Y., Yamashita, H., Recent strategies targeting efficient hydrogen production from chemical hydrogen storage materials over carbon-supported catalysts, NPG Asia Materials, 2018, 10, 277–292. [CrossRef]
- Enthaler, S., Von Langermann, J., Schmidt, T., Carbon dioxide and formic acid —the couple for environmental-friendly hydrogen storage, Energy Environ Sci, 2010, 3, 1207–1217. [CrossRef]
- Li, S.-J., Zho, Y-T., Kang, X.,Liu, D-X., Gu, L., Zhang, Q-H., Yan, J-M., Jiang, Q., A Simple and Effective Principle for a Rational Design of Heterogeneous Catalysts for Dehydrogenation of Formic Acid, Advanced Materials, 2019, 31, 1806781. [CrossRef]
- Wakai, C., Yoshida, K., Tsujino, Y., Matubayasi, N., Nakahara, M., Effect of concentration, acid, temperature, and metal on competitive reaction pathways for decarbonylation and decarboxylation of formic acid in hot water, Chem Lett, 2004, 33, 572–573. [CrossRef]
- Mellmann, D., Sponholz, P., Junge, H., Beller, M., Formic acid as a hydrogen storage material – development of homogeneous catalysts for selective hydrogen release, Chem Soc Rev, 2016, 45, 3954–3988. [CrossRef]
- Sordakis, K., Tang, C., Vogt, L.K., Junge, H., Dyson, P.j., Beller, M., Laurenczy, G., Homogeneous Catalysis for Sustainable Hydrogen Storage in Formic Acid and Alcohols, Chem Rev, 2018, 118, 372–433. [CrossRef]
- Onishi, N., Laurenczy, G., Beller, M., Himeda, Y., Recent progress for reversible homogeneous catalytic hydrogen storage in formic acid and in methanol, Coord Chem Rev, 2018, 373, 317–332. [CrossRef]
- Sun, Q., Wang, N., Xu, Q., Yu, J., Nanopore-Supported Metal Nanocatalysts for Efficient Hydrogen Generation from Liquid-Phase Chemical Hydrogen Storage Materials, Adv Mater, 2020, 32. [CrossRef]
- Xie, W., Schlücker, S., Surface-enhanced Raman spectroscopic detection of molecular chemo- and plasmo-catalysis on noble metal nanoparticles, Chemical Communications, 2018, 54, 2326–2336. [CrossRef]
- He, N., Li, Z. H., Palladium-atom catalyzed formic acid decomposition and the switch of reaction mechanism with temperature, Physical Chemistry Chemical Physics, 2016, 18, 10005–10017. [CrossRef]
- Navlani-García, M., Mori, K., Salinas-Torres, D., Kuwahara, Y., Yamashita, H., New approaches toward the hydrogen production from formic acid dehydrogenation over pd-based heterogeneous catalysts, Front Mater, 2019, 6, 439363. [CrossRef]
- Mori, K., Masuda, S., Tanaka, H., Yoshizawa, K., Che, M., Yamashita, H., Phenylamine-functionalized mesoporous silica supported PdAg nanoparticles: a dual heterogeneous catalyst for formic acid/CO2 -mediated chemical hydrogen delivery/storage, Chemical Communications, 2017, 53, 4677–4680. [CrossRef]
- Jin, M. H., Park, J. H., Oh, D., Park, J. S., Lee, K. Y, Lee, D. W., Effect of the amine group content on catalytic activity and stability of mesoporous silica supported Pd catalysts for additive-free formic acid dehydrogenation at room temperature, Int J Hydrogen Energy, 2019, 44, 4737–4744. [CrossRef]
- Bulut, A., Yurderi, M., Karatas, Y., Zahmakiran, M., Kivrak, H., Gulcan, M., Kaya, M., Pd-MnOx nanoparticles dispersed on amine-grafted silica: Highly efficient nanocatalyst for hydrogen production from additive-free dehydrogenation of formic acid under mild conditions, Appl Catal B, 2015, 164, 324–333.
- Wen, M., Mori, K., Kuwahara, Y., Yamashita, H., Plasmonic Au@Pd nanoparticles supported on a basic metal-organic framework: Synergic boosting of H2 production from formic acid, ACS Energy Lett, 2017, 2, 1–7. [CrossRef]
- Zhang, A., Xia, J., Yao, Q., Lu, Z. H., Pd–WOx heterostructures immobilized by MOFs-derived carbon cage for formic acid dehydrogenation, Appl Catal B, 2022, 309.
- Sun, Q., Chen, B., Wang, N, He, Q., Chang, A., Yang, C.M,. Asakura, H., Tanaka, T., Hülsey, M.J, Yu, J, Yan, N., Zeolite-Encaged Pd–Mn Nanocatalysts for CO2 Hydrogenation and Formic Acid Dehydrogenation, Angewandte Chemie International Edition, 2020, 59, 20183–20191.
- J. Li, Chen, W., Zhao, H., Zheng, X., Wu, L., Pan, H., Zhu, J., Chen, Y., Lu, j., Size-dependent catalytic activity over carbon-supported palladium nanoparticles in dehydrogenation of formic acid, J Catal, 2017, 352, 371–381. [CrossRef]
- Mori, K., Hara T., Mizugaki, T., Ebitani, K., Kaneda, K., Hydroxyapatite-supported palladium nanoclusters: A highly active heterogeneous catalyst for selective oxidation of alcohols by use of molecular oxygen, J Am Chem Soc, 2004, 126, 10657–10666. [CrossRef]
- Navlani-García, M., Mori, K., Wen, M., Kuwahara, Y., Yamashita,H., Size Effect of Carbon-Supported Pd Nanoparticles in the Hydrogen Production from Formic Acid, BCSJ, 2015, 88, 1500–1502. [CrossRef]
- Navlani-García, M., Mori, K., Nozaki, A., Kuwahara, Y., Yamashita, H., Investigation of Size Sensitivity in the Hydrogen Production from Formic Acid over Carbon-Supported Pd Nanoparticles, ChemistrySelect, 2016, 1, 1879–1886. [CrossRef]
- Navlani-García, M., Salinas-Torres,D., Mori, K., Léonard, A., Kuwahara, Y., Job, N., Yamashita, H., Insights on palladium decorated nitrogen-doped carbon xerogels for the hydrogen production from formic acid, Catal Today, 2019, 324, 90–96. [CrossRef]
- Chaparro-Garnica, J.A., Navlani-García, M., Salinas-Torres, D., Morallón, E., Cazorla-Amorós, D., Highly Stable N-Doped Carbon-Supported Pd-Based Catalysts Prepared from Biomass Waste for H2 Production from Formic Acid, ACS Sustain Chem Eng, 2020, 8, 15030–15043. [CrossRef]
- Chaparro-Garnica, J.A., Navlani-García, M., Salinas-Torres, D., Morallón, E., Cazorla-Amorós, D., H2 production from formic acid using highly stable carbon-supported Pd-based catalysts derived from soft-biomass residues: Effect of heat treatment and functionalization of the carbon support, Materials, 2021, 14, 6506. [CrossRef]
- Shao, X., Miao, X., Tian, F., Bai, M., Guo, X., Wang, W., Zhao, Z., Ji, X., Li, M., Deng, F., Amine-functionalized hierarchically porous carbon supported Pd nanocatalysts for highly efficient H2 generation from formic acid with fast-diffusion channels, Journal of Energy Chemistry, 2023, 76, 249–258. [CrossRef]
- Masuda, S., Mori, K., Futamura, Y., Yamashita, H., PdAg Nanoparticles Supported on Functionalized Mesoporous Carbon: Promotional Effect of Surface Amine Groups in Reversible Hydrogen Delivery/Storage Mediated by Formic Acid/CO2, ACS Catal, 2018, 8, 2277–2285. [CrossRef]
- Kim, Y., Kim, D. H., Hydrogen production from formic acid dehydrogenation over a Pd supported on N-doped mesoporous carbon catalyst: A role of nitrogen dopant, Appl Catal A Gen, 2020, 608, 117887. [CrossRef]
- Navlani-García M., Mori, K., Kuwahara, Y., . Yamashita, H., Recent strategies targeting efficient hydrogen production from chemical hydrogen storage materials over carbon-supported catalysts, NPG Asia Materials, 2018, 10, 277–292.
- Salinas-Torres, D., Navlani-García, M., Mori, K., Kuwahara, Y., Yamashita, H., Nitrogen-doped carbon materials as a promising platform toward the efficient catalysis for hydrogen generation, Appl Catal A Gen, 2019, 571, 25–41. [CrossRef]
- Nishchakova, A. D., Bulushev, D.A., Stonkus, O.A., Asanov, I.P., Ishchenko, A.V., Okotrub, A.V., Bulusheva, L.G., Effects of the Carbon Support Doping with Nitrogen for the Hydrogen Production from Formic Acid over Ni Catalysts, Energies, 2019, 12, 4111. [CrossRef]
- Yao, M., Liang, W., Chen, H., Zhang X., Efficient Hydrogen Production from Formic Acid Using Nitrogen-Doped Activated Carbon Supported Pd, Catal Letters, 2020, 150, 2377–2384. [CrossRef]
- Wang, H., Gu, X.K., Zheng, X., Pan, H., Zhu, J., Chen, S., Cao, L., Li, W-X., Lu, J., “Disentangling the size-dependent geometric and electronic effects of palladium nanocatalysts beyond selectivity,” Sci Adv, 2019, 5. [CrossRef]
- Jeon, M., Han, D.J., Lee, K-S., Choi, S.H, Han, J., Nam, S.W., Jang, S.C., Park, H.S., Yoon, C.W., Electronically modified Pd catalysts supported on N-doped carbon for the dehydrogenation of formic acid, Int J Hydrogen Energy, 2016, 15453–15461. [CrossRef]
- Chen, Y., Li, X., Wei, Z., Mao, S., Deng, J., Cao, Y., Wang, Y., Efficient synthesis of ultrafine Pd nanoparticles on an activated N-doping carbon for the decomposition of formic acid, Catal Commun, 2018, 108, 55–58. [CrossRef]
- Shao, X., Miao, X., Tian, F., Bai, M., Guo, X., Wang, W., Zhao, Z., Ji, X., Li, M., Deng, F., Amine-functionalized hierarchically porous carbon supported Pd nanocatalysts for highly efficient H2 generation from formic acid with fast-diffusion channels, Journal of Energy Chemistry, 2023, 76, 249–258. [CrossRef]
- Bulushev, D.A., Zacharska, M.,Shlyakhova, E.V., Chuvilin, A.L., Guo Y., Beloshapkin, S., Okotrub, A.V., Bulesheva, L.G., “Single Isolated Pd2+ Cations Supported on N-Doped Carbon as Active Sites for Hydrogen Production from Formic Acid Decomposition,” ACS Catal, 2016, 6, 681–691. [CrossRef]
- Bulushev, D. A., Golub, F.S., Trubina, S.V, Zvereva, V.V, Gerasimov, E.Y., Prosvirin, I.P, Navlani-García, M., Jena, H.S., Pd Active Sites on Covalent Triazine Frameworks for Catalytic Hydrogen Production from Formic Acid, ACS Appl Nano Mater, 2023, 6, 13551–13560. [CrossRef]
- Liu, D., Gao, Z. Y., Wang, X. C., Zeng, J., Li, Y. M., DFT study of hydrogen production from formic acid decomposition on Pd-Au alloy nanoclusters, Appl Surf Sci, 2017, 426, 194–205. [CrossRef]
- Navlani-García, M., Salinas-Torres, D., Cazorla-Amorós, D., Hydrogen Production from Formic Acid Attained by Bimetallic Heterogeneous PdAg Catalytic Systems, Energies, 2019,12, 4027. [CrossRef]
- Navlani-García, M., Mori, K., Nozaki, A., Kuwahara, Y., Yamashita, H., Screening of Carbon-Supported PdAg Nanoparticles in the Hydrogen Production from Formic Acid, Ind Eng Chem Res, 2016, 55, 7612–7620. [CrossRef]
- Kim Y., Kim D. H., Elucidating the alloying effect of PdAg/CNT catalysts on formic acid dehydrogenation with kinetic isotope effect, Molecular Catalysis, 2023, 547, 113343.
- Nabid, M. R., Bide, Y., Etemadi, B., Ag@Pd nanoparticles immobilized on a nitrogen-doped graphene carbon nanotube aerogel as a superb catalyst for the dehydrogenation of formic acid, New Journal of Chemistry, 2017, 41, 10773–10779. [CrossRef]
- Chaparro-Garnica, J., Navlani-García, M., Salinas-Torres, D., Berenguer-Murcia, Á., Morallón, E., Cazorla-Amorós, D., Efficient production of hydrogen from a valuable CO2-derived molecule: Formic acid dehydrogenation boosted by biomass waste-derived catalysts, Fuel, 2022, 320, 123900. [CrossRef]
- Jiang, Y., Fan, X., Chen, M., Xiao, X., Zhang, Y., Wang, C., Chen, L., AuPd Nanoparticles Anchored on Nitrogen-Decorated Carbon Nanosheets with Highly Efficient and Selective Catalysis for the Dehydrogenation of Formic Acid, Journal of Physical Chemistry C, 2018, 122, 4792–4801. [CrossRef]
- Hong, W., Kitta, M., Tsumori, N., Himeda Y., Autrey T., Xu Q., Immobilization of highly active bimetallic PdAu nanoparticles onto nanocarbons for dehydrogenation of formic acid, J Mater Chem A Mater, 2019, 7, 18835–18839. [CrossRef]
- Navlani-García, M., Salinas-Torres, D., Mori, K., Kuwahara, Y., Yamashita, H., Enhanced formic acid dehydrogenation by the synergistic alloying effect of PdCo catalysts supported on graphitic carbon nitride, Int J Hydrogen Energy, 2019, 44, 28483–28493. [CrossRef]
- Tamarany, R., Shin, D.Y., Kang, S., Jeong, H., Kim, J., Kim, J., Yoon, C.W., Lim, D-H., Formic acid dehydrogenation over PdNi alloys supported on N-doped carbon: synergistic effect of Pd–Ni alloying on hydrogen release, Phys. Chem. Chem. Phys., 2021, 23, 11515–11527. [CrossRef]
- 66. Mori, K., Tanaka, H., Dojo, M., Yoshizawa, K., Yamashita, H., “Synergic Catalysis of PdCu Alloy Nanoparticles within a Macroreticular Basic Resin for Hydrogen Production from Formic Acid, Chemistry – A European Journal, 2015, 21, 12085–12092. [CrossRef]
- Yurderi, M., Bulut, A., Zahmakiran, M., Kaya, M., Carbon supported trimetallic PdNiAg nanoparticles as highly active, selective and reusable catalyst in the formic acid decomposition, Appl Catal B, 2014, 160–161, 514–524.
- Wang, Z. L., Ping, Y., Yan, J. M., Wang, H. L., Jiang, Q., Hydrogen generation from formic acid decomposition at room temperature using a NiAuPd alloy nanocatalyst, Int J Hydrogen Energy, 2014, 39, 4850–4856. [CrossRef]
- Dong, Z., Li, F., He, Q., Xiao, X., Chen, M., Wang, C., Fan, X., Chen, L., PdCoNi nanoparticles supported on nitrogen-doped porous carbon nanosheets for room temperature dehydrogenation of formic acid, Int J Hydrogen Energy, 2019, 44, 11675–11683. [CrossRef]
- Liu, D. X., Zhou, Y. T., Zhu, Y. F., Chen, Z. Y., Yan, J. M., Jiang, Q., Tri-metallic AuPdIr nanoalloy towards efficient hydrogen generation from formic acid, Appl Catal B, 2022, 309, 121228. [CrossRef]
- Yan, N., Philippot, K., Transformation of CO2 by using nanoscale metal catalysts: cases studies on the formation of formic acid and dimethylether, Curr Opin Chem Eng, 2018, 20, 86–92. [CrossRef]
- Verma, P., Zhang, S., Song, S., Mori, K., Kuwahara, Y., Wen, M., Yamashita, H., An, T.,, Recent strategies for enhancing the catalytic activity of CO2 hydrogenation to formate/formic acid over Pd-based catalyst, Journal of CO2 Utilization, 2021, 54, 101765. [CrossRef]
- Kumarave, V. l., Bartlett, J., Pillai, S. C., Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products, ACS Energy Lett, 2020, 486–519. [CrossRef]
- Sun, Z., Ma T., Tao, H., Fan, Q., Han, B., Fundamentals and Challenges of Electrochemical CO2 Reduction Using Two-Dimensional Materials, Chem, 2017, 3, 560–587. [CrossRef]
- Bulushev, D. A., H. Ross, J. R., Heterogeneous catalysts for hydrogenation of CO2 and bicarbonates to formic acid and formates, Catalysis Reviews, 2018, 60, 566–593.
- Jessop, P. G., Ikariya, T., Noyori, R., Homogeneous Hydrogenation of Carbon Dioxide, Chem Rev, 1995, 95, 259–272. [CrossRef]
- Sun, R., Liao, Y., Bai, S-T., Zhen, M., Zhou, C., Zhang, T., Sels, B.F., Heterogeneous catalysts for CO2 hydrogenation to formic acid/formate: from nanoscale to single atom, Energy Environ Sci, 2021, 14, 1247–1285. [CrossRef]
- Jessop, P. G., Joó F., Tai, C. C., Recent advances in the homogeneous hydrogenation of carbon dioxide, Coord Chem Rev, 2004, 248,. 2425–2442. [CrossRef]
- Álvarez, A., Bansode, A., Urakawa, A., Bavykina, A.V, Wezendonk, T.A, Makkee, M., Gascon, J., Kapteijn, F., Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes, Chem Rev, 2017, 117, 9804–9838.
- Shao, X., Xu, J., Huang, Y., Su, X., Duan, H., Wang, X., Zhang, T., Pd@C3N4 nanocatalyst for highly efficient hydrogen storage system based on potassium bicarbonate/formate, AIChE Journal, 2016, 62, 2410–2418.
- Lee, J.H., Ryu, J., Kim, J.Y., Nam, S-W., Han, J.H., Lim, T-H., Gautam, S., Chae, K.H., Yoon, C.W., Carbon dioxide mediated, reversible chemical hydrogen storage using a Pd nanocatalyst supported on mesoporous graphitic carbon nitride, J Mater Chem A Mater, 2014, 2, 9490–9495. [CrossRef]
- Wang, F., Xu, J., Shao, X., Su, X., Huang, Y., Zhang, T., Palladium on Nitrogen-Doped Mesoporous Carbon: A Bifunctional Catalyst for Formate-Based, Carbon-Neutral Hydrogen Storage, ChemSusChem, 2016, 9, 246–251.
- Song, H., Zhang, N., Zhong, C., Liu, Z., Xiao, M., Gai, H., Hydrogenation of CO2 into formic acid using a palladium catalyst on chitin, New Journal of Chemistry, 2017, 41, 9170–9177. [CrossRef]
- Koh, K., Jeon, M., Chevrier, D. M., Zhang, P., Yoon, C. W., Asefa, T. Novel nanoporous N-doped carbon-supported ultrasmall Pd nanoparticles: Efficient catalysts for hydrogen storage and release, Appl Catal B, 2017, 203, 820–828. [CrossRef]
- Yang, G., Kuwahara, Y., Mori, K., Louis, C., Yamashita, H., Pd-cu alloy nanoparticles confined within mesoporous hollow carbon spheres for the hydrogenation of CO2 to formate, Journal of Physical Chemistry C, 2021, 125, 3961–3971. [CrossRef]
- Yang, G., Kuwahara, Y., Mori, K., Louis, C., Yamashita, H., PdAg alloy nanoparticles encapsulated in N-doped microporous hollow carbon spheres for hydrogenation of CO2 to formate, Appl Catal B, 2021, 283, 119628. [CrossRef]
- S. Masuda, K. Mori, Y. Futamura, and H. Yamashita, “PdAg Nanoparticles Supported on Functionalized Mesoporous Carbon: Promotional Effect of Surface Amine Groups in Reversible Hydrogen Delivery/Storage Mediated by Formic Acid/CO2,” ACS Catal, 2018, 8, 2277–2285. [CrossRef]
- Nguyen, L.T.M., Park, H., Banu, M., Kim, J.Y., Youn, D. H., Magesh, G., Kim, W. Y., Lee, J.S., Catalytic CO2 hydrogenation to formic acid over carbon nanotube-graphene supported PdNi alloy catalysts, RSC Adv, 2015, 5, 105560–105566. [CrossRef]
- Yang, G., Kuwahara, Y., Mori, K., Louis, C., Yamashita, H., PdAg alloy nanoparticles encapsulated in N-doped microporous hollow carbon spheres for hydrogenation of CO2 to formate, Appl Catal B, 2021, 283, 119628. [CrossRef]
- Su, J., Yang, L., Lu, M., Lin., H., Highly Efficient Hydrogen Storage System Based on Ammonium Bicarbonate/Formate Redox Equilibrium over Palladium Nanocatalysts, ChemSusChem, 2015, 8, 813–816. [CrossRef]
- Zhou, Y., Huang, Y., Jin, B., Luo, X., Liang, Z., Pd Nanoclusters-Based Catalysts with Schiff Base Modifying Carrier for CO2 Hydrogenation to Formic Acid, Ind Eng Chem Res, 2019, 58, 44–52. [CrossRef]
- Bi, Q-Y., Lin, J-D., Liu, Y-M., Du, X-L., Wang, J-Q., He, H-Y., Cao, Y., An Aqueous Rechargeable Formate-Based Hydrogen Battery Driven by Heterogeneous Pd Catalysis, Angewandte Chemie International Edition, 2014, 53, 13583–13587. [CrossRef]
- Soma-Noto, Sachtler, Y. W. M. H., Infrared spectra of carbon monoxide adsorbed on supported palladium and palladium-silver alloys, J Catal, 1974, 32, 315–324.
- Tedsree, K., Li, T., Jones, S., Chan, C. W. A., Yu, K. M. K., Bagot, P. A. J., Marquis, E.A., Smith, G.D.W., Tsang, S. C. E., Hydrogen production from formic acid decomposition at room temperature using a Ag-Pd core-shell nanocatalyst, Nat Nanotechnol, 2011, 6, 302–307. [CrossRef]
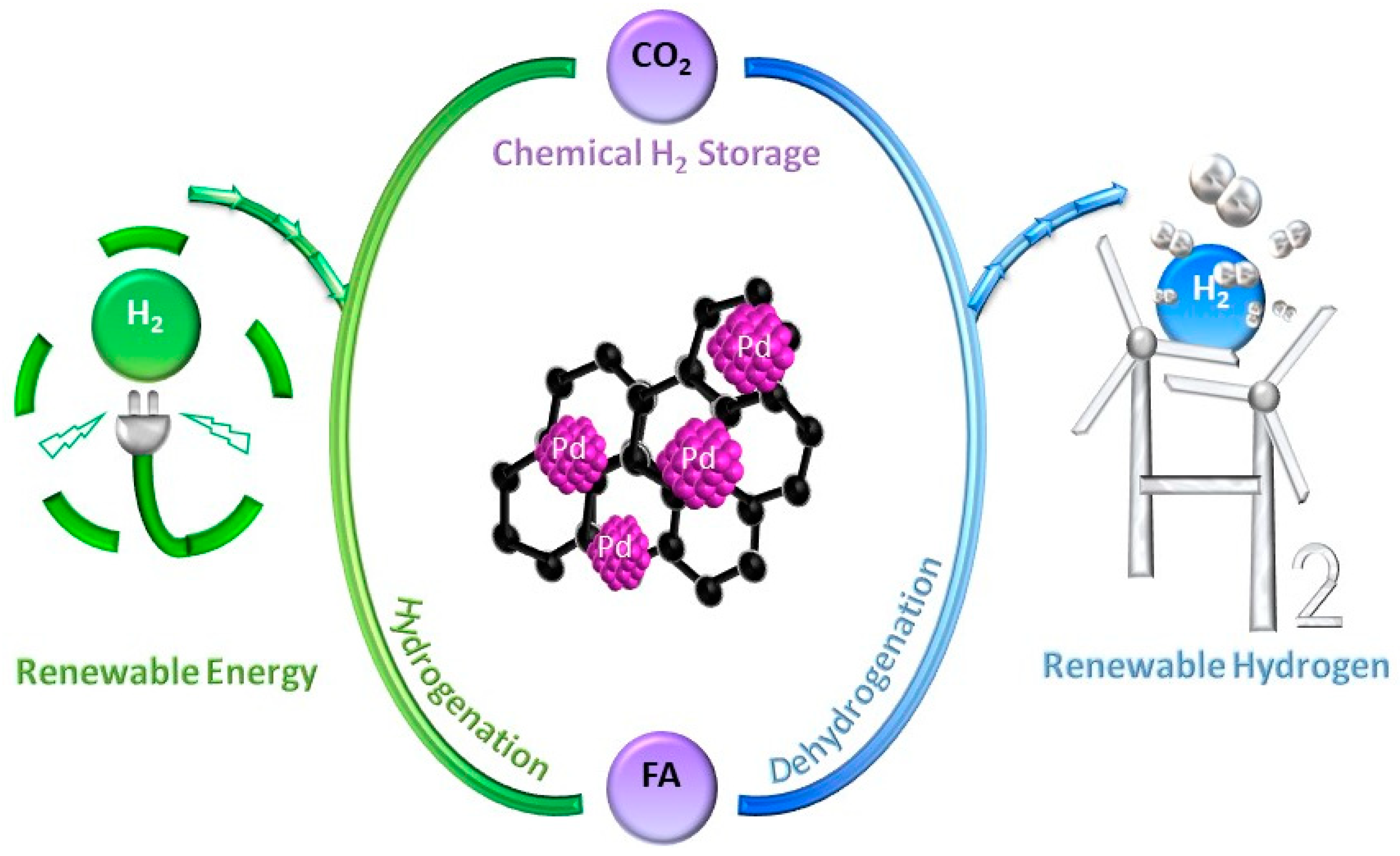
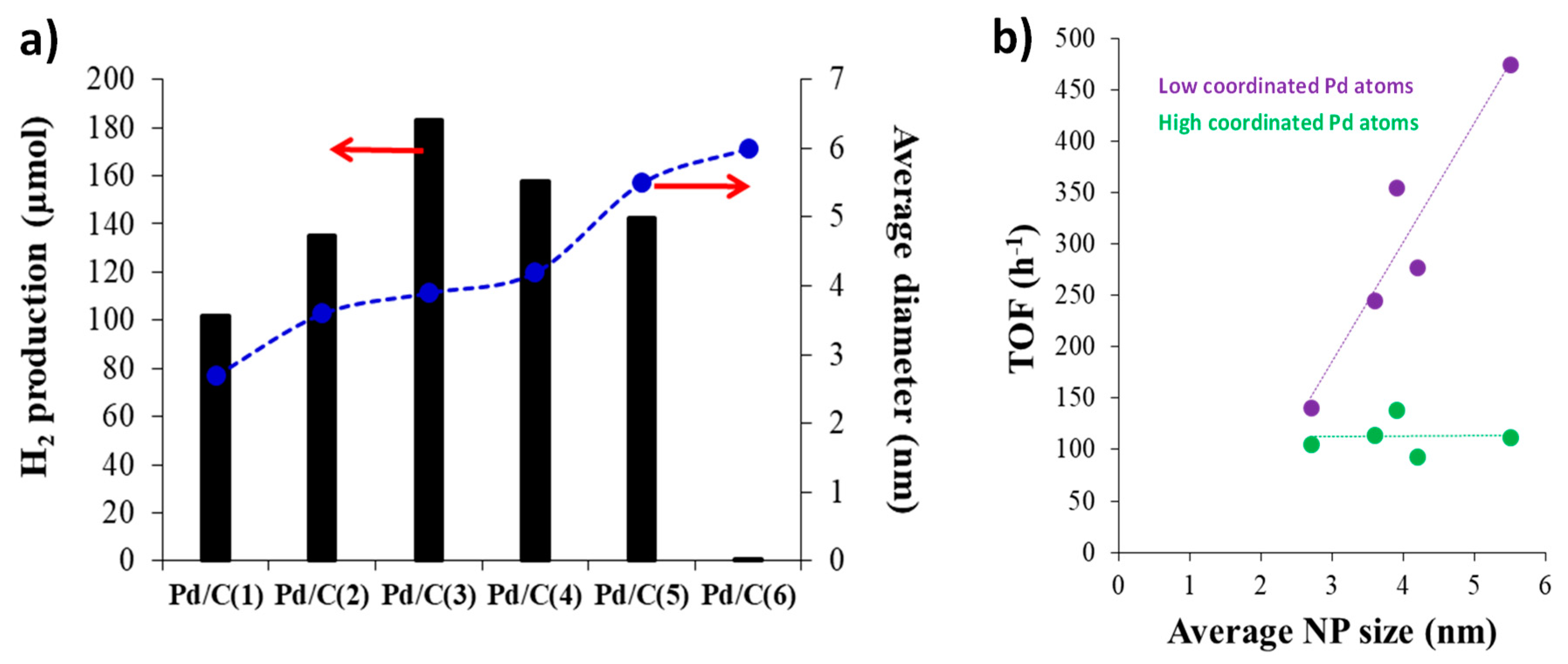
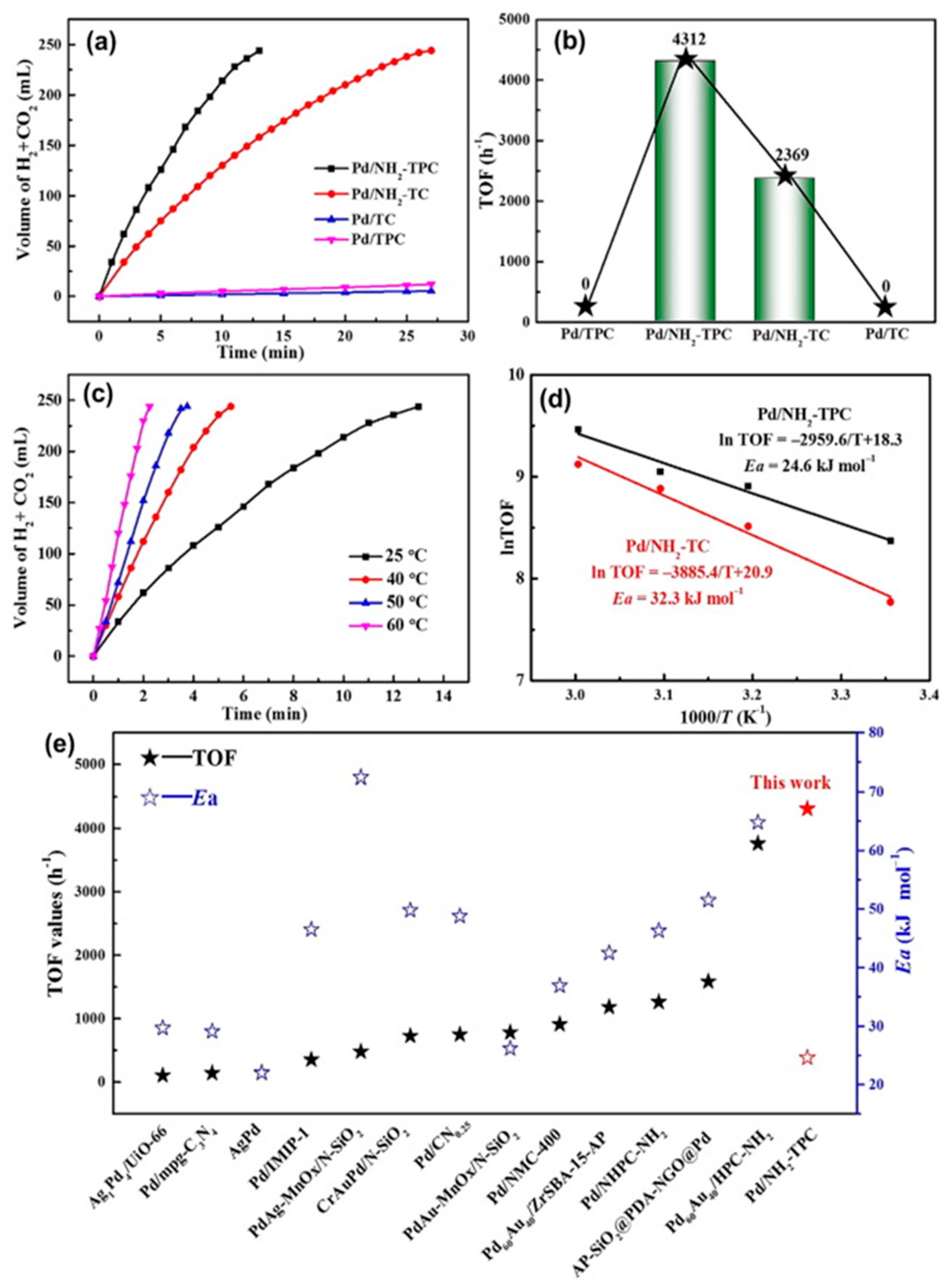

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




