Submitted:
04 December 2023
Posted:
05 December 2023
You are already at the latest version
Abstract
Keywords:
Introduction
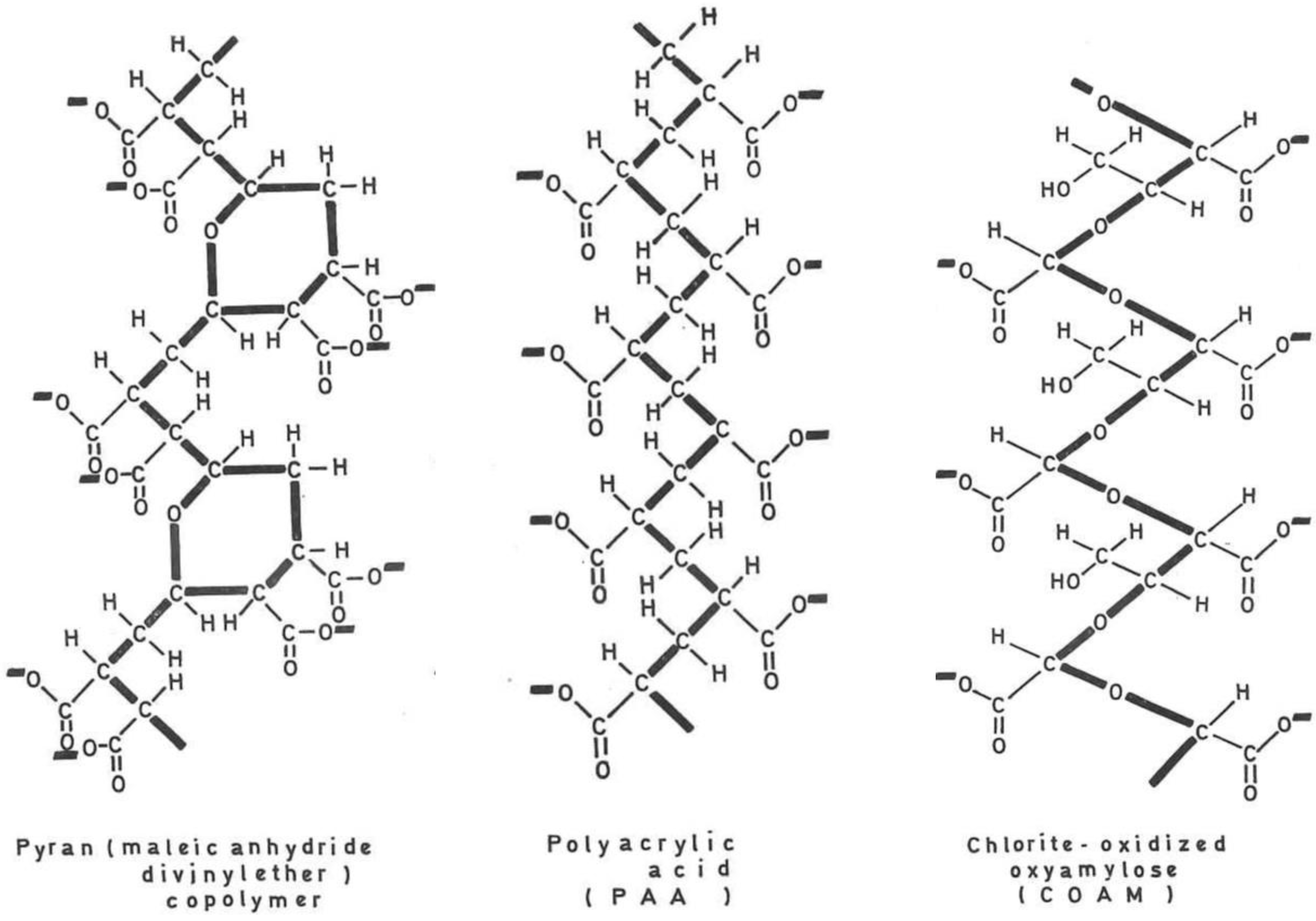
1. Interferon inducers (Figure 1)
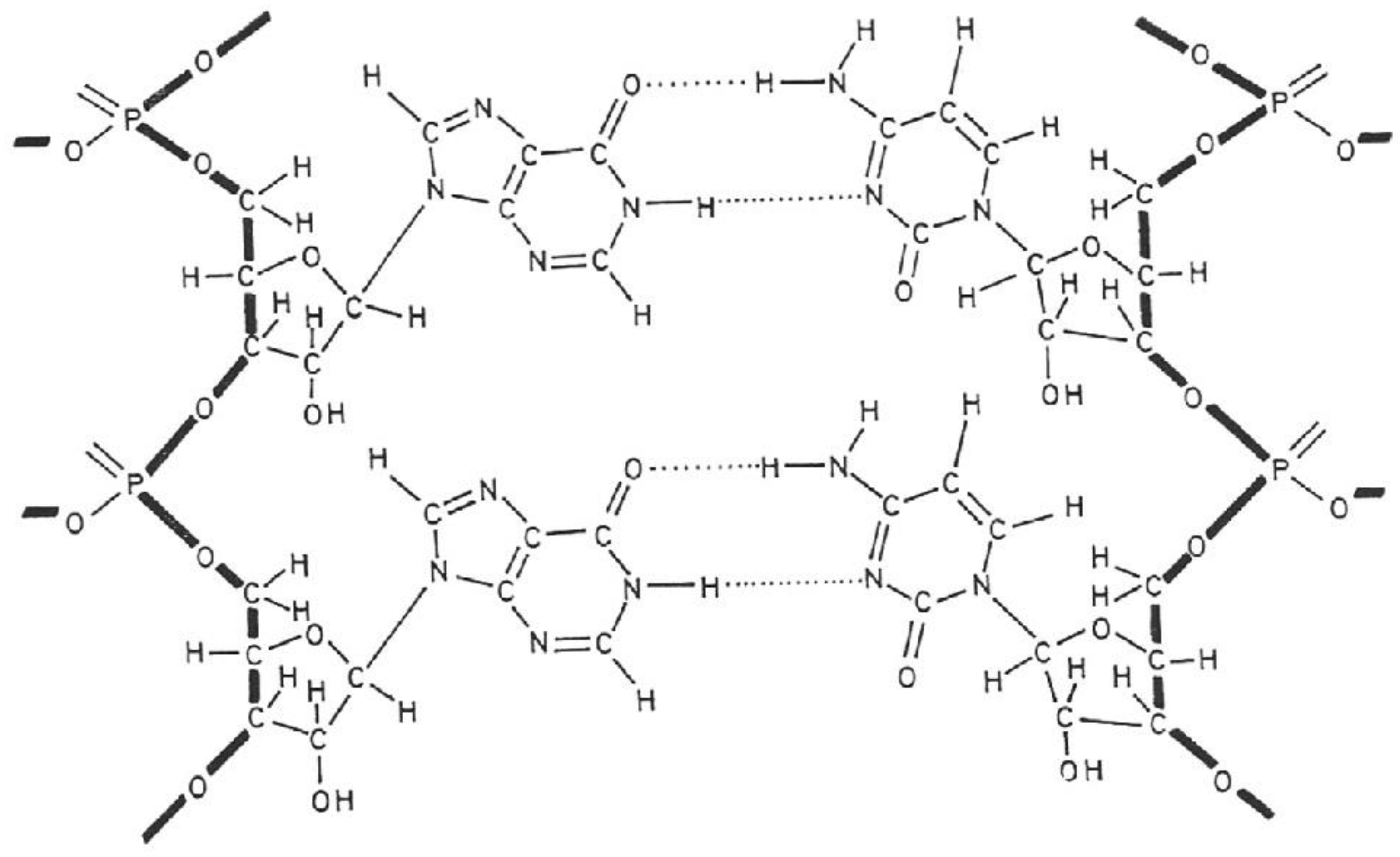
2. Poly(I).poly(C) (Figure 2)
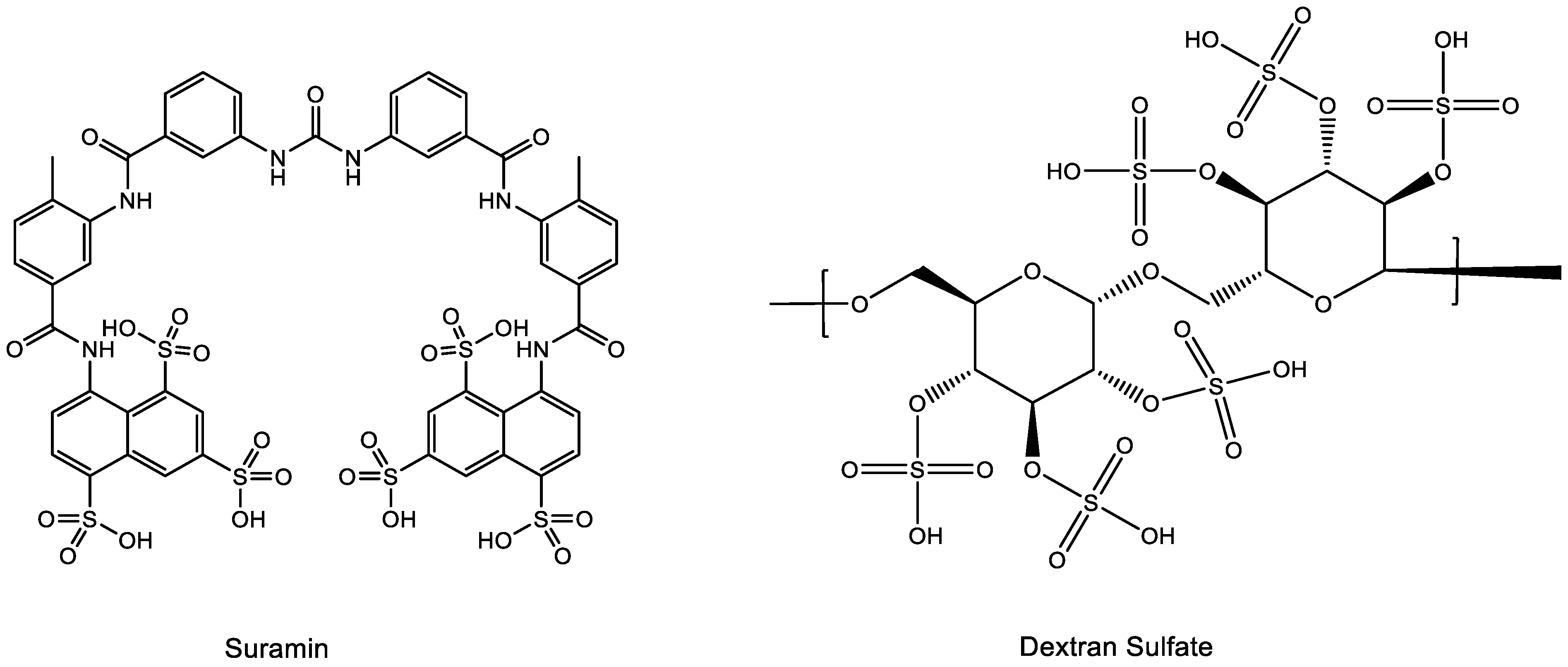
3. Suramin (Figure 3)
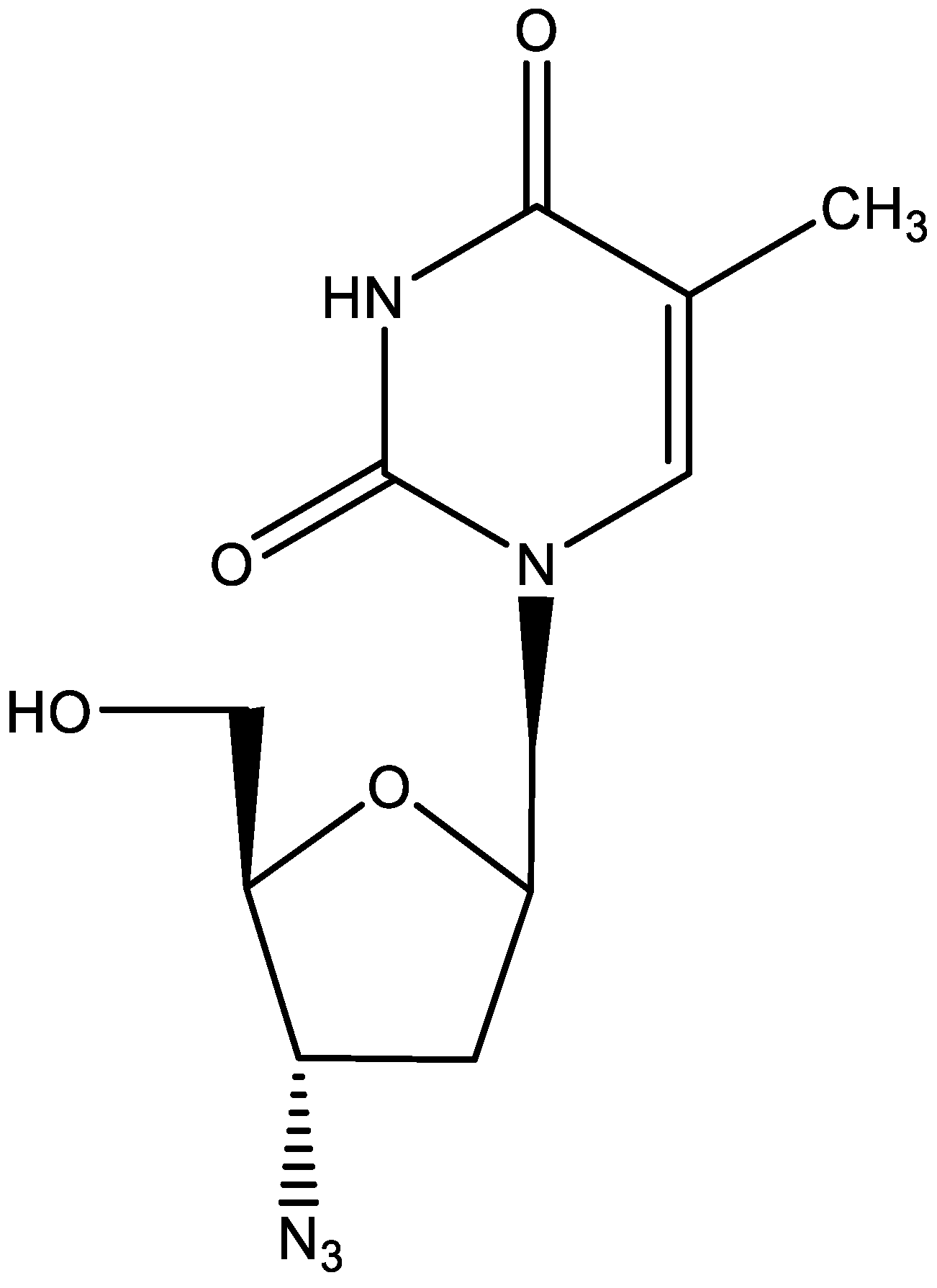
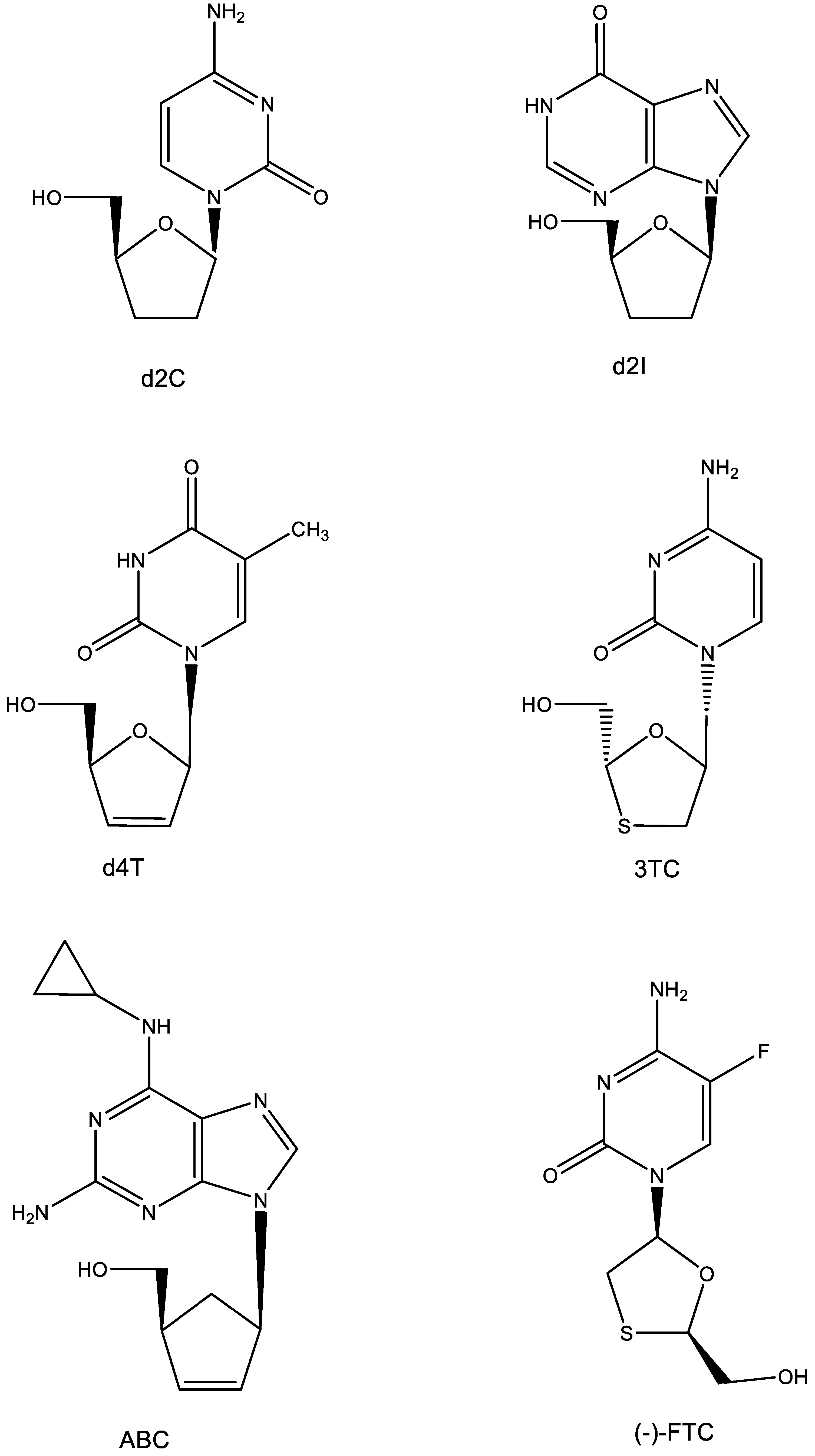
4. AZT (Figure 4)
5. d4T (Figure 5)
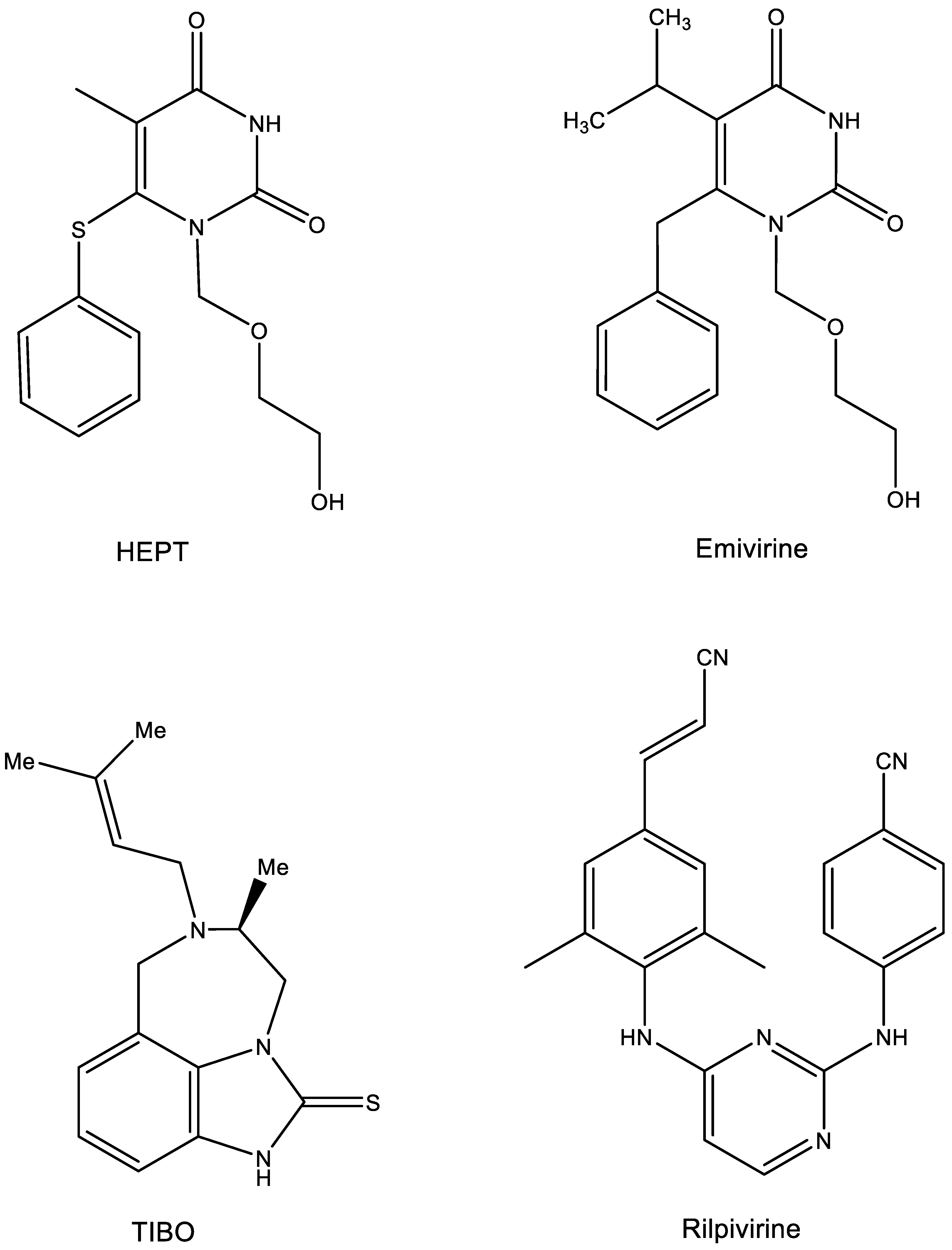
6. HEPT and TIBO (Figure 6)
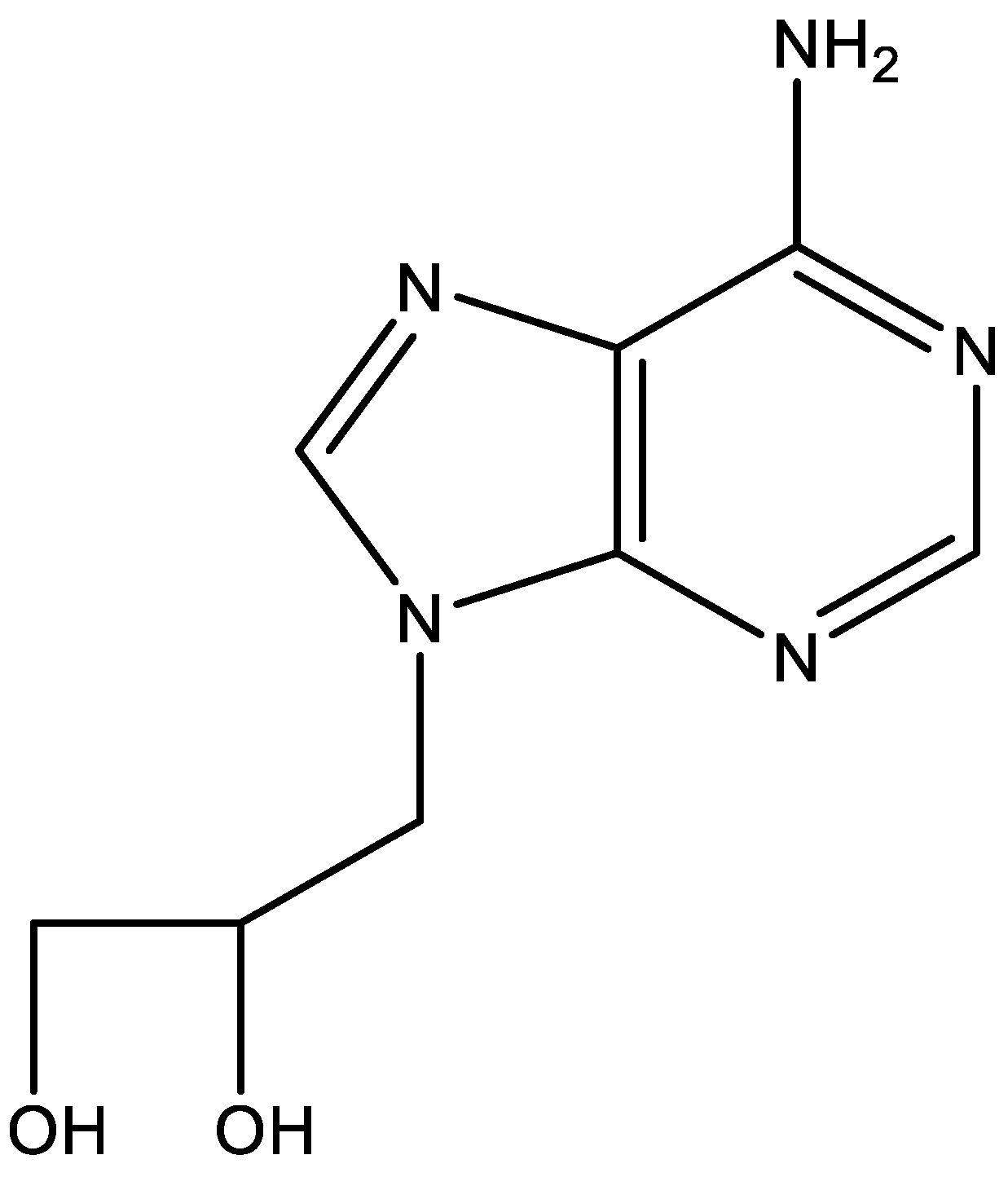
7. DHPA (Figure 7)
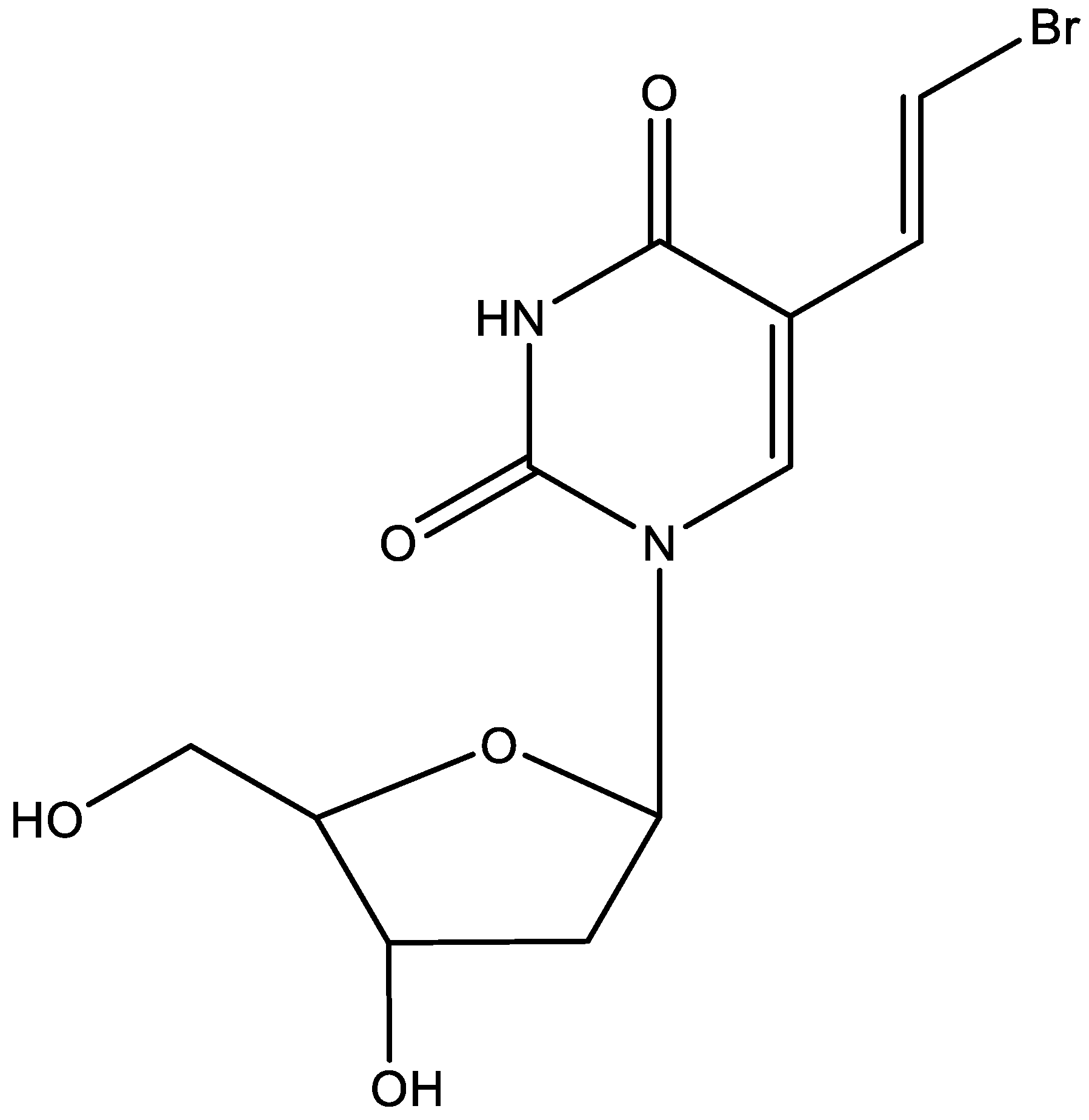
8. BVDU (Figure 8)
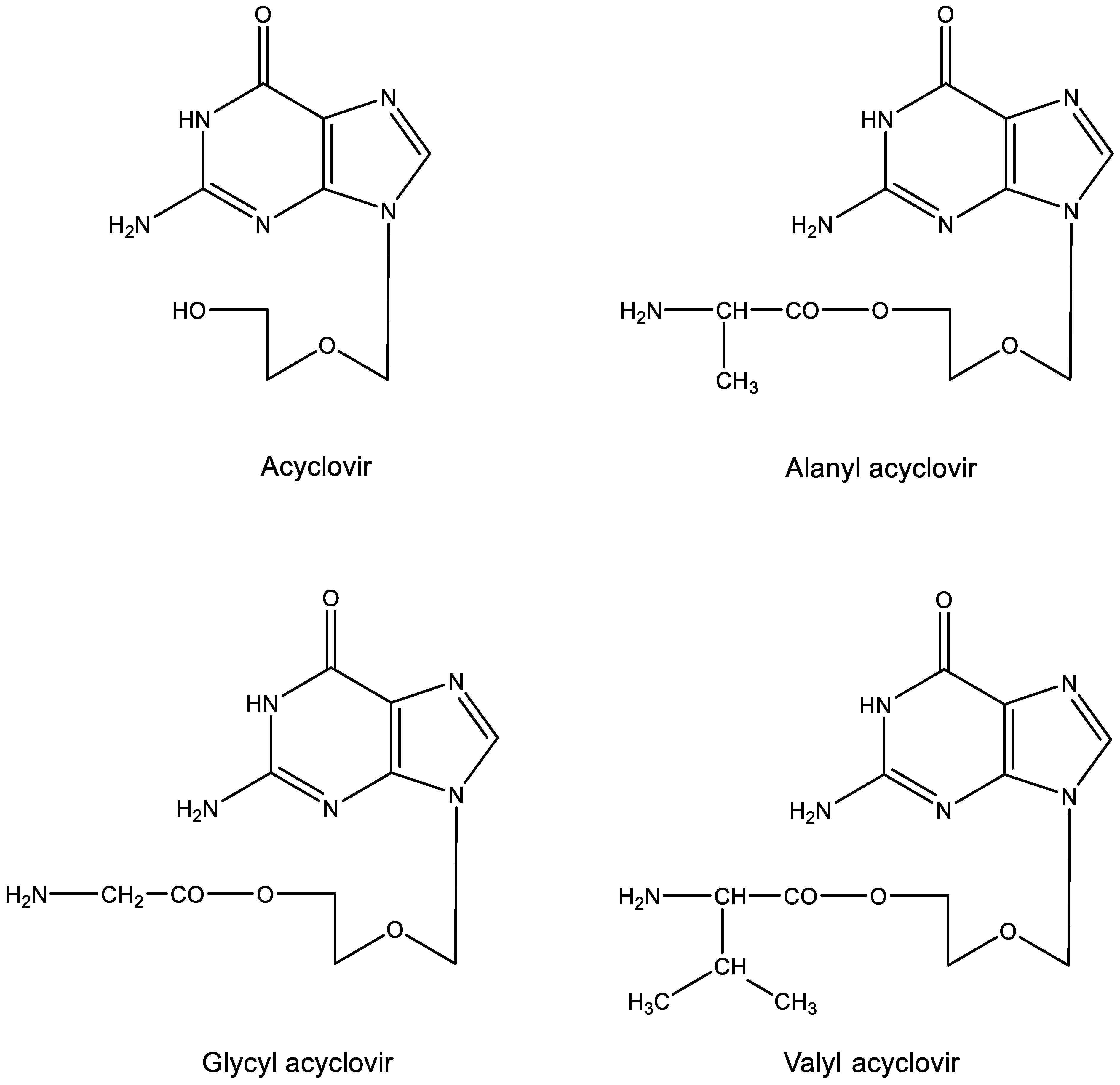
9. Aminoacyl esters of acyclovir (Figure 9)
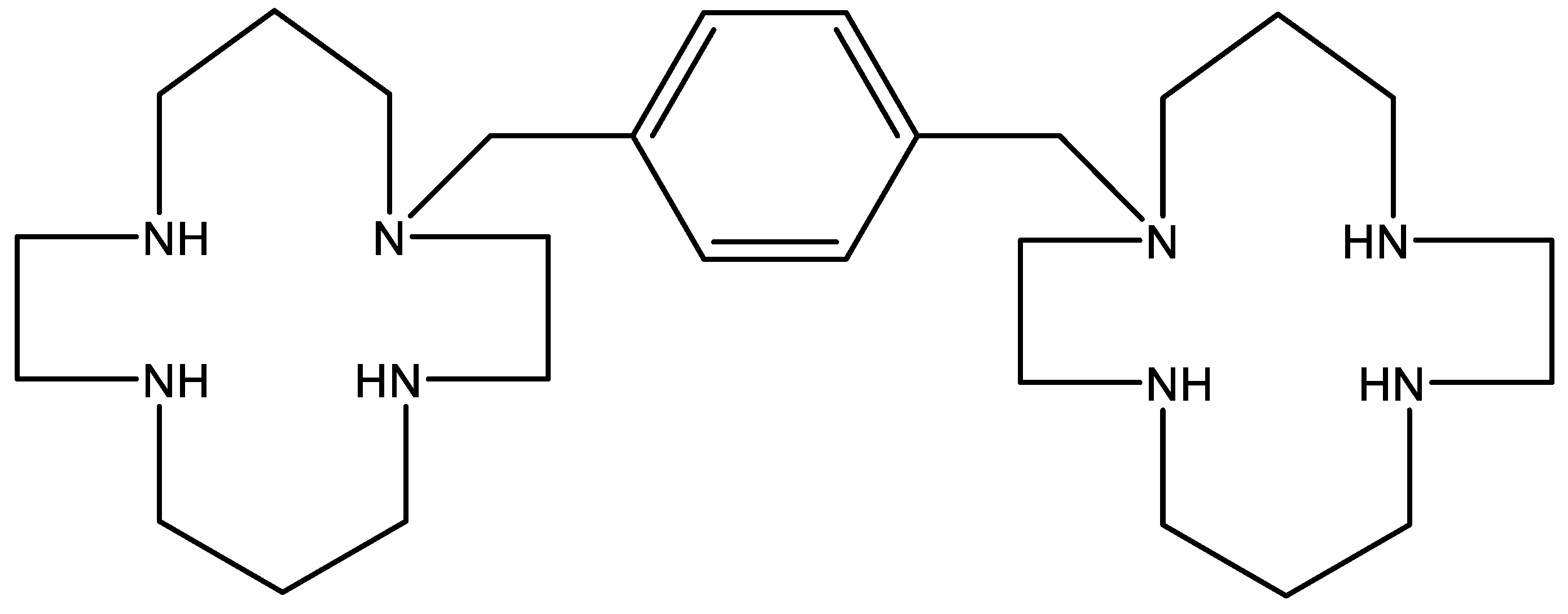
10. AMD-3100 (Figure 10)
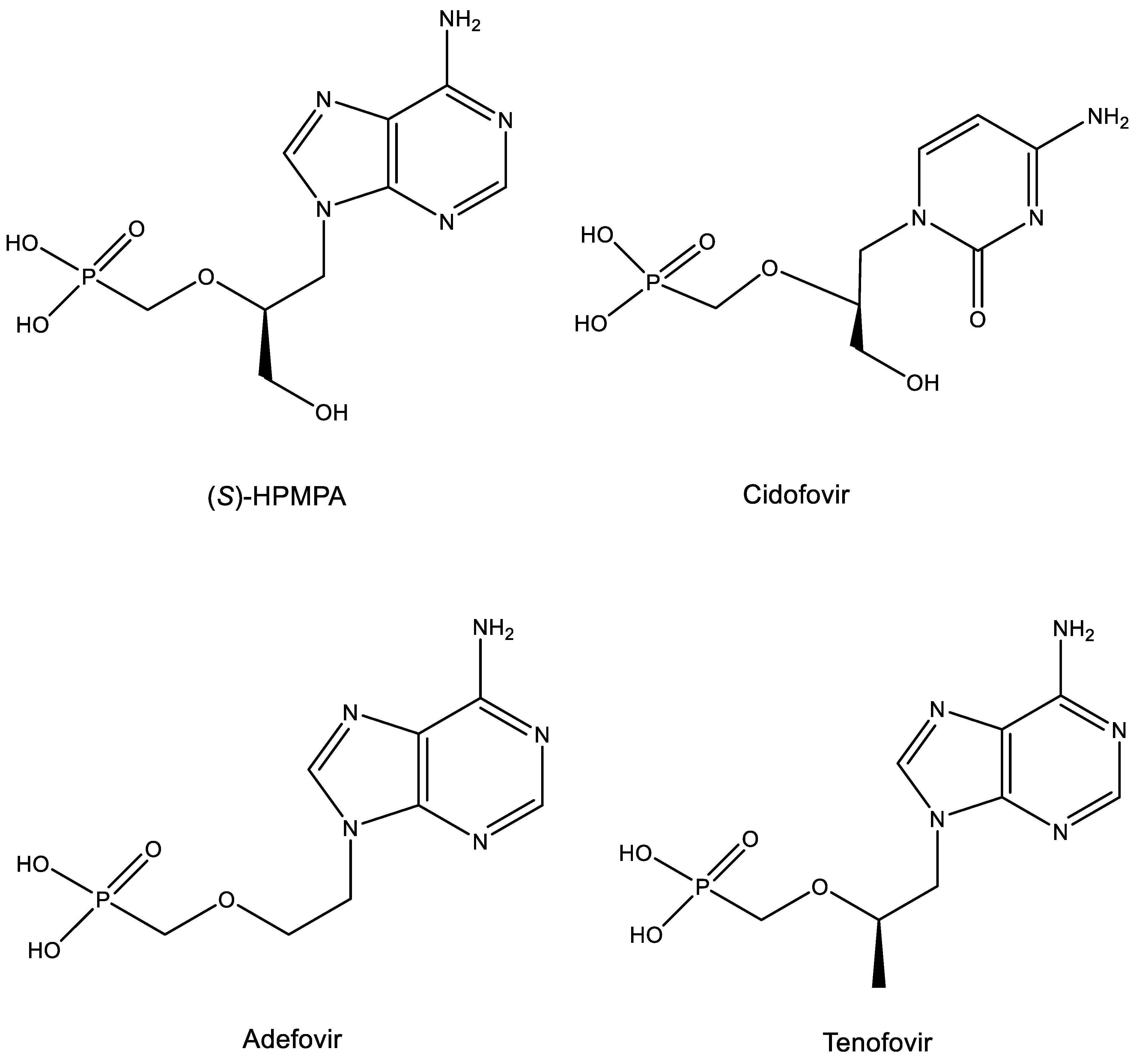
11. ANPs (Acyclic Nucleoside Phosphonates) (Figure 11)
Conclusion
Acknowledgments
References
- Isaacs, A.; Lindenmann, J., Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci 1957, 147, (927), 258-67.
- Temin, H. M.; Mizutani, S., RNA-dependent DNA polymerase in virions of Rous sarcoma virus. Nature 1970, 226, (5252), 1211-3.
- Baltimore, D., RNA-dependent DNA polymerase in virions of RNA tumour viruses. Nature 1970, 226, (5252), 1209-11. [CrossRef]
- Ohno, T.; Sweet, R. W.; Dejak, D.; Spiegelman, S., Purification and characterization of the DNA polymerase of human breast cancer particles. Proc Natl Acad Sci U S A 1977, 74, (2), 764-8. [CrossRef]
- Viola, M. V.; Frazier, M.; Wiernik, P. H.; McCredie, K. B.; Spiegelman, S., Reverse transcriptase in leukocytes of leukemic patients in remission. N Engl J Med 1976, 294, (2), 75-80. [CrossRef]
- Witkin, S. S.; Ohno, T.; Spiegelman, S., Purification of RNA-instructed DNA polymerase from human leukemic spleens. Proc Natl Acad Sci U S A 1975, 72, (10), 4133-6. [CrossRef]
- Spiegelman, S., Evidence for viruses in human neoplasias. Haematologica 1975, 60, (3), 339-72.
- Balda, B. R.; Hehlmann, R.; Cho, J. R.; Spiegelman, S., Oncornavirus-like particles in human skin cancers. Proc Natl Acad Sci U S A 1975, 72, (9), 3697-700. [CrossRef]
- Spiegelman, S., Viruses and human cancer. Prog Hematol 1975, 9, 305-30.
- Spiegelman, S.; Baxt, W.; Kufe, D.; Peters, W. P.; Schlom, J., Sequences related to the RNA tumor viruses in the RNA and DNA of human leukemias and lymphomas. Bibl Haematol 1975, (40), 3-25.
- Cuatico, W.; Cho, J. R.; Spiegelman, S., Evidence of particle-associated RNA-directed DNA polymerase and high molecular weight RNA in human gastrointestinal and lung malignancies. Proc Natl Acad Sci U S A 1974, 71, (8), 3304-8. [CrossRef]
- Cuatico, W.; Cho, J. R.; Spiegelman, S., Particles with RNA of high molecular weight and RNA-directed DNA polymerase in human brain tumors. Proc Natl Acad Sci U S A 1973, 70, (10), 2789-93. [CrossRef]
- Kufe, D.; Magrath, I. T.; Ziegler, J. L.; Spiegelman, S., Burkitt’s tumors contain particles encapsulating RNA-instructed DNA polymerase and high molecular weight virus-related RNA. Proc Natl Acad Sci U S A 1973, 70, (3), 737-41. [CrossRef]
- Baxt, W. G.; Spiegelman, S., Nuclear DNA sequences present in human leukemic cells and absent in normal leukocytes. Proc Natl Acad Sci U S A 1972, 69, (12), 3737-41. [CrossRef]
- Gulati, S. C.; Axel, R.; Spiegelman, S., Detection of RNA-instructed DNA polymerase and high molecular weight RNA in malignant tissue. Proc Natl Acad Sci U S A 1972, 69, (8), 2020-4. [CrossRef]
- De Clercq, E., Suramin: a potent inhibitor of the reverse transcriptase of RNA tumor viruses. Cancer Lett 1979, 8, 9-22.
- De Somer, P.; De Clercq, E.; Billiau, A.; Schonne, E.; Claesen, M., Antiviral activity of polyacrylic and polymethacrylic acids. I. Mode of action in vitro. J Virol 1968, 2, 878-885. [CrossRef]
- De Somer, P.; De Clercq, E.; Billiau, A.; Schonne, E.; Claesen, M., Antiviral activity of polyacrylic and polymethacrylic acids. II. Mode of action in vivo. J Virol 1968, 2, 886-893. [CrossRef]
- Merigan, T. C., Interferons of mice and men. N Engl J Med 1967, 276, (16), 913-20. [CrossRef]
- Merigan, T. C.; Finkelstein, M. S., Interferon-stimulating and in vivo antiviral effects of various synthetic anionic polymers. Virology 1968, 35, (3), 363-74. [CrossRef]
- De Clercq, E.; De Somer, P., Protective effect of interferon and polyacrylic acid in newborn mice infected with a lethal dose of vesicular stomatitis virus. Life Sci 1968, 7, 925-933. [CrossRef]
- De Clercq, E., Vesicular stomatitis virus (VSV) as a paradigm for predicting antiviral activity against Ebola virus (EBOV). Marmara Pharmaceutical Journal 2015, 19, 141-152.
- De Clercq, E.; De Somer, P., Effect of interferon, polyacrylin acid, and polymethacrylic acid on tail lesions on mice infected with vaccinia virus. Appl Microbiol 1968, 16, 1314-1319.
- De Clercq, E., Vaccinia virus inhibitors as a paradigm for the chemotherapy of poxvirus infections. Clin Microbiol Rev 2001, 14, 382-397.
- De Clercq, E.; De Somer, P., Prolonged antiviral protection by interferon inducers. Proc. Soc. Exp. Biol. Med 1969, 132, 699-703. [CrossRef]
- Claes, P.; Billiau, A.; De Clercq, E.; Desmyter, J.; Schonne, E.; Vanderhaeghe, H.; De Somer, P., Polyacetal carboxylic acids: a new group of antiviral polyanions. J Virol 1970, 5, (3), 313-20. [CrossRef]
- Kleinschmidt, W. J.; Cline, J. C.; Murphy, E. B., Interferon production induced by statolon. Proc Natl Acad Sci U S A 1964, 52, (3), 741-4. [CrossRef]
- Lampson, G. P.; Tytell, A. A.; Field, A. K.; Nemes, M. M.; Hilleman, M. R., Inducers of interferon and host resistance. I. Double-stranded RNA from extracts of Penicillium funiculosum. Proc Natl Acad Sci U S A 1967, 58, (2), 782-9. [CrossRef]
- Field, A. K.; Tytell, A. A.; Lampson, G. P.; Hilleman, M. R., Inducers of interferon and host resistance. II. Multistranded synthetic polynucleotide complexes. Proc Natl Acad Sci U S A 1967, 58, (3), 1004-10. [CrossRef]
- Tytell, A. A.; Lampson, G. P.; Field, A. K.; Hilleman, M. R., Inducers of interferon and host resistance. 3. Double-stranded RNA from reovirus type 3 virions (reo 3-RNA). Proc Natl Acad Sci U S A 1967, 58, (4), 1719-22. [CrossRef]
- Field, A. K.; Lampson, G. P.; Tytell, A. A.; Nemes, M. M.; Hilleman, M. R., Inducers of interferon and host resistance, IV. Double-stranded replicative form RNA (MS2-Ff-RNA) from E. coli infected with MS2 coliphage. Proc Natl Acad Sci U S A 1967, 58, (5), 2102-8. [CrossRef]
- De Clercq, E.; De Somer, P., Are cytotoxicity and interferon inducing activity of poly(I).poly(C) invariably linked in interferon-treated L cells? J Gen Virol 1975, 27, 35-44.
- De Clercq, E.; Edy, V. G.; Torrence, P. F.; Waters, J. A.; Witkop, B., Antiviral activity of poly(7-deazainosinic acid)-derived complexes in vitro and in vivo. Mol Pharmacol 1976, 12, 1045-1051.
- Derynck, R.; Content, J.; DeClercq, E.; Volckaert, G.; Tavernier, J.; Devos, R.; Fiers, W., Isolation and structure of a human fibroblast interferon gene. Nature 1980, 285, (5766), 542-7. [CrossRef]
- Derynck, R.; Remaut, E.; Saman, E.; Stanssens, P.; De Clercq, E.; Content, J.; Fiers, W., Expression of human fibroblast interferon gene in Escherichia coli. Nature 1980, 287, (5779), 193-7. [CrossRef]
- Content, J.; De Wit, L.; Pierard, D.; Derynck, R.; De Clercq, E.; Fiers, W., Secretory proteins induced in human fibroblasts under conditions used for the production of interferon beta. Proc Natl Acad Sci U S A 1982, 79, (9), 2768-72. [CrossRef]
- Mitsuya, H.; Popovic, M.; Yarchoan, R.; Matsushita, S.; Gallo, R. C.; Broder, S., Suramin protection of T cells in vitro against infectivity and cytopathic effect of HTLV-III. Science 1984, 226, (4671), 172-4. [CrossRef]
- Broder, S.; Yarchoan, R.; Collins, J. M.; Lane, H. C.; Markham, P. D.; Klecker, R. W.; Redfield, R. R.; Mitsuya, H.; Hoth, D. F.; Gelmann, E.; et al., Effects of suramin on HTLV-III/LAV infection presenting as Kaposi’s sarcoma or AIDS-related complex: clinical pharmacology and suppression of virus replication in vivo. Lancet 1985, 2, (8456), 627-30. [CrossRef]
- De Clercq, E., Suramin in the treatment of AIDS: mechanism of action. Antiviral Res 1987, 7, 1-10.
- Mitsuya, H.; Looney, D. J.; Kuno, S.; Ueno, R.; Wong-Staal, F.; Broder, S., Dextran sulfate suppression of viruses in the HIV family: inhibition of virion binding to CD4+ cells. Science 1988, 240, (4852), 646-9. [CrossRef]
- Baba, M.; Pauwels, R.; Balzarini, J.; Arnout, J.; Desmyter, J.; De Clercq, E., Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc Natl Acad Sci U S A 1988, 85, (16), 6132-6. [CrossRef]
- Witvrouw, M.; De Clercq, E., Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen Pharmac 1997, 29, (4), 497-511. [CrossRef]
- Mitsuya, H.; Weinhold, K. J.; Furman, P. A.; St Clair, M. H.; Lehrman, S. N.; Gallo, R. C.; Bolognesi, D.; Barry, D. W.; Broder, S., 3’-Azido-3’-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A 1985, 82, (20), 7096-100. [CrossRef]
- Furman, P. A.; Fyfe, J. A.; St Clair, M. H.; Weinhold, K.; Rideout, J. L.; Freeman, G. A.; Lehrman, S. N.; Bolognesi, D. P.; Broder, S.; Mitsuya, H.; et al., Phosphorylation of 3’-azido-3’-deoxythymidine and selective interaction of the 5’-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci U S A 1986, 83, (21), 8333-7. [CrossRef]
- Fischl, M. A.; Richman, D. D.; Grieco, M. H.; Gottlieb, M. S.; Volberding, P. A.; Laskin, O. L.; Leedom, J. M.; Groopman, J. E.; Mildvan, D.; Schooley, R. T.; et al., The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med 1987, 317, (4), 185-91. [CrossRef]
- Richman, D. D.; Fischl, M. A.; Grieco, M. H.; Gottlieb, M. S.; Volberding, P. A.; Laskin, O. L.; Leedom, J. M.; Groopman, J. E.; Mildvan, D.; Hirsch, M. S.; et al., The toxicity of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med 1987, 317, (4), 192-7. [CrossRef]
- Horwitz, J. P.; Chua, J.; Noel, M., Nucleosides. V. The monomesylates of 1-(2’-deoxy-β-D-lyxofuranosyl)thymine1,2. J Org Chem 1964, 29, (7), 2076-2078.
- De Clercq, E.; Balzarini, J.; Descamps, J.; Eckstein, F., Antiviral, antimetabolic and antineoplastic activities of 2’- or 3’-amino or -azido-substituted deoxyribonucleosides. Biochem Pharmacol 1980, 29, 1849-1851.
- De Clercq, E.; Fukui, T.; Kakiuchi, N.; Ikehara, M.; Hattori, M.; Pfleiderer, W., Influence of various 2- and 2’-substituted polyadenylic acids on murine leukemia virus reverse transcriptase. Cancer Lett 1979, 7, 27-37. [CrossRef]
- De Clercq, E.; Merigan, T. C., Moloney sarcoma virus-induced tumors in mice: inhibition or stimulation by (poly rI)·(poly rC). Proc Soc Exp Biol Med 1971, 137, (2), 590-593.
- Mitsuya, H.; Broder, S., Inhibition of the in vitro infectivity and cytopathic effect of human T-lymphotrophic virus type III/lymphadenopathy-associated virus (HTLV-III/LAV) by 2’,3’-dideoxynucleosides. Proc Natl Acad Sci U S A 1986, 83, (6), 1911-5. [CrossRef]
- Horwitz, J. P.; Chua, J.; Da Rooge, M. A.; Noel, M.; Klundt, I. L., Nucleosides. IX. The formation of 2’,3’-unsaturated pyrimidine nucleosides via a novel β-elimination reaction1,2. J Org Chem 1966, 31, (1), 205-211.
- Baba, M.; Pauwels, R.; Herdewijn, P.; De Clercq, E.; Desmyter, J.; Vandeputte, M., Both 2’,3’-dideoxythymidine and its 2’,3-unsaturated derivative (2’,3’-dideoxythymidinene) are potent and selective inhibitors of human immunodeficiency virus replication in vitro. Biochem Biophys Res Commun 1987, 142, 128-134.
- Hamamoto, Y.; Nakashima, H.; Matsui, T.; Matsuda, A.; Ueda, T.; Yamamoto, N., Inhibitory effect of 2’,3’-didehydro-2’,3’-dideoxynucleosides on infectivity, cytopathic effects, and replication of human immunodeficiency virus. Antimicrob Agents Chemother 1987, 31, (6), 907-910.
- Lin, T. S.; Schinazi, R. F.; Prusoff, W. H., Potent and selective in vitro activity of 3’-deoxythymidin-2’-ene (3’-deoxy-2’,3’-didehydrothymidine) against human immunodeficiency virus. Biochem Pharmacol 1987, 36, (17), 2713-8.
- Baba, M.; Tanaka, H.; De Clercq, E.; Pauwels, R.; Balzarini, J.; Schols, D.; Nakashima, H.; Perno, C.-F.; Walker, R. T.; Miyasaka, T., Highly specific inhibition of human immunodeficiency virus type 1 by a novel 6-substituted acyclouridine derivative. Biochem Biophys Res Commun 1989, 165, 1375-1381. [CrossRef]
- Miyasaka, T.; Tanaka, H.; Baba, M.; Hayakawa, H.; Walker, R. T.; Balzarini, J.; De Clercq, E., A novel lead for specific anti-HIV-1 agents: 1- [(2-hydroxyethoxy)methyl]-6-(phenylthio)thymine. J Med Chem 1989, 32, 2507-2509. [CrossRef]
- Pauwels, R.; Andries, K.; Desmyter, J.; Schols, D.; Kukla, M. J.; Breslin, H. J.; Raeymaeckers, A.; Van Gelder, J.; Woestenborghs, R.; Heykants, J.; et al., Potent and selective inhibition of HIV-1 replication in vitro by a novel series of TIBO derivatives. Nature 1990, 343, (6257), 470-4. [CrossRef]
- Debyser, Z.; Pauwels, R.; Andries, K.; Desmyter, J.; Kukla, M.; Janssen, P. A.; De Clercq, E., An antiviral target on reverse transcriptase of human immunodeficiency virus type 1 revealed by tetrahydroimidazo-[4,5,1-jk] [1,4]benzodiazepin-2 (1H)-one and -thione derivatives. Proc Natl Acad Sci U S A 1991, 88, (4), 1451-5. [CrossRef]
- Baba, M.; De Clercq, E.; Tanaka, H.; Ubasawa, M.; Takashima, H.; Sekiya, K.; Nitta, I.; Umezu, K.; Nakashima, H.; Mori, S.; et al., Potent and selective inhibition of human immunodeficiency virus type 1 (HIV-1) by 5-ethyl-6-phenylthiouracil derivatives through their interaction with the HIV-1 reverse transcriptase. Proc Natl Acad Sci U S A 1991, 88, (6), 2356-60. [CrossRef]
- Baba, M.; De Clercq, E.; Tanaka, H.; Ubasawa, M.; Takashima, H.; Sekiya, K.; Nitta, I.; Umezu, K.; Walker, R. T.; Mori, S.; et al., Highly potent and selective inhibition of human immunodeficiency virus type 1 by a novel series of 6-substituted acyclouridine derivatives. Mol Pharmacol 1991, 39, (6), 805-10.
- De Clercq, E., Non-nucleoside reverse transcriptase inhibitors (NNRTIs): past, present and future. Chemistry & Biodiversity 2004, 1, 44-64.
- Ding, J.; Das, K.; Moereels, H.; Koymans, L.; Andries, K.; Janssen, P. A.; Hughes, S. H.; Arnold, E., Structure of HIV-1 RT/TIBO R 86183 complex reveals similarity in the binding of diverse nonnucleoside inhibitors. Nat Struct Biol 1995, 2, (5), 407-15. [CrossRef]
- Das, K.; Ding, J.; Hsiou, Y.; Clark, A. D., Jr.; Moereels, H.; Koymans, L.; Andries, K.; Pauwels, R.; Janssen, P. A.; Boyer, P. L.; Clark, P.; Smith, R. H., Jr.; Kroeger Smith, M. B.; Michejda, C. J.; Hughes, S. H.; Arnold, E., Crystal structures of 8-Cl and 9-Cl TIBO complexed with wild-type HIV-1 RT and 8-Cl TIBO complexed with the Tyr181Cys HIV-1 RT drug-resistant mutant. J Mol Biol 1996, 264, (5), 1085-100. [CrossRef]
- Baba, M.; Shigeta, S.; Yuasa, S.; Takashima, H.; Sekiya, K.; Ubasawa, M.; Tanaka, H.; Miyasaka, T.; Walker, R. T.; De Clercq, E., Preclinical evaluation of MKC-442, a highly potent and specific inhibitor of human immunodeficiency virus type 1 in vitro. Antimicrob Agents Chemother 1994, 38, (4), 688-92. [CrossRef]
- Janssen, P. A.; Lewi, P. J.; Arnold, E.; Daeyaert, F.; de Jonge, M.; Heeres, J.; Koymans, L.; Vinkers, M.; Guillemont, J.; Pasquier, E.; Kukla, M.; Ludovici, D.; Andries, K.; de Béthune, M. P.; Pauwels, R.; Das, K.; Clark, A. D., Jr.; Frenkel, Y. V.; Hughes, S. H.; Medaer, B.; De Knaep, F.; Bohets, H.; De Clerck, F.; Lampo, A.; Williams, P.; Stoffels, P., In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2-pyrimidinyl]amino]benzonitrile (R278474, rilpivirine). J Med Chem 2005, 48, (6), 1901-9.
- De Clercq, E.; Descamps, J.; De Somer, P.; Holy, A., (S)-9-(2,3-dihydroxypropyl)adenone: an aliphatic nucleoside analog with broad-spectrum antiviral activity. Science 1978, 200, 563-565.
- Schaeffer, H. J.; Beauchamp, L.; de Miranda, P.; Elion, G. B.; Bauer, D. J.; Collins, P., 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature 1978, 272, (5654), 583-5. [CrossRef]
- Elion, G. B.; Furman, P. A.; Fyfe, J. A.; de Miranda, P.; Beauchamp, L.; Schaeffer, H. J., Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A 1977, 74, (12), 5716-20.
- De Clercq, E., S-Adenosylhomocysteine hydrolase inhibitors as broad-spectrum antiviral agents. Biochem Pharmacol 1987, 36, 2567-2575.
- King, G. S. D.; Sengier, L., Crystal structure of two forms of 9-(2,3-dihydroxypropyl)adenine. J Chem Res Synop 1981, 121, 1501 - 1538.
- Birnbaum, G. I.; Shugar, D., Biologically Active Nucleosides and Nucleotides: Conformational Features and Interactions with Enzymes. In Topics in Nucleic Acid Structure: Part 3, Neidle, S., Ed. Palgrave Macmillan UK: London, 1987; pp 1-70.
- De Clercq, E.; Descamps, J.; De Somer, P.; Barr, P. J.; Jones, A. S.; Walker, R. T., (E)-5-(2-Bromovinyl)-2’-deoxyuridine: a potent and selective anti-herpes agent. Proc Natl Acad Sci U S A 1979, 76, (6), 2947-51. [CrossRef]
- De Clercq, E.; Verhelst, G.; Descamps, J.; Bergstrom, D. E., Differential inhibition of herpes simplex viruses, type 1 (HSV-1) and type 2 (HSV-2), by (E)-5-(2-x-vinyl)-2’-deoxyuridines. Acta Microbiol. Acad. Sci. Hung 1981, 28, 307-312.
- De Clercq, E.; Degreef, H.; Wildiers, J.; De Jonge, G.; Drochmans, A.; Descamps, J.; De Somer, P., Oral (E)-5-(2-bromovinyl)-2’-deoxyuridine in severe herpes zoster. Br. Med. J 1980, 281, 1178-1178. [CrossRef]
- Maudgal, P. C.; De Clercq, E.; Descamps, J.; Missotten, L., Comparative evaluation of BVDU ((E)-5-(2-bromovinyl)-2’-deoxyuridine) and IDU (5-iodo-2’-deoxyuridine) in the treatment of experimental herpes simplex keratitis in rabbits. Bull Soc Belge Ophthalmol 1979, 186, 109-118.
- Maudgal, P. C.; De Clercq, E.; Descamps, J.; Missotten, L.; De Somer, P.; Busson, R.; Vanderhaeghe, H.; Verhelst, G.; Walker, R. T.; Jones, A. S., (E)-5-(2-Bromovinyl)-2’-deoxyuridine in the treatment of experimental herpes simplex keratitis. Antimicrob Agents Chemother 1980, 17, 8-12. [CrossRef]
- Maudgal, P. C.; Missotten, L.; De Clercq, E.; Descamps, J.; De Meuter, E., Efficacy of (E)-5-(2-bromovinyl)-2’-deoxyuridine in the topical treatment of herpes simplex keratitis. Albrecht von Graefes Arch Klin Ophthalmol 1981, 216, 261-268. [CrossRef]
- Maudgal, P. C.; De Clercq, E.; Descamps, J.; Missotten, L.; Wijnhoven, J., Experimental stromal herpes simplex keratitis. Influence of treatment with topical bromovinyldeoxyuridine and trifluridine. Arch Ophthalmol 1982, 100, 653-656.
- Maudgal, P. C.; Uyttebroeck, W.; De Clercq, E.; Missotten, L., Oral and topical treatment of experimental herpes simplex iritis with bromovinyldeoxyuridine. Arch Ophthalmol 1982, 100, 1337-1340. [CrossRef]
- Maudgal, P. C.; De Clercq, E.; Missotten, L., Efficacy of bromovinyldeoxyuridine in the treatment of herpes simplex virus and varicella-zoster virus eye infections. Antiviral Res 1984, 4, 281-291. [CrossRef]
- Maudgal, P. C.; De Clercq, E., Evaluation of bromovinyldeoxyuridine-related compounds in the treatment of experimental herpes simplex keratitis. Arch Ophthalmol 1985, 103, 1393-1397.
- Van Bijsterveld, O. P.; Meurs, P. J.; De Clercq, E.; Maudgal, P. C., Bromovinyldeoxyuridine and interferon treatment in ulcerative herpetic keratitis: a double masked study. Br J Ophthalmol 1989, 73, 604-607. [CrossRef]
- Dullaert, H.; Maudgal, P. C.; Leys, A.; Dralands, L.; De Clercq, E., Bromovinyldeoxyuridine treatment of outer retinal necrosis due to varicella-zoster virus: a case-report. Bull Soc Belge Ophthalmol 1997, 262, 107-113.
- De Clercq, E., Discovery and development of BVDU (brivudin) as a therapeutic for the treatment of herpes zoster. Biochem Pharmacol 2004, 68, 2301-2315. [CrossRef]
- Colla, L.; De Clercq, E.; Busson, R.; Vanderhaeghe, H., Synthesis and antiviral activity of water-soluble esters of acyclovir [9-[(2-hydroxyethoxy)methyl]guanine]. J Med Chem 1983, 26, 602-604. [CrossRef]
- Maudgal, P. C.; De Clercq, E.; Descamps, J.; Missotten, L., Topical treatment of experimental herpes simplex keratouveitis with 2’-O-glycylacyclovir. Arch Ophthalmol 1984, 102, 140-142. [CrossRef]
- De Clercq, E.; Yamamoto, N.; Pauwels, R.; Baba, M.; Schols, D.; Nakashima, H.; Balzarini, J.; Debyser, Z.; Murrer, B. A.; Schwartz, D.; et al., Potent and selective inhibition of human immunodeficiency virus (HIV)-1 and HIV-2 replication by a class of bicyclams interacting with a viral uncoating event. Proc Natl Acad Sci U S A 1992, 89, (12), 5286-90. [CrossRef]
- De Clercq, E.; Yamamoto, N.; Pauwels, R.; Balzarini, J.; Witvrouw, M.; De Vreese, K.; Debyser, Z.; Rosenwirth, B.; Peichl, P.; Datema, R.; et al., Highly potent and selective inhibition of human immunodeficiency virus by the bicyclam derivative JM3100. Antimicrob Agents Chemother 1994, 38, (4), 668-74. [CrossRef]
- de Vreese, K.; Kofler-Mongold, V.; Leutgeb, C.; Weber, V.; Vermeire, K.; Schacht, S.; Anné, J.; de Clercq, E.; Datema, R.; Werner, G., The molecular target of bicyclams, potent inhibitors of human immunodeficiency virus replication. J Virol 1996, 70, (2), 689-96. [CrossRef]
- Schols, D.; Esté, J. A.; Henson, G.; De Clercq, E., Bicyclams, a class of potent anti-HIV agents, are targeted at the HIV coreceptor fusin/CXCR-4. Antiviral Res 1997, 35, (3), 147-56. [CrossRef]
- Schols, D.; Struyf, S.; Van Damme, J.; Esté, J.; Henson, G.; De Clercq, E., Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med 1997, 186, (8), 1383-1388. [CrossRef]
- Donzella, G. A.; Schols, D.; Lin, S. W.; Esté, J. A.; Nagashima, K. A.; Maddon, P. J.; Allaway, G. P.; Sakmar, T. P.; Henson, G.; De Clercq, E.; Moore, J. P., AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med 1998, 4, (1), 72-7. [CrossRef]
- De Clercq, E., The bicyclam AMD3100 story. Nat Rev Drug Discov 2003, 2, (7), 581-7.
- Hendrix, C. W.; Flexner, C.; MacFarland, R. T.; Giandomenico, C.; Fuchs, E. J.; Redpath, E.; Bridger, G.; Henson, G. W., Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother 2000, 44, (6), 1667-73. [CrossRef]
- De Clercq, E., The AMD3100 story: The path to the discovery of a stem cell mobilizer (Mozobil). Biochem Pharmacol 2008.
- De Clercq, E., Recent advances on the use of the CXCR4 antagonist plerixafor (AMD3100, Mozobil) and potential of other CXCR4 antagonists as stem cell mobilizers. Pharmacol Ther 2010, 128, (3), 509-518.
- De Clercq, E., AMD3100/CXCR4 inhibitor. Front Immunol 2015, 6, 276.
- De Clercq, E., Mozobil® (Plerixafor, AMD3100), 10 years after its approval by the US Food and Drug Administration. Antivir Chem Chemother 2019, 27, 2040206619829382.
- De Clercq, E.; Holy, A.; Rosenberg, I.; Sakuma, T.; Balzarini, J.; Maudgal, P. C., A novel selective broad-spectrum anti-DNA virus agent. Nature 1986, 323, (464), 467. [CrossRef]
- De Clercq, E.; Sakuma, T.; Baba, M.; Pauwels, R.; Balzarini, J.; Rosenberg, I.; Holy, A., Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res 1987, 8, 261-272. [CrossRef]
- Balzarini, J.; Holy, A.; Jindrich, J.; Naesens, L.; Snoeck, R.; Schols, D.; De Clercq, E., Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob Agents Chemother 1993, 37, 332-338. [CrossRef]
- Tsai, C. C.; Follis, K. E.; Sabo, A.; Beck, T. W.; Grant, R. F.; Bischofberger, N.; Benveniste, R. E.; Black, R., Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science 1995, 270, (5239), 1197-9. [CrossRef]
- Robbins, B. L.; Srinivas, R. V.; Kim, C.; Bischofberger, N.; Fridland, A., Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother 1998, 42, (3), 612-7.
- Naesens, L.; Bischofberger, N.; Augustijns, P.; Annaert, P.; Van den Mooter, G.; Arimilli, M. N.; Kim, C. U.; De Clercq, E., Antiretroviral efficacy and pharmacokinetics of oral bis(isopropyloxycarbonyloxymethyl)-9-(2-phosphonylmethoxypropyl)adenine in mice. Antimicrob Agents Chemother 1998, 42, 1568-1573. [CrossRef]
- De Clercq, E., Tenofovir at the crossroad of the therapy and prophylaxis of HIV and HBV infections. J Cell Immunol 2020, 2, (1), 23-30.
- Lee, W. A.; He, G. X.; Eisenberg, E.; Cihlar, T.; Swaminathan, S.; Mulato, A.; Cundy, K. C., Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother 2005, 49, (5), 1898-906. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




