Preprint
Article
Urinalysis Results and Resistance to Hospital Treatment with Injectable Antimalarials in Angola
Altmetrics
Downloads
124
Views
52
Comments
0
Submitted:
01 December 2023
Posted:
04 December 2023
You are already at the latest version
Alerts
Abstract
Background: Resistance to antimalarial drugs often used in emerging countries, including combination therapies, has forced scientists to search for and develop drugs with novel mechanisms of action, especially resistance to Plasmodium falciparum and Plasmodium vivax, which are highly prevalent in Southeast Asia, Africa, and South America.
Objective: evaluate whether there is a relationship between urinalysis and resistance to in-hospital treatment of malaria in Angola.
Methodology: This was a cross-sectional, prospective study with a quantitative approach.
Results: of the 214 patients, the resistance rate was 24.1%, men (53.6%), between 21 and 40 years old (72.7%), employees (46.4%), from peri-urban regions (77.7%), treated with artemether (90.9), with high parasitemia (57.7%) and after 5 days of treatment, remained hospitalized (61.4%). Was a significant relationship between resistance in unemployed individuals [OR: 0.03 (95% CI: 0.01-0.29), p =0.003] and high levels of parasitemia [OR: 1.09 (95% CI: 1.09-3.95), p=0.040], remained hospitalized for more than 5 days [OR: 5.28 (95% CI: 0.65-43.1), p=0.121] and death [OR: 2.59 (95% CI: 0.32-20.9), p=0.371] when compared with other subgroups. Was a significant relationship between resistance to clear urine [OR: 5.55 (95% CI: 0.72-42.7), p =0.016], few urinary crystals [OR: 11.3 (95% CI: 5.07-25.3), p <0.001] and who presented some microorganisms that were not bacteria or fungi [OR: 3.02 (95% CI: 1 .32-6.90), p=0.009].
Conclusion: urine results, especially the appearance of cloudy urine, the presence of few crystals, and the presence of other microorganisms that are not bacteria or fungi, may be clear signs of resistance to hospital treatment with injectable antimalarials.
Keywords:
Subject: Medicine and Pharmacology - Clinical Medicine
Background
According to the Ministry of Health of Angola, in 2022, 9,211,346 cases of malaria were reported (41,886 more cases than in 2021), of which 30% were under 15 years of age, and 12,480 people died as a result of the disease. disease, not to mention the unreported cases, demonstrating how malaria is still a serious health problem in Angola and around the world [1].
Resistance to antimalarial drugs often used in emerging countries, including combination therapies, has forced scientists to search and develop drugs with novel mechanisms of action, especially with regards to resistance to Plasmodium falciparum and Plasmodium vivax, which are highly prevalent in Southeast Asia, Africa, and South America[2]. African multicentric study that stated resistance to Plasmodium falciparum at medium level increased in Tanzania (64.0%), Sudan(55.4%), Mozambique (45.7%), Kenya (29.7%) and Malawi (8.7%) between 2000 to 2010 and suffered a slight decline in Sudan (76.0%), Kenya (65.7%) and Tanzania (17.4%), from 2010 to 2020. In the same study, in Central Africa, levels increased in Equatorial Guinea (28.9%) and Congo (85.3%) from 2000 to 2020[3].
Our findings suggested that sociodemographic, clinical, and pharmacological procedures could be related to antimalarial resistance among patients with malaria in Angola, however, the use of adjuvant drugs such as dipyrone, metoclopramide, ciprofloxacin and diazepam during malaria treatment could reduce the chances of resistance to in-hospital treatment of malaria[4].
Studies show that early detection of changes in urine can allow early screening of different organ damage, especially liver or kidney damage, as dark urine has been associated with conditions of hemoglobinuria, myoglobinuria, hematuria, and bilirubinuria, however, other changes in the urine of malaria patients have been little explored as danger signs [4,6,7,8,9,10,11].
Malaria has a significant effect on urine chemical composition with bilirubin positively correlated with parasite density, dipstick urinalysis, and light microscopy in malaria-endemic areas with limited resources that can serve to accurately diagnose falciparum malaria infection[10]. Studies in Nigeria and Sudan found that urine abnormalities could report parasite density using the semi-quantitative system or the extent to which the degree of malaria parasitemia affects urinary composition in children from Ghana, where endemicity, mortality, and morbidity were associated with urine results, particularly in children and pregnant women[11,12].
The emergence of parasites resistant to antimalarials already posed a threat to efforts to control malaria, with the emergence of the COVID-19 pandemic, most African governments, including the government of Angola, reduced investments in the fight against malaria and other diseases, to pay more attention to the pandemic, which in a way, contributed to the increase in the number of malaria cases and deaths in Africa.
Although some studies have associated urinalysis with morbidity, mortality, and diagnosis of malaria, there is very little scientific evidence that demonstrates that urinalysis results can be markers of resistance to the treatment of malaria malaria at the hospital level, this study aimed to evaluate whether there is relationship between urinalysis and resistance to in-hospital treatment of malaria in Angola.
Methodology
Study design and ethical statement
This was a cross-sectional, prospective study with a quantitative approach. Before its execution, the study was submitted and approved by the Ethics Committee for Research on Human Beings of the Instituto de Ciências da Saúde (118/GD/ICISA/UAN/2021) and approved by the clinical management of Hospital Josina Machel - Maria Pia in Luanda (36/DPC/HJM/2023). Only patients who agreed and signed the informed consent form after being informed of the nature and objectives of the study were included.
Patient recruitment
Patients were recruited between December 2018 and January 2020, a sample of 214 of the 410 patients hospitalized for malaria at the Hospital Josina Machel - Maria Pia in that period was obtained, where we obtained a confidence index of 95% with a 5% margin of error. Sociodemographic information was collected through an open and closed questionnaire and the age of the patients was between 12 and 66 years old, where only patients who were hospitalized for more than 5 days could be included. All patients with a history of hypertensive disease, diabetes, chronic kidney disease, cerebral malaria, other chronic diseases, urinary infections, low immunity, and other pathologies that contribute to resistance to treatment were excluded to avoid bias in data analysis.
Laboratory procedure
The diagnosis of malaria was carried out using rapid malaria antigen tests (SD-Bioline Malaria AG Pf/PAN) and confirmed with a microscopic technique for direct visualization of the parasite by thickening the peripheral blood stained with Giemsa[13]. The parasitemic condition was classified as follows: patients who presented parasitemia less than or equal to 1,000 p/mm3 were classified as moderate parasitemia; patients who presented parasitemia above 1,000 p/mm3 were classified as high parasitemia [14,15]. The classification of resistance to malaria treatment was carried out by evaluating the parasitemia of patients within 5 days after the end of antimalarial treatment (Arthemeter or Artesunate), which can reduce parasitemia from 100,000 to 0 (p/mm3) in a maximum of 5 days of hospital treatment, as demonstrated in different studies [12,13,14]. Patients who did not have a complete reduction in parasitemia after 5 days of treatment were classified as resistant and patients who had a complete reduction in parasitemia were considered non-resistant.
Statistical analysis
Data obtained was categorized and analyzed using IBM® SPSS Statistics v25. Absolute and relative frequencies were determined. The chi-square (X2) and logistic regression tests were used to assess the relationship between categorical variables. The odds ratio (OR) and its 95% confidence intervals (CI) were calculated to assess the strength and direction of the relationship. All reported p-values are two-tailed and deemed significant when p<0.05. Pearson’s Chi-Square test is generally used to compare two categorical variables and check whether they are homogeneous with each other and allowed us to compare the phenomena we studied concerning treatment failure (with resistance/without resistance) and the Odds Ratios were used to verify the strength of association between different phenomena that we studied and the presence or absence of treatment restence.
Results
Urinary analysis and resistance treatment
The results of urinary analysis of patients with malaria about data on resistance to hospital treatment with injectable antimalarials are shown in Table 2. It was found that concerning urine color, the majority of individuals included had type 1 yellow urine(95.7%), and among individuals with type 2 yellow urine, only 9.1% were resistant to antimalarial treatment. Regarding the appearance of urine, it was found that the majority of individuals (92%) presented clear urine, however, only 5% of individuals resistant to treatment presented urine with a cloudy appearance. Regarding urinary pH, it was found that the majority of patients presented urine with a normal pH (84.9%), among individuals with increased urinary pH, only 8.8% were resistant to hospital treatment with antimalarial. Regarding urinary density, the majority of patients had high urinary density, among individuals with high urinary density, 25.2% of them were resistant to hospital treatment with the use of injectable antimalarials the number of individuals while for the unemployed, no resistance to treatment was observed. Regarding the presence of bacteria in urine, we found that the majority of patients had numerous bacteria in their urine (56.1%), however, only 18.5% of individuals resistant to antimalarial treatment had numerous bacteria in their urine. Regarding the presence of crystals in the urine, we found that the majority of patients studied (83.9%) had few urinary crystals, however, an interesting fact was that among the individuals who had many urinary crystals, 63.9% were resistant patients to antimalarial treatment. When evaluating the presence of other microorganisms that were not bacteria, the majority presented some microorganisms that were neither fungi nor bacteria, in reduced quantities, however, among the patients who presented fungi in their urine, 41.4% of them were individuals resistant to antimalarial treatment. When evaluating the presence of leukocytes in the urine, we found that the majority of patients had many urinary leukocytes (66.5%), among these individuals, only 20.6% were resistant to treatment. When evaluating urinary lymphocytes, we noticed that the majority of patients included in the study (82.5%), however, only 21.1% of patients resistant to antimalarial treatment had many urinary leukocytes. Despite all the data mentioned above, the statistical evaluation showed that there was no relationship between urinary chlorination, urinary density, the presence of bacteria, leukocytes, and lymphocytes in urine, and hospital resistance to antimalarial treatment (P<0.05), however, it showed that there is a relationship between the appearance of urine, pH, presence of crystals and the presence of other microorganisms in urine with resistance to injectable antimalarial hospital treatment (P>0.05).
In the univariate analysis it was found that although there was no significant relationship between the risk of resistance to hospital treatment and urine chlorination, individuals with urine with yellow chlorination 1, which is considered normal, had less chance of resistance [OR: 0.34 (OR: 0.34 (OR: 0.34 (OR: 0.34) 95% CI: 0.04-2.73), p=0.311], with normal pH [OR: 0.29 (95% CI: 0.08-1.02), p=0.055], with a low number of bacteria [OR: 0.63 (95% CI: 0.33-1.21), p=0.173], few urinary leukocytes [OR: 0.79 (95% CI: 0.40-1.54), p =0.468] and few urinary lymphocytes [OR: 0.77 (95% CI: 0.34-1.73), p=0.540], were less likely to be resistant to hospital treatment with injectable antimalarial when compared to their subgroups, however, individuals with low urine density [OR: 1.19 (95% CI: 0.24-5.87), p=0.825] and normal urine density [OR: 1.36 (95% CI: 0.55-3. 34), p=0.493], were more likely to present resistance to treatment than individuals who had high urinary density. On the other hand, there was a significant relationship between resistance to injectable hospital treatment with antimalarials and aspects of urine where individuals had clear urine [OR: 5.55 (95% CI: 0.72-42.7), p =0.016], which presented few urinary crystals [OR: 11.3 (95% CI: 5.07-25.3), p <0.001] and individuals who presented some microorganisms that were not fungi [OR: 3.02 (95% CI: 1 .32-6.90), p=0.009], were more likely to develop resistance to hospital treatment with injectable antimalarials, when compared to other subgroups.
The results showed that biochemical markers such as glucose, prothrombin, and Ascorbic acid were also evaluated in urine (Figure 1) and we found that the majority of patients with malaria did not present these markers, however, for some reason that requires further studies, only patients not resistant to hospital treatment with injectable antimalarials, presented glucose (n=7/167), prothrombin (8/167) and ascorbic acid (5/167).
Discussion
In the present study, it was found that the majority of patients were male and aged under 40 years, although there was no statistical relationship between gender and age and resistance to hospital treatment with injectable antimalarials (Table 1), similar data were observed in previous studies carried out by our team published in 2020, where men represented 71% of the population and the average age of patients was 21.3 years[15,16] and in 2021 and 2023, where men represented 59% of the population and the average age was 29.8 years[17,18].
In this study we found that individuals from urban and peri-urban regions and individuals treated with Arthemeter presented treatment resistance rates that exceeded 20%, although this was not statistically significant, similar data already found were observed in other studies carried out by our team previously. , where these resistance figures reach more than 24%[4,19]. a multicentric study carried out on the African continent showed that resistance to Plasmodium falciparum has been increasing, especially in countries such as Tanzania (64.0%), Sudan (55.4%), Mozambique (45.7%), Kenya (29.7%) and Malawi (8.7%), however, some countries managed to show a certain decrease in resistance rates, such as Sudan (76.0%), Kenya (65.7%) and Tanzania (17 .4%), others have increased the number of resistance cases, including Equatorial Guinea (28.9%) and Congo (85.3%)[20]. Occupation, degree of parasitemia, and clinical stages were associated with resin and hospital treatment with injectable antimalarial (p<0.05) in the present study (Table 1), similar data had already been observed in previous studies[4,19].
In the present study, we found (Table 2) that the light yellow color of urine, low or normal urine density, the presence of bacteria, the presence of few leukocytes and few lymphocytes in the urine can signal an efficient response to treatment with injectable antimalarials, because these factors appeared to be protective of hospital resistance to antimalarial treatment (P<0.05), but showed the cloudy appearance of urine, high pH, presence of few crystals and presence of other microorganisms in the urine may be signs of resistance to hospital treatment injectable with antimalarial (P>0.05). These data reinforce what was said by Kalantari and collaborators (2015) and Chai & Chua (2022), that urine, as it is an ultrafiltrate of the blood, if it contains the majority of specific plasma or serum proteins and low molecular weight, can be a suitable sample for investigating the pathological process not only of renal diseases, but also of systemic or infectious diseases [21,22].
Studies developed by some scholars have shown that drugs such as artesunate have a diuretic effect, and this can be a double-edged weapon that can modify the course of acute renal failure in malaria, as the increase in urinary loss of water and electrolytes can worsen the condition renal failure in hypovolemic patients with pyrexia and tachypnea, however, artesunate administration may also be useful in converting oliguric to non-oliguric renal failure in patients with volume overload, and there appears to be a rapid improvement in pulmonary function in a patient with respiratory distress syndrome due to the diuretic effect of artesunate[23,24]. Studies carried out by our team have shown that AKI is a common complication in patients with malaria and Angola the incidence is around 22% to 40%, and may be associated with the patients’ levels of parasitemia, in addition, it has an effect significant impact on mortality and length of hospital stay in patients with malaria, especially in patients with advanced stage kidney damage [15,17,18,25].
The present study showed the presence of biochemical markers in patients not resistant to injectable hospital treatment with antimalarials (Figure 1), perhaps this data may be associated with what was reported by Bi and collaborators (2022) found results as they realized that patients with a variant with a high level of albumin, α-hydroxybutyrate dehydrogenase and ferritin had a longer duration of parasite elimination compared to those who had a low level of these markers [26].
Although malaria is one of the most discussed topics in medical and health science literature, from different points of view, from infection, hematological, hepatic, renal, neuronal and other disorders, few studies were found evaluating patients’ urine to understand the clinical phenomena behind the disease, this appears to be one of the few studies worldwide in general and in Africa in particular to evaluate the results of urinalysis tests on resistance to hospital treatment of malaria, using injectable antimalarials.
Conclusion
Our results lead us to conclude that some sociodemographic conditions such as occupation and clinical conditions such as the degree of parasitemia and clinical outcomes can influence or be influenced by resistance to hospital treatment even when injectable antimalarial is used and that urine results, especially the appearance cloudy urine, the presence of few crystals and the presence of other microorganisms that are not bacteria or fungi, may be clear signs of resistance to hospital treatment with injectable antimalarials, however, more in-depth studies on this subject need to be carried out to obtain more data robustly.
Author Contributions
ENMS : 1. Conception and design or analysis and interpretation of data; ENMS,TPPP, and CSS: 2. Writing of the article or relevant critical review of the intellectual content; EKS, CSS and ENMS: 3. Final approval of the version to be published; ENMS: 4. Be responsible for all aspects of the work to ensure the accuracy and completeness of any part of the work.
Financing
This project was funded by the European Union(EU) and the African Union(AU) through the 2022 ARISE-PP-13 Project, which is coordinated by the African Academy of Sciences(AAS).
Availability of Data and Materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors would like to thank the patients for their participation and the management and the workers from the Josina Machel Hospital for institutional support. We are grateful for the logistic and scientific support given by the INIS and ICISA researchers.
Interest Conflicts
The authors declare that there is no conflict of interest for the publication of this article.
References
- ANGOLA. Ministério da Saúde (MINSA). 2022. Angola notificou 9,2 milhões de casos de malária em 2022, representando um ligeiro aumento de 0,4 por cento face ao ano anterior. [cited 2023 April 26]. https://www.verangola.net/va/pt/042023/Saude/35323/Angola-notifica-mais-casos-de-mal%C3%A1ria-em-2022-mas-mortalidade-diminui-10-por-cento.htm.
- Shibeshi MA, Kifle ZD, Atnafie SA. Antimalarial Drug Resistance and Novel Targets for Antimalarial Drug Discovery. Infect Drug Resist. 2020;13:4047-4060. [CrossRef]
- Amimo F, Lambert B, Magit A, Sacarlal J, Hashizume M, Shibuya K. (2020). Plasmodium falciparum resistance to sulfadoxine-pyrimethamine in Africa: a systematic analysis of national trends. 5(11), e003217. [CrossRef]
- Sacomboio ENM, S Sebastião C, Antonio JLF, Vezo ÁK, Bapolo DVS, Morais J. Factors Associated With Resistance to In-Hospital Treatment of Malaria in Angolan Patients. Infectious Diseases: Research and Treatment. January 2022. [CrossRef]
- Tobón-Castaño, ADM Cortina, AF Miranda e S. Blair Trujillo, “[Urina escura e icterícia como sinais de alerta na malária por Plasmodium falciparum na Colômbia”, Revista Cubana de Medicina Tropical, vol. 2, não. 1, pp. 28-35, 2010.
- Tobón-Castaño, C. Giraldo-Castro e S. Blair-Trujillo, “Utilidade dos sinais clínicos e parasitológicos no prognóstico da malária grave na Colômbia”, Biomedica , vol. 32, não. 2, pp. 79-94, 2012.
- AT Tobón-Castaño, JG Piñeros Jiménez, S. Blair-Trujillo e J. Carmona-Fonseca, “Apresentação clínica da malária grave por Plasmodiun falciparum. Estudo caso-controle em Tumaco e Turbo (Colômbia)”, Iatreia, vol. 19, não. 4, pp. 339–355, 2006.
- O’Donnell, DJ Weatherall, AM Taylor, JC Reeder e SJ Allen, “Lesão de células musculares, hemólise e urina escura em crianças com malária falciparum em Papua Nova Guiné”, Transações da Sociedade Real de Medicina Tropical e Higiene, volume 100, não. 9, pp. 817-825, 2006.
- EI Ugwuja e NC Ugwu, “achados anormais no exame de urina com tira reagente de pacientes ambulatoriais com malária em Abakaliki, Nigéria”, Journal of Vector Borne Diseases, vol. 48, nº. 4, pp. 205–209, 2011.
- G. Campuzano Maya e M. Arbeláez Gomez, “Análise de urina: mais do que um teste comum”, Medicina y Laboratorio, vol. 12, não. 11, pp. 511-556, 2006.
- Alberto Tobón-Castaño, Sebastián Barrera Escobar, Cecilia Giraldo Castro, “Urinalysis and Clinical Correlations in Patients with P. vivax or P. falciparum Malaria from Colombia”, Journal of Tropical Medicine, vol. 2017, Article ID 7868535, 12 pages, 2017. [CrossRef]
- Ephraim RK, Tashie W, Agbodzakey H, Sakyi SA, Essien-Baidoo S, Adoba P, Adu P, Ampong J. Dipstick urinalysis findings in children with Plasmodium falciparum in the South Tongu District: A case-control study. Niger Med J 2015;56:292-6. [CrossRef]
- Ugwuja EI, Ugwu NC. Abnormal findings on dipstick urinalysis of out-patients with malaria in Abakaliki, Nigeria. J Vector Borne Dis. 2011;48:205–9.
- Uzuegbu UE, Emeka CB. Changes in liver function biomarkers among malaria infected patients in Ikeja Lagos State, Nigeria. Curr Res J Biol Sci. 2011;3:172–4.
- Sacomboio ENM, Sebastião CS, Tchivango AT, Pecoits-Filho R, Calice-Silva V. Does parasitemia level increase the risk of acute kidney injury in patients with malaria? Results from an observational study in Angola. Sci Afr. 2020;7:e00232. [CrossRef]
- Manuel Sacomboio E, Bande J, Doqui Zua S. Effect of social and clinical conditions on blood pressure variation in angolans hospitalized with malaria. Acta Med Int. 2020;7:121.
- Sacomboio ENM, Campos LH, Daniel FN, Ekundi-Valentin E. Can vital signs indicate acute kidney injury in patients with malaria? Results of an observational study in Angola. Scientific African. 2021; 14e01021. [CrossRef]
- Wu Q, Sacomboio E, Valente de Souza L, Martins R, Kitoko J, Cardoso S, Ademolue TW, Paixão T, Lehtimäki J, Figueiredo A, Norden C, Tharaux PL, Weiss G, Wang F, Ramos S, Soares MP. Renal control of life-threatening malarial anemia. Cell Rep. 2023 Feb 28;42(2):112057. Epub 2023 Feb 2. PMID: 36735532. [CrossRef]
- Sacomboio ENM, dos Santos Sebastião C, Salvador STdC, João JA, Bapolo DVS, Francisco NM, et al. (2022) Evaluation of blood cell count parameters as predictors of treatment failure of malaria in Angola: An observational study. PLoS ONE 17(5): e0267671. [CrossRef]
- Da Veiga LS, Ward D, Campino S, et al. Drug resistance profile and clonality of Plasmodium falciparum parasites in Cape Verde: the 2017 malaria outbreak. Malar J 20, 172 (2021). [CrossRef]
- Kalantari S., Jafari A., Moradpoor R., Ghasemi E., Khalkhal E. Human Urine Proteomics: Analytical Techniques and Clinical Applications in Renal Diseases. Int. J. Proteom. 2015;2015:782798. [CrossRef]
- Chai, H. C., & Chua, K. H. (2022). Urine and Saliva: Relevant Specimens for Malaria Diagnosis?. Diagnostics (Basel, Switzerland), 12(12), 2989. [CrossRef]
- Campos SB, Rouch LH, Seguro AC. Effects of sodium artesunate, a new antimalarial drug, on renal function. Kidney Int. 2001;59:1044–51.
- Zaki, S. A., Shanbag, P., Lad, V., & Shenoy, P. (2011). Sodium artesunate-induced diuresis in a patient with malaria. Indian journal of pharmacology, 43(4), 472–473. [CrossRef]
- Calice-Silva, V., Sacomboio, E., Raimann, J. G., Evans, R., Dos Santos Sebastião, C., Tchivango, A. T., Kotanko, P., Levin, N., & Pecoits-Filho, R. (2018). Diagnostic performance of salivary urea nitrogen dipstick to detect and monitor acute kidney disease in patients with malaria. Malaria Journal, 17(1), Article 477. [CrossRef]
- Bi, D., Lin, J., Luo, X., Lin, L., Tang, X., Luo, X., Lu, Y., & Huang, X. (2022). Biochemical characteristics of patients with imported malaria. Frontiers in cellular and infection microbiology, 12, 1008430. [CrossRef]
Figure 1.
Biochemical markers in urine to data on resistance to hospital treatment with injectable antimalarials.
Figure 1.
Biochemical markers in urine to data on resistance to hospital treatment with injectable antimalarials.
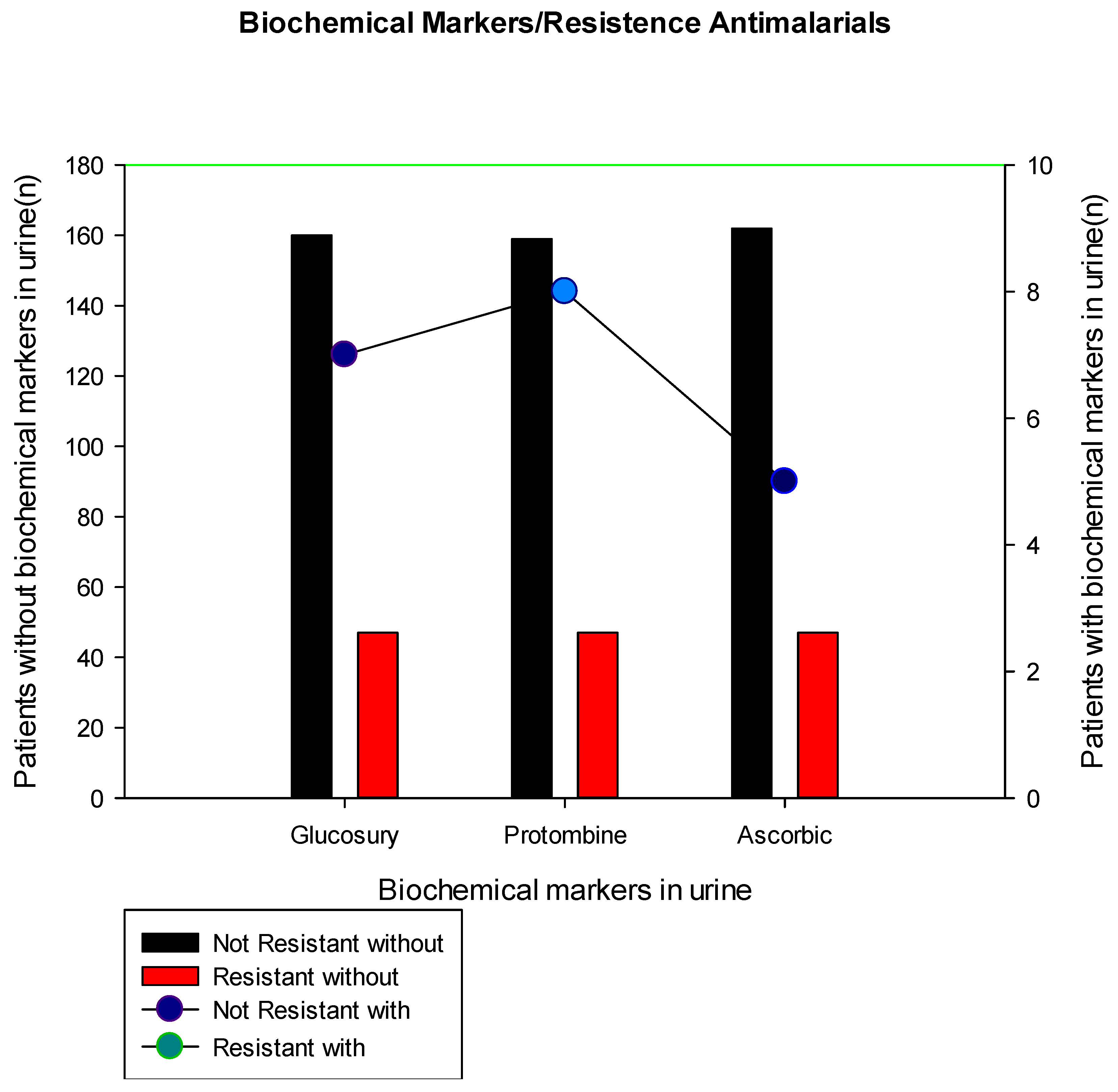
Table 1.
Characteristics of sociodemographic and clinical of patients with antimalarial resistance treatment in Luanda, Angola.
Table 1.
Characteristics of sociodemographic and clinical of patients with antimalarial resistance treatment in Luanda, Angola.
| Characteristic | N (%) | Antimalaric Resistance | Univariate analysis | ||||
| No (%) | Yes (%) | p-value | OR (95% CI) | p-value | |||
| Overall | 214 (100) | 167 (75.9) | 47 (24.1) | ||||
| Gender | |||||||
| Female | 100 (46.4) | 77 (78.4) | 23 (21.6) | 0.869 | 1 | - | |
| Male | 114(53.6) | 89 (79.6) | 24 (20.4) | 1.01 (0.50– 1.93) | 0.976 | ||
| Age Group (years) | |||||||
| <21y | 39 (18.2) | 30 (80.0) | 9 (20.0) | 0.172 | 1 | - | |
| 21-40y | 156 (72.7) | 119 (76.9) | 37 (23.1) | 4.55 (0.48– 39-1) | 0.149 | ||
| >40y | 19 (9.1) | 18 (95.0) | 1 (5.0) | 5.83 (0.77– 47.9) | 0.078 | ||
| Occupation Activity | |||||||
| Employed | 99 (46.4) | 70(71.6) | 30 (28.4) | <0.001 | 1 | - | |
| Unemployed | 55 (25.5) | 55 (100) | 0 (0.0) | 0.03 (0.01– 0.29) | 0.003 | ||
| Student | 60 (28.2) | 43 (73.8) | 17 (26.2) | 1.04 (0.49 – 2.01) | 0.876 | ||
| Residence Region | |||||||
| Urban | 64 (30.0) | 49 (78.8) | 15 (21.2) | 0.212 | 1 | - | |
| Peri-Urban | 102 (47.7) | 76 (75.2) | 25(24.8) | 1.87 (0.64– 5.39) | 0.236 | ||
| Rural | 48 (22.3) | 41 (87.8) | 7 (12.2) | 2.42 (0.87– 6.87) | 0.062 | ||
| Antimalarial | |||||||
| Artemether | 195 (90.9) | 152 (78.5) | 43(21.5) | 0.847 | 1 | - | |
| Artesunate | 19 (9.1) | 15 (80.0) | 4 (20.0) | 1.09 (0.29– 3.51) | 0.856 | ||
| Parasitemic Degree | |||||||
| Moderate | 91 (42.3) | 65 (72.8) | 27 (27.2) | 0.021 | 1 | - | |
| High | 123 (57.7) | 102 (84.3) | 20 (15.7) | 1.99 (1.09– 3.95) | 0.040 | ||
| Clinical Outcome | |||||||
| Discharged | 70 (32.7) | 48 (69.4) | 22 (30.6) | 0.036 | 1 | - | |
| Kept | 131 (61.4) | 108 (83.6) | 23 (16.4) | 5.28 (0.65 – 43.1) | 0.121 | ||
| Dead | 13 (5.9) | 11 (92.3) | 2 (7.7) | 2.59 (0.32– 20.9) | 0.371 | ||
Table 2.
Urinary analysis of patients with malaria to data on resistance to hospital treatment with injectable antimalarials.
Table 2.
Urinary analysis of patients with malaria to data on resistance to hospital treatment with injectable antimalarials.
| Characteristic | N (%) | Resistence | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| No (%) | Yes (%) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |||
| Overall | 214 (100) | 167 (75.9) | 47 (24.1) | ||||||
| Color (Reference: Yellow 1) | |||||||||
| Yellow 1 | 203 (95.7) | 157 (77.3) | 46 (22.7) | 0.290 | 0.34 (0.04 – 2.73) | 0.311 | 0.34 (0.04 – 2.73) | 0.311 | |
| Yellow 2 | 11 (4.3) | 10 (90.9) | 1 (9.1) | 1 | - | 1 | - | ||
| Aspect (Reference: Clear) | |||||||||
| Clear | 195 (92.0) | 149 (76.4) | 46 (23.6) | 0.015 | 5.55 (0.72 – 42.7) | 0.016 | 5.55 (0.72 – 42.7) | 0.016 | |
| Cloudy | 19 (8.0) | 18 (94.7) | 1(5.3) | 1 | - | 1 | - | ||
| pH (Reference: 5.5-7.0) | |||||||||
| Normal | 180 (84.9) | 136 (75.6) | 44 (24.4) | 0.044 | 0.29 (0.08 – 1.02) | 0.055 | 0.29 (0.08 – 1.02) | 0.055 | |
| High | 34 (15.1) | 31 (91.2) | 3(8.8) | 1 | - | 1 | - | ||
| Density (Reference: 1.005 e 1.030) | |||||||||
| Low | 10 (4.7) | 8 (80.0) | 2 (20.0) | 0.779 | 0.83 (0.17 – 4.10) | 0.825 | 1.19 (0.24 – 5.87) | 0.825 | |
| Normal | 39 (18.4) | 32 (82.1) | 7 (17.9) | 0.73 (0.29 – 1.78) | 0.493 | 1.36 (0.55 – 3.34) | 0.493 | ||
| High | 163 (76.9) | 122(74.8) | 38 (25.2) | 1 | - | 1 | - | ||
| Bacteria (Reference: Absent) | |||||||||
| Low | 95 (43.9) | 70 (73.7) | 25 (26.3) | 0.169 | 0.63 (0.33 – 1.21) | 0.171 | 0.63 (0.33 – 1.21) | 0.173 | |
| Many | 119 (56.1) | 97 (81.5) | 22 (18.5) | 1 | - | 1 | - | ||
| Crystals (Reference: Absent) | |||||||||
| Low | 178 (83.9) | 154 (86.5) | 24 (13.5) | <0.001 | 11.3 (5.07 – 25.3) | <0.001 | 11.3 (5.07 – 25.3) | <0.001 | |
| Many | 36 (16.1) | 13 (36.1) | 23 (63.9) | 1 | - | 1 | - | ||
| Others (Reference: Absent) | |||||||||
| Some | 185 (72.2) | 150 (81.1) | 35 (18.9) | 0.007 | 3.02 (1.32 – 6.90) | 0.009 | 3.02 (1.32 – 6.90) | 0.009 | |
| Fungi | 29 (13.8) | 17 (58.6) | 12 (41.4) | 1 | - | 1 | - | ||
| Leucocity (Reference: Absent) | |||||||||
| Low | 73 (33.5) | 55 (75.3) | 18 (24.7) | 0.493 | 0.79 (0.40 – 1.54) | 0.468 | 0.79 (0.40 – 1.54) | 0.468 | |
| Many | 141 (66.5) | 112 (79.4) | 29 (20.6) | 1 | - | 1 | - | ||
| Linfocite (Reference: Absent) | |||||||||
| Low | 39 (17.5) | 29 (74.4) | 10 (25.6) | 0.493 | 0.77 (0.34 – 1.73) | 0.540 | 0.77 (0.34 – 1.73) | 0.540 | |
| Many | 175 (82.5) | 138 (78.9) | 37 (21.1) | 1 | - | 1 | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






