1. Introduction
Coastal areas are highly dynamic regions influenced by human and natural factors [
1].With the changing global climate patterns, coastal areas are likely to experience increased shoreline change due to increased erosion and accretion processes[
2] thus greater risk of flooding and destruction of coastal infrastructure [
3]. Such changes are often amplified by loss and degradation of natural capital, including mangroves [
4]. A study by [
5] estimated 16% of Kenya coastline as highly exposed to natural hazards; and projecting a 41% escalation if marine ecosystems continue to be degraded . Global Projections indicate that the intensity of coastal hazards including storms and cyclones is likely to increase by 1-10% by 2030 -2080 [
1,
6]. In the past, responses to such hazards included construction of protective structures like seawalls and breakwaters (“grey” engineering). However, these kind of interventions are rigid, challenging to maintain, lack a natural component, and contribute to the depletion of natural habitats as well as “coastal squeeze” [
1,
7,
8]. Consequently, attention has recently shifted to ecosystem-based interventions in responding to coastal hazards [
9]. Ecosystem-based interventions, also known as nature-based solutions, involve the management, conservation, and restoration of natural ecosystems to minimize disaster risks and promote sustainability and resilience [
1,
10,
11].
Mangroves and associated ecosystems provide the first line of defense to storm damage, and other coastal hazards [
12,
13,
14,
15]. Mangroves are capable of minimizing wave energy, stabilizing sediment and facilitating sediment accretion[
1,
12,
13,
16,
17,
18]. For instance, a 100m width of mangroves has a potential to reduce incoming wave energy by 66% of the waves height[
19]. Their root system form a network that stabilize sediments preventing erosion [
13]. The vegetation cover reduces the speed of the flowing water and wind promoting sediment accretion which stimulate the production of below ground root system[
20] further improving soil cohesion. The thick mangrove vegetation together with the reduced wind speed contribute to reduced wave vigor [
19,
21,
22,
23] , thus protecting the shoreline from wave damage. In the event of sea level rise, mangroves are able to accrete sediments, thereby, adapting to the rising sea levels [
24,
25]. The role of mangrove in coastal protection was well illustrated in the event of Indian Ocean Tsunami (IOT) of 2004 where areas with degraded mangroves experienced high losses of life and properties compared to areas with intact ecosystems [
26,
27]. The loss of life was estimated to exceed 200,000 deaths and with property loss of more than 9.9 billion dollars [
28,
29,
30].
Despite their significance role in coastal protection and other services, mangrove throughout the world continue to be lost and degraded as a result of human and natural causes [
31]. It is estimated that the world is losing 1-3 % of mangrove areas per year [
32]. In Kenya ,least 40% of the 60,000 ha of mangroves were lost and degraded between 1990 and 2005 [
33]. Whereas the rate of loss has declined in some areas, the remaining mangrove forests are still threatened by illegal harvesting, land encroachment and climate change effects [
34] .
According to[
35], there are about 800,000 ha of potential mangrove restoration sites around the world. Unfortunately, the success rates of restoration efforts varies from one project to another[
36,
37,
38] depending on site history, choice of the species to be planted, and restoration approach used. Areas exposed to high energy experience poor performance due to dislodging of propagules by wave actions [
39]. Conventional restoration techniques involving direct planting, use of nursery raised seedlings, or natural regeneration seems to fail where habitats have been destroyed and ecological conditions altered [
40]. At Gazi bay in Kenya where this study was based, for instance, large contiguous blank areas left since 1970s massive mangrove deforestation for industrial wood energy failed to regenerate naturally [
41]. Earlier attempts by [
39] to rehabilitate these sites using nursery raised saplings and wildlings failed as well. Here, we used a modified Riley Encasement Methodology (REM) initially proposed by [
42] to re-establish the lost mangroves in Gazi Bay. Under REM the planting materials are installed in Polyvinyl Chloride (PVC) tubing’s to protect them from being dislodged by waves [
42]. In the current study, we varied the diameter of encasements (7 to 10 cm) as well as materials types (bamboos and PVC pipes). Research questions were as follows: What are the biophysical characteristics of degraded high-energy mangrove site in Gazi? How does survival and growth of the replanted mangroves compare among different treatments?
2. Materials and Methods
2.1. Description of the Study Area
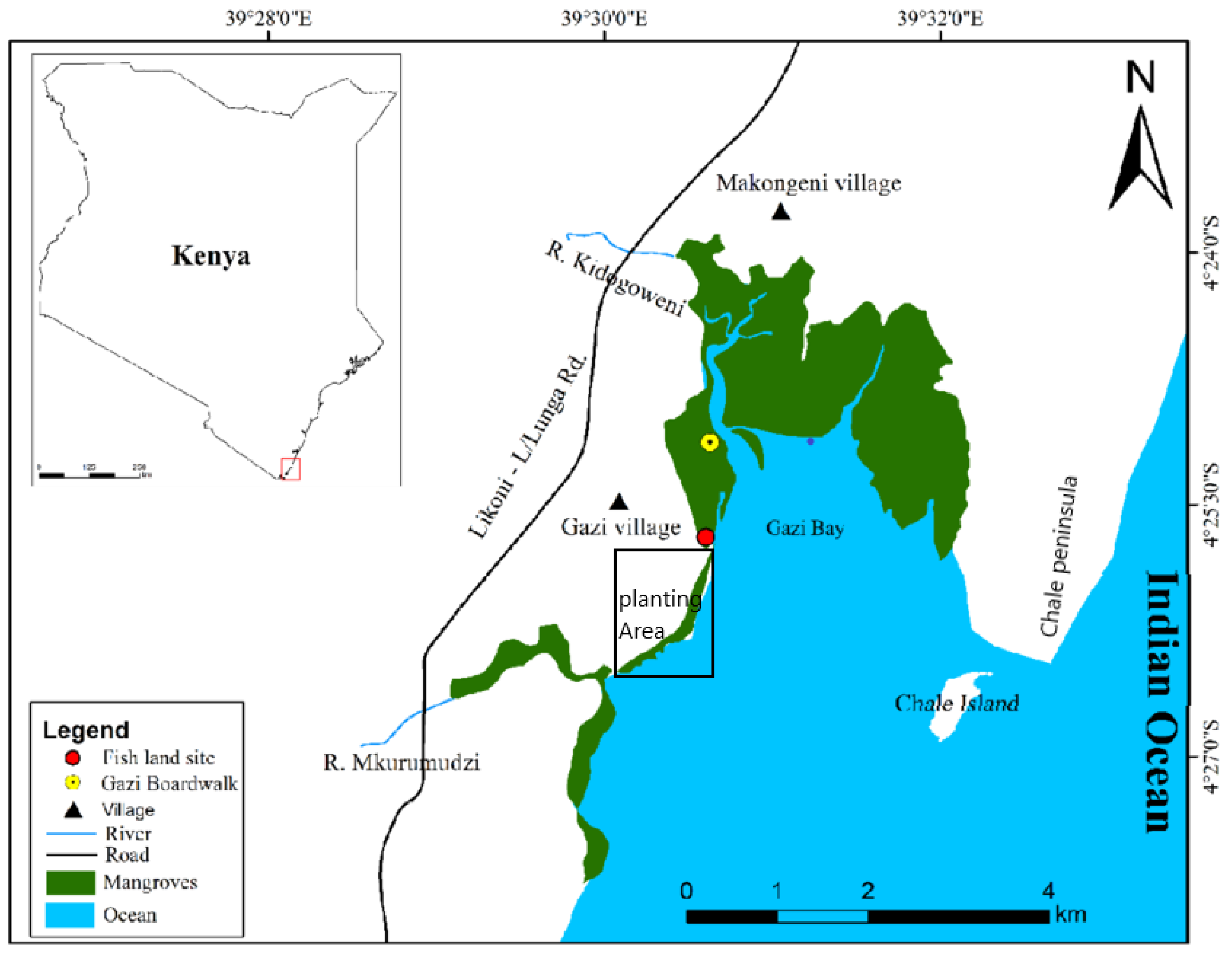
The study was carried out in the south coast of Kenya at Gazi Bay (39° 30' E, 04° 26' S), located about 55km from Mombasa. The Bay is characterized by the eastern and western creeks, fringing mangroves, seagrass beds, and long intertidal area. Two seasonal rivers; Kidogoweni and Mukurumudzi, drains into the western creek and provide freshwater input into the Bay while groundwater seepage happens in a few points [
43]. The bay is protected to the east by Chale Peninsula and to the south a fringing coral reef.
Figure 1.
Map of Gazi bay showing activity area.
Figure 1.
Map of Gazi bay showing activity area.
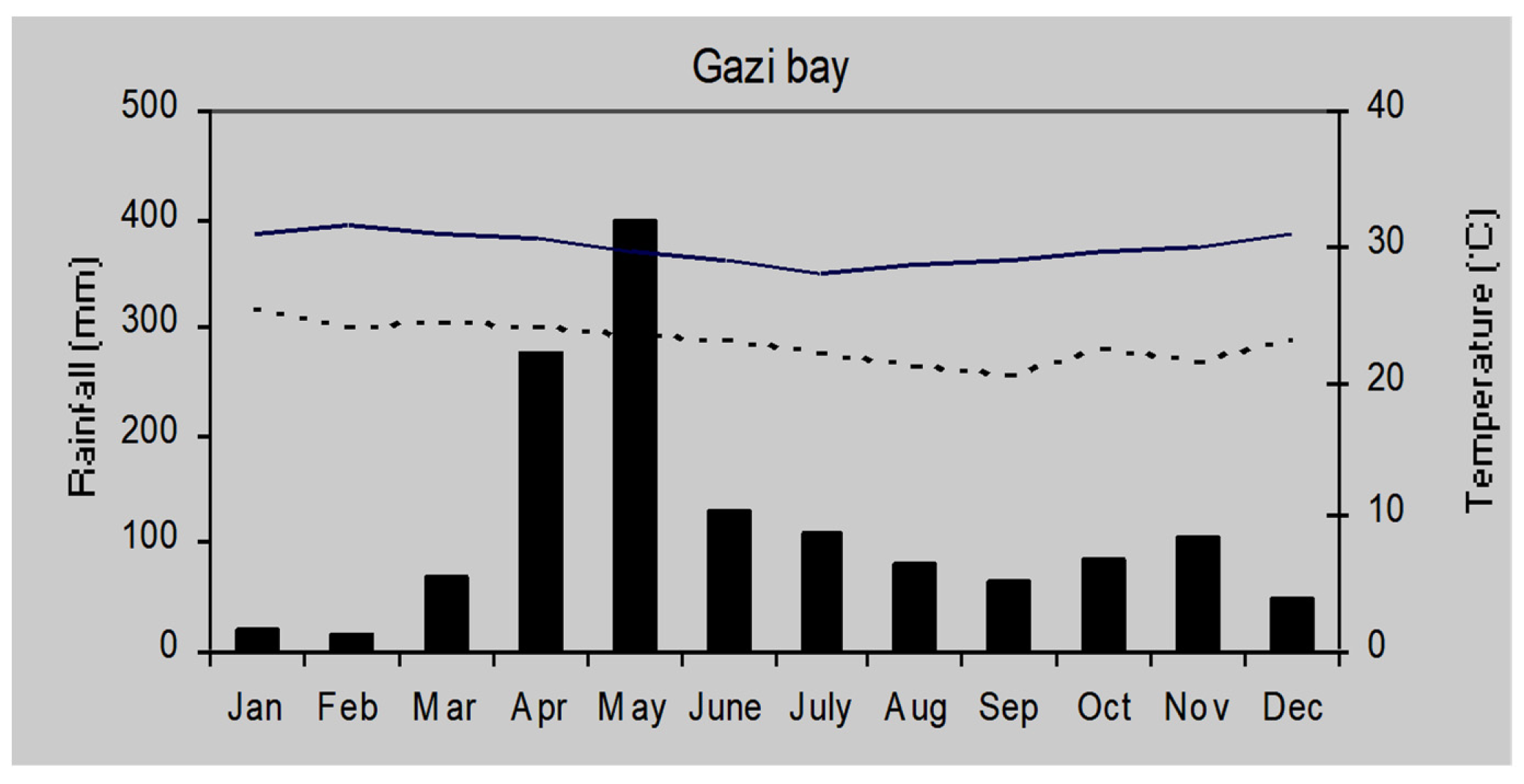
The climate of Gazi Bay may be classified as tropical wet/dry and is heavily influenced by South East monsoon winds. There are two rainy seasons. The long rains associated with South East Monsoons falls from March to August (
Figure 2). This is followed by the short rains form November to March that is associated with the North East monsoon winds. Annual total rainfall in Gazi ranges from 1000 mm to 1,600 mm; with temperature ranging from 19 to 34
0C throughout the year [
44]. Humidity is high, and averages at about 80 % all year round [
45].
2.1.1. Mangroves in Gazi Bay
There are 710ha of mangroves in Gazi bay; represented by nine species [
46,
47]. The most important mangrove species in Gazi bay are
Rhizophora mucronata, Ceriops tagal and
Avicennia marina that occupies more than 80% of forest formation [
48]. Gazi mangroves exhibit a zonation pattern that is similar to other mangrove forests in Kenya. The seaward side is occupied by
Sonneratia alba. This is followed by
Rhizophora mucronata -
Bruguiera gymnorrhiza in the mid-zone and the
Ceriops tagal,
Avicennia marina and
Lumnitzera racemose on the landward side [
49].
2.1.2. History of Mangrove Exploitation and Management Interventions in Gazi
Historically, mangroves in Gazi bay have been exploited for wood and non-wood resources [
49,
50]. In the 1970s and 1980s industrial exploitation of mangrove wood for energy in Gazi left large contiguous blank areas, some of which have failed to regenerate naturally [
41,
51]. As a result, the area experiences coastal erosion due to wave exposure prompting to the uprooting of coconut trees adjacent to the community agricultural field. This exposes the community to the risk of coastal hazards.
Reforestation program to rehabilitate degraded mangrove areas in Gazi was initiated in 1991 by the Kenya Marine and Fisheries Research Institute (KMFRI). In 1994 more than 200 000 different mangrove seedlings were planted [
49] and by 2004, about 100 ha of the deforested areas had been replanted. In 2013, a joint management program involving communities, forest agency and donors was initiated through a carbon offset program, Mikoko Pamoja [
52]. Under Mikoko Pamoja community in Gazi restore and protect mangrove through sale of carbon credits in the Voluntary Carbon Market (VCM); and is the world’s first community-led conservation project funded by carbon credits.
Despite the enormous efforts towards mangrove restoration in Gazi over the years, some sites failed to recover as very low success rates are reported. This failure is attributed to changes in site conditions and exposure to high wave action [
39]. Poor mangrove performance have been reported elsewhere, where unshielded coastlines are prone to wave actions [
53,
54]. As such, the current study focused on improving restoration success of mangroves replanted in high energy degraded site of the bay (
Figure 1, planting area).
2.2. Vegetation Surveys
Systematic random sampling design was adopted in the study. Square quadrats of 100m
2 were established along a 50 m belt transects established perpendicular to the waterline at an interval of 20 m. In the quadrats, all mangroves with a stem diameter at breast height (dbh) ≥ 2.5 were identified, counted and recorded. Vegetation attributes including trees height (m) and Dbh (cm) were collected. Dbh measurements for all species were taken at 130 cm above the ground [
55]; except for
Rhiziphora mucronata in which they were taken at 30 cm above the highest grounded prop root [
56]. From the field data, the following were derived; trees basal area (m
2/ha), stand density (stems/ha) and Importance value (IV) following procedures discussed in [
57] and [
58].
2.3. Sediment Characteristics
Within the 100m
2 quadrats established for vegetation assessment, two sediment cores were collected using a 7.0 cm diameter half-arc soil corer. The soil corer was vertically inserted into the soil until a maximum depth was reached and pulled out gently. Each core was partitioned using a ruler into sections representative of various depths (0-15, 15-30, 30-50, and 50-100). Sub-samples measuring 5 cm were collected from midpoints of the sections as follows; 5-10, 20-25, 37.5-42.5 and 72.5-77.5 cm [
57]. The samples were then placed into labeled containers and transported to the laboratory and stored at 4
oC for further analysis of particle size, bulk density, and sediment organic matter.
2.3.1. Determination of Sediment Particle Sizes
Dry sieve method was used to determine the distribution of soil grain sizes. A standard weight of 100g of the dried sample was measured, homogenized and then passed through a series of sieves ranging from 2 mm to 38 µm mesh-size. Subsequent statistical analysis was done following [
59] method on GRADISTAT computer program to determine the percentage proportions of silt, clay and sand [
60].
2.3.2. Determination of Bulk Density and Sediment Organic Matter
To calculate bulk density, samples were placed on pre-weighed aluminum foils and oven dried at 60
oC until a constant dry weight, weighed and recorded. The bulk density was calculated by dividing the mass of the oven-dried sample by the volume of the sample as illustrated in the equation below [
61].
Where,
To determine soil organic matter (SOM), loss-on-ignition (LOI) method was used. It is a semi-quantitative method in which soil organic matter is lost in the process of combustion in a furnace. To maximize the efficacy of the results, samples used in computing for bulky density were used. The dried samples were homogenized and grinded using an electronic grinder machine for ten (10) minutes and then sieved to separate the fine sediment from debris. Samples were then combusted at 450
0C in an induction furnace for 8 hours in three replicates of 5.0g each, after which they were cooled in a desiccator and weighed to obtain weight after combustion. Percentage soil organic matter (SOM) was then calculated following the equation below:
A relation of LOI% to SOC% was used to estimate SOC from SOM [
62] as follows:
2.4. Experimental Mangrove Planting
2.4.1. Site Selection
The choice of the planting site was motivated by both natural and socio-ecological factors. In terms of natural suitability, the site was selected due to the previous presence of mangrove vegetation. Additionally, there were signs of secondary growth and limited vegetation, featuring a few noteworthy species. The location is also characterized by erosion due to exposure to strong waves, currents, and winds. Regarding socio-ecological suitability, we expect improved protection against coastal hazards, such as flooding, through the rehabilitation of mangroves. Majority of the community in Gazi supports the idea of replanting mangroves to control shoreline change in the designated area. Mangrove rehabilitation efforts are anticipated to align harmoniously with both current and future land use and development needs in the area.
2.4.2. Propagule Installations in PVC and Bamboo Pipes
Though traditional methods are generally effective for mangrove restoration in low-energy areas, they may not suffice in areas that face challenges such as wave action and soil erosion/accretion. They often provide minimal protection from waves and other physical forces [
63]. In response to such conditions, alternative methods, such as the use of PVC pipes (Riley encased method) and bamboo pipes, are incorporated to provide protection during the early developmental stages [
42]. This physical protection serves to mitigate the impacts of wave and wind action. Importantly, the PVC pipe is intended to be removed once the saplings have established [
64].
Mature propagules of Rhizophora mucronata were collected from forest floor or by shaking the mother plant and picking the falls. To initiate rooting, the propagules were spread on top of moistened sawdust in a dark room and covered with a wet heavy blanket. Propagules which did not root after 7 days were considered non-viable and discarded. Viable propagules were then assigned to treatments.
2.4.3. Encasements Preparation
Using an electric driller, the 3” and 4” PVC plus the bamboo piping’s were drilled to make holes along the length for allowing water to flush in and out. A vertical slit was then made on the PVC pipes to allow easy removal when mangroves are fully established.
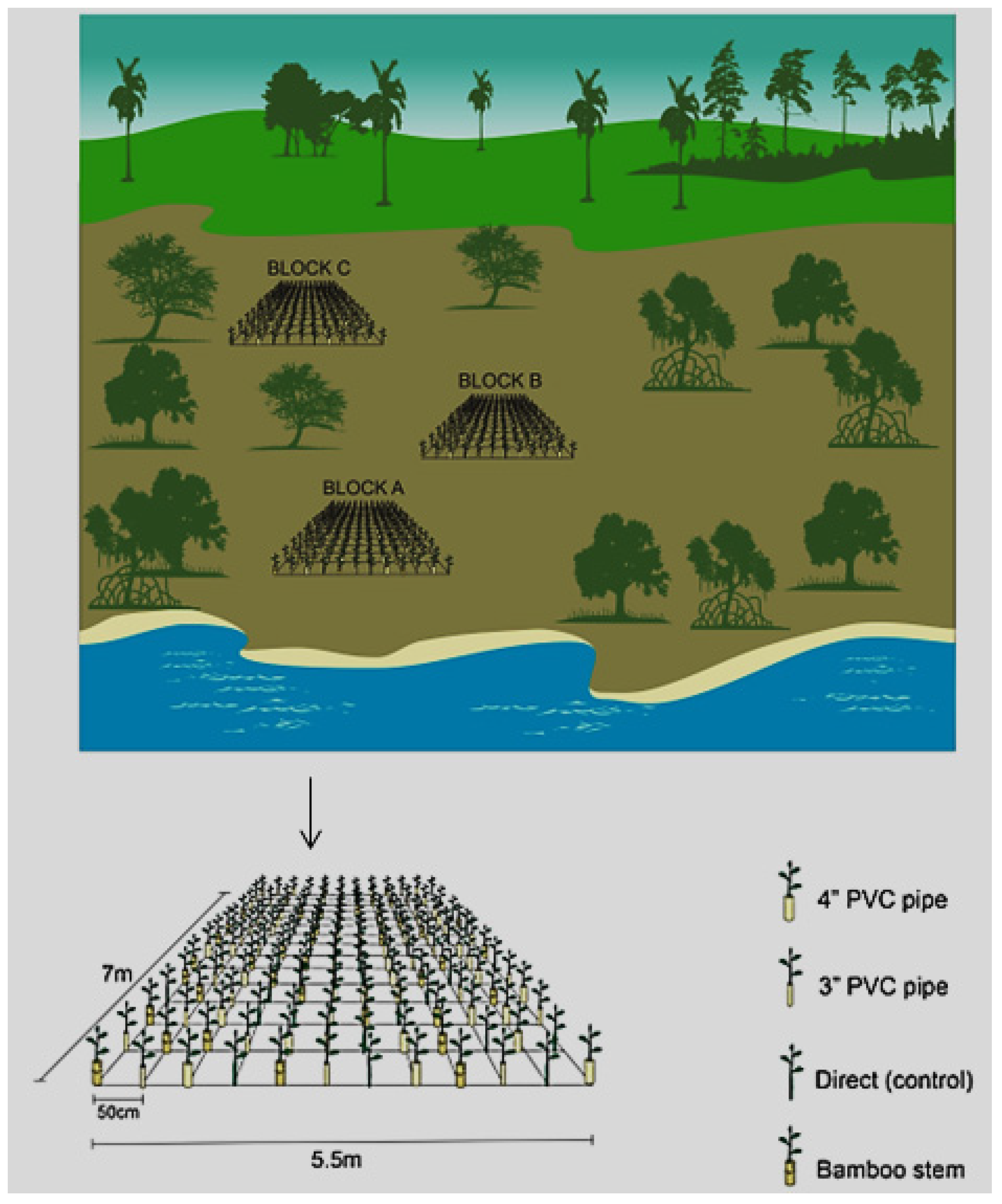
2.4.4. Experimental Design
The randomized complete block design (RCBD) was employed in setting up the experiment. Three rectangular blocks measuring 5.5 m by 7 m were established perpendicular to the shore. Treatments including 3”PVC (7 cm in diameter), 4”PVC (10 cm in diameter), bamboo tubing and control were randomized separately for each block using the RAND function in excel 2016 package.
On every block pits were dug at a spacing of 50 cm for encasements installation as the bedrock was shallow, making it impossible to directly push encasements on the ground as anticipated. After digging pits encasements were tightly fixed on the ground and cut into height corresponding to the elevation of existing
Rhizophora mucronata mangroves closest to the planting site [
49]. The pipes were then filled with sufficient mangrove sediments to set the seedling at an elevation consistent with the mean-high-water mark. On each encasement, one propagule was planted. Every block contained 15 rows at spacing of 50 cm and every line contained 3 units of each of the treatment which resulted to 45 units of every treatment in a block. This added up to a total of 180 propagules in every block and a total of 540 propagules in all blocks (
Figure 3).
2.4.5. Monitoring of Growth Performance
Monitoring of planted mangrove propagules was done monthly for eight (8) months by collecting survival and growth data using [
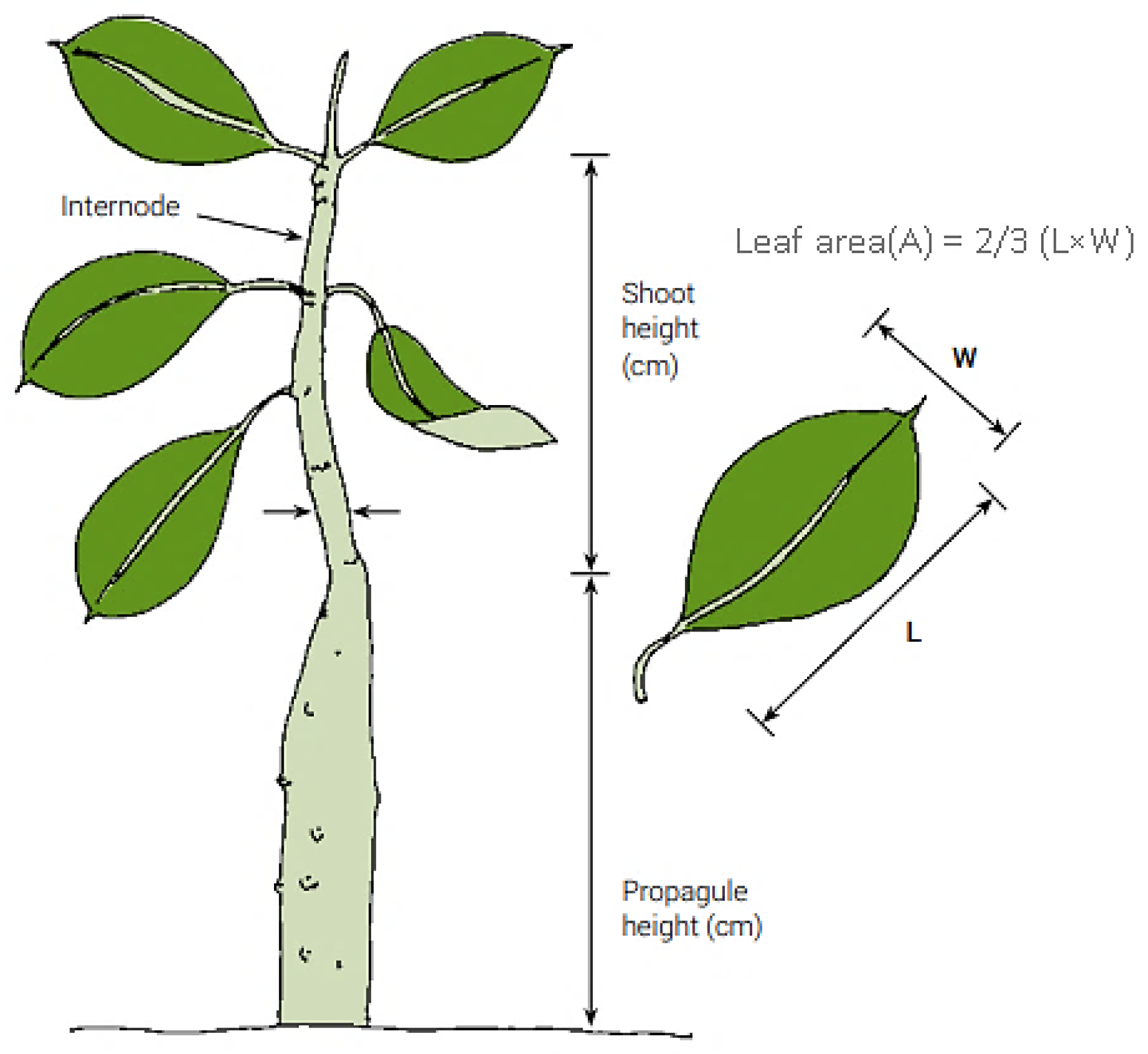
38] protocol. The following vegetation data was generated (
Table 1); shoot height (cm), number of leaves, number of internodes, number of branches and leaf area (
Figure 4).
2.5. Data Analysis
Statistical analysis was done using the SPSS version 26.0, GRADISTAT computer program and Microsoft Excel 2016.Tabular and graphic presentations of data allowed ease of visualization. Normality of the data was tested using Kolmogorov-Smirnov test and Shapiro-wilk test and data were normalized where necessary to meet parametric assumptions. All data on survival and growth parameters were subjected to repeated measures of Analysis of Variance (ANOVA) followed by a Post-hoc Tukey HSD (P< 0.05) to compare means among treatments and blocks. The bamboo and control groups were not included in the growth performance analysis due to the low survival rates (high mortality) thus small sample size that would compromise the results of the study. Measures of soil properties were also subjected to ANOVA followed by a Tukey HSD Post-hoc to realize variations in depth.
3. Results
3.1. Biophysical Characteristcs
Soils in the degraded areas of Gazi are characterized by high proportions of sand accounting for
96.97 ± 0.17 % with very small proportions of silt and clay (
3.03 ± 0.17 %). No clear pattern is depicted in percentage sand as well as clay and silt with depth (
Table 2). However, the difference in the values across the depth intervals are not statistically significant
(p>0.05). The average bulk density across the different depth intervals ranged from 0.065 to 0.335 g/cm
3 (mean: 0.255 ± 0.007 g/cm
3). Bulk density varied significantly with depth
(p<0.05) as values decreased with increasing depth. Proportions of soil organic matter (SOM) ranged from 5.948 to 7.315 % (mean: 6.334
± 0.24 %); while those of soil organic carbon (SOC) ranged from 5.358 to 5.926 % (mean: 5.519 ± 0.10) with no significant differences between the depth intervals
(p>0.05).
3.2. Mangrove Forest Structure
Three species of mangroves were encountered in the project site. Based on their Importance Value (IV) index, the most dominant species in the site is
Sonneratia alba (IV = 293.23%); followed by
Rhizophora mucronata (16.25) and Avicennia marina (13.25) -
Table 3
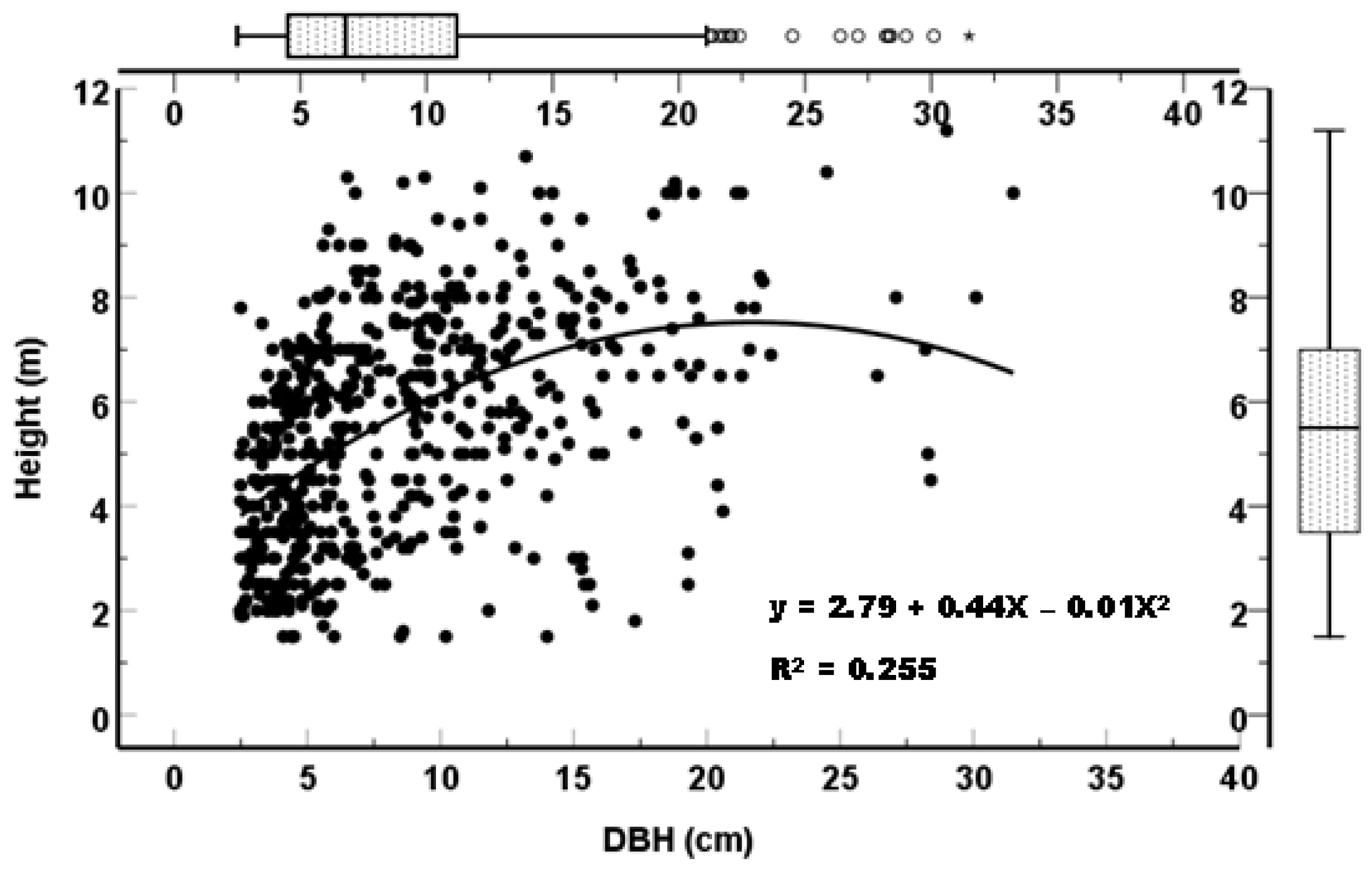
The overall stand density of the mangroves in the study site at Gazi was estimated at 2659 ± 460.13 stems ha-1 (range: 500 - 10,000 stems ha-1); with mean basal area, tree height and DBH of 21.06 ± 2.45 m2 ha-1 (range: 3.28 - 39.96 m2ha-1), 5.49 ± 0.09 m (range; 1.5-11.2 m) and 8.52 cm (range; 2.5-31.5 cm) respectively.
Figure 5 shows a Scattergram of heights against stems diameters of mangroves in Gazi. As expected, there is a positive correlation (R
2 = 0.2203) between stem diameter and height. Some 50% of trees in the study sites had diameters and height range from 4.5 - 11.5 cm and 3.5 - 7 m respectively.
3.3. Trial Mangrove Growing Experiments in High Energy Site of Gazi
3.3.1. Survival Rates of the Replanted Mangroves
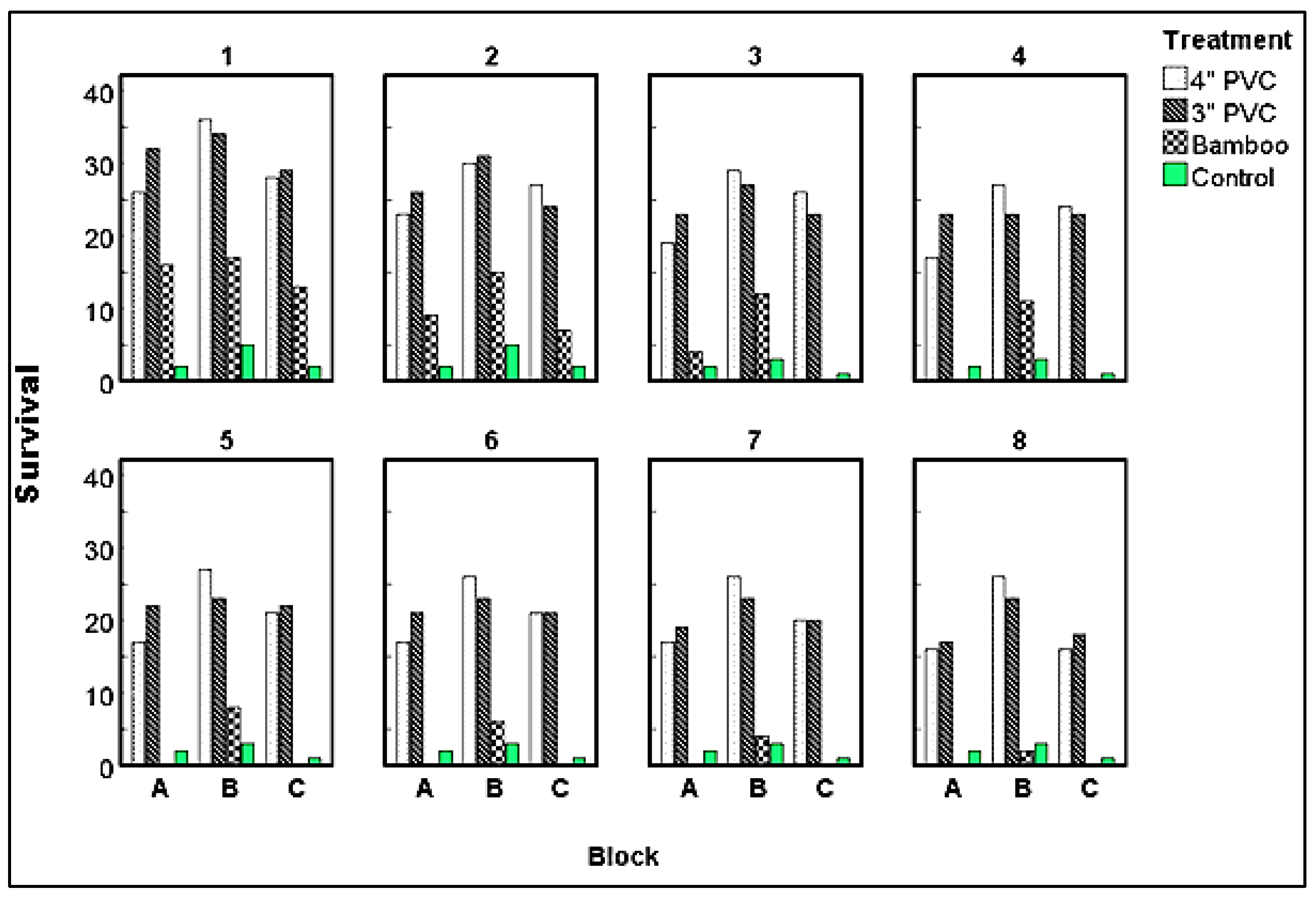
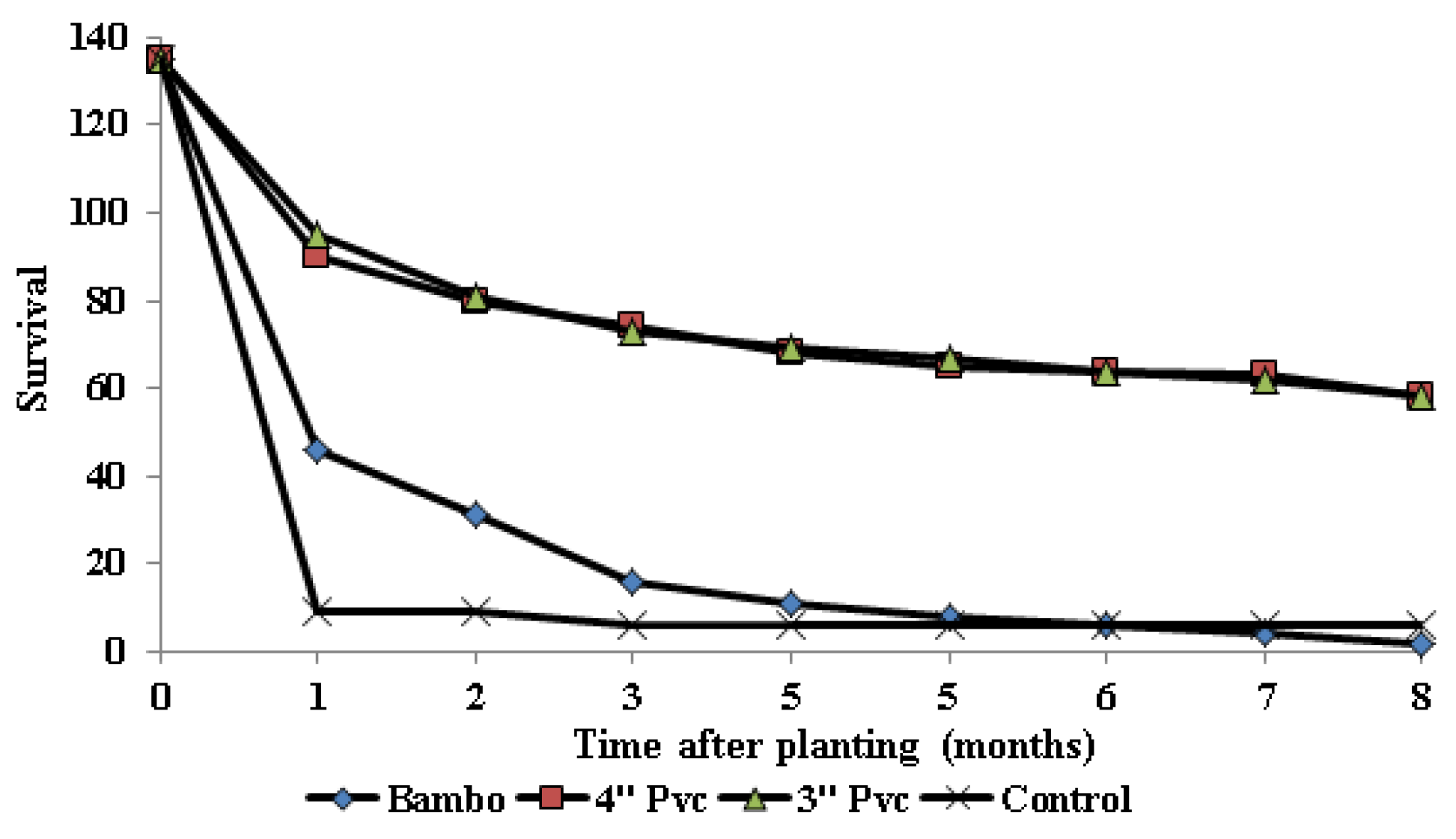
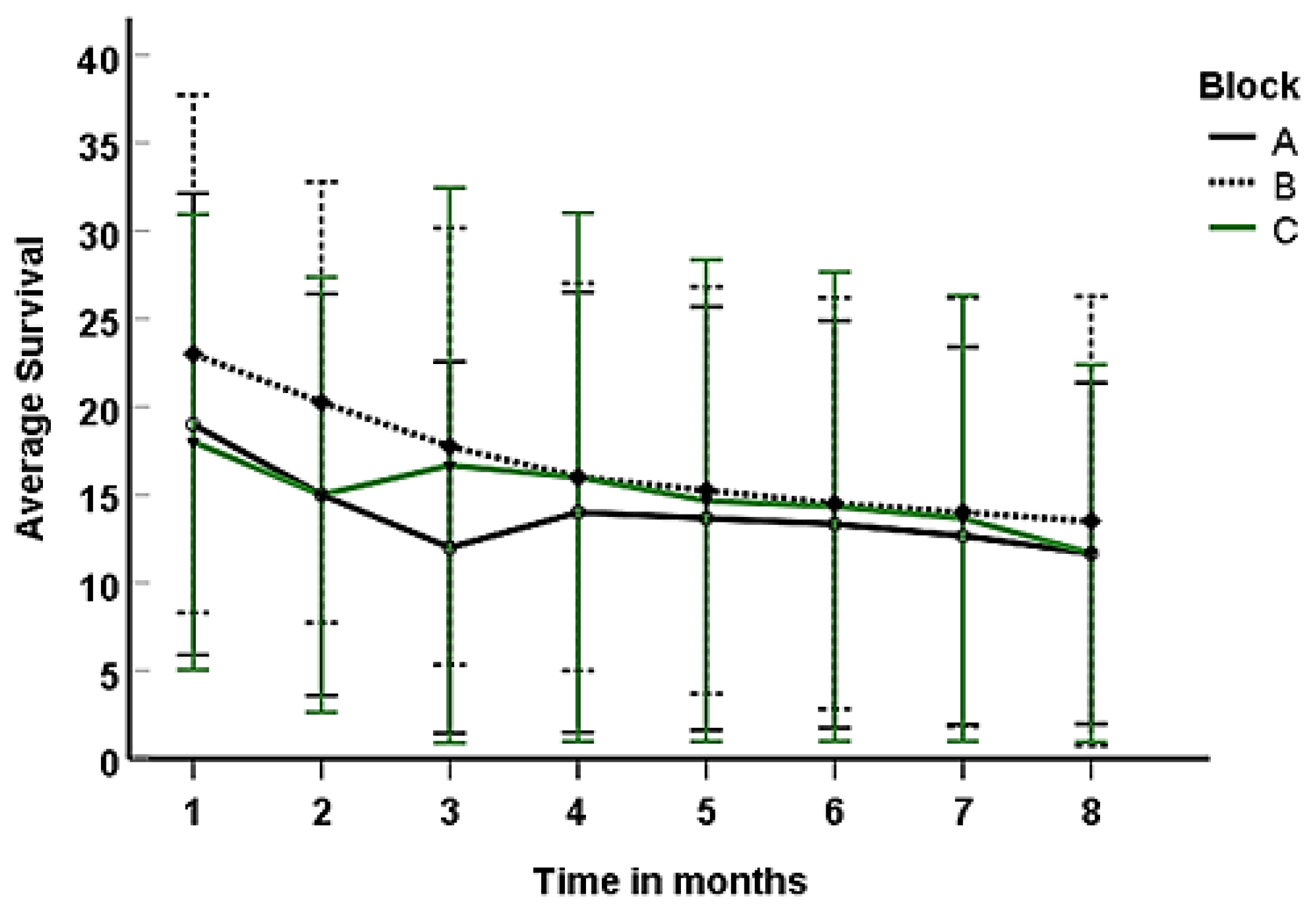
Three months after planting (the last month at which mangroves encased within bamboo poles were present), Block A exhibited survival rates ranging from 2 to 23 (4-52%). Among these, those encased in 3” PVC pipes displayed the highest survival rate at 52%, followed by those in 4” PVC at 42%, and bamboo pipes at 9%. The control group, consisting of directly planted seedlings, recorded a 4% survival rate during the same period. At the end of the eighth month, survival rates varied from 2 to 17 (4-38%). Mangroves in 3” PVC achieved the highest survival rate at 38%, followed by those in 4” PVC at 36%, and the directly planted seedlings at 4% (
Figure 6).
Eight months after planting, mangroves grown in Block B recorded survival rates ranging from 2 to 26 (4-58%); with those established in 4” PVC recording the highest survival rate of 58%; followed by those in 3” PVC (51%), and bamboo pipes (4%). Over the same growth period, direct planted propagules had a survival rate of 7% only (
Figure 6).
Mangroves sown in Block C had survival rates ranging from 2 to 27 (4-60%) two months after planting (the last month at which mangroves encased within bamboo poles were present); with seedlings encased in 4”PVC having the highest survival (60%), followed by those encased within 3” PVC (53), and bamboo pipes 16%. The direct planted propagules had the least survival of 4%. At the end of the experiment (8 month after planting), survival rates ranged from 2-40 % (1-18) with mangroves planted within 3” PVC recording higher survival rate of 40% followed by those planted in 4” PVC (36%) and control with 2% survival (
Figure 6).
At the end of the experiment, mangroves encased in 3” and 4” PVC recorded survival rates higher than 42% (58) when compared to the bamboo grown and control that recorded survival rates of less than 5% (
Figure 7).
Mangroves sowed in Block B recorded a mean survival rate of 16.78 ± 2.0 % followed block C (15.12 ± 2.05) and Block A (14.07 ± 1.8%). Overall, the survival rates were not significantly different between blocks
(F=0.5, p=0.608) ((
Figure 8).
3.3.2. Growth Performance of the Replanted Mangroves
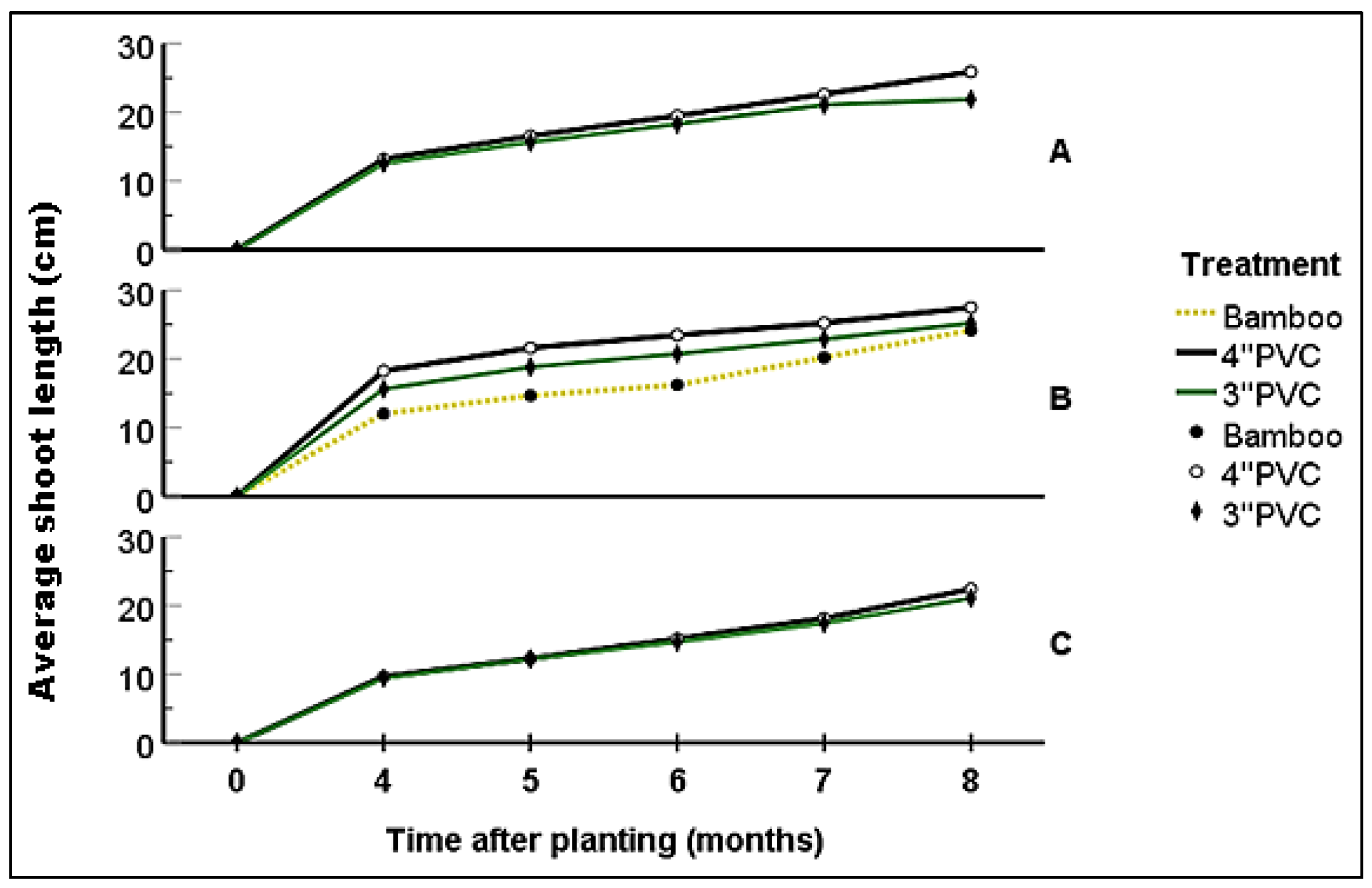
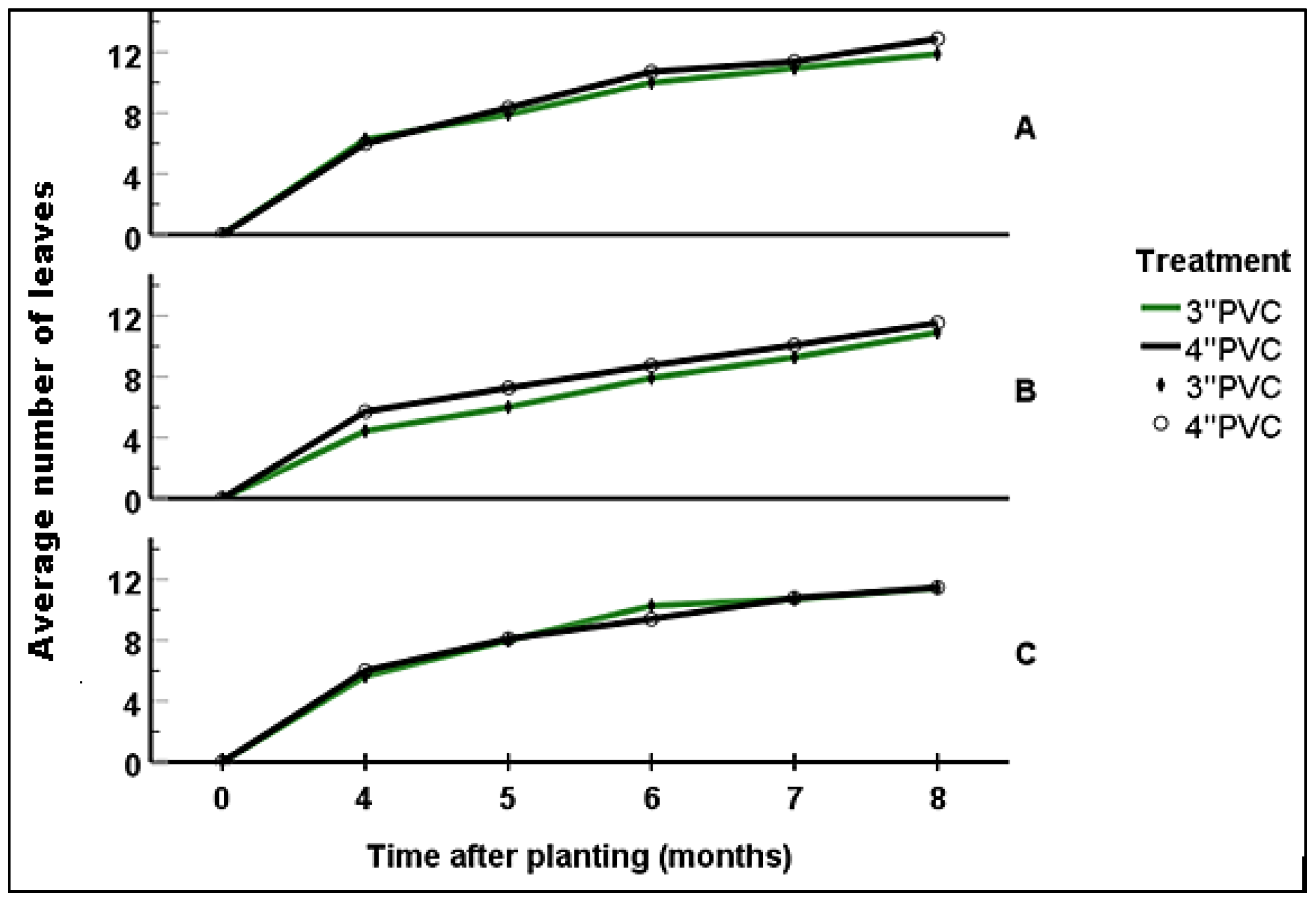
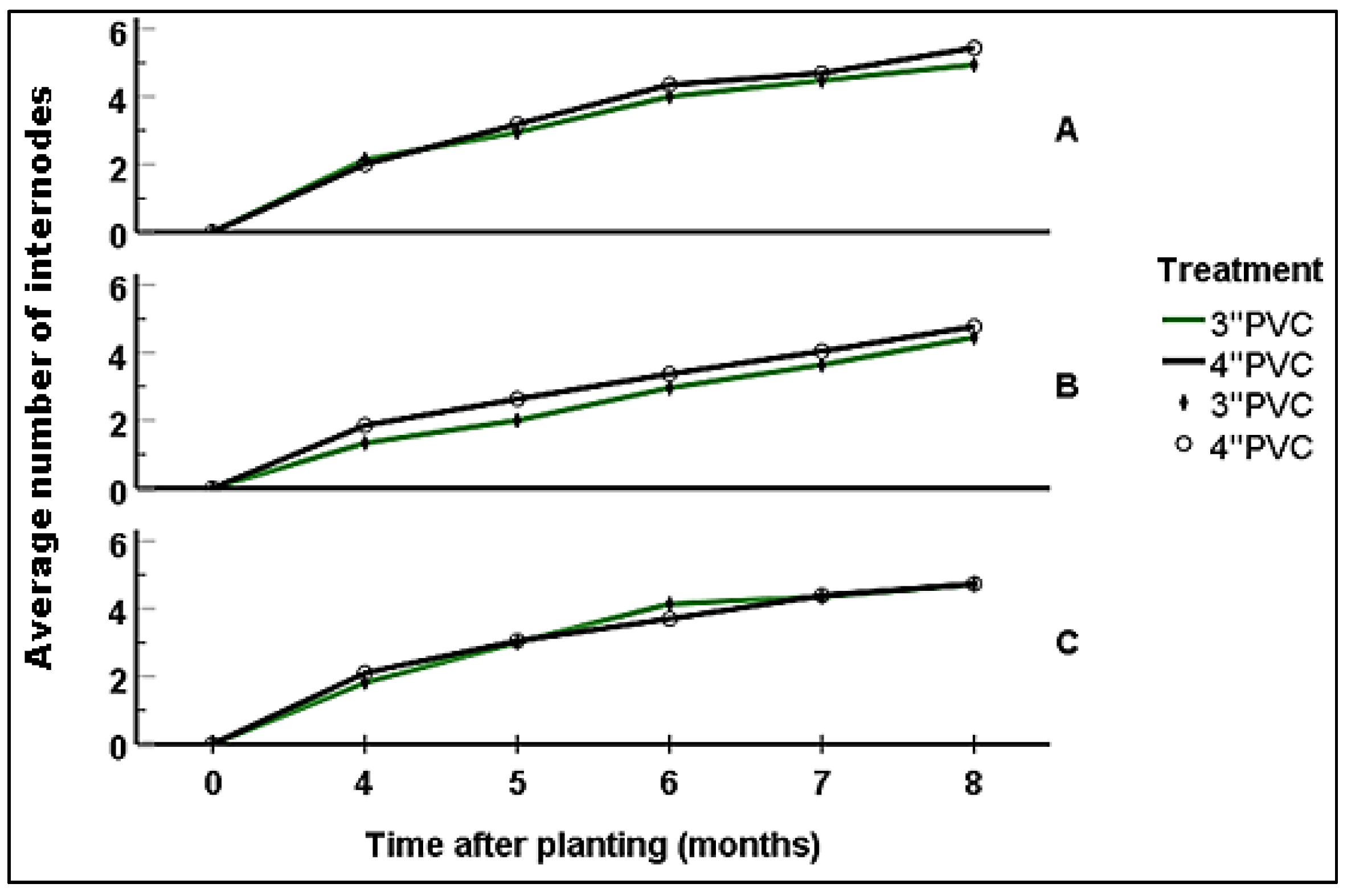
Four months after planting, seedlings growing in Block A had attained a shoot length of 7.3 - 22.2 cm (12.82 ± 0.52); with 4-8 leaves (6.15 ± 0.27) and 1-3 internodes (2.08±0.13). At this stage, there were no significant statistical differences in vegetation attributes among seedlings grown in 3'' and 4'' PVC. At six months, seedlings had shoot length ranging from 11.8-28 cm (18.83±0.62); with 8-12 (10.31 ± 0.2) leaves and 3-5 (4.5 ± 0.1) internodes. Still, there were no significant statistical differences in vegetation attributes among seedling established within the different-sized PVC encasement (p>0.05). By the eighth month, the seedlings’ shoot ranged from 15.5 - 37.2 cm (mean: 25.38 ± 0.83); with those encased in 4''PVC and 3''PVC attaining a mean of 25.91± 1.43 cm and 24.88 ± 0.90 cm respectively. Number of leaves and internodes ranged between 10-14 (12.36 ± 0.2) and 4-6 (5.18 ± 0.1) respectively; with seedlings encased in 4'' PVC recording an average of 12.88 ± 0.32 (10-14) leaves and 5.44 ± 0.16 (4-6) internodes while those encased in 3’’PVC had 10-14 (11.88 ± 0.21) leaves and 4-6 (4.94 ± 0.10) internodes (
Figure 9,
Figure 10 and
Figure 11). The differences recorded between the two treatments were not statistically significant (p>0.05).
By the fourth month of growing, seedlings planted in Block B had attained shoot length ranging from 4.9-24.3 cm (16.36 ± 0.63); with 2-8 (5.12 ± 0.21) leaves and 1-3 (1.63 ± 0.10) internodes. In six months, the shoot length was ranging between12.4-33 (21.58 ± 0.71) while the number of leaves and internodes ranged between 4-12 (8.36± 0.21) and 1-5 (3.18±0.11) respectively. On reaching the age of eight months, seedlings had attained a shoot length of 26.33 ± 0.67 (15.1-38.1), 8-14 (11.25 ±0.19) leaves and 3-6 (4.62 ± 0.10) internodes. Seedlings encased in 4” PVC had an average shoot length of 27.49 ± 0.86 cm, 11.54.88 ± 0.28 leaves and 4.77 ± 0.14 internodes; while those encased in 3” PVC recorded an average of 25.21 ± 1.08 cm in shoot length, 10.91 ± 0.25 leaves and 4.45 ± 0.13 internodes (
Figure 9,
Figure 10 and
Figure 11). At all the above stages, there were no significant differences in vegetation attributes among seedlings grown in 3’’ and 4’’ PVC encasement.
At four months of growing, seedlings in Block C attained a mean shoot length of 13.28 ± 0.42 cm (range: 4.3-18), 5.81 ± 0.27 (2-8) leaves and 1.95 ± 0.13 (1-3) internodes. After six months, seedlings attained a shoot length of 8-24.5 cm (18.76 ± 0.46), with 4-12 (9.86 ± 0.21) leaves and 1-5 (3.93 ± 0.1) internodes. By the eighth month, the shoot length reached 24.73 ± 0.48 cm (range: 12.3-35.3 cm) with 11.45 ± 0.22 (8-14) leaves and 4.73 ± 0.11 (3-6) internodes. Seedlings grown in the 3'' PVC recorded an average shoot length of 21.09 ± 1.17 cm (range: 12.6 - 31.5 cm), with 11.44 ± 0.27 leaves (range: 10 to 14) and 4.72 ± 0.14 internodes (range: 4 to 6). In contrast, those enclosed within the 4'' PVC achieved a shoot length of 22.39 ± 1.42 cm (range: 12.3 to 35.3 cm). These seedlings also displayed 8 to 14 leaves (mean: 11.47 ± 0.36) and 3 to 6 internodes (mean: 4.73 ± 0.18) (
Figure 9,
Figure 10 and
Figure 11).
The overall leaf area per plant for seedlings encased in 3'' and 4'' PVC varied within the ranges of 41.33-149.29 cm
2 and 41.79-146.29 cm
2, respectively, at the six-month mark. In Blocks A, B, and C, leaf area ranged from 47.20-149.29, 48.10-142.91, and 41.33-145.51 respectively. On average, when considering all blocks together, the leaf area per plant was 100.48 ± 2.05 (with a range of 41.33-149.29). At the conclusion of the experiment, the growth performances of mangroves enclosed in 3'' and 4'' PVC showed minor differences, but these variances were not statistically significant (p≤0.05), as indicated in
Table 4.
4. Discussion
The motivation of this study emanated from the past failed attempts to restore mangroves in a degraded high energy shoreline of Gazi Bay [
65] through conventional planting methods. The site was designated for annual planting by communities involved in carbon offset scheme – Mikoko Pamoja. Based on technical specifications, community is expected to replant 4000 new mangrove seedlings each year in the degraded areas of Gazi bay. Previous planting experiments by [
39] recorded more than 90% mortality for mangrove installed in eroding high energy areas at Gazi. The current study was, therefore, sought to address these challenges by developing innovative approaches to enhance mangrove growth in high energy areas. Restored mangroves is expected to protect the shoreline from further erosion at the same time contribute to the return of other ecosystem services [
38]. Managing mangroves for shoreline protection has been practiced elsewhere in the world, including South East Asia and Latin America [
66].
4.1. Sediment Characteristics
Sediment particle size distribution is among the most critical physical properties that affects soil properties in regards to erosion [
67,
68]. The very low amount of percentage silt-clay (3.03 ± 0.17) in sediment compared to those of forested sites (38.38 %) in Gazi [
69] is an indication of a highly degraded and low vegetation cover site. The proportions of fine sand (53.68 ± 1.35%) are comparable to those of non-reforested sites in Gazi (58.85± 6.11%) [
69] further indicating a situation of a degraded site. The relatively small amounts of SOM (6.33 ± 0.24 %) and SOC (5.52 ± 0.10 %) in the sediment compared to values obtained in Gazi forested sites (38.38, 31.04, 17.38 %) by [
69] suggests near zero sediment accretion. Mangrove vegetation enhances the build-up of SOM by of accreting sediments [
69]. Mangrove vegetation boosts sediment trapping and settlement by reducing the speed of water movement [
20,
70]. With the high proportions of sand (
96.97 ± 0.17 %) in the sediments, there is a high possibility of decreased soil stability which increases the risk of erosion [
71,
72] by high tides and other extreme weather events.
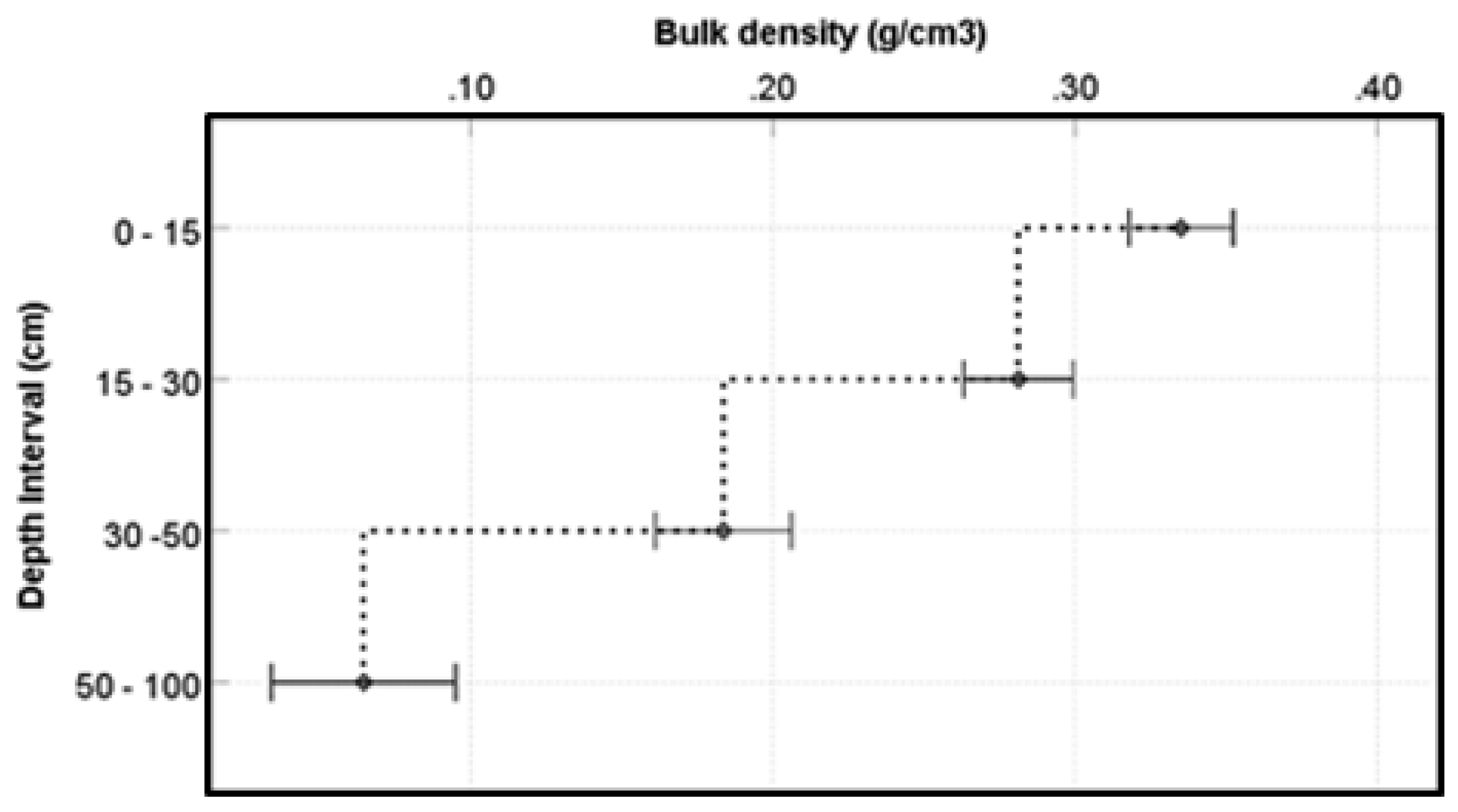
Soil bulk density is considered as the soil’s dry weight per unit volume and it indicates soil compaction. Normally, as the soil depth increases bulk density is known to increase [
73] as the weight of the top layer sediments effect soil compaction [
74]. However, the results of this study indicate an exact opposite situation where bulk density significantly decreases with increase in soil depth (
Figure 12). We would imagine of a situation where the top layers of mangrove sediment have experienced physical compaction due to the action of waves, tides and other forms of disturbance. Such a situation could lead to increased bulk density in the shallow layers as the impact of compaction would diminish with depth resulting in lower bulk density in deeper layers.
4.1. Vegetation Attributes of Mangrove in Gazi
The designated mangrove planting area in Gazi is dominated by
Sonneratia alba. The species naturally occur in the low lying intertidal areas, in the inundation Class 1 of [
75]. The fringing forest in Gazi is heavily fragmented due to mangrove harvesting for fuelwood in the 1970’s [
51] that left blank contiguous areas with no natural regeneration to date [
41]. Other mangrove species in the zone are
Rhizophora mucronata and
Avicennia marina that exist as both adults and juveniles [
48,
69]. The presence of mature established species of
Rhizophora mucronata in the study area prompted us use it in the restoration experiment.
Figure 13.
A photo taken from the study site showing mature Rhizophora stand establishing in combination with the native species.
Figure 13.
A photo taken from the study site showing mature Rhizophora stand establishing in combination with the native species.
Adult Rhizophora species have prop roots for anchorage and breathing [
76]. The rooting systems forms strong mesh system that enable them to create wave barriers [
66]. As such Rhizophora have high proficiency in wave attenuation compared to other mangroves species due to the complexity of the roots’ system; creating greater friction to incoming waves thus a higher drag coefficient [
66,
77,
78]. It has been reported that, 80 m width of Rhizophora mangroves can dissipate incoming wave energy by 80% while a 100 m width Sonneratia stand can only reduce 50 % of the waves energy [
79,
80].
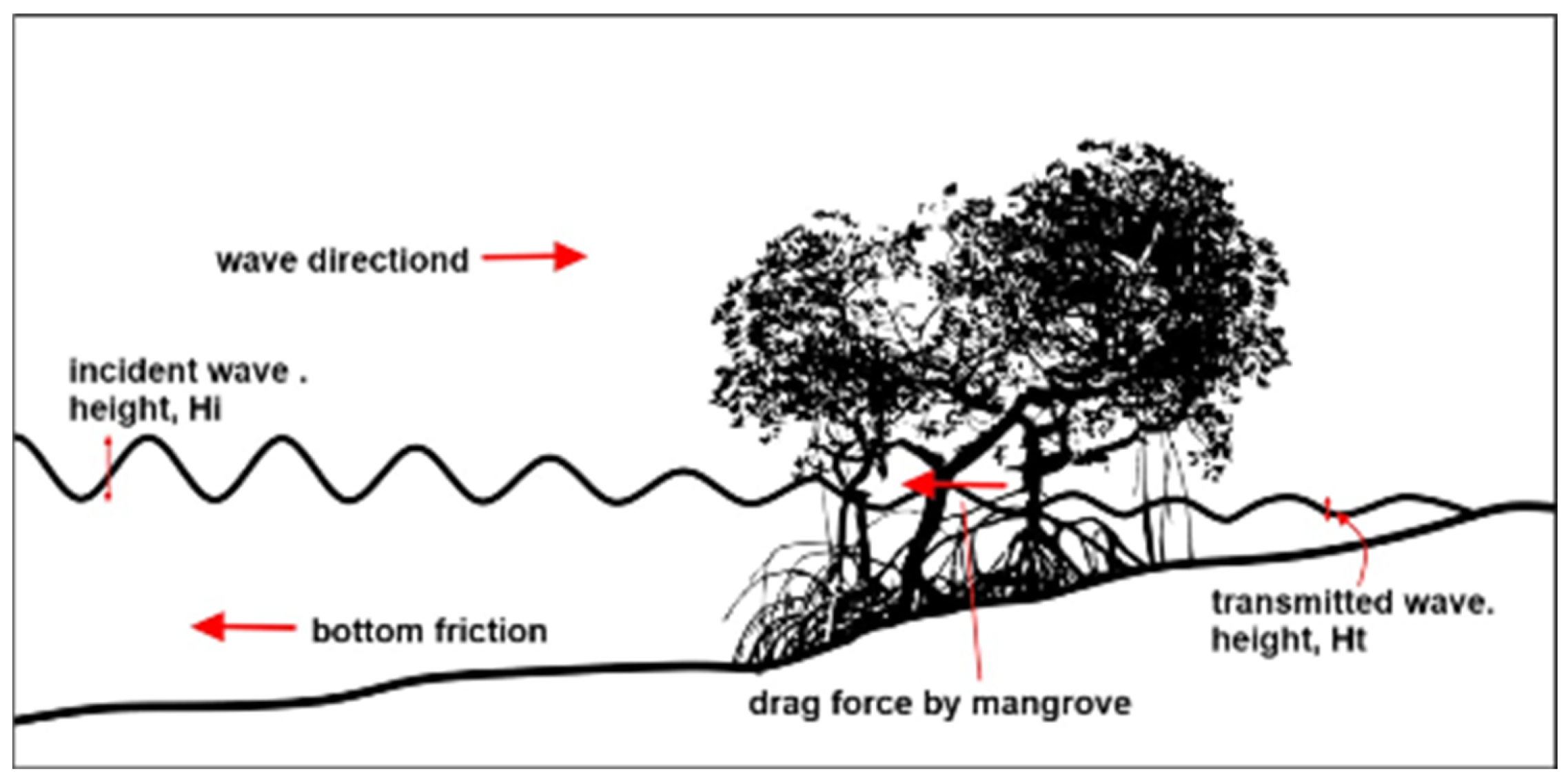
Figure 14.
Wave dissipation by mangroves [
66].
Figure 14.
Wave dissipation by mangroves [
66].
The rooting system also accretes sediments and facilitates natural recruitment of the indigenous species. The genus Rhizophora is also known to be a land-builder through sediment accretion and stabilizations [
81] thus the ability to keeping pace with rising sea-levels [
17,
82,
83]. We, therefore, postulate that replanted mangroves through the current study will develop and accrete sediments and facilitate natural recruitment of new mangrove seedlings.
Overall, conditions along the shoreline of Gazi Bay depict a picture of a highly degraded and exposed mangrove site that is in daring need of restoration. The fact that this fringing mangrove belt stands between land and the sea, validates the need for restoration to ensure that communities are protected. In the face of changing climate and sea-level rise, shoreline protection has become a major concern [
84] since shorelines safeguard coastal communities as well as coastal ecosystems.
4.3. Survival and Mortality of the Planted Mangroves
Major losses occurred during the first and second months after planting through washing by tides. One month after planting, the highest mortality (94%) was recorded in direct planted propagules, which is expected. These propagules were exposed to strong wave energy with no protection [
65]. Similar results were observed in high energy mangrove planting areas of Florida [
85]. Results also align with a study by [
39] that demonstrated up to 90% mortality for mangrove seedlings exposed to high energy. High mortality for propagules encased in bamboo tubes was also occasioned by washing by tides as a result of small diameter tube that could not hold enough soils. Survival rates were highest in propagules installed in 3” and 4” PVC tubes. Despite the differences in PVC diameters, there were no significant differences (p>0.05) in survival rates across the blocks. Actually at the end of the experiment they recorded similar survival rates (43%) while bamboo tubes and control had survivals of 1% and 4% respectively. This could be attributed to the PVC’s ability to hold more sediments thus offering adequate support to the seedlings. In comparison to the control (no encasement), there is potential in the use of PVC encasement for mangrove restoration in high energy areas. Modifications to prevent sediment loss from within the pipes could further enhance the efficacy of PVC. In addition, techniques such as crafting physical barriers around the restoration blocks could help in minimizing the impacts of ocean tides, waves and currents thereby enhancing success [
86,
87]
. These defenses have the ability of attenuating incoming waves [
88] thus reduced energy reaching the planted mangroves.
4.4. Growth Performance of the Planted Mangroves
There were no significance differences (p>0.05) in sapling’s shoot length, numbers of leaves, internodes and branches for mangroves encased in 3” and 4” PVC. The average number of leaves per plant by the fourth month of growing (5.67 ± 0.65), slightly compares with that of seedlings planted under field conditions (6.17 ± 3.86) by [
49] in Gazi. This is an indication that PVC encasements might have the capability of imitating the natural environment under which mangroves establish. However, other parameters including shoot growth and number of internodes were relatively high under field conditions (35.11±3.86; 7.29±3.78) by [
49] compared to PVC environment (14.15±0.53; 1.87±0.12) in this study. Additionally, values obtained for the total leaf area per plant at the age of 6 months (100.5±2.1 cm
2 (41.3-149.3 cm
2) are significantly lower compared to those obtained by [
49] at the age of 12 months (893.1± 71.3 cm
2 (195.2-1615.5 cm
2) under field conditions. This necessitates for optimization of environmental conditions within the PVC pipes may be through better nutrient management. This is so as washing away of sediments from PVC pipes was evident over time in the current experiment, which could have led to nutrient scarcity thus slowed growth and reduced leaf area.
5. Conclusions
Over the eight months of observation, seedlings encased in 3” and 4” PVC pipes recorded similar survival and growth rates. Though bamboo encasements were not effective in this study, there is still potential in their use if large diameters are available. A deep substrate would be suitable for bamboo pipes due to ease installation. Established mangroves are likely to return the ecosystem functions by facilitating soil accretion, minimizing wave energy and encouraging biodiversity thus return on investment [
89,
90]. However, successful restoration demands obligation from the communities that utilize and are associated with mangroves to ensure long term monitoring and maintenance of the restored areas [
91,
92]. This study offers future research opportunities on ecological restoration of mangroves under the changing climate. The results of the study have direct application in areas mangrove restoration is needed for shoreline protection and disaster risk reductions; under the UN’s Decade on Ecosystem Restoration [
93].
Author Contributions
Conceptualization, J.G.K., J.N.G. and G.K.; methodology, J.G.K., J.N.G., B.K.G and G.K.; software, G.K; validation, J.G.K. and G.K.; formal analysis, J.N.G and G.K.; investigation, J.G.K. ,J.N.G. and G.K.; resources, J.G.K., J.N.G., B.K.G and G.K.; data curation, G.K.; writing— original draft preparation, G.K.; writing—review and editing, , J.G.K., G.K.; M.N.G., and R.N.N; visualization,G.K. and J.G.K.; supervision, M.N.G., R.N.N and J.G.K.; project administration, G.K. and J.G.K.; funding acquisition, G.K.,J.N.G and J.G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Marine Research Grant (MARG I) Programme of Western Indian Ocean Marine Science Association (WIOMSA), under the support of the Swedish Government, Contract Number MARG-I-2021_CO-16. Additional financial support was through 1st Rufford Small Grant, Contract Number 32852-1 and Pew Charitable Trust’s Contract: 00032875.
Institutional Review Board Statement
Not applicable
Data Availability Statement
The data supporting the findings of this study are available upon request from the corresponding author. The data are not publicly available due to privacy reasons.
Acknowledgments
The authors recognize the technical support received during the entire duration of the study by the team at KMFRI. We also thank the Western Indian Ocean Marine Science Association (WIOMSA), The Rufford Organization and PEW Charitable Trust’s for their financial support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Steven, A.D.L.; Appeaning Addo, K.; Llewellyn, G.; Vu, T.C. et al. Coastal Development: Resilience, Restoration and Infrastructure Requirements. Washington, DC: World Resources Institute 2020.
- Bandeira, S.; and Balidy, H. Limpopo estuary mangrove transformation, rehabilitation and management,” in Estuaries: A Lifeline of Ecosystem Services in the Western Indian Ocean. Springer 2016, pp. 227–237. [CrossRef]
- Brooks, S.M.; and Spencer, T. Shoreline retreat and sediment release in response to accelerating sea level rise: Measuring and modelling cliffline dynamics on the Suffolk Coast, UK. Glob. Planet. Change 2012, vol. 80, pp. 165–179. [CrossRef]
- Hochard, J. P.; Hamilton, S.; Barbier, E.B. Mangroves shelter coastal economic activity from cyclones. Proc. Natl. Acad. Sci. 2019, vol. 116, no. 25, pp. 12232–12237. [CrossRef]
- Hamza, A.J.; Esteves, L. S.; Cvitanović, M. Changes in mangrove cover and exposure to coastal hazards in Kenya. Land 2022, vol. 11, no. 10, p. 1714. [CrossRef]
- Kossin, J. P.; Knapp, K. R.; Olander, T. L.; Velden, C. S. Global increase in major tropical cyclone exceedance probability over the past four decades. Proc. Natl. Acad. Sci. 2020, vol. 117, no. 22, pp. 11975–11980. [CrossRef]
- Hsu, T.W.; Tseng, I.F.; Lin, T.Y.; Shin, C.Y.; Ou, S.H. Review of countermeasures against beach erosion on the Taiwanese coast. Coast. Manag.2008, vol. 36, no. 3, pp. 274–293. [CrossRef]
- Martínez, M. L.; Mendoza-Gonzalez, G.; Silva-Casarín, R.; Mendoza-Baldwin, E. Land use changes and sea level rise may induce a ‘coastal squeeze’ on the coasts of Veracruz, Mexico. Glob. Environ. Chang.2014, vol. 29, pp. 180–188. [CrossRef]
- Kitazato H.; Matsuda, O.; Oyarzun, E. S.; Kuanui, P.; Wibisono, Y.; Yasukawa, S.; Oki, Y. Ecosystem-based Disaster Risk Reduction (Eco-DRR): Toward Better Restoration of Coastal Environments, Resources and Livelihoods from big earthquake and tsunamis. EGU General Assembly Conference Abstracts 2018, p. 2873.
- Sudmeier-Rieux K.; Arce-Mojica, T.; Boehmer, H. J.; Doswald, N.; Emerton, L.; Friess, D. A.; Galvin, S.; Hagenlocher, M.; James, H.; Laban, P. Scientific evidence for ecosystem-based disaster risk reduction. Nat. Sustain. 2021 vol. 4, no. 9, pp. 803–810. [CrossRef]
- Wickramasinghe, D. Ecosystem-Based Disaster Risk Reduction. Oxford Research Encyclopedia of Natural Hazard Science 2021. https://oxfordre.com/naturalhazardscience/view/10.1093/acrefore/9780199389407.001.0001/acrefore-9780199389407-e-360.
- Gedan, K. B.; Kirwan, M. L.; Wolanski, E.; Barbier, E. B.; Silliman, B. R. The present and future role of coastal wetland vegetation in protecting shorelines: answering recent challenges to the paradigm. Clim. Change 2011, vol. 106, no. 1, pp. 7–29. [CrossRef]
- Shepard, C. C.; Crain, C. M.; Beck, M. W. The protective role of coastal marshes: a systematic review and meta-analysis. PLoS One 2011, vol. 6, no. 11, p. e27374. [CrossRef]
- Sandilyan, S.; Kathiresan, K. Mangroves as bio-shield: an undisputable fact. Ocean Coast. Manag.2015, vol. 103, pp. 94–96.
- Indarsih, R.; Masruri, M. S. Mangrove conservation as an abration strategy risk reduction based on ecosystem in the coastal area of the Rembang Regency. IOP Conference Series: Earth and Environmental Science 2019, vol. 271, no. 1, p. 12021. [CrossRef]
- Lewis III, R. R. Ecological engineering for successful management and restoration of mangrove forests,” Ecol. Eng.2005, vol. 24, no. 4, pp. 403–418. [CrossRef]
- Alongi, D. M. Mangrove forests: resilience, protection from tsunamis, and responses to global climate change. Estuar. Coast. Shelf Sci. 2008, vol. 76, no. 1, pp. 1–13. [CrossRef]
- Ostling, J. L.; Butler, D. R.; Dixon, R. W. The bio geomorphology of mangroves and their role in natural hazards mitigation,” Geogr. Compass 2009, vol. 3, no. 5, pp. 1607–1624. [CrossRef]
- Mclvor, A.; Moller, I.; Spencer, T.; Spalding, M. Reduction of wind and swell waves by mangroves. Natural Coastal Protection Series: Report 1. Cambridge Coastal Research Unit Working Paper 40. The Nature Conservancy and Wetlands International 2012, 27 pp. ISSN 2050–7941.
- McKee, K. L.; Cherry, J. A. Hurricane Katrina sediment slowed elevation loss in subsiding brackish marshes of the Mississippi River delta. Wetlands 2009, vol. 29, no. 1, pp. 2–15. [CrossRef]
- Wolanski, E. Thematic paper: Synthesis of the protective functions of coastal forests and trees against natural hazards. Coast. Prot. aftermath Indian Ocean tsunami: What role for forests and trees 2006, pp. 157–179.
- Day J. W.; Boesch, D. F.; Clairain, E. J.; Kemp, G. P.; Laska, S. B.; Mitsch, W. J.; Orth, K.; Mashriqui, H.; Reed, D. J.; Shabman, L. Restoration of the Mississippi Delta: lessons from hurricanes Katrina and Rita. Science 2007, vol. 315, no. 5819, pp. 1679–1684. [CrossRef]
- Sanderman, J.; Hengl, T.; Fiske, G.; Solvik, K.; Adame, M. F.; Benson, L.; Bukoski, J. J.; Carnell, P.; Cifuentes-Jara, M.; Donato, D. A global map of mangrove forest soil carbon at 30 m spatial resolution. Environ. Res. Lett. 2018, vol. 13, no. 5, p. 55002.. [CrossRef]
- McKee, K. L.; Cahoon, D. R.; Feller, I. C. Caribbean mangroves adjust to rising sea level through biotic controls on change in soil elevation. Glob. Ecol. Biogeogr.2007, vol. 16, no. 5, pp. 545–556. [CrossRef]
- Krauss, K. W.; McKee, K. L.; Lovelock, C. E.; Cahoon, D. R.; Saintilan, N.; Reef, R.; Chen, L. How mangrove forests adjust to rising sea level. New Phytol.2014, vol. 202, no. 1, pp. 19–34. [CrossRef]
- Dahdouh-Guebas, F.; Jayatissa, L. P.; Di Nitto, D.; Bosire, J. O.; Lo Seen, D.; Koedam, N. How effective were mangroves as a defence against the recent tsunami. Curr. Biol.2005, vol. 15, no. 12, pp. R443–R447. [CrossRef]
- Patel, D. M.; Patel, V. M.; Bhupesh, K.; Patel, K. A. Performance of mangrove in tsunami resistance. Int. J. Emerg. Technol. Res.2014, vol. 1, no. 3, pp. 29–32.
- Fehr, I.; Grossi, P.; Hernandez, S.; Krebs, T.; McKay, S.; Muir-Wood, R.; Pomonis, A.; Del Re, D.; Souch, C.; Windeler, D. .Managing tsunami risk in the aftermath of the 2004 Indian Ocean earthquake & tsunami. RMS Incorporated 2006. http://www.disastersrus.org/emtools/tsunami/indianoceantsunamireport.pdf.
- Hawkes, A. D.; Bird, M.; Cowie, S.; Grundy-Warr, C.; Horton, B. P.; Hwai, A. T. S.; Law, L.; Macgregor, C.; Nott, J.; Ong, J. E. Sediments deposited by the 2004 Indian Ocean tsunami along the Malaysia–Thailand Peninsula. Mar. Geol.2007, vol. 242, no. 1–3, pp. 169–190. [CrossRef]
- Athukorala, P.C. Disaster, generosity and recovery: Indian Ocean tsunami. Departmental Working Papers 2012-04, The Australian National University, Arndt-Corden Department of Economics 2012.
- Carugati , L.; Gatto, B.; Rastelli, E.; Lo Martire, M.; Coral, C.; Greco, S.; Danovaro, R. Impact of mangrove forests degradation on biodiversity and ecosystem functioning. Sci. Rep.2018, vol. 8, no. 1, pp. 1–11. [CrossRef]
- Hamilton, S. E.; Casey, D. Creation of a high spatio-temporal resolution global database of continuous mangrove forest cover for the 21st century (CGMFC-21). Glob. Ecol. Biogeogr.2016 vol. 25, no. 6, pp. 729–738. [CrossRef]
- GoK. National Mangrove Ecosystem Management Plan. Kenya Forest Service, Nairobi, Kenya, 2017. https://wiomn.org/wp-content/uploads/2017/10/National-Mangrove-Ecosystem-Management-Plan_Final_170628.pdf.
- Bosire J.O.; Mangora, M.M.; Bandeira, S.O.; Rajkaran, A.; Ratsimbazafy, R.; Appadoo, C.; Kairo, J.G. (Ed.). Mangroves of the Western Indian Ocean: Status and management. Western Indian Ocean Marine Science Association (WIOMSA): Zanzibar 2015, ISBN 978-9987-9559-4-7, xxviii, 157 pp.
- Zimmer, M.; Ajonina, G. N.; Amir, A. A.; Cragg, S. M.; Crooks, S.; Dahdouh-Guebas, F.; Duke, N. C.; Fratini, S.; Friess, D. A.; Helfer, V. When nature needs a helping hand: Different levels of human intervention for mangrove (re-) establishment. Front. For. Glob. Chang.2022, vol. 5, p. 784322. [CrossRef]
- Kodikara, K. A.; Mukherjee, S.N.; Jayatissa, L. P.; Dahdouh-Guebas, F.; Koedam, N. Have mangrove restoration projects worked? An in-depth study in Sri Lanka,” Restor. Ecol.2017, vol. 25, no. 5, pp. 705–716. [CrossRef]
- Wodehouse, D. C. J.; Rayment, M. B. Mangrove area and propagule number planting targets produce sub-optimal rehabilitation and afforestation outcomes. Estuar. Coast. Shelf Sci.2019, vol. 222, pp. 91–102. [CrossRef]
- UNEP-Nairobi Convention/USAID/WIOMSA. Guidelines on Mangrove Ecosystem Restoration for the Western Indian Ocean Region. UNEP, Nairobi 2020, 71 pp. A digital copy of this report is available at: www.nairobiconvention.org/; www.wiomn.org; www. wiomsa.org.
- Kairo, J. G.; Dahdouh-Guebas, F.; Bosire, J.; and Koedam, N. Restoration and management of mangrove systems—a lesson for and from the East African region. South African J. Bot. 2001, vol. 67, no. 3, pp. 383–389. [CrossRef]
- Otero, V.; Van De Kerchove, R.; Satyanarayana, B.; Mohd-Lokman, H.; Lucas, R.; Dahdouh-Guebas, F. An analysis of the early regeneration of mangrove forests using Landsat time series in the Matang Mangrove Forest Reserve, Peninsular Malaysia. Remote Sens.2019, vol. 11, no. 7, p. 774. [CrossRef]
- Kirui, K. B.; Kairo, J. G.; Bosire, J.; Viergever, K. M.; Rudra, S.; Huxham, M.; Briers, R. A. Mapping of mangrove forest land cover change along the Kenya coastline using Landsat imagery. Ocean Coast. Manag.2013, vol. 83, pp. 19–24. [CrossRef]
- Riley, R. W.; Kent, C. P. S. Riley encased methodology: principles and processes of mangrove habitat creation and restoration. Mangroves Salt Marshes 1999, vol. 3, no. 4, pp. 207–213. [CrossRef]
- Tack, J.; Polk, P. The influence of tropical catchments upon the coastal zone: modelling the links between groundwater and mangrove losses in Kenya, India/Bangladesh and Florida. Sustain. Manag. Trop. catchments.1999, pp. 359–371.
- UNEP/Institute of Marine Sciences, University of Dar es Salaam/FAQ/SIDA: Overview of Land-based Sources and Activities Affecting the Marine, Coastal and Associated Freshwater Environment in the Eastern African Region. UNEP Regional Seas Reports and Studies 1998, No. 167.
- Kitheka, J. U. Water circulation and coastal trapping of brackish water in a tropical mangrove-dominated bay in Kenya,” Limnol. Oceanogr.1996, vol. 41, no. 1, pp. 169–176. [CrossRef]
- Kirui, B.; Kairo, J. G.; Karachi, M. Allometric equations for estimating above ground biomass of Rhizophora mucronata Lamk.(Rhizophoraceae) mangroves at Gazi Bay, Kenya. West. Indian Ocean J. Mar. Sci. 2006, vol. 5, no. 1, pp. 27–34. [CrossRef]
- Neukermans, G.; Dahdouh-Guebas, F.; Kairo, J. G.; Koedam, N. Mangrove species and stand mapping in Gazi Bay (Kenya) using Quick bird satellite imagery. J. Spat. Sci. 2008, vol. 53, no. 1, pp. 75–86. [CrossRef]
- Kairu, A.; Kotut, K.; Mbeche, R.; Kairo, J.G. Participatory forestry improves mangrove forest management in Kenya,” Int. For. Rev. 2021, vol. 23, no. 1, pp. 41–54. [CrossRef]
- Kairo, J. G. Artificial regeneration and sustainable yield management of mangrove forests at Gazi Bay, Kenya. University of Nairobi, Kenya, 1995.
- Bosire, J. O.; Dahdouh-Guebas, F.; Kairo, J. G.; Koedam, N. Colonization of non-planted mangrove species into restored mangrove stands in Gazi Bay, Kenya. Aquat. Bot. 2003, vol. 76, no. 4, pp. 267–279. [CrossRef]
- Dahdouh-Guebas, F.; Van Pottelbergh, I.; Kairo, J. G.; Cannicci, S.; Koedam, N. Human-impacted mangroves in Gazi (Kenya): predicting future vegetation based on retrospective remote sensing, social surveys, and tree distribution. Mar. Ecol. Prog. Ser.2004, vol. 272, pp. 77–92. [CrossRef]
- Huff, A.; Tonui, C. Making ‘Mangroves Together: Carbon, conservation and co-management in Gazi Bay, Kenya, STEPS Working Paper 95, Brighton: STEPS Centre 2017.
- Kamali, K.; Hashim, R. Mangrove restoration without planting. Ecol. Eng.2011, vol. 37, no. 2, pp. 387–391. [CrossRef]
- Schmitt, K.; Duke, N.C. Mangrove management, assessment and monitoring. Trop. For. Handb.2015, pp. 1–29. [CrossRef]
- Cintron, G.; Schaeffer Novelli, Y. Methods for studying mangrove structure. Monogr. Oceanogr. Methodol.1984, vol. 8, pp. 91–113.
- Komiyama, A.; Poungparn, S.; Kato, S. Common allometric equations for estimating the tree weight of mangroves. J. Trop. Ecol.2005, vol. 21, no. 4, pp. 471–477. [CrossRef]
- Kauffman, J.B.; Donato, D. C. Protocols for the measurement, monitoring and reporting of structure ,biomass and carbon stocks in mangrove forests. Working Paper 86. CIFOR, Bogor, Indonesia 2012. [CrossRef]
- Kershaw Jr, J.A; Ducey, M.J.; Beers, T.W.; Husch, B. Forest mensuration, Ed.5, John Wiley & Sons. https://books.google.co.ke/books?id=SGVJDQAAQBAJ, 2016.
- Folk, R. L.; Ward, W. C. Brazos River bar [Texas]: A study in the significance of grain size parameters. J. Sediment. Res.1957, vol. 27, no. 1, pp. 3–26. [CrossRef]
- Blott, S. J.; Pye, K. GRADISTAT: a grain size distribution and statistics package for the analysis of unconsolidated sediments. Earth Surf. Process. Landforms 2001, vol. 26, no. 11, pp. 1237–1248. [CrossRef]
- Howard, J.; Hoyt, S.; Isensee, K.; Telszewski, M.; Pidgeon, E. Coastal Blue Carbon: Methods for assessing carbon stocks and emissions factors in mangroves, tidal salt marshes, and sea grasses. Conservation International, Intergovernmental Oceanographic Commission of UNESCO, International Union for Conservation of Nature. Arlington, Virginia, USA, 2014.
- Kauffman, J. B.; Heider, C.; Cole, T. G.; Dwire, K. A.; Donato, D. C. Ecosystem carbon stocks of Micronesian mangrove forests. Wetlands 2011, vol. 31, pp. 343–352. [CrossRef]
- Krumholz, J.; Jadot, C. Demonstration of a new technology for restoration of Red Mangrove (Rhizophora mangle) in high-energy environments. Mar. Technol. Soc. J. 2009. vol. 43, no. 1, pp. 64–72. [CrossRef]
- Clarke, A.; Johns, L. Mangrove Nurseries: Construction, Propagation and Planting: Fisheries Guidelines, Department of Primary Industries, Queensland, Fish Habitat Guideline FHG 004 2002, 32 pp.
- Ndirangu, M. D. Bio-Shields: Wave Energy Reduction and Sediment Stabilization by Mangroves in Gazi Bay, Kenya. University of Nairobi, Kenya, 2016.
- Kamil, E. A.; Takaijudin, H.; Hashim, A. M. Mangroves as coastal bio-shield: a review of mangroves performance in wave attenuation,” Civ. Eng. J. 2021, vol. 7, no. 11, pp. 1964–1981. [CrossRef]
- Rahardjo, H.; Indrawan, I. G. B.; Leong, E. C.; Yong, W. K. Effects of coarse-grained material on hydraulic properties and shear strength of top soil. Eng. Geol. 2008, vol. 101, no. 3–4, pp. 165–173. [CrossRef]
- Zhao, Y.; Cui, Y.; Zhou, H.; Feng, X.; Huang, Z. Effects of void ratio and grain size distribution on water retention properties of compacted in filled joint soils. Soils Found.2017, vol. 57, no. 1, pp. 50–59. [CrossRef]
- Kairo, J. G.; Lang’at, J. K. S.; Dahdouh-Guebas, F.; Bosire, J.; Karachi, M. Structural development and productivity of replanted mangrove plantations in Kenya. For. Ecol. Manage.2008, vol. 255, no. 7, pp. 2670–2677. [CrossRef]
- Tue, N. T.; Quy, T. D.; Amano, A.; Hamaoka, H.; Tanabe, S.; Nhuan, M. T.; Omori, K. Historical profiles of trace element concentrations in mangrove sediments from the Ba Lat Estuary, Red River, Vietnam. Water, Air, Soil Pollut.2012, vol. 223, pp. 1315–1330, 2012. [CrossRef]
- Al-Shayea, N. A. The combined effect of clay and moisture content on the behavior of remolded unsaturated soils,” Eng. Geol.2001, vol. 62, no. 4, pp. 319–342. [CrossRef]
- Dimitrova, R. S.; Yanful, E. K. Factors affecting the shear strength of mine tailings/clay mixtures with varying clay content and clay mineralogy. Eng. Geol. 2012, vol. 125, pp. 11–25. [CrossRef]
- Cerón-Bretón, J. G.; Cerón-Bretón, R. M.; Rangel-Marrón, M.; Estrella-Cahuich, A. Evaluation of carbon sequestration potential in undisturbed mangrove forest in Términos Lagoon, Campeche. Dev. Energy Environ. Econ. 2010, vol. 1, pp. 295–300.
- Calderón, F. J.; Reeves, J. B.; Collins, H. P.; Paul, E. A. Chemical Differences in Soil Organic Matter Fractions Determined by Diffuse-Reflectance Mid-Infrared Spectroscopy. Soil Sci. Soc. Am. J. 2011, vol. 75, no. 2, pp. 568–579. [CrossRef]
- Watson, J. Do. Mangrove forests of the Malay Peninsula. Malayan Forest Records 6, Forest Department, Federated Malay States, Kuala Lumpur. Malay For. Rec.1928 vol. 6, pp. 1–275.
- Srikanth, S.; Lum, S. K. Y.; Chen, Z. Mangrove root: adaptations and ecological importance. Trees 2016, vol. 30, pp. 451–465. [CrossRef]
- Tanaka, N.; Sasaki, Y.; Mowjood, M. I. M.; Jinadasa, K.; Homchuen, S. Coastal vegetation structures and their functions in tsunami protection: experience of the recent Indian Ocean tsunami. Landsc. Ecol. Eng.2007, vol. 3, pp. 33–45, 2007. [CrossRef]
- Ohira, W.; Honda, K.; Nagai, M.; Ratanasuwan, A. Mangrove stilt root morphology modeling for estimating hydraulic drag in tsunami inundation simulation. Trees 2013, vol. 27, pp. 141–148. [CrossRef]
- Mazda, Y.; Magi, M.; Ikeda, Y.; Kurokawa, T.; Asano, T. Wave reduction in a mangrove forest dominated by Sonneratia sp. Wetl. Ecol. Manag. 2006, vol. 14, no. 4, p. 365. [CrossRef]
- Hashim, A. M.; Catherine, S. M. P. A laboratory study on wave reduction by mangrove forests. APCBEE procedia. 2013, vol. 5, pp. 27–32. [CrossRef]
- Primavera J. H., Dela Cruz, M.; Montilijao, C.; Consunji, H.; Dela Paz, M.; Rollon, R. N.; Maranan, K.; Samson, M. S.; Blanco, A. Preliminary assessment of post-Haiyan mangrove damage and short-term recovery in Eastern Samar, central Philippines. Mar. Pollut. Bull.2016, vol. 109, no. 2, pp. 744–750. [CrossRef]
- Temmerman, S.; Meire, P.; Bouma, T. J.; Herman, P. M. J.; Ysebaert, T.; De Vriend, H. J. Ecosystem-based coastal defence in the face of global change. Nature 2013, vol. 504, no. 7478, pp. 79–83. [CrossRef]
- Lovelock C. E.; Cahoon, D. R.; Friess, D. A.; Guntenspergen, G. R.; Krauss, K. W.; Reef, R.; Rogers, K.; Saunders, M. L.; Sidik, F.; Swales, A. Sea level and turbidity controls on mangrove soil surface elevation change. Estuar. Coast. Shelf Sci.2015, vol. 153, pp. 1–9. [CrossRef]
- Prasad, D. H.; Kumar, N. D. Coastal Erosion Studies—A Review. Int. J. Geosci.2014, vol. 2014. [CrossRef]
- Teas, H. J. Ecology and restoration of mangrove shorelines in Florida. Environ. Conserv.1977, vol. 4, no. 1, pp. 51–58. [CrossRef]
- Hashim, R.; Kamali, B.; Tamin, N. M.; Zakaria, R. An integrated approach to coastal rehabilitation: mangrove restoration in Sungai Haji Dorani, Malaysia. Estuar. Coast. Shelf Sci.2010 vol. 86, no. 1, pp. 118–124. [CrossRef]
- Furukawa K.; Primavera, J. H.; Loma, R. J.; Montilijao, C. L.; Coching, J. D.; Maria, Y. Y.; Siringan, F. P. A community based mangrove rehabilitation of high energy coasts in Pedada Bay, Philippines. J. Trop. For. Res. 2019, vol. 3, pp. 36–53.
- Shu, A.; Zhu, J.; Cui, B.; Wang, L.; Zhang, Z.; Pi, C. Coastal wave-energy attenuation by artificial wooden fences deployed for mangrove restoration: an experimental study. Front. Mar. Sci.2023, vol. 10, p. 1165048. [CrossRef]
- Su, J.; Friess, D. A.; Gasparatos, A. A meta-analysis of the ecological and economic outcomes of mangrove restoration. Nat. Commun.2021 vol. 12, no. 1, p. 5050. [CrossRef]
- Beck M. W.; Heck, N.; Narayan, S.; Menéndez, P.; Reguero, B. G.; Bitterwolf, S.; Torres-Ortega, S.; Lange, G.-M.; Pfliegner, K.; McNulty, V. P. Return on investment for mangrove and reef flood protection. Ecosyst. Serv.2022, vol. 56, p. 101440. [CrossRef]
- Ounanian, K.; Carballo-Cárdenas, E.; van Tatenhove, J. P. M.; Delaney, A.; Papadopoulou, K. N.; Smith, C. J. Governing marine ecosystem restoration: the role of discourses and uncertainties. Mar. Policy 2018, vol. 96, pp. 136–144. [CrossRef]
- Gann G. D.; McDonald, T.; Walder, B.; Aronson, J.; Nelson, C. R.; Jonson, J.; Hallett, J. G.; Eisenberg, C., Guariguata, M. R., Liu, J. International principles and standards for the practice of ecological restoration. Restor. Ecol. 2019, vol. 27, no. S1, pp. S1–S46. [CrossRef]
- Waltham N. J.; Elliott, M.; Lee, S. Y.; Lovelock, C.; Duarte, C. M.; Buelow, C.; Simenstad, C.; Nagelkerken, I.; Claassens, L.; Wen, C. K. C. UN decade on ecosystem restoration 2021–2030—what chance for success in restoring coastal ecosystems? Front. Mar. Sci. 2020, vol. 7, p. 71. [CrossRef]
Figure 2.
Climate diagram of Gazi
Figure 2.
Climate diagram of Gazi
Figure 3.
Schematic presentation of the experimental design showing randomized blocks and treatments in the planting area.
Figure 3.
Schematic presentation of the experimental design showing randomized blocks and treatments in the planting area.
Figure 4.
Biometry of mangrove sapling [
38].
Figure 4.
Biometry of mangrove sapling [
38].
Figure 5.
Height-diameter distribution of mangroves in Gazi degraded site. .
Figure 5.
Height-diameter distribution of mangroves in Gazi degraded site. .
Figure 6.
Survival rates of mangroves established in three blocks (A, B, C) under different treatments eight (8) months after planting. 1 to 8 represents time in months after planting.
Figure 6.
Survival rates of mangroves established in three blocks (A, B, C) under different treatments eight (8) months after planting. 1 to 8 represents time in months after planting.
Figure 7.
Overall survival rates of mangroves established under different treatments.
Figure 7.
Overall survival rates of mangroves established under different treatments.
Figure 8.
Variations in survival rates of mangroves grown in Blocks A, B, and C. Error bars represent the standard error of the mean.
Figure 8.
Variations in survival rates of mangroves grown in Blocks A, B, and C. Error bars represent the standard error of the mean.
Figure 9.
Average shoot growth (in cm) in blocks per treatment. A, B and C represents the blocks.
Figure 9.
Average shoot growth (in cm) in blocks per treatment. A, B and C represents the blocks.
Figure 10.
Average number of leaves in blocks per treatment. A, B and C represents the blocks.
Figure 10.
Average number of leaves in blocks per treatment. A, B and C represents the blocks.
Figure 11.
Average number of internodes in blocks per treatment. A, B and C represents the blocks.
Figure 11.
Average number of internodes in blocks per treatment. A, B and C represents the blocks.
Figure 12.
The relationship between depth and bulky density. Error bars represent the standard error of mean.
Figure 12.
The relationship between depth and bulky density. Error bars represent the standard error of mean.
Table 1.
Monitoring schedule adopted in assessing survival and growth performance of the planted seedlings.
Table 1.
Monitoring schedule adopted in assessing survival and growth performance of the planted seedlings.
| Time after planting |
Parameters measured |
| 0 to 3 months |
Percentage Survival |
| 4 to 6 months |
Percentage Survival
Shoot height ( from first node to the base of top-most leaves)
Number of leaves (for all individuals)
Number of internodes (for all individuals) |
| 7 to 8 months |
Percentage Survival
Shoot Height
Number of leaves (for all individuals)
Number of internodes (for all individuals) |
Table 2.
Sediment physico-chemical properties in the different depth intervals of Gazi eroded site. The values are presented as percentage mean ± S.E.
Table 2.
Sediment physico-chemical properties in the different depth intervals of Gazi eroded site. The values are presented as percentage mean ± S.E.
| Sample Depth (cm) |
Coarse Sand (%) |
Medium sand (%) |
Fine Sand (%) |
Silt-clay
(%) |
Bulk Density
(g/cm3) |
SOM
(%) |
SOC
(%) |
| 0 – 15 |
13.54±0.98 |
27.68±2.30 |
55.33±2.60 |
3.45±0.30 |
0.335±0.009 |
6.419±0.35 |
5.554±0.15 |
| 15 – 30 |
19.50±1.41 |
27.60±1.87 |
50.05±2.30 |
2.86±0.24 |
0.281±0.010 |
5.948±0.48 |
5.358±0.20 |
| 30 -50 |
18.74±1.53 |
24.66±2.08 |
53.76±2.55 |
2.84±0.43 |
0.184±0.008 |
6.251±0.55 |
5.484±0.23 |
| 50 – 100 |
19.23±1.73 |
19.51±1.75 |
58.65±2.99 |
2.60±0.34 |
0.065±0.004 |
7.315±0.58 |
5.926±0.24 |
| Average |
17.19±0.70 |
26.10±1.13 |
53.68±1.35 |
3.03±0.17 |
0.255±0.007 |
6.334±0.24 |
5.519±0.10 |
Table 3.
Species composition and importance values of mangroves encountered in Gazi Bay eroding shoreline.
Table 3.
Species composition and importance values of mangroves encountered in Gazi Bay eroding shoreline.
| Species |
Relative values (%) |
|
| |
Dominance |
Frequency |
Density |
IV (%) |
| Avicennia marina |
2.45 |
9.09 |
1.709 |
13.25 |
| Rhizophora mucronata |
1.25 |
13.64 |
1.368 |
16.25 |
| Sonneratia alba |
96.30 |
100 |
96.923 |
293.23 |
Table 4.
Overall growth performance of the replanted mangroves after eight months of growing. Values are reported as mean ± se.
Table 4.
Overall growth performance of the replanted mangroves after eight months of growing. Values are reported as mean ± se.
| Treatment |
Shoot
growth (cm) |
Number of
leaves |
Number of
internodes |
Number of
branches |
| 3’’PVC |
23.84 ± 0.66 |
11.37 ± 0.15 |
4.68 ± 0.08 |
1.87 ± 0.13 |
| 4’’PVC |
25.65 ± 0.72 |
11.89 ± 0.20 |
4.95 ± 0.10 |
2.45 ± 0.18 |
| Total |
24.74 ± 0.69 |
11.63 ± 0.13 |
4.82 ± 0.06 |
2.27 ± 0.13 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).