Submitted:
01 December 2023
Posted:
04 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Data and Methods
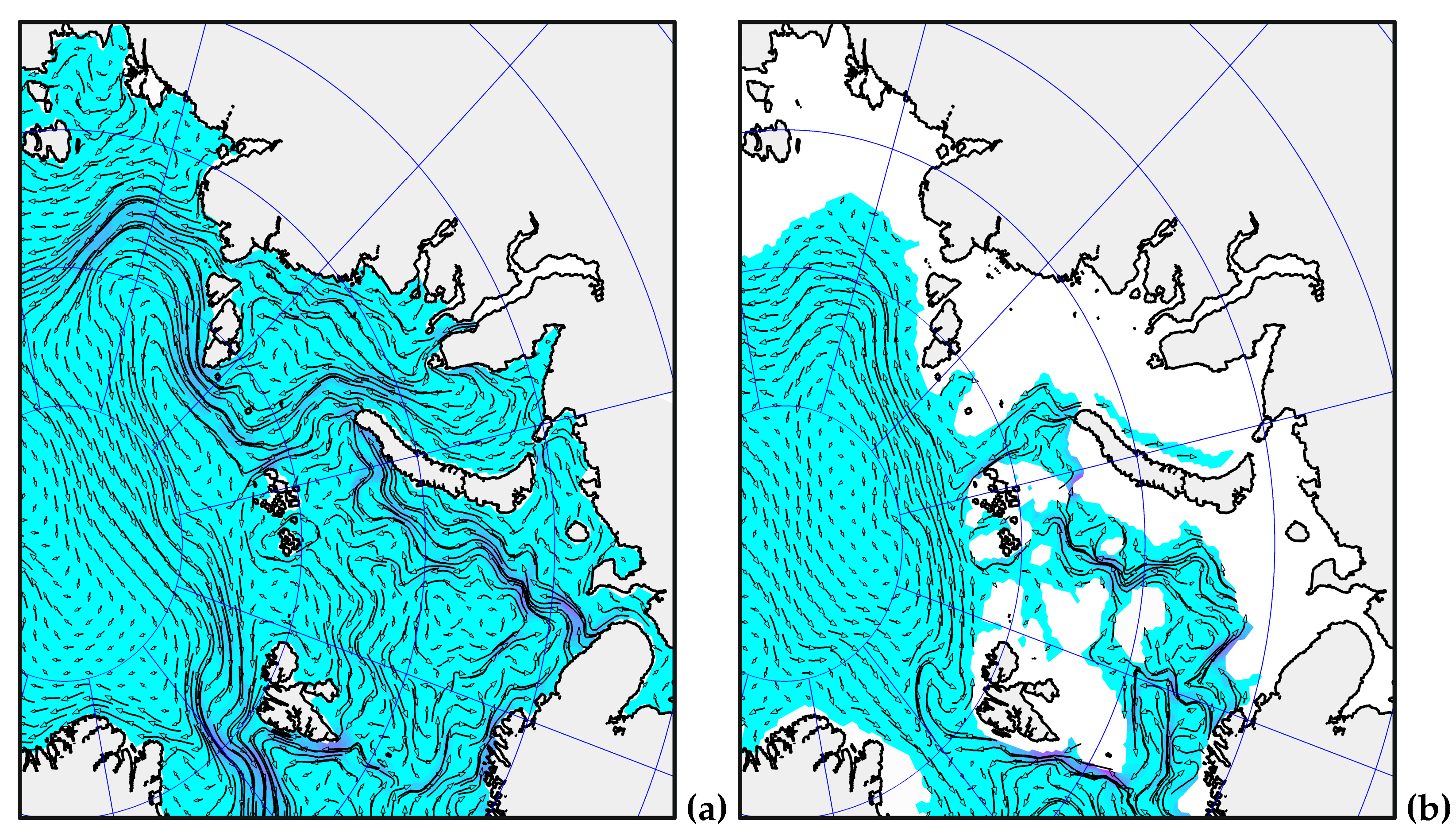
2.1. Method of numerical simulation of the Arctic Ocean and sea ice state
2.2. Method of simulation of microplastic transport
2.2.1. Ice freezing and melting
2.2.2. Biofouling
2.3. Experimental design
3. Results
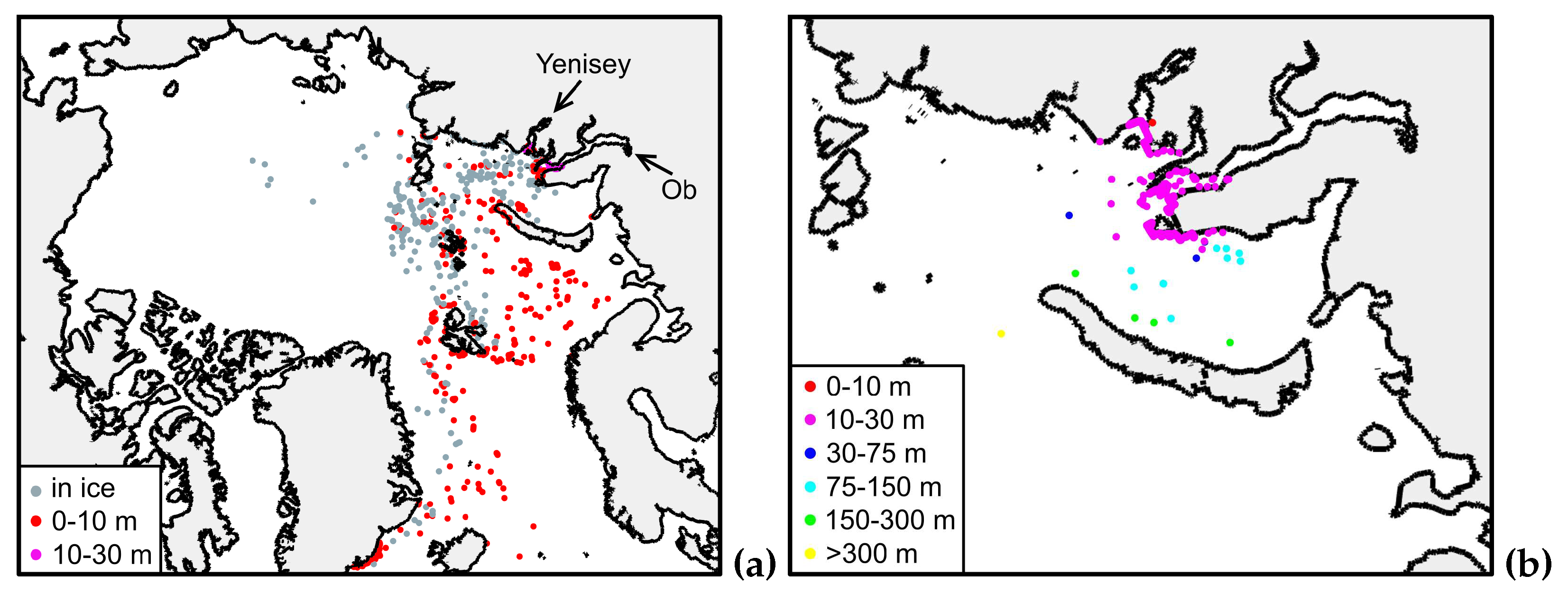
3.1. Control experiment. Biofouling excluded
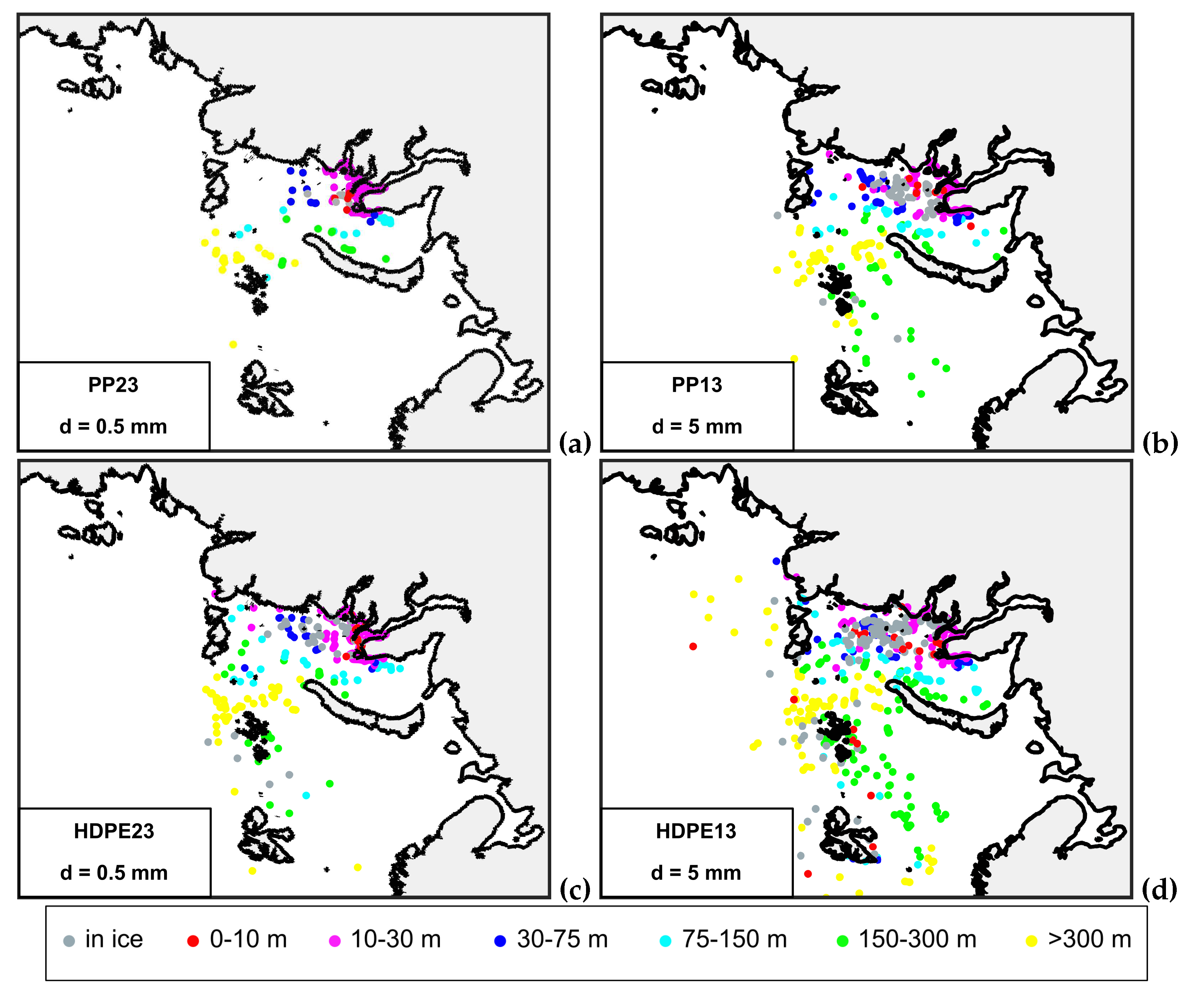
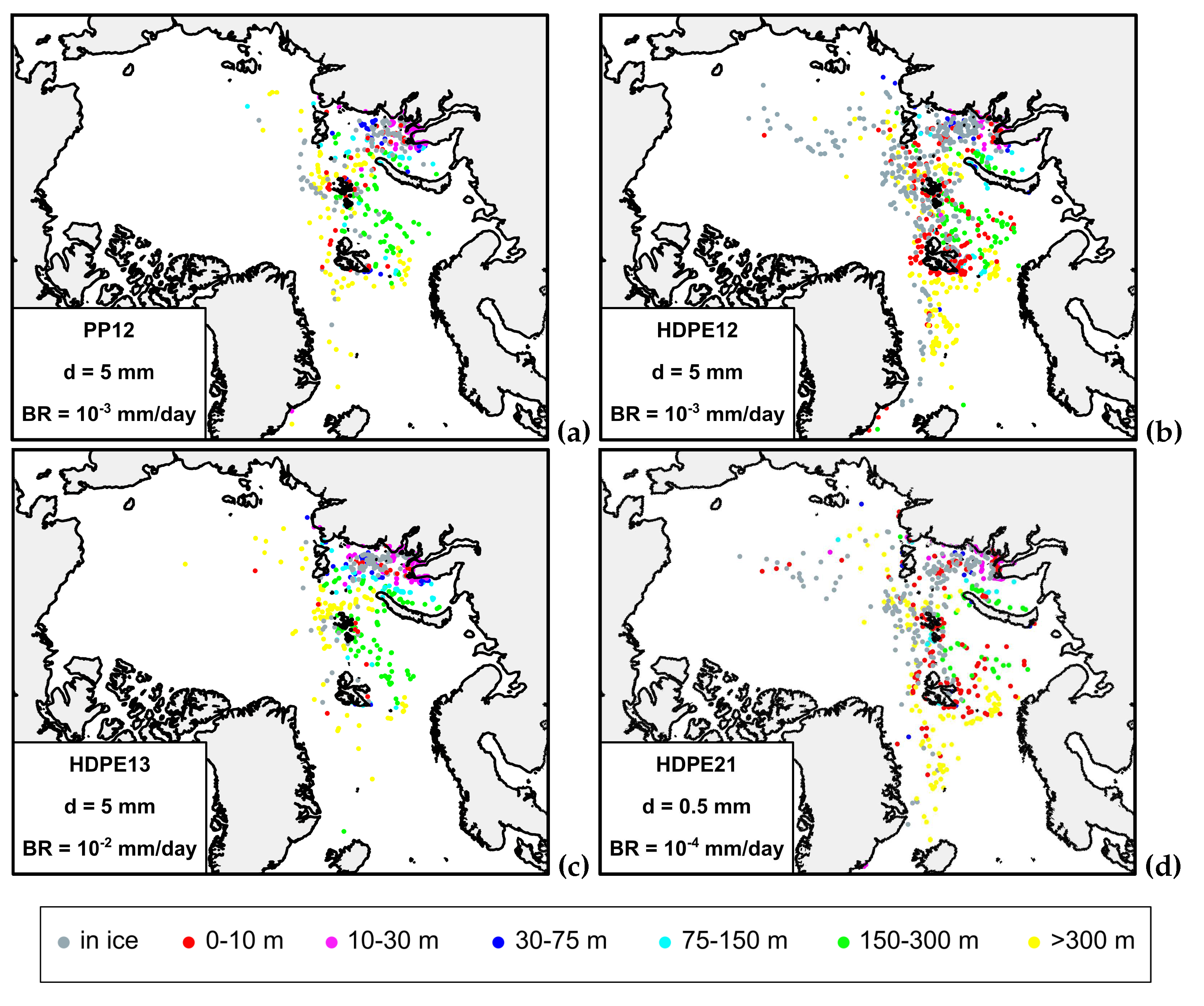
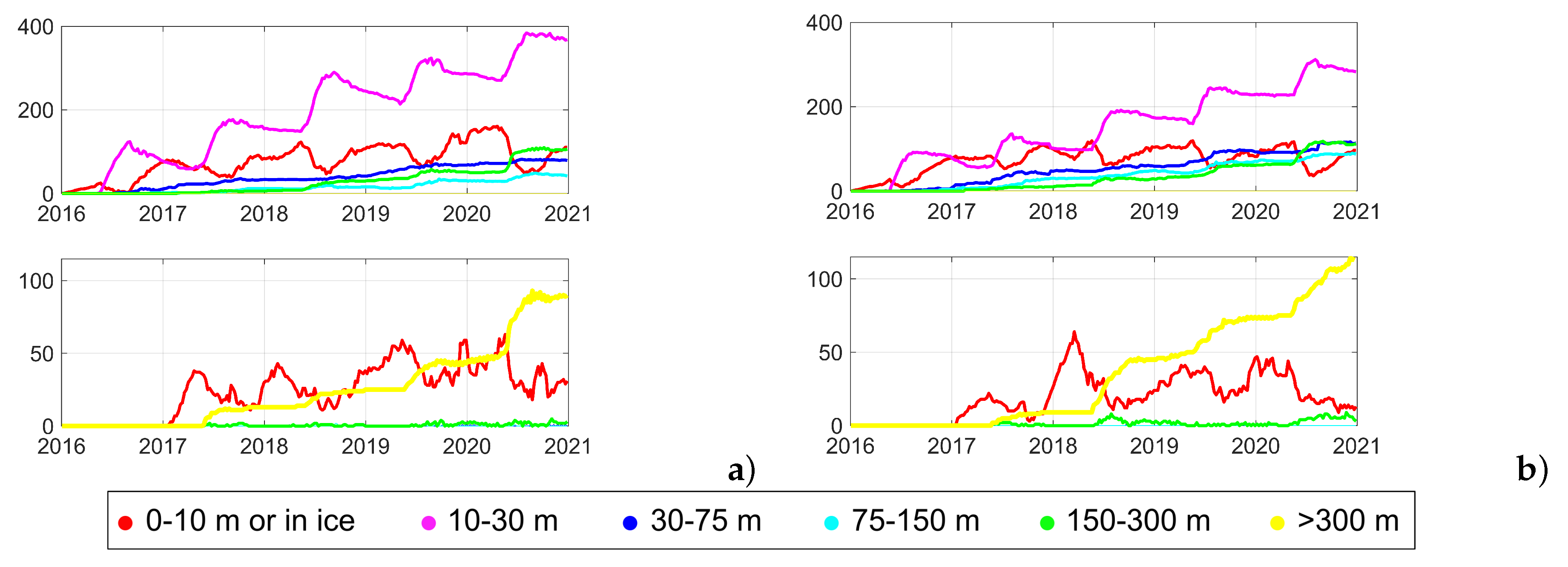
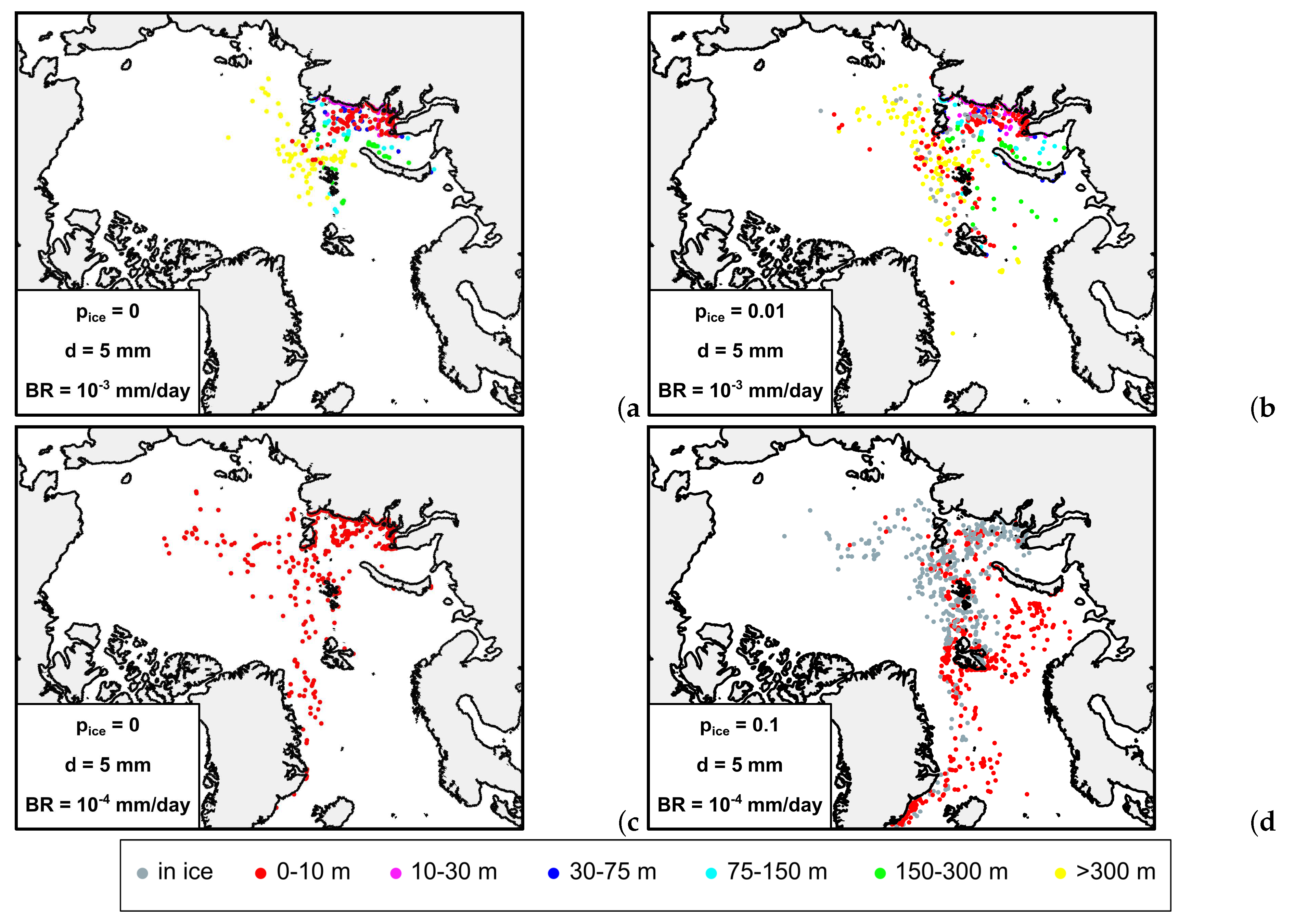
3.2. Sensitivity tests to biofouling rate and particle size
3.3. Sensitivity MP tranfer to freezing into sea ice
4. Discussion
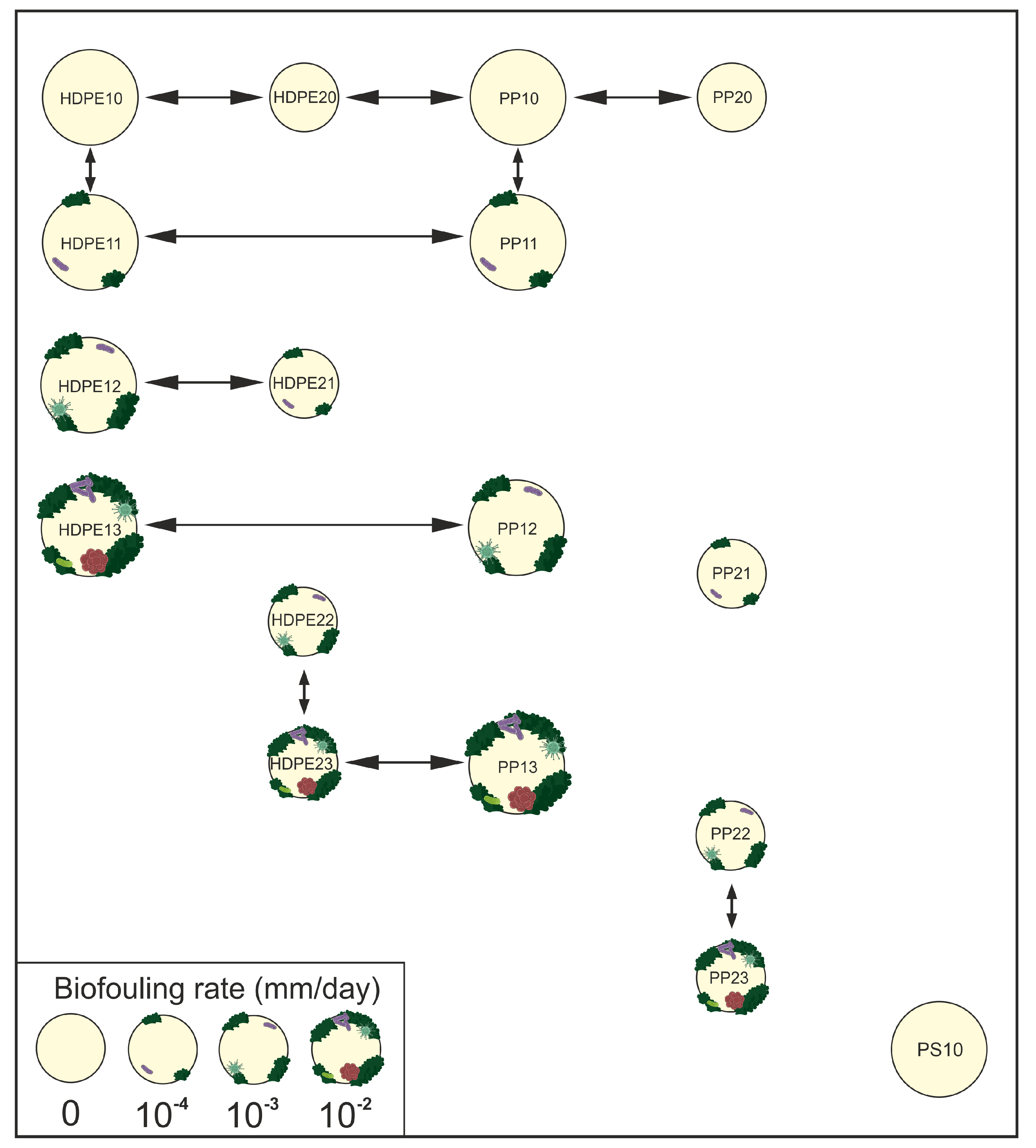
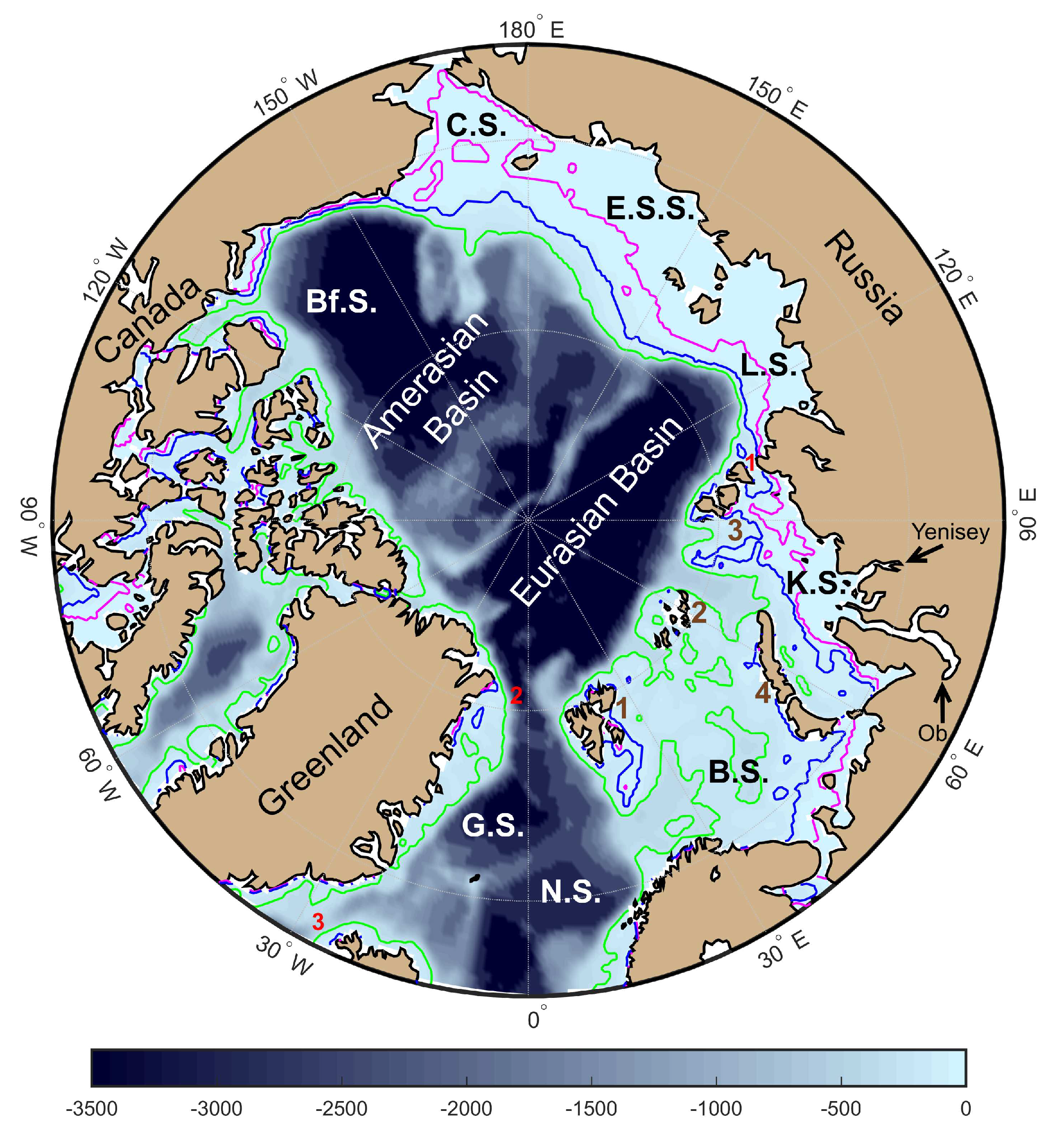
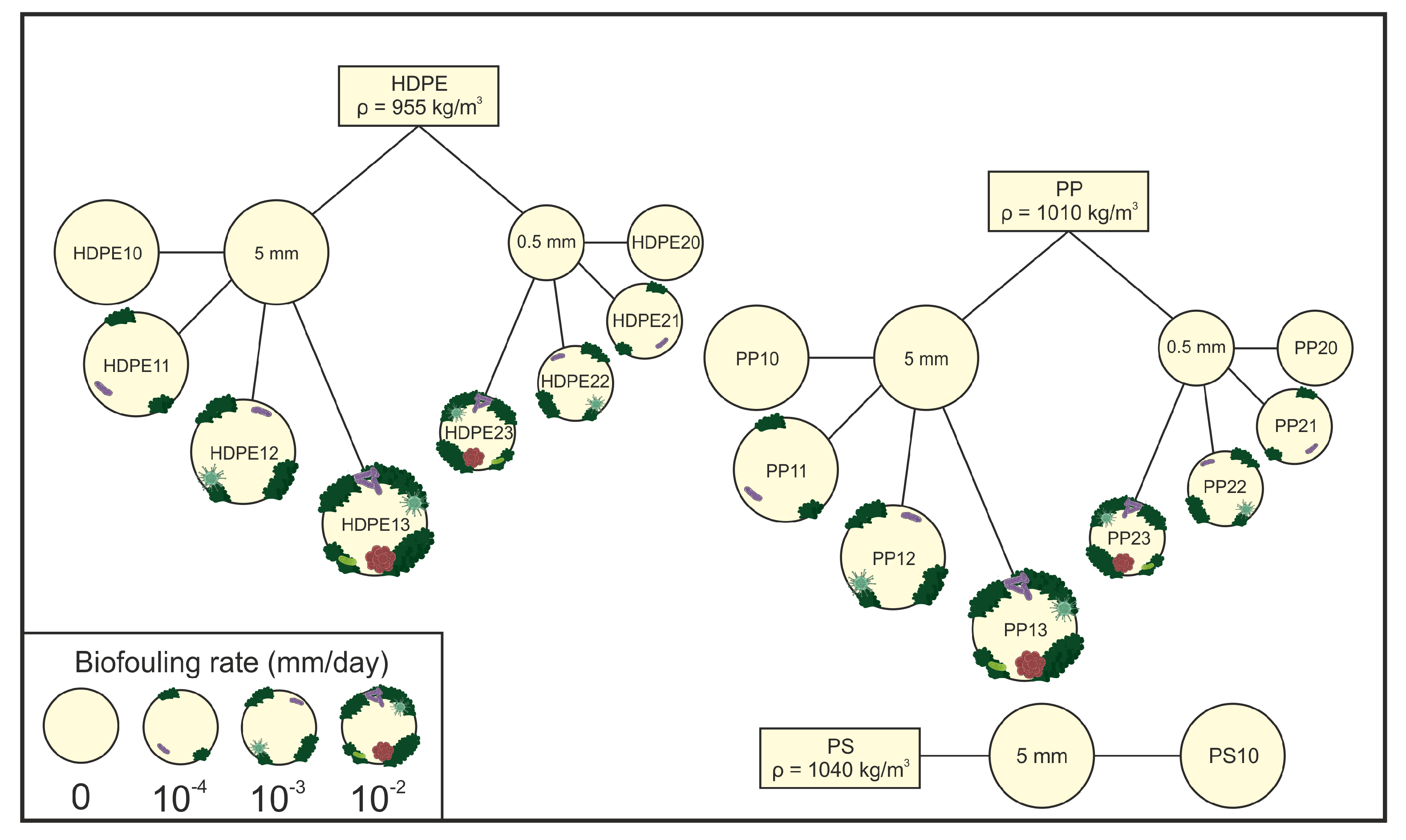
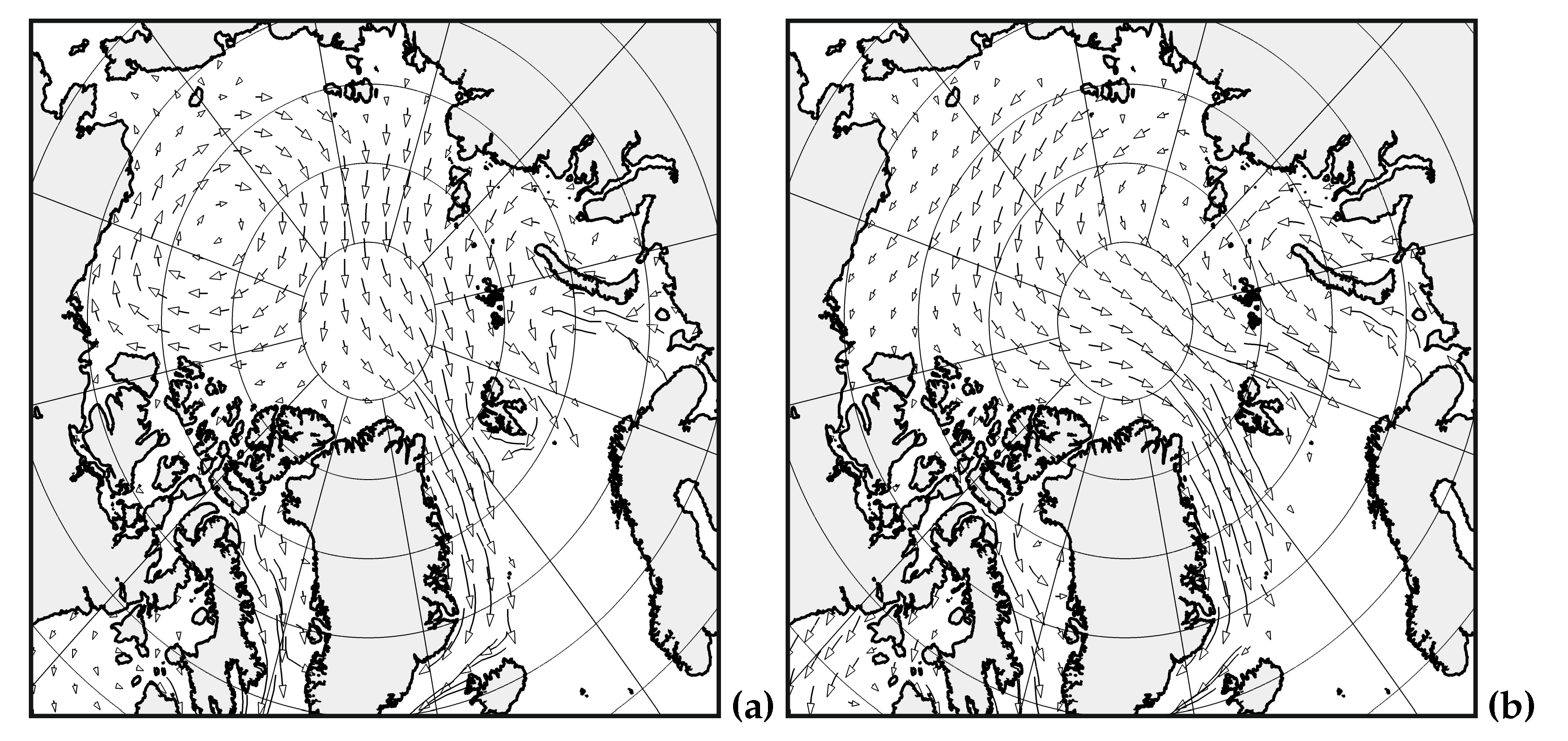
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cózar, A.; et al. Plastic debris in the open ocean. Proc. Nat. Acad. Sci. 2014, 111, 10239–10244. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Persistence of Plastic Litter in the Oceans. In Marine Anthropogenic Litter. Eds. Bergmann M., Gutow L., Klages M. Springer, Cham. 2015. [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos Trans R Soc Lond B Biol Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Tekman, M.; Gutow, L. Sea change for plastic pollution. Nature 2017, 544, 297. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Allen, S.; Krumpen, T.; Allen, D. High Levels of Microplastics in the Arctic Sea Ice Alga Melosira arctica, a Vector to Ice-Associated and Benthic Food Webs. Sci. Technol. 2023, 57, 6799–6807. [Google Scholar] [CrossRef] [PubMed]
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic pollution in the marine environment. Heliyon 2020, 27, e04709. [Google Scholar] [CrossRef] [PubMed]
- Law, K.L. Plastics in the Marine Environment. Annu. Rev. Mar. Sci. 2017, 9, 205–29. [Google Scholar] [CrossRef] [PubMed]
- Carbery, M.; O’Connor, W.; Palanisami, T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health. Environment International 2018, 115, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Schaafsma, F.L.; van Werven, B.; et al. Plastic ingestion by juvenile polar cod (Boreogadus saida) in the Arctic Ocean. Polar Biol 2018, 41, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Woodall, L.C.; Sanchez-Vidal, A.; Canals, M.; Paterson, G.L.; Coppock, R.; Sleight, V.; Calafat, A.; Rogers, A.D.; Narayanaswamy, B.E.; Thompson, R.C. The deep sea is a major sink for microplastic debris. R. Soc. Open Sci. 2014, 1, 140317. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Lee, H.J.; Kim, J.S.; Kang, S.H.; Yang, E.J.; Cho, K.H.; Tian, Z.; Andrady, A. Importance of seasonal sea ice in the western Arctic ocean to the Arctic and global microplastic budgets. J. Hazard. Mater. 2021, 418, 125971. [Google Scholar] [CrossRef] [PubMed]
- Peeken, I.; Primpke, S. Beyer, B; Gütermann, J; Katlein, C.; Krumpen, T.; Bergmann, M.; Hehemann, L.; Gerdts, G. Arctic sea ice is an important temporal sink and means of transport for microplastic. Nat. Commun. 2018, 9, 1505. [Google Scholar] [CrossRef] [PubMed]
- Obbard, R.W.; et al. Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth’s Future 2014, 2, 315–320. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Allen, D.; Wang, Z.; Luo, X.; Yang, L.; Chen, J.; Hu, Z.; Chen, P.; Du, W.; Allen, S. Cryosphere as a temporal sink and source of microplastics in the Arctic region, Geoscience Frontiers 2023, 14, 101566. 14. [CrossRef]
- Yakushev, E.; et al. Microplastics distribution in the Eurasian Arctic is affected by Atlantic waters and Siberian rivers. Earth Environ. 2021, 2, 23. [Google Scholar] [CrossRef]
- Lusher, A.L.; et al. Microplastics in Arctic polar waters: the first reported values of particles in surface and sub-surface samples. Sci. Rep. 2015, 5, 14947. [Google Scholar] [CrossRef] [PubMed]
- Cózar, A.; et al. The Arctic Ocean as a dead end for floating plastics in the North Atlantic branch of the Thermohaline Circulation. Sci.Adv. 2017, 3, e1600582. [Google Scholar] [CrossRef] [PubMed]
- Kanhai, D.K.; Gårdfeldt, K.; Lyashevska, O.; Hassellöv, M.; Thompson, R.C.; O’Connor, I. Microplastics in sub-surface waters of the Arctic Central Basin. Mar Pollut Bull. 2018, 130, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Kanhai, D.K.; Johansson, C.; Frias, J.P.G.L.; Gardfeldt, K.; Thompson, R.C.; O’Connor, I. Deep sea sediments of the Arctic Central Basin: A potential sink for microplastic. Deep-Sea Research, 2019; 145 Part I, 137–142. [Google Scholar] [CrossRef]
- Mu, J.; Qu, L.; Jin, F.; Zhang, S.; Fang, C.; Ma, X.; Zhang, W.; Huo, C.; Cong, Y.; Wang, J. Abundance and distribution of microplastics in the surface sediments from the northern Bering and Chukchi Seas. Environmental Pollution 2019, 245, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Collard, F.; Fabres, J.; et al. Plastic pollution in the Arctic. Nat Rev Earth Environ 2022, 3, 323–337. [Google Scholar] [CrossRef]
- Mu, J.; et al. Microplastics abundance and characteristics in surface waters from the Northwest Pacific, the Bering Sea, and the Chukchi Sea. Mar. Pollut. Bull. 2019, 143, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Frank, Y.A.; Vorobiev, D.S. Kayler,O.A.; Vorobiev, E.D.; Kulinicheva, K.S.; Trifonov, A.A.; Hunter, T.S. Evidence for microplastics contamination of the remote tributary of the Yenisey River Siberia-the pilot study results. Water 2021, 13, 3248. [Google Scholar] [CrossRef]
- Pogojeva, M.; et al. Distribution of floating marine macro-litter in relation to oceanographic characteristics in the Russian Arctic Seas. Mar. Pollut. Bull. 2021, 166, 112201. [Google Scholar] [CrossRef] [PubMed]
- Van Sebille, E.; et al. The physical oceanography of the transport of floating marine debris. Environ. Res. Lett. 2020, 15, 023003. [Google Scholar] [CrossRef]
- Kaiser, D.; Kowalski, N.; Waniek, J.J. Effects of biofouling on the sinking behavior of microplastics. Environ. Res. Lett. 2017, 12, 124003. [Google Scholar] [CrossRef]
- Morét-Ferguson, S.; Law, K.L.; Proskurowski, G.; Murphy, E.K.; Peacock, E.E.; Reddy, C.M. The size, mass, and composition of plastic debris in the western North Atlantic Ocean. Mar Pollut Bull. 2010, 60, 1873–1878. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiong, X.; Jiang, X.; Shi, H.; Wu, C. Sinking of floating plastic debris caused by biofilm development in a freshwater lake. Chemosphere 2019, 222, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Fazey, F.M.; Ryan, P.G. Biofouling on buoyant marine plastics: An experimental study into the effect of size on surface longevity. Environ Pollut. 2016, 210, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Chubarenko, I. Physical processes behind interactions of microplastic particles with natural ice. Environ. Res. Commun. 2022, 4, 012001. [Google Scholar] [CrossRef]
- Chubarenko, I.; Bocherikova, I.; Esiukova, E.; Isachenko, I.; Kupriyanova, A.; Lobchuk, O.; Fetisov, S. Microplastics in sea ice: A fingerprint of bubble flotation. Science of The Total Environment 2023, 164611. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, L.; et al. Interactions between the ice algae Fragillariopsis cylindrus and microplastics in sea ice. Environment International 2020, 139, 105697. [Google Scholar] [CrossRef]
- Pradel, A.; Gautier, M.; Bavay, D.; Gigault, J. Micro- and nanoplastics’ transfer in freezing saltwater: Implications for their fate in polar waters. Environ. Sci.: Processes Impacts 2021, 23, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Mountford, A.S.; Morales Maqueda, M.A. Eulerian Modeling of the Three-Dimensional Distribution of Seven Popular Microplastic Types in the Global Ocean. JGR Oceans 2019, 124, 8558–8573. [Google Scholar] [CrossRef]
- Mountford, A. S.; Morales Maqueda, M.A. Modeling the accumulation and transport of microplastics by sea ice. JGR Oceans 2020, 126, e2020JC016826. [Google Scholar] [CrossRef]
- Huserbråten, M.B.O.; Hattermann, T.; Broms, C.; et al. Trans-polar drift-pathways of riverine European microplastic. Sci. Rep. 2022, 12, 3016. [Google Scholar] [CrossRef] [PubMed]
- Golubeva, E. N.; Platov, G. A. On improving the simulation of Atlantic Water circulation in the Arctic Ocean. J. Geophys. Res. 2007, 112, C04S05. [Google Scholar] [CrossRef]
- Golubeva, E.N.; Platov, G.A. Numerical modeling of the Arctic Ocean ice system response to variations in the atmospheric circulation from 1948 to 2007. Izv. Atmospheric and Oceanic Physics 2009, 45, 137–151. [Google Scholar] [CrossRef]
- Leonard, B.P. A stable and accurate convective modeling procedure based on quadratic upstream interpolation. Computer Methods Applied Mechanics and Engineering 1979, 19, 59–98. [Google Scholar] [CrossRef]
- Leonard, B.P.; Lock, A.P.; MacVean, M.K. Conservative explicit unrestricted-timestep multidimensional constancy-preserving advection schemes. Mon. Weather Rev. 1996, 124, 2588–2606. [Google Scholar] [CrossRef]
- Platov, G.A. Numerical modeling of the Arctic Ocean deepwater formation: Part II. Results of regional and global experiments. Izv. Atmospheric and Oceanic Physics 2011, 47, 377–392. [Google Scholar] [CrossRef]
- Hunke, E. C.; Dukowicz, J. K. An elastic-viscous-plastic model for ice dynamics. J. Phys. Oceanography 1997, 27, 1849–1867. [Google Scholar] [CrossRef]
- Bitz, C.M.; Lipscomb, W.H. An energy-conserving thermodynamic model of sea ice. J. Geophys. Res. 1999, 104, 15669–15677. [Google Scholar] [CrossRef]
- Lipscomb, W.H.; Hunke, E.C. Modeling Sea Ice Transport Using Incremental Remapping. Mon. Weather Rev. 2004, 132, 1341–1354. [Google Scholar] [CrossRef]
- Kalnay, E.; Kanamitsu, M.; Kistler, R.; Collins, W.; Deaven, D.; Gandin, L.; Iredell, M.; Saha, S.; White, G.; Woollen, J.; Zhu, Y.; Chelliah, M.; Ebisuzaki, W.; Higgins, W.; Janowiak, J.; Mo, K. C.; Ropelewski, C.; Wang, J.; Leetmaa, A.; Reynolds, R.; Jenne, R.; Joseph, D. The NCEP/NCAR 40-Year Reanalysis Project. Bull. Amer. Meteor. Soc. 1996, 77, 437–471. [Google Scholar] [CrossRef]
- Platov, G.A.; Golubeva, E.N.; Kraineva, M.V.; Malakhova, V.V. Modeling of climate tendencies in Arctic seas based on atmospheric forcing EOF decomposition. Ocean Dynamics 2019, 69, 747–767. [Google Scholar] [CrossRef]
- Woodgate, R.A. Increases in the Pacific inflow to the Arctic from 1990 to 2015, and insights into seasonal trends and driving mechanisms from year-round Bering Strait mooring data. Prog. Oceanogr. 2018, 160, 124–154. [Google Scholar] [CrossRef]
- Vorosmarty, C.J.; Fekete, B.M.; Tucker, B.A. 1998. Global River Discharge, 1807-1991, V[ersion]. 1.1 (RivDIS). ORNL DAAC, Oak Ridge, Tennessee, USA. [CrossRef]
- McClelland, J.W.; Tank, S.E.; Spencer, R.G.M.; Shiklomanov, A.I.; Zolkos, S.; Holmes, R.M.; Arctic Great Rivers Observatory. Discharge Dataset, Version 20230810. Available online: https://arcticgreatrivers.org/discharge/ (accessed on 01 December 2023).
- Whitefield, J., Winsor, P., McClelland, J., Menemenlis, D. A new river discharge and river temperature climatology data set for the pan-Arctic region. Ocean Modelling 2015, 88, 1–15, ISSN 1463-5003. [CrossRef]
- Forum for Arctic Modeling and Observational Synthesis. Available online: https://web.whoi.edu/famos (accessed on 01 December 2023).
- Proshutinsky, A.; Aksenov, Y.; Clement Kinney, J.; Gerdes, R.; Golubeva, E.; Holland, D.; Holloway, G.; Jahn, A.; Johnson, M.; Popova, E.; Steele, M.; Watanabe, E. Recent advances in Arctic ocean studies employing models from the Arctic Ocean Model Intercomparison Project. Oceanography 2011, 24, 102–113. [Google Scholar] [CrossRef]
- Proshutinsky, A.; Krishfield, R.; Toole, J.M.; Timmermans, M.-L.; Williams, W.; Zimmermann, S.; et al. Analysis of the Beaufort Gyre freshwater content in 2003–2018. JGR Oceans 2019, 124. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, Y.; Karcher, M.; Proshutinsky, A.; Gerdes, R.; de Cuevas, B.; Golubeva, E.; Kauker, F.; Nguyen, A.T.; Platov, G.A.; Wadley, M.; Watanabe, E.; Coward, A.C.; Nurser, A.J.G. Arctic pathways of Pacific Water: Arctic Ocean Model Intercomparison experiments. JGR Oceans 2016, 121, 27–59. [Google Scholar] [CrossRef] [PubMed]
- Platov, G.A.; Golubeva, E.N.; Krupchatnikov, V.N.; et al. Numerical Study of Interaction between the Climate System Components and Their Role in Arctic Amplification of Climate Change. Water Resour 50 2023, 664–674. [Google Scholar] [CrossRef]
- Golubeva, E.; Kraineva, M.; Platov, G.; Iakshina, D.; Tarkhanova, M. Marine heatwave in Siberian Arctic Seas and adjacent region. Remote Sens. 2021, 13, 4436. [Google Scholar] [CrossRef]
- Zhiyao, S.; Tingting, W.; Fumin, X.; Ruijie, L. A simple formula for predicting settling velocity of sediment particles. Water Science and Engineering 2008, 1, 37–43. [Google Scholar] [CrossRef]
- Chubarenko, I.; Bagaev, A.; Zobkov, M.; Esiukova, E. On some physical and dynamical properties of microplastic particles in marine environment. Mar. Pollut. Bull. 2016, 108, 105–12. [Google Scholar] [CrossRef] [PubMed]
- Fisher, N.S.; Bjerregaard, P.; Fowler, S.W. Interactions of marine plankton with transuranic elements. 1. Biokinetics of neptunium, plutonium, americium, and californium in phytoplankton. Limnol. Oceanogr. 1983, 28, 432–447. [Google Scholar] [CrossRef]
- Wassmann, P.; Reigstad, M. Future Arctic Ocean seasonal ice zones and implications for pelagic-benthic coupling. Oceanography 2011, 24, 220–231. [Google Scholar] [CrossRef]
- Schmidt, C.; Krauth, T.; Wagner, S. Export of Plastic Debris by Rivers into the Sea. Environ Sci Technol. 2017, 51, 12246–12253. [Google Scholar] [CrossRef] [PubMed]
- Jalón-Rojas, I.; Wang, X.H.; Fredj, E. A 3D numerical model to Track Marine Plastic Debris (TrackMPD): Sensitivity of microplastic trajectories and fates to particle dynamical properties and physical processes. Mar Pollut Bull. 2019, 141, 256–272. [Google Scholar] [CrossRef] [PubMed]
- Gradova, M.A.; Golubeva, E.N. Evaluation of the Riverine Heat Influx Impact on the Arctic Ice Cover Based on Numerical Modeling. Russ. Meteorol. Hydrol. 2023, 48, 639–644. [Google Scholar] [CrossRef]
- Korsnes, R.; Pavlova, O.; Godtliebsen, F. Assessment of potential transport of pollutants into the Barents Sea via sea ice – an observational approach. Mar Pollut Bull. 2002, 44, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Emberson-Marl, H.R.L.; Coppock, M.; Cole, B.J.; Godley, N.; Mimpriss, S.E.; Nelms, P.K.; Lindeque, P.K. Microplastics in the Arctic: a transect through the Barents Sea. Front. Mar. Sci. Sec. Marine Pollution 2023, 10. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




