Introduction
Cancer is a public health problem worldwide, new cases of cancer in Angola according to Globocan in 2020 were about 20,327 and 12,599 deaths [
1]. Tumor markers have been widely applied in early diagnosis, treatment, treatment response assessment, and recurrence monitoring [
2,
3,
4,
5]. There are different cancer risk factors, fifty percent of cancer deaths are caused by modifiable risk factors, and infections play an important role in many sub-Saharan African countries [
6,
7]. Luanda is the largest city in Angola with more than 9 million inhabitants 4,471,310 male and 4,608,501 female [
8].
Plasma/serum biomarkers have great potential in cancer screening and their role could extend further from general population risk assessment to treatment response estimation and recurrence monitoring. The rich content of different molecular and cellular elements in blood, which give information about the health status of an individual, make it a perfect tool to develop noninvasive diagnostics approaches for cancer [
9].
Due to the proven effectiveness of screening approaches, immunodiagnostic methods for quantitation-specific tumor markers are widely used in screening and clinical diagnosis. Different tumor markers have been applied for screening and early detection of cancer, these tumor markers include carcinogenic antigen 125 (CA125) in ovarian cancer (OC) the most deadly cancer of the female reproductive system, unfortunately up to now there is no an effective screening method for early detection of OC10,11. Serum carcinoembryonic antigen (CEA) is a set of glycoproteins involved in cell adhesion, it can be elevated in colorectal, breast (BC), lung, and pancreatic cancers [
12,
13]. Serum CEA is an important biomarker for the detection of different disease conditions [
14]. Another important tumor marker is Alpha Fetoprotein (AFP) produced in the fetal liver and yolk sac, AFP gene is methylated in neonates in adults the levels are undetectable or present in small quantities at normal conditions [
15]. AFP is a specific serological marker for Hepatocellular carcinoma (HCC) and Colorectal cancers [
16,
17] and represents the most prominent biomarker for early diagnosis, monitoring, prognosis assessment, and therapeutic response [
18].
Cancer Antigen 15-3 (CA 15-3) antigen is the most valuable serum tumor marker in BC, it helps in the early detection of recurrence and monitor response to the treatment. CA 15-3 levels can be higher some kinds of cancer [
13], CA 15.3 may be increased in lung, pancreas, OC, and prostate cancer and some non-cancerous conditions. Carbohydrate antigen 19-9 (CA 19-9) and Carbohydrate antigen 72-4 (CA 72-4) have become common tumor markers for digestive system tumors, elevated levels can be used to assist in diagnosis [
19,
20]. Updated data on Prostate-specific antigen (PSA) screening are available, PSA screening increases prostate cancer (PC) diagnosis and reduces mortality [
21,
22,
23,
24].
Just because a person has tumor markers, it does not always mean cancer is present or has come back. Conditions besides cancer can raise tumor marker levels but tumor markers can be used to predict the response of a tumor to treatment and prognosis, researchers expected that they might also be useful in screening tests that aim to detect cancer early before there are any symptoms. However, studies to see whether circulating tumor markers can be used to screen for cancer have generally found that these markers don't identify everyone with the disease (they are not sensitive enough) or that they indicate the possible presence of cancer in people who don't have it. When a test has low specificity, more tests are needed to determine whether cancer is present or not, and some screening tests based on tumor markers have been shown to lead to overdiagnosis, which happens when people are diagnosed with cancers that would never have affected them during their lifetimes. For example, the PSA test was used routinely in the past to screen men for prostate cancer. However, as more was learned about the limitations of the test (including relatively low specificity), medical groups began to recommend against using it for routine population screening New approaches like Several liquid biopsy-based assays that test for multiple tumor markers to detect cancer early, in people without symptoms, are in development, this test focuses on cancer-related alterations in DNA obtained from body fluids.
The development of screening and early detection test methods, are of great importance to improve the efficacy of therapies and to reduce cancer mortality. In this sense, precision medicine has gained particular attention in the oncology field. liquid biopsy is a minimally or non-invasive detection approach for circulant tumor-derived components in biofluids, such as blood, urine or saliva liquid biopsy is a revolutionary approach with significant potential for the management of cancer. Genomic and transcriptomic alterations can be accurately detected through liquid biopsies, which provide a more comprehensive characterization of the heterogeneous tumor profile [
25]. Recently research in oncology has focused on liquid biopsies (LB), which consist of the detection of cancer-derived components, including circulating tumor cells (CTCs) [
26,
27,
28], circulating tumor DNA (ctDNA) [
29] RNA, extracellular vesicles (EVs) a promising approach to detect early-stage, curable cancers uses biomarkers present in circulating EVs [
30,
31] and tumor educated platelets (TEPs) [
32], in the biofluids of patients, providing genomic [
33] epigenetic [
34] transcriptomic, and proteomic [
35] information about tumors and metastatic sites. The use of LB as a clinical tool will improve cancer screening [
36].
Liquid biopsies (LB) hold the potential to inform cancer patient prognosis and to guide treatment decisions at a time when direct tumor biopsy may be impractical due to its invasive nature, inaccessibility, and associated complications. Specifically, circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) have shown promising results as companion diagnostic biomarkers for screening, prognostication, and/or patient surveillance in many cancer types. In ovarian cancer (OC), CTC and ctDNA analysis allows comprehensive molecular profiling of the primary, metastatic, and recurrent tumors [
37]. These biomarkers also correlate with overall tumor burden and thus, they provide minimally-invasive means for patient monitoring during clinical course to ascertain therapy response and timely treatment modification in the context of disease relapse [
38]. Liquid biopsies (LB) are defined as the analysis of either corpuscular (circulating tumor cells, extracellular vesicles) or molecular (circulating DNA or RNA) tumor-derived material. LB could more precisely identify clinically relevant alterations that characterize the metastatic potential of tumors, predict response to specific treatments or actively monitor for the emergence of resistance. These tests can potentially be repeated as often as deemed necessary and can detect real-time responses to treatment with minimal inconvenience to the patient [
39].
Other inexpensive and non-invasive methods, the fecal occult blood test (FOBT) based screening, have also been developed in the past, but with lower sensitivity and specificity [
40,
41,
42], but have been used nowadays [
43,
44]. According to the CDC (Center for Disease Control and Prevention), the new cases of cancer expected for 2020 will be more than 1.8 million, with 606,520 deaths from cancer, where fortunately, some of the types of cancer, for example, colon, and lung, cervix, breast cancer, could be detected with screening, which helped to delay or even stop the progression of cancer [
61], given the fact that most risk factors for cancer are preventable, simply eliminating or reducing the use of tobacco products and exposure to secondhand smoke, getting vaccinated (HPV-Human Papilloma Virus), avoiding indoor tanning, maintaining a healthy weight, staying physically active, avoiding processed or red meat, having a healthy diet high in fruit and vegetables and other measures can substantially lower a person's lifetime risk of developing or dying from cancer [
62].
In recent years, with the advanced knowledge of tumor mechanisms and the development of molecular biology technology, the detection of tumor markers has been commonly used for early screening and diagnosis of cancer, guidance of cancer treatment, evaluation of curative effect, monitoring of cancer recurrence and metastasis, and the judgment of prognosis and survival.
Knowing the importance of cancer screening and prevention in the Angolan population in particular, which faces several difficulties in cancer treatment, is of great importance. Putting apart our limitations, Identifying individuals suspected of developing cancer in the future using an inexpensive and non-invasive technique can change the approaches to patient care, improving early diagnosis, clinical and pathological response as well as patient survival.
Although there are more efficient and recent techniques for cancer screening, trained human resources are needed, in developing countries the costs can be high and make the technique unfeasible from an economic point of view. So our research group is looking forward to bringing all this knowledge and technology to start studies focusing on LB at a low cost.
The present study aimed to screen the most frequent tumor markers in the Angolan population according to age and gender.
Methodology
Study design and setting
This was an analytical, retrospective study, with a quantitative approach, where tumor markers were evaluated in individuals submitted to examination of tumor markers in MEDIAG Lab located in Luanda, the capital city of Angola. The studied population consisted of 18,222 individuals, regardless of gender and age among those who agree to participate in the study after being informed about the objectives and nature of the study. The data analyzed in the present study were obtained from the MEDIAG laboratory database that was attended between January 2019 and December 2021.
Ethics statement
To carry out the study, the project was submitted, analyzed, and approved by the Ethics Committee in Research on Human Beings of the Instituto Superior de Ciências da Saúde, Universidade Agostinho Neto (nr.234/GD/ISCISA/UAN/2019), subsequently the study was accepted by the Directorate of Laboratorio Mediag (nr.004/DG/MEDIAG/2019). All patients gave oral and in writing their informed consent before being included in the study. All methods were carried out by the relevant guidelines and regulations.
Sample collection and laboratory procedure
A whole blood sample estimated at 2 mL was collected for each patient by the venipuncture technique and the samples were placed in test tubes containing ethylenediaminetetraacetic acid (EDTA). Serum levels were measured using the electrochemiluminescence method, using the automated Cobas e411 equipment (Roche®) according to the manufacturer's instructions. Individuals were classified as not suspected when they presented values of tumor markers between the reference values and suspected when they presented tumor markers above the reference values, which depends on the type of marker analyzed. For the AFP (a marker for tumors in the stomach, intestine, ovaries, or presence of liver metastases), individuals who presented results below 10 ng/mL were considered non-suspected, and individuals who presented values greater than 12.5% of 10 ng/mL were considered suspect. For CA 125 (a marker for tumors in organs such as the liver, lung, breast, rectum, and stomach), individuals who presented values below 35 U/mL were considered non-suspected, and individuals who presented values greater than 12.5% of 35 U/mL were considered suspect. For CA 15.3 (a marker for several other neoplasms, such as ovarian cancer, breast cancer, lung cancer, colon cancer, hepatocellular carcinoma, and lymphomas), individuals who presented values below 25 U/mL were considered non-suspected, and individuals who presented values greater than 12.5% of 25 U/mL were considered suspect. For CA 19.9 (marker for pancreatic cancer, colorectal cancer, gallbladder cancer, and liver cancer), individuals who presented values below 37 U/mL were considered non-suspected, and individuals who presented values greater than 12.5% of 37 U/mL were considered suspect. For CA 72.4(markers for colon cancer, stomach cancer, pancreas, and biliary tract cancer, and mucinous ovarian carcinoma), individuals who presented values below 6.9 U/mL were considered non-suspected and individuals who presented values greater than 12.5% of 6,9 U/mL were considered suspect. For the CEA (markers for tumors of gastrointestinal origin), which can also be elevated in other types of tumors, such as breast, lung, and ovary), individuals who presented results below 3.5 ng/mL were considered non-suspected, and individuals who presented values greater than 12.5% of 3.5 ng/mL were considered suspect. For the TPS (a marker for prostate cancer), individuals who presented results below 4.0 ng/mL were considered non-suspected, and individuals who presented values greater than 12.5% of 4.0 ng/mL were considered suspect.
Statistical analysis
Initially, the data obtained in the study were compiled in an Excel 2017 database and subsequently transferred, categorized, and analyzed in SPSS v25. Absolute and relative frequencies were determined. The data were categorized as suspicious and not suspicious and for each of the categories the data were presented in frequencies and percentages in cross tables, created in the SPSS v25 program. Graphs were produced using the Sigmaplot 12.0 program (Systat Software, Inc.), using mean ± standard deviation for the categories of suspects and non-suspects about gender (male and female) and concerning the age groups that were categorized according to the social reality of Angola, where children (under 13 years old), adolescents (from 13 to 18 years old), young people (from 19 to 40 years old and adults (over 40 years old and under 65 years old) are considered.
Results
The data in
Table 1 show the incidence of suspected cases of cancer according to age groups. It can be seen that most of the 18,222 individuals found in the database were adults (68.7%, n=12531), in this group 23.6% (n=2950) were suspected individuals, the most common carcinogenic markers were CA 15.3 (50.8%), followed by CEA (24.8%), TPSA (24.4%) and CA 125 (22.6%). Young people represented about 22.6% (n=4120), where 27.9% (n=1150) were suspected cases, where the most verified markers were CA 15.3 (50, 4%), followed by AFP (42.6%) and CA. 125(24.3%). Adolescents represented 1.14% (n=208) of the population, of which 23.1% (n=48) were considered suspicious, where the most common marker was CA 15.3 (68.7%), followed by CA 125(29.7%) and TPSA (20%). Children represented about 0.77%(n=136) of the studied population, where 25.7% (n=28) were considered suspects, and the most frequent tumor markers in these groups were CA 125(34.5%), CA 15.3 (33.3%), TPSA (25.0%), AFP (21.3%) and CA 19.9 (20%). In individuals with unknown age, they represented about 6.8% (n=1234) of the studied population and in this group, 27.9 (n=101) of them were considered suspicious. It was found that the most identified markers were CA 15.3 (54.2%), followed by AFP (46.6%), CEA (29.2%), CA 125 (24.2%), CA 19.9 (21.1%) and TPSA (19.7%).
In
Table 2, we present the incidence of cases by gender. It can be seen that the majority are male, representing about 73.7%(n=13428) of the entire population studied, in this group, the number of suspected cases was 23.6%(n=3173), where the most frequently identified were AFP (30.2%, n=451), TPSA (23.6%, n=2018), CA 125 (23.1%, n=348), CEA (22.5%, n=203) and CA 15.3 (21.0%, n=41) only for CA 19.9 the incidence of suspected cases was 15.0%(n=112), for CA 72.4 no suspected cases were identified. Female individuals represented about 26.3% (n=4794), with 28.1%(n=1347) of suspected cases, where the highest incidence of suspected cases was found in the CA.15.3 assessment (71.8%, n=201), in seconds of AFP (31.9%, n=401), CEA (24.5%, n=135) and CA 125 (24.4%, n=558), however, for CA 19.9 the percentage was less than 12% and for CA 72.4 and TPSA, as expected, no cases were identified.
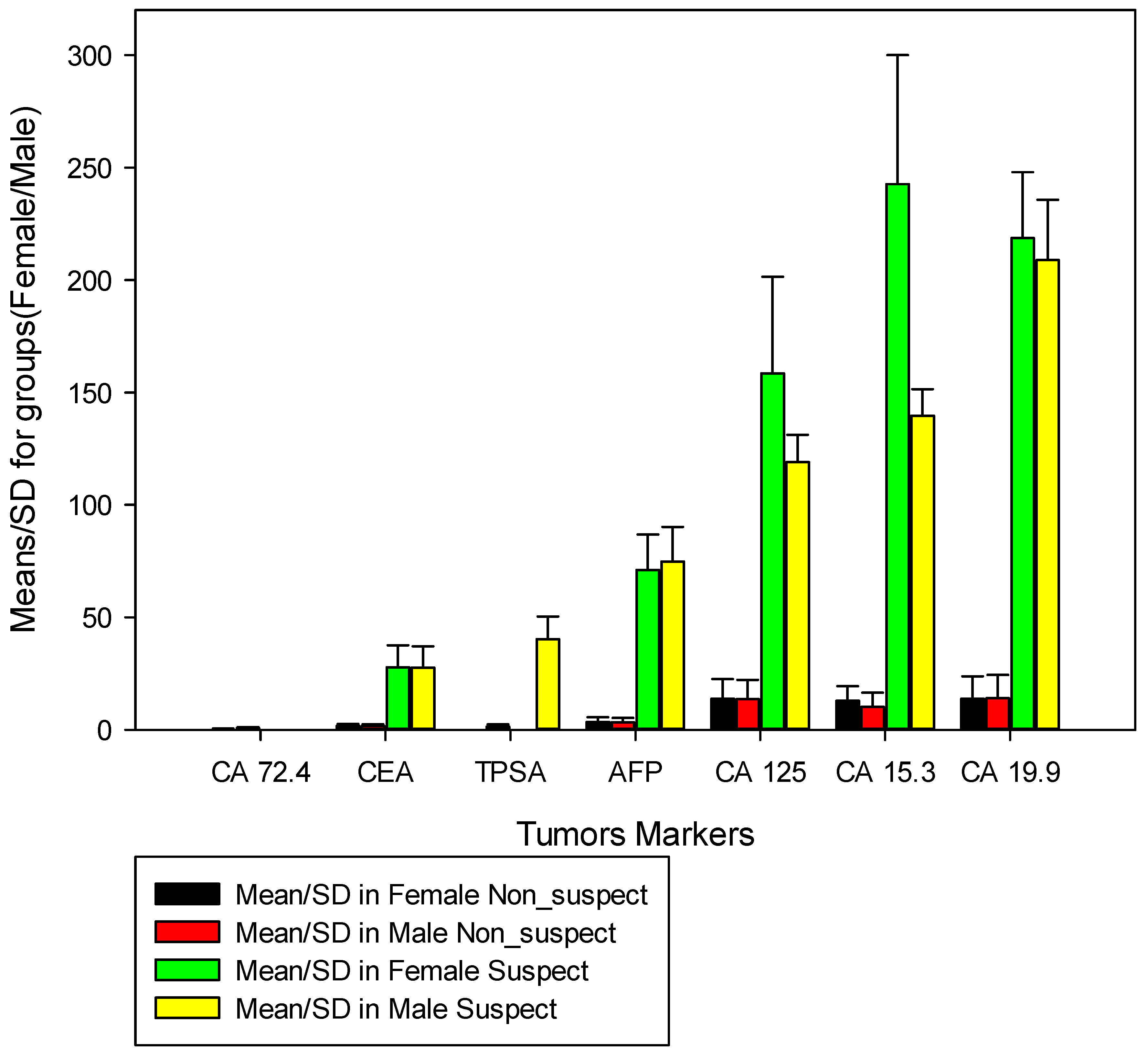
Graph 1 illustrates the difference between the mean of the tone markers, between individuals considered non-suspect VS suspected individuals concerning gender. In the evaluation of the average of tumor markers in female individuals, we verified that in women not suspected of suspected VS, the average of AFP was 3.5ng/ml (SD=2.0) VS 71.1ng/ml (SD=15.7 ), for CA 125 the mean was 13.8 U/ml (SD=8.7) VS 158.6 U/ml (SD=42.9) for CA 15.3 the mean was 12.8 U/ml ml (SD=6.5) VS 242.6 U/ml (SD=57.4), for CA 19.9 the mean was 13.7 U/ml (SD=9.9) VS 218.7 U /ml (SD=9.3), for CA 72.4 the average was 0.4 U/ml (SD=0.2) VS 0.0 (SD=0.0) since there were no positive cases, for CEA the mean was 1.8 ng/ml (SD=0.8) vs. 27.8 ng/ml (SD=9.7). TPSA was not evaluated in this group because it is a specific marker for prostate cancer. In unsuspected vs suspected men, the mean AFP was 3.3 ng/ml (SD=2.0) VS 74.7 ng/ml (SD=15.5), for CA 125 the mean was 13.6 U/ml (SD=8.5) VS 119.0 U/ml (SD=12.1), for CA 15.3 the mean value was 10.2 U/ml (SD=6.3) VS 139 .6 U/ml (SD=11.9), for CA 19.9 the average was 14.0 U/ml (SD=10.4 ) VS 208.9 U/ml (SD=26.7), for CA 72.4 the mean was 0.8 U/ml (0.4) VS 0.0 (0.0) as no suspected case occurred, for CEA the mean was 1.7 ng/ml (SD =0.8) VS 27.6 ng/ml (SD=9.4) and for TPSA the mean was 1.4 ng/ml (SD=1.02) VS 40.3 ng/ml (SD=10.0).
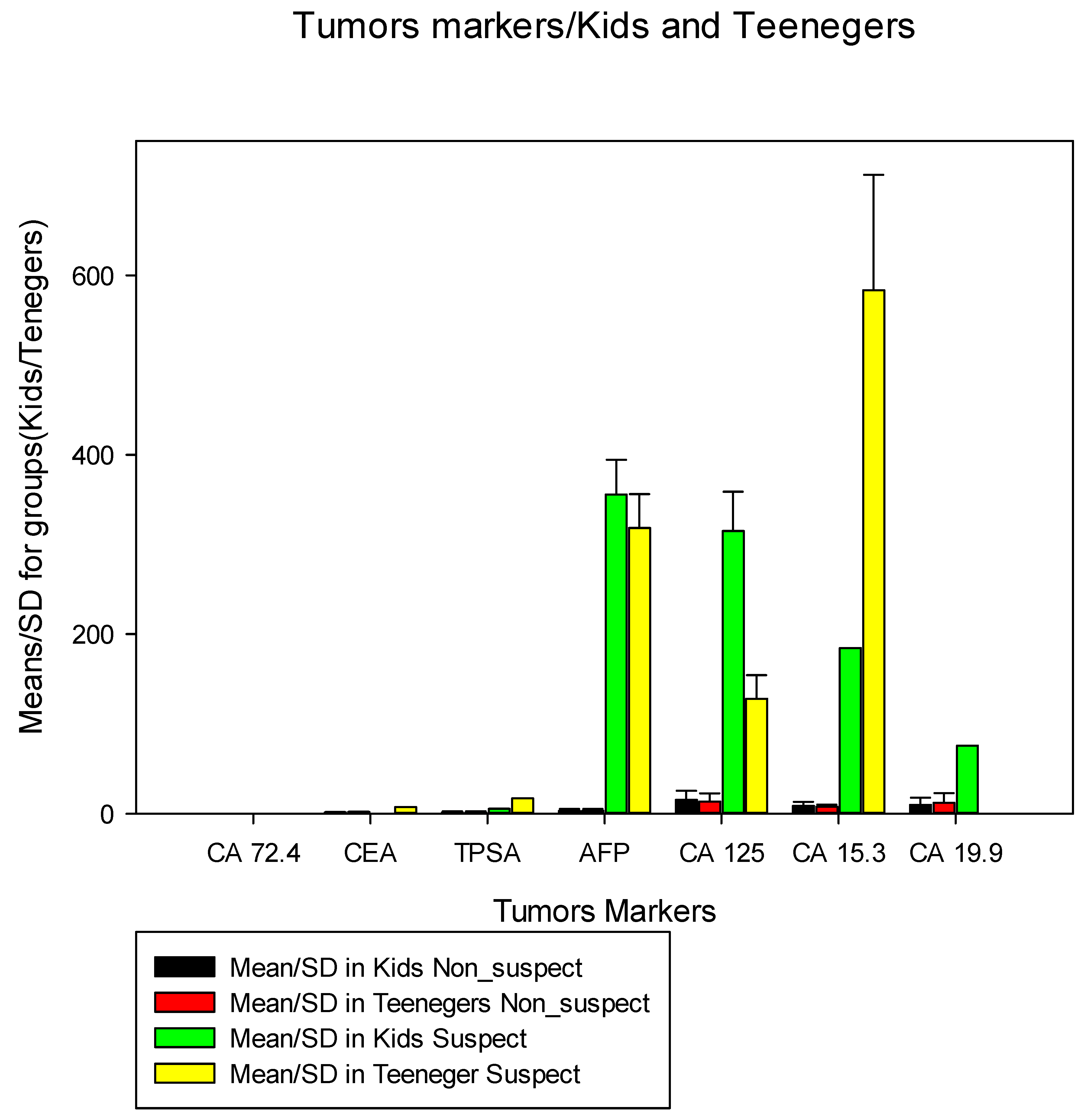
We compared the average of each marker by groups of non-suspected VS suspected for children and adolescents (
Figure 2), we verified that in non-suspected children SV suspected children, the mean AFP was 3.0 ng/ml (SD=2.1) VS 355.7 ng/ml (SD=38.7), for CA 125 the average was 15.4 U/ml (SD=10.3) VS 314.9 U/ml (SD=44.0), for CA 15.3 the mean was 8.6 U/ml (SD=4.5) VS 184.3 U/ml (SD=0.5), for CA 19.9 the mean was 9.85 U/ml ml (SD=8.1) VS 75.9 U/ml (0.5) and for CEA the mean was 1.37 ng/ml (SD=0.5) VS without suspected cases. There was no indication for CA 72.4 assessment in children. In adolescents not suspected VS suspected, the average of AFP proteins was 3.34 ng/ml (SD=1.9) VS 318.3 ng/ml (SD=37.8), for CA 125 the average was 13 .4 U/ml (SD=9.1) VS 127.7 U/ml (SD=26.3), for CA 15.3 the average was 7.6 U/ml (SD=2.33) VS 583.5 U/ml (SD=128.8), for CA 19 the average was 12.1 U/ml (SD= 10.7) VS 0.0 (SD=0.0) since there were no suspected cases, for CA 72.4 the average was 0.2 U/ml (SD=0.0) VS 0.0(SD=0.0) since there were no suspected cases, for CEA the average was 1.7 ng/ml (SD=0 .65) VS 7.2 ng/ml (SD=0.0), for TPSA the mean was 1.2 ng/ml (SD=1.5) VS 17.1 (SD=0.0).
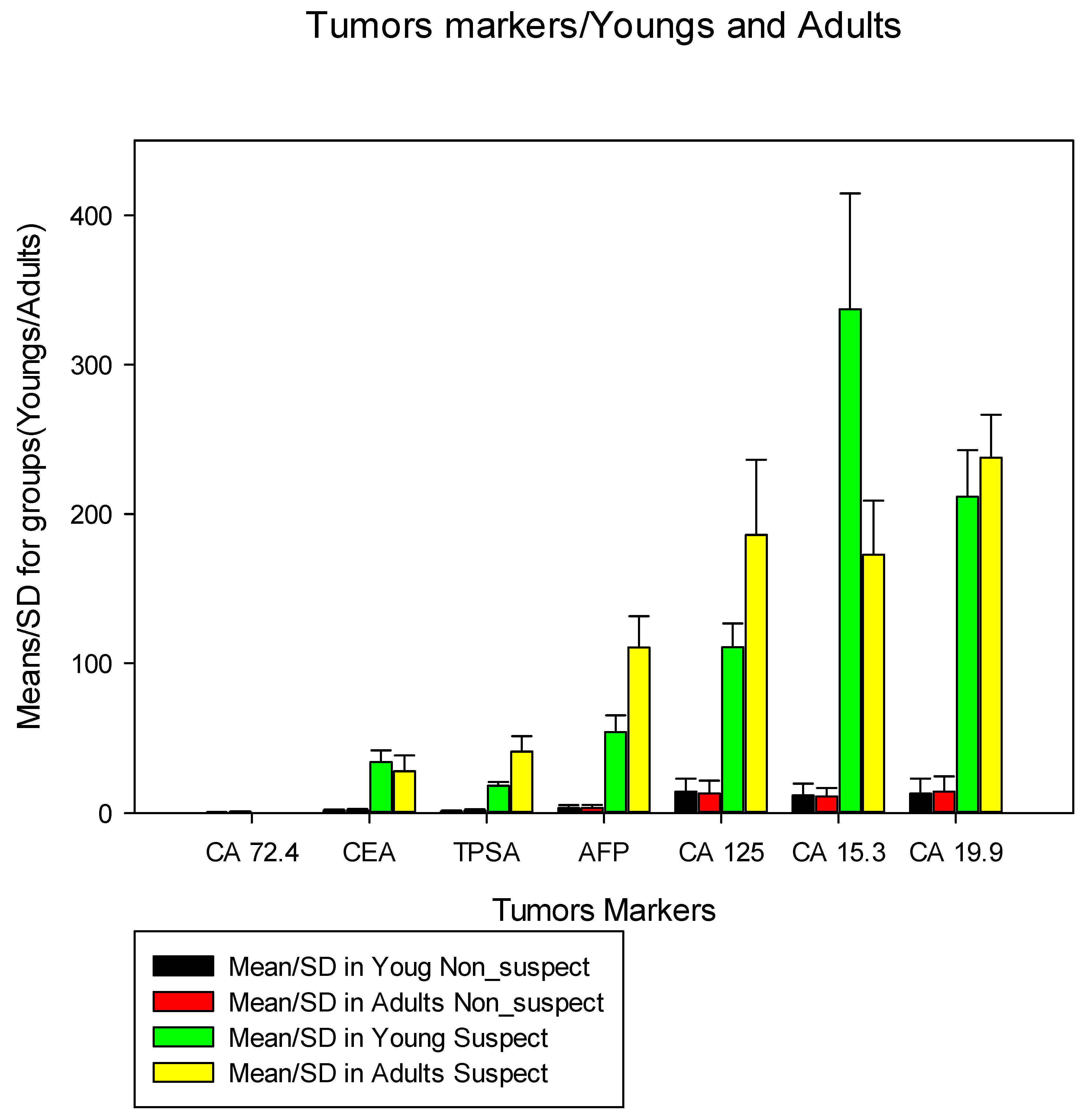
In the evaluation of markers in suspected and non-suspected youths and adults (
Figure 3), we noted that for suspect vs non-suspected youths the mean AFP was 3.3ng/ml (SD=2.0) VS 54.2ng/ ml (SD=11.1), for CA 125 the average was 14.3 U/ml (SD=8.6) vs 110.8 U/ml (SD=15.9), for CA 15.3 a mean was 11.7 (SD=7.87) VS 337.1 (SD=77.6), for CA 19.9 the mean was 12.8 (SD=10.0) VS 211.7 (SD =31.1), for CA 72.4 the mean was 0.45 (0.1) VS 0.0(SD=0.0) since there were no suspected cases, for CEA the mean was 1.6 ng/ml (SD=0.8) VS 34.1 ng/ml (SD=7.8), for TPSA the mean was 0.9 ng/ml (SD=0.6) VS 18.2 ng/ml (SD =2.6). In unsuspected adults vs suspected adults, the mean AFP was 3.4 ng/ml (SD=2.0) VS 110.5 ng/ml (SD=21.1), for CA 125 the mean was 12 .9 U/ml (SD=8.6) VS 186.0 U/ml (SD=50.3), for CA 15.3 the mean was 10.9 (5.8) VS 172.6 (SD =36.3), for CA 19.9 the mean was 14.3 U/ml (SD=10.2) VS 237.8 U/ml (SD=28.7), for CA 72.4 the mean was 0.8 U/ml (SD=0.3) VS 0.0(SD=0.0) since there were no suspected cases, for CEA the mean was 1.78 ng/ml (SD=0.8) VS 27.9 ng/ml (SD=10.6) and for TPSA the mean was 1.43 ng/ml (SD=1.0) VS 41.1 ng/ml (SD=10.3).
Discussion
Cancer screening has contributed to reducing cancer morbidity and mortality and this has been verified all over the world, the results of the present study reinforce this, since about 36.3% (n=6620) of the total population, 18,222 individuals (
Table 1 and
Table 2), were considered suspicious. These data are similar to a study carried out at the National Institute of Oncology of Angola, by the team of one of our authors, where they identified a total of 4,791 patients during the entire study period, with an annual average of 958 cases of cancer, where the Most common were breast cancer (20.5%), cervical cancer (16.5%), and head and neck cancer (10.6%), followed by lymphoma (7.2%), Kaposi's sarcoma (6.1%) and prostate cancer (4%) [
64]. But in some cases, this test is not recommended as a screening procedure for cancer detection in the general population.
The data showed (
Table 1) that among individuals of unknown age, the highest incidence of suspected cases was found in the CA.15.3 assessment (54.2%, n=13/24) which is an important biomarker for several neoplasms, such as ovarian cancer, breast cancer, lung cancer, colon cancer, hepatocellular carcinoma, and lymphomas; however, Most current guidelines do not recommend the serial analysis of the CA 15.3 tumor marker in the follow-up of asymptomatic patients treated for some types of cancer such as breast cancer in the early stages, for example. These guidelines are based on small-scale studies conducted in an era with more limited treatment options than today. In large centers, the assessment of CA 15.3 is routinely used in the fellow up, while more sophisticated and costly approaches, such as the detection of distant metastases, are performed only on medical advice48. AFP, one of the fetal serum proteins in the developing embryo, is synthesized mainly by the liver and yolk sac45, and its levels are often increased in malignancies. AFP is well established as a useful diagnostic tumor marker for hepatocellular carcinomas (HCC), embryonal, yolk sac tumors (YSTs), carcinoma of the ovary, and some testicular cancers, it can be useful as a tumor marker for detecting malignancies such as YSTs, which often occur in young women. In our study we detected high suspected cases in young
Table 1, this data corroborates with literature studies that demonstrated increased expression of AFP in young women, our dates in
Table 2 and
Figure 1 show an increase in suspect cases in young women but without statistical significance. Generally, AFP tumor maker is useful for Follow-up management of patients undergoing cancer therapy, especially for testicular and ovarian tumors and for hepatocellular carcinoma. Satisfactory results of these studies have led to recommendations that AFP should be integrated with other factors by some guidelines for HCC screening [
46,
47].
Liver biopsy provides relevant information about the biology of the tumor, but it is an invasive procedure, not routinely used and not representative of all neoplasms due to intratumoral heterogeneity, hence the importance of screening for tumor markers [
49], for the reality of Angola, screening of cancer is relevant because it is a cheaper, faster approach and does not require specialized personnel, since this is one of the major problems in Angola, which has few dedifferentiated units for diagnosis and still lacks specialized labor to perform more complex tests.
An interesting fact in the studied children was that we observed a 34.4% incidence of suspected cases of ovarian cancer antigen (CA. 125), which has been described that its elevation may be associated with benign or malignant situations, being ovarian cancer is the second deadliest gynecological neoplasm50. Elevated CA 125 levels occur in about 29% of patients with non-gynecologic diseases and some benign gynecologic conditions, such as pelvic inflammatory disease, endometriosis, uterine leiomyoma, and early pregnancy, and ovarian fibroma/fibrosarcoma with elevated serum CA 125 levels is not so common in clinical practice and for this reason, it can be misdiagnosed as epithelial ovarian cancer [
52]. In 2021, an ovarian cancer screening in the United Kingdom "Cancer Screening (UKCTOCS)" to evaluate the effect of screening in reducing deaths from the disease revealed that multimodal screening or long-term ultrasound did not reduce deaths from the disease. ovarian cancer, since there was a decrease in the incidence of stages III and IV with stages I and II53, thus, the CA-125 marker for the diagnosis of ovarian cancer in another study proved to be effective with good clinical application, being considered useful for clinical clinical actions and may be an indispensable tool for screening [
54].
In adolescents (13 to 19 years old), the highest incidence of suspected cases was verified in the CA15-3 assessment (68.7%, n= 4/6), with data similar to individuals of unknown age followed by the CA assessment. 125(29.7%, n=35/118) and TPSA (20.0%,n=1/5), although no studies were found that demonstrate the susceptibility of adolescents to cancers that express CA15.3 in Angola, however, lack of HPV vaccination measures in adolescents may contribute to an increased risk for ovarian cancer, cervical cancer and other types of cancer. Data from the Istituto Nacional de Estatitica de Angola show that the population is mostly young, perhaps explaining the high incidence in this group 55. The normal level of this biomarker in the blood is less than 30 U mL, but if the total serum level of CA 15.3 increases significantly, this may be indicative of breast cancer56. As with almost all serum markers, the lack of sensitivity for in situ or low-stage invasive disease and the lack of specificity for cancer reduce the use of CA 15.3 for screening, as recent data demonstrate that current strategies for screening for epithelial ovarian cancer (EOC) use a combination of blood biomarkers, primarily CA 125; mucin 16 (MUC16), human epididymal protein (HE)-4 and transvaginal ultrasound56. However, results from randomized screening trials and prospective population cohorts have demonstrated insufficient sensitivity and specificity for CA 125 and HE4, which are the two best markers currently available for detecting early-stage ovarian tumors, thus substantial efforts are being directed toward the search for additional protein biomarkers that, individually or in combination with CA 125 and other markers, may increase sensitivity and specificity to detect ovarian cancer at an earlier and more treatable stage [
57].
Our results showed that in the age group of 20 to 40 years, there were more suspicions linked to liver, ovarian, and testicular cancer, several factors may be associated with this condition in our population, however, the lack of studies related to this subject limits our approach. The large population fringe of Luanda is under 40 years old, but already published in some studies by the Instituto Angolano de Controlo do Cancer (IACC) and estimates by Globocan show that prostate cancer is the most frequent among Angolan men, and in women cervical cancer followed by breast [
1,
59,
64].
It was noticeable in the study that for adults over 41 years of age, the most suspected cancers are related to CA 15.3(50.8%), CEA(24.8%), TPSA(24.6%), and to the markers AFP and CA 19.9 the incidence of suspected cases was less than 17% and for CA 72.4 no suspected cases were found. Studies show that the cancer antigen (CA 15.3) is used to monitor response to breast cancer treatment and disease recurrence, although the diagnostic threshold varies according to the laboratory and the kit used, data from the present study corroborate with those found in the literature, where we observed a high incidence of breast cancer in women, although CA 15.3 has not been used specifically for breast cancer screening, it can be implemented to reduce costs and high risks with mammography at these ages. A recent study showed that the combination of neutrophil lymphocytes (NLR), prognostic nutritional index (PNI), D-dimer (D-D), CD3+ T lymphocytes (CD3+ T) and CEA has a high clinical application value for colorectal cancer and can provide a reference for early screening and auxiliary diagnosis of colorectal cancer [
60]. CEA is the most widely used tumor marker for clinical screening and diagnosis of colorectal cancer. It has great advantages as it is a simple, non-invasive test with good reproducibility, but due to insufficient sensitivity and low organ specificity, its single detection has some limitations, these suspected cases are in line with Globocan data where they demonstrate that for 2020 the incidence of colorectal cancer in men appears in second place behind only prostate cancer and women in third place; these data are very interesting a prospective study to validate these data can be done in the future to elucidate and validate this data [
59]. Prostate cancer is the most common type of cancer in Angola and the TPSA assessment is already routinely applied in clinical practice for screening, diagnosis, and monitoring of response to therapy [
64]. Although the TPSA is widely applied for screening, a recent study showed that different clinical indicators should also be used for prostate cancer screening at different ages, they recommend that different clinical indicators be used in the screening and diagnosis of prostate cancer at different ages [
61].
The study identified that in women (
Table 2) the highest incidence of suspected cases was found in the evaluation of CA15.3 (71.8%), which is used to monitor the response to breast cancer treatment and evaluating recurrence, these data corroborate data from the literature that indicate that breast cancer is the most frequent in almost all cases and Angola in particular it is the second most incident type of cancer only behind cervical cancer [
59,
64], the use of this marker for cancer screening of breast cancer in the Angolan population can reduce mammography costs and women's continued exposure to low doses of radiation that these devices can mimic. AFP was the second marker with the highest number of suspected cases in women (32.5%) and has been the most used for HCC (hepatocellular carcinoma) which, according to data from Globocan, liver cancer represents the fifth most incident in Angola women59, understanding that early screening can reduce the risk and eliminate the chances of advanced cases, as well as improve the clinical and pathological response and make the neoplasm curative, we believe that its applicability in hospital units could help a lot in the timely diagnosis and immediate treatment. In the same data from the present study, AFP appears in men with about 30.2% of suspected cases, and may be associated with other factors or other liver diseases, whose causes or associated factors may turn out to be socio-demographic conditions not explored in this study, however, and the suspicion of prostate cancer by the TPSA screen was 23.6% in male individuals included in the study, which may be a sign that prostate cancer may be common in young Angolans and studies deeper studies are needed to understand the reality of the disease in the Angolan context.
We believe that the most interesting data in the present study is in the comparison between the markers in different groups, in the case of data between men and women (
Figure 1), observing the difference between the means of each marker in non-suspected VS suspects, we found that the difference between the averages of the AFP, CA 125, CA 15.3, CA 19.9, CA 72.4 and CEA markers between non-suspected/suspected individuals was greater than 70% in both sexes, which may indicate that most diagnoses happen randomly very late, certainly because of the difficulty that patients have in receiving the diagnosis, or the late search for medical services for diagnosis. In general, the data in
Figure 1 shows a high number of suspected cases in men and women compared to non-suspected cases, except for the TPSA marker, which is evaluated only in men; therefore, the highest suspected cases in both sexes were CA 19.9, CA 15.3, CA 125 and AFP, this data is contradictory because when we compare it with Globocan estimates for 2020 [
59,
64], cervical cancer appears in first, followed by breast and colorectal cancer and in males, prostate cancer followed by colorectal cancer and lymphoma. These data need to be refined, as our study is based on tumor markers that are not always used for screening, and Globocan estimates are based on data from the region and not always on hospital record bases.
Surprisingly, the data in
Figure 2 drew attention, since when we compared the average markers in non-suspected/suspected children and adolescents, we observed that the average markers CA 15.3, CA 125, and AFP in non-suspected and suspected children and adolescents were greater than 1000%, these data, in addition to showing a high prevalence of suspected cases in this group of individuals, also demonstrate that little attention is paid to the health of children and adolescents, which may be associated with public policies and inefficient health programs in the diagnosis and fight against cancer which means that children and adolescents are being submitted to screening only upon medical request, however, no national study has demonstrated the incidence of HCC in children and adolescents, which makes this study the first in this regard. This preliminary work will open the opportunity for further study to elucidate different points not clarified here. The CA 125 marker also appears in our study in both relatively high intervals, this demonstrates that in children and adolescents, the suspicion of ovarian cancer is high. risk of developing ovarian and uterine cancer is non-existent or incipient, therefore the CA 125 marker is essential in the screening of ovarian cancer and could be routinely applied51. CA 15-3 showed very high suspicious cases in adolescents, this is another interesting data that needs to be explored in future studies.
When comparing the evaluation of markers in suspected and non-suspected youths and adults (
Figure 3), the mean AFP, CA 125, CA 15.3, CA 19.9, CEA, and TPSA in suspected individuals was greater than 500% when compared to non-suspected individuals, these data illustrate how robust this condition of suspicion is and the severity of cancer in the studied individuals. Thus, for young people and adults of both sexes, the greatest suspicions were for ovarian cancer, breast cancer, lung cancer, and uterine colon cancer, data that contradict the local incidence of other studies [
54,
64] that highlight cervical cancer uterine cancer, female breast cancer, followed by pancreatic cancer, colorectal cancer, true biliary cancer and liver cancer and markers for ovarian cancer also have a very high average of suspected cases of liver cancer, ovarian cancer, testicles.
Despite prostate cancer being the most frequent in men in our study, the means of this marker in suspected cases did not exceed CA19.9, AFP, and CA125, this fact can be justified by our population pyramid, which demonstrates a mostly young population and our reality, few young people and adults are not screened for prostate cancer. Individuals often undergo tests and show symptoms, which can translate into an already advanced stage of the disease.
Author Contributions
Conceptualization: EKC, JF, ENMS. Data curation: EKC, JF, ENMS, MSE, LQ, AA, SRS. Formal analysis: ENMS, CSS, EKC, and JF. Investigation: ENMS, EKC, JF, LQ. Project administration: EKC, JF, ENMS, CSS, MSE, LQ, AA, SRS. Supervision: CAPS and LQ. Validation: ENMS, EKC, and JF. Writing – original draft: EKC, ENMS, and JF. Writing – review & editing: EKC, JF, ENMS, CSS, CAPS, MSE, LQ, AA, SRS. All authors approved the submitted version of this manuscript.