The prevalence of microplastics (plastic particles < 5 mm) pollution in the world and its potential animal, plant and human health risks have attracted great attention in recent years (Kumar et al., 2023; Zhang et al., 2022). Terrestrial ecosystems are an important gathering place of microplastics, so the impact of microplastics on soil ecosystems and plant growth and development has become a research hotspot (Yang et al., 2021). The possible sources of microplastics in the soil environment mainly include plastic film mulching, sludge landfill, compost application and irrigation (Chae and An, 2018). Sugarcane is widely planted in Southern China, with large economic benefits, and is an important industry for local farmers. Plastic film mulching is an effective measure used to increase crop yield. Sugarcane mulching has been applied in China for more than 30 years, and there is basically no recovery of plastic film in sugarcane fields (Li and Yang, 2015). Our investigation in the sugarcane area showed that the amount of plastic film used in sugarcane fields was 75-150 kg/ha/year. Assuming that all the plastic film was converted into microplastics and remained in the soil, the cumulative concentration of microplastics in the soil of the sugarcane area would have reached 0.1%-0.2% in 30 years, which indicates that the pollution of microplastics in sugarcane fields is serious. At the same time, sugarcane has the characteristics of a long growth cycle, high yield and high nutrient demand (Li and Yang, 2015). However, the soil types in sugarcane areas in China are mainly red soil and lateritic red soil, which are usually acidic and have a low nutrient content, especially available P, so ensuring the supply of soil P nutrients is essential for the good quality and high yield of sugarcane (Su et al., 2021; Wu et al., 2020). Therefore, it is particularly necessary to explore the effects of microplastic pollution on sugarcane growth and soil nutrient content.
When microplastic particles enter the soil, they can combine with soil particles, thereby affecting the physical and chemical properties of the soil, the distribution of soil microorganisms and the growth and development of plants (Jacques and Prosser, 2021; Yao et al., 2022). Rillig is one of the earliest researchers in the world to study the harm of microplastics to soil; he believes that the accumulation of microplastics at a certain concentration will affect the nature and function of soil (Rillig, 2012). Further researches have found that microplastics affect soil physical and chemical properties, including soil porosity and moisture, pH, organic matter and N, P and potassium (K) nutrient content (Chae and An, 2018; de Souza Machado et al., 2019). Polyethylene (PE) microplastics have been found to have increased soil pH and the content of soluble organic matter and ammonium N by microcosm incubation (Gao et al., 2021). For different types of soils, the addition of 0.1% and 1% polyvinyl chloride (PVC) microplastics significantly increased the content of available P in red soil but led to a decrease in available P in paddy soil (Yan et al., 2021). Microplastics have also been found by many studies to affect soil microbial community structure and function (Feng et al., 2022; Huang et al., 2019). Microplastics can directly or indirectly change soil properties, thereby affecting the composition and diversity of microbial communities (Fei et al., 2020; Zhou et al., 2021). At the same time, microplastics themselves can provide specific substrates or adsorption sites for microorganisms, and microorganisms on the surface of microplastics are enriched and form biofilms (Chen et al., 2020). The microbial community structure in the biofilm is clearly different from the microbial composition in the soil, thus changing the functional properties of the soil (Huang et al., 2019). Soil microbial community composition is an important indicator of soil quality, and microbial activities also affect the recycling of N, P and K nutrients (Das et al., 2022). For example, PVC microplastics containing plasticizers have been found to increase N-fixing microorganisms, urea-decomposing bacteria and nitrate-reducing bacteria and reduce nitrifying bacteria, thereby affecting the soil N cycle (Zhu et al., 2022). In addition, microplastics can also cause changes in rhizosphere microbial communities, which may affect plant growth and development to a certain extent (de Souza Machado et al., 2019). However, there are few studies focus on the effects of microplastics accumulation on soil microorganisms, especially those related to P cycling, and the relationship between microbial changes and sugarcane growth.
Biochar is formed by the pyrolysis of biomass under anaerobic conditions. Its carbon (C) content can reach 40%-75%, and it is rich in mineral nutrients, functional groups and pore structure. Biochar is stable and not easy to degrade, so it has great application prospects in promoting crop growth, soil improvement and soil pollution remediation (Kuppusamy et al., 2016). Biochar can significantly improve the yield of maize, rice and tobacco (Arif et al., 2017; Wu et al., 2021). For tobacco-growing soil, the application of biochar increases the content of soil organic matter, available P and total N and slows soil acidification, which has a significant effect on improving the plant growth environment (Ren et al., 2022). Additionally, studies on soil contaminated by heavy metals such as cadmium and arsenic found that the application of biochar can reduce the harm of toxic elements and reduce their bioavailability and environmental risks (Abbas et al., 2017; Gregory et al., 2014). Biochar can also alleviate the impact of microplastic pollution on soil microbial diversity (Khalid et al., 2023; Ran et al., 2023). It has been found that PVC microplastic pollution has adverse effects on crop yield, soil enzyme activity and microorganisms, while biochar mitigates the adverse effects (Khalid et al., 2023). However, to date, the effects and mechanisms of biochar application on sugarcane growth, soil nutrients and microbial communities in soils contaminated with different concentrations of microplastics are still unclear.
In this study, sugarcane planting soil was collected to carry out greenhouse pot experiments and six treatments were set: 0.1% (w/w, soil dry weight) and 1% polyethylene microplastic (Low PE, High PE), 1% corn straw biochar (BC), 0.1% and 1% polyethylene microplastic combined with 1% corn straw biochar (Low PE + BC, High PE + BC), and the control (without microplastics or biochar, CK). The study aims to: (1) compare the differences in the growth of sugarcane, soil physio-chemical properties and microbial community characteristics under PE microplastics adding alone and PE microplastics combined with corn straw biochar, (2) explore the relationship among sugarcane growth, soil physio-chemical properties and microbial community characteristics and their mechanism. Based on previous research results (Wang et al., 2022a) and sugarcane field survey data, we hypothesized that PE microplastics adding alone will inhibit the growth of sugarcane and have a negative impact on soil physicochemical and microbial communities, while the addition of biochar may alleviate the negative effects of soil microplastic pollution on plant growth and microbial community structure and function, as biochar has rich nutrients and a large specific surface area. The purpose of this study is to provide a reference for the control of microplastic pollution in sugarcane fields and promote the green development of the sugarcane industry.
1. Materials and Methods
1.1. Experimental Soil, Biochar and Microplastics
The tested red soil was collected from the sugarcane experimental base in Wengyuan, Guangdong (E 113.94 °, N 24.28 °), and the soil physico-chemical properties were as follows: pH 4.14, organic matter 14.2 g/kg, available N 158.67 mg/kg, available P 17.47 mg/kg, and available K 89.50 mg/kg. Corn stalk biochar (BC) was purchased from Henan Lize Environmental Protection Technology Co., Ltd. and was obtained by pyrolysis at 600 ℃. Its main properties were pH 9.0, organic carbon 51.1%, total N 0.9%, total phosphorus 0.2%, and total potassium 1.6%. High-density polyethylene microplastics (PE) were purchased from Shanghai Aladdin Company. According to the data provided by the manufacturer, the polyethylene particles have a random spherical structure, a purity of 99.99%, a density of 0.96 g/cm3, and a melting point of 132°. In this study, polyethylene spherical particles with a particle size of approximately 180 µm were selected for the experiment. PE microplastics were first placed in a solution to remove possible heavy metals, then washed with deionized water and air-dried, sterilized to eliminate microbial contamination, and stored in a refrigerator at 4 °C for later use.
1.2. Experimental Setup and Design
The pot experiment was conducted in a greenhouse, and six treatments were set up: adding 0.1% PE microplastic (w/w, soil dry weight, Low PE), adding 1% PE microplastic (High PE), adding 1% BC, 0.1% PE microplastic combined with 1% biochar (Low PE + BC), 1% PE microplastic combined with 1% biochar (High PE + BC), and control (no addition of PE microplastic and biochar, CK). Each treatment was repeated three times, with a total of 18 pots arranged randomly. Urea was used as the main source of N at the rate of 300 mg N/kg soil, and potassium dihydrogen phosphate was used as the source of P and K at the rate of 150 mg K/kg soil. Soil micronutrient additions were (mg/kg soil): CaCl2 125.67, MgSO4·7H2O 43.34, EDTA-FeNa 5.80, MnSO4·4H2O 6.67. Before potting, all the above microplastics, biochar and nutrients were fully mixed with the soil. On March 1, 2022, one healthy sugarcane seedling (ROC22) of similar growth was transplanted into each pot. During the experiment, the weighing method was used to keep the water content at 70-80% of the field capacity to meet the water demand of sugarcane. It was harvested on December 30, 2022.
1.3. Sample Collection and Analysis
1.3.1. Plant and Soil Collection
Plant samples were collected, including aboveground and underground parts. After cleaning, the sugarcane plants were quenched at 105 °C for 30 min and dried at 70 °C to constant weight, and the dry biomass was weighed. Collection and treatment of soil samples: the rhizosphere soil was obtained by shaking the soil, and the test plants were gently shaken to remove the larger soil clumps from the root system. Then, the soil attached to the root system was shaken off and put into a sterile self-sealing bag. Soil samples were quickly brought back to the laboratory, and one part of the fresh sample of rhizosphere soil was stored in a refrigerator at -40 °C for analysis of soil microorganisms. The other part of the rhizosphere soil was naturally air-dried and then used for determining the physicochemical properties of the soil after impurities were removed.
1.3.2. Measurement of Soil Physicochemical Properties
Soil pH was measured with a pH meter (1: 2.5 soil/water). The electrical conductivity (EC) was determined by an electrical conductivity meter. Soil organic carbon (SOC) was measured by the digestion method. Soil total N and total K were determined using micro-Kjeldahl digestion and flame photometry methods, respectively. Soil available N was determined using the alkaline diffusion method. Available K was extracted with 1 M NH4OAc and then measured by a flame photometer. Total P was extracted with an acid solution (H2SO4-HClO4), and the available P was extracted with 0.5 NaHCO3 (Olsen P) and then measured through molybdenum blue colorimetry (Page, 1982).
1.3.3. Soil DNA Extraction and Microbial Community Analysis
Soil DNA was extracted from 0.5 g of fresh soil samples using a Power Soil DNA isolation kit (MoBio Laboratories, USA) following the instructions in the accompanying manual, and each treatment contained 3 biological replicates. The quality and concentration of the extracted DNA were determined with a Qubit™ 3.0 fluorometer (Thermo Fisher Scientific Inc., Waltham, USA). The extracted DNA samples were stored at -40 ℃.
The 16S rRNA gene was amplified with the primer F515/R907 (GTGCCAGCMGCCGCGG/CCGTCAATTCMTTTRAGTTT) using the qualified DNA of the assay sample as the template. PCR was carried out on a MasterCycler gradient (Eppendorf, Germany) using 25 μL reaction volumes containing 5×reaction buffer 5μL, 5×GC buffer 5μL, dNTP(2.5 mM)2μL, forward primer(10 µM)1μL, reverse primer(10 µM)1μL, DNA template 2μL, ddH2O 8.75μL, Q5 DNA Polymerase 0.25μL. The cycling parameters were 95 °C for 5 min, followed by 28 cycles of 95 °C for 45 s, 55 °C for 50 s and 72 °C for 45 s with a final extension at 72 °C for 10 min. Each sample was mixed after three replicates at the same time. Agarose gel electrophoresis (1%) was used to detect the quality of PCR amplification products, an Omega gel recovery and purification kit was used to purify them, and Qubit 2.0 was used to determine the concentration of purified PCR products. The PCR amplification products of different samples were mixed according to equimolar amounts, and the quality of the mixed PCR products was detected at the same time. Then, sequencing was completed by the Illumina MiSeq-PE300 sequencing platform.
In addition, primers ALPS-F730/ALPS-R1101 (CAGTGGGACGACCACGAGGT/GAGGCCGATCGGCATGTCG) were used for phoD gene amplification (Sakurai et al., 2008). PCR was carried out on a Mastercycler gradient thermal cycler (Eppendorf, Germany) using 25 μL reaction volumes containing 5×reaction buffer 5μL, 5×GC buffer 5μL, dNTP(2.5 mM)2μL, forward primer(10 µM)1μL, reverse primer(10 µM)1μL, DNA template 2μL, ddH2O 8.75μL, Q5 DNA Polymerase 0.25μL. The cycling parameters were 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 50 °C for 30 s and 72 °C for 60 s with a final extension at 72 °C for 7 min. Sequencing was completed by the Illumina MiSeq-PE250 sequencing platform after they were qualified.
1.4. Data Analysis
All data were collated using Excel; SASV9 software was used for one-way analysis of variance, and the Duncan (SSR) method was used to test the significance of the mean value (3 replicates) of each index among different treatments (P < 0.05).
2. Results and Analysis
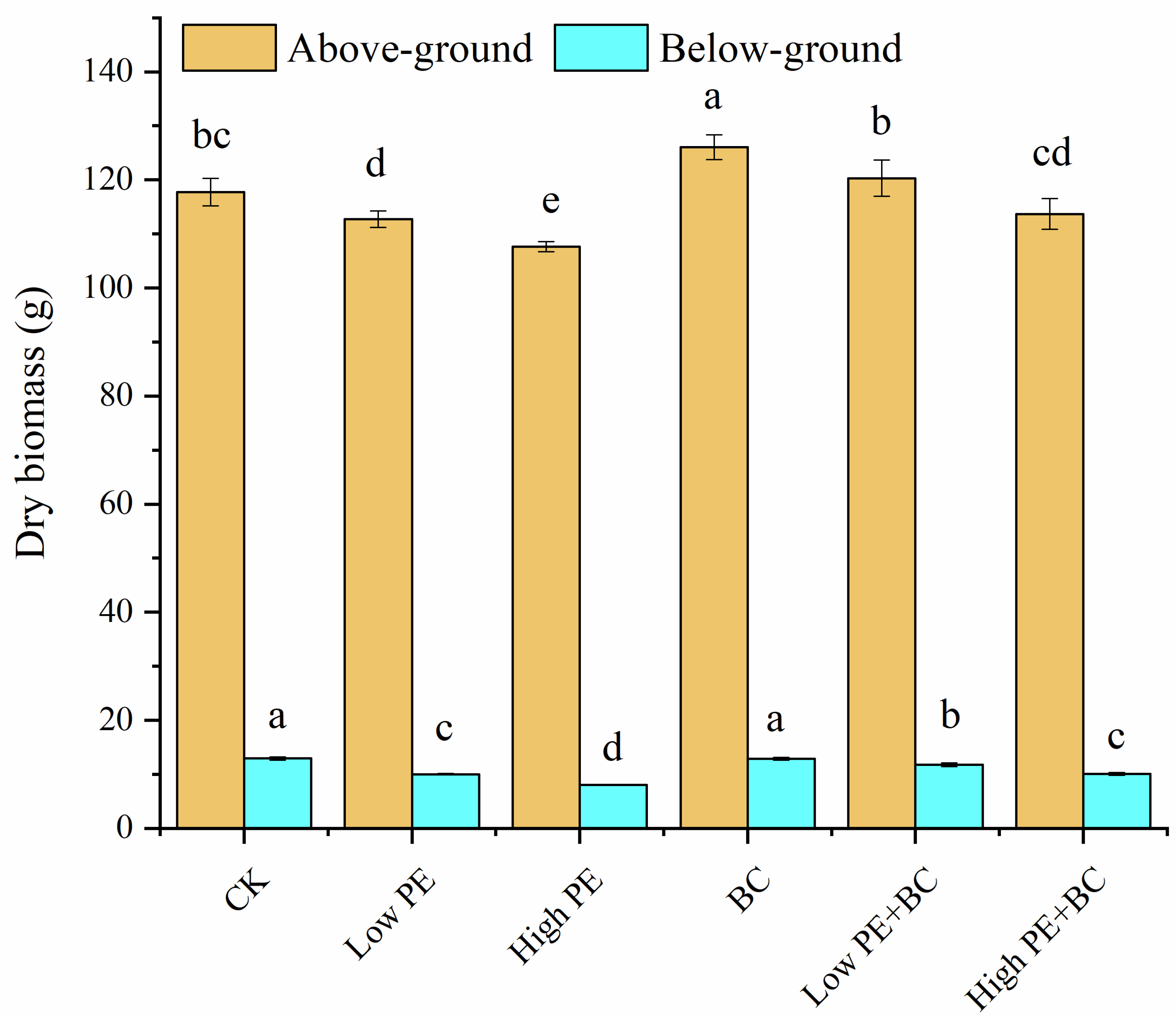
2.1. Biomass of Sugarcane
The aboveground biomass (AGB) and below-ground biomass (BGB) of sugarcane in different treatments showed some differences (
Figure 1). Compared with the CK treatment, the AGB of the BC treatment increased significantly, the AGB and BGB of the Low PE and High PE treatments decreased significantly, and the BGB of the Low PE + BC and High PE + BC treatments decreased significantly (
P < 0.05). In addition, the total biomass of sugarcane (the sum of BGB and BGB) was in the order of BC > Low PE + BC > CK > High PE + BC > Low PE > High PE. The AGB and BGB in the treatments with microplastics combined with biochar (Low PE + BC and High PE + BC) were significantly higher than those in the treatments with microplastics alone (Low PE and High PE).
2.2. Physical and Chemical Properties of Soil
Compared with CK, treatments with microplastic alone slightly decreased the pH values, while treatments with BC amendment (Low PE + BC, High PE + BC and BC) increased the pH values (
Table 1). All the other five treatments increased the SOC contents compare to CK, only treatments with BC amendment were significantly higher than CK (
P < 0.05). Compared with CK, the contents of total N, P and K, available N and P decreased slightly while the available K contents increased slightly in treatments with microplastic alone; these corresponding indices in treatments with BC amendment significantly increased. In addition, the contents of pH, EC, SOC, total N, total K and available N, P and K in the treatments of microplastics combined with biochar were significantly higher than those in treatments with microplastics alone.
2.3. Soil Microorganisms
2.3.1. Diversity of Soil Microbial Communities
For the total bacteria (16S rRNA gene-based bacteria), the observed_OTUs, Chao1 and Shannon index of Low PE and High PE were decreased compared with CK, while these three indices were increased for Low PE + BC, High PE + BC and BC treatments (
Table 2). Moreover, compared with the treatment with microplastics alone, the treatments with microplastics combined with biochar could significantly increase the observed_OTUs, Chao1 and Shannon indices. For the
phoD-harbouring bacteria, compared with CK, the observed_OTUs, Chao1 and Shannon indices of the other five treatments were significantly increased, and these indices were the highest in High PE and lowest in Low PE treatment. In addition, the treatments of microplastics combined with biochar could reduce the observed_OTUs, Chao1 and Shannon indices compared with the treatment with microplastics alone.
2.3.2. Soil Microbial Community Composition
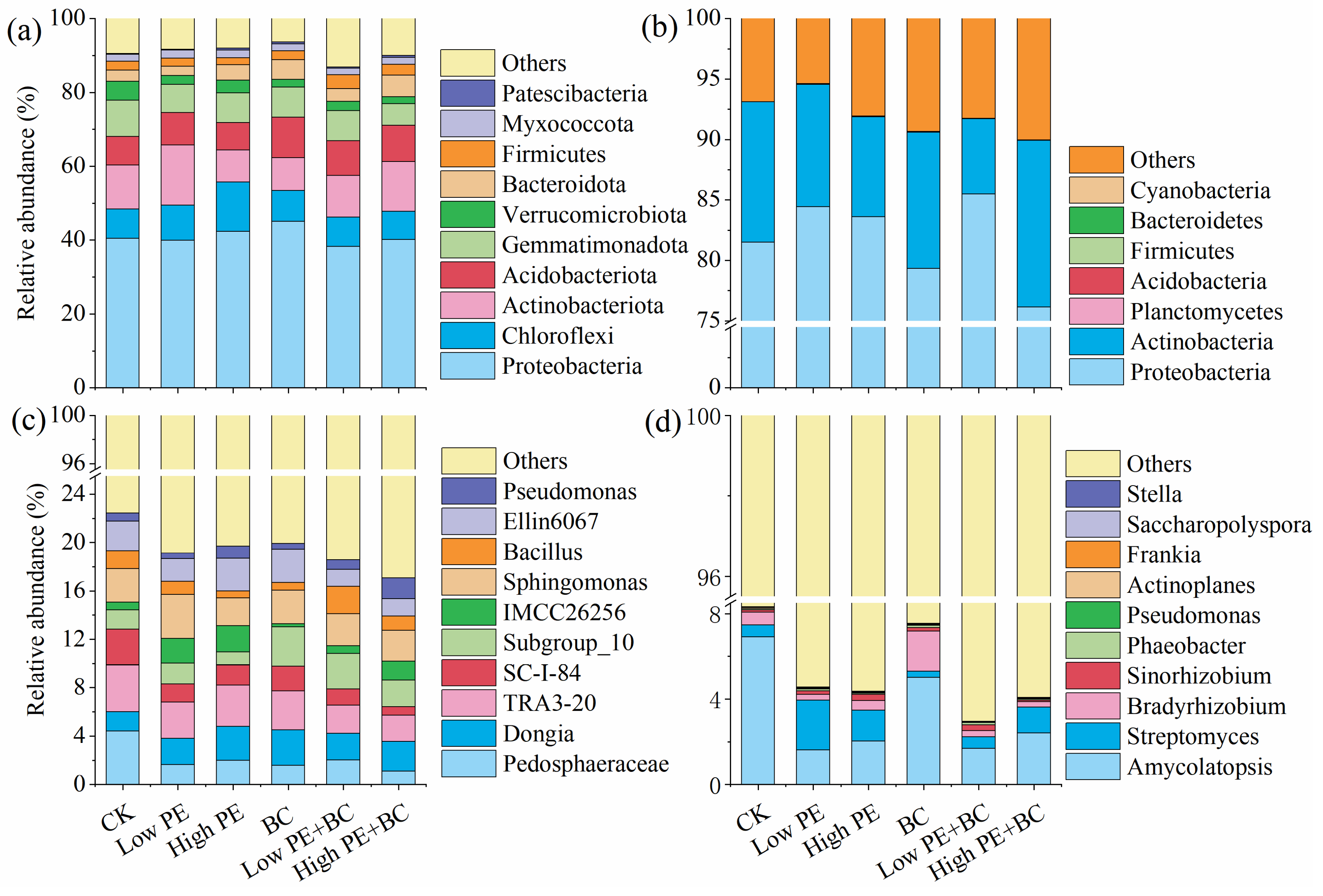
There were significant differences in the composition and proportion of dominant bacterial species in soil samples from different treatments (
Figure 2). For total bacteria (16S rRNA gene-based bacteria), at the phylum level, the dominant bacteria were Proteobacteria, Chloroflexi, Actinobacteria, Acidobacteria and Gemmatimonadota in all treatments, with the average abundances of 41.0%, 9.1%, 11.8%, 9.0%, and 8.0%, respectively. There were significant differences in the relative abundances of two of the top ten bacterial phyla (
P < 0.05) among the six treatments (
Table S1). Compared with CK, the other five treatments significantly decreased the relative abundance of Gemmatimonadota, and increased abundance of Acidobacteria was observed in BC treatment. In addition, the treatments of microplastics combined with biochar increased the relative abundance of Acidobacteria compared with the treatments adding microplastics alone. At the genus level, the top five genera were
Pedosphaeraceae,
Dongia,
TRA3-20,
Sphingomonas and
Subgroup_10 among all treatments, and the average abundances were 2.12%, 2.36%, 3.00%, 2.78% and 2.13%, respectively. There were significant differences in the relative abundances of seven of the top ten bacterial genera (
P < 0.05) among the six treatments (
Table S2). However, for the top 10 genera, there is no significant difference between Low PE or High PE with CK, only the treatments of Low PE + BC and High PE + BC significantly increased the relative abundance of
Subgroup_10 while significantly decreased abundance of
TRA3-20,
SC-I-84 and
Ellin6067 compare to CK. In addition, treatments of microplastics combined with biochar increased the relative abundance of
Subgroup_10,
Bacillus and
Pseudomonas while decreased the abundance of
TRA3-20,
SC-I-84 and
IMCC26256 compared with the treatments with microplastics alone.
For
phoD-harbouring bacteria, the dominant phyla in all treatments were Proteobacteria and Actinobacteria at the phylum level, and the sum of their relative abundance was in the range of 89.9% -94.6%. There was no significant difference in the relative abundance of Proteobacteria and Actinobacteria between other five treatments with CK (
Table S1). At the genus level,
Amycolatopsis,
Streptomyces and
Bradyrhizobium were abundant in all treatments (at least one treatment > 1%). There was no significant difference in the relative abundances of
Amycolatopsis and
Bradyrhizobium between other five treatments with CK, only the Low PE and High PE treatments significantly increased the relative abundance of
Streptomyces compared with CK (
Table S2). In addition, the treatments with microplastics combined with biochar increased the relative abundance of
Amycolatopsis and
Bradyrhizobium while decreased abundance of
Streptomyces compared with the treatment with microplastics alone.
2.3.3. Soil Microbial Function
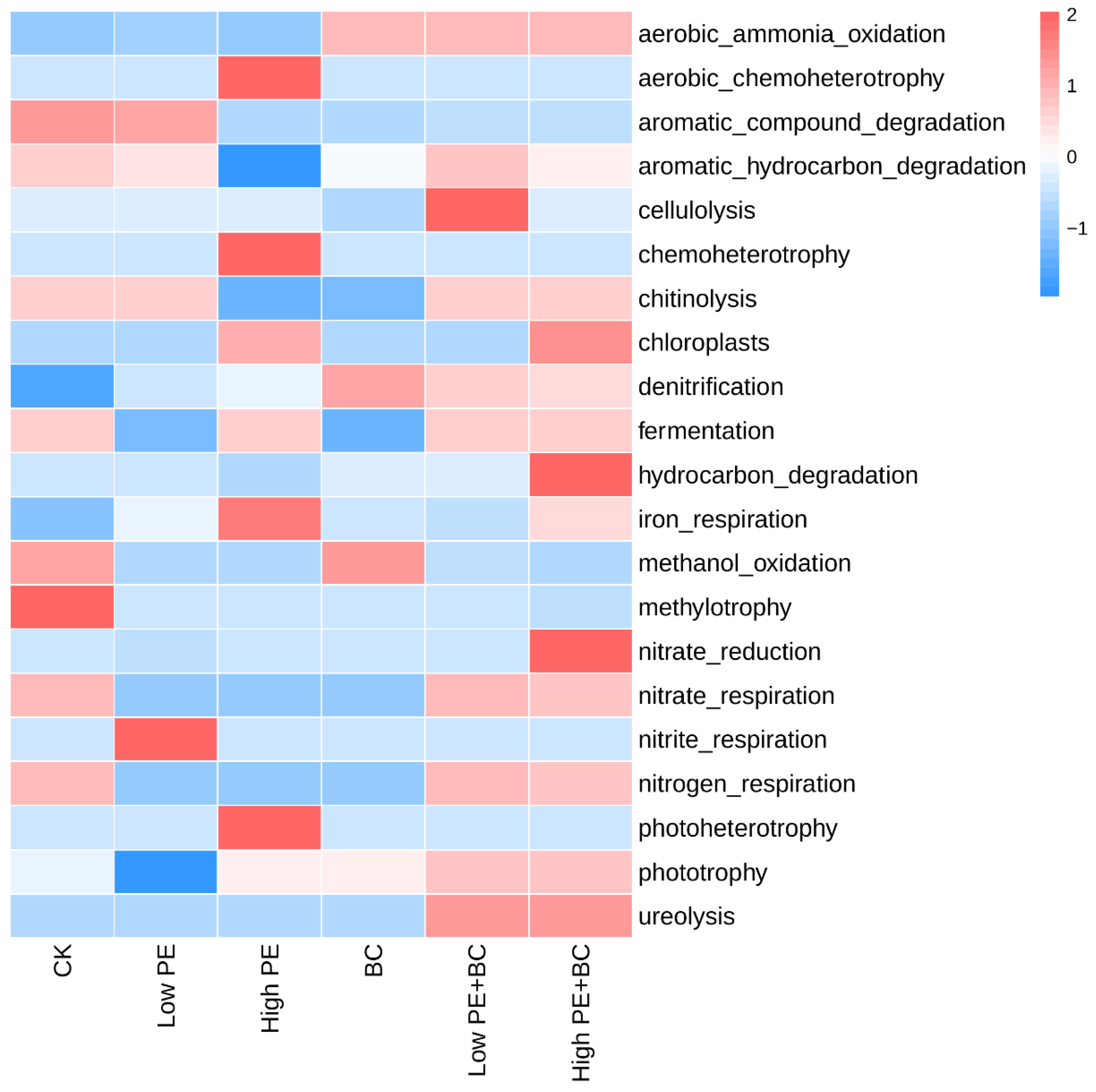
FAPROTAX was used to predict the potential functions of total bacterial communities. In general, bacterial functions were mainly related to the cycling of C and N in the ecosystem, and there were obvious differences in the abundance of functional genes in different treatments (
Figure 3). Compared to CK, other five treatments increased the abundance of functional genes such as aerobic_ammonia _oxidation, denitrification and iron respiration, while decreased the aromatic_compound_degradation, methylotrophy, nitrate_respiration and nitrogen_ respiration. Moreover, High PE had a significantly higher abundance of functional genes of aerobic_chemoheterotrophy, chemoheterotrophy, iron respiration and photoheterotrophy while had a significantly lower abundance of functional genes of aromatic_hydrocarbon_degradation and chitinolysis than CK. In addition, compared to the treatments with microplastics alone, treatments with microplastics combined with biochar increased the abundance of aerobic_ammonia_oxidation, denitrification, nitrate_respiration and nitrogen_respiration.
2.4. Correlations between Plant Biomass, Soil Microbes and Soil Physicochemical Properties
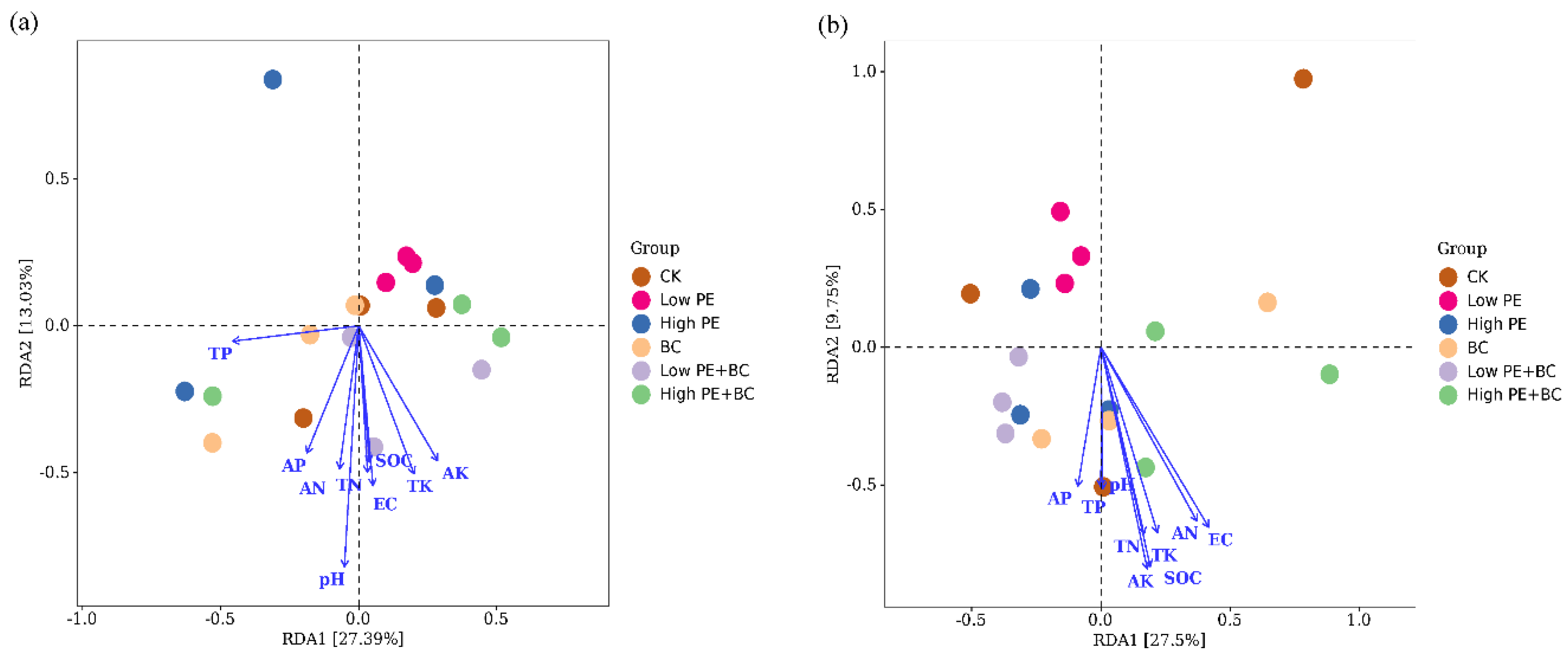
Redundancy analysis (RDA) was used to analyse the relationship between bacterial community structure and soil properties (
Figure 4). For total bacteria (16S rRNA gene-based bacteria), pH, EC, SOC, TN, TP, TK, AN, AP, and AK together accounted for 53.1% of the bacterial community structure variation, with the first two axes accounting for 27.4% and 13.0% of the variation, respectively. The total bacterial (16S rRNA gene-based bacteria) community structure was mainly affected by pH, EC and AK and was significantly negatively correlated with pH (
P < 0.05). For
phoD-harbouring bacteria, the main physicochemical properties of the soil explained 40.3% of the bacterial community changes, and the first two axes explained 27.5% and 9.8% of the changes, respectively. The community structure of
phoD-harbouring bacteria was significantly negatively correlated with SOC, EC and AK (
P < 0.05).
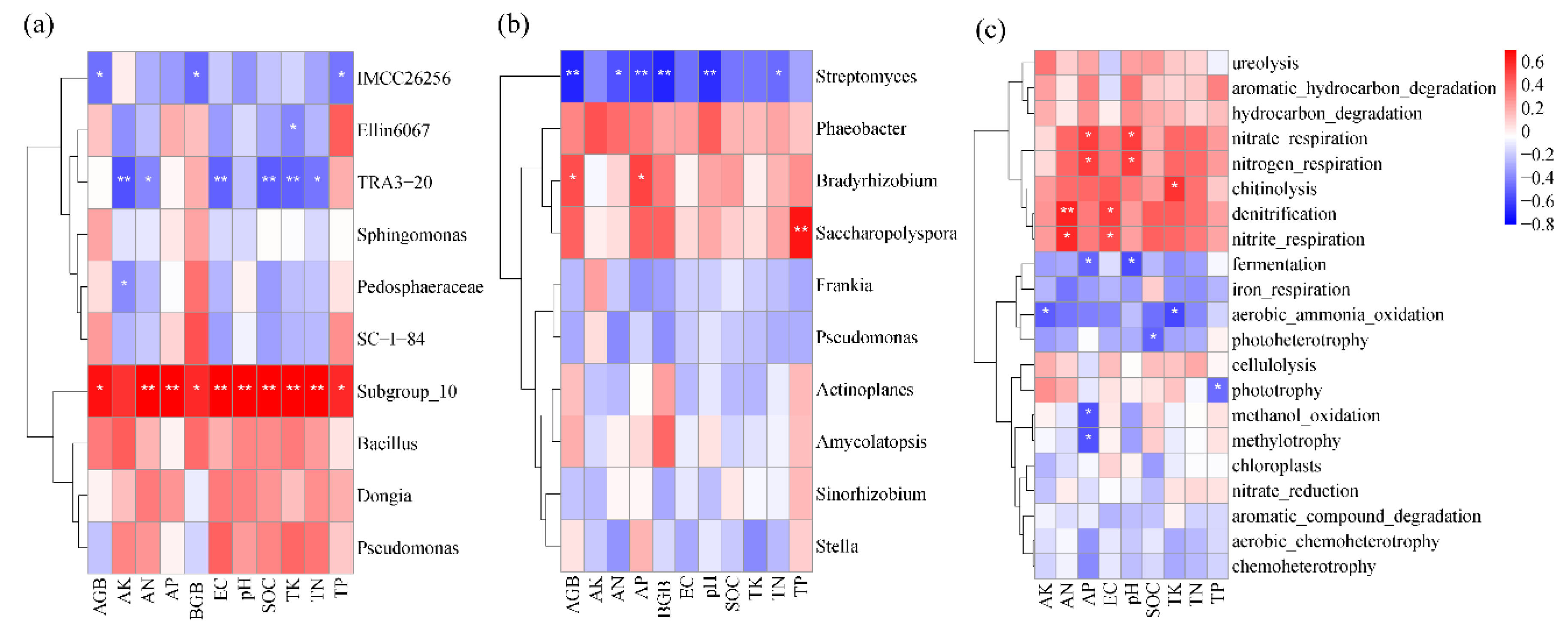
The correlation between sugarcane biomass, soil bacterial community composition, functional gene abundance and soil physical and chemical properties was further analysed (
Figure 5). For total bacteria (16S rRNA gene-based bacteria), the relative abundance of
Subgroup_10 was significantly and positively correlated with sugarcane AGB and BGB, soil pH, EC, SOC, and N, P, and K nutrient contents, the relative abundance of
IMCC26256 was significantly negatively correlated with the AGB and BGB of sugarcane and soil TP, and the relative abundance of
TRA3-20 was significantly negatively correlated with soil SOC, EC, TN, TK, AN and AK (
P < 0.05). For
phoD-harbouring bacteria,
Streptomyces had a significant negative correlation with the AGB and BGB of sugarcane, soil pH, TN, AN and AP, while
Bradyrhizobium had a significant positive correlation with the AGB of sugarcane and soil AP. There was a significant positive correlation between
Saccharopolyspora and soil TP (
P < 0.05). For the abundance of functional genes, nitrate_respiration and nitrogen_respiration were positively correlated with soil pH and AP, while fermentation was negatively correlated with soil pH and AP. Denitrification and nitrite respiration were positively correlated with soil AN and EC, while methanol_oxidation and methylotrophy were negatively correlated with AP. Aerobic_ammonia_oxidation was negatively correlated with AK and TK.
3. Discussion
3.1. Effects of Microplastics and Biochar on Sugarcane Biomass and Soil Physical and Chemical Properties
In this study, the treatments with microplastics alone significantly decreased the AGB and BGB of sugarcane compared with the CK, and the decreasing amplitude in the High PE treatment was greater (
Figure 1), indicating that the accumulation of microplastics would inhibit the biomass of sugarcane and that the degree of influence increased with the increasing concentration of microplastics. This is similar to the findings of many previous studies that the accumulation of microplastics can inhibit plant growth and development, thereby reducing plant biomass (de Souza Machado et al., 2019; Yao et al., 2022). Compared with the treatments with microplastics alone, the treatments of microplastics combined with biochar significantly increased the total biomass, which confirmed that the addition of biochar could reduce the inhibition of microplastic accumulation on sugarcane growth. This is consistent with the results of other studies (Hammer et al., 2015; Ran et al., 2023). It has been reported that biochar addition could increase the shoot dry matter production in different concentrations of PVC-contaminated soil (Khalid et al., 2023). At the same time, compared with CK, treatments with microplastics alone slightly decreased the pH, total and available N and P contents (
Table 1), indicating that the accumulation of microplastics would lead to soil acidification and reduce soil nutrients. Many previous studies have reported the effects of PE microplastics on soil physical and chemical properties, but the results vary (Wang et al., 2022a). For example, PE microplastics increased soil pH and soluble organic matter content in microcosm incubation (Gao et al., 2021). In red soil, the addition of PE microplastics reduced soil pH and organic matter content (Yan et al., 2021). The differences in the effects of PE microplastics on soil properties may be related to the concentration of microplastics, soil type and climate conditions (Chae and An, 2018; Wang et al., 2022a). Additionally, treatments with BC amendment had significantly higher soil pH, SOC and nutrients than treatments with microplastics alone, which may be due to the high pH, SOC and nutrients contents of corn straw biochar itself (Kavitha et al., 2018). In addition, the correlation analysis showed that the total biomass of sugarcane was significantly positively correlated with soil pH, SOC, total and available N and P contents, indicating that promotion of sugarcane growth by the addition of biochar may due to its improvement on soil quality (Song et al., 2019).
3.2. Effects of Microplastics and Biochar Addition on Bacterial Community Diversity and Composition
The diversity and composition of total bacteria (16S rRNA gene-based bacteria) were significantly affected by the addition of microplastics or biochar. Previous studies have also reported that microplastics accumulation or biochar amendment changed the diversity of bacteria (Fei et al., 2020; Kavitha et al., 2018). Compared with CK, the treatment with microplastics alone decreased the observed_OTUs, Chao1 and Shannon, while treatments with microplastics combined with biochar increased the three indices (
Table 2). From the perspective of the abundance and diversity of soil microbial communities, biochar addition can increase the abundance and diversity of soil total bacteria, thereby enhancing the stability and functional diversity of soil ecosystems (Bahram et al., 2018; Hartmann and Six, 2023). For total bacteria (16S rRNA gene-based bacteria), the other five treatments significantly decreased the relative abundance of Gemmatimonadota compared with CK (
Figure 2 and
Table S1), which may due to the inhibition of microplastics accumulation on underground growth of sugarcane. Several bacteria in Gemmatimonadota form symbiotic relationships with plant roots (Mujakic et al., 2022), and the underground biomass of sugarcane treated with microplastics in this study was lower than that of CK, resulting in a decrease in the relative abundance of Gemmatimonadota in these treatments. The BC treatment significantly increased the relative abundance of Acidobacteria compared with CK, which may relate to the obvious increase in soil pH, SOC and nutrients in BC (Kalam et al., 2020). At the genus level, compared with CK, only treatments with microplastics combined with biochar significantly increased the relative abundance of
Subgroup_10 while significantly decreased abundance of
TRA3-20. Correlation analysis showed that the relative abundance of
Subgroup_10 was significantly positively correlated with the AGB and BGB of sugarcane and the main soil physico-chemical properties, while the relative abundance of
TRA3-20 was significantly negatively correlated with the majority of soil physico-chemical properties. Additionally, compared with the treatment of microplastics alone, the treatment of microplastics combined with biochar increased the relative abundance of
Bacillus and
Pseudomonas while decreased the abundance of
IMCC26256.
Bacillus and
Pseudomonas are important bacterial groups with biological control functions in plant rhizosphere soil that can inhibit harmful bacteria and pathogens and promote plant growth. Therefore, the promoting effect of microplastics combined with biochar on sugarcane growth may be related to changes in the total bacterial community composition in soil (Fan et al., 2021).
The diversity and composition of
phoD-harbouring bacteria in different treatments were also significantly different. The
phoD gene of bacteria encodes alkaline phosphatase, which plays an important role in soil organic P decomposition (Wei et al., 2021). Compared with CK, the observed_OTUs, Chao1 and Shannon indices of the other five treatments were significantly increased, and the index of the High PE treatment was the highest. In this study, the AP in the High PE treatment was the lowest while the abundance and diversity of
phoD-harbouring bacteria were the highest, which may be explained by sugarcane requiring a certain amount of P provided by organic P decomposition for growth (Chen et al., 2019). For
phoD-harbouring bacteria community composition, Proteobacteria and Actinobacteria were the dominant phyla. Many currently known P solubilizing bacteria belong to these two phyla (Liu et al., 2020). At the genus level, only the Low PE and High PE treatments significantly increased the relative abundance of
Streptomyces compared with CK (
Figure 2 and
Table S2). The higher relative abundance of
Streptomyces at higher concentrations of microplastics (such as High PE) may be due to its greater stress tolerance (Wang et al., 2022b). Moreover, compared with the treatment with microplastics alone, the treatment with microplastics combined with biochar increased the relative abundance of
Amycolatopsis and
Bradyrhizobium, both of which are plant growth-promoting bacteria (Luo et al., 2017). Correlation analysis also confirmed that
Bradyrhizobium was significantly positively correlated with sugarcane biomass and soil AP content, while
Streptomyces was significantly negatively correlated with sugarcane biomass, soil pH, TN, AN and AP (
P < 0.05). These confirmed that the addition of biochar could change the microbial composition and thus alleviate the inhibitory effect of microplastics on plant growth (Palansooriya et al., 2022).
3.3. Effects of Microplastics and Biochar Addition on Microbial Community Structure and Function
Changes in soil physical and chemical properties can cause changes in the soil microbial community structure and further change the function of soil microorganisms (Ali et al., 2021; Zhang et al., 2023). RDA showed that the community structure of total bacteria (16S rRNA gene-based bacteria) was mainly affected by pH, EC, TN and AK, and the structure of the total bacterial community was significantly negatively correlated with pH (
P < 0.05). Soil pH, EC, TN and AK increased significantly with biochar addition, which in turn drove significant changes in the total bacterial community structure (
Table 1 and
Figure 4). Many previous studies also showed that soil total bacterial community structure was affected by soil pH, SOC, AP and other physical and chemical properties (Liu et al., 2020; Zhou et al., 2015). A study on black soil in Northeast China found that pH and NO
3- concentration played an important role in the formation of bacterial community structure (Zhou et al., 2015), while the bacterial community structure on loess was significantly correlated with dissolved organic C, AP and TP (Liu et al., 2020). These results confirmed that there were some differences in the factors affecting the structure of the total bacterial community in soil, which may due to differences in soil types and fertilization management measures. The structure of
phoD-harbouring bacteria community was significantly negatively correlated with SOC, EC and AK (
P < 0.05). The results of different studies on the relationship between the structure of
phoD-harbouring bacteria community and soil physico-chemical properties are quite different (Chen et al., 2019; Wei et al., 2021). Chen et al. (2019) has reported that soil pH and AP were related to changes in the community structure of
phoD-harbouring bacteria in soil, while Liu et al. (2020) has found that various physical and chemical properties had little effect on the structure of
phoD-harbouring bacteria community. These variable findings may imply that the structure of
phoD-harbouring bacteria community is quite different under different soil types and climatic conditions (Liu et al., 2020).
Correlation analysis showed that AP and pH significantly affected soil microbial species and the abundance of functional genes (
Figure 5). Soil pH was significantly correlated with
Subgroup_10,
Streptomyces, as well as nitrate_respiration, nitrogen_respiration, and fermentation. Similarly, Zhang et al. (2023) also found that pH was significantly correlated with nitrogen_respiration, nitrate_respiration. Furthermore, there were significant differences in the abundance of functional genes among the different treatments. Compared with CK, the High PE treatments significantly changed the abundance of functional genes related to C cycling such as erobic_chemoheterotrophy, chemoheterotrophy, aromatic_hydrocarbon_degradation and chitinolysis (
Figure 3). The high functional genes related to C cycling in High PE treatment may be related to the changes in the content and form of C in soil after adding high concentration of microplastics, for microplastics are high-C polymers with a C content of more than 90% (Rillig, 2012). Moreover, compared with the treatment with microplastics alone, the treatment with microplastics combined with biochar increased the abundance of functional genes such as aerobic_ammonia_oxidation, denitrification, nitrate_respiration and nitrogen_respiration. These genes participate in the soil N cycle and may affect soil N content available to plant (Zhang et al., 2023). And in this study, the content of soil AN and the AGB and BGB of sugarcane were significantly higher in treatment of microplastics combined with biochar than those in treatment with microplastics alone.
4. Conclusions
The accumulation of polyethylene microplastics decreased the aboveground biomass and underground biomass of sugarcane, soil pH and nitrogen and phosphorus content, as well as decreased the observed_OTUs, Chao1 and Shannon indices of soil total bacteria (16s rRNA gene-based bacteria) while increasing them in phoD-harbouring bacteria compared to the control. In contrast, microplastics combined with biochar could alleviate the negative effects of microplastic accumulation on sugarcane growth and soil quality. There were significant differences in the bacterial community compositions among different treatments. Compared with treatments with microplastics alone, the treatments with microplastics combined with biochar increased the relative abundance of Subgroup_10, Bacillus, Pseudomonas in soil total bacteria and Amycolatopsis and Bradyrhizobium in phoD-harbouring bacteria, all of which can inhibit harmful bacteria and promote plant growth. In addition, different treatments also changed the abundance of potential microbial functional genes. Compared with the treatment with microplastic alone, the treatments with microplastics combined with biochar increased the abundance of functional genes involved in the nitrogen cycle, such as aerobic_ammonia_oxidation, denitrification, nitrate_respiration and nitrogen_respiration, which may increase soil nitrogen content available to plant, thereby promoting the growth of sugarcane. In general, microplastics combined with biochar could alleviate the negative effects of microplastic accumulation on plant growth by improve soil nutrients and microbial community structure and function.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Table S1: The relative abundances of the most abundant phyla in different treatments. Table S2: The relative abundances of the 10 most abundant genera in different treatments.
References
- Abbas, T., Rizwan, M., Ali, S., Zia-ur-Rehman, M., Farooq Qayyum, M., Abbas, F., Hannan, F., Rinklebe, J., Sik Ok, Y., 2017. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotox. Environ. Safe. 140, 37-47. [CrossRef]
- Ali, A., Ghani, M.I., Elrys, A.S., Ding, H., Iqbal, M., Cheng, Z., Cai, Z., 2021. Different cropping systems regulate the metabolic capabilities and potential ecological functions altered by soil microbiome structure in the plastic shed mono-cropped cucumber rhizosphere. Agr. Ecosyst. Environ. 318, 107486. [CrossRef]
- Arif, M., Ilyas, M., Riaz, M., Ali, K., Shah, K., Ul Haq, I., Fahad, S., 2017. Biochar improves phosphorus use efficiency of organic-inorganic fertilizers, maize-wheat productivity and soil quality in a low fertility alkaline soil. Field. Crop Res. 214, 25-37. [CrossRef]
- Bahram, M., Hildebrand, F., Forslund, S.K., Anderson, J.L., Soudzilovskaia, N.A., Bodegom, P.M., Bengtsson-Palme, J., Anslan, S., Coelho, L.P., Harend, H., Huerta-Cepas, J., Medema, M.H., Maltz, M.R., Mundra, S., Olsson, P.A., Pent, M., Põlme, S., Sunagawa, S., Ryberg, M., Tedersoo, L., Bork, P., 2018. Structure and function of the global topsoil microbiome. Nature 560(7717), 233-237. [CrossRef]
- Chae, Y., An, Y.-J., 2018. Current research trends on plastic pollution and ecological impacts on the soil ecosystem: A review. Environ. Pollut. 240, 387-395. [CrossRef]
- Chen, X., Chen, X., Zhao, Y., Zhou, H., Xiong, X., Wu, C., 2020. Effects of microplastic biofilms on nutrient cycling in simulated freshwater systems. Sci. Total Environ. 719, 137276. [CrossRef]
- Chen, X., Jiang, N., Condron, L.M., Dunfield, K.E., Chen, Z., Wang, J., Chen, L., 2019. Impact of long-term phosphorus fertilizer inputs on bacterial phoD gene community in a maize field, Northeast China. Sci. Total Environ. 669, 1011-1018. [CrossRef]
- Das, P.P., Singh, K.R.B., Nagpure, G., Mansoori, A., Singh, R.P., Ghazi, I.A., Kumar, A., Singh, J., 2022. Plant-soil-microbes: A tripartite interaction for nutrient acquisition and better plant growth for sustainable agricultural practices. Environ. Res. 214, 113821. [CrossRef]
- de Souza Machado, A.A., Lau, C.W., Kloas, W., Bergmann, J., Bachelier, J.B., Faltin, E., Becker, R., Görlich, A.S., Rillig, M.C., 2019. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 53(10), 6044-6052. [CrossRef]
- Fan, K., Delgado-Baquerizo, M., Guo, X., Wang, D., Zhu, Y.-g., Chu, H., 2021. Biodiversity of key-stone phylotypes determines crop production in a 4-decade fertilization experiment. ISME J. 15(2), 550-561. [CrossRef]
- Fei, Y., Huang, S., Zhang, H., Tong, Y., Wen, D., Xia, X., Wang, H., Luo, Y., Barceló, D., 2020. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 707, 135634. [CrossRef]
- Feng, X., Wang, Q., Sun, Y., Zhang, S., Wang, F., 2022. Microplastics change soil properties, heavy metal availability and bacterial community in a Pb-Zn-contaminated soil. J. Hazard. Mater. 424, 127364. [CrossRef]
- Gao, B., Yao, H., Li, Y., Zhu, Y., 2021. Microplastic addition alters the microbial community structure and stimulates soil carbon dioxide emissions in vegetable-growing soil. Environ. Toxicol. Chem. 40(2), 352-365. [CrossRef]
- Gregory, S.J., Anderson, C.W.N., Camps Arbestain, M., McManus, M.T., 2014. Response of plant and soil microbes to biochar amendment of an arsenic-contaminated soil. Agr. Ecosyst. Environ. 191, 133-141. [CrossRef]
- Hammer, E.C., Forstreuter, M., Rillig, M.C., Kohler, J., 2015. Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl. Soil Ecol. 96, 114-121. [CrossRef]
- Hartmann, M., Six, J., 2023. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Env. 4(1), 4-18. [CrossRef]
- Huang, Y., Zhao, Y., Wang, J., Zhang, M., Jia, W., Qin, X., 2019. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 254, 112983. [CrossRef]
- Jacques, O., Prosser, R.S., 2021. A probabilistic risk assessment of microplastics in soil ecosystems. Sci. Total Environ. 757, 143987. [CrossRef]
- Kalam, S., Basu, A., Ahmad, I., Sayyed, R.Z., El-Enshasy, H.A., Dailin, D.J., Suriani, N.L., 2020. Recent understanding of soil acidobacteria and their ecological significance: A critical review. Front. Microbiol. 11. [CrossRef]
- Kavitha, B., Reddy, P.V.L., Kim, B., Lee, S.S., Pandey, S.K., Kim, K.-H., 2018. Benefits and limitations of biochar amendment in agricultural soils: A review. J. Environ. Manage. 227, 146-154. [CrossRef]
- Khalid, A.R., Shah, T., Asad, M., Ali, A., Samee, E., Adnan, F., Bhatti, M.F., Marhan, S., Kammann, C.I., Haider, G., 2023. Biochar alleviated the toxic effects of PVC microplastic in a soil-plant system by upregulating soil enzyme activities and microbial abundance. Environ. Pollut. 332, 121810. [CrossRef]
- Kumar, A., Mishra, S., Pandey, R., Yu, Z.G., Kumar, M., Khoo, K.S., Thakur, T.K., Show, P.L., 2023. Microplastics in terrestrial ecosystems: Un-ignorable impacts on soil characterises, nutrient storage and its cycling. Trac-Trend Anal. Chem. 158, 116869. [CrossRef]
- Kuppusamy, S., Thavamani, P., Megharaj, M., Venkateswarlu, K., Naidu, R., 2016. Agronomic and remedial benefits and risks of applying biochar to soil: Current knowledge and future research directions. Environ. Int. 87, 1-12. [CrossRef]
- Li, Y.-R., Yang, L.-T., 2015. Sugarcane agriculture and sugar industry in China. Sugar Tech 17(1), 1-8. [CrossRef]
- Liu, J., Ma, Q., Hui, X., Ran, J., Ma, Q., Wang, X., Wang, Z., 2020. Long-term high-P fertilizer input decreased the total bacterial diversity but not phoD-harboring bacteria in wheat rhizosphere soil with available-P deficiency. Soil Biol. Biochem. 149, 107918. [CrossRef]
- Luo, G., Ling, N., Nannipieri, P., Chen, H., Raza, W., Wang, M., Guo, S., Shen, Q., 2017. Long-term fertilisation regimes affect the composition of the alkaline phosphomonoesterase encoding microbial community of a vertisol and its derivative soil fractions. Biol. Fert. Soils 53. [CrossRef]
- Mujakic, I., Piwosz, K., Koblizek, M., 2022. Phylum Gemmatimonadota and its role in the Environment. Microorganisms 10, 151. [CrossRef]
- Page, A.L., 1982. Methods of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy/Soil Science Society of America, Madison, WI, USA.
- Palansooriya, K.N., Sang, M.K., Igalavithana, A.D., Zhang, M., Hou, D., Oleszczuk, P., Sung, J., Ok, Y.S., 2022. Biochar alters chemical and microbial properties of microplastic-contaminated soil. Environ. Res. 209, 112807. [CrossRef]
- Ran, T., Li, J., Liao, H., Zhao, Y., Yang, G., Long, J., 2023. Effects of biochar amendment on bacterial communities and their function predictions in a microplastic-contaminated Capsicum annuum L. soil. Environ. Technol. Inno. 31, 103174. [CrossRef]
- Ren, T., Feng, H., Xu, C., Xu, Q., Fu, B., Azwar, E., Wei, Y., Lam, S.S., Liu, G., 2022. Exogenous application and interaction of biochar with environmental factors for improving functional diversity of rhizosphere's microbial community and health. Chemosphere 294, 133710. [CrossRef]
- Rillig, M.C., 2012. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 46(12), 6453-6454. [CrossRef]
- Sakurai, M., Wasaki, J., Tomizawa, Y., Shinano, T., Osaki, M., 2008. Analysis of bacterial communities on alkaline phosphatase genes in soil supplied with organic matter. Soil Sci. Plant Nutr. 54, 62-71. [CrossRef]
- Song, D., Xi, X., Zheng, Q., Liang, G., Zhou, W., Wang, X., 2019. Soil nutrient and microbial activity responses to two years after maize straw biochar application in a calcareous soil. Ecotox. Environ. Safe. 180, 348-356. [CrossRef]
- Su, M., Meng, L., Zhao, L., Tang, Y., Qiu, J., Tian, D., Li, Z., 2021. Phosphorus deficiency in soils with red color: Insights from the interactions between minerals and microorganisms. Geoderma 404, 115311. [CrossRef]
- Wang, F., Wang, Q., Adams, C.A., Sun, Y., Zhang, S., 2022a. Effects of microplastics on soil properties: Current knowledge and future perspectives. J. Hazard. Mater. 424, 127531. [CrossRef]
- Wang, J., Li, Y., Pinto-Tomás, A., Cheng, K., Huang, Y., 2022b. Habitat adaptation drives speciation of a Streptomyces species with distinct habitats and disparate geographic origins. Mbio 13. [CrossRef]
- Wei, X., Hu, Y., Cai, G., Yao, H., Ye, J., Sun, Q., Veresoglou, S.D., Li, Y., Zhu, Z., Guggenberger, G., Chen, X., Su, Y., Li, Y., Wu, J., Ge, T., 2021. Organic phosphorus availability shapes the diversity of phoD-harboring bacteria in agricultural soil. Soil Biol. Biochem. 161, 108364. [CrossRef]
- Wu, L., Zhang, S., Chen, M., Liu, J., Ding, X., 2021. A sustainable option: Biochar addition can improve soil phosphorus retention and rice yield in a saline–alkaline soil. Environ. Technol. Inno. 24, 102070. [CrossRef]
- Wu, Q., Zhou, W., Chen, D., Cai, A., Ao, J., Huang, Z., 2020. Optimizing soil and fertilizer phosphorus management according to the yield response and phosphorus use efficiency of sugarcane in southern China. J. Soil Sci. Plant Nut. 20(4), 1655-1664. [CrossRef]
- Yan, Y., Chen, Z., Zhu, F., Zhu, C., Wang, C., Gu, C., 2021. Effect of polyvinyl chloride microplastics on bacterial community and nutrient status in two agricultural soils. B. Environ. Contam. Tox. 107(4), 602-609. [CrossRef]
- Yang, L., Zhang, Y., Kang, S., Wang, Z., Wu, C., 2021. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environ. 780, 146546. [CrossRef]
- Yao, Y., Lili, W., Shufen, P., Li, G., Hongmei, L., Weiming, X., Lingxuan, G., Jianning, Z., Guilong, Z., Dianlin, Y., 2022. Can microplastic mediate soil properties, plant growth and carbon/nitrogen turnover in the terrestrial ecosystem? Ecosyst. Health Sust. 8, 1. [CrossRef]
- Zhang, J., Ren, S., Xu, W., Liang, C., Li, J., Zhang, H., Li, Y., Liu, X., Jones, D.L., Chadwick, D.R., Zhang, F., Wang, K., 2022. Effects of plastic residues and microplastics on soil ecosystems: A global meta-analysis. J. Hazard. Mater. 435, 129065. [CrossRef]
- Zhang, M., Liang, G., Ren, S., Li, L., Li, C., Li, Y., Yu, X., Yin, Y., Liu, T., Liu, X., 2023. Responses of soil microbial community structure, potential ecological functions, and soil physicochemical properties to different cultivation patterns in cucumber. Geoderma 429, 116237. [CrossRef]
- Zhou, J., Guan, D., Zhou, B., Zhao, B., Ma, M., Qin, J., Jiang, X., Chen, S., Cao, F., Shen, D., Li, J., 2015. Influence of 34-years of fertilization on bacterial communities in an intensively cultivated black soil in northeast China. Soil Biol. Biochem. 90, 42-51. [CrossRef]
- Zhou, J., Gui, H., Banfield, C.C., Wen, Y., Zang, H., Dippold, M.A., Charlton, A., Jones, D.L., 2021. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 156, 108211. [CrossRef]
- Zhu, F., Yan, Y., Doyle, E., Zhu, C., Jin, X., Chen, Z., Wang, C., He, H., Zhou, D., Gu, C., 2022. Microplastics altered soil microbiome and nitrogen cycling: The role of phthalate plasticizer. J. Hazard. Mater. 427, 127944. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).