1. Introduction
When compared to other fuels, natural gas produces
fewer greenhouse gases during combustion. According to the International Energy Agency (IEA) [1], it covers over one-third of
global energy consumption and is expected to expand substantially in all scenarios. Although natural gas is considered a pure fuel, it contains carbon
dioxide (CO2), hydrogen sulfide (H2S), and other sulfur compounds such as mercaptans (R-SH), carbonyl sulfide (COS), and carbon
disulfide (CS2) that need to be removed. The term “sour natural gas” refers to natural gas that contains hydrogen sulfide [2]. Because H2S is toxic and corrosive in nature, even a trace amount of it should be removed or reduced substantially. To meet the natural gas sales specification, the H2S concentration must be less than 4 ppmv [3]. The procedure of sweetening of natural gas
entails the extraction of acid gases, with a primary focus on hydrogen sulfide
(H2S). There exist four distinct methodologies employed for the
removal of H2S, including absorption, adsorption, membrane
separation, and cryogenic distillation. Absorption and adsorption are widely
recognized processes employed in the natural gas sector for the removal of acid
gases. This methodology primarily relies on the selective absorption of H2S
and CO2 gases using various solvents. Alkanolamine solvents and
their blends are extensively utilized as solvent [4,5].
The process of absorption involves a chemical reaction between acid gases and
solvents; hence, it is referred to as chemisorption. The process is
characterized by high energy consumption, as well as issues related to
equipment corrosion and solvent loss. Adsorption is the process by which
molecules adhere to the adsorbent’s surface. In this process, activated carbon [6–8], MOFs [9,10],
metal oxides [11,12] and zeolites [13,14] are commonly used to eliminate H2S
from various gases. The adsorbents that were described before each have their
own set of drawbacks, which prevents them from being utilized in industrial
settings. In the case of activated carbon, for instance, it possesses a high
adsorption capacity and is superior to other adsorbents; yet its regeneration
process is very challenging. Metal oxides exhibit a high affinity for H2S,
but they have a poor surface area and a lack of pores [15]. Furthermore, they are able to demonstrate
their activity at high temperatures, which leads to increased costs related to
energy consumption and obstacles in the process. However, zeolites are
preferable to other adsorbents for their characteristics, such as high temperature
stability, large surface area, regenerability, and low cost. They are
crystalline aluminosilicates of alkaline and alkaline earth metals. Their open
3-dimensional framework structures are made of corner-sharing AlO4
and SiO4 tetrahedra. Adsorbents such as Linde Type A (4A, 5A) and
Faujasite (13X) molecular sieves are widely used in industry to remove acid
gases. The use of 13X Faujasite zeolite for the extraction of H2S
from different combination mixtures has shown promising results [16–19].
As a result of the increased demand for natural gas
usage, the sour components contained in it will have to be reduced appreciably.
This requires the synthesis of new adsorbents or improvements in the adsorption
properties of the existing ones. The introduction of various metals, such as
Cu, Zn, Co, and Ag, into zeolites has been extensively studied to increase the
adsorption performance toward H2S. Barelli et. al. [20] conducted a study on the use of Cu exchanged
13X for the removal of H2S from biogas and found that it exhibits
high adsorption capacity in a wide range of operating conditions. Chen et.al. [21] studied the adsorption performance of AgX, CoX
and ZnX zeolite synthesized by ion-exchanging of X zeolite for Claus tail gas
desulfurization. The authors reported that AgX has a high adsorption capacity
for H2S and COS in comparison to other samples. Kumar et.al. [22] investigated Ag and Cu modified X and Y
Faujasite to remove H2S from gas streams containing He, N2,
CO2, CO, and H2O. The experiments were carried out at
both room temperature and 150℃. AgX and AgY were capable to adsorb H2S
as despite the presence of other gases while CuX and CuY failed in the presence
of 2% CO. It was found that Ag exchanged Faujasite has strong selectivity
towards H2S whereas Cu exchanged Faujasite is susceptible to CO
adsorption. Kulawong et al. [23] examined Ag
exchanged NaX zeolite as a means of removing H2S from an anaerobic
digestor reactor. The author’s findings revealed that an increase in the
loading of Ag positively impacts the adsorption of H2S. As mentioned
earlier, H2S is mainly found with methane in various gases including
biogas, natural gas, refinery gas, coal gas and other gases. Therefore, in
order to assess adsorbents in a real-world setting, it is crucial to conduct
tests using real gas mixtures. The aforementioned research attempts utilized a
diverse range of synthetic gases instead of natural gas. To our knowledge,
there has been an absence of study pertaining to the adsorption of H2S
from methane.
In this work, we conducted experiments with a real
natural gas mixture to study the effect of Ag-modified 13X molecular sieves on
the removal of H2S. Ag-modified 13X samples were prepared by
ion-exchange method. In addition, we investigated the effect of the inlet H2S
concentrations and exchange rates of the Ag ions on the adsorption operation.
The current study shown significant efficacy in the development of a silver
exchanged 13X molecular sieve utilized for the extraction of H2S
from natural gas.
2. Materials and Methods
2.1. Reagents and materials
The substrate materials were conventionally
available 13X molecular sieve (from Hurtland LLC, Poland), AgNO3 (from
Stanlab LLC, Poland), Methane 2.5 (Siad Poland LLC, Poland), 5000 ppm H2S
in CH4 (from Air Liquide Polska LLC) and deionized water.
2.2. Synthesis of Ag ion exchanged 13X
Ag ion exchanged 13X was prepared by stirring 10 g
of 13X molecular sieve in various molar concentrations of AgNO3 water
solution (0.02M, 0.05M, and 0.1M in 200 ml) for 24 h. Ag ion exchanged 13X
molecular sieves were labelled as AgI-13X, AgII-13X, and AgIII-13X,
respectively. Then the samples were washed with deionized water, filtered, and
dried at 110℃ for 12 h. Calcination was done at 600℃ for overnight in the oven.
Samples were cooled and kept in a desiccator.
2.3. Characterization
The phase composition of samples was determined
using a powder X-ray diffractometer (Seifert 3003TT) with a Cu X-ray tube (kλ1 =1.540598Å, kλ2 =1.544426Å, kβ=1,39225Å). The powder samples were analyzed between 5° and 80° of 2Theta with
0.05° step. In order to validate the crystal structures, the X-ray diffraction
patterns that were acquired were compared with the information that was
collected from the Joint Committee on Powder Diffraction Standards (JCPDS).
Morphological features of the sample surface were obtained by Scanning Electron
Microscope (SEM) images using a Phenom ProX SEM (Phenom-World BV, Eindhoven,
The Netherlands). The elemental analysis of the samples were also carried out
by energy dispersive X-ray spectroscopy (EDS) during SEM image acquisition. The
BET surface of the samples was measured using a Micromeritics ASAP 2020
adsorption analyzer, Micromeritics Inc., Norcross, GA, USA.
2.4. H2S gas separation
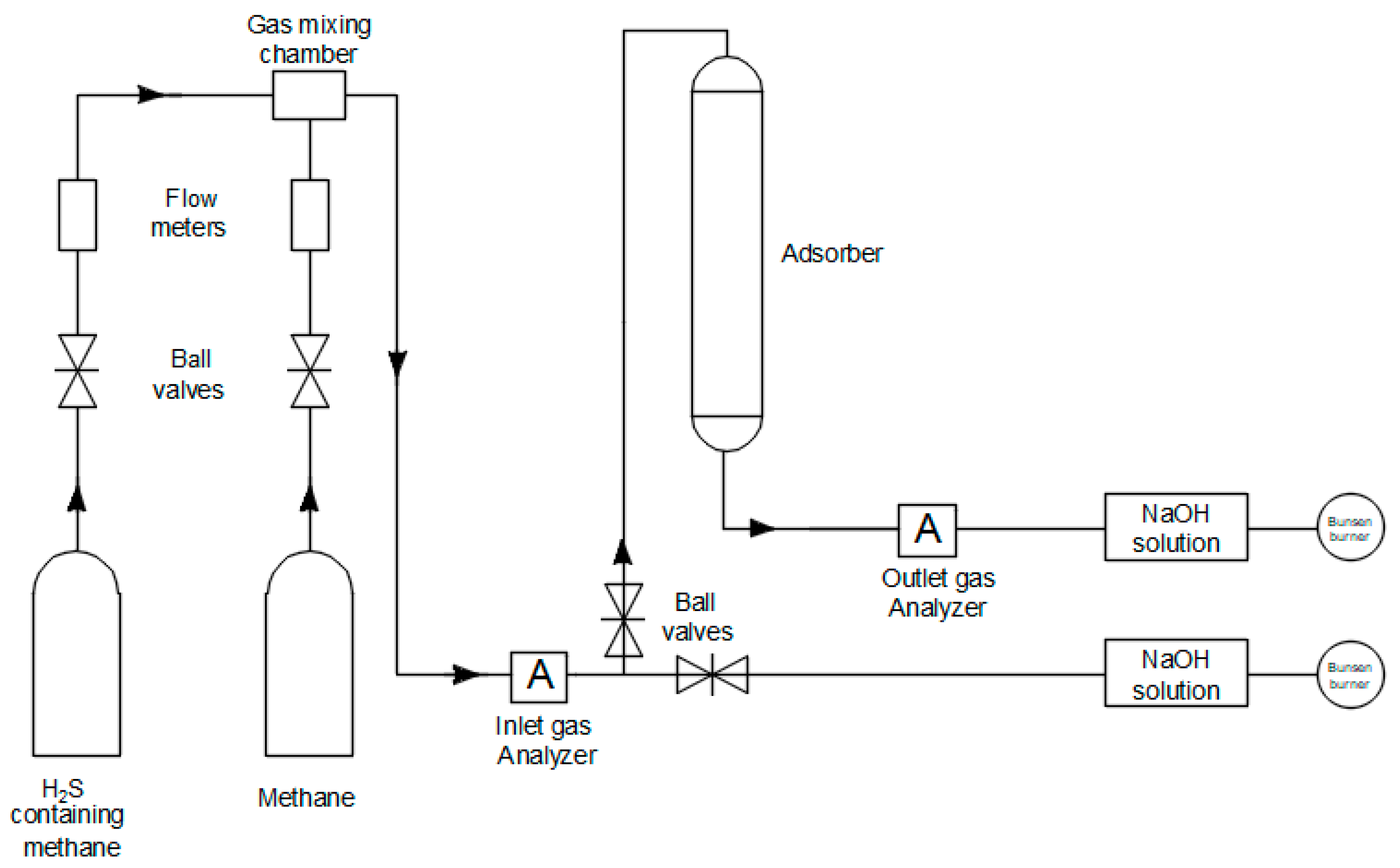
A laboratory scale set-up was used to carry out H
2S
adsorption, as shown in
Figure 1. 10 g of
adsorbent was placed in the Teflon adsorber (40 mm long and 1.5 mm internal
diameter) and attached to the system. The primary objective of employing 10 g
of adsorbent is to replicate the authentic process, as the flow rate of 400
mL/min may prevent H
2S molecules present in methane coming into
contact with the surface of the adsorbent. As a result, analyzers in the outlet
flow may quickly detect the presence of H
2S gas that is not in
direct contact with the adsorbent, allowing for the early detection of
breakthrough time. The installation was carefully checked to ensure all
connections had no leakage. The mixture was fed directly to a scrubber unit
until the desired H
2S concentration in methane was achieved.
Mixtures composed of various concentrations of H
2S were introduced
at the top of the adsorption column with a flow rate of 400 mL/min under
atmospheric pressure. The flow rate of the mixed gas was controlled by the
rotameter to maintain a flow rate of 400 mL/min. To measure initial and
breakthrough H
2S concentrations, two analyzers (Southland Sensing
Ltd., USA) were installed prior to and after the adsorber. Analyzers are able
to measure the concentration of hydrogen sulfide in a broad range, from 0 to
2000 ppm. H
2S concentrations were recorded in the input and output
every second to obtain accurate results. Outlet gas was treated with a NaOH
solution and burned before being released. Phenolphthalein was used to indicate
the H
2S saturation of the NaOH solution. Pipelines and fittings made
of stainless steel were used to prevent corrosion.
2.5. Adsorption capacity
Adsorption capacity is defined as the ratio of
adsorbed molecular amount to adsorbent mass, and it is typically represented in
units of mmol/g or mg/g [14]. The efficiency
of the adsorbent is assessed by finding its adsorption capacity. There are two
types of adsorption capacity effective and saturated adsorption capacity. The
former is calculated when the outlet concentration of H
2S is 1 ppm,
regardless of what the inlet concentration is. However, the latter is
calculated when the outlet H
2S concentration reaches the initial
concentration. Consequently, the saturated adsorption capacity is always
greater than the effective adsorption capacity. Since, in most cases, effective
adsorption capacity is important, in this study we were limited to its
calculation alone. The following equation was used to calculate
effective adsorption capacity.
where, Qtot=total gas flow rate (
Nl/h)
MW=molecular weight of H2S
(g/mol)
Cin=inlet H2S
concentration (ppmv)
t1=breakthrough time when the
outlet concentration is 1 ppmv (h)
t0=breakthrough time at the last detection
of 0 ppmv (h)
Vm=molar volume (24,414 Nl/mol)
m=mass of adsorbent material (g).
2.6. Methodology
To evaluate the material’s efficacy in removing H2S,
dynamic tests were conducted. 13X spherical pellets with an average particle
diameter in the 3–5 mm range and its Ag- ion-exchanged samples were used for
the main part of the experiments. Ag The zeolite adsorbents were heated in an
oven at 110 ℃ overnight in order to remove any residual gases and traces
of humidity that were present inside the pores. After the heating process was
complete, the zeolite adsorbents were cooled and stored in a desiccator. The
amount of samples was 10.00 g for each test measured after thermal treatment.
Adsorption runs were carried out on zeolite samples, obtaining for each set of
operating conditions the corresponding breakthrough curve. To produce an
adequate concentration of H2S (i.e., 150ppm, 300 pm, 500ppm), 5000
ppm of H2S in methane is diluted with methane. Within a certain
amount of time, the generated gas was flown to the H2S scrubber in
order to guarantee that the proper concentration of H2S was reached.
Afterwards, a gas mixture containing the desired concentration of H2S
was passed through the adsorber and inlet and outlet H2S
concentrations were measured at every secund to achieve accurate results.
3. Results and discussion
3.1. XRD analysis
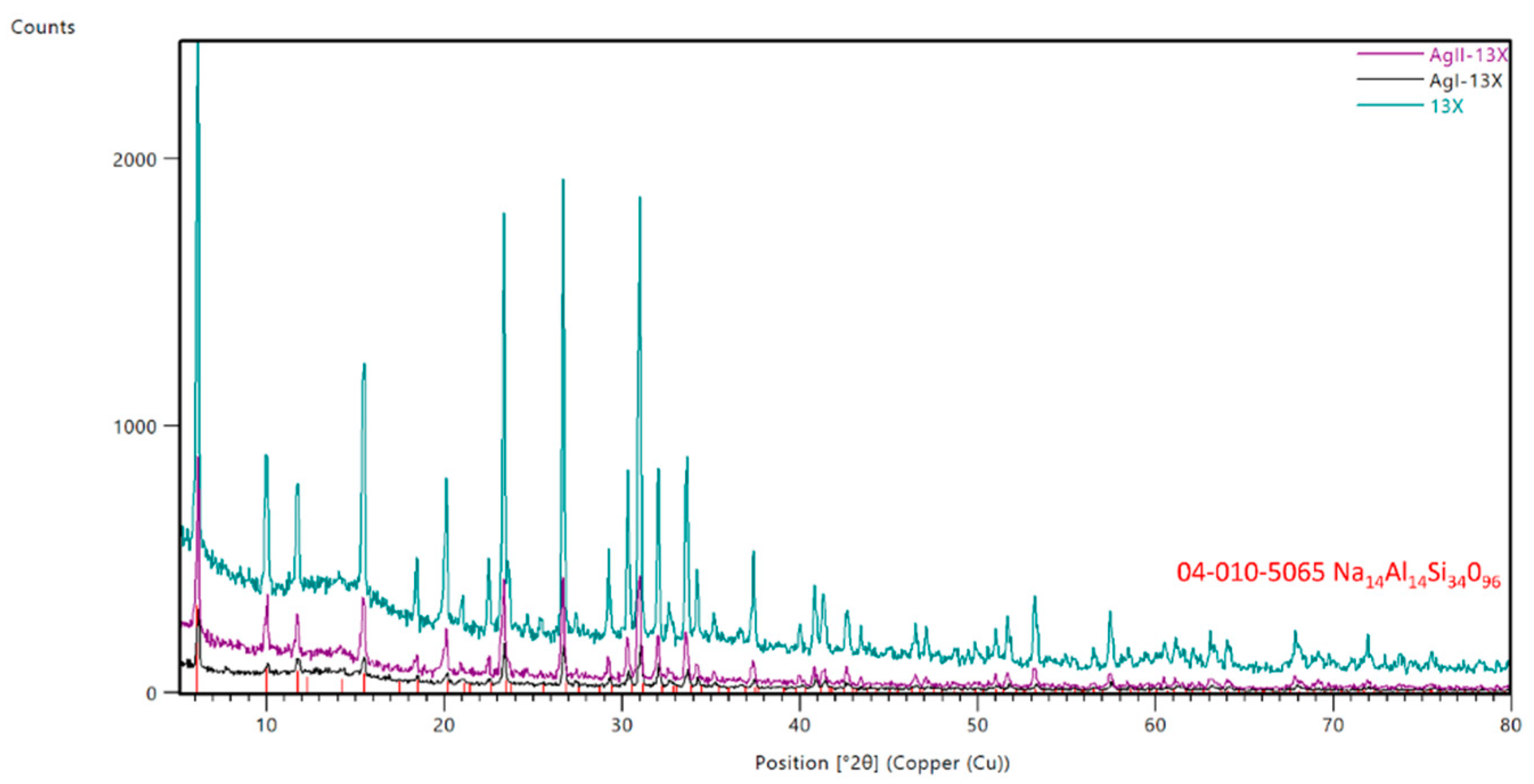
The XRD patterns corresponding to 13X and AgI-13X,
AgII-13X were presented in
Figure 2. The
samples feature significant crystallinity, as shown by the strength and
broadening of the XRD peaks. The investigated samples showed mainly a
crystalline phase composed of sodium aluminum silicate (Na
14Al
14Si
34O
96)
according to the PDF card no. 04-010-5065. The main diffraction peaks at 2 θ =
6.1, 10.0, 11.9, 15.2, 18.3, 20.1l 23.2, 26.9, 31.0 are characteristic of the
Faujasite structure (JCPDS No: 12-0228) [24].
Between 5° and 15° of 2Theta the characteristic bump was identified
characteristic for the amorphous phase. In this part, some of the Ag-based
compounds could be identified, however not in the crystalline phase. No
significant difference was observed between fresh 13X and the Ag modified
samples. It should be noted, however, that the intensity of some peaks for the
samples that had been exchanged with silver was diminished. It indicates that
the crystal structure of the 13X molecular sieve remains intact after Ag
ion-exchange treatment.
3.2. SEM images
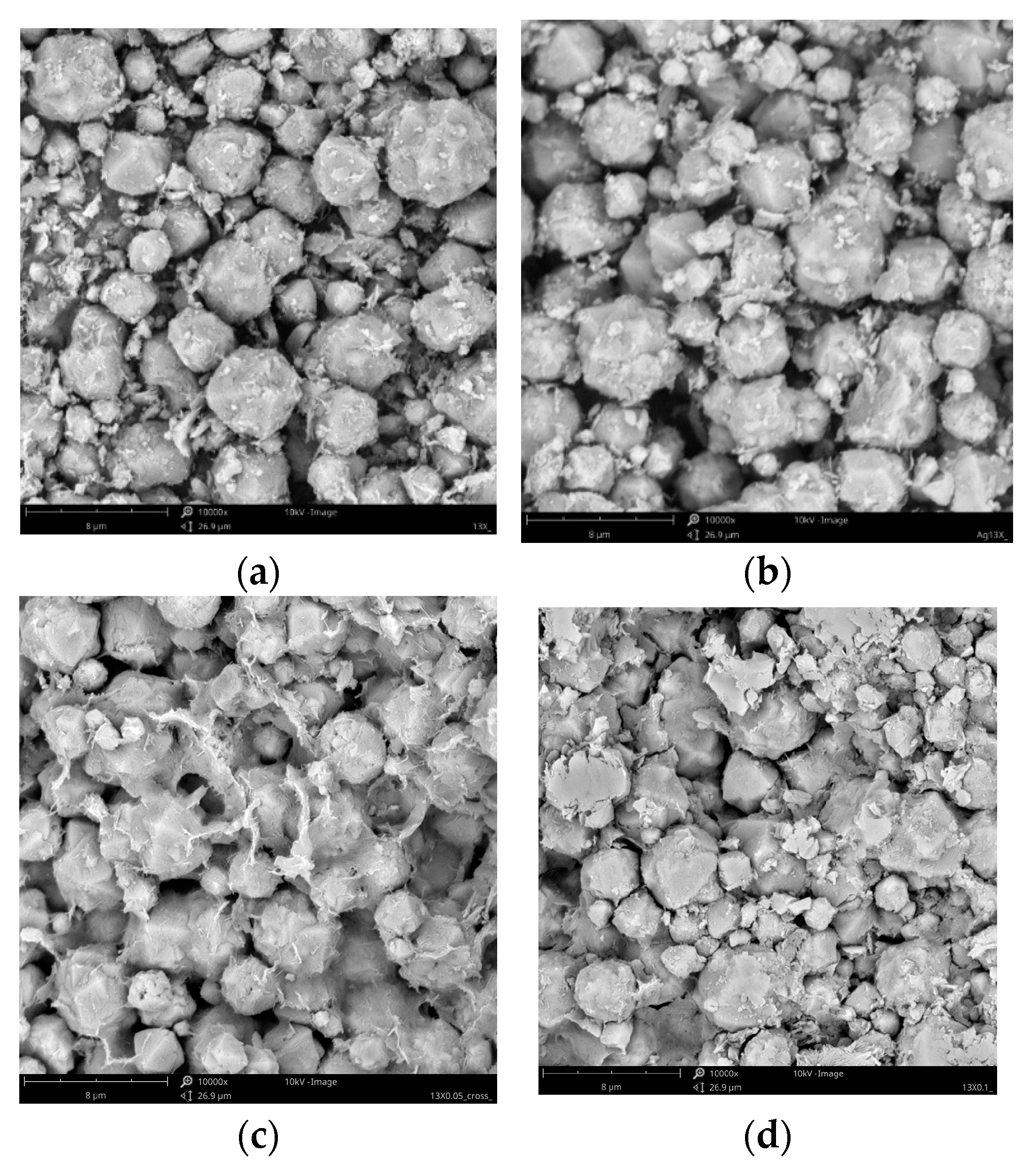
Figure 3
shows the SEM images of the samples. The framework of the Faujasite zeolite is
built by connecting sodalite cages by six rings [25].
SEM analysis verified that both silver modified and fresh 13X are composed of
very well- shaped crystallites with a spherical (octahedral) morphology.
However, the fresh 13X molecular sieve shows much smoother surfaces as compared
to the silver ion-exchanged 13X particles. Moreover, after the ion-exchange
process, some of 13X particles cracked, suggesting that lattice destruction
might happen during the ion-exchange or high-temperature calcination. These
findings are also compatible with the findings of the XRD investigation.
Additionally, it is evident that when the ion exchange rate rose in silver
modified 13X, the quantity of minor additives in its surface morphology also
increased. It is possible to attribute the smaller particles that are
found between the zeolite crystals to the binder, which is composed of clay and
is utilized in the process of shaping the crystals into beads.
Compositional characterization of the samples was
done by energy dispersive X-ray spectroscopy (EDS) during SEM image
acquisition. The element content in the samples was shown in
Table 1. It can be seen that the high weight
concentration of silver in AgI-13X (10.24 wt. %), AgII-13X (21.38 wt. %)
and AgIII-13X (32.38 wt. %) confirmed ion exchange was carried out
successfully. Since alkaline metals tend to exchange with silver ions, it can
be confirmed that Na
+ cations contained in zeolite are replaced by
Ag
+ cations. This can be confirmed by a decrease in Na
+
cations and an increase in Ag
+ cations (
Table 1 ).
3.3. BET analysis analysis
The specific surface area was determined by low
temperature nitrogen adsorption (ASAP 2020, Micromeritics Inc., Norcross, GA,
USA) using the Brunauer–Emmett–Teller equation [26].
Prior to taking the nitrogen adsorption measurements, each selected sample was
outgassed for 24 h at 350
℃. The BET surface area of the samples is given
in
Table 2. The BET surface area was calculated 501.33 m
2/g
for 13X. However, BET surface area was influenced by the ion-exchange of Ag. An
increase in the quantity of silver ions most likely contributed to a decrease
in the BET surface area, since the silver ion-exchange of 13X resulted in a
marginal decrease in the specific surface area of AgIII-13X from 501.33 to 405
m
2/g. Similar findings were given by Chen et.al.: the BET-specific
surface area of silver exchanged X was reduced by 33% [21].
3.4. H2S adsorption
The effects of inlet concentration were measured in
samples with H
2S concentration of 150 ppm, 300 ppm and 500 ppm at
ambient temperature. The breakthrough curves for 13X and modified zeolites were
demonstrated in
Figure 4. The
concentration of H
2S in the outlet stream is zero for a significant
amount of time before it breaks through. The experiments were stopped when the
outlet concentration reached 10% of the initial concentration, indicating an
effective adsorption time [27]. Adsorption
capacity of the 13X, AgI-13X, AgII-13X, and AgIII-13X was calculated from the
experimental breakthrough curves and was shown in
Table 3. Breakthrough time was determined when
the outlet concentration is 1 ppm. Experiments were run until the outlet
concentration reached 10% of its initial concentration to show how the
breakthrough curve evolved after breakthrough time. Increased inlet H
2S
concentration resulted in earlier breakthrough times for all samples, as
anticipated. The 13X molecular sieve exhibited the earliest breakthrough time
over all concentration range (150-500). It is noteworthy to mention that the
breakthrough time for AgIII-13X was detected earlier than that of AgII-13X.
However, the breakthrough curves demonstrated that AgIII-13X is capable of
adsorbing substantial quantities of H
2S molecules even after
breakthrough time. Across all ranges, the most lates breakthrough time for
AgII-13X samples was discovered, indicating their high effective adsorption
capacity.
3.5. Effect of inlet gas composition
The influence of H
2S concentration in
the inlet gas composition was evaluated to determine its impact on adsorption
capacity. Initially, three concentrations of H
2S in natural gas, 150
ppm, 300 ppm, and 500 ppm were considered. The breakthrough curve, the
corresponding breakthrough time, and the effective H
2S adsorption
capacity are shown in
Figure 4 and
Table 3, respectively. When high H
2S
concentrations 500 ppm were used, the breakthrough was reached, as expected,
significantly earlier (
Figure 4c) with respect
to the concentration of 150 ppm. At a 300 ppm H
2S inlet
concentration, a greater adsorption capacity of 13.05 mg/g was achieved for
AgII-13X. The lowest adsorption capacity of 0.238 mg/g was observed for
non-modified 13X when the H
2S inlet concentration was 150 ppm.
Table 4 provides the breakthrough times for the
samples.
3.6. Effect of Ag concentration
The effect of the concentration of Ag ions on the adsorption
capacity of modified zeolite towards H
2S uptake was investigated in
the range of molar concentrations of 0.02-0.1M AgNO
3 water solution.
The results are reported in
Figure 4 and
Table 3 respectively. It can be seen in
Figure 4 that the increase of silver ions on
zeolite samples led to an increase in breakthrough time and H
2S
adsorption capacity. However, when the silver ion concentration is too large (
Figure 4a for AgIII-13X, 0.1 M AgNO
3)
the breakthrough time was observed earlier with respect to AgII-13X, resulting
in decrease in the adsorption performance. In spite of observing earlier
breakthrough time for AgIII-13X, breakthrough curve changed marginally compared
to other samples. It is understandable that excessive metal ions might clog zeolites’
pores and prevent H
2S from adsorbing on AgIII-13X samples. The use
of high AgNO
3 solution concentration results in an increase in the
cost of the adsorbent. Therefore, modification of the 13X molecular sieve using
0.05 M AgNO
3 solution was believed appropriate. It was determined
that AgII-13X possessed a greater adsorption capacity, measuring 13.06 mg/g.
AgII-13X showed about 50 times more adsorption capacity than non-modified 13X,
which only had 0.238 mg/g of adsorption capacity.
3.7. Adsorption mechanism
After the procedure was started, it was observed
that the initially white adsorbent surface underwent a color change,
transitioning to a darker shade. This alteration in coloration served as an
indication that chemical adsorption was taking place. Sodium (Na+)
ions present in 13X replace silver (Ag+) cations, leading to a
subsequent chemical reaction with hydrogen sulfide (H2S) molecules,
resulting in the formation of black silver sulfide (Ag2S).
π-complexation and sulfur-metal (S-M) bond formation may take place between
sulfur compounds and metal ions. Previous research also stated that [21] the S-M bond was found to exist between the
metal ion and H2S. The Ag-sulfide bond was found to have the highest
strength according to the Mayer bond order (0.639), which was determined by
employing Density Functional Theory (DFT).
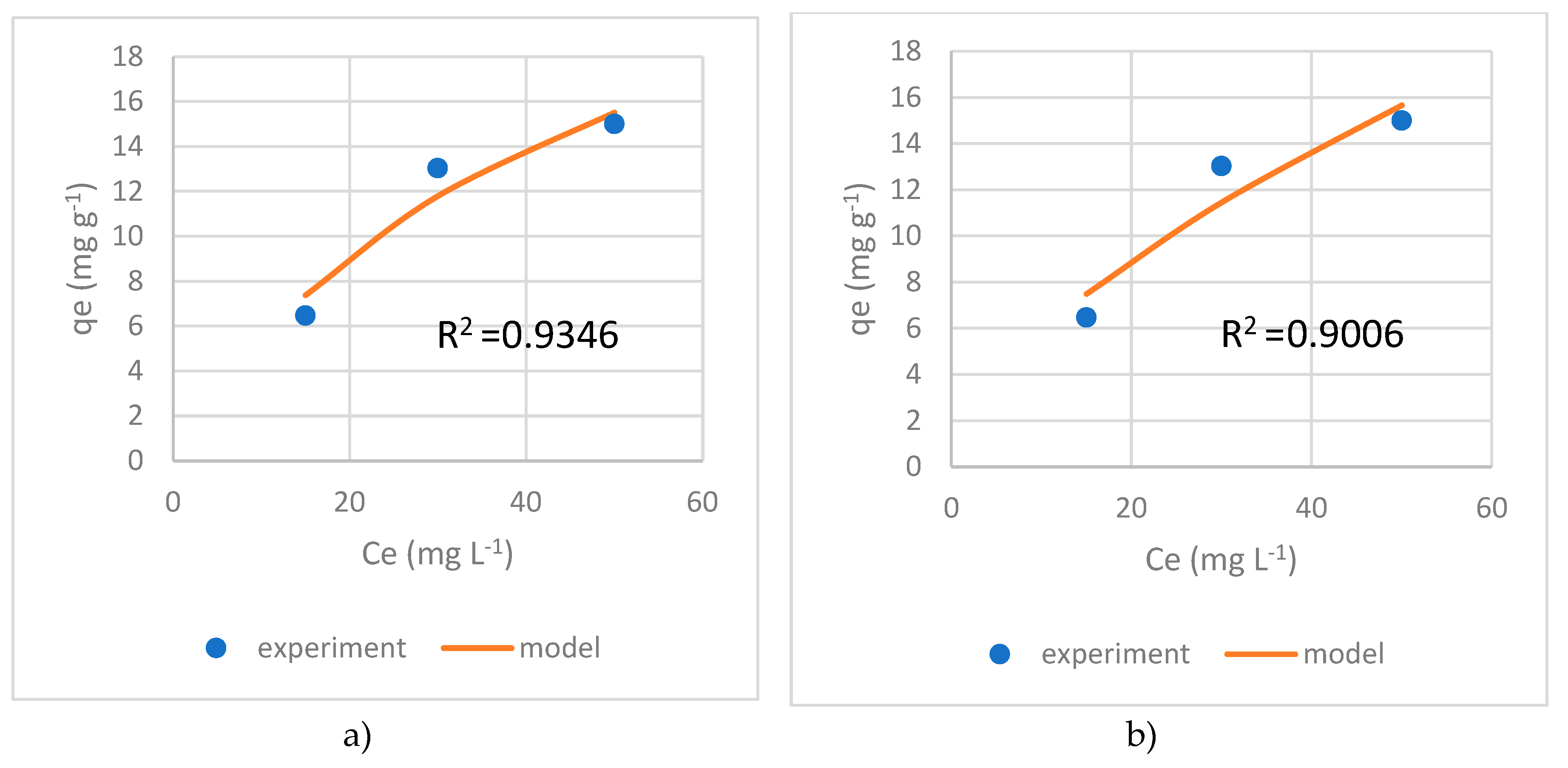
3.8. Adsorption isotherms
The Langmuir and Freundlich adsorption models were
applied to assess adsorption parameters and to investigate adsorption
mechanisms at ambient temperature. The Langmuir model describes monolayer
adsorption of adsorbate onto homogenous solid surface sites, while the
Freundlich model does not have a maximum adsorption limit. Two adsorption
models were implemented for AgII-13X since it showed high adsorption capacity (
Figure 5 ).
Following Langmuir isotherm equation was used:
where,
qe and
Ce are the H
2S uptake and equilibrium concentration,
respectively, K
L is the Langmuir isotherm constant related to the
binding energy, and
qmax is the theoretically calculated
adsorption capacity.
The Freundlich model adsorption parameters were
obtained using following equation (4):
where,
KF is a Freundlich constant or maximum adsorption capacity, C
e is the concentration of adsorbate under equilibrium condition (mg/L), q
e is the amount of adsorbate adsorbed per unit mass of adsorbent (mg/g), is the value indicating the degree of linearity between the adsorbate solution and the adsorption process.
Table 4.
Adsorption isotherm parameters for AgII-13X.
Table 4.
Adsorption isotherm parameters for AgII-13X.
| Langmuir |
Value |
Freundlich |
Value |
| KL (µM-1) |
0.002229 |
KF (mmol m-2 µM-1/n) |
0.347795 |
| R2 (Ce vs qe) |
0.9346 |
R2 (Ce vs qe) |
0.9006 |
| qmax (mg g-1) |
29.42685 |
n |
1.632228 |
The average determination coefficient (R2) for AgII-13X zeolites was 0.9346 in Langmuir and 0.9006 in Freundlich, indicating that Langmuir’s isothermal model is better in our case. The maximum adsorption capacity was calculated 29.42 mg/g that is higher then effective adsorption capacity of 13.06 mg/g.
4. Conclusions
Several promising results have been reported on the modification of the commercially available 13X molecular sieve adsorbent with silver ions and its application in the separation of H2S from various mixtures. However, there is no comprehensive study in the available literature on the extraction of H2S from natural gas using Ag-modified adsorbents. In this work, 13X molecular sieve was modified with different concentrations of AgNO3 solution and it was used to purify natural gas from H2S.
The results showed that the ion exchange of 13X molecular sieve with silver ions has a positive effect on the increase in adsorbent capacity. The highest adsorption capacity of 13.05 mg/g was reached using AgII-13X zeolite and is highly effective at removing H2S from a variety of gases that contain methane.
Author Contributions
Conceptualization, M.A., M.H.A.-R. and J.W.; Supervision, M.H.A.-R. and J.W.; Visualization, M.A., M.H.A.-R. and J.W.; Writing—original draft, M.A.; Writing—reviewing and editing M.H.A.-R. and J.W.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- International Energy Agency (2023), World Energy Outlook 2023, IEA,. Licence: Creative Commons Attribution CC BY-NC-SA 4.0.
- Sebastien Duval, “Natural gas sweetening,” in Surface Process, Transportation, and Storage, 2023, pp. 37–78. [CrossRef]
- S. Mokhatab, W. A. Poe, and J. Y. Mak, Handbook of natural gas transmission and processing: Principles and practices. 2018. [CrossRef]
- M. Hedayat, M. Soltanieh, and S. A. Mousavi, “Simultaneous separation of H2S and CO2 from natural gas by hollow fiber membrane contactor using mixture of alkanolamines,” J. Memb. Sci., vol. 377, no. 1–2, pp. 191–197, 2011. [CrossRef]
- M. S. Shah, M. Tsapatsis, and J. I. Siepmann, “Hydrogen Sulfide Capture: From Absorption in Polar Liquids to Oxide, Zeolite, and Metal-Organic Framework Adsorbents and Membranes,” Chem. Rev., vol. 117, no. 14, pp. 9755–9803, Jul. 2017. [CrossRef]
- L. H. de Oliveira, J. G. Meneguin, M. V. Pereira, J. F. do Nascimento, and P. A. Arroyo, “Adsorption of hydrogen sulfide, carbon dioxide, methane, and their mixtures on activated carbon,” Chem. Eng. Commun., vol. 206, no. 11, pp. 1544–1564, 2019. [CrossRef]
- M. C. Castrillon et al., “CO2 and H2S Removal from CH4-Rich Streams by Adsorption on Activated Carbons Modified with K2CO3, NaOH, or Fe2O3,” Energy and Fuels, vol. 30, no. 11, pp. 9596–9604, 2016. [CrossRef]
- N. N. Zulkefli, M. S. Masdar, W. N. Roslam Wan Isahak, J. M. Jahim, S. A. Md Rejab, and C. C. Lye, “Removal of hydrogen sulfide from a biogas mimic by using impregnated activated carbon adsorbent,” PLoS ONE, vol. 14, no. 2. 2019. [CrossRef]
- Y. Belmabkhout et al., “Natural gas upgrading using a fluorinated MOF with tuned H2S and CO2 adsorption selectivity,” Ind. Eng. Chem. Res., vol. 25, no. 16, p. 111886, Jul. 2020. [CrossRef]
- G. Liu et al., “Enabling Fluorinated MOF-Based Membranes for Simultaneous Removal of H2S and CO2 from Natural Gas,” Angew. Chemie - Int. Ed., vol. 57, no. 45, pp. 14811–14816, 2018. [CrossRef]
- Mohammadi, Z. Saadati, and S. Joohari, “Comparison of the adsorption of H2S by ZnO–TiO2 and Ni–ZnO–TiO2 nanoparticles: An adsorption isotherm and thermodynamic study,” Environ. Prog. Sustain. Energy, vol. 38, no. 6, 2019. [CrossRef]
- D. Jiang et al., “Cu-Zn-Al mixed metal oxides derived from hydroxycarbonate precursors for H2S removal at low temperature,” Appl. Surf. Sci., vol. 256, no. 10, pp. 3216–3223, 2010. [CrossRef]
- T. Yu, Z. Chen, Z. Liu, J. Xu, and Y. Wang, “Review of Hydrogen Sulfide Removal from Various Industrial Gases by Zeolites,” Separations, vol. 9, no. 9, 2022. [CrossRef]
- M. Abdirakhimov, M. H. Al-Rashed, and J. Wójcik, “Recent Attempts on the Removal of H2S from Various Gas Mixtures Using Zeolites and Waste-Based Adsorbents,” Energies, vol. 15, no. 15, p. 5391, Jul. 2022. [CrossRef]
- Y. Huang, W. Su, R. Wang, and T. Zhao, “Removal of typical industrial gaseous pollutants: From carbon, zeolite, and metal-organic frameworks to molecularly imprinted adsorbents,” Aerosol Air Qual. Res., vol. 19, no. 9, pp. 2130–2150, 2019. [CrossRef]
- L. Sigot, M. Fontseré Obis, H. Benbelkacem, P. Germain, and G. Ducom, “Comparing the performance of a 13X zeolite and an impregnated activated carbon for H2S removal from biogas to fuel an SOFC: Influence of water,” Int. J. Hydrogen Energy, vol. 41, no. 41, pp. 18533–18541, 2016. [CrossRef]
- F. Bandarchian and M. Anbia, “Conventional hydrothermal synthesis of nanoporous molecular sieve 13X for selective adsorption of trace amount of hydrogen sulfide from mixture with propane,” J. Nat. Gas Sci. Eng., vol. 26, pp. 1380–1387, 2015. [CrossRef]
- K. Yang, B. K. Yang, B. Su, L. Shi, H. Wang, and Q. Cui, “Adsorption Mechanism and Regeneration Performance of 13X for H2S and SO2,” Energy & Fuels, vol. 32, no. 12, pp. 12742–12749, Dec. 2018. [Google Scholar] [CrossRef]
- Starke, C. Pasel, C. Bläker, T. Eckardt, J. Zimmermann, and D. Bathen, “Investigation of the Adsorption of Hydrogen Sulfide on Faujasite Zeolites Focusing on the Influence of Cations,” ACS Omega, vol. 7, no. 48, pp. 43665–43677, 2022. [CrossRef]
- L. Barelli, G. Bidini, L. Micoli, E. Sisani, and M. Turco, “13X Ex-Cu zeolite performance characterization towards H2S removal for biogas use in molten carbonate fuel cells,” Energy, vol. 160, pp. 44–53, 2018. [CrossRef]
- X. Chen, B. Shen, H. Sun, and G. zhan, “Ion-exchange modified zeolites X for selective adsorption desulfurization from Claus tail gas: Experimental and computational investigations,” Microporous Mesoporous Mater., vol. 261, no. November 2017, pp. 227–236, 2018. [CrossRef]
- P. Kumar et al., “H2S adsorption by Ag and Cu ion exchanged faujasites,” Microporous Mesoporous Mater., vol. 146, no. 1–3, pp. 127–133, 2011. [CrossRef]
- S. Kulawong, R. Artkla, P. Sriprapakhan, and P. Maneechot, “Biogas purification by adsorption of hydrogen sulphide on NaX and Ag-exchanged NaX zeolites,” Biomass and Bioenergy, vol. 159, no. November 2021, p. 106417, 2022. [CrossRef]
- S. A. Bradley, R. W. Broach, T. M. Mezza, S. Prabhakar, and W. Sinkler, Zeolite Characterization. 2010. [CrossRef]
- C. Baerlocher and L.B.McCusker, “Database of Zeolite Structures, http://www.iza-structure.org/databases/,” Database of Zeolite Structures. [Online]. Available online: http://www.iza-structure.org/databases/.
- S. Brunauer, P. H. Emmett, and E. Teller, “Adsorption of Gases in Multimolecular Layers,” J. Am. Chem. Soc., vol. 60, no. 2, pp. 309–319, 1938. [CrossRef]
- L. Zhu et al., “Modification of zeolite by metal and adsorption desulfurization of organic sulfide in natural gas,” J. Nat. Gas Sci. Eng., vol. 69, no. February, p. 102941, 2019. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).