Submitted:
21 November 2023
Posted:
01 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Diagnostic Assays
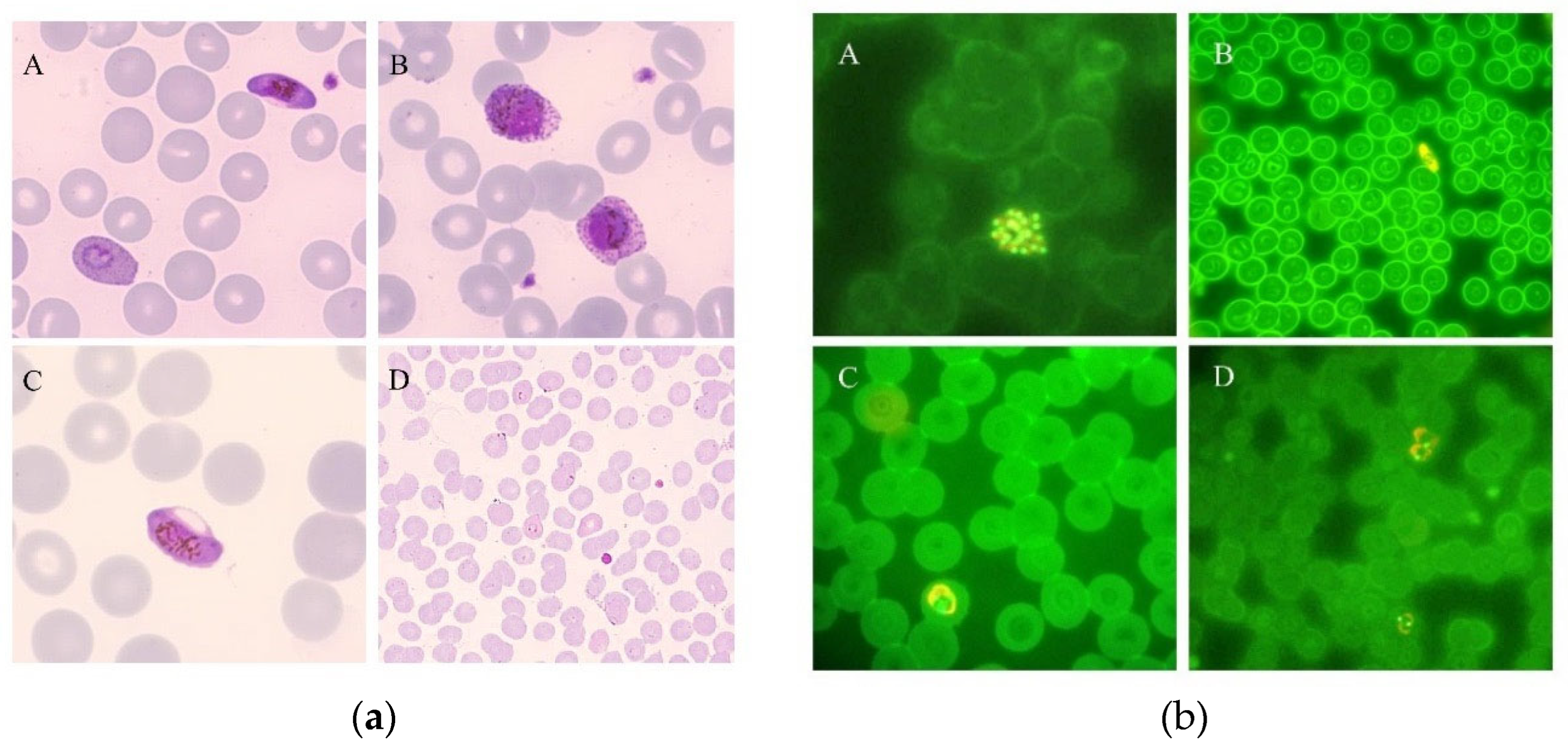
2.1. Microscopy
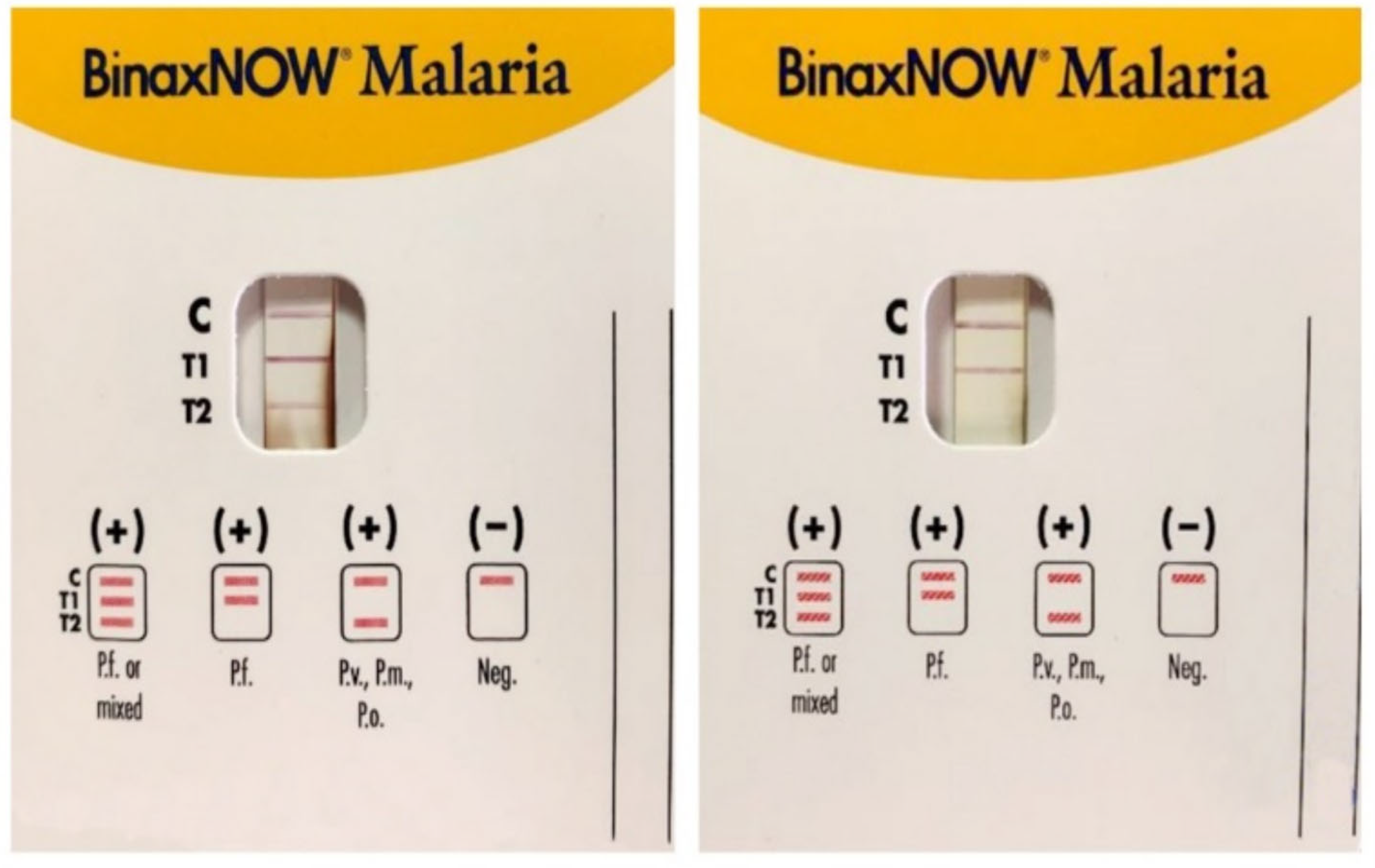
2.2. Rapid Diagnostic Tests
2.3. Flow Cytometry: Hemozoin-Based Diagnosis
2.4. Serodiagnosis
2.5. Molecular Methods
2.5.1. Molecular-Based Point of Care Test
2.6. Innovative Recently Developed Methods
3. Discussion
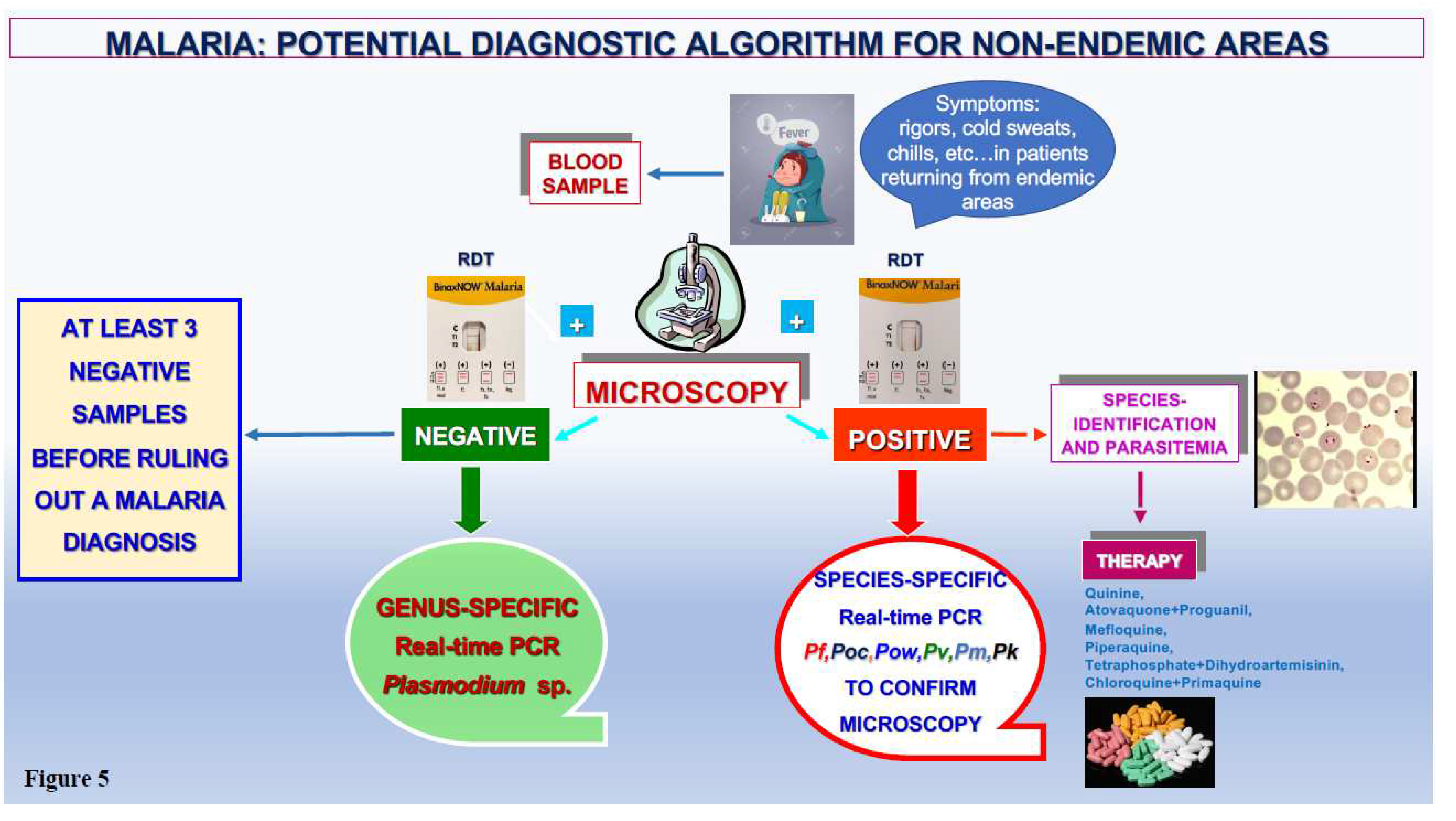
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interests
References
- Ashley, E.A.; Pyae Phyo, A.; Woodrow, C.J. Malaria. Lancet 2018, 21, 1608–1621. [Google Scholar] [CrossRef]
- Anstey, N.M.; Grigg, M.J. Zoonotic Malaria: The Better You Look, the More You Find. J. Infect. Dis. 2019, 15, 679–681. [Google Scholar] [CrossRef]
- Imwong, M.; Madmanee, W.; Suwannasin, K.; Kunasol. C.; Peto, T.J.; Tripura, R.; von Seidlein, L.; Nguon, C.; Davoeung, C.; Day, N.P.J.; Dondorp, A.M.; White, N.J. Asymptomatic Natural Human Infections With the Simian Malaria Parasites Plasmodium cynomolgi and Plasmodium knowlesi. J. Infect. Dis. 2019, 5, 695–702. [CrossRef]
- Grigg, M.J.; Snounou, G. Plasmodium simium: a Brazilian focus of anthropozoonotic vivax malaria? Lancet Glob. Health. 2017, 5, e961–e962. [Google Scholar] [CrossRef] [PubMed]
- Zekar, L.; Sharman, T. Plasmodium falciparum Malaria. 2023, 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 32310422.
- The “World malaria report 2019” at a glance. Available online: https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019.
- Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria.
- Garcia, L.S. Malaria. Clin Lab Med. 2010, 30, 93–129. [Google Scholar] [CrossRef] [PubMed]
- Global Technical Strategy for Malaria 2016-2030. Available online: https://www.who.int/docs/default-source/documents/global-technical-strategy-for-malaria-2016-2030.pdf.
- Medical Microbiology 4th edition. Chapter 83. Malaria. Available online: https://www.ncbi.nlm.nih.gov/books/NBK8584/.
- Mbanefo, A.; Kumar, N. Evaluation of Malaria Diagnostic Methods as a Key for Successful Control and Elimination Programs. Trop. Med. Infect. Dis. 2020, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Kamaliddin, C.; Le Bouar, M.; Berry, A.; Fenneteau, O.; Gillet, P.; Godineau, N.; Candolfi, E.; Houzé, S. Assessment of diagnostic methods for imported malaria in mainland France. Med. Mal. Infect. 2020, 50, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; Gorrini, C.; Peruzzi, S.; Piccolo, G.; Dettori, G.; Chezzi, C. An 8-year survey on the occurrence of imported malaria in a nonendemic area by microscopy and molecular assays. Diagn. Microbiol. Infect. Dis. 2008, 61, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; Montecchini, S.; Buttrini, M.; Piccolo, G.; Rossi, S.; Arcangeletti, M.C.; Farina, B.; De Conto, F.; Chezzi, C. Malaria Diagnosis in Non-Endemic Settings: The European Experience in the Last 22 Years. Microorganisms. 2021, 31, 2265. [Google Scholar] [CrossRef] [PubMed]
- Global Malaria Programme. Available online: https://www.who.int/teams/global-malaria-programme/case-management/diagnosis.
- Malaria Microscopy. Quality Assurance Manual. Available online: https://www.who.int/docs/default-source/documents/publications/gmp/malaria-microscopy-quality-assurance-manual.pdf.
- Malaria. CDC Yellow Book 2024. Available online: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/malaria.
- Malaria. Treatment Guidelines for Clinicians. Available online: https://www.cdc.gov/malaria/diagnosis_treatment/clinicians1.
- Moody, A. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 2002, 15, 66–78. [Google Scholar] [CrossRef]
- Huber, J.H.; Elliott, M.; Koepfli, C.; Perkins, T.A. The Impact of Emerging Plasmodium knowlesi on Accurate Diagnosis by Light Microscopy: A Systematic Review and Modeling Analysis. Am. J. Trop. Med. Hyg. 2022, 12, 61–68. [Google Scholar] [CrossRef]
- Fitri, L.E.; Widaningrum, T.; Endharti. A.T.; Prabowo, M.H.; Winaris, N.; Nugraha, R.Y.B. Malaria diagnostic update: From conventional to advanced method. J. Clin. Lab. Anal. 2022, 36, e24314. [CrossRef]
- Gitta, B.; Kilian, N. Diagnosis of Malaria Parasites Plasmodium spp. in Endemic Areas: Current Strategies for an Ancient Disease. Bioessays. 2020, 42:e1900138. [CrossRef]
- Prescott, W.R.; Jordan, R.G.; Grobusch, M.P.; Chinchilli, V.M.; Kleinschmidt, I.; Borovsky, J.; Plaskow, M.; Torrez, M.; Mico, M.; Schwabe, C. Performance of a malaria microscopy image analysis slide reading device. Malar. J. 2012, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Florin, K.; Maelegheer, K.; Muyldermans, A.; Van Esbroeck, M.; Nulens, E.; Emmerechts, J. Evaluation of the CellaVision DM96 advanced RBC application for screening and follow-up of malaria infection. Diagn. Microbiol. Infect. Dis. 2018, 90, 253. [Google Scholar] [CrossRef] [PubMed]
- Lenz, D.; Kremsner, P.G.; Lell, B.; Biallas, B.; Boettcher, M.; Mordmuller, B.; Adegnika, A.A. Assessment of LED fluorescence microscopy for the diagnosis of Plasmodium falciparum infections in Gabon. 2011;10:194. https://doi.org/10.1186/1475-2875-10-194. Malar. J. 2011, 10, 194. [CrossRef]
- Kimura, M.; Teramoto, I.; Chan, C.W.; Idris, Z.M.; Kongere, J.; Kagaya, W.; Kawamoto, F.; Asada, R.; Isozumi, R.; Kaneko, A. Improvement of malaria diagnostic system based on acridine orange staining. Malar. J. 2018, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; Piccolo, G.; Montecchini, S.; Buttrini, M.; Rossi, S.; Dell’Anna, M.L.; De Remigis, V.; Arcangeletti, M.C.; Chezzi, C.; De Conto, F. High prevalence of malaria in a non-endemic setting: comparison of diagnostic tools and patient outcome during a four-year survey (2013-2017). Malar J. 2018, 5, 63. [Google Scholar] [CrossRef]
- Global Malaria Programme. Rapid diagnostic tests. Available online: https://www.who.int/teams/global-malaria-programme/case-management/diagnosis/rapid-diagnostic-tests.
- WHO Guidelines for malaria. Available online: https://www.who.int/publications/i/item/guidelines-for-malaria.
- Berthod, D.; Rochat, J.; Voumard, R.; et al. Self-diagnosis of malaria by travelers: a cohort study on the use of malaria rapid diagnostic tests provided by a Swiss travel clinic. Malar. J. 2017, 16, 436. [Google Scholar] [CrossRef]
- Martiáñez-Vendrell, X.; Skjefte, M.; Sikka, R.; Gupta, H. Factors Affecting the Performance of HRP2-Based Malaria Rapid Diagnostic Tests. Trop. Med. Infect. Dis. 2022, 7, 265. [Google Scholar] [CrossRef]
- Kavanaugh, M.J.; Azzam, S.E.; Rockabrand, D.M. Malaria Rapid Diagnostic Tests: Literary Review and Recommendation for a Quality Assurance, Quality Control Algorithm. Diagnostics (Basel). 2021, 11, 768. [Google Scholar] [CrossRef]
- Bronner, U.; Karlsson, L.; Evengård, B. Evaluation of rapid diagnostic tests for malaria in Swedish travelers. APMIS. 2011, 119, 88–92. [Google Scholar] [CrossRef]
- Pasricha, J.M.; Juneja, S.; Manitta, J.; Whitehead, S.; Maxwell, E.; Goh, W.K.; Pasricha, S.R.; Eisen, D.P. Is serial testing required to diagnose imported malaria in the era of rapid diagnostic tests? Am. J. Trop. Med. Hyg. 2013, 88, 20–23. [Google Scholar] [CrossRef]
- Houzé, S.; Hubert, V.; Cohen, D.P.; Rivetz, B.; Le Bras, J. Evaluation of the Clearview® Malaria pLDH Malaria Rapid Diagnostic Test in a non-endemic setting. Malar. J. 2011, 10, 284. [Google Scholar] [CrossRef]
- Maltha, J.; Gillet, P.; Bottieau, E.; Cnops, L.; van Esbroeck, M.; Jacobs, J. Evaluation of a rapid diagnostic test (CareStart Malaria HRP-2/pLDH (Pf/pan) Combo Test) for the diagnosis of malaria in a reference setting. Malar. J. 2010, 9, 171. [Google Scholar] [CrossRef]
- Maltha, J.; Gillet, P.; Cnops, L.; Bottieau, E.; Van Esbroeck, M.; Bruggeman, C.; Jacobs, J. Evaluation of the rapid diagnostic test SDFK40 (Pf-pLDH/pan-pLDH) for the diagnosis of malaria in a non-endemic setting. Malar. J. 2011, 10, 7. [Google Scholar] [CrossRef]
- van der Palen, M.; Gillet, P.; Bottieau, E.; Cnops, L.; Van Esbroeck, M.; Jacobs, J. Test characteristics of two rapid antigen detection tests (SD FK50 and SD FK60) for the diagnosis of malaria in returned travelers. Malar. J. 2009, 8, 90. [Google Scholar] [CrossRef]
- Gillet, P.; van Dijk, D.P.; Bottieau, E.; Cnops, L.; Van Esbroeck, M.; Jacobs, J. Test characteristics of the SD FK80 Plasmodium falciparum/Plasmodium vivax malaria rapid diagnostic test in a non-endemic setting. Malar. J. 2009, 8, 262. [Google Scholar] [CrossRef]
- Heutmekers, M.; Gillet, P.; Cnops, L.; Bottieau, E.; Van Esbroeck, M.; Maltha, J.; Jacobs, J. Evaluation of the malaria rapid diagnostic test SDFK90: detection of both PfHRP2 and Pf-pLDH. Malar. J. 2012, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Eibach, D.; Traore, B.; Bouchrik, M.; Coulibaly, B.; Coulibaly, N.; Siby, F.; Bonnot, G.; Bienvenu, A.L.; Picot, S. Evaluation of the malaria rapid diagnostic test VIKIA malaria Ag Pf/Pan™ in endemic and non-endemic settings. Malar. J. 2013, 12, 188. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, D.P.; Gillet, P.; Vlieghe, E.; Cnops, L.; Van Esbroeck, M.; Jacobs, J. Evaluation of the Immunoquick+4 malaria rapid diagnostic test in a non-endemic setting. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 577–83. [Google Scholar] [CrossRef] [PubMed]
- Houzé, S.; Boly, M.D.; Le Bras, J.; et al. Pf HRP2 and Pf LDH antigen detection for monitoring the efficacy of artemisinin-based combination therapy (ACT) in the treatment of uncomplicated falciparum malaria. Malar. J. 2009, 8, 211. [Google Scholar] [CrossRef] [PubMed]
- Barber, B.E.; William, T.; Grigg, M.J.; Piera, K.; Yeo, T.W.; Anstey, N.M. Evaluation of the Sensitivity of a pLDH-Based and an Aldolase-Based Rapid Diagnostic Test for Diagnosis of Uncomplicated and Severe Malaria Caused by PCR-Confirmed Plasmodium knowlesi, Plasmodium falciparum, and Plasmodium vivax. J. Clin. Microbiol. 2013, 51, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Bisoffi, Z.; Sirima, S.B.; Menten, J.; Pattaro, C.; Angheben, A.; Gobbi, F.; Tinto, H.; Lodesani, C.; Neya, B.; Gobbo, M.; Van den Ende, J. Accuracy of a rapid diagnostic test on the diagnosis of malaria infection and of malaria-attributable fever during low and high transmission season in Burkina Faso. Malar. J. 2010, 9, 192. [Google Scholar] [CrossRef]
- Vásquez, A.M.; Medina, A.C.; Tobón-Castaño, A.; Posada, M.; Vélez, G.J.; Campillo, A.; González, I.J.; Ding, X. Performance of a highly sensitive rapid diagnostic test (HS-RDT) for detecting malaria in peripheral and placental blood samples from pregnant women in Colombia. PLoS One. 2018, 2, e0201769. [Google Scholar] [CrossRef]
- Mohapatra, S.; Ghosh, A.; Singh, R.; Singh, D.P.; Sharma, B.; Samantaray, J.C.; Deb, M.; Gaind, R. Hemozoin Pigment: An Important Tool for Low Parasitemic Malarial Diagnosis. Korean J. Parasitol. 2016, 54, 393–7. [Google Scholar] [CrossRef]
- Tangpukdee, N.; Duangdee, C.; Wilairatana, P.; Krudsood, S. Malaria diagnosis: a brief review. Korean J. Parasitol. 2009, 47, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.K.; Kong, T.F.; Ng, C.S.; Chen, L.; Huang, Y.; Bhagat, A.A.; Nguyen, N.T.; Preiser, P.R.; Han, J. Micromagnetic resonance relaxometry for rapid label-free malaria diagnosis. Nat. Med. 2014, 20, 1069–73. [Google Scholar] [CrossRef]
- Kong, T.F.; Ye, W.; Peng, W.K.; Hou, H.W., Marcos; Preiser, P.R.; Nguyen, N.T.; Han, J. Enhancing malaria diagnosis through microfluidic cell enrichment and magnetic resonance relaxometry detection. Sci. Rep. 2015, 5, 11425. [Google Scholar] [CrossRef] [PubMed]
- Thamarath, S.S.; Xiong, A.; Lin, P.H.; Preiser, P.R.; Han, J. Enhancing the sensitivity of micro magnetic resonance relaxometry detection of low parasitemia Plasmodium falciparum in human blood. Sci. Rep. 2019, 9, 2555. [Google Scholar] [CrossRef]
- Mens, P.F.; Matelon, R.J.; Nour, B.Y.; Newman, D.M.; Schallig, HD. Laboratory evaluation on the sensitivity and specificity of a novel and rapid detection method for malaria diagnosis based on magneto-optical technology (MOT). Malar. J. 2010, 9, 207. [Google Scholar] [CrossRef]
- Orbán, Á.; Rebelo, M.; Molnár, P.; Albuquerque, I.S.; Butykai, A.; Kézsmárki, I. Efficient monitoring of the blood-stage infection in a malaria rodent model by the rotating-crystal magneto-optical method. Sci. Rep. 2016, 6, 23218. [Google Scholar] [CrossRef]
- Roch, A.; Prodéo, J.; Pierart, C.; Muller, R.N.; Duez, P. The paramagnetic properties of malaria pigment, hemozoin, yield clues to a low-cost system for its trapping and determination. Talanta. 2019, 197, 553–557. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, A.K.; Shrivas, S.; Thota, P.; Singh, M.P.; Rajasubramaniam, S.; Das, A.; Bharti, P.K. First successful field evaluation of new, one-minute haemozoin-based malaria diagnostic device. EClinicalMedicine. 2020, 22, 100347. [Google Scholar] [CrossRef]
- McBirney, S.E.; Chen, D.; Scholtz, A.; Ameri, H.; Armani, A.M. Rapid Diagnostic for Point-of-Care Malaria Screening. ACS Sens. 2018, 3, 1264–1270. [Google Scholar] [CrossRef]
- Garrett, N.L.; Sekine, R.; Dixon, M.W.; Tilley, L.; Bambery, K.R.; Wood, B.R. Bio-sensing with butterfly wings: naturally occurring nanostructures for SERS-based malaria parasite detection. Phys. Chem. Chem. Phys. 2015, 17, 21164–21168. [Google Scholar] [CrossRef] [PubMed]
- Butykai, A.; Orbán, A.; Kocsis, V.; Szaller, D.; Bordács, S.; Tátrai-Szekeres, E.; Kiss, L.F.; Bóta, A.; Vértessy, B.G.; Zelles, T.; Kézsmárki, I. Malaria pigment crystals as magnetic micro-rotors: key for high-sensitivity diagnosis. Sci. Rep. 2013, 3, 1431. [Google Scholar] [CrossRef]
- Tripathy, U.; Giguère-Bisson, M.; Sangji, M.H.; Bellemare, M.J.; Bohle, D.S.; Georges, E.; Wiseman, P.W. Optimization of malaria detection based on third harmonic generation imaging of hemozoin. Anal. Bioanal. Chem. 2013, 405, 5431–5440. [Google Scholar] [CrossRef]
- Catarino, S.O.; Felix, P.; Sousa, P.J.; Pinto, V.; Veiga, M.I.; Minas, G. Portable Device for Optical Quantification of Hemozoin in Diluted Blood Samples. IEEE Trans. Biomed. Eng. 2020, 67, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.S.; Baptista, V.; Ferreira, G.M.; Lima, D.; Minas, G.; Veiga, M.I.; Catarino, S.O. Multilayer Thin-Film Optical Filters for Reflectance-Based Malaria Diagnostics. Micromachines (Basel). 2021, 12, 890. [Google Scholar] [CrossRef] [PubMed]
- Rifaie-Graham, O.; Pollard, J.; Raccio, S.; Balog, S.; Rusch, S.; Hernández-Castañeda, M.A.; Mantel, P.Y.; Beck, H.P.; Bruns, N. Hemozoin-catalyzed precipitation polymerization as an assay for malaria diagnosis. Nat. Commun. 2019, 10, 1369. [Google Scholar] [CrossRef]
- Cai, C.; Carey, K.A.; Nedosekin, D.A.; Menyaev, Y.A.; Sarimollaoglu, M.; Galanzha, E.I.; Stumhofer, J.S.; Zharov, V.P. In vivo photoacoustic flow cytometry for early malaria diagnosis. Cytometry A. 2016, 89, 531–42. [Google Scholar] [CrossRef]
- Menyaev, Y.A.; Carey, K.A.; Nedosekin, D.A.; Sarimollaoglu, M.; Galanzha, E.I.; Stumhofer, J.S. , Zharov, V.P. Preclinical photoacoustic models: application for ultrasensitive single cell malaria diagnosis in large vein and artery. Biomed. Opt. Express. 2016, 7, 3643–3658. [Google Scholar] [CrossRef]
- Lukianova-Hleb, E.Y.; Campbell, K.M.; Constantinou, P.E. , Braam, J.; Olson, J.S.; Ware, R.E.; Sullivan, D.J. Jr.; Lapotko, D.O. Hemozoin-generated vapor nanobubbles for transdermal reagent- and needle-free detection of malaria. Proc. Natl. Acad. Sci. U.S.A. 2014, 111, 900–5. [Google Scholar] [CrossRef]
- Wang, S.; Yang, C.; Preiser, P.; Zheng, Y. A photoacoustic-surface-acoustic-wave sensor for ring-stage malaria parasite detection. IEEE Transactions on Circuits and Systems II: Express Briefs, 2020 ieeexplore.ieee.org.
- Rodrigues, M.H.; Cunha, M.G.; Machado, R.L.; Ferreira, O.C. Jr, Rodrigues, M.M.; Soares, I.S. Serological detection of Plasmodium vivax malaria using recombinant proteins corresponding to the 19-kDa C-terminal region of the merozoite surface protein-1. Malar. J. 2003, 14, 2. [CrossRef]
- Doderer, C.; Heschung, A.; Guntz, P.; Cazenave, J.P.; Hansmann, Y.; Senegas, A.; Pfaff, A.W.; Abdelrahman, T.; Candolfi, E. A new ELISA kit which uses a combination of Plasmodium falciparum extract and recombinant Plasmodium vivax antigens as an alternative to IFAT for detection of malaria antibodies. Malar. J. 2007, 21, 19. [Google Scholar] [CrossRef]
- Slater, L.; Ashraf, S.; Zahid, O.; Ali, Q.; Oneeb, M.; Akbar, M.H.; Riaz, M.I.; Afshan, K.; Sargison, N.; Chaudhry, U. Current methods for the detection of Plasmodium parasite species infecting humans. Curr. Res. Parasitol. Vector Borne Dis. 2022, 2, 100086. [Google Scholar] [CrossRef] [PubMed]
- Malaria. Diagnostic Tools. Available online: https://www.cdc.gov/malaria/diagnosis_treatment/diagnostic_tools.html.
- Opoku Afriyie, S.; Addison, T.K.; Gebre, Y.; Mutala, A.H.; Antwi, K.B.; Abbas, D.A.; Addo, K.A.; Tweneboah, A.; Ayisi-Boateng, N.K.; Koepfli, C.; Badu, K. Accuracy of diagnosis among clinical malaria patients: comparing microscopy, RDT and a highly sensitive quantitative PCR looking at the implications for submicroscopic infections. Malar. J. 2023, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Snounou, G.; Viriyakosol, S.; Jarra, W.; Thaithong, S.; Brown, K.N. Identification of the four human malaria parasite species in field samples by the polymerase chain reaction and detection of a high prevalence of mixed infections. Mol. Biochem. Parasitol. 1993, 58, 283–92. [Google Scholar] [CrossRef]
- Snounou, G.; Viriyakosol, S.; Zhu, X.P.; Jarra, W.; Pinheiro, L.; do Rosario, V.E.; et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 1993, 61, 315–20. [Google Scholar] [CrossRef] [PubMed]
- Calderaro, A.; Piccolo, G.; Perandin, F.; Gorrini, C.; Peruzzi, S.; Zuelli, C.; Ricci, L.; Manca, N.; Dettori, G.; Chezzi, C.; Snounou, G. Genetic polymorphisms influence Plasmodium ovale PCR detection accuracy. J. Clin. Microbiol. 2007, 45, 1624–7. [Google Scholar] [CrossRef] [PubMed]
- Polley, S.D.; Mori, Y.; Watson, J.; Perkins, M.D.; González, I.J.; Notomi, T.; Chiodini, P.L.; Sutherland, C.J. Mitochondrial DNA targets increase sensitivity of malaria detection using loop-mediated isothermal amplification. J. Clin. Microbiol. 2010, 48, 2866–2871. [Google Scholar] [CrossRef] [PubMed]
- Niang, M.; Yan Yam, X.; Preiser, P.R. The Plasmodium falciparum STEVOR multigene family mediates antigenic variation of the infected erythrocyte. PLoS Pathog. 2009, 5, e1000307. [Google Scholar] [CrossRef]
- Reed, J.; Kirkman, L.A.; Kafsack, B.F.; Mason, C.E.; Deitsch, K.W. Telomere length dynamics in response to DNA damage in malaria parasites. iScience. 2021, 20, 102082. [Google Scholar] [CrossRef]
- Patel, J.C.; Oberstaller, J.; Xayavong, M.; Narayanan, J.; DeBarry, J.D.; Srinivasamoorthy, G.; Villegas, L.; Escalante, A.A.; DaSilva, A.; Peterson, D.S.; Barnwell, J.W.; Kissinger, J.C.; Udhayakumar, V.; Lucchi, N.W. Real-time loop-mediated isothermal amplification (RealAmp) for the species-specific identification of Plasmodium vivax. PLoS One. 2013, 8, e54986. [Google Scholar] [CrossRef]
- Dafalla, O.M.; Alzahrani, M.; Sahli, A.; Al Helal, M.A.; Alhazmi, M.M.; Noureldin, E.M.; Mohamed, W.S.; Hamid, T.B.; Abdelhaleem, A.A.; Hobani, Y.A.; Arif, O.A.; Bokar, I.M.; Hakami, A.M.; Eisa, Z.M. Kelch 13-propeller polymorphisms in Plasmodium falciparum from Jazan region, southwest Saudi Arabia. Malar. J. 2020, 10, 397. [Google Scholar] [CrossRef]
- Chavalitshewinkoon-Petmitr, P. Laboratory diagnosis of malaria. Siriraj Med. J. 2020, 62 (2 SE-Special issue), 98-102.
- Calderaro, A.; Piccolo, G.; Gorrini, C.; Montecchini, S.; Rossi, S.; Medici, M.C.; Chezzi, C.; Snounou, G. A new real-time PCR for the detection of Plasmodium ovale wallikeri. PLoS One. 2012, 7, e48033. [Google Scholar] [CrossRef]
- Calderaro, A.; Piccolo, G.; Gorrini, C.; Rossi, S.; Montecchini, S.; Dell’Anna, M.L.; De Conto, F.; Medici, M.C.; Chezzi, C.; Arcangeletti, M.C. Accurate identification of the six human Plasmodium spp. causing imported malaria, including Plasmodium ovale wallikeri and Plasmodium knowlesi. Malar. J. 2013, 12, 321. [Google Scholar] [CrossRef] [PubMed]
- Perandin, F.; Manca, N.; Calderaro, A.; Piccolo, G.; Galati, L.; Ricci, L.; Medici, M.C.; Arcangeletti, M.C.; Snounou, G.; Dettori, G.; Chezzi, C. Development of a real-time PCR assay for detection of Plasmodium falciparum, Plasmodium vivax, and Plasmodium ovale for routine clinical diagnosis. J. Clin. Microbiol. 2004, 42, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Rougemont, M.; Van Saanen, M.; Sahli, R.; Hinrikson, H.P.; Bille, J.; Jaton, K. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J. Clin. Microbiol. 2004, 42, 5636–5643. [Google Scholar] [CrossRef] [PubMed]
- Mangold, K.A.; Manson, R.U.; Koay, E.S.; Stephens, L.; Regner, M.; Thomson, R.B. Jr, Peterson LR, Kaul KL. Real-time PCR for detection and identification of Plasmodium spp. J. Clin. Microbiol. 2005, 43, 2435–2440. [Google Scholar] [CrossRef] [PubMed]
- Tajebe, A.; Magoma, G.; Aemero, M.; Kimani, F. Detection of mixed infection level of Plasmodium falciparum and Plasmodium vivax by SYBR Green I-based real-time PCR in North Gondar, north-west Ethiopia. Malar. J. 2014, 18, 411. [Google Scholar] [CrossRef]
- Vincent, J.P.; Existe, A.V.; Komaki-Yasuda, K.; Boncy, J.; Kano, S. Performance of the procedure for ultra-rapid extraction and loop-mediated isothermal amplification (PURE-LAMP) method to detect malaria in Haiti. Infect. Dis. Poverty. 2023, 22, 53. [Google Scholar] [CrossRef]
- Malpartida-Cardenas, K.; Moser, N.; Ansah, F.; Pennisi, I.; Ahu Prah, D.; Amoah, L.E.; Awandare, G.; Hafalla, J.C.R.; Cunnington, A.; Baum, J.; Rodriguez-Manzano, J.; Georgiou, P. Sensitive Detection of Asymptomatic and Symptomatic Malaria with Seven Novel Parasite-Specific LAMP Assays and Translation for Use at Point-of-Care. Microbiol. Spectr. 2023, 15, 11:e0522222. [Google Scholar] [CrossRef] [PubMed]
- Febrer-Sendra, B.; Crego-Vicente, B.; Nindia, A.; Martínez-Campreciós, J.; Aixut, S.; Mediavilla, A.; Silgado, A.; Oliveira-Souto, I.; Salvador, F.; Molina, I.; Muro, A.; Sulleiro, E.; Fernández-Soto, P. First field and laboratory evaluation of LAMP assay for malaria diagnosis in Cubal, Angola. Parasit. Vectors. 2023; 16, 343. [CrossRef]
- Surabattula, R.; Vejandla, M.P.; Mallepaddi, P.C.; Faulstich, K.; Polavarapu, R. Simple, rapid, inexpensive platform for the diagnosis of malaria by loop mediated isothermal amplification (LAMP). Exp. Parasitol. 2013, 134, 333–40. [Google Scholar] [CrossRef] [PubMed]
- Haanshuus, C.G.; Mørch, K.; Blomberg, B.; Strøm, G.E.A.; Langeland, N.; Hanevik, K.; Mohn, S.C. Assessment of malaria real-time PCR methods and application with focus on low-level parasitaemia. PLoS One. 2019, 5, e0218982. [Google Scholar] [CrossRef] [PubMed]
- Gadalla, A.A.H.; Siciliano, G.; Farid, R.; Alano, P.; Ranford-Cartwright, L.; McCarthy, J.S.; Thompson, J.; Babiker, H.A. Real-time PCR assays for detection and quantification of early P. falciparum gametocyte stages. Sci. Rep. 2021, 27, 19118. [Google Scholar] [CrossRef] [PubMed]
- Padley, D.J.; Heath, A.B.; Sutherland, C.; Chiodini, P.L.; Baylis, S.A. Establishment of the 1st World Health Organization International Standard for Plasmodium falciparum DNA for nucleic acid amplification technique (NAT)-based assays. Malar. J. 2008, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase polymerase amplification: Basics, applications and recent advances. Trends Analyt. Chem. 2018, 98, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Kersting, S.; Rausch, V.; Bier, F.F.; von Nickisch-Rosenegk, M. Rapid detection of Plasmodium falciparum with isothermal recombinase polymerase amplification and lateral flow analysis. Malar. J. 2014, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Biondi, M.J.; Feld, J.J.; Chan, W.C. Clinical Validation of Quantum Dot Barcode Diagnostic Technology. ACS Nano. 2016, 26, 4742–4753. [Google Scholar] [CrossRef]
- de la Escosura-Muñiz, A.; Baptista-Pires, L.; Serrano, L.; Altet, L.; Francino, O.; Sánchez, A.; Merkoçi, A. Magnetic Bead/Gold Nanoparticle Double-Labeled Primers for Electrochemical Detection of Isothermal Amplified Leishmania DNA. Small. 2016, 13, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Koo, K.M.; Wee, E.J.; Wang, Y.; Trau, M. A nanoplasmonic label-free surface-enhanced Raman scattering strategy for non-invasive cancer genetic subtyping in patient samples. Nanoscale. 2017, 9, 3496–3503. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.Y.; Lau, Y.L. Detection of Plasmodium knowlesi using recombinase polymerase amplification (RPA) combined with SYBR Green I. Acta Trop. 2020, 15, 105511. [Google Scholar] [CrossRef] [PubMed]
- Rubio, J.M.; Benito, A.; Berzosa, P.J.; Roche, J.; Puente, S.; Subirats, M.; López-Vélez, R.; García, L.; Alvar, J. Usefulness of seminested multiplex PCR in surveillance of imported malaria in Spain. J. Clin. Microbiol. 1999, 37, 3260–3264. [Google Scholar] [CrossRef] [PubMed]
- Paglia, M.G.; Vairo, F.; Bevilacqua, N.; Ghirga, P.; Narciso, P.; Severini, C.; Nicastri, E. Molecular diagnosis and species identification of imported malaria in returning travellers in Italy. Diagn. Microbiol. Infect. Dis. 2012, 72, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Schallig, H.D.; Schoone, G.J.; Lommerse, E.J.; Kroon, C.C.; de Vries, P.J.; van Gool, T. Usefulness of quantitative nucleic Acid sequence-based amplification for diagnosis of malaria in an academic hospital setting. Eur. J. Clin. Microbiol. Infect. Dis. 2003, 22, 555–557. [Google Scholar] [CrossRef]
- Mens, P.F.; Schoone, G.J.; Kager, P.A. , Schallig, H.D. Detection and identification of human Plasmodium species with real-time quantitative nucleic acid sequence-based amplification. Malar. J. 2006, 3, 80. [Google Scholar] [CrossRef]
- Dakić, Z.; Ivović, V.; Pavlović, M.; Lavadinović, L.; Marković, M.; Djurković-Djaković, O. Clinical significance of molecular methods in the diagnosis of imported malaria in returning travelers in Serbia. Int. J. Infect. Dis. 2014, 29, 24–30. [Google Scholar] [CrossRef]
- Strøm, G.E.; Haanshuus, C.G.; Fataki, M.; Langeland, N.; Blomberg, B. Challenges in diagnosing paediatric malaria in Dar es Salaam, Tanzania. Malar. J. 2013, 3, 228. [Google Scholar] [CrossRef]
- Grignard, L.; Nolder, D.; Sepúlveda, N.; Berhane, A.; Mihreteab, S.; Kaaya, R.; Phelan, J.; Moser, K.; van Schalkwyk, D.A.; Campino, S.; Parr, J.B.; Juliano, J.J.; Chiodini, P.; Cunningham, J.; Sutherland, C.J.; Drakeley, C.; Beshir, K.B. A novel multiplex qPCR assay for detection of Plasmodium falciparum with histidine-rich protein 2 and 3 (pfhrp2 and pfhrp3) deletions in polyclonal infections. EBioMedicine. 2020, 55, 102757. [Google Scholar] [CrossRef]
- Bourgeois, N.; Boutet, A.; Bousquet, P.J., Basset, D.; Douard-Enault, C.; Charachon, S.; Lachaud, L. Comparison of three real-time PCR methods with blood smears and rapid diagnostic test in Plasmodium sp. infection. Clin. Microbiol. Infect. 2010, 16, :1305–1311. [CrossRef]
- Marti, H.; Stalder, C.; González, I.J. Diagnostic accuracy of a LAMP kit for diagnosis of imported malaria in Switzerland. Travel. Med. Infect. Dis. 2015, 13, 167–71. [Google Scholar] [CrossRef]
- Cuadros, J.; Martin Ramírez, A.; González, I.J.; Ding, X.C.; Perez Tanoira, R.; Rojo-Marcos, G.; Gómez-Herruz, P.; Rubio, J.M. LAMP kit for diagnosis of non-falciparum malaria in Plasmodium ovale infected patients. Malar. J. 2017, 16, 20. [Google Scholar] [CrossRef]
- Charpentier, E.; Benichou, E.; Pagès, A.; Chauvin, P.; Fillaux, J.; Valentin, A.; Guegan, H.; Guemas, E.; Salabert, A.S.; Armengol, C.; Menard, S.; Cassaing, S.; Berry, A.; Iriart, X. Performance evaluation of different strategies based on microscopy techniques, rapid diagnostic test and molecular loop-mediated isothermal amplification assay for the diagnosis of imported malaria. Clin. Microbiol. Infect. 2020, 26, 115–121. [Google Scholar] [CrossRef]
- Rei Yan, S.L.; Wakasuqui, F.; Wrenger, C. Point-of-care tests for malaria: speeding up the diagnostics at the bedside and challenges in malaria cases detection. Diagn. Microbiol. Infect. Dis. 2020, 98, 115122. [Google Scholar] [CrossRef]
- Mahendran, P.; Liew, J.W.K.; Amir, A.; Ching, X.T.; Lau, Y.L. Droplet digital polymerase chain reaction (ddPCR) for the detection of Plasmodium knowlesi and Plasmodium vivax. Malar. J. 2020, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Tessema, S.K.; Raman, J.; Duffy, C.W.; Ishengoma, D.S.; Amambua-Ngwa, A.; Greenhouse, B. Applying next-generation sequencing to track falciparum malaria in sub-Saharan Africa. Malar. J. 2019, 3, 268. [Google Scholar] [CrossRef] [PubMed]
- Talundzic, E.; Ravishankar, S.; Kelley, J.; Patel, D.; Plucinski, M.; Schmedes, S.; Ljolje, D.; Clemons, B.; Madison-Antenucci, S.; Arguin, P.M.; Lucchi, N.W.; Vannberg, F.; Udhayakumar, V. Next-Generation Sequencing and Bioinformatics Protocol for Malaria Drug Resistance Marker Surveillance. Antimicrob. Agents Chemother. 2018, 27, e02474-17. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, N.J.; Menting, S.; Wentink-Bonnema, E.M.S.; Broekhuizen-van Haaften, P.E.; Withycombe, E.; Schallig, H.D.F.H.; Mens, P.F. Laboratory evaluation of the miniature direct-on-blood PCR nucleic acid lateral flow immunoassay (mini-dbPCR-NALFIA), a simplified molecular diagnostic test for Plasmodium. Malar. J. 2023, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- Madhu, G.; Mohamed, A.W.; Kautish, S.; Shah, M.A.; Ali, I. Intelligent diagnostic model for malaria parasite detection and classification using imperative inception-based capsule neural networks. Sci. Rep. 2023, 17, 13377. [Google Scholar] [CrossRef] [PubMed]
- Aqeel, S., Haider, Z.; Khan, W. Towards digital diagnosis of malaria: How far have we reached? J. Microbiol. Methods. 2023, 204, 106630. [CrossRef]
- Stauning, M.A.; Jensen, C.S.; Staalsøe, T.; Kurtzhals, J.A.L. Detection and quantification of Plasmodium falciparum in human blood by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry: a proof of concept study. Malar. J. 2023, 26, 285. [Google Scholar] [CrossRef] [PubMed]
- Obeng-Aboagye, E.; Frimpong, A.; Amponsah, J.A.; Danso, S.E.; Owusu, E.D.A.; Ofori, M.F. Inflammatory cytokines as potential biomarkers for early diagnosis of severe malaria in children in Ghana. Malar. J. 2023, 31, 220. [Google Scholar] [CrossRef] [PubMed]
- Askling, H.H.; Bruneel, F.; Burchard, G.; Castelli, F.; Chiodini, P.L.; Grobusch, M.P.; Lopez-Vélez, R.; Paul, M.; Petersen, E.; Popescu, C.; Ramharter, M.; Schlagenhauf, P. European Society for Clinical Microbiology and Infectious Diseases Study Group on Clinical Parasitology. Management of imported malaria in Europe. Malar. J. 2012, 11, 328. [Google Scholar] [CrossRef]
- Iwelunmor, J.; Belue, R.; Nwosa. I.; Adedokun, A.; Airhihenbuwa, C.O. Case-management of malaria in children attending an outpatient clinic in southwest Nigeria. Int. Q. Community Health. Educ. 2013, 34, 255–267. [CrossRef]
- van Bergen, K.; Stuitje, T.; Akkers, R.; Vermeer, E.; Castel, R.; Mank, T. Evaluation of a novel real-time PCR assay for the detection, identification and quantification of Plasmodium species causing malaria in humans. Malar. J. 2021, 12, 314. [Google Scholar] [CrossRef]
- Siłka, W.; Wieczorek, M.; Siłka, J.; Woźniak, M. Malaria Detection Using Advanced Deep Learning Architecture. Sensors (Basel). 2023, 29, 1501. [Google Scholar] [CrossRef] [PubMed]
- Marletta, S.; L’Imperio, V.; Eccher, A.; Antonini, P.; Santonicco, N.; Girolami, I.; Dei Tos, A.P.; Sbaraglia, M.; Pagni, F.; Brunelli, M.; Marino, A.; Scarpa, A.; Munari, E.; Fusco, N.; Pantanowitz, L. Artificial intelligence-based tools applied to pathological diagnosis of microbiological diseases. Pathol. Res. Pract. 2023, 243, 154362. [Google Scholar] [CrossRef] [PubMed]

| Species tested | ||||||
|---|---|---|---|---|---|---|
| P. falciparum | P. vivax | P. ovale | P. malariae | *Pan | References | |
| MalaQuick (R-Biopharm, Germany) | X | X | [33] | |||
| BinaxNOWTM MALARIA (AbbottTM, Italy) | X | X | [34] | |||
| Clearview® malaria (Orgenics Ltd., Alere Diagnostics, Yavne, Israel) | X | X | X | X | [35] | |
| CarestartTM Malaria (AccessBio Inc., USA) | X | X | X | X | [36] | |
| SD Bioline Malaria Ag 05FK40 (Standard Diagnostics Inc., Korea) | X | [37] | ||||
| SD Bioline Malaria Ag Pf FK50 (Standard Diagnostics Inc., Korea) | X | [38] | ||||
| SD FK70 Malaria Antigen Pv test (Standard Diagnostics Inc., Korea) | X | [39] | ||||
| SD FK80 Pf/Pv Malaria Antigen Rapid Test (Standard Diagnostics Inc., Korea) | X | X | [39] | |||
| SD Malaria Antigen Pf 05FK90-02-0 (Standard Diagnostics, Inc., Korea) | X | [40] | ||||
| VIKIA Malaria (Biomerieux, France) | X | X | [41] | |||
| Core Malaria (Core Diagnostics, United Kingdom) | X | X | X | [40] | ||
| PALUTOP®+4 OPTIMA (BioSynex, France) | X | X | X | [35] | ||
| OptiMal-IT® (DiaMed, Switzerland) | X | X | [35] | |||
| Immunoquick+4 (BioSynex, France) | X | X | X | X | [42] | |
| Technology | Limit of detection | References |
|---|---|---|
| Electromagnetic | ||
| Magnetic Resonance Relaxometry (MRR) | 0.002% of Pf culture | [49] |
| Microfluidic separation followed saponin lysis and MRR | 0.0005% of Pf culture | [50] |
| Saponin lysis and MRR | 0.0001% of Pf culture | [51] |
| Magneto-optic | ||
| Magneto-optical technology | 50-100 Pf culture/µl | [52] |
| Rotating-crystal magneto-optical technique | 40 -10 Pf culture /µl | [53] |
| Magneto-chromatographic online system | 55 parasite (Pf)/µl | [54] |
| Gazzelle | 50 parasite (Pf)/µl | [55] |
| Portable optical diagnostic system | 25 parasites (Pf)/µl | [56] |
| Surface-enhanced Raman spectroscopy | 30 parasites (Pf)/µl | [57] |
| Optical features | ||
| Polarized light microscopy | 30 parasites (Pf)/µl | [58] |
| Third-Harmonic Generation Imaging | nondefined | [59] |
| Optical Absorbance Diagnostic Method | 100%sensitivity-96.3%specificity until 1µg hemozoin | [60] |
| Optical Reflectance Diagnostic Method | 12 parasites (Pf)/µl | [61] |
| Polymerization-based Assay | 10 parasites (Pf)/µl | [62] |
| Photoacoustic properties | ||
| In vivo photoacoustic flow cytometry | less than 5 P.yoelii-infected mice/µl | [63] |
| In vivo photoacoustic flow cytometry | 5 P.yoelii-infected mice/µl | [64] |
| Hemozoin-generated vapor nanobubbles | 5 parasites (Pf)/µl | [65] |
| Photoacoustic excited surface acoustic wave | 1000 parasites (Pf)/µl | [66] |
| Molecular Assay | Type of Amplification | Target | Reference |
|---|---|---|---|
| In-house genus/species-specific PCR | Nested-PCR | 18S-rRNA | [83,100] |
| In-house species-specific PCR | Semi nested-PCR | 18S-rRNA | [101] |
| In-house genus/species-specific PCR | QT-NASBA* | 18S-rRNA | [102,103] |
| In-house genus/species-specific PCR | TaqMan | 18S-rRNA/mitochondrial DNA sequences | [81,82,83] |
| In-house genus/species-specific qPCR | TaqMan | 18S-rRNA/mitochondrial DNA sequences | [84,104,105,106] |
| In-house genus/species specific qPCR | Sybr Green | Pf CoxI gene Plasmodium mitochondrial sequence 18S-rRNA | [107] |
| Pan and Pf Loop AMP® (Eiken Chemical Co.,Japan) | Loop mediated isothermal amplification | Mitochondrial DNA sequence | [108,109,110] |
| RPA | Recombinase polymerase amplification | 18S-rRNA | [99] |
| Molecular-based point of care test | RPA/LAMP | 18S-rRNA | [21,111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





