Submitted:
01 December 2023
Posted:
05 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- Bioinformatic analysis
- Literature search and data selection
- Inclusion and Exclusion criteria
3. Results
3.1. Data from the Swiss Target Prediction
3.2. Analysis of Gene Ontology and Metabolic Pathways
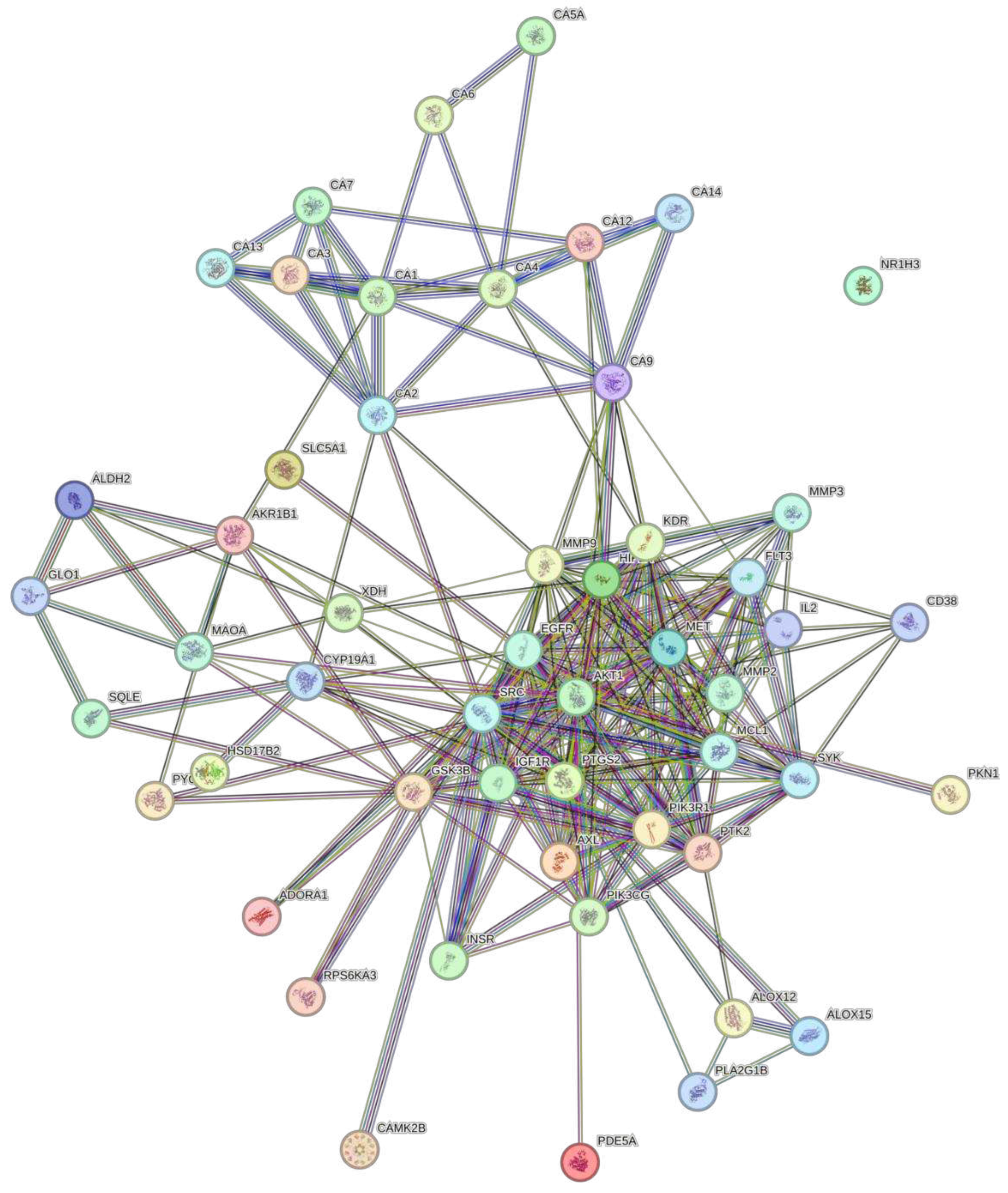
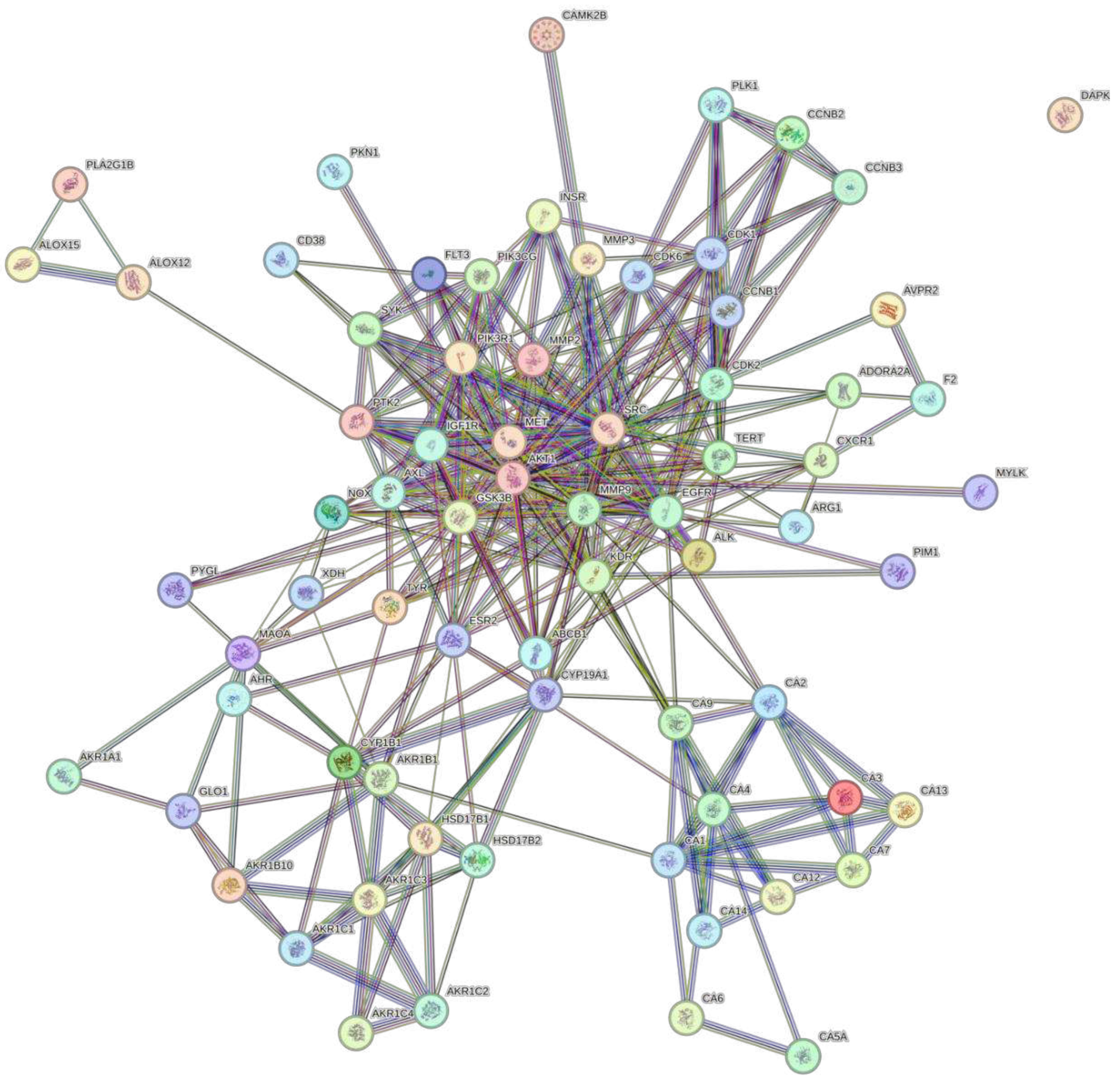
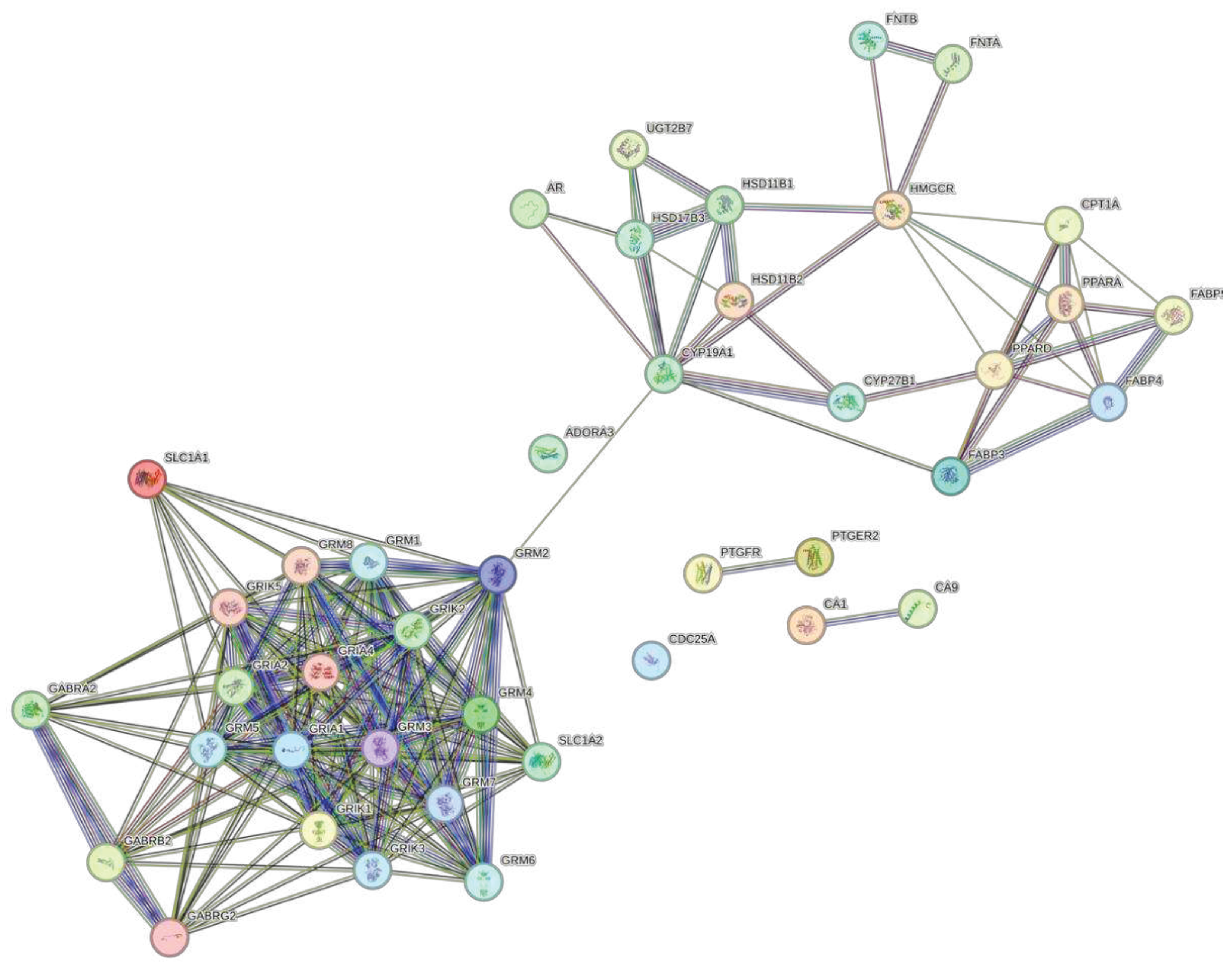
3.3. Protein-Protein Interaction Network
3.4. Data Recollection from the Evidence of the Hub Genes with the Biocompounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ojulari OV, Lee SG, Nam JO. Beneficial Effects of Natural Bioactive Compounds from Hibiscus sabdariffa L. On obesity. Molecules. 2019, 24, 1–14. [Google Scholar]
- Amos A, Khiatah B. Mechanisms of Action of Nutritionally Rich Hibiscus sabdariffa’s Therapeutic Uses in Major Common Chronic Diseases: A Literature Review. J Am Nutr Assoc. 2021, 41, 116–124. [Google Scholar]
- Long Q, Chen H, Yang W, Yang Li and Zhang L. Delphinidin-3-sambubioside from Hibiscus sabdariffa. L attenuates hyperlipidemia in high fat diet-induced obese rats and oleic acid-induced steatosis in HepG2 cells. Bioengineered. 2021, 12, 3837–3849. [Google Scholar] [CrossRef] [PubMed]
- Fridrich D, Teller N, Esselen M, Pahlke G, Marko D. Comparison of delphinidin, quercetin and (-)-epigallocatechin-3-gallate as inhibitors of the EGFR and the ErbB2 receptor phosphorylation. Mol Nutr Food Res. 2008, 52, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Lee MJ, Chou FP, Tseng TH, Hsieh MH, Lin MC, Wang CJ. Hibiscus protocatechuic acid or esculetin can inhibit oxidative LDL induced by either copper ion or nitric oxide donor. J Agric Food Chem [Internet]. 2002, 50, 2130–2136, Available from: https://pubmed.ncbi.nlm.nih.gov/11902968/. [Google Scholar] [CrossRef] [PubMed]
- Syed DN, Afaq F, Sarfaraz S, Khan N, Kedlaya R, Setaluri V, et al. Delphinidin inhibits cell proliferation and invasion via modulation of Met receptor phosphorylation. Toxicol Appl Pharmacol. 2008, 231, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Teller N, Thiele W, Boettler U, Sleeman J, Marko D. Delphinidin inhibits a broad spectrum of receptor tyrosine kinases of the ErbB and VEGFR family. Mol Nutr Food Res. 2009, 53, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- 8. Pal HC, Sharma S, Strickland LR, Agarwal J, Athar M, Elmets CA, et al. Delphinidin Reduces Cell Proliferation and Induces Apoptosis of Non-Small-Cell Lung Cancer Cells by Targeting EGFR/VEGFR2 Signaling Pathways. PLoS One. 2013 Oct 4;8(10).
- Mohammadi-Bardbori A, Bengtsson J, Rannug U, Rannug A, Wincent E. Quercetin, Resveratrol, and Curcumin Are Indirect Activators of the Aryl Hydrocarbon Receptor (AHR). Chem Res Toxicol. 2012, 25, 1878–1884. [Google Scholar] [CrossRef] [PubMed]
- Ciolino HP, Daschner PJ, Yeh GC. Dietary flavonols quercetin and kaempferol are ligands of the aryl hydrocarbon receptor that affect CYP1A1 transcription differentially. Biochem J. 1999, 340, 715–722. [Google Scholar] [CrossRef]
- Škarydová L, Živná L, Xiong G, Maser E, Wsól V. AKR1C3 as a potential target for the inhibitory effect of dietary flavonoids. Chem Biol Interact. 2009, 178, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Chou CC, Yang JS, Lu HF, Ip SW, Lo C, Wu CC, et al. Quercetin-mediated cell cycle arrest and apoptosis involving activation of a caspase cascade through the mitochondrial pathway in human breast cancer MCF-7 cells. Arch Pharm Res. 2010, 33, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Yousuf M, Khan P, Shamsi A, Shahbaaz M, Hasan GM, Haque QMR, et al. Inhibiting CDK6 activity by quercetin is an attractive strategy for cancer therapy. ACS Omega. 2020, 5, 27480–27491. [Google Scholar] [CrossRef] [PubMed]
- Mense SM, Chhabra J, Bhat HK. Preferential induction of cytochrome P450 1A1 over cytochrome P450 1B1 in human breast epithelial cells following exposure to quercetin. J Steroid Biochem Mol Biol. 2008, 110, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Liu Y, Li Y, Xu L, Shi J, Yu X, Wang X, et al. Quercetin Attenuates Podocyte Apoptosis of Diabetic Nephropathy Through Targeting EGFR Signaling. Front Pharmacol. 2022, 12. [Google Scholar]
- Chen K, Rekep M, Wei W, Wu Q, Xue Q, Li S, et al. Quercetin Prevents In Vivo and In Vitro Myocardial Hypertrophy Through the Proteasome-GSK-3 Pathway. Cardiovasc Drugs Ther. 2018, 32, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Chen WJ, Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH. Quercetin blocks the aggressive phenotype of triple-negative breast cancer by inhibiting igf1/ igf1r-mediated emt program. J Food Drug Anal. 2021, 29, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Bandaruk Y, Mukai R, Terao J. Cellular uptake of quercetin and luteolin and their effects on monoamine oxidase-A in human neuroblastoma SH-SY5Y cells. Toxicol Rep. 2014, 1, 639–649.
- 19. Cao HH, Cheng CY, Su T, Fu XQ, Guo H, Li T, et al. Quercetin inhibits HGF/c-Met signaling and HGFstimulated melanoma cell migration and invasion. Mol Cancer.
- Tu H, Ma D, Luo Y, Tang S, Li Y, Chen G, et al. Quercetin alleviates chronic renal failure by targeting the PI3k/Akt pathway. Bioengineered. 2021, 12, 6538–6558. [Google Scholar] [CrossRef] [PubMed]
- Gfeller D, Michielin O, Zoete V. Shaping the interaction landscape of bioactive molecules. Bioinformatics. 2013, 29, 3073–3079. [Google Scholar] [CrossRef] [PubMed]
- Yan L, Zhang Z, Liu Y, Ren S, Zhu Z, Wei L, et al. Anticancer Activity of Erianin: Cancer-Specific Target Prediction Based on Network Pharmacology. Front Mol Biosci. 2022 Mar 17;9.
- Ge SX, Jung D, Yao R. ShinyGO: a graphical gene-set enrichment tool for animals and plants. Bioinformatics. 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Vaez S, Parivr K, Amidi F, Rudbari NH, Moini A, Amini N. Quercetin and polycystic ovary syndrome; inflammation, hormonal parameters and pregnancy outcome: A randomized clinical trial. Am J Reprod Immunol. 2023, 89, e13644. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Soto A, Alejandra Chavez-Santoscoy R, Porras O, Hidalgo-Ledesma M, Serrano-Medina A, Alejandra Ramírez-Rodríguez A, et al. Epicatechin and quercetin exhibit in vitro antioxidant effect, improve biochemical parameters related to metabolic syndrome, and decrease cellular genotoxicity in humans. Food Research International. 2021, 142, 110101. [Google Scholar] [CrossRef] [PubMed]
- Zheoat AM, Gray AI, Igoli JO, Ferro VA, Drummond RM. Hibiscus acid from Hibiscus sabdariffa (Malvaceae) has a vasorelaxant effect on the rat aorta. Fitoterapia [Internet]. 2019, 134, 5–13, Available from: https://pubmed.ncbi.nlm.nih.gov/30690125/. [Google Scholar] [CrossRef] [PubMed]
- Lin WL, Hsieh YJ, Chou FP, Wang CJ, Cheng MT, Tseng TH. Hibiscus protocatechuic acid inhibits lipopolysaccharide-induced rat hepatic damage. Arch Toxicol [Internet]. 2003, 77, 42–47, Available from: https://pubmed.ncbi.nlm.nih.gov/12491040/. [Google Scholar] [CrossRef] [PubMed]
- Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2012, 41, D808–15. [Google Scholar] [CrossRef] [PubMed]
- 29. von Mering C, Jensen LJ, Kuhn M, Chaffron S, Doerks T, Kruger B, et al. STRING 7--recent developments in the integration and prediction of protein interactions. Nucleic Acids Res.
- Szklarczyk D, Franceschini A, Kuhn M, Simonovic M, Roth A, Minguez P, et al. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2011, 39, D561–8. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017, 45, D362–8. [Google Scholar] [CrossRef] [PubMed]
- Jensen LJ, Kuhn M, Stark M, Chaffron S, Creevey C, Muller J, et al. STRING 8--a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res. 2009, 37, D412–6. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–13. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, et al. The STRING database in 2021: customizable protein–protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–12. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk D, Kirsch R, Koutrouli M, Nastou K, Mehryary F, Hachilif R, et al. The STRING database in 2023: protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–46. [Google Scholar] [CrossRef] [PubMed]
- Ojeda D, Jiménez-Ferrer E, Zamilpa A, Herrera-Arellano A, Tortoriello Jaime and Alvarez L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J Ethnopharmacol. 2009, 127, 7–10.
- Huang YT, Hwang JJ, Lee PP, Ke FC, Huang JH, Huang CJ, et al. Effects of luteolin and quercetin, inhibitors of tyrosine kinase, on cell growth and metastasis-associated properties in A431 cells overexpressing epidermal growth factor receptor. Br J Pharmacol. 1999, 128, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Syed DN, Afaq F, Sarfaraz S, Khan N, Kedlaya R, Setaluri V, et al. Delphinidin inhibits cell proliferation and invasion via modulation of Met receptor phosphorylation. Toxicol Appl Pharmacol. 2008, 231, 52–60. [Google Scholar] [CrossRef] [PubMed]
| Enrichment FDR | nGenes | Pathway Genes | Fold Enrichment | Pathway | Genes |
|---|---|---|---|---|---|
| 4.20E-18 | 10 | 17 | 141.151703 | Nitrogen metabolism | CA2 CA9 CA14 CA6 CA1 CA3 CA4 CA7 CA5A CA13 |
| 1.49E-09 | fifteen | 354 | 10.1677074 | PI3K-Akt signaling pathway | GSK3B PIK3CG MET IL2 FLT3 PKN1 KDR IGF1R AKT1 MCL1 PIK3R1 EGFR SYK PTK2 INSR |
| 2.21E-09 | 9 | 79 | 27.3369754 | EGFR tyrosine kinase inhibitor resistance | GSK3B MET KDR IGF1R AKT1 PIK3R1 EGFR AXL SRC |
| 2.73E-09 | 27 | 1527 | 4.24287044 | Metabolic pathways | CD38 PTGS2 CA12 AKR1B1 HSD17B2 PYGL CA2 SQLE PIK3CG CA9 ALOX12 ALDH2 CA14 GLO1 CA6 CA1 CYP19A1 PDE5A XDH ALOX15 CA3 CA4 CA7 PLA2G1B CA5A CA13 MAOA |
| 1.98E-07 | 8 | 95 | 20.2069806 | Endocrine resistance | MMP2 MMP9 IGF1R AKT1 PIK3R1 EGFR PTK2 SRC |
| 3.94E-06 | 7 | 108 | 15.5528265 | Insulin resistance | NR1H3 GSK3B PYGL AKT1 PIK3R1 INSR RPS6KA3 |
| 4.89E-06 | 6 | 70 | 20.5678196 | Central carbon metabolism in cancer | HIF1 A MET FLT3 AKT1 PIK3R1 EGFR |
| 2.28E-05 | 8 | 214 | 8.97038859 | Lipid and atherosclerosis | CAMK2B GSK3B MMP9 AKT1 PIK3R1 MMP3 PTK2 SRC |
| 2.52E-05 | 5 | 56 | 21.424812 | Regulation of lipolysis in adipocytes | PTGS2 AKT1 PIK3R1 ADORA1 INSR |
| 0.0001554 | 8 | 294 | 6.52946652 | MAPK signaling pathway | MET FLT 3 KDR IGF1R AKT1 EGFR INSR RPS6KA3 |
| 0.00042661 | 5 | 112 | 10.712406 | TNF signaling pathway | PTGS 2 MMP 9 AKT1 PIK3R1 MMP3 |
| 0.0008788 | 5 | 137 | 8.7575874 | Insulin signaling pathway | GSK3 B PYGL AKT1 PIK3R1 INSR |
| 0.00134156 | 5 | 155 | 7.74057725 | Non-alcoholic fatty liver disease | NR1H 3 GSK 3B AKT1 PIK3R1 INSR |
| 0.0024404 | 3 | 47 | 15.3164614 | Carbohydrate digestion and absorption | SLC5A1 AKT1 PIK3R1 |
| 0.00373995 | 4 | 120 | 7.99859649 | AMPK signaling pathway | IGF1R AKT1 PIK3R1 INSR |
| 0.01950816 | 3 | 107 | 6.72779144 | Glucagon signaling pathway | CAMK2B PYGL AKT1 |
| 0.02834824 | 2 | 46 | 10.432952 | Type II diabetes mellitus | PIK3R1 INSR |
| 0.02929805 | 2 | 47 | 10.2109742 | Pyruvate metabolism | ALDH2 GLO1 |
| Enrichment FDR | nGenes | Pathway Genes | Fold Enrichment | Pathway | Genes |
|---|---|---|---|---|---|
| 4.83E-18 | 10 | 17 | 138.241358 | Nitrogen metabolism | CA2 CA9 CA14 CA6 CA1 CA3 CA4 CA7 CA5A CA13 |
| 3.54E-08 | 7 | 51 | 32.256317 | Ovarian steroidogenesis | HSD17B2 HSD17B1 CYP19A1 CYP1B1 IGF1R INSR AKR1C3 |
| 5.91E-09 | 8 | 61 | 30.8210242 | Steroid hormone biosynthesis | HSD17B2 HSD17B1 CYP19A1 CYP1B1 AKR1C2 AKR1C1 AKR1C3 AKR1C4 |
| 1.77E-09 | 9 | 79 | 26.7733264 | EGFR tyrosine kinase inhibitor resistance | GSK3B MET KDR IGF1R AKT1 PIK3R1 EGFR AXL SRC |
| 6.38E-09 | 9 | 95 | 22.2641346 | Endocrine resistance | MMP2 MMP9 ESR2 IGF1R AKT1 PIK3R1 EGFR PTK2 SRC |
| 9.14E-09 | 9 | 100 | 21.1509278 | Progesterone-mediated oocyte maturation | CDK2 CCNB1 IGF1R AKT1 PIK3R1 CCNB3 CCNB2 PLK1 CDK1 |
| 9.27E-07 | 7 | 84 | 19.5841924 | ErbB signaling pathway | CAMK2B GSK3B AKT1 PIK3R1 EGFR PTK2 SRC |
| 1.38E-07 | 8 | 97 | 19.3822935 | prostate cancer | GSK3B MMP9 CDK2 IGF1R AKT1 PIK3R1 EGFR MMP3 |
| 6.03E-09 | 10 | 131 | 17.9397183 | FoxO signaling pathway | CDK2 CCNB1 IGF1R AKT1 PIK3R1 EGFR CCNB3 CCNB2 PLK1 INSR |
| 4.57E-11 | 14 | 223 | 14.7540104 | Chemical carcinogenesis | NOX4 MET AHR AKR1A1 CYP1B1 AKT1 PIK3R1 EGFR AKR1C2 PTK2 AKR1C1 AKR1C3 SRC AKR1C4 |
| 1.98E-07 | 9 | 148 | 14.2911675 | Phospholipase D signaling pathway | PIK3CG AVPR2 AKT1 PIK3R1 EGFR CXCR1 SYK INSR F2 |
| 1.98E-08 | eleven | 202 | 12.7975911 | Proteoglycans in cancer | CAMK2B MMP2 MMP9 MET KDR IGF1R AKT1 PIK3R1 EGFR PTK2 SRC |
| 2.87E-06 | 8 | 148 | 12.70326 | Gastric cancer | GSK3B ABCB1 MET CDK2 AKT1 PIK3R1 EGFR TERT |
| 3.85E-06 | 8 | 156 | 12.0518107 | Cellular senescence | CDK6 CDK2 CCNB1 AKT1 PIK3R1 CCNB3 CCNB2 CDK1 |
| 1.98E-07 | 10 | 200 | 11.7505155 | Focal adhesion | MYLK GSK3B MET KDR IGF1R AKT1 PIK3R1 EGFR PTK2 SRC |
| 4.65E-06 | 8 | 161 | 11.6775309 | MicroRNAs in cancer | ABCB1 MMP9 CDK6 MET PIM1 CYP1B1 PIK3R1 EGFR |
| 3.35E-06 | 9 | 210 | 10.0718704 | Rap1 signaling pathway | MET KDR ADORA2A IGF1R AKT1 PIK3R1 EGFR INSR SRC |
| 1.13E-09 | fifteen | 354 | 9.95806395 | PI3K-Akt signaling pathway | GSK3B CDK6 PIK3CG MET FLT3 PKN1 CDK2 KDR IGF1R AKT1 PIK3R1 EGFR SYK PTK2 INSR |
| 4.57E-11 | 19 | 530 | 8.42489788 | Pathways in cancer | CAMK2B GSK3B MMP2 MMP9 CDK6 MET FLT3 CDK2 PIM1 ESR2 IGF1R AKT1 PIK3R1 EGFR TERT PTK2 ALK F2 DAPK1 |
| 8.98E-12 | 31 | 1527 | 4.77100169 | Metabolic pathways | CD38 CA12 TYR AKR1B1 HSD17B2 PYGL CA2 PIK3CG CA9 HSD17B1 ALOX12 AKR1A1 CA14 ARG1 GLO1 CA6 CA1 CYP19A1 |
| Enrichment FDR | nGenes | Pathway Genes | Fold Enrichment | Pathway | Genes |
|---|---|---|---|---|---|
| 2.73E-08 | 6 | 40 | 55.1516129 | Nicotine addiction | GABRG2 GRIA2 GABRB2 GABRA2 GRIA4 GRIA1 |
| 1.31E-23 | 17 | 114 | 54.82908885 | Glutamatergic synapse | GRIK5 SLC1A1 SLC1A2 GRM6 GRIA2 GRM4 GRIA4 GRM1 GRIA1 GRIK3 GRM2 GRIK2 GRM5 GRIK1 GRM8 GRM7 GRM3 |
| 0.000306466 | 3 | 22 | 50.13782991 | Terpenoid backbone biosynthesis | HMGCR FNTA FNTB |
| 0.005845035 | 2 | 17 | 43.25616698 | Nitrogen metabolism | CA9 CA1 |
| 9.26E-06 | 5 | 61 | 30.13749339 | Steroid hormone biosynthesis | HSD11B1 HSD17B3 CYP19A1 UGT2B7 HSD11B2 |
| 8.88E-07 | 6 | 75 | 29.41419355 | PPAR signaling pathway | CPT1A PPARD FABP3 FABP5 FABP4 PPARA |
| 0.002418552 | 3 | 49 | 22.51086241 | Cocaine addiction | GRIA2 GRM2 GRM3 |
| 7.85E-09 | 9 | 148 | 22.35876199 | Phospholipase D signaling pathway | GRM6 PTGFR GRM4 GRM1 GRM2 GRM5 GRM8 GRM7 GRM3 |
| 2.25E-21 | twenty-one | 350 | 22.06064516 | Neuroactive ligand-receptor interaction | GRIK5 GRM6 GABRG2 GRIA2 PTGFR GRM4 PTGER2 GABRB2 GABRA2 GRIA4 GRM1 GRIA1 GRIK3 GRM2 GRIK2 GRM5 GRIK1 GRM8 GRM7 GRM3 ADORA3 |
| 0.000324344 | 4 | 67 | 21.95089071 | Long-term potentiation | GRIA2 GRM1 GRIA1 GRM5 |
| 1.27E-07 | 8 | 148 | 19.8744551 | Retrograde endocannabinoid signaling | GABRG2 GRIA2 GABRB2 GABRA2 GRIA4 GRM1 GRIA1 GRM5 |
| 0.004067259 | 3 | 60 | 18.38387097 | Long-term depression | GRIA2 GRM1 GRIA1 |
| 0.005712018 | 3 | 69 | 15.98597475 | Amphetamine addiction | GRIA2 GRIA4 GRIA1 |
| 0.008700522 | 3 | 85 | 12.97685009 | Taste transduction | GRM4 GABRA2 GRM1 |
| 0.009404411 | 3 | 89 | 12.39362088 | GABAergic synapse | GABRG2 GABRB2 GABRA2 |
| 0.009520584 | 3 | 91 | 12.12123361 | Morphine addiction | GABRG2 GABRB2 GABRA2 |
| 0.001753072 | 5 | 197 | 9.331914197 | Chemical carcinogenesis | VDR CDC25A AR UGT2B7 PPARA |
| 0.002418552 | 5 | 219 | 8.394461629 | CAMP signaling pathway | GRIA2 PTGER2 GRIA4 GRIA1 PPARA |
| 0.008334 | 5 | 306 | 6.00780097 | Huntington’s disease | SLC1A2 GRIA2 GRIA4 GRIA1 GRM5 |
| 0.000129008 | fifteen | 1527 | 3.611762469* | Metabolic pathways | FOLH1 HAO1 CA9 HMGCR HSD11B1 HSD17B3 ACLY CA1 CYP19A1 PGD PTGES G6PD UGT2B7 HSD11B2 AKR1B10 |
| Gene symbol | Protein name | Protein-Function |
|---|---|---|
| AKT1 | RAC-alpha serine/threonine-protein kinase | Regulates many processes including metabolism, proliferation, cell survival, growth, and angiogenesis. |
| PTK2 | Focal adhesion Kinase 1 | Related to the increase in glucose uptake and glycogen synthesis in insulin-sensitive tissues. |
| IL2 | Interleukin-2 | Required for T-cell proliferation and other cells of the immune system |
| PIK3R1 | Phosphoinositide-3-kinase regulatory subunit alpha/beta/delta | Necessary for the insulin-stimulated increase in glucose uptake and glycogen synthesis |
| SYK | Spleen-associated tyrosine kinase | Regulates biological processes including immunity, cell adhesion, vascular development, and others. |
| PTGS2 | Prostaglandin G/H synthase 2 | Plays a role in the production of inflammatory prostaglandins |
| MMP9 | Matrix metalloproteinase-9 | Key in local proteolysis of the extracellular matrix and leukocyte migration |
| HIF1A | Hypoxia-inducible factor 1-alpha | Master transcriptional regulation in response to hypoxia |
| MMP2 | Matrix metalloproteinase-2 (gelatinase a) | Involved in angiogenesis, tissue repair, tumor invasion, inflammation, and atherosclerotic plaque rupture |
| KDR | Vascular endothelial growth factor receptor 2 | Essential in the regulation of angiogenesis, promotes the proliferation, survival, and migration of endothelial cells |
| MET | Hepatocyte growth factor receptor | Regulates processes like proliferation, scattering, morphogenesis, and survival |
| HGF | Hepatocyte growth factor | Growth factor for a broad spectrum of tissues and cell types |
| EGFR | Epidermal growth factor receptor | Converts extracellular cues into appropriate cellular responses |
| IGF1R | Insulin-like growth factor 1 receptor | Involved in cell growth and survival control |
| CA9 | Carbonic anhydrase 9 | Involved in pH regulation |
| BLNK | B-cell linker protein | Important for the activation of NF-kappa-B and NFAT |
| Gene symbol | Protein name | Protein-function |
|---|---|---|
| ABCB1 | ATP-dependent translocase | Translocates drugs and phospholipids across the membrane. |
| AHR | Aryl hydrocarbon receptor | Ligand-activated transcriptional activator |
| AKR1A1 | Aldo-keto reductase family 1 member A | Displays enzymatic activity towards endogenous metabolites such as aromatic and aliphatic aldehydes, ketones, monosaccharides and bile acids, with a preference for negatively charged substrates, such as glucuronate and succinic semialdehyde. |
| AKR1B1 | Aldo-keto reductase family 1 member B1 | Displays enzymatic activity towards endogenous metabolites such as aromatic and aliphatic aldehydes, ketones, monosaccharides and bile acids, with a preference for negatively charged substrates, such as glucuronate and succinic semialdehyde. |
| AKR1B10 | Aldo-keto reductase family 1 member B10 | Catalyzes the NADPH-dependent reduction of a wide variety of carbonyl-containing compounds to their corresponding alcohols. |
| AKR1C1 | Aldo-keto reductase family 1 member C1 | Converts progesterone to its inactive form, 20-alpha-dihydroxyprogesterone (20-alpha-OHP). In the liver and intestine, may have a role in the transport of bile |
| AKR1C2 | Aldo-keto reductase family 1 member C2 | Works in concert with the 5-alpha/5-beta-steroid reductases to convert steroid hormones into the 3-alpha/5-alpha and 3-alpha/5-beta-tetrahydrosteroids. |
| AKR1C3 | Aldo-keto reductase family 1 member C3 | ; Catalyzes the conversion of aldehydes and ketones to alcohols. Catalyzes the reduction of prostaglandin (PG) D2, PGH2 and phenanthrenequinone (PQ) and the oxidation of 9-alpha,11-beta-PGF2 to PGD2. |
| AKR1C4 | Aldo-keto reductase family 1 member C4 | ;Catalyzes the transformation of the potent androgen dihydrotestosterone (DHT) into the less active form, 5-alpha-androstan- 3-alpha,17-beta-diol (3-alpha-diol). |
| AKT1 | RAC-alpha serine/threonine-protein kinase | Regulate many processes including metabolism, proliferation, cell survival, growth and angiogenesis. This is mediated through serine and/or threonine phosphorylation of a range of downstream substrates. |
| ALK | ALK tyrosine kinase receptor | Important role in the genesis and differentiation of the nervous system. Transduces signals from ligands at the cell surface, through specific activation of the mitogen-activated protein kinase (MAPK) pathway. |
| ALOX12 | Arachidonate 12-lipoxygenase 12S-type | Mainly converts arachidonic acid to (12S)-hydroperoxyeicosatetraenoic acid/(12S)-HPETE but can also metabolize linoleic acid. In contrast does not react towards methyl esters of linoleic and arachidonic acids (By similarity). |
| ALOX15 | Arachidonate 15-lipoxygenase | ; Non-heme iron-containing dioxygenase that catalyzes the stereo-specific peroxidation of free and esterified polyunsaturated fatty acids generating a spectrum of bioactive lipid mediators. |
| AXL | Tyrosine-protein kinase UFO receptor | Receptor tyrosine kinase that transduces signals from the extracellular matrix into the cytoplasm by binding growth factor GAS6 and which is thus regulating many physiological processes including cell survival, cell proliferation, migration and differentiation. |
| CA1 | Carbonic anhydrase 1 | Reversible hydration of carbon dioxide |
| CA12 | Carbonic anhydrase 12 | Reversible hydration of carbon dioxide |
| CA13 | Carbonic anhydrase 13 | Reversible hydration of carbon dioxide |
| CA14 | Carbonic anhydrase 14 | Reversible hydration of carbon dioxide |
| CA2 | Carbonic anhydrase 2 | Essential for bone resorption and osteoclast differentiation. Reversible hydration of carbon dioxide. |
| CA3 | Carbonic anhydrase 3 | Reversible hydration of carbon dioxide |
| CA4 | Carbonic anhydrase 4 | Reversible hydration of carbon dioxide |
| CA7 | Carbonic anhydrase 7 | Reversible hydration of carbon dioxide |
| CA9 | Carbonic anhydrase 9 | Reversible hydration of carbon dioxide |
| CAMK2B | Calcium/calmodulin-dependent protein kinase type II subunit beta | Calcium/calmodulin-dependent protein kinase that functions autonomously after Ca(2+)/calmodulin-binding and autophosphorylation, ansport in skeletal muscle. |
| CCNB1 | G2/mitotic-specific cyclin-B1 | Essential for the control of the cell cycle at the G2/M (mitosis) transition |
| CCNB2 | G2/mitotic-specific cyclin-B2 | Essential for the control of the cell cycle at the G2/M (mitosis) transition |
| CCNB3 | G2/mitotic-specific cyclin-B3 | Plays an essential role in the control of the cell cycle, notably via their destruction during cell division. Its tissue specificity suggest that it may be required during early meiotic prophase I. |
| CDK1 | Cyclin dependent kinase 1 | Cyclin-dependent kinase 1; Plays a key role in the control of the eukaryotic cell cycle by modulating the centrosome cycle as well as mitotic onset; promotes G2-M transition, and regulates G1 progress and G1-S transition via association with multiple interphase cyclins. Required in higher cells for entry into S-phase and mitosis. Q |
| CDK2 | Cyclin dependent kinase 2 | Cyclin-dependent kinase 2; Serine/threonine-protein kinase involved in the control of the cell cycle; essential for meiosis, but dispensable for mitosis. |
| CDK6 | Cyclin dependent kinase 6 | Cyclin-dependent kinase 6; Serine/threonine-protein kinase involved in the control of the cell cycle and differentiation; promotes G1/S transition. |
| CXCR1 | CXC chemokine receptor type 1; | Receptor to interleukin-8, which is a powerful neutrophils chemotactic factor. |
| CYP19A1 | Aromatase | A cytochrome P450 monooxygenase that catalyzes the conversion of C19 androgens, androst-4-ene-3,17-dione (androstenedione) and testosterone to the C18 estrogens, estrone and estradiol, respectively. |
| CYP1B1 | Cytochrome P450 1B1 | A cytochrome P450 monooxygenase involved in the metabolism of various endogenous substrates, including fatty acids, steroid hormones and vitamins. |
| DAPK1 | Death-associated protein kinase 1 | Calcium/calmodulin-dependent serine/threonine kinase involved in multiple cellular signaling pathways that trigger cell survival, apoptosis, and autophagy. |
| EGFR | Epidermal growth factor receptor | Receptor tyrosine kinase binding ligands of the EGF family and activating several signaling cascades to convert extracellular cues into appropriate cellular responses. |
| ESR2 | Estrogen receptor beta | Nuclear hormone receptor. Binds estrogens with an affinity similar to that of ESR1, and activates expression of reporter genes containing estrogen response elements (ERE) in an estrogen-dependent manner. |
| FLT3 | Receptor-type tyrosine-protein kinase FLT3 | Tyrosine-protein kinase that acts as cell-surface receptor for the cytokine FLT3LG and regulates differentiation, proliferation and survival of hematopoietic progenitor cells and of dendritic cells. |
| GLO1 | Lactoylglutathione lyase | Involved in the regulation of TNF-induced transcriptional activity of NF-kappa-B. |
| GSK3B | Glycogen synthase kinase-3 beta | Constitutively active protein kinase that acts as a negative regulator in the hormonal control of glucose homeostasis, |
| HSD17B1 | Estradiol 17-beta-dehydrogenase 1 | Estradiol 17-beta-dehydrogenase 1; Favors the reduction of estrogens and androgens. |
| HSD17B2 | Estradiol 17-beta-dehydrogenase 2 | Estradiol 17-beta-dehydrogenase 2; Capable of catalyzing the interconversion of testosterone and androstenedione, as well as estradiol and estrone. |
| IGF1R | Insulin-like growth factor 1 receptor alpha chain | Receptor tyrosine kinase which mediates actions of insulin-like growth factor 1 (IGF1). Binds IGF1 with high affinity and IGF2 and insulin (INS) with a lower affinity. |
| INSR | Insulin receptor subunit alpha | Receptor tyrosine kinase which mediates the pleiotropic actions of insulin. Binding of insulin leads to phosphorylation of several intracellular substrates, including, insulin receptor substrates (IRS1, 2, 3, 4), SHC, GAB1, CBL and other signaling intermediates. |
| KDR | Vascular endothelial growth factor receptor 2 | Tyrosine-protein kinase that acts as a cell-surface receptor for VEGFA, VEGFC and VEGFD. Plays an essential role in the regulation of angiogenesis, vascular development, vascular permeability, and embryonic hematopoiesis. |
| MAOA | Amine oxidase [flavin-containing] A | Catalyzes the oxidative deamination of biogenic and xenobiotic amines and has important functions in the metabolism of neuroactive and vasoactive amines in the central nervous system and peripheral tissues. |
| MET | Hepatocyte growth factor receptor | Receptor tyrosine kinase that transduces signals from the extracellular matrix into the cytoplasm by binding to hepatocyte growth factor/HGF ligand. |
| MMP2 | 72 kDa type IV collagenase | Ubiquitinous metalloproteinase that is involved in diverse functions such as remodeling of the vasculature, angiogenesis, tissue repair, tumor invasion, inflammation, and atherosclerotic plaque rupture. |
| MMP3 | Stromelysin-1 | Can degrade fibronectin, laminin, gelatins of type I, III, IV, and V; collagens III, IV, X, and IX, and cartilage proteoglycans. Activates procollagenase |
| MMP9 | 67 kDa matrix metalloproteinase-9 | May play an essential role in local proteolysis of the extracellular matrix and in leukocyte migration. Could play a role in bone osteoclastic resorption. |
| NOX4 | NADPH oxidase 4 | Constitutive NADPH oxidase which generates superoxide intracellularly upon formation of a complex with CYBA/p22phox. Regulates signaling cascades probably through phosphatases inhibition. |
| PIK3CG | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform; | Phosphoinositide-3-kinase (PI3K) that phosphorylates PtdIns(4,5)P2 (Phosphatidylinositol 4,5-bisphosphate) to generate phosphatidylinositol 3,4,5-trisphosphate (PIP3). |
| PIK3R1 | Phosphatidylinositol 3-kinase regulatory subunit alpha | Necessary for the insulin-stimulated increase in glucose uptake and glycogen synthesis in insulin-sensitive tissues. |
| PLA2G1B | Phospholipase A2 | PA2 catalyzes the calcium-dependent hydrolysis of the 2-acyl groups in 3-sn-phosphoglycerides, this releases glycerophospholipids and arachidonic acid that serve as the precursors of signal molecules. |
| PLK1 | Serine/threonine-protein kinase PLK1 | Serine/threonine-protein kinase that performs several important functions throughout M phase of the cell cycle, including the regulation of centrosome maturation and spindle assembly, the removal of cohesins from chromosome arms, the inactivation of anaphase-promoting complex/cyclosome (APC/C ) inhibitors, and the regulation of mitotic exit and cytokinesis. |
| PTK2 | Focal adhesion kinase 1 | Non-receptor protein-tyrosine kinase that plays an essential role in regulating cell migration, adhesion, spreading, reorganization of the actin cytoskeleton, formation and disassembly of focal adhesions and cell protrusions, cell cycle progression, cell proliferation and apoptosis. |
| PYGL | Glycogen phosphorylase, liver form | Phosphorylase is an important allosteric enzyme in carbohydrate metabolism. |
| CRS | Proto-oncogene tyrosine-protein kinase Src | Non-receptor protein tyrosine kinase which is activated following engagement of many different classes of cellular receptors including immune response receptors, integrins and other adhesion receptors, receptor protein tyrosine kinases, G protein-coupled receptors as well as cytokine receptors. |
| SYK | Tyrosine-protein kinase SYK | Non-receptor tyrosine kinase which mediates signal transduction downstream of a variety of transmembrane receptors including classical immunoreceptors like the B-cell receptor (BCR). Regulates several biological processes including innate and adaptive immunity, cell adhesion, osteoclast maturation, platelet activation and vascular development. |
| TERT | Telomerase reverse transcriptase | Telomerase is a ribonucleoprotein enzyme essential for the replication of chromosome termini in most eukaryotes. Active in progenitor and cancer cells. Inactive, or very low activity, in normal somatic cells. |
| TYR | Tyrosinase | This is a copper-containing oxidase that functions in the formation of pigments such as melanins and other polyphenolic compounds. Catalyzes the initial and rate limiting step in the cascade of reactions leading to melanin production from tyrosine. Yo |
| Gene symbol | Protein | Protein-Function |
|---|---|---|
| CPT1A | Carnitine O-palmitoyltransferase 1, liver isoform | Catalyzes the transfer of the acyl group of long-chain fatty acid-CoA conjugates onto carnitine, an essential step for the mitochondrial uptake of long-chain fatty acids and their subsequent beta-oxidation in the mitochondrion. |
| CYP19A1 | Aromatase | A cytochrome P450 monooxygenase that catalyzes the conversion of C19 androgens, androst-4-ene-3,17-dione (androstenedione) and testosterone to the C18 estrogens, estrone and estradiol, respectively. |
| CYP27B1 | 25-hydroxyvitamin D-1 alpha hydroxylase, mitochondrial | A cytochrome P450 monooxygenase involved in vitamin D metabolism and in calcium and phosphorus homeostasis. |
| FABP3 | Fatty acid-binding protein, heart | FABP are thought to play a role in the intracellular transport of long-chain fatty acids and their acyl-CoA esters |
| FABP4 | Fatty acid-binding protein, adipocyte | Lipid transport protein in adipocytes. Binds both long chain fatty acids and retinoic acid. Delivers long-chain fatty acids and retinoic acid to their cognate receptors in the nucleus. Belongs to the calycin superfamily. Fatty-acid binding protein (FABP) family. (132 years) |
| FABP5 | Fatty acid-binding protein 5 | Intracellular carrier for long-chain fatty acids and related active lipids, such as the endocannabinoid, that regulates the metabolism and actions of the ligands they bind. In addition to the cytosolic transport, it selectively delivers specific fatty acids from the cytosol to the nucleus, wherein they activate nuclear receptors. |
| GABRA2 | Gamma-aminobutyric acid receptor subunit alpha-2 | Ligand-gated chloride channel which is a component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the brain (By similarity). Plays an important role in the formation of functional inhibitory GABAergic synapses in addition to mediating synaptic inhibition as a GABA-gated ion channel (By similarity). |
| GABRB2 | Gamma-aminobutyric acid receptor subunit beta-2 | Ligand-gated chloride channel which is a component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the brain. Plays an important role in the formation of functional inhibitory GABAergic synapses in addition to mediating synaptic inhibition as a GABA-gated ion channel. |
| GABRG2 | Gamma-aminobutyric acid receptor subunit gamma-2 | Ligand-gated chloride channel which is a component of the heteropentameric receptor for GABA, the major inhibitory neurotransmitter in the brain. Plays an important role in the formation of functional inhibitory GABAergic synapses in addition to mediating synaptic inhibition as a GABA-gated ion channel. |
| GRIA1 | Glutamate receptor 1 | Ionotropic glutamate receptor. L-glutamate acts as an excitatory neurotransmitter at many synapses in the central nervous system. |
| GRIA2 | Glutamate receptor 2 | Receptor for glutamate that functions as ligand-gated ion channel in the central nervous system and plays an important role in excitatory synaptic transmission. L-glutamate acts as an excitatory neurotransmitter at many synapses in the central nervous system. |
| GRIA4 | Glutamate receptor 4 | Receptor for glutamate that functions as ligand-gated ion channel in the central nervous system and plays an important role in excitatory synaptic transmission. L-glutamate acts as an excitatory neurotransmitter at many synapses in the central nervous system. |
| GRIK1 | Glutamate ionotropic receptor, kainate 1 | Ionotropic glutamate receptor. L-glutamate acts as an excitatory neurotransmitter at many synapses in the central nervous system. b |
| GRIK2 | Glutamate ionotropic receptor, kainate 2 | Ionotropic glutamate receptor. L-glutamate acts as an excitatory neurotransmitter at many synapses in the central nervous system. |
| GRIK3 | Glutamate ionotropic receptor, kainate 3 | Receptor for glutamate that functions as ligand-gated ion channel in the central nervous system and plays an important role in excitatory synaptic transmission. |
| GRIK5 | Ionotropic receptor glutamate, kainate 5 | Receptor for glutamate. L-glutamate acts as an excitatory neurotransmitter at many synapses in the central nervous system. |
| GRM1 | Metabotropic glutamate receptor 1 | G-protein coupled receptor for glutamate. |
| GRM2 | Metabotropic glutamate receptor 2 | G-protein coupled receptor for glutamate. |
| GRM3 | Metabotropic glutamate receptor 3 | G-protein coupled receptor for glutamate. |
| GRM4 | Metabotropic glutamate receptor 4 | G-protein coupled receptor for glutamate. |
| GRM5 | Metabotropic glutamate receptor 5 | G-protein coupled receptor for glutamate. |
| GRM6 | Metabotropic glutamate receptor 6 | G-protein coupled receptor for glutamate. |
| GRM7 | Metabotropic glutamate receptor 7 | G-protein coupled receptor for glutamate. |
| GRM8 | Metabotropic glutamate receptor 8 | G-protein coupled receptor for glutamate. |
| HMGCR | 3-hydroxy-3-methylglutaryl-coenzyme A reductase | Transmembrane glycoprotein that is the rate-limiting enzyme in cholesterol biosynthesis as well as in the biosynthesis of nonsterol isoprenoids that are essential for normal cell function including ubiquinone and geranylgeranyl proteins. |
| HSD11B1 | Corticosteroid 11-beta-dehydrogenase isozyme 1 | Catalyzes reversibly the conversion of cortisol to the inactive metabolite cortisone. Catalyzes reversibly the conversion of 7-ketocholesterol to 7-beta-hydroxycholesterol. |
| HSD11B2 | Corticosteroid 11-beta-dehydrogenase isozyme 2 | Catalyzes the conversion of cortisol to the inactive metabolite cortisone. |
| HSD17B3 | Testosterone 17-beta-dehydrogenase 3 | Favors the reduction of androstenedione to testosterone. |
| PPARA | Peroxisome proliferator-activated receptor alpha | Ligand-activated transcription factor. Key regulator of lipid metabolism. Activated by the endogenous ligand 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphocholine (16:0/18:1-GPC). Activated by oleylethanolamide, a naturally occurring lipid that regulates satiety. |
| PPARD | Peroxisome proliferator-activated receptor delta | Ligand-activated transcription factor. Receptor that binds peroxisome proliferators such as hypolipidemic drugs and fatty acids. |
| SLC1A1 | Excitatory amino acid transporter 3 | Sodium-dependent, high-affinity amino acid transporter that mediates the uptake of L-glutamate and also L-aspartate and D-aspartate. |
| SLC1A2 | Excitatory amino acid transporter 2 | Sodium-dependent, high-affinity amino acid transporter that mediates the uptake of L-glutamate and also L-aspartate and D-aspartate. |
| HSD17B3 | Testosterone 17-beta-dehydrogenase 3 | Favors the reduction of androstenedione to testosterone. |
| PPARA | Peroxisome proliferator-activated receptor alpha | Ligand-activated transcription factor. Key regulator of lipid metabolism. |
| PPARD | Peroxisome proliferator-activated receptor delta | Ligand-activated transcription factor. Receptor that binds peroxisome proliferators such as hypolipidemic drugs and fatty acids. Has a preference for poly-unsaturated fatty acids, such as gamma-linoleic acid and eicosapentaenoic acid. |
| SLC1A1 | Excitatory amino acid transporter 3 | Sodium-dependent, high-affinity amino acid transporter that mediates the uptake of L-glutamate and also L-aspartate and D-aspartate. |
| SLC1A2 | Excitatory amino acid transporter 2 | Sodium-dependent, high-affinity amino acid transporter that mediates the uptake of L-glutamate and also L-aspartate and D-aspartate. |
| Genes | Results at the gene expression level | Results at the protein level | Results of pathway impact |
|---|---|---|---|
| MET | Deeba N. 2008 (6): Suppress the phosphorylation of the protein | ||
| IGF1R | Teller et al, 2009 (7): Inhibition of its kinase activity | ||
| EGFR | Harish Chandra Pal(8), et al, 2013: Reduction in the expression of the gene | Fridrich D, Et all, 2008 (4): Suppress phosphorylation of the protein. | Harish Chandra Pal, et al, 2013 (8): Inhibition of the PI3K-Akt pathway |
| Genes | Results at the gene expression level | Results at the protein level | Results of pathway impact |
|---|---|---|---|
| AHR | Mohammadi-Bardbori (9), 2012: reduces protein activation | Ciolino H, 1999(10): Generates changes in the pathway for Chemical carcinogenesis. Hamidullah , 2015: Changes in the Akt- mTor pathway |
|
| AK1RC3 | Skarydová et al, 2014(11): Inhibition of the activity of the enzyme | ||
| CDK2 | Chou, et al, 2010 (12): Decreased levels of the protein | ||
| CDK6 | Mohd et al, 2020 (13): Decreases the expression of the gene. | Teler et al, 2009 (4): Inhibition of its kinase activity | |
| CYP1B1 | Mense S, 2008 (14): Increase in RNA transcription | Mense S, 2008 (14): Increase in levels of the protein in epithelial cells | |
| EGFR | Yiqi et al, 2022 (15): Decreases the phosphorylation of the protein/receptor Huang Y, 1999: Decreses the phosphorylation of the protein/receptor |
||
| GSK3B | Chen K, et al, 2018(16): Decrease in the phosphorylation, promotes the activity of the protein | ||
| IGF1R | Wei-Jen, 2021(17): Reduces the phosphorylation of the protein | ||
| MAOA | Yauhen, 2014(18): The activity of the protein is decreased, it is probable that quercetin inhibits the protein, there is no decrease in the levels of protein | ||
| MET | Hui et al, 2015 (19): Reduction on the transcription of the gene | Hui et al, 2015 (19) Reduced phosphorylation of the protein. | |
| PIK3R1 | Haitao et al, 2021(20): Inhibits the function of the protein (is this protein level |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





