Submitted:
04 December 2023
Posted:
05 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
2.2.1. Preparation of Organo Clay
2.2.2. Preparation of Melamine Formaldehyde Pre-Polymer (MF) and MF-Organo-Clay Nanocomposite
2.2.3. Batch Adsorption Experiments
2.2.4. Zeta Potential Measurements
2.2.5. Spectroscopic and Microscopic Analyzes
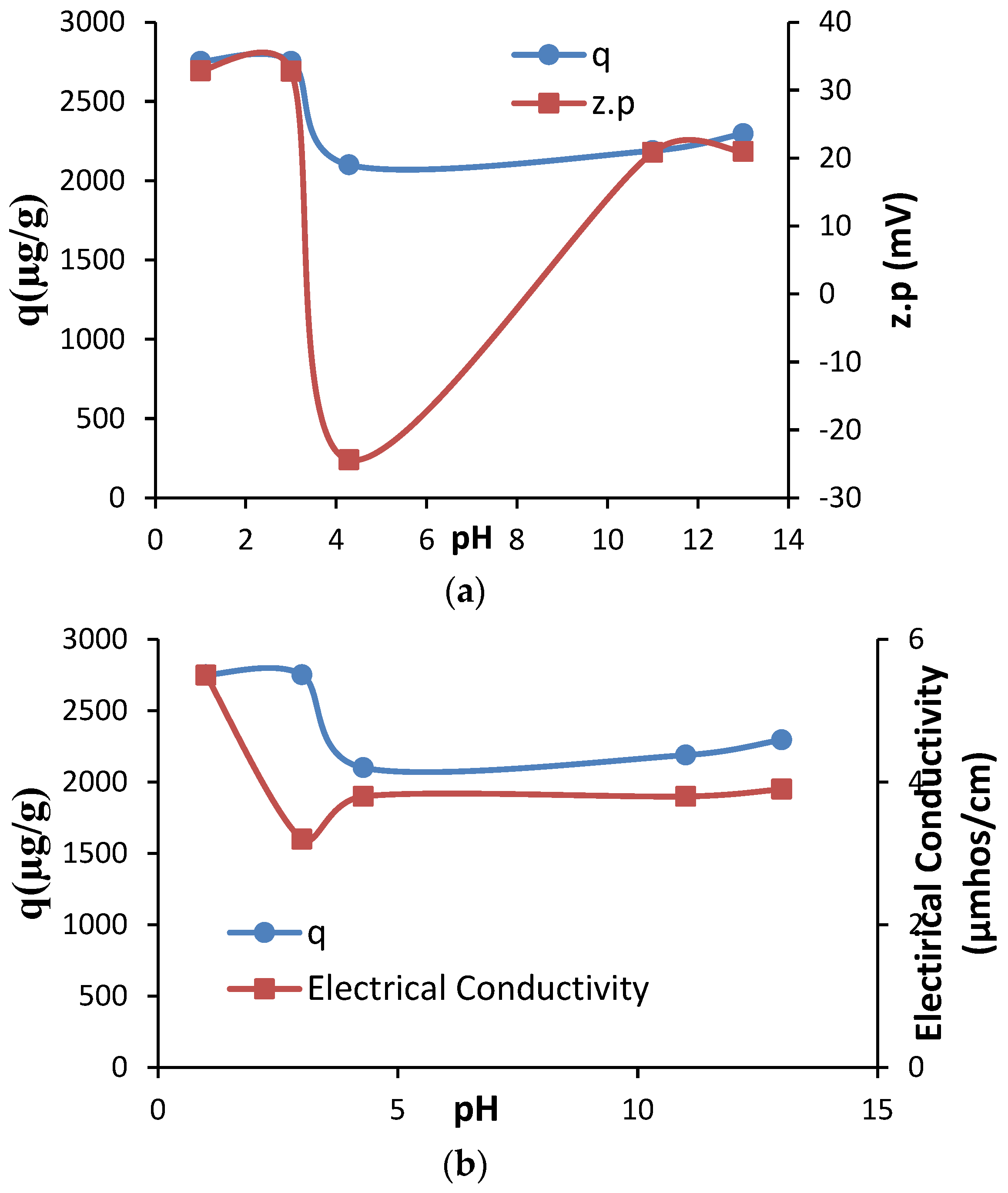
3. Results and Discussion
3.1. Textural, Structural and Crystallographic Characterization of Raw Montmorillonite (MMT), Organo-Montmorillonite (OMMT), Melamine Formaldehyde Foam (MF) and Melamine Formaldehyde Organo-Clay Nanocomposite Foam (MFCNC)
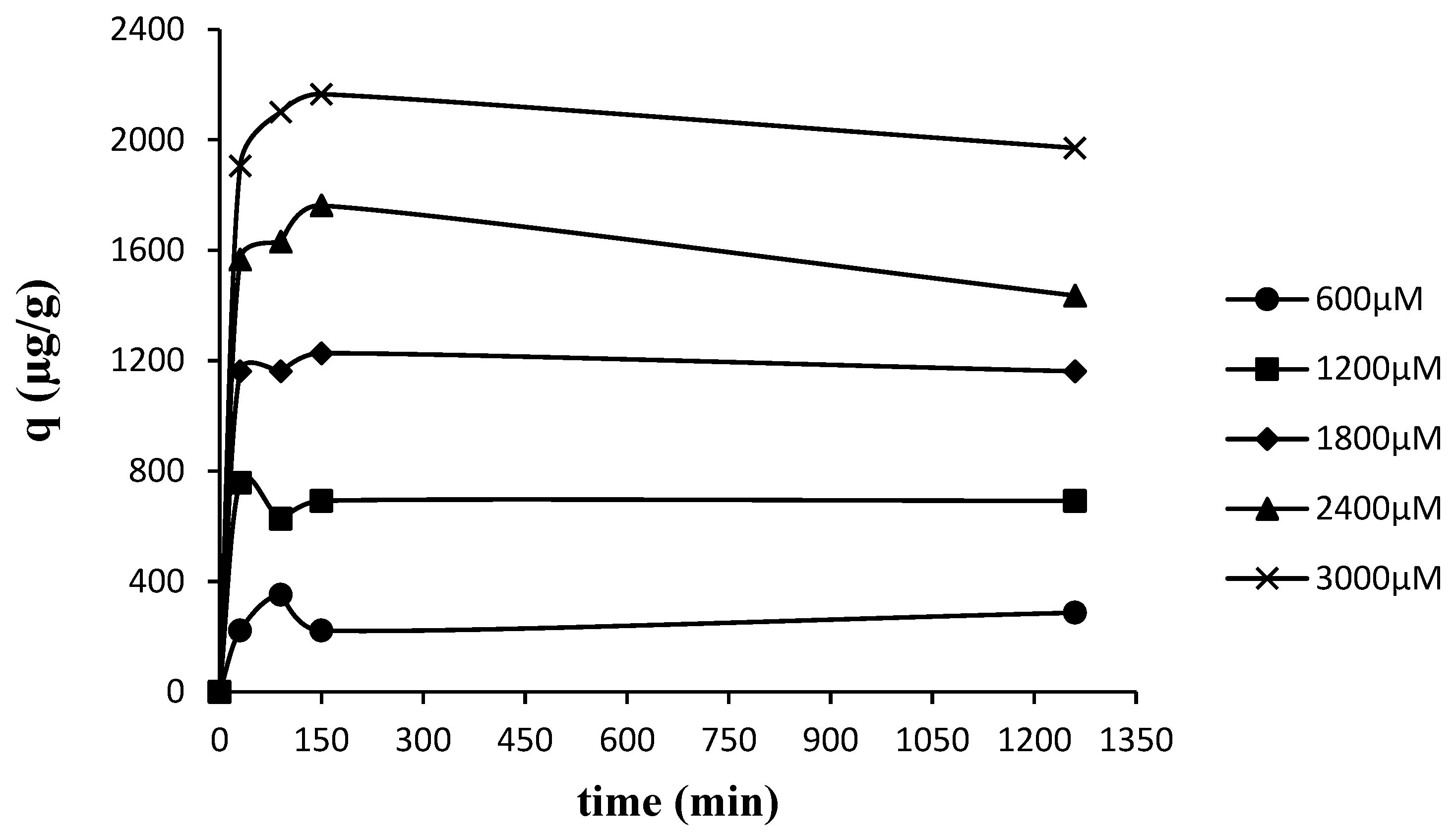
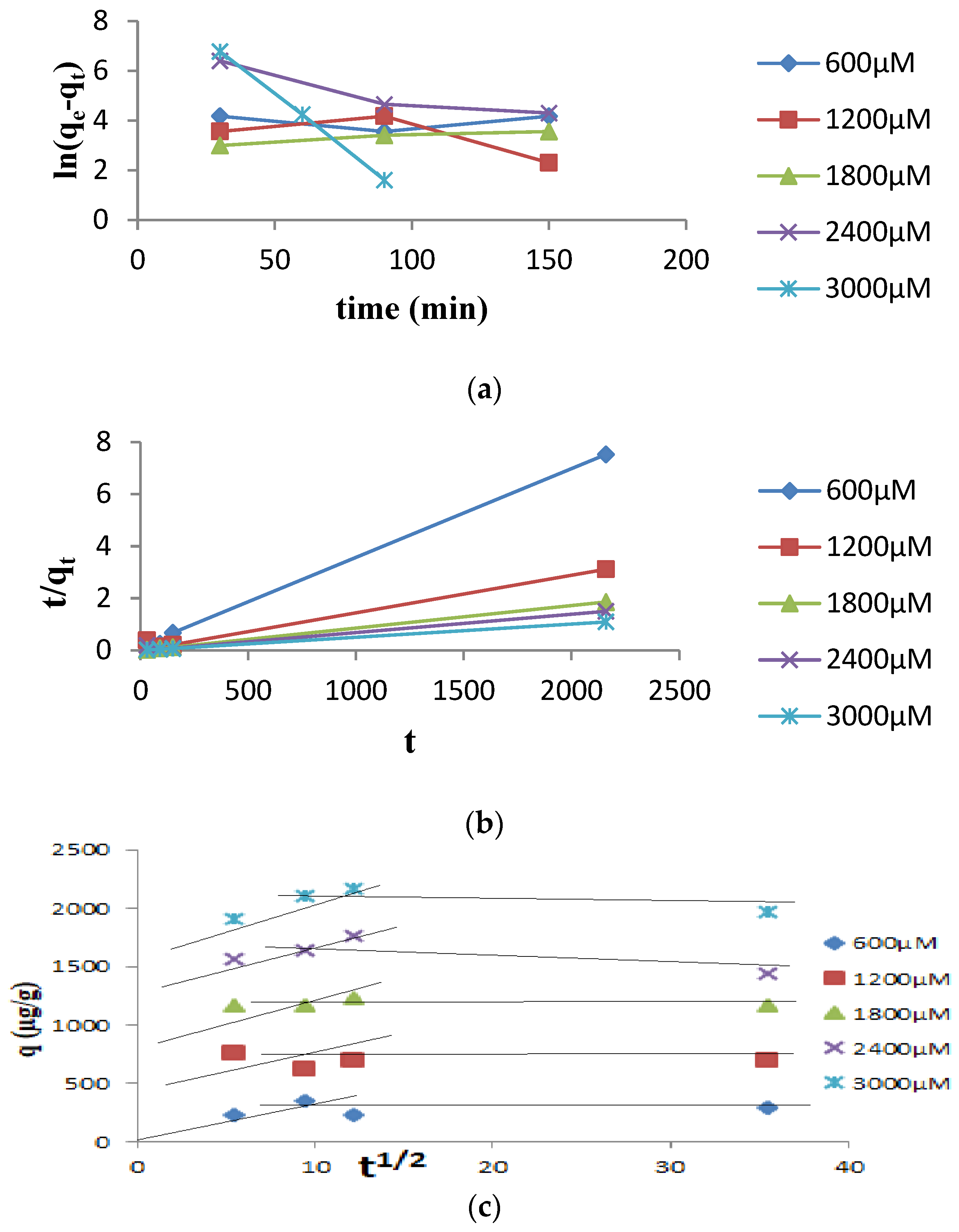
3.2. Kinetic Studies
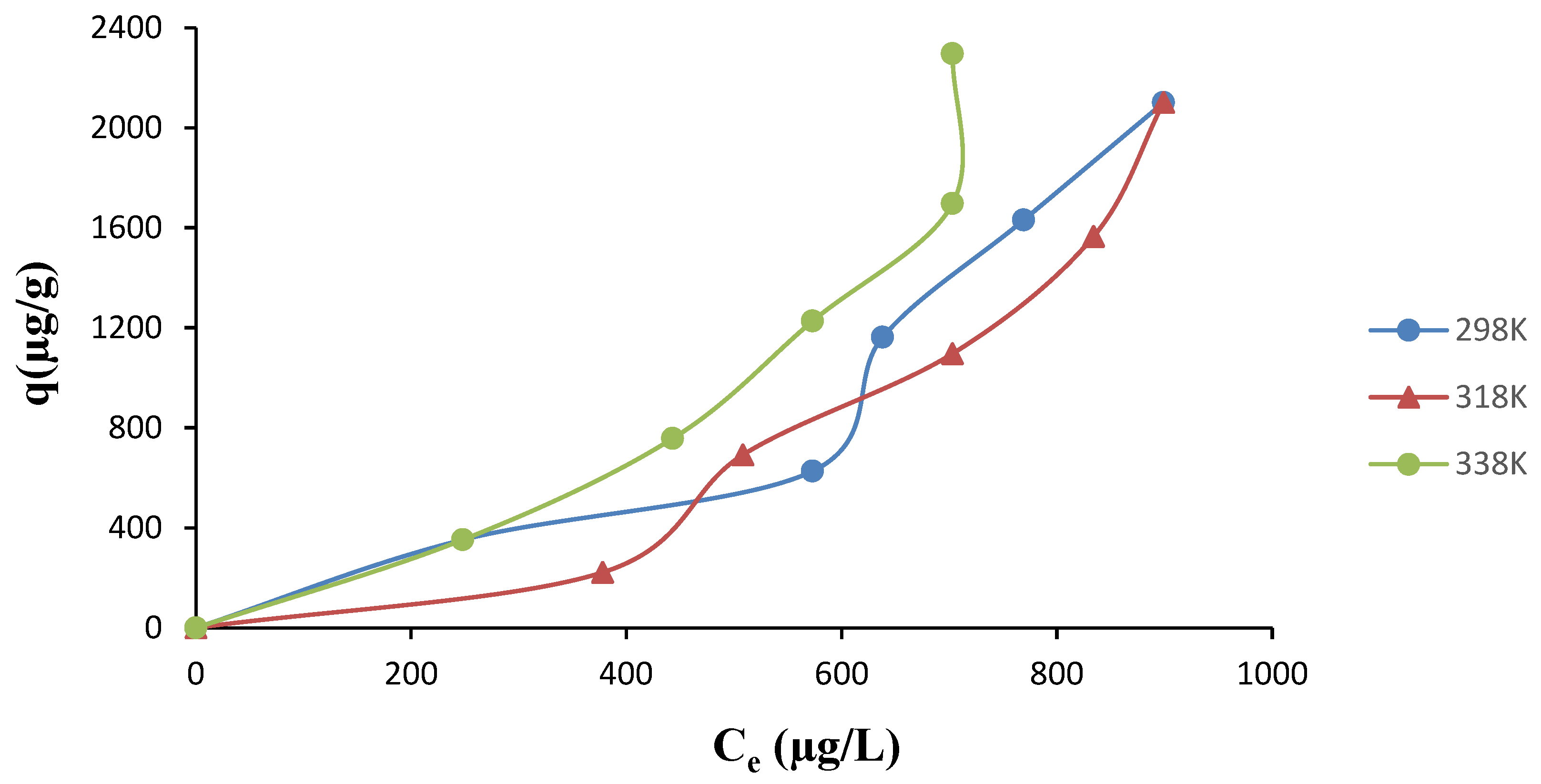
3.3. Adsorption Isotherms and the Effect of Temperature
3.4. Adsorption Thermodynamics
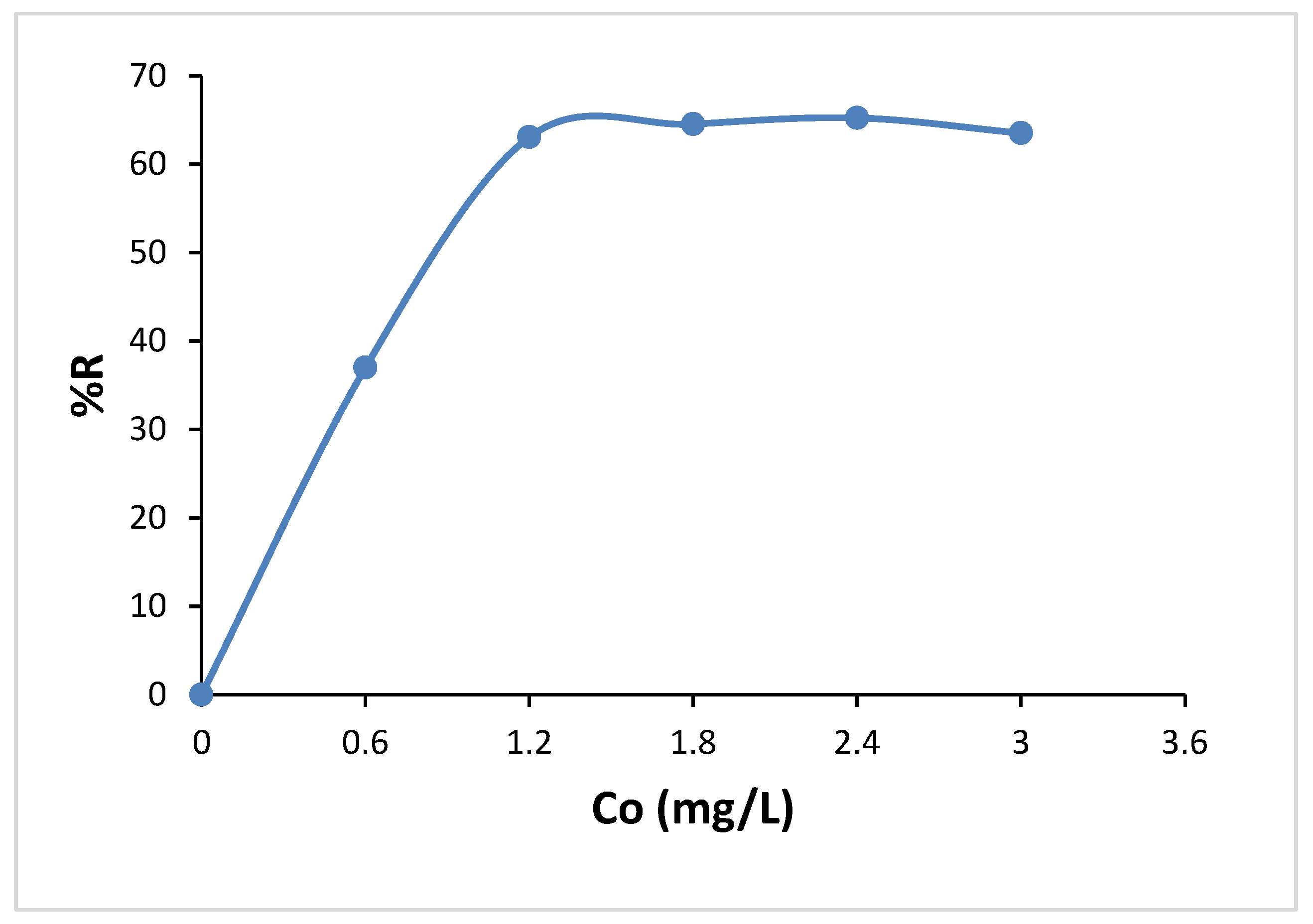
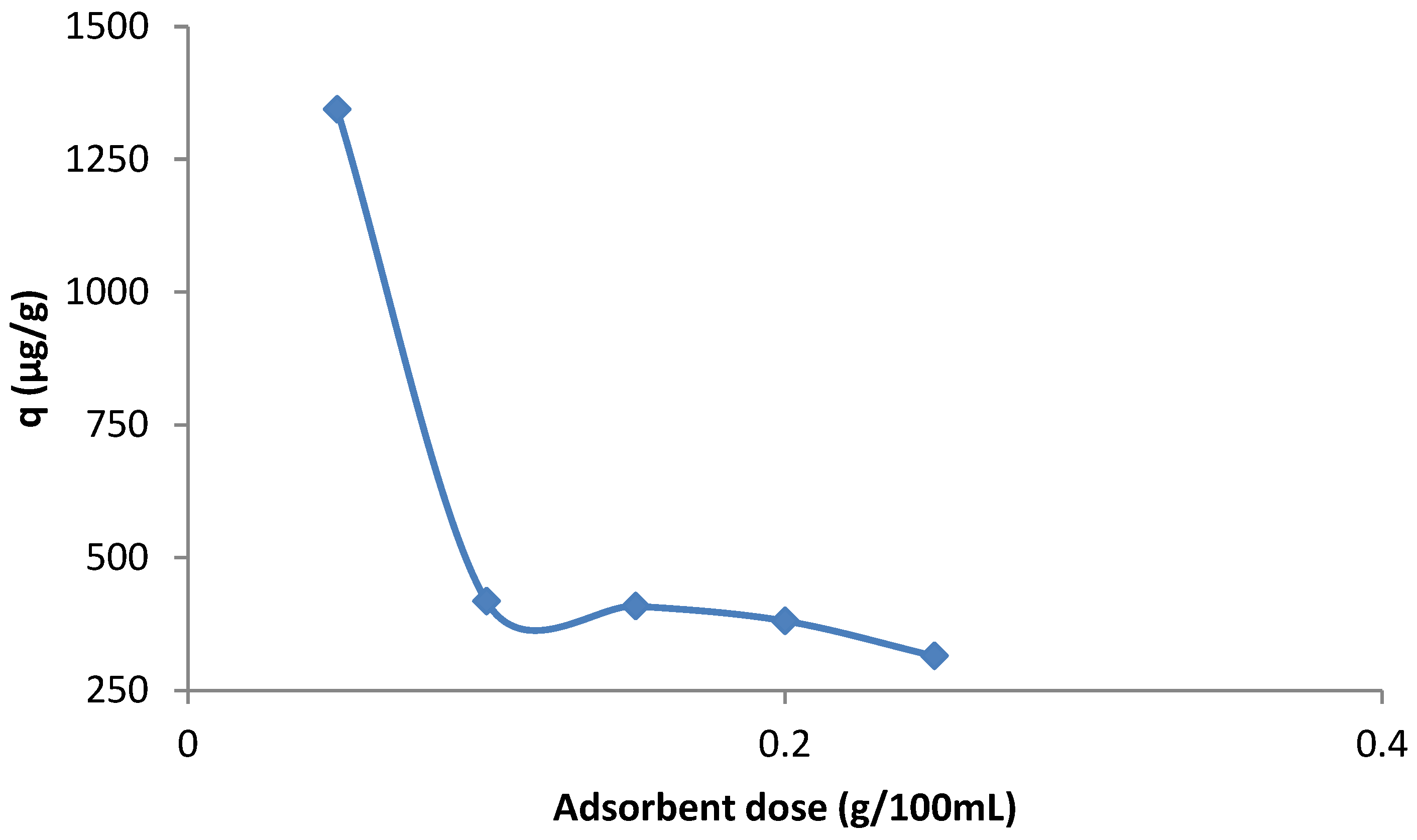
3.6. Effect of Adsorbent Dose
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhalara, P.D.; Punetha, D.; Balasubramanian, K. A review of potential remediation techniques for uranium (VI) ion retrieval from contaminated aqueous environment. Journal of Environmental Chemical Engineering 2014, 2, 1621–1634. [Google Scholar] [CrossRef]
- Benedict, M., Pigford, T. H., & Levi, H. W. (1981). Nuclear chemical engineering (Vol. 2). New York: McGraw-Hill.
- Burns, P.C.; Finch, R.J. Wyartite: crystallographic evidence for the first pentavalent-uranium mineral. American Mineralogist 1999, 84, 1456–1460. [Google Scholar] [CrossRef]
- Dinis MD, L.; Fiúza, A. Mitigation of uranium mining impacts—a review on groundwater remediation technologies. Geosciences 2021, 11, 250. [Google Scholar] [CrossRef]
- Sellin, P.; Leupin, O.X. The use of clay as an engineered barrier in radioactive-waste management–a review. Clays and Clay Minerals 2013, 61, 477–498. [Google Scholar] [CrossRef]
- Schnurr, A.; Marsac, R.; Rabung, T.; Lützenkirchen, J.; Geckeis, H. Sorption of Cm (III) and Eu (III) onto clay minerals under saline conditions: Batch adsorption, laser-fluorescence spectroscopy and modeling. Geochimica et Cosmochimica Acta 2015, 151, 192–202. [Google Scholar] [CrossRef]
- Bradbury, M.H.; Baeyens, B. Sorption modelling on illite. Part II: Actinide sorption and linear free energy relationships. Geochimica et Cosmochimica Acta 2009, 73, 1004–1013. [Google Scholar] [CrossRef]
- Abney, C.W.; Mayes, R.T.; Saito, T.; Dai, S. Materials for the recovery of uranium from seawater. Chemical reviews 2017, 117, 13935–14013. [Google Scholar] [CrossRef]
- Tsang, C.F.; Bernier, F.; Davies, C. Geohydromechanical processes in the Excavation Damaged Zone in crystalline rock, rock salt, and indurated and plastic clays—in the context of radioactive waste disposal. International Journal of Rock Mechanics and Mining Sciences 2005, 42, 109–125. [Google Scholar] [CrossRef]
- Johnson, D.; Leake, J.R.; Read, D.J. Transfer of recent photosynthate into mycorrhizal mycelium of an upland grassland: short-term respiratory losses and accumulation of 14C. Soil Biology and Biochemistry 2002, 34, 1521–1524. [Google Scholar] [CrossRef]
- Joseph, C.; Van Loon, L.R.; Jakob, A.; Steudtner, R.; Schmeide, K.; Sachs, S.; Bernhard, G. Diffusion of U (VI) in Opalinus Clay: Influence of temperature and humic acid. Geochimica et Cosmochimica Acta 2013, 109, 74–89. [Google Scholar] [CrossRef]
- Keith, L. S., & Faroon, O. M. (2022). Uranium. In Handbook on the Toxicology of Metals (pp. 885-936). Academic Press. [CrossRef]
- Fernandes, M.M.; Baeyens, B.; Dähn, R.; Scheinost, A.C.; Bradbury, M.H. U (VI) sorption on montmorillonite in the absence and presence of carbonate: a macroscopic and microscopic study. Geochimica et Cosmochimica Acta 2012, 93, 262–277. [Google Scholar] [CrossRef]
- Belloni, F.; Kütahyali, C.; Rondinella, V.V.; Carbol, P.; Wiss, T.; Mangione, A. Can carbon nanotubes play a role in the field of nuclear waste management. Environ. Sci. Technol. 2009, 43, 1250–1255. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X.; Liang, S.; Li, Z.; Yang, W.; Wang, H. Large reversible capacity of high quality graphene sheets as an anode material for lithium-ion batteries. Electrochimica Acta 2010, 55, 3909–3914. [Google Scholar] [CrossRef]
- Chen, S.; Hong, J.; Yang, H.; Yang, J. Adsorption of uranium (VI) from aqueous solution using a novel graphene oxide-activated carbon felt composite. Journal of Environmental Radioactivity 2013, 126, 253–258. [Google Scholar] [CrossRef]
- Ibrahim WA, W.; Nodeh, H.R.; Sanagi, M.M. Graphene-based materials as solid phase extraction sorbent for trace metal ions, organic compounds, and biological sample preparation. Critical reviews in analytical chemistry 2016, 46, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Chianella, I.; Goel, S.; Nezhad, H.Y. (2022). Exploring the Nexus Between Structure, Properties and Applications in Graphene Conductors. Biomaterials and Polymers Horizon (2022) 1(4).
- Wang, Y.; Gu, Z.; Yang, J.; Liao, J.; Yang, Y.; Liu, N.; Tang, J. Amidoxime-grafted multiwalled carbon nanotubes by plasma techniques for efficient removal of uranium (VI). Applied surface science 2014, 320, 10–20. [Google Scholar] [CrossRef]
- Xue, J.H.; Zhang, H.; Ding, D.X.; Hu, N.; Wang, Y.D.; Wang, Y.S. Linear β-cyclodextrin polymer functionalized multiwalled carbon nanotubes as nanoadsorbent for highly effective removal of U (VI) from aqueous solution based on inner-sphere surface complexation. Industrial & Engineering Chemistry Research 2019, 58, 4074–4083. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Z.; Yang, J.; Liao, J.; Yang, Y.; Liu, N.; Tang, J. Amidoxime-grafted multiwalled carbon nanotubes by plasma techniques for efficient removal of uranium (VI). Applied surface science 2014, 320, 10–20. [Google Scholar] [CrossRef]
- Shaw, S.; Neill, T.S.; Bryan, N.; Sherriff, N.; Natrajan, L.S.; Wilson, H.; Lopez-Odriozola, L.; Rigby, B.; Haigh, S.J.; Zou, Y.-C.; Harrison, R.; Morris, K. Hydrotalcite Colloidal Stability and Interactions with Uranium (VI) at Neutral to Alkaline pH. Langmuir 2022, 38, 2576–2589. [Google Scholar] [CrossRef]
- Sadeghi, M.; Amiri, M.; Roshanfarzad, P.; Avila, M.; Tenreiro, C. Radiochemical studies relevant to the no-carrier-added production of 61, 64Cu at a cyclotron. Radiochimica Acta 2008, 96, 399–402. [Google Scholar] [CrossRef]
- Metwally, E.; Saleh, A.S.; El-Naggar, H.A. Extraction and separation of uranium (VI) and thorium (IV) using tri-n-dodecylamine impregnated resins. Journal of Nuclear and Radiochemical Sciences 2005, 6, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Sh, S.A.; El-Naggar, H. A. Extraction and Separation of Uranium (VI) and Thorium (IV) Using Tri-n-dodecylamine Impregnated Resins. Journal of nuclear and radiochemical sciences 2005, 6, 119–126. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, P.; Liu, Y. Decontamination of radioactive wastewater: State of the art and challenges forward. Chemosphere 2019, 215, 543–553. [Google Scholar] [CrossRef]
- Peng, H.; Guo, J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: a review. Environmental Chemistry Letters 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Aly, M.M.; Hamza, M.F. A review: studies on uranium removal using different techniques. Overview. Journal of dispersion science and technology 2013, 34, 182–213. [Google Scholar] [CrossRef]
- Fomina, M.; Gadd, G.M. Biosorption: current perspectives on concept, definition and application. Bioresource technology 2014, 160, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Michard, P.; Guibal, E.; Vincent, T.; Le Cloirec, P. Sorption and desorption of uranyl ions by silica gel: pH, particle size and porosity effects. Microporous materials 1996, 5, 309–324. [Google Scholar] [CrossRef]
- Tsuruta, S.; Teter, M.A.; Takatsuka, T.; Tatsumi, T.; Tamagaki, R. Confronting neutron star cooling theories with new observations. The Astrophysical Journal 2002, 571, L143. [Google Scholar] [CrossRef]
- Mirjalili, K.; Roshani, M. Resin-in-pulp method for uranium recovery from leached pulp of low grade uranium ore. Hydrometallurgy 2007, 85, 103–109. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, D.K.; Kumar, R.; Gupta, A. Controlled release of the fungicide thiram from starch–alginate–clay based formulation. Applied Clay Science 2009, 45, 76–82. [Google Scholar] [CrossRef]
- Nakajima, A.; Sakaguchi, T. Recovery of uranium from uranium refining waste water by using immobilized persimmon tannin. Journal of radioanalytical and nuclear chemistry 1999, 242, 623–626. [Google Scholar] [CrossRef]
- Kilislioglu, A.; Bilgin, B. Adsorption of uranium on halloysite. Radiochimica Acta 2002, 90, 155–160. [Google Scholar] [CrossRef]
- Atun, G.; Kilislioglu, A. The adsorption behavior of natural sand in contact with uranium contaminated seawater. Journal of Environmental Science and Health, Part A 2002, 37, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Merdivan, M.; Düz, M.Z.; Hamamci, C. Sorption behaviour of uranium (VI) with N, N-dibutyl-N′-benzoylthiourea impregnated in Amberlite XAD-16. Talanta 2001, 55, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, J.B.; Zolfonoun, E. Simultaneous spectrophotometric determination of trace amounts of uranium, thorium, and zirconium using the partial least squares method after their preconcentration by α-benzoin oxime modified Amberlite XAD-2000 resin. Talanta 2010, 80, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Metilda, P.; Sanghamitra, K.; Gladis, J.M.; Naidu GR, K.; Rao, T.P. Amberlite XAD-4 functionalized with succinic acid for the solid phase extractive preconcentration and separation of uranium (VI). Talanta 2005, 65, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Karve, M.; Rajgor, R.V. Amberlite XAD-2 impregnated organophosphinic acid extractant for separation of uranium (VI) from rare earth elements. Desalination 2008, 232, 191–197. [Google Scholar] [CrossRef]
- Qadeer, R.; Hanif, J. Adsorption of dysprosium ions on activated charcoal from aqueous solutions. Carbon 1995, 33, 215–220. [Google Scholar] [CrossRef]
- Rahmati, A.; Ghaemi, A.; Samadfam, M. Kinetic and thermodynamic studies of uranium (VI) adsorption using Amberlite IRA-910 resin. Annals of Nuclear Energy 2012, 39, 42–48. [Google Scholar] [CrossRef]
- Zhang, Z.; Clifford, D.A. Exhausting and regenerating resin for uranium removal. Journal-American Water Works Association 1994, 86, 228–241. [Google Scholar] [CrossRef]
- Ahn, K.H.; Song, K.G. Treatment of domestic wastewater using microfiltration for reuse of wastewater. Desalination 1999, 126, 7–14. [Google Scholar] [CrossRef]
- Huikuri, P.; Salonen, L. Removal of uranium from Finnish groundwaters in domestic use with a strong base anion resin. Journal of Radioanalytical and Nuclear chemistry 2000, 245, 385–393. [Google Scholar] [CrossRef]
- Chellam, S.; Clifford, D.A. Physical–chemical treatment of groundwater contaminated by leachate from surface disposal of uranium tailings. Journal of Environmental Engineering 2002, 128, 942–952. [Google Scholar] [CrossRef]
- Ladeira, A.C.Q.; Morais, C.A.D. Uranium recovery from industrial effluent by ion exchange—column experiments. Minerals engineering 2005, 18, 1337–1340. [Google Scholar] [CrossRef]
- Semnani, F.; Asadi, Z.; Samadfam, M.; Sepehrian, H. Uranium (VI) sorption behavior onto amberlite CG-400 anion exchange resin: effects of pH, contact time, temperature and presence of phosphate. Annals of nuclear energy 2012, 48, 21–24. [Google Scholar] [CrossRef]
- Kütahyalı, C.; Eral, M. Sorption studies of uranium and thorium on activated carbon prepared from olive stones: kinetic and thermodynamic aspects. Journal of Nuclear Materials 2010, 396, 251–256. [Google Scholar] [CrossRef]
- Akperov, E.O.; Maharramov, A.M.; Akperov, O.G. Uranyl ion adsorption using novel cross-linked maleic anhydride–allyl propionate–styrene terpolymer. Hydrometallurgy 2009, 100, 76–81. [Google Scholar] [CrossRef]
- Korichi, S.; Bensmaili, A. Sorption of uranium (VI) on homoionic sodium smectite experimental study and surface complexation modeling. Journal of hazardous materials 2009, 169, 780–793. [Google Scholar] [CrossRef]
- Hritcu, D.; Humelnicu, D.; Dodi, G.; Popa, M.I. Magnetic chitosan composite particles: evaluation of thorium and uranyl ion adsorption from aqueous solutions. Carbohydrate Polymers 2012, 87, 1185–1191. [Google Scholar] [CrossRef]
- Fernandes, M.M.; Vér, N.; Baeyens, B. Predicting the uptake of Cs, Co, Ni, Eu, Th and U on argillaceous rocks using sorption models for illite. Applied Geochemistry 2015, 59, 189–199. [Google Scholar] [CrossRef]
- Stockmann, M.; Fritsch, K.; Bok, F.; Fernandes, M.M.; Baeyens, B.; Steudtner, R.; Schmeide, K. New insights into U (VI) sorption onto montmorillonite from batch sorption and spectroscopic studies at increased ionic strength. Science of The Total Environment 2022, 806, 150653. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, T.; Bauer, A.; Coppin, F.; Fanghänel, T.; Kim, J.I. Inner-sphere, outer-sphere and ternary surface complexes: a TRLFS study of the sorption process of Eu (III) onto smectite and kaolinite. Radiochimica Acta 2002, 90, 345–349. [Google Scholar] [CrossRef]
- Geckeis, H.; Lützenkirchen, J.; Polly, R.; Rabung, T.; Schmidt, M. Mineral–water interface reactions of actinides. Chemical reviews 2013, 113, 1016–1062. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, M.H.; Baeyens, B. Sorption of Eu on Na-and Ca-montmorillonites: Experimental investigations and modelling with cation exchange and surface complexation. Geochimica et Cosmochimica Acta 2002, 66, 2325–2334. [Google Scholar] [CrossRef]
- Guillaumont, R., & Mompean, F. J. (2003). Update on the chemical thermodynamics of uranium, neptunium, plutonium, americium and technetium (Vol. 5, pp. 64–70). Amsterdam: Elsevier.
- Zachara, J.M.; McKinley, J.P. Influence of hydrolysis on the sorption of metal cations by smectites: Importance of edge coordination reactions: Dedicated to Paul W. Schindler on his retirement. Aquatic Sciences 1993, 55, 250–261. [Google Scholar] [CrossRef]
- McKinley, J.P.; Zachara, J.M.; Smith, S.C.; Turner, G.D. The influence of uranyl hydrolysis and multiple site-binding reactions on adsorption of U (VI) to montmorillonite. Clays and clay minerals 1995, 43, 586–598. [Google Scholar] [CrossRef]
- Pabalan, R.T.; Turner, D.R. Uranium (6+) sorption on montmorillonite: Experimental and surface complexation modeling study. Aquatic Geochemistry 1996, 2, 203–226. [Google Scholar] [CrossRef]
- Kowal-Fouchard, A.; Drot, R.; Simoni, E.; Ehrhardt, J.J. Use of spectroscopic techniques for uranium (VI)/montmorillonite interaction modeling. Environmental science & technology 2004, 38, 1399–1407. [Google Scholar] [CrossRef]
- Bradbury, M.H.; Baeyens, B. Experimental measurements and modeling of sorption competition on montmorillonite. Geochimica et Cosmochimica Acta 2005, 69, 4187–4197. [Google Scholar] [CrossRef]
- Bachmaf, S.; Planer-Friedrich, B.; Merkel, B.J. Effect of sulfate, carbonate, and phosphate on the uranium (VI) sorption behavior onto bentonite. Radiochimica Acta 2008, 96, 359–366. [Google Scholar] [CrossRef]
- Dent, A.J.; Ramsay, J.D.; Swanton, S.W. An EXAFS study of uranyl ion in solution and sorbed onto silica and montmorillonite clay colloids. Journal of colloid and interface science 1992, 150, 45–60. [Google Scholar] [CrossRef]
- Chisholm-Brause, C.; Conradson, S.D.; Buscher, C.T.; Eller, P.G.; Morris, D.E. Speciation of uranyl sorbed at multiple binding sites on montmorillonite. Geochimica et Cosmochimica Acta 1994, 58, 3625–3631. [Google Scholar] [CrossRef]
- Sylwester, E.R.; Hudson, E.A.; Allen, P.G. The structure of uranium (VI) sorption complexes on silica, alumina, and montmorillonite. Geochimica et Cosmochimica Acta 2000, 64, 2431–2438. [Google Scholar] [CrossRef]
- Catalano, J.G.; Brown, G.E., Jr. Uranyl adsorption onto montmorillonite: Evaluation of binding sites and carbonate complexation. Geochimica et Cosmochimica Acta 2005, 69, 2995–3005. [Google Scholar] [CrossRef]
- Joseph, L.; Jun, B.M.; Flora, J.R.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano) materials for sustainable water treatment: A review. Carbohydrate polymers 2021, 251, 116986. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Negro, C.; Blanco, A. Chitosan grafted/cross-linked with biodegradable polymers: A review. International Journal of Biological Macromolecules 2021, 178, 325–343. [Google Scholar] [CrossRef] [PubMed]
- Singh, P. P. (2021). Natural polymer-based hydrogels for adsorption applications. In Natural polymers-based green adsorbents for water treatment (pp. 267-306). Elsevier. [CrossRef]
- Baraka, A. (2006). Removal of heavy metals from aqueous solutions by novel melamine-formaldehyde-polyaminopolycarboxylic acid chelating adsorbents.
- Xie, Y.; Chen, C.; Ren, X.; Wang, X.; Wang, H.; Wang, X. Emerging natural and tailored materials for uranium-contaminated water treatment and environmental remediation. Progress in Materials Science 2019, 103, 180–234. [Google Scholar] [CrossRef]
- Wei, K.; Wang, Q.; Huang, L.; Sun, L. Amino-functionalized urea–formaldehyde framework mesoporous silica for U (VI) adsorption in wastewater treatment. Industrial & Engineering Chemistry Research 2016, 55, 12420–12429. [Google Scholar] [CrossRef]
- Rudresh, B.M.; Ravi Kumar, B.N.; Madhu, D. Combined effect of micro- and nano-fillers on mechanical, thermal, and morphological behavior of glass–carbon PA66/PTFE hybrid nano-composites. Adv. Compos. Hybrid Mater. 2019, 2, 176–188. [Google Scholar] [CrossRef]
- Rudresh, B.M.; Ravi Kumar, B.N.; Lingesh, B.V. Fibridization effect on the mechanical behavior of PA66/PTFE blend based fibrous composites. Trans. Indian Inst. Met. 2017, 70, 2683–2694. [Google Scholar] [CrossRef]
- Morreale, M.; Liga, A.; Mistretta, M.C.; Ascione, L.; La Mantia, F.P. Mechanical, Thermomechanical and Reprocessing Behavior of Green Composites from Biodegradable Polymer and Wood Flour. Materials 2015, 8, 7536–7548. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Deepa, J.R. Synthesis and characterization of multi-carboxyl-functionalized nanocellulose/nanobentonite composite for the adsorption of uranium (VI) from aqueous solutions: Kinetic and equilibrium profiles. Chemical Engineering Journal 2015, 273, 390–400. [Google Scholar] [CrossRef]
- Gürses, A.; Güne¸s, K.; Mindivan, F.; Korucu, M.E.; Açıkyıldız, M.; Do˘gar, Ç. The investigation of electrokinetic behaviour of micro-particles produced by CTA+ ions and Na-montmorillonite. Appl. Surf. Sci. 2014, 318, 79–84. [Google Scholar] [CrossRef]
- Singla, P.; Mehta, R.; Upadhyay, S.N. Clay Modification by the Use of Organic Cations. Green Sustain. Chem. 2012, 2, 21–25. [Google Scholar] [CrossRef]
- dos Santos, E.C.; Bandeira, R.M.; Vega, M.L.; Junior, J.R.d.S. Poly(melamine-formaldehyde-silica) Composite Hydrogel for Methylene Blue Removal. Mater. Res. 2021, 24. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. Preparation and characterization of microencapsulated LDHs with melamine-formaldehyde resin and its flame retardant application in epoxy resin. Polym. Adv. Technol. 2018, 29, 2147–2216. [Google Scholar] [CrossRef]
- Gürses, A.; Barın, T.B. Preparation, Structural Characterization and Evaluation of Some Dynamic and Rheological Properties of a New Type of Clay Containing Mastic Material, Clay-Mastic. Minerals 2023, 13, 705. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Yu, F.; Zhang, Z.; Gao, X. Preparation and properties of graphene oxide-modified poly (melamineformaldehyde) microcapsules containing phase change material n-dodecanol for thermal energy storage. J. Mater. Chem. A 2015, 3, 11624–11630. [Google Scholar] [CrossRef]
- Gürses, A.; Do˘gar, Ç.; Köktepe, S.; Mindivan, F.; Güne¸s, K.; Aktürk, S. Investigation of Thermal Properties of PUF/colored Organoclay Nanocomposites. Acta Phys. Pol. A 2015, 127, 979–983. [Google Scholar] [CrossRef]
- Wanyika, H.; Maina, E.; Gachanja, A.; Marika, D. Instrumental characterization of montmorillonite clays by X-ray fluorescence spectroscopy, fourier transform infrared spectroscopy, X-ray diffraction and uv/visible spectrophotometry. J. Agric. Sci. Technol. 2016, 17, 224–239. [Google Scholar]
- Yu, C.; Xu, W.; Zhao, X.; Xu, J.; Jiang, M. Effects of the reaction degree of melamine-formaldehyde resin on the structures and properties of melamine-formaldehyde/polyvinyl alcohol composite fiber. Fibers Polym. 2014, 15, 1828–1834. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of adsorption on carbon from solution. Journal of the sanitary engineering division 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process biochemistry 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Gürses, A.; Doğar, Ç.; Yalçın, M.; Açıkyıldız, M.; Bayrak, R.; Karaca, S. The adsorption kinetics of the cationic dye, methylene blue, onto clay. Journal of Hazardous Materials 2006, 131, 217–228. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Coconut husk derived activated carbon via microwave induced activation: effects of activation agents, preparation parameters and adsorption performance. Chemical Engineering Journal 2012, 184, 57–65. [Google Scholar] [CrossRef]
- Oguntimein, G.B. Biosorption of dye from textile wastewater effluent onto alkali treated dried sunflower seed hull and design of a batch adsorber. Journal of Environmental Chemical Engineering 2015, 3, 2647–2661. [Google Scholar] [CrossRef]
- El Maguana, Y.; Elhadiri, N.; Benchanaa, M.; Chikri, R. Activated carbon for dyes removal: modeling and understanding the adsorption process. Journal of Chemistry 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Azizian, S.; Douven, S. Implications of apparent pseudo-second-order adsorption kinetics onto cellulosic materials: A review. BioResources 2019, 14. [Google Scholar] [CrossRef]
- Ugbe, F.A.; Abdus-Salam, N. Kinetics and thermodynamic modelling of natural and synthetic goethite for dyes scavenging from aqueous systems. Arabian Journal of Chemical and Environmental Research 2020, 7, 12–28. [Google Scholar]
- Galamini, G.; Ferretti, G.; Medoro, V.; Tescaro, N.; Faccini, B.; Coltorti, M. Isotherms, kinetics, and thermodynamics of nh4+ adsorption in raw liquid manure by using natural chabazite zeolite-rich tuff. Water 2020, 12, 2944. [Google Scholar] [CrossRef]
- Majd, M.M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption isotherm models: A comprehensive and systematic review (2010−2020). Science of The Total Environment 2022, 812, 151334. [Google Scholar] [CrossRef] [PubMed]
- Adusei, J.K.; Agorku, E.S.; Voegborlo, R.B.; Ampong, F.K.; Awarikabey, E.; Danu, B.Y.; Amarh, F.A. Zero Valent Iron Impregnated Sodium Alginate Grafted (Acrylamide-Co-Acrylic Acid) Adsorbents for the Removal of Methylene Blue in Aqueous Systems. Journal of Macromolecular Science, Part B 2023, 62, 265–279. [Google Scholar] [CrossRef]
- Gürses, A.; Güneş, K.; Şahin, E.; Açıkyıldız, M. Investigation of the removal kinetics, thermodynamics and adsorption mechanism of anionic textile dye, Remazol Red RB, with powder pumice, a sustainable adsorbent from waste water. Frontiers in Chemistry 2023, 11, 1156577. [Google Scholar] [CrossRef]
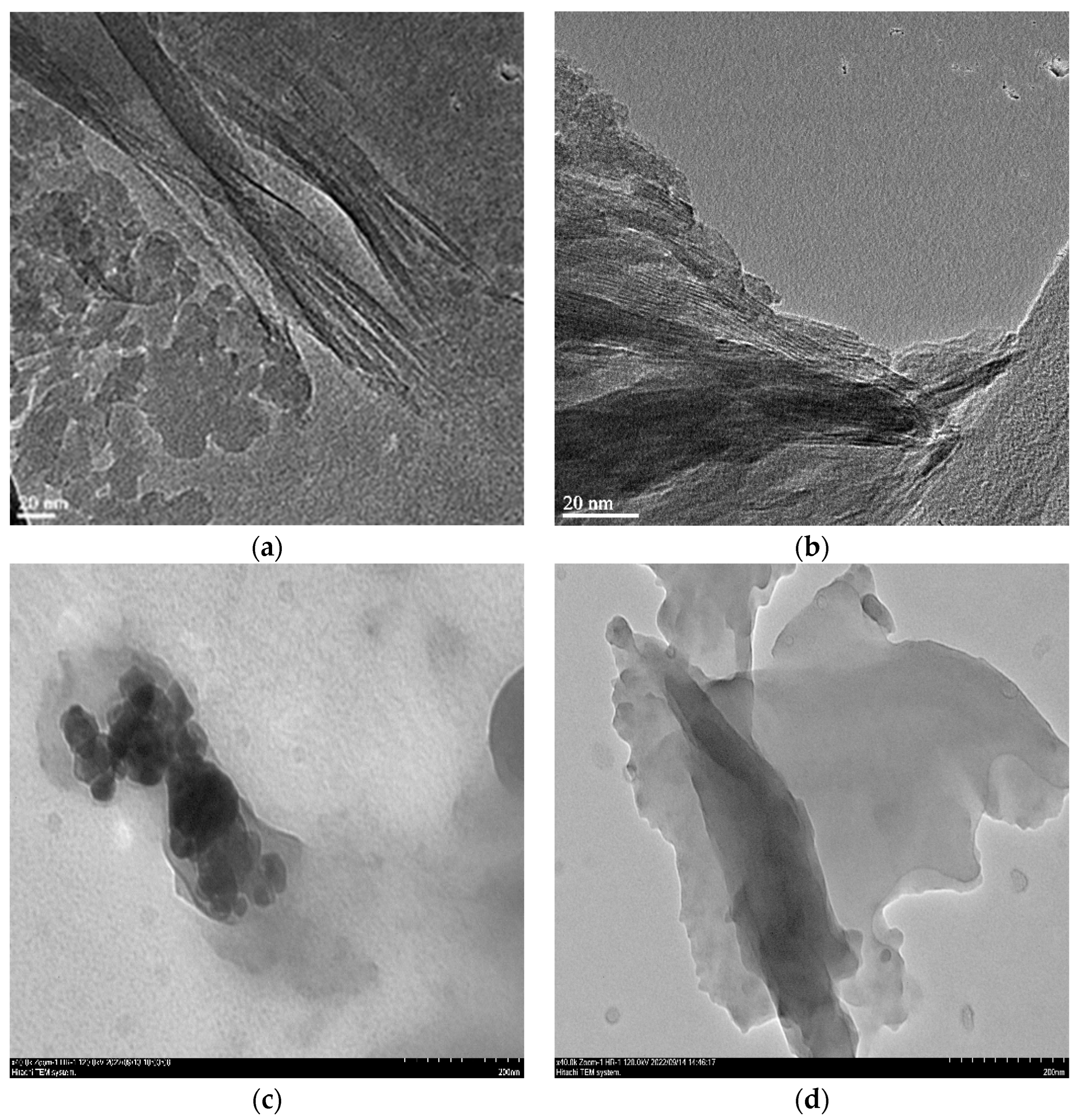
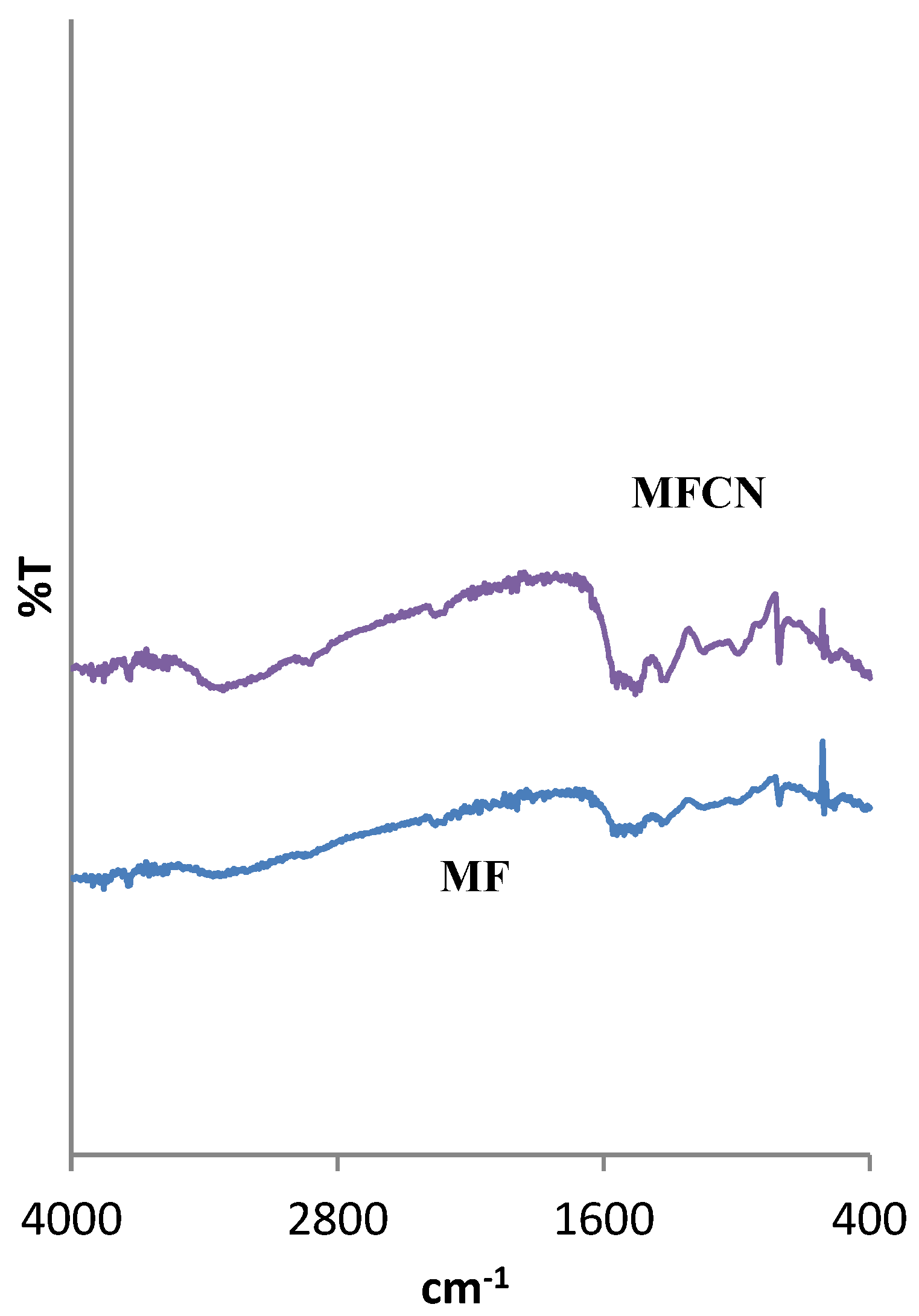
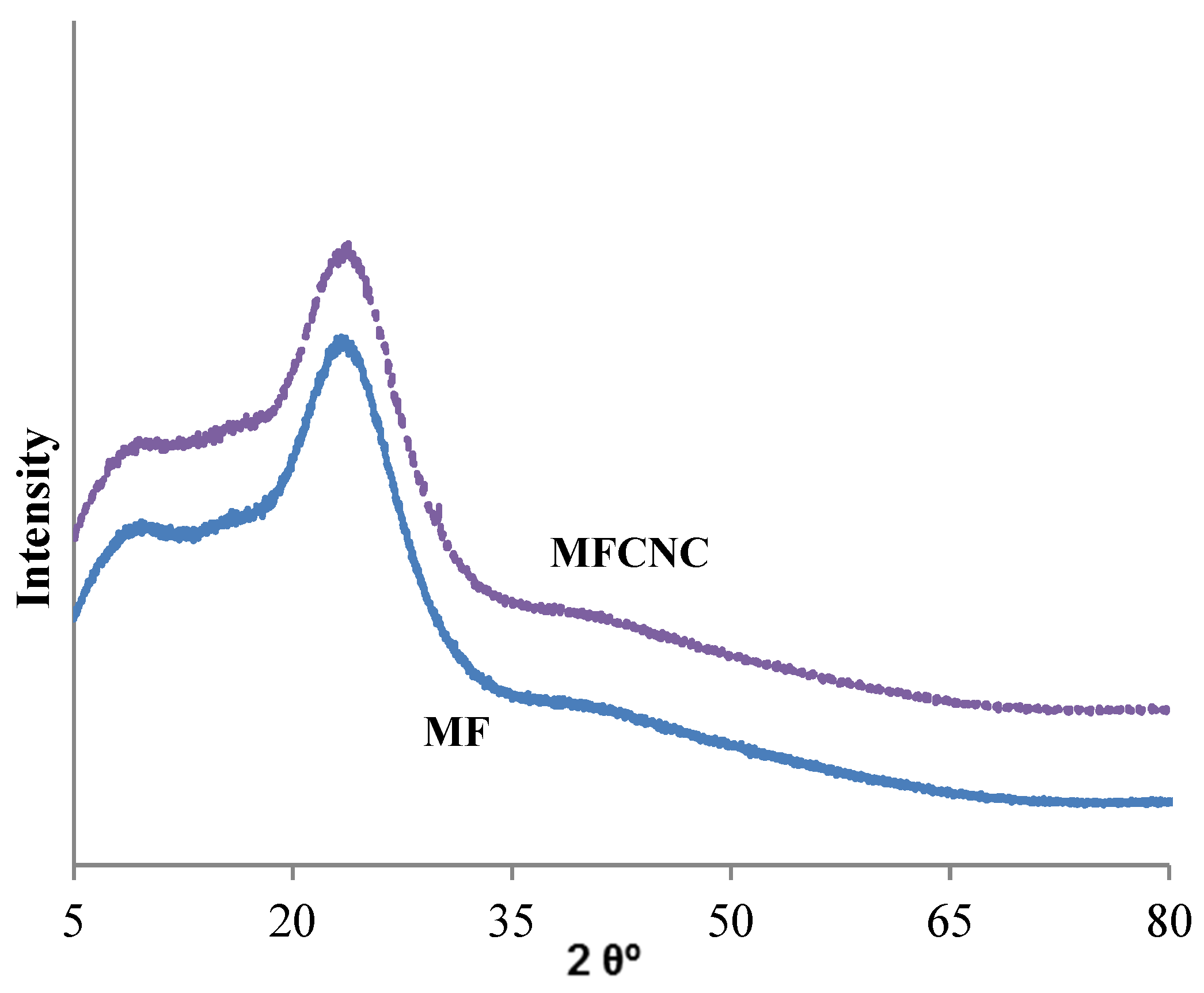
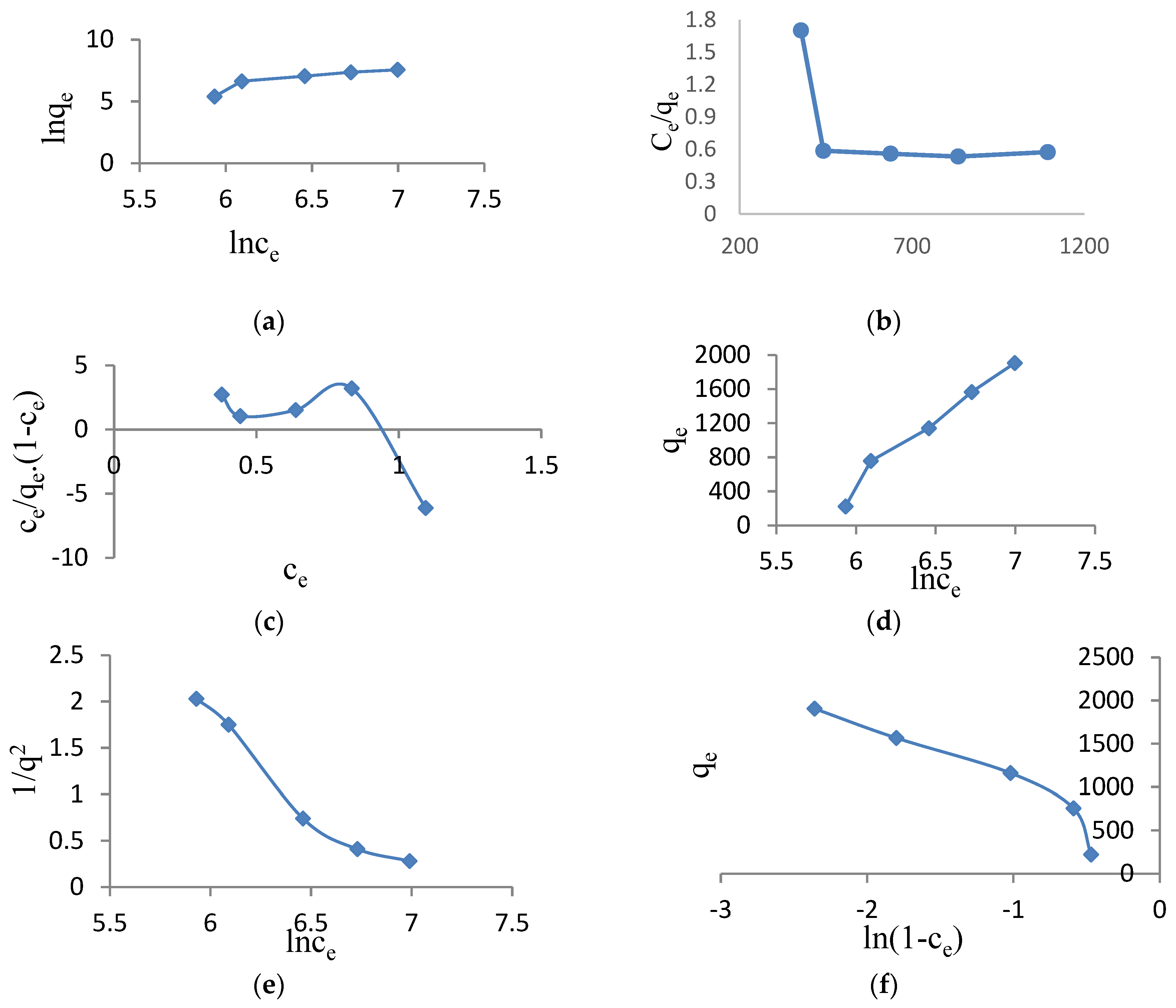
| Component (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | TiO2 | SO3 | Other | |||
| Montmorillonite | 59.32 | 17.19 | 5.95 | 3.63 | 2.21 | 1.68 | 0.97 | 0.74 | 0.51 | 7.81 | ||
| Density (15 °C), kg/m3 |
Calorific Value MJ/kg |
Flash Point °C | Water by Distillation, wt. % |
C | H | N | S | Ash |
|---|---|---|---|---|---|---|---|---|
| 990.7 | 42.74 | 105.8 | 0.1 | 83.4 | 11.9 | 0.8 | 1.5 | 0.03 |
| Cint (µg L−1) |
Temperature K |
Pseudo-first-order | Pseudo-second-order | Intra particle diffusion (first part) | Intra particle diffusion (second part) | ||||
|---|---|---|---|---|---|---|---|---|---|
| k1 | R2 | k2 | R2 | kid,1 mg s−1/2 g−1 |
R2 | kid,2 mg s−1/2 g−1 |
R2 | ||
| 600 | 298 | - | - | 0.029 | 0.999 | 39.14 | 0.9697 | 6.32 | 0.8261 |
| 1200 | 298 | - | - | 0.002 | 0988 | 28.03 | 0.9137 | 10.04 | 0.7638 |
| 1800 | 298 | - | - | 0.001 | 1.000 | 11.24 | 0.6531 | 1.09 | 0.1714 |
| 2400 | 298 | - | - | 0.001 | 0.952 | 8.91 | 03469 | 1.53 | 0.3379 |
| 3000 | 298 | - | - | 0.007 | 1.000 | 2.34 | 0.0112 | 0.44 | 0.0093 |
| Isotherm equations | Constant parameter | Regression coefficients(R2) | |
|---|---|---|---|
| Freundlich | n | 0.569 | 0.8110 |
| ln q=1/n ln Ce + lnKf | Kf | 1.506 | |
| Langmuir | qm | 1000 | 0.3352 |
| Ce/q = 1/qmK +Ce/qm | K | 0.001 | |
| BET | qm | 5.649 | 0.5088 |
| C/q(1-Ce)=1/qmk + (k-1)Ce/qmk | k | 1.170 | |
| Halsey | nH | 0.0005 | 0.9734 |
| qe = 1/nHlnKH-1/nH lnCe | KH | 1.445 | |
| Harkins-Jura | A | 0.566 | 0.9431 |
| 1/q2= (B/A) -1/A lnCe | B | 7.031 | |
| Smith | Wb | -327.3 | 0.7137 |
| q =Wb - Wln(1-Ce) | W | 564.5 |
| Temperature (K) |
ΔH°ads (kJ/mol) For the first plateau |
D1 |
ΔH°ads (kJ/mol) for the second plateau |
D1 |
ΔS°ads (J/mol K) for the first plateau |
D2 |
ΔS°ads (J/molK) for the second plateau |
D2 |
|---|---|---|---|---|---|---|---|---|
| 298 |
3.65 |
1.08 |
-51.02 |
3.59 |
1.77 |
1.71 |
-167.28 |
5.92 |
| 338 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




