Introduction
Leishmanioses are diseases caused by protozoan flagellate parasites of the genus
Leishmania. Some of them are causative agents of disease in humans, dogs, cats, horses, rodents etc., but most of them, 20 out of 30 species are zoonotic [
1]. The life cycle of
Leishmania parasites is dixenous i.e. it includes vertebrate host (final host) and arthropod host (biological vector) although they may be transmitted directly from the final host without the arthropod vector [
2,
3,
4,
5,
6].
In vertebrates,
Leishmania sp. parasitise intracellularly in mononuclear phagocytes as amastigote forms while in the biological vector, e.g. sandflies of the genera
Phlebotomus and
Lutzomyia, they live extracellularly as promastigote forms [
7]. Infections with
Leishmania parasites occur mainly in tropical and poor areas of Africa, Asia and Latin America, but also increasingly in countries of southern Europe. They can cause cutaneous (CL) or visceral leishmaniasis (VL) in humans and canine leishmaniosis (CanL) in dogs [
8,
9].
From the aspect of veterinary medicine, CanL is most important, although the disease can be diagnosed in other domestic and wild animals [
10]. Dogs are important reservoirs of the
Leishmania parasites, i. e. the causative agent of CanL and human leishmaniasis, which in Europe is caused by the most prevalent species –
L. infantum [
11], while in Brazil the disease has been related to eight species of
Leishmania, mainly
L. infantum and
L. brasiliensis [
1]. Although wild animals may be involved in the transmission of
Leishmania, dogs are the most relevant reservoir in urban areas due their close association with humans [
12]. The zoonotic nature of
L. infantum is a serious concern for animal and public health. In the Americas, Brazil is the country responsible for the endemic disease of leishmaniasis, with 96% of the VL cases occurring in this country. Although surveys that assess the VL/CanL situation in Brazil are scarce, the expansion of cities into forest areas, the high number of domestic dogs as well as the adaptability of sandflies are likely to be responsible for the spread of leishmaniasis in northeastern municipalities of Brazil [
13,
14,
15]. Thus, dogs have become the main target for disease control [
16]. In Europe, the import of
L. infantum into previously non-endemic countries is known for a long time but lately it became a problem and seems to be a consequence of the increased travelling with dogs into endemic areas and the import of infected puppies and stray dogs from endemic areas [
17,
18,
19,
20]. Further, global warming favors the occurrence of the sandfly vector in these countries with increasing the risk of transmission [
21]. Animal and human health authorities have recognized the emergence of
leishmaniasis in part of the European Union as a serious public health problem and have stressed the importance of surveillance, notification and control of this disease [
11].
CanL shows a variable, non-specific spectrum of clinical signs depending on the immune status, and infected dogs are often asymptomatic, which is a major challenge as they may contribute to the spread of the disease despite the lack of clinical signs; therefore, detection of such animals is important but remains mostly incidental [
22].
The serological tests already available such as the indirect fluorescent antibody test (IFAT), ELISA – , lineblot and lateral flow tests (LFT) play a central role in disease surveillance because they are inexpensive and in particular the LFT is easy to use [
23]. However, they often fail to detect subclinical infections, may crossreact with other infectious agents and do not always discriminate infected from vaccinated dogs, as many of these tests are based on whole
leishmania-antigens [
24,
25]. To ameliorate the sero-diagnosis of leishmaniosis, we recently developed a new recombinant kinesin antigen from
L. infantum (rKLi8.3) that has shown improved diagnostic performance in VL patients, independent of their endemic area origin [
26].
The aim of this study was to compare the diagnostic performance of different sero-diagnostic methods on panel of sera from Croatian and Brazilian dogs that have been classified as Leishmania positive or negative dogs by IFAT, parasitological examination and/or Dual Path Platform (DPP®) tests. The results demonstrate that ELISA, lineblot and LFT based on the rKLi8.3 antigen exhibit superior diagnostic performance with regard to symptomatic (SD), oligosymptomatic (OD), asymptomatic (AD), vaccinated dogs and those suffering from other canine infections.
Materials and methods
Canine serum sample
We analyzed serum samples from 232 Croatian and 112 from Brazilian adult dogs of both sexes. Croatian samples were obtained from a serum repository of the Serological Animal Laboratory, Department for Parasitology and Invasive Diseases with Clinics, Faculty of Veterinary Medicine, University of Zagreb and included asymptomatic and symptomatic animals with serologically proven leishmaniosis, healthy controls and sera from dogs with other infections.
Brazilian samples were obtained from a serum repository of the Laboratory of Immunopathology at the Federal University of Ouro Preto, Minas Gerais and comprised sera from parasitological confirmed (L. infantum), asymptomatic, oligosymptomatic, symptomatic dogs and non-infected, endemic controls (EC). VAC (n = 20) sera from healthy dogs immunized with Leish-Tec® vaccine (Ceva Hertape AS, Juatuba, Brazil) were received from the blood bank of the Santo Agostinho Veterinary Hospital, Belo Horizonte, Minas Gerais, Brazil (Table S1).
Clinical examinations
Dogs positive for CanL were classified according to the presence of clinical symptoms into three groups: asymptomatic (AD; n = 11), without clinical suggestive signs of the disease; oligosymptomatic (OD; n = 12), with a maximum of three symptoms indicative of CanL including dull hair and/or localized alopecia and/or moderate weight loss; symptomatic (SD; n = 13), with clinical signs characteristic of CanL, such as dull hair, severe weight loss, onychogryphosis, skin lesions, apathy and keratoconjunctivitis [
27].
Serological tests
IFAT classified sera were re-evaluated by two ELISAs and one lateral flow test (LFT): VetLine® Leishmania ELISA test (GSD Frankfurt, Germany) based on native
L. infantum antigens, recombinant KLi8.3 antigen based ELISA (rKLi8.3 ELISA, GSD Frankfurt, Germany) and the rKLi8.3 LFT (INgezim® Leishma CROM, GSD Madrid, Spain) [
26]. For ELISAs, samples were probed in duplicates and optical densities (OD) were read in a spectrophotometer. The mean was used to classify samples as positive, negative or- ambiguous (uncertain), following kit instructions. The principle of the LFT is shown in Figure S1 and tests were performed according to the manufacturer's instructions. Briefly, 10 μl of serum was added to the sample window of the test device, followed by 4 drops of buffer provided in the kits. The test was read 10 min after the addition of the buffer. The results were positive if two distinct red or pink lines appeared (test and control region), negative when control region was positive but test region negative and invalid if the control line failed to appear. LFT was performed twice, and critical sera (low antibody titers/asymptomatic dogs) were analyzed in triplicate.
For IFAT, the antigen was prepared from promastigotes of
L. infantum MON-1 (archive isolate from continuous
in vitro culture-MCAN/HR/2011/SO) for Croatia and
L. amazonensis (MHOM/BR/1960/BH6) and
L. chagasi (MHOM/BR/1972/BH46) for Brazil, and anti-
Leishmania antibodies were detected using goat or rabbit anti-dog FITC IgG. Cytoplasmic or membranous green fluorescence of promastigotes was scored positive with a cutoff dilution of 1/40. As controls, CanL positive and -negative sera were included [
28].
Recombinant antigens, rK28, rK39 and rLb6H were produced at the Infectious Disease Research Institute (IDRI), Seattle, USA. ELISAs with these antigens were performed as previously described [
29].
For lineblots (VetBlot® Leishmania LineBlot, GSD Frankfurt, Germany), recombinant rKLi8.3, rK39 and rKLO8 and a native Leishmania antigen were printed with a dispenser (FrontLine HR microliter contact; BioDot, Irvine, CA, USA) on a nitrocellulose membrane (GE Healthcare, Chicago, USA), together with a control line for sample loading and for conjugate function as shown in Figure S2. After drying, the membranes were cut into 3 mm stripes and stored at 4 °C until use. Prior use, stripes were equilibrated in 1 ml sample dilution buffer (10 mM phosphate buffer, pH 7.2; Samples were added in a dilution of 1:100 and the membranes were incubated gentle shanking for 1 hour at room temperature. After 3 washes with 1 ml washing buffer (0.2 M phosphate, pH = 7.2; for 5 min each, the membranes were incubated gentle shanking for 30 min with 1 ml horseradish peroxidase (HRP) labelled protein A/G conjugate at room temperature. The stripes were washed three times with 1 ml washing buffer for 5 min. The development of the signals took place by incubation of the membranes with 1 ml 3,3’,5,5’-tetramethylbenzidine (TMB) substrate solution for 15 min with gentle shaking at room temperature. The reaction was stopped by the addition of at least 1 ml distilled water. After drying the membranes for at least 30 min at room temperature, the stripes were evaluated.
Statistical analyses
Statistical analysis of the mean optical densities (ODs) of infected and healthy groups was performed using the Mann-Whitney U test. Comparisons of the mean ODs from asymptomatic, oligosymptomatic, symptomatic and healthy dogs as well as dogs with other diseases were performed using Kruskal–Wallis test, followed by Dunn’s Multiple Comparison Test (GraphPad Software 9.0, San Diego, CA, USA). Sensitivity and specificity were calculated with their 95% confidence intervals. P values of <0.05 were considered statistically significant.
Results
Comparative testing of IFAT, ELISA and LFT for CanL sero-diagnosis in Croatia
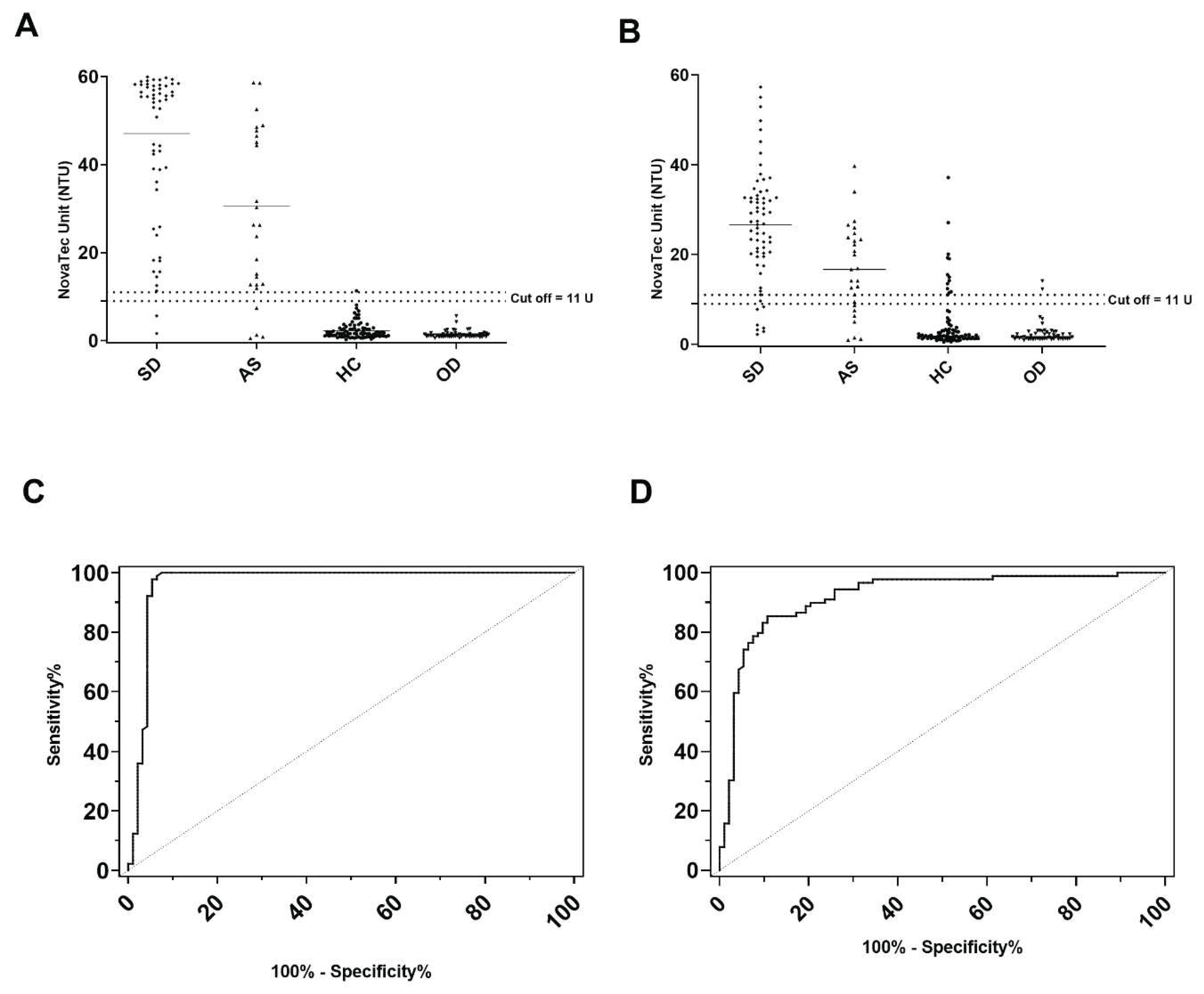
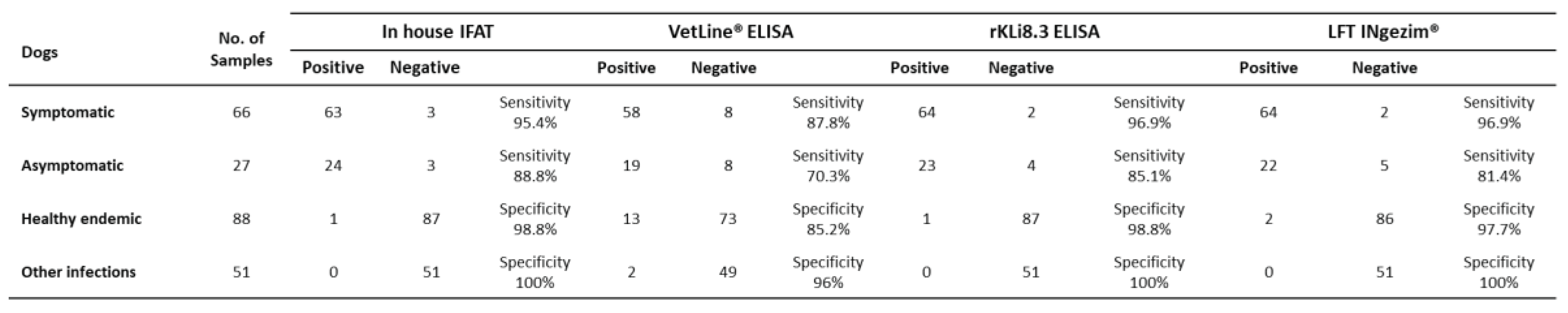
IFAT characterized sera from Croatian dogs were re-evaluated by the VetLine® ELISA, the rKLi8.3 ELISA and the LFT.
The rKLi8.3 based ELISA and LFT showed the highest diagnostic performance. Of 66 symptomatic dogs, 64 animals were positive in both, rKLi8.3 ELISA and LFT. This corresponds to a sensitivity of 96.9%. IFAT detected 63 and VetLine® ELISA 58 dogs, corresponding to 95.4% and 87.8% sensitivity, respectively. Of the 27 asymptomatic dogs, 19 sera were positive by the VetLine® ELISA, while 23 and 22 dogs were positive by the rKLi8.3 ELISA and the LFT, respectively. Interestingly, 24 animals were CanL positive by IFAT, corresponding to 88.8% sensitivity.
To assess the specificity of these tests, we analyzed 88 serum samples from healthy dogs originating from the same endemic area. The specificity was as follows: IFAT (98.8%), VetLine® ELISA (85.2%), rKLi8.3 ELISA (98.8%) and LFT (97.7%) (
Table 1).
Further, potential cross-reactivity of the test systems was assessed with 51 serum samples from dogs that were parasitological positive for canine babesiosis, Giardia duodenalis, Dirofilaria repens, Toxocara canis, Ehrlichia canis, Ancylostoma caninum and Anaplasmosis. While the VetLine® ELISA cross-reacted with 2 sera of canine babesiosis, none of the 51 sera cross-reacted with IFAT, rKLi8.3 ELISA and LFT.
Comparison of the ELISAs indicated that rKLi8.3 ELISA showed the highest area under the curve (AUC), with a value of 0.9664 and a confidence interval (CI) 95%: 0.9342 to 0.9986, whereas VetLine® ELISA showed an AUC of 0.9221, CI 95%: 0.8805 to 0.9638 (
Figure 1).
Evaluation of the rKLi8.3 ELISA and LFT in healthy, vaccinated and infected dogs from Brazil
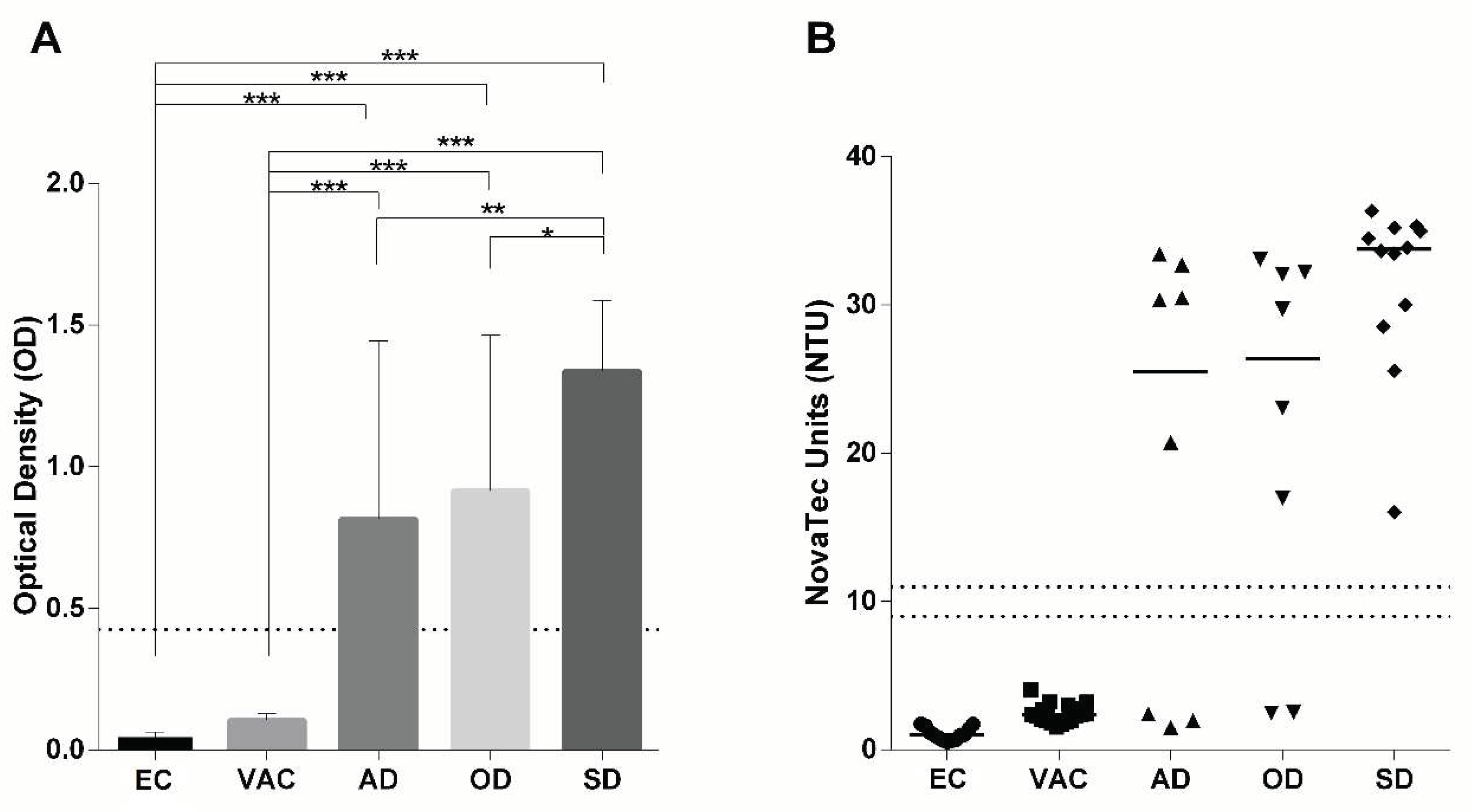
We next analyzed a cohort of Brazilan dogs which comprised parasitologically confirmed cases of CanL, including asymptomatic - (AD), oligosymptomatic - (OD) and symptomatic (SD) infected dogs. As controls, non-vaccinated (EC) and vaccinated (Leish-Tec
®) healthy dogs (VAC) from the same endemic area were investigated by the rKLi8.3 ELISA and LFT. All SD (12/12), 6/8 OD and 5/8 AD dogs were positive in ELISA as well as LFT and none of the healthy and vaccinated control animals showed any signals in ELISA (
Figure 2 and Table S2). It is interesting to note, that both rKLi8.3-based tests, ELISA and LFT, gave identical results in all investigated groups. Accordingly, the sensitivity of the ELISA and LTF for SD, OD and AD was 100%, 75% and 62%,respectively and their specificity for VAC and EC was 100%. Of note, rKLi8.3 based ELISA and LFT did not cross-react with autochtonous
T.cruzi infected dogs from Brazil (Figure S3, Table S3).
ELISAs using recombinant rK28, rK39 and rLb6H antigens were also performed on the same serum panel. With the rK28 antigen 13/13 SD, 9/12 OD and 8/11 AD were identified as positive. The corresponding sensitivities are 100 %, 75 % and 72.7 %. For the rK39 antigen, 12/13 SDs, 10/12 ODs and 8/11 ADs were positive, representing sensitivities of 92.3%, 83.3% and 72.7%. With the rLb6H antigen, 13/13 SD, 11/12 OD and 11/11 AD were positive, giving a sensitivity of 100% for SD and AD and 90.9% for OD. The rK28 exhibited 100% specificity, rK39 91.6% and rLb6H only 14.2%. (Figure S4).
Detection of CanL-specific IgG antibodies in dogs from Croatia and Brazil by rKLi8.3 lineblot
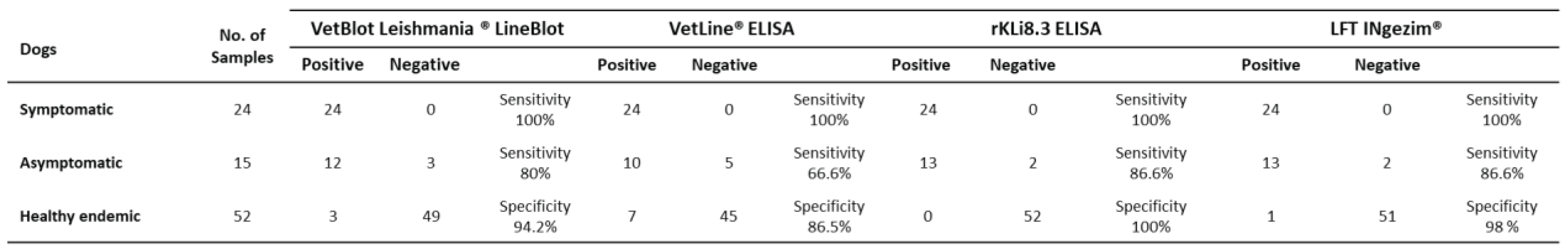
24 sera from SD, 15 from AD and 52 from EC dogs from Croatia previously tested positive or negative for CanL by IFAT, VetLine® ELISA, rKLi8.3 ELISA and LFT INgezim® were also tested with the rKLi8.3 lineblot (Table S4).
In SD, all above mentioned test systems showed 100% sensitivity. For AD, rKLi8.3 lineblot showed 80%, rKLi8.3 ELISA 86.6%, LFT INgezim® 86.6%, and VetLine® ELISA 66.6% sensitivity. The EC group exhibited 94.2% specificity with the rKLi8.3 lineblot, 100% with the rKLi8.3 ELISA, 98% with the LFT, and 86.5% with the VetLine® ELISA (
Table 2).
Further, we analyzed Brazilian dogs with parasitologically confirmed CanL cases, including AD, OD and SD dogs by lineblot. EC and vaccinated healthy dogs were used as controls (Table S5). In the SD group (n=13) all sera were positive, in the AD group (n=11), 6 sera were positive, showing a sensitivity of 54.5%. In the OD group (n=11), 8 sera were positive, exhibiting a sensitivity of 72.7%. All sera from the EC groups (n=13) and vaccinated dogs (n=16) were tested negative, showing 100% specificity.
Discussion
Leishmania infected dogs act as the main reservoir for the zoonotic disease and play a crucial role in the transmission to humans. Increased travel and the import of dogs by animal welfare organizations bears the risk of spreading leishmaniosis to countries where it has not previously occurred. Part of the problem is also that surveillance and notification of canine leishmaniosis has a low priority among animal health authorities [
11,
30].
To minimize the risk of spreading infections with
L. infantum, systematic infection control of dogs originating from or moved to endemic areas is required. Thus, there is a need for a simple and reliable, point of care test. Currently used test systems, e.g. ELISA, IFAT, PCR and parasitological examinations require a laboratory and are not suitable for fast and simple routine diagnostic [
31]. Further, some of the diagnostic tests show low specificity and cross-reactivity between
Leishmania s.p. and other canine pathogens, such as
Ehrlichia,
Babesia canis,
Toxoplasma gondii and
T. cruzi or are unable to differentiate between vaccinated and infected dogs [
29,
32].
An additional challenge of CanL diagnosis are infected but clinically asymptomatic dogs, because they represent a potential reservoir of the parasite [
22]. The detection of asymptomatic dogs often represent a problem, as
Leishmania-specific antibody levels are either very low or some animals never become sero-positive or revert to sero-negative while they still harbor the parasite [
33,
34,
35,
36].
IFAT is most commonly used to diagnose CanL in Mediterranean countries [
33,
37]. However, IFAT is a complex and time-consuming technique that relies on the experience of the observer, as positive antigen-antibody reactions are assessed by the fluorescence intensity. It needs great expertise and results may vary between different investigators. In our study, IFAT was highly sensitive for SD and AD and showed a high specificity in EC, while other studies reported sensitivities ranging from 68% to 100% and specificities from 60% to 90% [
38,
39].
Commercially available ELISA which are based on whole parasite antigens often show low reproducibility due to the use of different
Leishmania species and thus variant antigen compositions that react with different VL antibodies [
40].
Further, several recombinant antigens, such as rK39, rK26, rK28, rLb6H and rKLO8 have been used for sero-diagnosis in human and dogs [
26,
36,
41,
42,
43,
44]. Although the reported sensitivities of these antigens are generally high for symptomatic dogs, variable sensitivities (98%-64%) have been described for asymptomatic dogs [33,36,44)] These discrepancies illustrate the problems of comparing different studies, especially when carried out on different canine serum samples.
The rKLi8.3 kinesin antigen is characterized by a high content of charged amino acids from
L. infantum and an increased numbers of B cell epitopes. This antigen demonstrated superior diagnostic efficacy over rK39 in diagnosing human VL when used in the ELISA and LFT format [
26], however its diagnostic potential in dogs has not been investigated.
The CanL screening of 232 Croatian dogs revealed that both, rKLi8.3 ELISA and - LFT have a very similar diagnostic sensitivity with regard to symptomatic (both 96.9%) and asymptomatic (85.1%; 81,4%) dogs. Further, both tests showed 100% specificity for animals with other infections and about 98% when tested in healthy endemic controls. Similar results were also observed for the rKLi8.3 lineblot. It is interesting to note that the IFAT results were comparable to rKLi8.3 based test systems, with the difference that IFAT was slightly better in AD (88.8%) and marginally less sensitive in SD (93.9%). In contrast, the VetLine® ELISA showed a significantly poorer diagnostic efficiency, with a sensitivity of 87.8% in SD and 70.3% in AD groups.
The extension of the study with Brazilian dogs provided additional insight into the diagnostic performance of the rKLi8.3 based ELISA,- lineblot and - LFT in a different endemic area.
Screening of 72 sera from parasitologically tested dogs from Belo Horizonte, Brazil, showed that all three tests gave very similar results and that the performance of these tests was independent of the endemic area. Neither vaccinated nor endemic controls gave positive signals in either tests and rKLi8.3 did not cross-react with sera from T.cruzi infected dogs.
Notably, the same sera (except for vaccinated animals) were tested in ELISA with rK28, rK39 and rLb6H antigens, but none of these antigens outperformed the rKLi8.3 antigen in terms of SD, OD and AD group sensitivities or specificity for EC group.
In conclusion, the study shows that the rKLi8.3 -based test systems are highly sensitive and specific diagnostic tools for Leishmaniosis in humans and dogs, irrespective of the endemic area. However, it is important to note that, unlike parasitological methods, serological tests are fundamentally dependent on the amount of Leishmania-specific antibodies, which are not present in all asymptomatic animals. However, the high diagnostic performance, combined with simple use, rapid results and laboratory independence, make the rKLi8.3 LFT suitable for routine and large-scale diagnosis in veterinary practices and in the field.
Author Contributions
All authors had access to the data in this study and take responsibility for integrity and accuracy of data analysis. The authors contribution was as follows: Conceptualization R.M., F.M., U.S., H.T.; methodology, J.R-O., A.L., H.C.T., D.H., C.A., I.E.P., A.B; Formal analysis: F.M., R.M. H.C.T.; writing-original draft preparation, F.M., R.M., writing – review and editing: U.S. and R.M.; resources, F.M., H.C.T., A. L., C. A. and A.F-T; critical review of manuscript and data: all authors; supervision, U.S. H.C.T.; project administration, U.S.; funding acquisition, U.S.
Funding
The project was funded by the Loewe Center Druid (Project C4 to U.S.) within the Hessian Excellence Program. The work was also supported by Conselho Nacional de Pesquisa e Desenvolvimento Tecnológico (CNPq, Project 310313/2019-8 to H.C.T.) and Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG, Project RED-00313-16 to H.C.T.).
Acknowledgements
The authors thank Dr. Patrick Bastien for providing us Leishmania strains and Bärbel Camara and Anne Hellhund for their excellent technical support. We are grateful for the excellent support of Dr. Thomas Widmann (Transmit GmbH) for writing the patent and for bringing the license agreement with Gold Standard Diagnostics to a conclusion. We also thank Dr. Maria de Lourdes P. Araújo and the staff of Santo Agostinho Veterinary Hospital of Belo Horizonte, MG, Brazil, for the LeishTec-immunized dog sera and Dr. Marta de Lana (UFOP, Brazil) for the kind donation of sera from dogs infected with T. cruzi.
Conflicts of Interest
U.S. and R.M. are inventors on a patent application related to the use of rKLi8.3 that has been filed by the Philipps-University Marburg (EP22152398.8). The title of patent application: Diagnostic test for high sensitive detection of antibodies from visceral leishmaniasis patients. A. L., D.H. are employees of Gold Standard Diagnostics Frankfurt, A. F-T. and C.A. are employees of Gold Standard Diagnostics Madrid and were involved in the development of rKLi8.3 ELISA and lateral flow tests.
References
- Ashford, R. The leishmaniases as emerging and reemerging zoonoses. Int. J. Parasitol. 2000, 30, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Boggiatto, P.M.; Gibson-Corley, K.N.; Metz, K.; Gallup, J.M.; Hostetter, J.M.; Mullin, K.; Petersen, C.A. Transplacental Transmission of Leishmania infantum as a Means for Continued Disease Incidence in North America. PLoS Negl. Trop. Dis. 2011, 5, e1019. [Google Scholar] [CrossRef] [PubMed]
- Owens, S.D.; Oakley, D.A.; Marryott, K.; Hatchett, W.; Walton, R.; Nolan, T.J.; Newton, A.; Steurer, F.; Schantz, P.; Giger, U. Transmission of visceral leishmaniasis through blood transfusions from infected English Foxhounds to anemic dogs. J. Am. Veter- Med Assoc. 2001, 219, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Serafim TD, Coutinho-Abreu IV, Dey R, Kissinger R, Valenzuela JG, Oliveira F, Kamhawi S. 2021. Leishmaniasis: the act of transmission. Trends in Parasitology 37:976-987.
- Svobodova, V.; Svoboda, M.; Friedlaenderova, L.; Drahotsky, P.; Bohacova, E.; Baneth, G. Canine leishmaniosis in three consecutive generations of dogs in Czech Republic. Veter- Parasitol. 2017, 237, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Boggiatto, P.M.; Gibson-Corley, K.N.; Metz, K.; Gallup, J.M.; Hostetter, J.M.; Mullin, K.; Petersen, C.A. Transplacental Transmission of Leishmania infantum as a Means for Continued Disease Incidence in North America. PLoS Negl. Trop. Dis. 2011, 5, e1019. [Google Scholar] [CrossRef]
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef]
- Morales-Yuste, M.; Martín-Sánchez, J.; Corpas-Lopez, V. Canine Leishmaniasis: Update on Epidemiology, Diagnosis, Treatment, and Prevention. Veter- Sci. 2022, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. ; Who Leishmaniasis Control the WHO Leishmaniasis Control Team Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Quinnell, R.J.; Courtenay, O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology 2009, 136, 1915–1934. [Google Scholar] [CrossRef]
- Berriatua, E.; Maia, C.; Conceição, C.; Özbel, Y.; Töz, S.; Baneth, G.; Pérez-Cutillas, P.; Ortuño, M.; Muñoz, C.; Jumakanova, Z.; et al. Leishmaniases in the European Union and Neighboring Countries. Emerg. Infect. Dis. 2021, 27, 1723–1727. [Google Scholar] [CrossRef]
- Navea-Pérez HM, Díaz-Sáez V, Corpas-López V, Merino-Espinosa G, Morillas-Márquez F, Martín-Sánchez J. 2015. in wild rodents: reservoirs or just irrelevant incidental hosts? Parasitology Research 114:2363-2370.
- Nunes JB, Laurenti MD, Kanamura HY, Pereira AAC, Colombo FA, Marques MJ. 2016. Infection in Dogs from the Southern Region of State, Brazil. Revista Do Instituto De Medicina Tropical De Sao Paulo 58.
- Reguera, R.M.; Morán, M.; Pérez-Pertejo, Y.; García-Estrada, C.; Balaña-Fouce, R. Current status on prevention and treatment of canine leishmaniasis. Veter- Parasitol. 2016, 227, 98–114. [Google Scholar] [CrossRef] [PubMed]
- Werneck, G.L. Visceral leishmaniasis in Brazil: rationale and concerns related to reservoir control. Rev. de Saude publica 2014, 48, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, P.S.S.; Hiramoto, R.M.; Pereira, V.B.R.; Camprigher, V.M.; Taniguchi, H.H.; Barbosa, J.E.d.R.; Cortez, L.R.P.d.B.; Fonseca, E.d.S.; Guimarães, R.B.; Tolezano, J.E. Impact of the dog population and household environment for the maintenance of natural foci of Leishmania infantum transmission to human and animal hosts in endemic areas for visceral leishmaniasis in Sao Paulo state, Brazil. PLOS ONE 2021, 16, e0256534. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Cardoso, L. Spread of Leishmania infantum in Europe with dog travelling. Veter- Parasitol. 2015, 213, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, I.; Volkmann, M.; Beelitz, P.; Merle, R.; Müller, E.; Kohn, B. Retrospective evaluation of vector-borne infections in dogs imported from the Mediterranean region and southeastern Europe (2007–2015). Parasites Vectors 2019, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Menn, B.; Lorentz, S.; Naucke, T.J. Imported and travelling dogs as carriers of canine vector-borne pathogens in Germany. Parasites Vectors 2010, 3, 34–34. [Google Scholar] [CrossRef]
- Hamel, D.; Silaghi, C.; Pfister, K. Arthropod-borne infections in travelled dogs in Europe. Parasite 2013, 20, 9. [Google Scholar] [CrossRef]
- Oerther, S.; Jöst, H.; Heitmann, A.; Lühken, R.; Krüger, A.; Steinhausen, I.; Brinker, C.; Lorentz, S.; Marx, M.; Schmidt-Chanasit, J.; et al. Phlebotomine sand flies in Southwest Germany: an update with records in new locations. Parasites Vectors 2020, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moshfe, A.; Mohebali, M.; Edrissian, G.; Zarei, Z.; Akhoundi, B.; Kazemi, B.; Jamshidi, S.; Mahmoodi, M. Canine visceral leishmaniasis: Asymptomatic infected dogs as a source of L. infantum infection. Acta Trop. 2009, 112, 101–105. [Google Scholar] [CrossRef]
- Maia, C.; Campino, L. Methods for diagnosis of canine leishmaniasis and immune response to infection. Veter- Parasitol. 2008, 158, 274–287. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Cardoso, L.; Pennisi, M.G.; Petersen, C.; Bourdeau, P.; Oliva, G.; Miró, G.; Ferrer, L.; Baneth, G. Diagnostic Challenges in the Era of Canine Leishmania infantum Vaccines. Trends Parasitol. 2017, 33, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.M.F.d.S.; Furuta, P.I.; de Carvalho, D.; Machado, R.Z. Study of cross-reactivity in serum samples from dogs positive for Leishmania sp. , Babesia canis and Ehrlichia canis in enzyme-linked immunosorbent assay and indirect fluorescent antibody test. 2008, 17, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, R.; Shams-Eldin, H.; Witt, S.; Latz, A.; Heinz, D.; Fresco-Taboada, A.; Aira, C.; Hübner, M.P.; Sukyte, D.; Visekruna, A.; et al. Development of a Novel Enzyme-Linked Immunosorbent Assay and Lateral Flow Test System for Improved Serodiagnosis of Visceral Leishmaniasis in Different Areas of Endemicity. Microbiol. Spectr. 2023, 11, e0433822. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.B.; Teixeira-Carvalho, A.; Giunchetti, R.C.; Guerra, L.L.; Carvalho, M.G.; Mayrink, W.; Genaro, O.; Corrêa-Oliveira, R.; A Martins-Filho, O. Phenotypic features of circulating leucocytes as immunological markers for clinical status and bone marrow parasite density in dogs naturally infected by Leishmania chagasi. Clin. Exp. Immunol. 2006, 146, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Martinkovic, F.; Marinculic, A. Antibodies against Leishmania cross-react with Crithidia luciliae: indirect immunofluorescence and Dot-ELISA study in dogs. Parasitol. Res. 2005, 98, 378–380. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.E.; Silva, K.P.; Menegati, L.M.; Pinheiro, A.C.; Assunção, E.A.O.; Araújo, M.D.L.P.; Abass, E.; Duthie, M.S.; Steinhoff, U.; Teixeira, H.C. Performance of recombinant proteins in diagnosis and differentiation of canine visceral leishmaniasis infected and vaccinated dogs. Eur. J. Microbiol. Immunol. 2020, 10, 165–171. [Google Scholar] [CrossRef]
- Lachaud L, Dedet JP, Marty P, Faraut F, Buffet P, Gangneux JP, Ravel C, Bastien P, Lei WGNH. . Surveillance of leishmaniases in France, 1999 to 2012. Eurosurveillance 2013, 18, 38–44.
- Grimaldi G, Jr. , Tesh RB. . Leishmaniases of the New World: current concepts and implications for future research. Clin Microbiol Rev 1993, 6, 230–250. [Google Scholar]
- Zanette, M.F.; de Lima, V.M.F.; Laurenti, M.D.; Rossi, C.N.; Vides, J.P.; da Costa Vieira, R.F.; Biondo, A.W.; Marcondes, M. Serological cross-reactivity of Trypanosoma cruzi, Ehrlichia canis, Toxoplasma gondii, Neospora caninum and Babesia canis to Leishmania infantum chagasi tests in dogs. Rev. Soc. Bras. Med. Trop. 2014, 47, 105–107. [Google Scholar] [CrossRef]
- Mettler M, Grimm F, Capelli G, Camp H, Deplazes P. 2005. Evaluation of enzyme-linked immunosorbent assays, an immunofluorescent-antibody test, and two rapid tests (immunochromatographic-dipstick and gel tests) for serological diagnosis of symptomatic and asymptomatic Leishmania infections in dogs. J Clin Microbiol 43:5515-5519.
- Dye, C.; Vidor, E.; Dereure, J. Serological diagnosis of leishmaniasis: on detecting infection as well as disease. Epidemiology Infect. 1993, 110, 647–656. [Google Scholar] [CrossRef]
- Quinnell, R.J.; Courtenay, O.; Garcez, L.; Dye, C. The epidemiology of canine leishmaniasis: transmission rates estimated from a cohort study in Amazonian Brazil. Parasitology 1997, 115, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Porrozzi, R.; da Costa, M.V.S.; Teva, A.; Falqueto, A.; Ferreira, A.L.; dos Santos, C.D.; Fernandes, A.P.; Gazzinelli, R.T.; Campos-Neto, A.; Grimaldi, G. Comparative Evaluation of Enzyme-Linked Immunosorbent Assays Based on Crude and Recombinant Leishmanial Antigens for Serodiagnosis of Symptomatic and Asymptomatic Leishmania infantum Visceral Infections in Dogs. Clin. Vaccine Immunol. 2007, 14, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Santarem N, Sousa S, Amorim CG, de Carvalho NL, de Carvalho HL, Felgueiras O, Brito M, da Silva AC. 2020. Challenges in the serological evaluation of dogs clinically suspect for canine leishmaniasis. Sci Rep 10:3099.
- Ferreira Ede C, de Lana M, Carneiro M, Reis AB, Paes DV, da Silva ES, Schallig H, Gontijo CM. 2007. Comparison of serological assays for the diagnosis of canine visceral leishmaniasis in animals presenting different clinical manifestations. Vet Parasitol 146:235-241.
- Ferroglio, E.; Centaro, E.; Mignone, W.; Trisciuoglio, A. Evaluation of an ELISA rapid device for the serological diagnosis of Leishmania infantum infection in dog as compared with immunofluorescence assay and Western blot. Veter- Parasitol. 2007, 144, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Nolan, T.J.; Herman, R. Effects of Long-Term in Vitro Cultivation on Leishmania donovani Promastigotes. J. Protozool. 1985, 32, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.E.; Silva, K.P.; Menegati, L.M.; Pinheiro, A.C.; Assunção, E.A.O.; Araújo, M.D.L.P.; Abass, E.; Duthie, M.S.; Steinhoff, U.; Teixeira, H.C. Performance of recombinant proteins in diagnosis and differentiation of canine visceral leishmaniasis infected and vaccinated dogs. Eur. J. Microbiol. Immunol. 2020, 10, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Abad, L.P.M.; Almeida, C.S.; Mattos, A.M.M.; Mendonça, A.C.P.; Alves, M.J.; Pinheiro, A.C.; Porrozzi, R.; Abass, E.; Steinhoff, U.; Teixeira, H.C. Diagnostic accuracy of rKLO8 versus rK26 ELISAs for screening of canine visceral leishmaniasis. Acta Trop. 2017, 166, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Abass, E.; Kang, C.; Martinkovic, F.; Semião-Santos, S.J.; Sundar, S.; Walden, P.; Piarroux, R.; el Harith, A.; Lohoff, M.; Steinhoff, U. Heterogeneity of Leishmania donovani Parasites Complicates Diagnosis of Visceral Leishmaniasis: Comparison of Different Serological Tests in Three Endemic Regions. PLOS ONE 2015, 10, e0116408–e0116408. [Google Scholar] [CrossRef]
- da Costa, R.T.; França, J.C.; Mayrink, W.; Nascimento, E.; Genaro, O.; Campos-Neto, A. Standardization of a rapid immunochromatographic test with the recombinant antigens K39 and K26 for the diagnosis of canine visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 678–682. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).