1. Introduction
In the last years, consumers increased their awareness about healthy lifestyle and health promoting foods. Therefore, nowadays foods are not intended only to satisfy hunger and to provide necessary nutrients for humans, but also to prevent nutrition-related diseases and improve consumers’ health [
1]. These kinds of foods are considered functional foods which are ordinary foods with components or ingredients able to provide specific health effect other than purely nutritional effect. They are not intended as medicines, but they are existing traditional products, intended to be consumed as part of a normal and balanced diet [
2].
Among functional foods, dairy-based beverages are very important. Most of them are considered probiotic due to the presence of probiotic microorganisms which interact with the host, or which produce microbial metabolites, and others are enriched with bioactive components such as ω-3 fatty acids, bioactive peptides, Conjugated Linoleic Acid (CLA), vitamins or mineral [
2,
3]. Frequently, these beverages contain whey as principal component due to its nutritional characteristics. This product is often considered a by-product although its interesting nutritional values. Above all the nutrients, whey proteins are rich in essential amino acids such as lysine, methionine and cysteine, and in branched chain amino acids (BCAA) like isoleucine, leucine, and valine [
4,
5]. Furthermore, between whey protein fractions, there is also lactoferrin, a particular peptide interesting in the production of functional foods due to its beneficial role in consumer’s health. This protein has been demonstrated to possess antimicrobic, anti-inflammatory, immunogenic, antioxidant and anticarcinogenic activity [
6,
7]. Moreover, lactose is the major component of whey, and it represents about 70% of total solids, most of the lactose from the starting milk ends up in whey. In addition, whey is also a good source of electrolytes such as sodium and potassium. Even calcium, magnesium and phosphorus are present in solution and bound to proteins [
8]. In addition to these compounds, in these beverages there can be also probiotic microorganisms which are mainly lactic acid bacteria such as
Lactobacillus spp. and microorganisms belonging to Bifidobacterium genus [
9]. They can provide many beneficial effects including improvement of lactose digestibility, anticarcinogenic activity, reduction of serum cholesterol level, synthesis of B vitamins, control of inflammatory bowel diseases, facilitation in calcium absorption and control of pathogenic microorganisms in the intestinal tract [
10,
11,
12,
13,
14]. To exert these activities, they must be able to survive to the passage through the gut, to proliferate and to colonize the digestive tract. Moreover, they must be safe and effective along the entire shelf life of the product. So as to maintain the effectiveness, probiotic foods must contain over 10E6 UFC/mL live microbial cells at the time of ingestion [
15,
16].
Along with whey, fruit juices are often used to improve taste and acceptance of consumers and to add important nutrients to the beverage such as vitamins and polyphenols. Consumption of 100% fruit juices can increase the amount of precious nutrients like vitamin A and C, folate, carotenoids, magnesium, and potassium in diet helping to reach the recommended amount of those micronutrients [
17]. Among fruit juices those made with Aronia berries are more and more relevant due to a huge antioxidant activity if compared to other berries or fruits. Aronia juice exhibits the highest antioxidant capacity among the polyphenol-rich beverages. In vitro studies show that Aronia has antiproliferative or protective effect against some types of cancers, antimutagenic activity, hepatoprotective effect able to decrease the toxicity and the accumulation of cadmium in the liver and kidney, it decreases serum total LDL cholesterol, and it increases HDL cholesterol, and it has a role in the prevention and control of diabetes mellitus type II and diabetes associated complications [
18]. Another important fruit used to produce fruit juice is Ravèntse which is an autochthonous variety of apple cultivated in Aosta Valley that shows high amounts of polyphenols [
19].
Considering the relevance of functional foods and problems related to whey disposal, the aim of this project was to create a new functional beverage starting from YoAlp® -whey, a by-product derived from Aosta Valley cattle breed fermented milk obtained with autochthonous strains (Streptococcus thermophilus and Lactobacillus delbrueckii) inoculation, and fruit juices typically produced in Aosta Valley. Those two ingredients allow to combine many positive and health promoting nutrients such as polyphenols, antioxidants, vitamins, bioactive peptides as well as the natural probiotic lactic acid bacteria. This product could be obtained also by the Aosta Valley’s farms to increase their competitiveness and reduce their environmental impact by creating a sustainable process through the recovery of a common by-product.
2. Materials and Methods
2.1. Beverage formulation
Whey has been obtained from YoAlp® straining, a fermented milk developed by Institut Agricole Régional in a project founded by FESR (HEART VdA). YoAlp® has been realized using milk from Aosta Valley’s autochthonous breeds and local strains of lactic acid bacteria belonging to species Streptococcus thermophilus and Lactobacillus delbrueckii. These strains have been isolated during years in small dairies of alpine pastures in Aosta Valley. After the production, to increase the creaminess and concentration of nutrients, YoAlp® has been strained. The strain has been made by gravity suspending the mass in a towel until the 30% of the mass is lost. After that, preparation of beverages has been made by mixing whey with fruit juice in percentages of 60/40%. Fruit juices have been produced by local producers, using fruits typically cultivated in Aosta Valley. Three different fruit juices were used: Ravèntse juice, Renetta Canada– Aronia juice and Renetta Canada/Golden Delicious – Raspberry juice. Final beverages have not been pasteurized to preserve lactic acid bacteria, so the shelf life of these beverages has been monitored up to 21 days: 24 hours; 7 days; 14 days and 21 days. The product has been stored in sterilized bottles at refrigerated temperature (4°C).
2.2. Chemical analysis
2.2.1. Total acidity and pH
pH and total acidity for every sample at each shelf-life step were assessed. pH analysis was made through a pH meter. Total acidity was determined by titration with NaOH 0,1 N using phenolphthalein as an indicator.
2.2.2. Peptides
Samples preparation and analysis has been performed according to the method of Sforza et al. 2012 [
20] and Rizzello et al. 2005 [
21]. Briefly, samples have been centrifuged at 3800 rpm for 30 minutes at 4°C, then filtered with Whatman N° 2 Paper filters. Filtered samples were again centrifuged a 3000 rpm for 1,30 hours with a Cut-off 10 kDa with Amicon Ultra – 2 or 4 – UltraCel 10 K. After that, 5 ml of each sample was aliquoted and evaporated in Centrivap overnight. Evaporated samples were dissolved in 500 µL of mobile phase A and vortexed in a Thermomixer for 90 minutes. Samples were filtered again at 0,45 µm and injected (10 µl) into the column (Jupiter 4u Proteo 90A, 250’4.6 mm). Solvent used during the analysis were mobile phase A (0,2% CH
3CN e 0,1% HCOOH in H
2O v/v) and mobile phase B (0,2% H
2O e 0,1% HCOOH in CH
3CN). These samples have been analyzed by Reverse-phase high performance liquid chromatography coupled with a mass spectrometer (RP-HPLC-ESI (+)/MS). RP-HPLC was performed with ACCELA LC system (Thermofisher Scientific, Milan, Italy) equipped with an auto sampler maintained at 15°C. The output was directly interfaced with an ESI-Ion Trap mass spectrometry LTQ XL (Thermofisher). The positive ion mode has been used, and the mass scan was acquired in a range of 150-1900 m/z. Cone voltage and capillary voltage has been set at 30 V and 3,2 kV. This analysis has been replicated three times per sample. Finally, peptide identification has been performed using an internal database based on literature data and online databases (ESI PROT or BIOPEP). Each peptide has been also associated to a possible bioactive effect and the Peptide Ranker online software gave an indication on the probability of each sequence to exert a bioactive effect.
2.2.3. Polyphenols
Total polyphenolic content was determined by an optimized Folin-Ciocalteu method by Rigo et al. 2000. Briefly, to remove the organic acids, free sugars, free SO2, amino acids, proteins and other hydrophilic compounds that could cause interference, a preliminary cleanup of the phenols was performed with a Sep-Pak C18 (Waters, Milano, 500 mg) previously conditioned with 2 ml of MeOH followed by 5 ml of 5mM H2SO4.Samples were suspended in sulfuric acid 1N with appropriated dilution and analyzed in triplicates. One milliliter of each sample was then slowly loaded on the conditioned Sep-Pak, and the polar substances were removed with 2 ml of 5 mM H2SO4. The phenolic compounds were eluted into a 20 ml calibrated flask, with 2 ml of MeOH followed by 5 ml of distilled water. 0.5 milliliter of Folin-Ciocalteu reagent and, after 3 min. 1 ml of 20% Na2Co3 were added, and the solution was brought to 20 ml with distilled water. After 20 min at 70°C, samples were filtered at 0.45 μm) and their absorbance was read at 750 nm in a 1 mm cell, against a blank test prepared by using distilled water in place of sample. Concentration was determined by means of a calibration curve as (+) catechin standard (mg/L).
2.2.4. Antioxidant activity
Potential antioxidant activity has been evaluated using the method developed by Prieto, 2012 [
22] with some modifications. DPPH solution 0,2 mM have been prepared pured DPPH powder dissolved in EtOH. This solution must be kept in the fridge wrapped in foil when not in use, to reduce its degradation.
The analysis has been performed in microplate reader using a 96-well microplate in which has been added 100 µL of sample and 100 µL DPPH 0,2 mM. Serial dilutions (1:2) of samples can be done using EtOH. As standard has been used Vitamin C in different dilution. In addition, for each sample and standard, a blank has been prepared by adding only 100 µL EtOH instead of DPPH solution. Moreover, the maximum signal of the radical has been obtained by reacting 100 µL DPPH solution with 100 µL EtOH.
Then the plate has been covered with the lid to minimize evaporation and it has been incubated for 30 minutes. The absorbance (517 nm) has been then read in a microplate reader. The percentage of radical scavenging can be obtained using the following expression (1):
2.2.5. ACE inhibitory activity
Angiotensin I Converting Enzyme (ACE) Activity Assay Kit (Fluorometric) (Merck-Sigma-Aldrich, Milano, Italia) has been used to assess total ACE inhibitory activity of YoAlp
® whey-based beverages. Samples were diluted 1:2 in the Assay Buffer and ACE positive control has been used. The assay has been performed in excitation mode at 320 nm and emission mode at 405 nm at 37°C. Fluorescence has been read immediately in kinetic mode in 5 cycles for 5 minutes. Results has been obtained using a standard curve and a sample kinetic curve. The standard curve linear regression slope and the sample kinetic curve linear regression slope were used to transform values of samples from RFU/min to nmol/min (units) using the following formula (2):
A percentage of inhibition was then obtained from the following formula (3):
Where Fpc is the fluorescence of positive control; Fsample is the fluorescence of the sample and Fnc is the fluorescence of the negative control.
2.3. Microbiological analysis
3M Petrifilm™ Count Plate specific for different microorganisms (3M Company, St. Paul, MN, USA) have been used to assess total viable cell counts of bacteria, yeasts and mold, Escherichia coli and coliforms.
Plates have been incubated for 72 hours at 30° C for total aerobic and yeast and mold count, while an incubation for 24-48 hours at 37°C has been applied for E. coli/coliform count.
Furthermore, also probiotics have been evaluated. MRS Agar with Tween 80 and M17 Agar have been used for
Lactobacillus delbrueckii and
Streptococcus thermophilus count respectively. Plates have been incubated for 24-48 hours at 40°C in anaerobiotic conditions for
L. delbrueckii and for the same time at 45°C in aerobic conditions for
S. thermophilus. The minimum acceptance threshold for probiotic bacteria has been set to 10E6 CFU/mL for
L. delbrueckii and 10E7 CFU/mL for
S. thermophilus as reported by Ranhadeera et al., (2017) [
31].
2.4. Statistical analysis
All samples have been analyzed in three technical replicates. Results are expressed as the mean (n = 3) ± SD. All the statistical analyses have been performed using Jamovi software (The Jamovi project (2023). Jamovi Version 2.3, Sidney, Australia) and confirmed by JASP software (JASP Team (2023). JASP (Version 0.17.3), Amsterdam, The Netherlands). Data have been tested for normality using Shapiro-Wilk normality test and for homogeneity using the Levene’s Test for Homogeneity of Variance with default parameters. Since data resulted parametric, the analysis of variance test (ANOVA), followed by Tuckey’s post-hoc test (p < 0.05) has been performed to evaluate the differences among compared group.
3. Results and discussion
3.1. Chemical analysis
3.1.1. Peptides
Peptides analysis by LC-MS ESI+ on the fraction below 10 kDa of YoAlp® whey and all YoAlp® whey-based beverages detected the presence of 92 different peptides. Thanks to literature research and online database, these peptides have been linked to one or more identification. Each m/z can be related to one or more aminoacidic sequences, so a total of 174 possible aminoacidic sequences has been found. Those peptides were originated from all milk proteins: αs1-casein (αs1-CN), αs2-casein (αs2-CN), k-casein (k-CN), β-lactoglobulin (LGB), α-lactalbumin (LALBA) and from β-casein (β-CN).
A similar number of bioactive peptides have been found among beverages, while differences have been highlighted related to the type of peptides detected. This can be due to different pH detected in the samples at 24 hours (YWR: pH 3,81, YWL: pH 4,10, YWA: pH 3,90) which influence the proteolytic activity of LABs. Nielsen et al. [
23] have shown that
S. thermophilus and
L. lactis increase proteolytic activity with lower pH values from pH 4,6 to pH 4,3 and pH 3,5. Furthermore, the same author [
23] has reported an increase in ACE inhibitory activity.
Furthermore, it has been possible to correlate the identified peptides to a specific potential bioactive effect and to predict the probability of each sequence to exert a supposed bioactive effect.
Among the 174 aminoacidic sequences, 35 have been related to a possible bioactive effect. Most of them are considered ACE (Angiotensin-converting enzyme) inhibitory (about 85,29%), the others are supposed to be antioxidant (5,88%), mineral binding, opioid antagonist, cytomodulator, antimicrobial and DPP (Dypeptil peptidase) inhibitory. Peptide Ranker analysis shows that 62,64% of peptides has a low probability to exert a bioactive effect (probability lower than 50%) and 15,52% has a high probability to exert a bioactive effect (probability higher than 75%).
Moreover, the marker of β-Casein A2 variant, the beta-casomorphin BCM-9 (VYPFPGPIPN), related to possible positive effects on human health, has been detected and it has been highlighted the absence of betacasomorphin BCM-7, marker for the β-Casein A1 variant (BCM7), which is a potential risk factor for health diseases. The most interesting bioactive peptides and the marker of β-Casein A2 variant are shown in
Table 1. Some of these peptides are supposed to resist to the gastric digestion according to literature and YQEPVLGPVRGPFPIIV has been reported to has been found in blood plasma [
24,
25].
3.1.2. Polyphenols
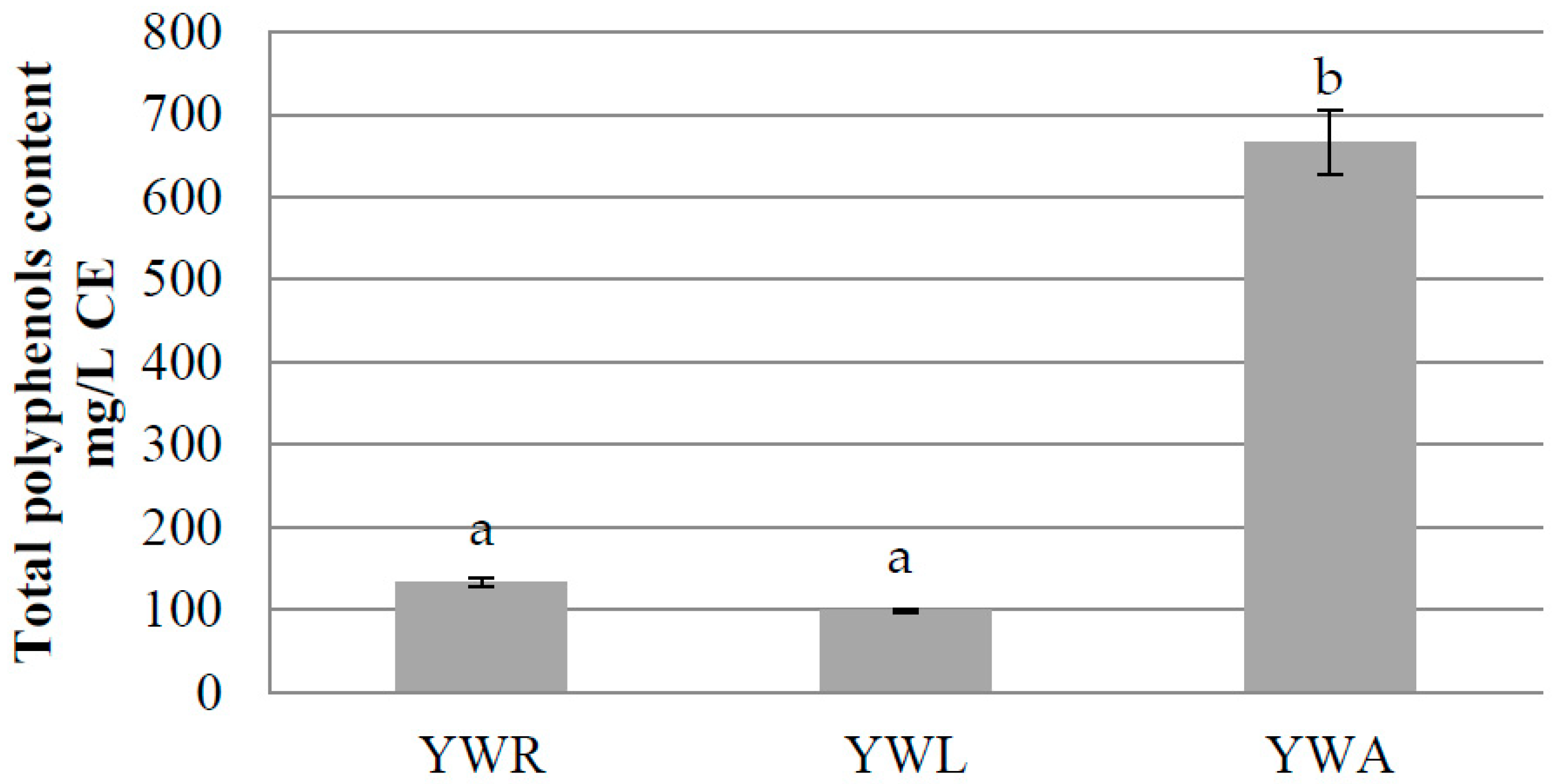
Total polyphenol content of YoAlp
® whey-based beverages has been assessed. The highest amount has been observed in YWA (665,99 ± 38,39 mg/L CE) than YWR (132,55 ± 5,18 mg/L CE) and YWL (98,55 ± 2,25 mg/L CE) (
Figure 1).
These results confirmed those obtained in the analysis performed on fruit juices. The richest fruit juice was Renetta/Aronia (2967,59 ± 98,92 mg/L CE), followed by Ravèntse juice (588,17 ± 7,45 mg/L CE) and Renetta/Golden/Raspberry juice (278,44 ± 37,32 mg/L CE).
Statistical significance has been highlighted between group a and group b (p < 0.001), while no statistical significance has been found among YWR and YWL.
Moreover, no significative differences on polyphenols content have been detected during the shelf-life period.
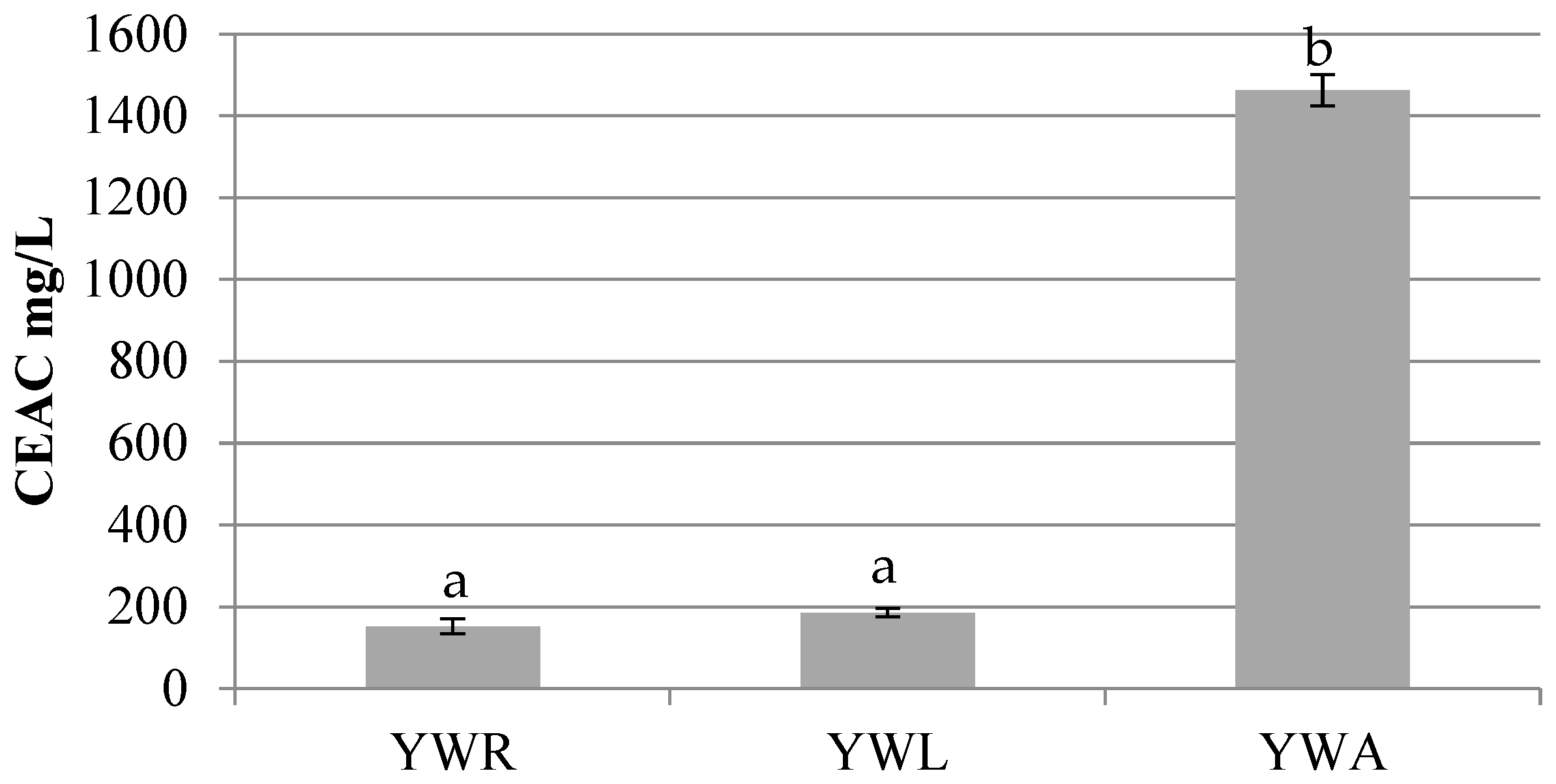
3.1.3. Antioxidant activity
Antiradical activity against DPPH was assessed in whey-based beverages and expressed in Vitamin C Antioxidant Capacity Equivalents (CEAC) mg/L. The values detected in all samples ranged from a mean value of 152,43 to 1463,13 mg/L CEAC.
YWA shows the highest mean value of inhibition reaching 1463,13 ± 38,25 mg/L CEAC, more than YWL (186,00 ± 10,17 mg/L CEAC) and YWR (152,43 ± 18,36 mg/L CEAC) (
Figure 2). Moreover, also YoAlp
® whey without the addition of fruit juices has been analyzed. The results show low values if compared to beverages reaching 20,79 ± 0,69 mg/L CEAC.
These results of YoAlp
® whey-based beverages suggest a good antioxidant activity if compared with other similar whey-based beverages studied. Jaworska et al., 2014 [
32] assessed 76,60 mg/L CEAC on a whey-based Apple beverage (result only available in mg/L CEAC).
Statistical significance has been highlighted between group a and group b (p < 0.001), while no statistical significance has been found among YWR and YWL.
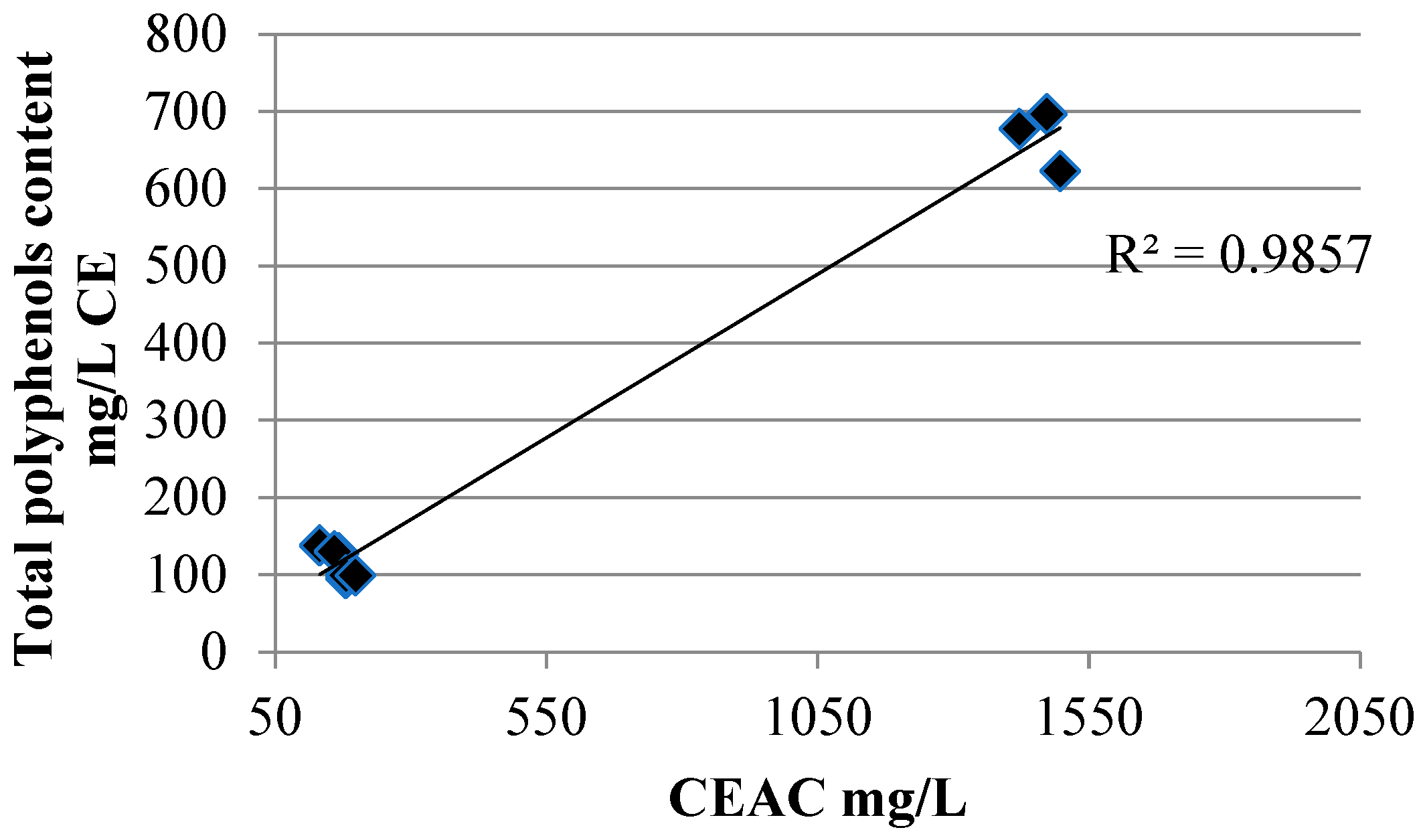
Finally, a great correlation between ARP (antiradical power) values and polyphenol content was found indicating that the antiradical power is mainly due to polyphenols present in fruit juices (
Figure 3).
3.1.4. ACE inhibitory activity
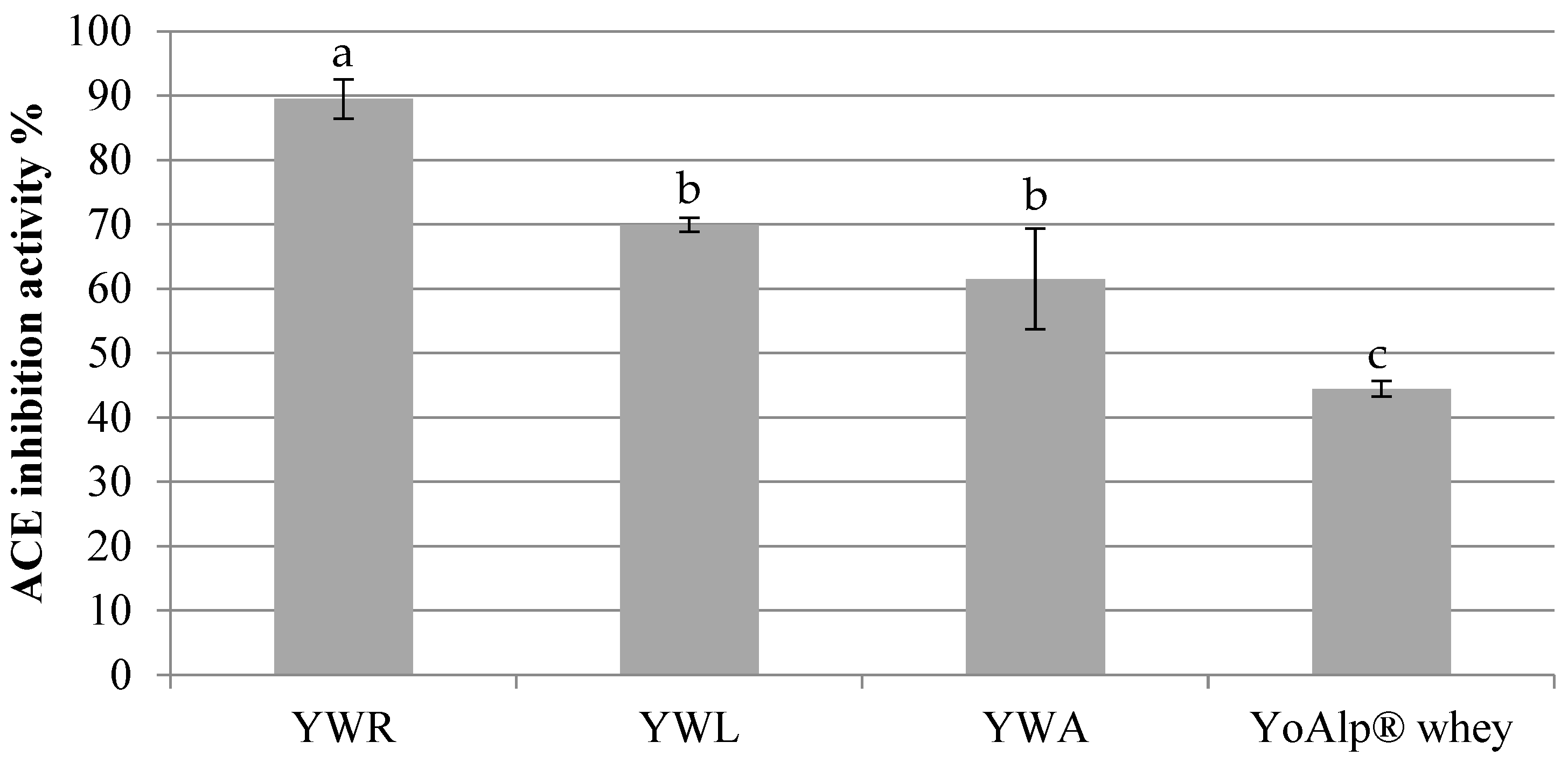
Angiotensin Converting Enzyme (ACE) inhibitory activity was tested in all samples (
Figure 4) to verify the biological activity potentially exert by biochemical components, in particular bioactive peptides.
Results showed by YoAlp
® whey-based beverages highlighted a percentage of ACE inhibition from 61,55% to 89,50%. The highest value has been reached by YWR (89,50 ± 3,03%), then YWL juice (69,99 ± 1,10%) and YWA (61,55 ± 7,79%). This can be due to a lower pH value of YWR if compared to YWA and YWL (YWR: pH 3,81, YWL: pH 4,10, YWA: pH 3,90), as reported by Nielsen et al. [
23]. Moreover, also YoAlp
® whey without addition of fruit juices has been analyzed. These results show good values of inhibition reaching 44,46%. So, it is possible to conclude that the ACE inhibitory activity is probably due to bioactive peptides present in whey together with phenolic compounds present in fruit juices.
These results were compared with ACE inhibitory activity data of similar studies showing that YoAlp
® whey beverages ACE inhibitory activity, in particular whey based Ravèntse beverage has a very high functionality compared with similar products [
26,
27,
28,
29,
30].
Statistical significance has been highlighted between group a, b and c (a and b p < 0.002, a and c p < 0.001, b and c p < 0.005), while no statistical significance has been found among YWA and YWL.
3.2. Microbiological analyses
Microbiological assessment has been conducted to determine the presence of different categories of microorganisms, and to monitor their variations along shelf life. YoAlp® starters were assessed to verify the vitality and viability of these probiotic bacteria. Spoilers and pathogens were also detected to avoid their presence along shelf life.
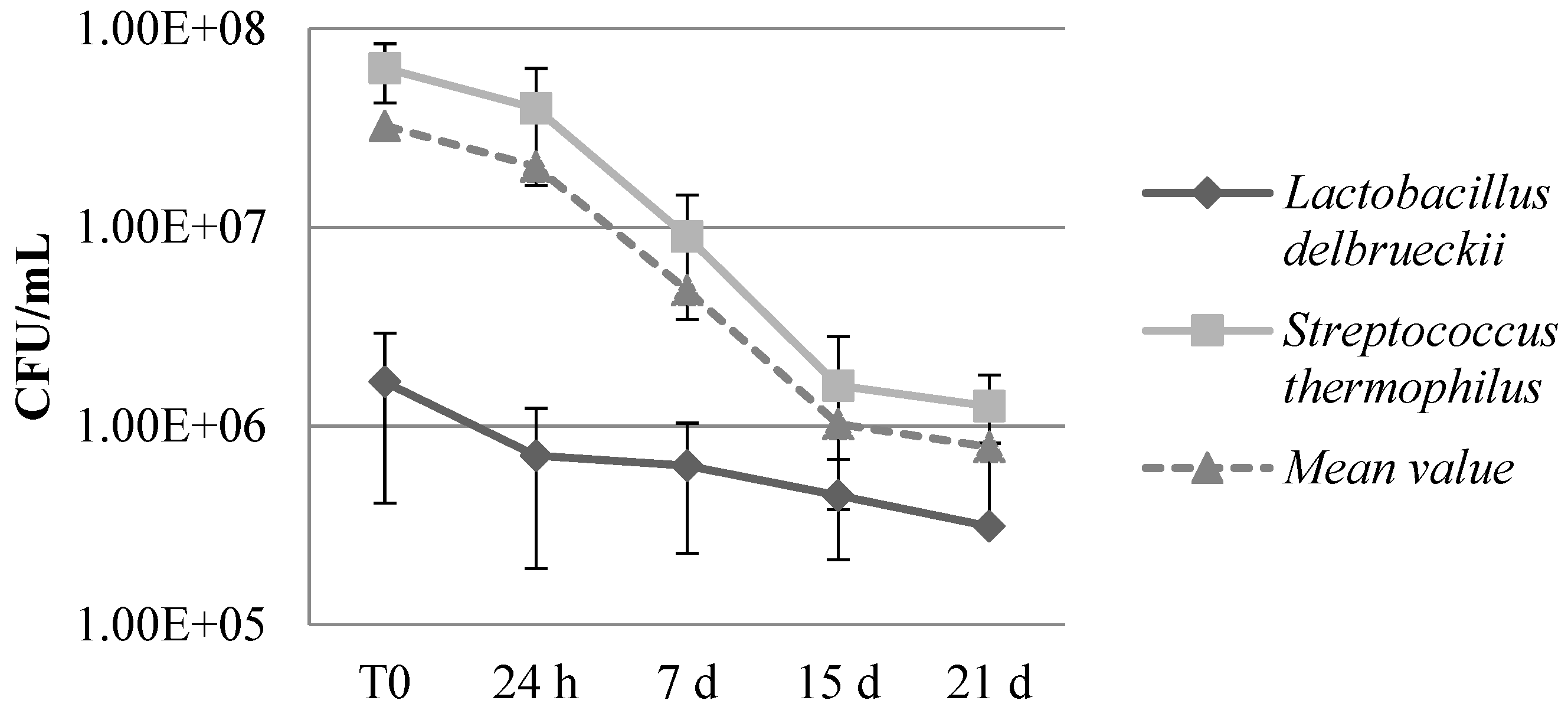
Microbial count of lactic acid bacteria has been detected along 4 different shelf-life steps from 24 hours to 21 days (
Figure 5). The vitality and viability of these bacteria used as starter in YoAlp
® making process must be monitored since they are probiotic organisms that confer possible biological activity to YoAlp
® whey-based beverages. The microbial charge has slowly decreased during the shelf-life period at storage temperature (4°C).
The count of
S. thermophilus remains high even after 7 days (9,00E+06 CFU/ml), in particular for YWA with 1,50E+07 a value that allow to exert a probiotic activity. On the contrary,
L. delbrueckii was below (6,33E+05 CFU/mL) the recommended therapeutic levels (10E+06 CFU/mL) able to assure the survivability of probiotics along the gastric tract [
31].
Figure 5.
Microbial count of lactic acid bacteria (L. delbrueckii, S. thermophilus and mean value) along 4 different shelf-life steps from 24 hours to 21 days related to the mean value of the YoAlp® whey based-beverages analyzed.
Figure 5.
Microbial count of lactic acid bacteria (L. delbrueckii, S. thermophilus and mean value) along 4 different shelf-life steps from 24 hours to 21 days related to the mean value of the YoAlp® whey based-beverages analyzed.
As far as other microorganisms are concerned, the total microbial count (CMT) was below 10E2 CFU/mL during the entire shelf-life. Coliforms were always under limits and no colonies of E. Coli have been detected in any sample.
Considering yeasts, during the first steps, they were under control maintaining always counts below 10E2 CFU/mL. However, after 15 days of shelf-life, they increased, reaching values over 10E3 CFU/mL. Yeasts at these concentrations could lead to unwanted fermentations during the product storage.
Considering these results, shelf-life period of these beverages has been established up to 7 days at storage temperature of 4°C to avoid the presence of yeast and to preserve natural whey probiotic bacteria.
4. Conclusions
The presence of bioactive peptides and phenolic compounds can give to the product a possible antiradical power and ACE inhibitory activity which seem to be higher than other similar dairy-based beverages present on the market or under formulation. Furthermore, the marker of allelic variant A2 of β-Casein and the absence of marker of allelic variant A1 of β-Casein variant suggest possible beneficial health effects leading to a lower incidence of cardiovascular disease and type 1 diabetes, and a reduction in cholesterol and triglycerides. Moreover, these beverages contain viable Lactic acid bacteria (Streptococcus thermophilus) which can give beneficial effects on the gastrointestinal tract and spoilage microorganisms stay under the regulation threshold up to 7 days of shelf-life at 4°C. Anyway, other studies should be carried out to increase the vitality and viability of Lactobacillus delbrueckii to gain a probiotic effect during the entire shelf-life period. Furthermore, in vivo studies should be performed to evaluate the bioavailability of bioactive peptides and phenolic compounds.
So, YoAlp® whey-based beverages could represent a huge opportunity for Aosta Valley’s farms to increase their income and competitiveness reducing environmental impact of dairy industry thanks to reuse of a byproduct.
Author Contributions
Conceptualization, S.V. and L.V.; methodology, T.F. and S.V.; validation, T.F., S.Z., and S.V.; formal analysis, S.V.; investigation, T.F., M.M., R.P., S.Z.; resources, S.V. and L.V.; data curation, T.F., M.M., R.P., S.Z., and S.V.; writing—original draft preparation, M.M. and S.V.; writing—review and editing, M.M. and S.V..; visualization, S.V.; supervision, S.V.; project administration, S.V.; funding acquisition, S.V. and L.V. All authors have read and agreed to the published version of the manuscript.
Funding
This experimental work was financially supported, through TYPICALP project, by European Union, European Regional Development Fund, Italian State, Swiss Confederation and Cantons co-financed operation, within the framework of the Intrreg V-A Italy-Switzerland Cooperation Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siro, I.; Kapolna, E.; Kapolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance: a review. Appetite 2008, 51, 456–467.
- Gobbetti, M.; Cagno, R.D.; De Angelis, M. Functional microorganisms for functional food quality. Crit. Rev. Food Sci. Nutr. 2010 50, 716–727. [CrossRef]
- Stanton, C.; Ross, R. P.; Fitzgerald, G. F.; Van Sinderen, D. (2005). Fermented functional foods based on probiotics and their biogenic metabolites. Curr. Opin. Biotechnol 2005, 16, 198–203. [CrossRef]
- Sherwood, S. Protein beverage and method for making the same. US Patent US 7906/160 B2 2007.
- Renner, E. Nutritional aspects of fermented milk products. Cultured Dairy Prod. J. 1986, 5, 6-13.
- Arranz, E.; Corrochano, A.R.; Shanahan, C.; Villalva, M.; Jaime, L.; Santoyo, S.; Giblin, L. Antioxidant activity and characterization of whey protein-based beverages: Effect of shelf life and gastrointestinal transit on bioactivity. Innov. Food Sci. Emerg. Technol. 2019, 57, 102209. [CrossRef]
- Gonzalez-Chavez, S.A.; Arevalo-Gallegos, S.; Rascon-Cruz, Q. Lactoferrin: structure, function and applications. Int. J. Antimicrob. Agents 2009, 33(4), 301.e1–301.e8.
- Durham, R.J.; Hourigan, J.A. Waste management and co-product recovery indairy processing. Editor: Keith Waldron. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Publisher: Woodhead Publishing, 2007, pp. 332-387.
- Saad, N.; Delattre, C.; Urdaci, M.; Schmitter, J.M.; Bressollier, P. An overview of the last advances in probiotic and prebiotic field, LWT - Food Sci. Technol. 2013, 50, Issue 1, 1-16. [CrossRef]
- Xu, R.; Shang, N.; Li, P. In vitro and in vivo antioxidant activity of exopolysaccharide fractions from Bifidobacterium animalis. Anaerobe 2011, 17, 226–231. [CrossRef]
- Linares, D.M.; Gomez, C.; Renes, E. Lactic Acid Bacteria and Bifidobacteria with Potential to Design Natural Biofunctional Health-Promoting Dairy Foods. Front Microbiol 2017, 8, 846. [CrossRef]
- Roy, D. Technological aspects related to the use of bifidobacteria in dairy Products. Le Lait 2005, 85(1–2), 39–56. [CrossRef]
- Shah, N.P. Functional cultures and health benefits. Int. Dairy J. 2007, 17(11), 1262–1277. [CrossRef]
- Ruas-Madiedo, P.; Gueimonde, M.; Fernández-García, M.; de los Reyes-Gavilán, C.G.; Margolles, A. Mucin degradation by Bifidobacterium strains isolated from the human intestinal microbiota. Appl. Environ. Microbiol. 2008, 74(6), 1936-1940. [CrossRef]
- Vinderola, C.G.; Prosello, W.; Ghiberto, D.; Reinheimer, J.A. Viability of probiotic (Bifidobacterium, Lactobacillus acidophilus and Lactobacillus casei) and non-probiotic microflora in Argentinian Fresco cheese. J. Dairy Sci. 2000, 83, 1905–1911.
- Watson, R.R.; Preedy, V.R. Probiotics, Prebiotics and Symbiotics: Bioactive Foods in Health Promotion. 1st Edition. Amsterdam: Elsevier, 2015.
- Nicklas, T.; Kleinman, R.E.; O’Neil, C.E. Taking into Account Scientific Evidence Showing the Benefits of 100% Fruit Juice. Am J Public Health 2012, 102. [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa) - A review on the characteristic components and potential health effects. Planta Med 2008, 74(13), 1625–1634. [CrossRef]
- Casazza, A.; Aliakbarian, B.; Mura, M.; Chasseur, M.; Freguglia, M.; Valentini, S.; Palombo, D.; Perego, P. Polyphenols from Grape and Apple Skin: a Study on Non-Conventional Extractions and Biological Activity on Endothelial Cell Cultures. Chem. Eng. Trans. 2015, 44, 205-210.
- Sforza, S.; Cavatorta, V.; Lambertini, F.; Galaverna, G.; Dossena, A.; Marchelli, R. Cheese peptidomics: A detailed study on the evolution of the oligopeptide fraction in Parmigiano-Reggiano cheese from curd to 24 months of aging. J. Dairy Sci. 2012, 95, 3514-3526. [CrossRef]
- Rizzello, C.G.; Losito, I.; Gobbetti, M.; Carbonara, T.; De Bari, M.D.; Zambonin, P.G. Antibacterial Activities of Peptides from the Water-Soluble Extracts of Italian Cheese Varieties. J. Dairy Sci. 2005, 88, 2348-2360. [CrossRef]
- Prieto, J.M. Procedure: Preparation of DPPH Radical, and antioxidant scavenging assay. 2012.
- Nielsen M.S., Martinussen T., Flambard B., Sørensen K.I. and Otte J., 2009. Peptide profiles and angiotensin-I-converting enzyme inhibitory activity of fermented milk products: effect of bacterial strain, fermentation pH, and storage time. Int. Dairy J. 2009, 19, 155-165. [CrossRef]
- Baptista, D.P.; Gigante, M.L. Bioactive peptides in ripened cheeses: release during technological processes and resistance to the gastrointestinal tract. J Sci Food Agric 2021, 101, 4010-4017. [CrossRef]
- Sanchez-Rivera, L.; Martinez-Maqueda, D.; Cruz-Huerta, E.; Miralles, B; Recio, I.Peptidomics for discovery, bioavailability and monitoring of dairy bioactive peptides”. Int. Food Res. J. 2014, 63, 1-11.
- Vieira, A.H.; Balthazar, C.F.; Guimaraes, J.T.; Rocha, R.S.; Pagani, M.M.; Esmerino, E.A.; Silva, M.C.; Raices, R.S.L.; Tonon, R.V.; Cabral, L.M.C.; Walter, E.H.M.; Freitas, M.Q.; Cruz, A.G. Advantages of microfiltration processing of goat whey orange juice beverage. Food Res. Int. 2020, 132, 109060. [CrossRef]
- Amaral, G.V.; Silva, E.K.; Cavalcanti, R.N.; Martins, C.P.C.; Andrade, L.G.Z.S.; Moraes, J.; Alvarenga, V.O.; Guimarães, J.T.; Esmerino, E.A.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Sant’Ana, A.S.; Meireles, M.A.A.; Cruz, A.G. Whey- grape juice drink processed by supercritical carbon dioxide technology: physicochemical characteristics, bioactive compounds and volatile profile. Food Chem 2017, 239, 697-703.
- Silveira, M.R.; Coutinho, N.M.; Esmerino, E.A.; Moraes, J.; Fernandes, L.M.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Senaka Ranadheera, C.; Borges, F.O.; Neto, R.P.C.; Tavares, M.I.B.; Fernandes, F.A.N.; Fonteles, T.V.; Nazzaro, F.; Rodrigues, S.; Cruz, A.G. Guava-flavored whey beverage processed by cold Plasma technology: bioactive compounds, fatty acid profile and volatile compounds. Food Chem 2018, 279, 120-127. [CrossRef]
- Ferreira, M.V.S.; Cappato, L.P.; Silva, R.; Rocha, R.S.; Guimarães, J.T.; Balthazar, C.F.; Esmerino, E.A.; Freitas, M.Q.; Rodrigues, F.N.; Granato, D.; Neto, R.P.C.; Tavares, M.I.B.; Silva, P.H.F.; Raices, R.S.L.; Silva, M.C.; Cruz, A.G.; Ohmic Heating for processing of whey-raspberry flavored beverage. Food Chem 2019, 297, 125018. [CrossRef]
- Souza, F.P.; Balthazar, C.F.; Guimarães, J.T.; Pimentel, T.C.; Esmerino, E.A.; Freitas, Mô.Q.; Raices, R.S.L.; Silva, Má.C., Cruz, A.G. The addition of xyloligoosaccharide in strawberry-flavored whey beverage. LWT - Food Sci. Technol 2019, 109, 118-122. [CrossRef]
- Ranadheera, C.S.; Chaminda, S.; Janak, K.; Vidanarachchi, R.S.; Rocha, A.G.C.; Said, A. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67.
- Jaworska, G.; Grega, T.; Sady, M.; Bernas ́, E.; Pogon ́, K. Quality of applewhey and apple beverages over 12-month storage period. J. Food Nutr. Res. 2014, 53, 117–126.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).