1. Introduction
Despite recent improvements in the methods used to eradicate and minimize mosquito populations, they still pose severe threats to human health. Mosquitoes are the primary transmitters of a number of deadly diseases, such as malaria, yellow fever, dengue fever, and encephalitis, in addition to the ongoing irritation they give humans and animals merely by their blood-sucking habit and itching (Cheng et al., 2004; Hemingway, 2004; Kuo et al., 2007). There are at least 3,450 different species of mosquitoes and an estimated 100 trillion of them worldwide (CDC, 2020).
Each year, one of the most frequent causes of death for humans worldwide is malaria, which is spread by mosquitoes (Nasrin, 2005). Mosquitoes undergo complete metamorphosis, much like other true flies. Both the larval and pupal stages are undergone by the juvenile form. Anopheles takes 7–12 days from the egg to the adult stage, depending on temperature and other environmental variable. Each batch of eggs—100 or more—that the female Anopheles produce at intervals of two or three days requires a blood meal. While they may consume nectar and fruit juices, they cannot lay eggs in the absence of bloodmeal (Jacobs-Lorena, 2003). The larvae have different anatomical characteristics from the adults, inhabit various habitats, and consume various kinds of meals (Clements, 1992). Many pathogens and parasites, such as viruses, bacteria, fungi, and nematodes, can be found in mosquitoes. Adult mosquitoes are susceptible to acquiring parasites or pathogens from one vertebrate host to another because of their blood-sucking habits, but several characteristics of the mosquito's ecology and physiology must be suitable for it to acquire, house, and transmit a specific organism (Haq et al., 2003). In addition to localized skin reactions, mosquito bites can also result in systemic allergic reactions in humans, including urticaria and angioedema (Peng et al., 1999).
In 106 nations and territories, malaria affects more than 3.3 billion people, approximately half of the world's population. According to the WHO, there were 216 million cases of malaria in 2010. After pneumonia and diarrheal illness, malaria is the third most common cause of death for children under five worldwide. Encephalitis, filariasis, yellow fever, dengue, and chikungunya continue to be mostly spread by mosquitoes. With 81% of fatalities in Africa attributable to it, malaria is still the second-leading infectious disease killer behind HIV/AIDS (WHO, 2012). In Nigeria, it is the cause of 300,000 deaths annually, as opposed to HIV/AIDS, which is the cause of 215,000 deaths. Hospitalizations account for between 10–30% of all hospitalizations, and 12–25% of all pediatric fatalities (Enato and Okhamefe, 2004). Organochlorides, organophosphates, and carbamates are examples of synthetic pesticides that are poisonous and have a negative impact on the environment by contaminating soil, water, and air. The number of adult mosquitoes and their breeding sites is growing as their resistance to man-made insecticides increases. The resurgence of these diseases has been caused by an increase in the vector (Mosquito) in urban agglomerations, which has led to an increase in mosquito resistance to these insecticides (WHO, 1996, Enyi and Ekpunobi, 2022)
Larvicides are typically used in mosquito control operations to target the larval stage in breeding sites because adulticides may only temporarily lower the adult population (Hag et al., 2003). Larvicides are applied to kill mosquito larvae before they spread to human habitations because, unlike adults, mosquito larvae cannot alter their behaviour to evade control measures that are directed at the larval environment (Charlwood and Graves, 1987). (Killeen et al., 2002). This may also lessen the overall amount of insecticides that must be applied to manage adult mosquito populations (Dharmagadda et al., 2005). This alone is a benefit that can be used to find fresh and innovative larvicides. The most promising source of new secondary metabolites continues to be natural products. According to several studies, over 2000 plants contain phytochemicals that can either kill or repel mosquitoes (Anupam et al., 2011).
The plant Ocimum gratissimum belongs to the Lamiaceae family. Its essential oil contains insecticidal and mosquito-repelling characteristics that have been employed locally (Mann et al., 2009). Peasant farmers in northern Nigeria utilize a variety of plant components to safeguard grains and legumes from pest damage while they are in storage (Mann, 2003). Whole plant remedies, particularly in India, are used to treat influenza, sunstroke, headaches, and stomachaches. The seeds are used to treat gonorrhoea and have laxative qualities. The essential oil is used to treat skin conditions, stomach aches, diarrhoea, and inflammations of the ears, eyes, throat, and skin (Afolabi et al, 2007).
Developing nations may be able to find unique answers to the challenges that malaria poses by using phytochemical active substances produced from the common Ocimum gratissimum. There is a need to create new, powerful botanical insecticides from a plant source that are efficient, environmentally benign, quickly biodegradable, and because of how common this disease and its vector are. This research aimed to investigate the Larvicidal potentials of silver nanoparticles of Ocimum gratissimum on wild Anopheles larvae from Lagos
2. Methods
2.1. Site of Study
The plant used in this study was collected from the southeastern part of Nigeria, Ibite-Olo in the Ezeagu Local Government Area of Enugu State. The methodology adopted for this study includes the use of a questionnaire and Interviews/meetings with residents of the Ezeagu Local Government Area to know the plants that were used in the control of mosquitoes This formed the basis of the choice of Ocimum gratissimum plants for the assessment of the Larvicidal potential of silver nanoparticles on wild Anopheles Larvae in Lagos, Nigeria.
2.2. Test Insect, Source and Stabilization
The laboratory was equipped with standard insectary conditions (25–29 °C; 78–82% relative humidity) after wild Anopheles mosquito larvae were collected from a field in Kosofe Local Government Area of Lagos State, Nigeria. This location was used because resistant anopheline populations have been observed in and around Lagos State (Awolola et al, 2007; Oduola et al., 2012; Adeogun et al, 2017).
Prior to their use in the experiment, the larvae were housed in a container for a full day in order to allow them to become acclimated to the laboratory environment. The standard taxonomic key (Gillies & Coetzee, 1987) was used to identify the Field Anopheles mosquito larvae through morphological means.
2.3. Preparation of Plant Extracts
Freshly collected newly collected leaves of
Ocimum gratissimum were taxonomically identified and were then washed thoroughly with distilled water, and dried with paper absorbents.
Figure 3.
Ocimum gratissimum.
Figure 3.
Ocimum gratissimum.
2.4. Synthesis of silver nanoparticles (AgNO3)
A magnetic stirrer was used to mix 160 ml of ionized water with 1.019 g of silver nitrate salt for five minutes, during which time 40 ml of extracts were added. After carefully covering the setup to prevent light from penetrating, it was left on the magnetic stirrer for 48 hours. Pale yellow to dark brown colour changes were seen, signifying the shift from Ag+ to Ago. Following an hour, the solution's colour changed from colorless to honey brown, signifying the formation of silver nanoparticles, which UV-visible spectroscopy verified. The silver nanoparticles were obtained as a brownish powder by oven-drying the leftover residue at 70 °C after discarding the supernatant (Umoren et al. 2014).
Figure 4.
Laboratory synthesis process of silver nanoparticles.
Figure 4.
Laboratory synthesis process of silver nanoparticles.
2.5. Larvicidal Bioassay
The static bioassay method, as detailed by Reish et al. (1987), was used in the experiment to determine the organism's reaction to the test toxicants under carefully regulated environmental conditions. In accordance with WHO guidelines, a bioassay test was conducted to evaluate the larvicidal activity at various concentrations. wherein thirty larvae were dropped using droppers into 500 ml glass beakers that held 250 ml of water. Larvae that were small, unhealthy, or damaged were taken out and replaced. For a duration of 72 hours, the test containers were maintained at a temperature of 27 ± 2oC with a photoperiod of 12 hours of light followed by 12 hours of darkness. Each extract was tested in six (6) different concentrations at a time, with four duplicates. Every set of experiments included a control, whose mortality was noted after a 24-hour period. Experiments were conducted on wild Anopheles larvae in a laboratory setting with controlled temperature conditions (27± 2oC). In order to determine the LC50 and values with a 95% confidence limit, the obtained values were subjected to log prohibit regression analysis.
Percentage mortality was calculated as follows:
Percentage mortality = (Number of dead individuals/Number of treated individuals)×100
2.6. Toxicity Testing Studies
A range-finding test was carried out as described by (Sogbanmu et al., 2020), where randomly selected mosquito larval populations were exposed to the different ranges of test concentrations and control to realize a suitable concentration gradient for the definitive tests.
2.7. Quantal Response
Throughout each exposure period, deaths and obvious abnormalities pertaining to behaviour and appearance were noted in all media at 24-hour intervals. Moribund larvae were counted and added to dead larvae following a 24-hour exposure in order to determine the percentage of mortality. When a needle is inserted into a siphon, dead larvae are those that cannot be made to move. When the water is disturbed, larvae that are unable to surface or do not exhibit the typical diving response are said to be moribund. The LC50 and LC95 values are noted along with the results that are recorded. To prevent contaminating the test medium, the number of dead organisms was counted and their carcass was promptly removed (Chukwu and Okhumale, 2009).
The exam was deemed faulty in the event that over 10% of the control larvae pupated or perished during the experiment, the test will be deemed invalid by larvae that pupated during the test period.
2.8. Data Analysis
Using the computer program SPSS (IBM SPSS v20.0), data from the experimental analysis were subjected to probit regression analysis in order to analyze the toxicological dose-response data involving cumulative quantal response (mortality) after 72 hours.
3. Result
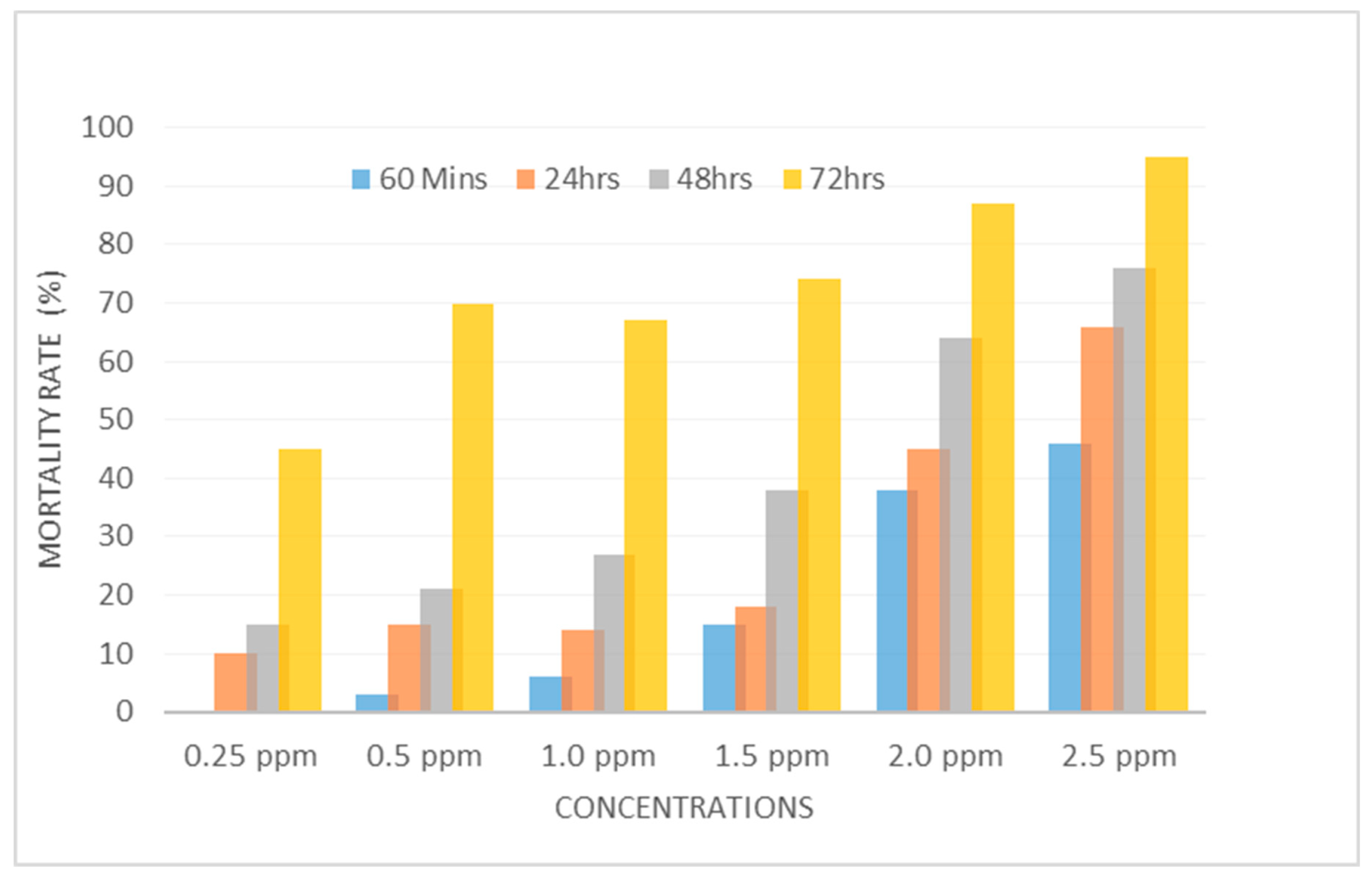
Larvicidal activities of the silver nanoparticles of Ocimum gratissimum against Anopheles species.
The results of the larvicidal activities of the silver nanoparticles of
Ocimum gratissimum are shown in
Figure 5. Mortality rate was found to be dose-dependent with the percentage mortality increasing in a directly proportional rate as with dose and time of exposure (
Table 1).
Utilizing IBMSPSSv20.0 as a basis, probit regression analysis was employed to examine the toxicological dose-response data pertaining to cumulative quantal response (mortality) following a 72-hour period. LC50, or median lethal concentration, which causes a 50% response (mortality) in exposed organisms, and LC95, or lethal concentration, which causes a 95% response (mortality) in exposed organisms, are the toxicity measurement indices that resulted from this analysis.
Using both descriptive and inferential statistics, data from the 72-hour larvicidal bioassay of Ocimum gratissimum silver nanoparticles against Anopheles mosquito larvae were gathered as shown in
Table 1.
4. Discussion and Conclusion
Many pharmacological alleles produced by plants have not yet had their physiological characteristics thoroughly investigated (Norduland and Sauls, 1981). It is well-recognized that phytochemicals selectively prevent pathogenic organisms from growing, forming, changing into new forms, and reproducing (Ahmad, 2007). The commercial development of plant-derived chemicals as eco-friendly insecticides is currently experiencing a resurgence of interest. Moreover, nanotechnology is one of the cutting-edge scientific fields that has made it possible to synthesize particles with a variety of sizes, shapes, and compositions. Pharmaceuticals and disease vector control have made extensive use of the creation of metal nanoparticles, including silver, gold, and platinum (Mittal et al., 2013).
When compared to other plants that possess antibacterial properties, Ocimum gratissum has demonstrated its ability to combat a range of microorganisms and parasites. According to Nsofor et al. (2014) and Prabu et al. (2017), the AgNP of Ocimum gratissimum in an aqueous gel basis has the ability to treat acne vulgaris. Its effectiveness against Escherichia Coli O157 was also demonstrated. Additionally, AgNPs have shown increased antibacterial effectiveness against Gram-positive (Staphylococcus aureus, Bacillus subtilis, and Micrococcus luteus) as well as Gram-negative (Escherichia coli, Klebsiella pneumoniae) bacteria (Sharma et al., 2020; Ekpunobi et al., 2023). It has also been demonstrated that Ocimum gratissimum's AgNP possesses larvicidal action.
The crude extracts of Ocimum gratissimum leaf using different solvent extracts were tested for mosquito larvicidal activity against Culex quinquifaciatus with both crude extracts and isolated fractions showing larvicidal activity (Egunyomi et al., 2010).
The larvicidal activity of Ocimum gratissimum's AgNP against Anopheles mosquitoes was investigated in this work. It was discovered that the effectiveness against Anopheles sp. was dose-dependent, with the percentage fatality rising with increasing exposure time and dose. Compared to previous research that just employed Ocimum gratissimum extracts, the potency of Ocimum gratissimum against mosquito larvae in this study is substantially increased in conjunction with AgNPs, which indicates an increase in toxicity against strains of Anopheles larvae. This discovery is consistent with recent reports (Rajasekharreddy and Rani, 2014; Jinu et al, 2018; Fouad et al., 2018) on the larvicidal activity of biosynthesized AgNPs against several mosquito vectors.
The enhanced effectiveness of Ocimum gratissimum's AgNP may be explained by the particles' ability to pass through larval membranes and bind to protein compounds that contain sulfur and phosphorus, such as DNA, which causes some organelles and enzymes to become denatured (Rai et al., 2009). An additional benefit of AgNPs is their minuscule size, which allows them to easily penetrate the insect's exoskeleton and enter its alimentary canal cells, interfering with physiological functions (Suresh et al., 2018).
This work presents an efficient and low-cost synthesis method for AgNPs.The synthetically produced silver nanoparticles are crystalline, spherical, and soluble in water. The constituent parts have an unadulterated silver appearance. When applied to wild Anopheles larvae in Lagos, Ocimum gratissimum silver nanoparticles' larvicidal activity was enhanced by biosynthesized silver nanoparticles. As a result, this study demonstrates that Ocimum gratissimum silver nanoparticles are good candidates for the creation of novel natural pesticides and may be employed against mosquito vectors that are resistant to insecticides. To determine whether stored silver nanoparticles from Ocimum gratissimum samples are stable larvicidal agents and whether they are cytotoxic to non-target species and epithelial cells, more research is required.
Conflicts of Interest
All the authors declares no conflicts in interest.
References
- Adeogun, A. O., Popoola, K. O. K., Oduola, A. O., Olakiigbe, A. K., & Awolola, T. S. (2017). High level of DDT resistance and reduced susceptibility to Deltamethrin in Anopheles gambiae, Anopheles coluzzi, and Anopheles arabiensis from Urban communities in Oyo State, South-West Nigeria. Journal of Mosquito Research, 7, 125–133. [CrossRef]
- Afolabi C.A.,Ibukun E. O., Afor, E., Obuotor, E. M. and Farombi, E.O. (2007).Phytochemical constituent and antioxidant activity of extract from the leaves of Ocimum gratissimum. Scientific Research and Essay 2(5): 163-166.
- Ahmad, M. (2007). Insecticide resistance mechanisms and their Management in Helicoverpaarmigera (Hübner) -A review. Journal of Agricultural Research,45(4):319-335.
- Anupam G., Nandita C. and Goutan C. (2011). Review Artticle, Plant extracts as potential mosquito larvicides. Indian Journal of Medical Research 135: 581-598.
- Awolola, T. S., Oduola, O. A., Obansa, J. B., Chukwurah, N. J., & Unyimadu, J. P. (2007). Anopheles gambiae s.s. breeding in polluted water bodies in urban Lagos, Southwestern Nigeria. Journal of Vector-Borne Diseases, 44, 241–244.
- CDC, 2020. What is a mosquito? https://www.cdc.gov/mosquitoes/about/what-is-a-mosquito.html#:~:text=Over%203%2C500%20types%20of%20mosquitoes,Some%20mosquitoes%20can%20be%20vectors.0.
- Charlwood, J.D. and Graves, P.M. (1987), The effect of permethrin-impregnated bednets on a population of Anopheles farauti in coastal Papua New Guinea. Medical and Veterinary Entomology, 1: 319-327. [CrossRef]
- Cheng S. S., Liu J. Y., Tsai K. H., Chen W. J. and Chang S. T. (2004). Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. Journal of Agricultural Food Chemisrty. 52: 4395–4400. [CrossRef]
- Chukwu, L. O and Okhumale, B. O., 2009. Mode of joint action response to binary mixtures of three refined petroleum products by Nile tilapia, Oreochromis niloticus fingerlings. Scientific Research and Essay, 4, (8): 806-811.
- Clements, A. N. (1992). The Biology of Mosquitoes: Development, Nutrition and Reproduction. Volume 1 of The Biology of Mosquitoes, Alan Neville Clements. [CrossRef]
- Dharmagadda, V.S.S., Naik, S., Mittal, P., and Vasudevan, P. (2005). Larvicidal activity of Tagetes patula essential oil against three mosquito species. Bioresource technology. 96. 1235-1240. [CrossRef]
- Egunyomi, A., Gbadamosi, I.T. and Osiname K.O. (2010). Comparative effectiveness of ethnobotanical mosquito repellents used in Ibadan, Nigeria. Journal of Applied Biosciences (36): 2383- 2388.
- Ekpunobi, N., Akinsuyi, O., Ariri, T., Ogunmola, T. (2023). The Reemergence of Monkeypox in Nigeria. Challenges 14, 22. [CrossRef]
- Enato, E.F. and Okhamafe, A.O. (2004). Plasmodium falciparum malaria and antimalarial interventions in sub-Saharan Africa: Challenges and Opportunities. African Journal of Food, Agriculture, Nutrition and Development, 4(13). [CrossRef]
- Enyi, E. O., Ekpunobi, N. F. (2022). Secondary metabolites from endophytic fungi of moringa oleifera: antimicrobial and antioxidant properties. J Microbiol Exp. 10(5):150 ‒ 154.
- Fouad, H., Hongjie, L., Hosni, D., Wei, J., Abbas, G.; Ga’al, H.; Jianchu, M. (2018) Controlling Aedesalbopictusand Culexpipienspallensusing silver nanoparticles synthesized from aqueous extract of Cassia fistula fruit pulp and its mode of action. Artif. Cells Nanomed. Biotechnol., 46, 558–567. [CrossRef]
- Gillies, M. T., and Coetzee, M. (1987). A supplement to the Anophelinae of Africa south of the Sahara (Afro- An annotated checklist and bibliography of the most tropical Region). Johannesburg: South African Institute for Medical Research. Interior Health, 2009. Pest management plan for control of mosquito larvae that are potential West Nile virus vectors.
- Haq, S., Yadav, R.S. and Kohli, V.K., (2003). Developing larvivorous fish network for mosquito in urban areas: a case study. ICMR Bull., 33: 69-73.
- Hemingway J. (2004). Taking aim at mosquitoes. Nature. 430: 936-941. [CrossRef]
- Jacobs-Lorena, M. (2003). Interrupting malaria transmission by genetic manipulation of anopheline mosquitoes. J Vector Borne Dis 40(3-4):73-77.
- Jinu, U.; Rajakumaran, S.; Senthil-Nathan, S.; Geetha, N.; Venkatachalam, P. (2018) Potential larvicidal activity of silver nanohybrids synthesized using leaf extracts of Cleistanthuscollinus (Roxb.) Benth. ex Hook.f. and Strychnos nux-vomica L. nux-vomica against dengue, Chikungunya and Zika vectors. Physiol. Mol. Plant Pathol. 101, 163–171. [CrossRef]
- Killeen, G. F., Fillinger, U., and Knols, B. G. (2002). Advantages of larval control for African malaria vectors: low mobility and behavioural responsiveness of immature mosquito stages allow high effective coverage. Malaria journal, 1, 8. [CrossRef]
- Kuo P. M., Chu, F. H., Chang, S. Y., Hsiao, W. F. and Wang, S. Y. (2007). Insecticidal activity of essential oil from Chamaecyparis formosensis. Holzforschung 61: 595–599. [CrossRef]
- Mann, A., Gbate, M., and Nda-Umar, A. (2003). Medicinal and Economic Plants of Nupeland, Jube-Evans Books and Publications, Bida, Niger State, Nigeria.
- Mann, A., Ibrahim, K., Oyewale, A., Amupitan, J.And Okogun, J. (2009). Antimycobacterial activity of some medicinal plants in Niger state, Nigeria. African Journal of Infectious Diseases, 3(2). [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C.(2013). Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv., 31, 346–356. [CrossRef]
- Norduland, D.A. and Sauls, G.E. (1981). Kairomones and their use for the management of entomophagous insects. J Gen Ecol 7:1057-1061.
- Nsofor CA, Chikezie UN, Azuwuike CO.(2014) Antibacterial activity of ocimumgratissimumleaves extract on Escherichia ColiO157. Palgo Journal of Medicine and Medical Science.1(2):15-18.
- Oduola, A. O., Idowu, E. T., Oyebola, M. K., Adeogun, A. A., Olojede, J. B., Otubanjo, O. A., & Awolola, T. S. (2012). Evidence of carbamate resistance in urban populations of Anopheles gambiae s.s. Mosquitoes resistant to DDT and deltamethrin insecticides in Lagos. Parasites & Vectors, 5, 1–9. [CrossRef]
- Peng, Z., Yang, J., Wang, H., and Simons, F. E. (1999). Production and characterisation of monoclonal antibodies to two new mosquito Aedes aegypti salivary proteins. Insect Biochem Mol Biol. 29:909–914. [CrossRef]
- Prabu, S. L., Umamaheswari, A., Rajakumar, S., Bhuvaneswari, P. L, and Muthupetchi, S. (2017). Development and Evaluation of Gel Incorporated with Synthesized Silver Nanoparticles from Ocimumgratissimum for the Treatment of Acne Vulgaris. American Journal of AdvancedDrug Delivery 5(03):107-117.
- Rai, M., Yadav, A., & Gade, A. (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances, 27, 76–83. [CrossRef]
- Rajasekharreddy, P.; Rani, P.U. (2014). Biofabrication of Ag nanoparticles using SterculiafoetidaL. seed extract and their toxic potential against mosquito vectors and HeLa cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 39, 203–212. [CrossRef]
- Reish, D.J. and Oshida, P.S. (1987). Manual of methods in aquatic environmental research. Part 10. Procedures for short-term static bioassays. FAO Fish. Tech. 247;62.
- Sharma, K., Guleria, S. &Razdan, V.K.(2020). Green synthesis of silver nanoparticles using Ocimumgratissimumleaf extract: characterization, antimicrobial activity and toxicity analysis. J. Plant Biochem. Biotechnol. 29, 213–224. [CrossRef]
- Sogbanmu T. O., Aitsegame S. O., Otubanjo O. A., Odiyo J. O. (2020). Drinking water quality and human health risk evaluations in rural and urban areas of Ibeju-Lekki and Epe local government areas, Lagos, Nigeria. Hum. Ecol. Risk Assess. Int. J. 26 (4), 1062–1075. [CrossRef]
- Suresh, U.; Murugan, K.; Panneerselvam, C.; Rajaganesh, R.; Roni, M.; Aziz, A.T.; Naji Al-Aoh, H.A.; Trivedi, S.; Rehman, H.; Kumar, S. (2018). Suaedamaritima-based herbal coils and green nanoparticles as potential biopesticides against the dengue vector Aedes aegypti and the tobacco cutworm Spodopteralitura. Physiol. Mol. Plant Pathol. 101, 225–235. [CrossRef]
- Umoren, S. A., Obot, I. B., & Gasem, Z. M. (2014). Green synthesis of silver nanoparticles using apple (Malus domestica) fruit extract at room temperature. Journal of Materials and Environmental Science, 5, 907–914.
- WHO. (1996). Report of WHO informal consultation on the evaluation and testing insecticides.WHO CTD/WHO PES/IC/; 96(1):69. 5. Jacobson M. Insecticides from plants.
- World Health Organization. World Malaria Report. Geneva: WHO; 2012.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).