Submitted:
04 December 2023
Posted:
05 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Strain and Culture Medium
2.2.Main Reagents and Instruments
2.3.Training Method
2.4.Magnetic Field Treatment
2.5.Analytical Method
2.5.1. Detection of Biomass and Lycopene Yield
2.5.2. Determination of Process Curve
2.5.3. Determination of Cell Permeability of Bacteria
2.5.5. Scanning Electron Microscope (SEM) Observation
2.5.6. Transcriptome Analysis
2.5.7. RT-qPCR
2.5.8. Data Analysis
3. Results
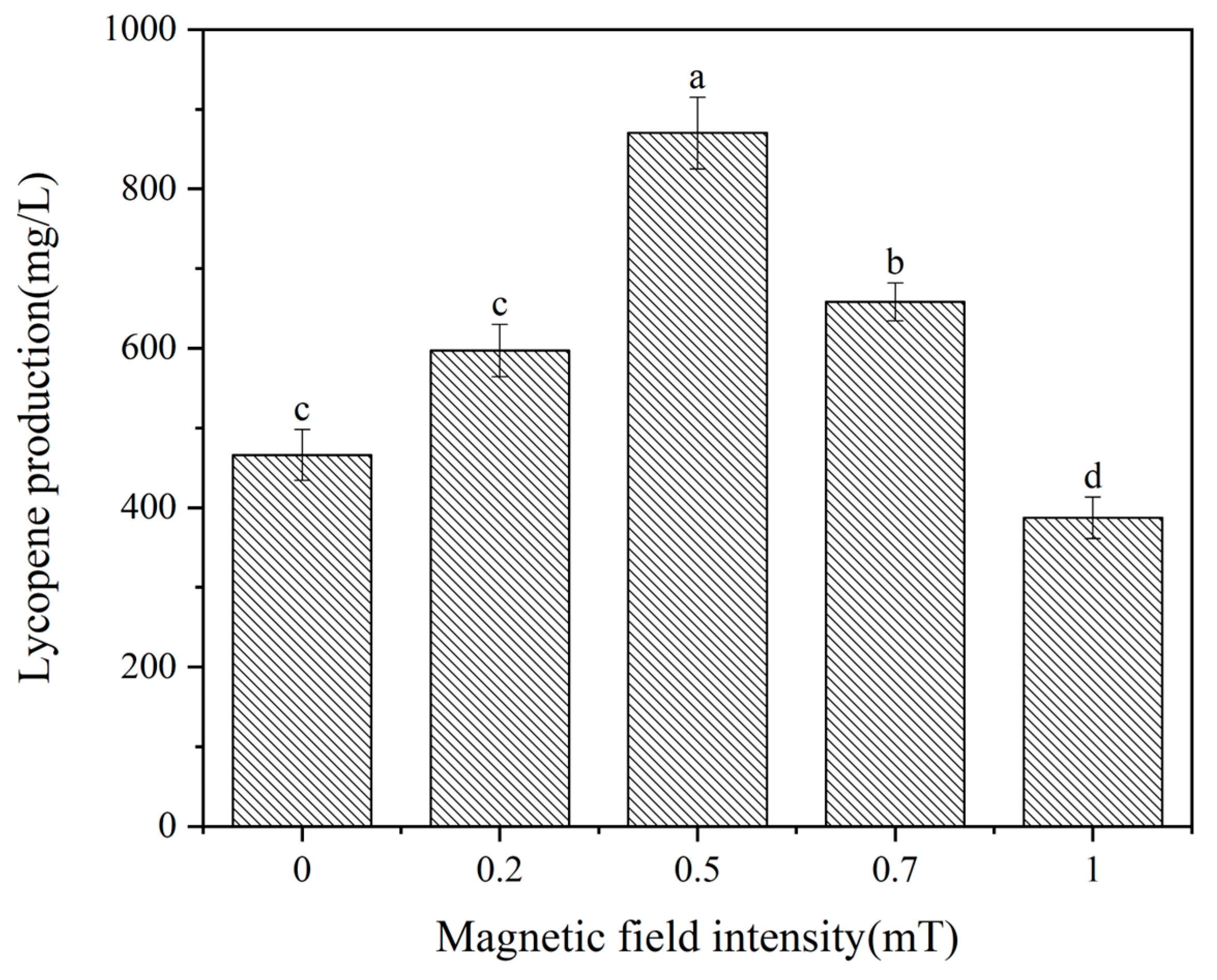
3.1. Magnetic Field Intensity
3.2. Magnetic Field Processing Time
| Exposure period(d) | Exposure time(h) | Dry cell weight(g/L) | Lycopene yield(mg/L) |
|---|---|---|---|
| Control | 0 | 63.3±3.40 | 466.46±23.2 |
| 1 | 4 12 24 48 72 |
60.4±4.30 58.3±3.50 56.1±3.80 57.2±4.20 58.3±5.30 |
411.36±22.28e 467.45±16.52b 437.63±18.37c 492.17±14.23a 421.32±21.41d |
| 2 | 4 12 24 48 72 |
59.3±2.70 58.1±3.40 56.3±4.50 58.1±4.60 60.2±3.20 |
575.25±19.48e 723.26±25.25d 759.53±21.37c 1473.16±29.42a 892.13±19.64b |
| 3 | 4 12 24 48 72 |
59.3±2.80 61.2±2.40 58.5±5.20 60.2±3.20 57.1±4.60 |
598.26±18.33e 672.44±19.22d 692.48±15.53c 911.76±18.43a 742.21±17.53b |
3.3. Process Metabolic Curve
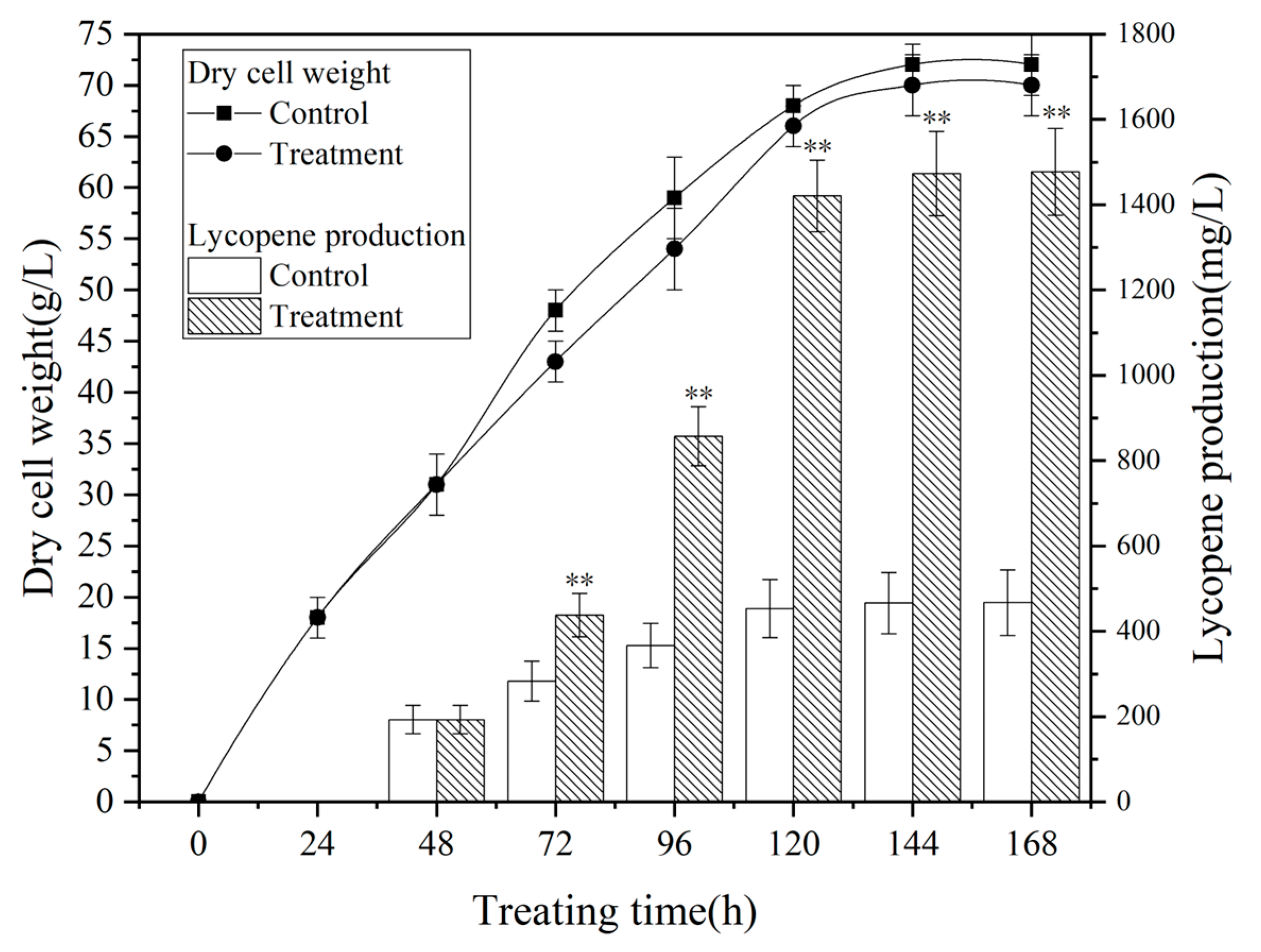
3.4. Transcriptome Analysis of the Effect of Magnetic Field on Bacteriae
3.4.1. Differentially Expressed Genes
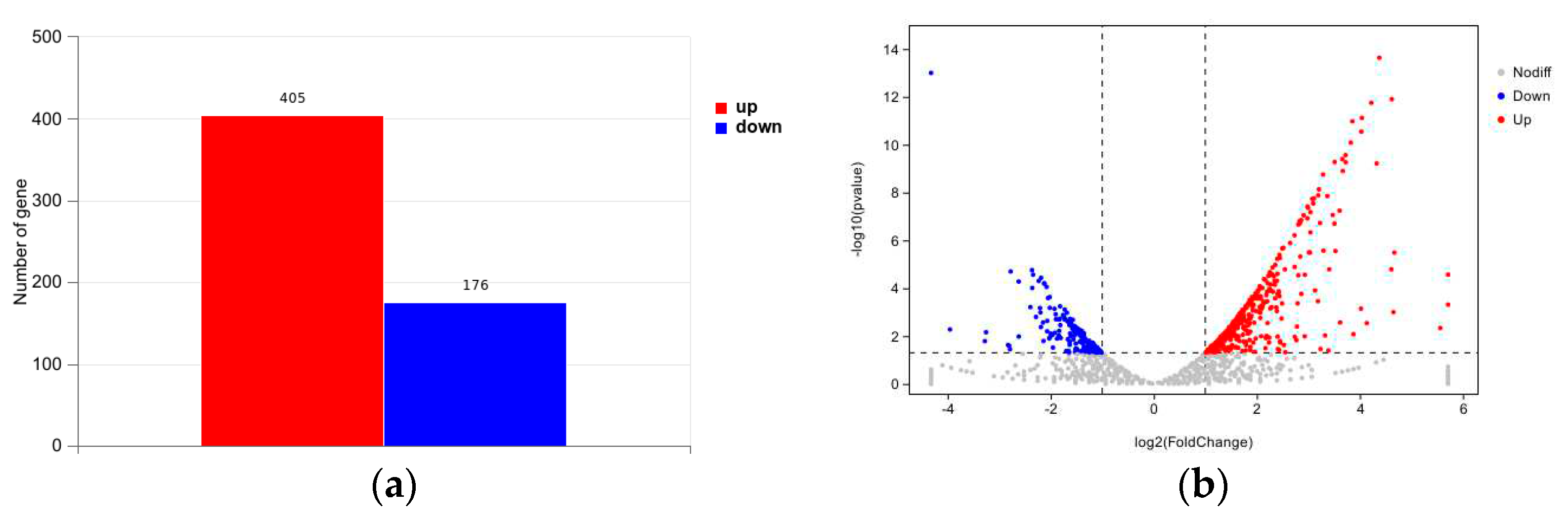
3.4.2. Differentially Expressed Genes
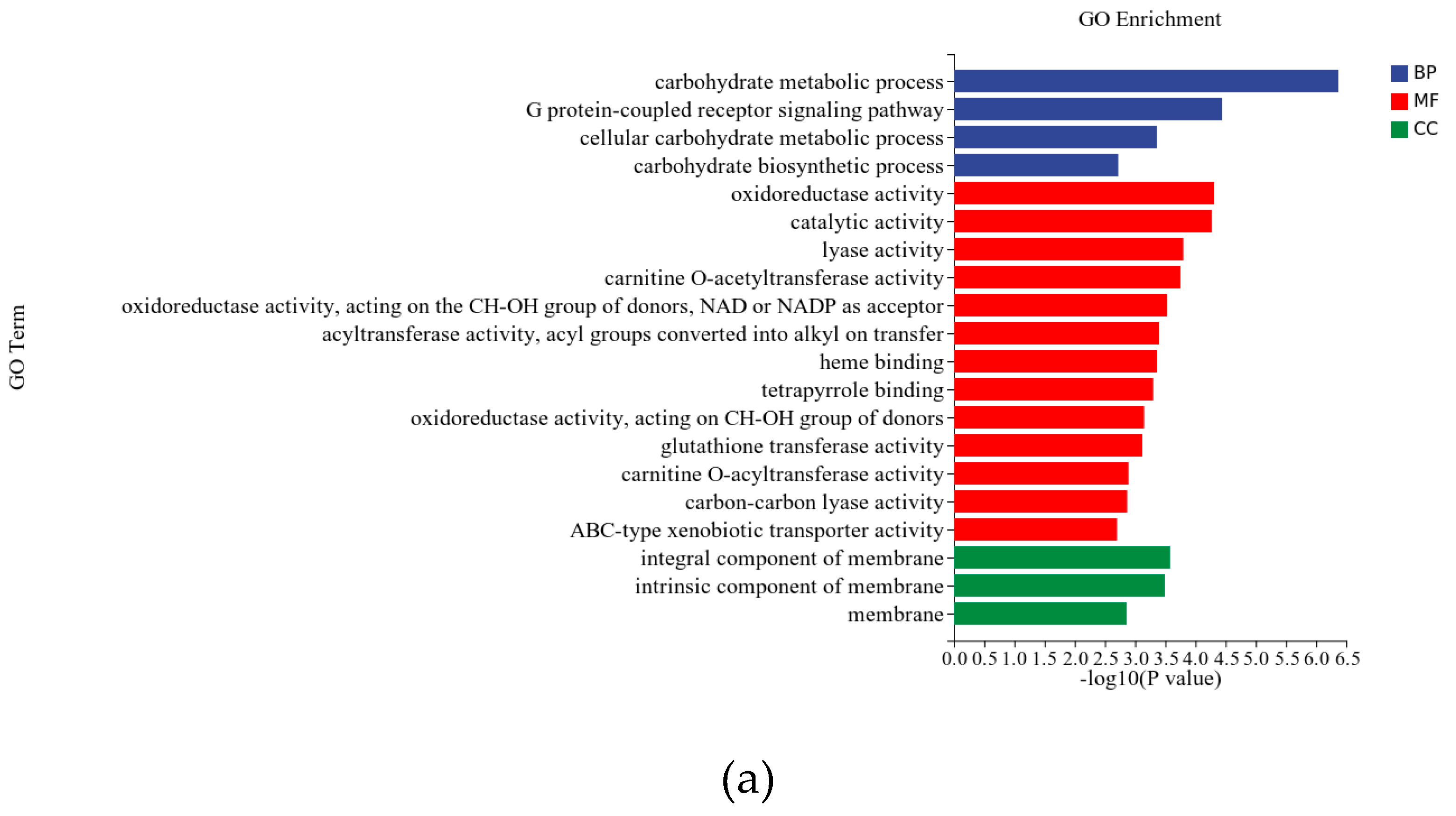
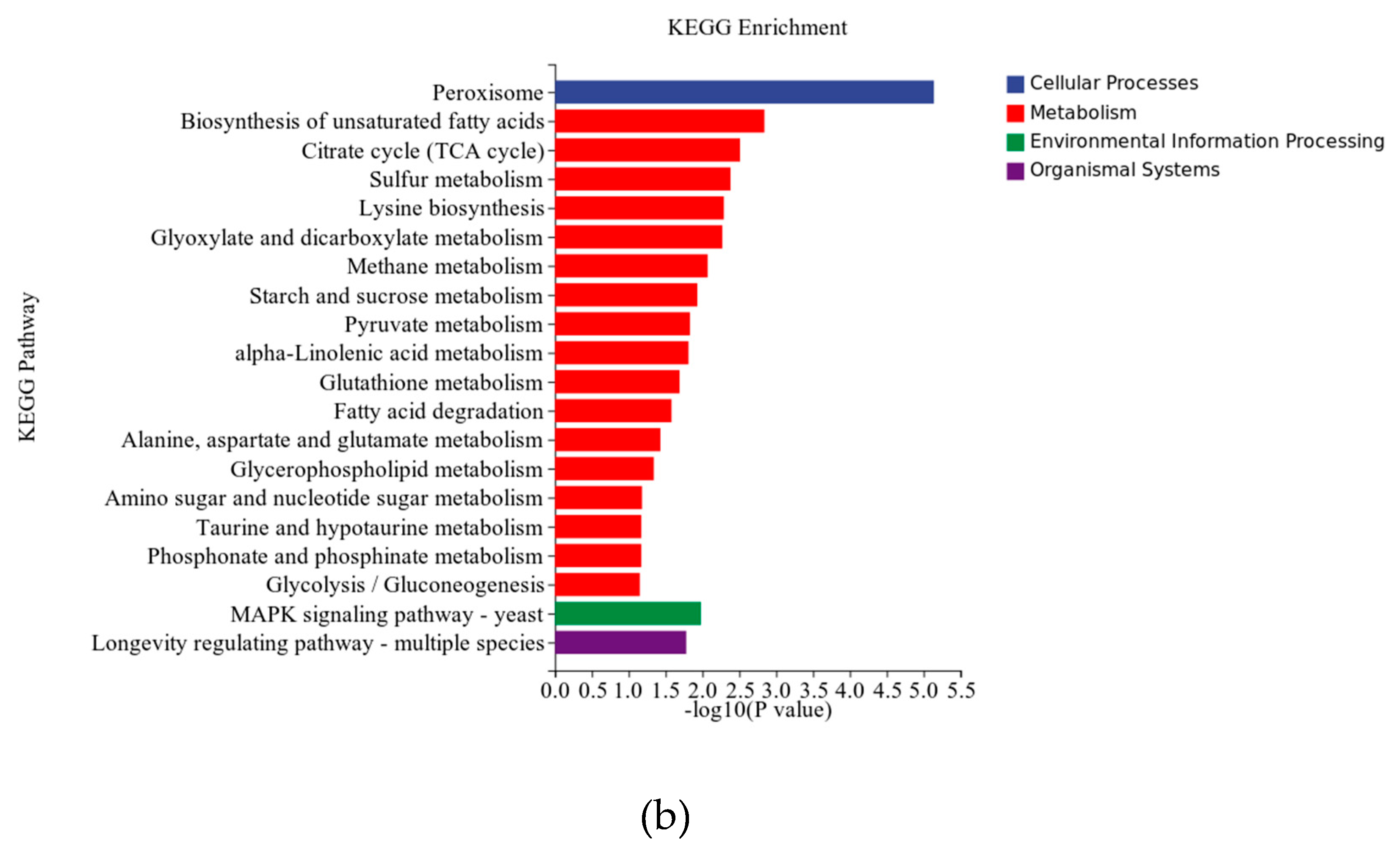
3.5. RT-qPCR Analysis
3.6. Mycelial Morphologye
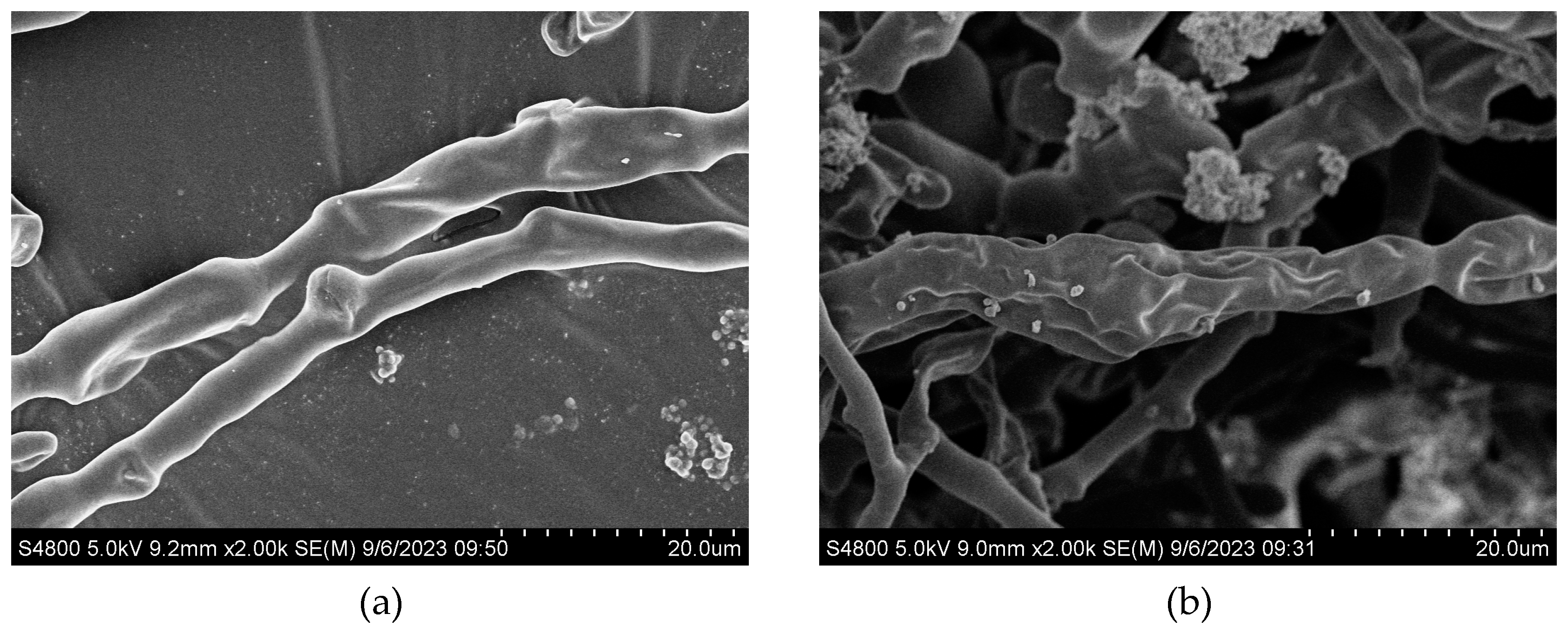
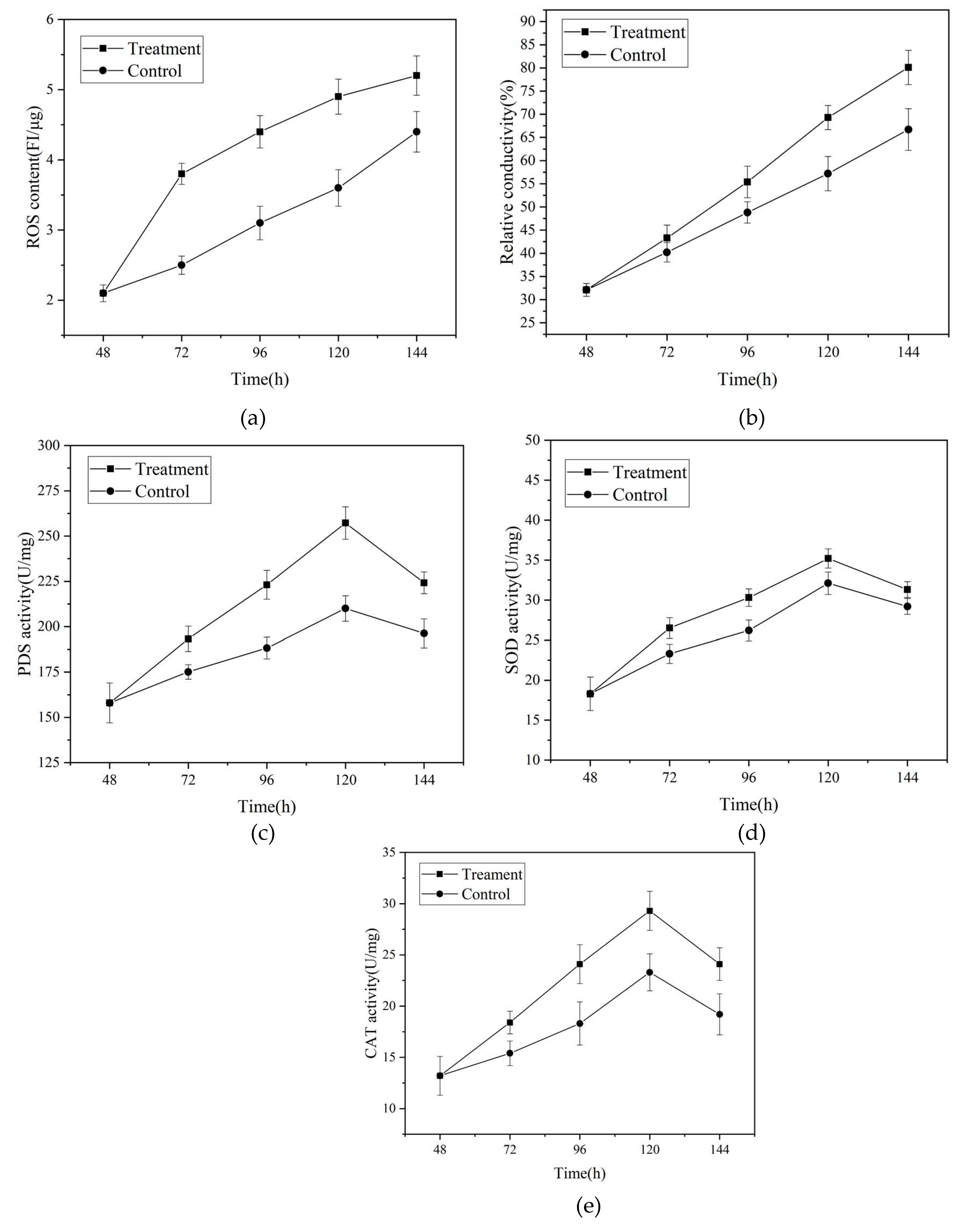
3.7. Effects of Magnetic Field Treatment on Intracellular Reactive Oxygen Species, Cell Membrane Permeability and Activities of Key Enzymes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Yarema, K.; Xu, A. Impact of Static Magnetic Field (SMF) on Microorganisms, Plants and Animals. Springer Singapore 2017, 10.1007/978-981-10-3579–1, 133–172.
- Zhang, J.; Zeng, D.; Xu, C.; Gao, M. Effect of low-frequency magnetic field on formation of pigments of Monascus purpureus. European Food Research and Technology 2014, 240, 577–582. [Google Scholar] [CrossRef]
- Makarov, I.O.K. Effect of Low-Frequency Pulsed Magnetic Field and Low-Level Laser Radiation on Oxidoreductase Activity and Growth of FungiActive Destructors of Polymer Materials. Microbiology 2019, 88. [Google Scholar] [CrossRef]
- Aboneima, S.; El-Metwally, M.M. Effect of extremely low frequency magnetic field in growth, CMCase, electric conductivity and DNA of Aspergillus niger. National Information and Documentation Center (NIDOC), Academy of Scientific Research and Technology (ASRT) 2020.
- Tagging, A.N.I.M. Re: Tomato and Lycopene and Multiple Health Outcomes: Umbrella Review. The Journal of Urology 2022, 207. [Google Scholar]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Hindawi Limited, 2021. [Google Scholar]
- Nigussie, A.; Tura, A.K.; Sisay, M.; Hagos, B.; Motbaynor, B. Anti-Cancer Effects of Lycopene in Animal Models of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Frontiers in pharmacology 2020, 11. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Crowe-White, K.M.; Phillips, T.A.; Ellis, A.C. Lycopene and cognitive function. 2019.
- Srivastava, S.; Srivastava, A.K. Lycopene; chemistry, biosynthesis, metabolism and degradation under various abiotic parameters. Journal of Food Science & Technology 2015, 52, 41–53. [Google Scholar] [CrossRef]
- Qiang, W.; Ling-Ran, F.; Luo, W.; Han-Guang, L.; Lin, W.; Ya, Z.; Xiao-Bin, Y. Mutation Breeding of Lycopene-Producing Strain Blakeslea Trispora by a Novel Atmospheric and Room Temperature Plasma (ARTP). Applied Biochemistry and Biotechnology 2014. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Feng, L.R.; Luo, W.; Li, H.G.; Zhou, Y.; Yu, X.B. Effect of Inoculation Process on Lycopene Production by Blakeslea trispora in a Stirred-Tank Reactor. Applied Biochemistry & Biotechnology 2015, 175, 770–779. [Google Scholar] [CrossRef]
- 13. Sun; Cheng; Mueller; Erich; Meffert; Matthias; Gerthsen; Dagmar On the Progress of Scanning Transmission Electron Microscopy (STEM) Imaging in a Scanning Electron Microscope. Microscopy & Microanalysis the Official Journal of Microscopy Society of America Microbeam Analysis Society Microscopical Society of Canada 2018.
- André D., Schmidt; Heinekamp, T.; Matuschek, M.; Liebmann, B.; Bollschweiler, C.; Brakhage, A.A. Analysis of mating-dependent transcription of Blakeslea trispora carotenoid biosynthesis genes carB and carRA by quantitative real-time PCR. Applied Microbiology & Biotechnology 2005, 67, 549–555. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. 2013.
- Liu, D.; Zhu, L.; Guo, Y.; Zhao, Y.; Betchem, G.; Yolandani, Y.; Ma, H. Enhancing submerged fermentation of Antrodia camphorata by low-frequency alternating magnetic field. Innovative Food Science & Emerging Technologies 2023, 86. [Google Scholar] [CrossRef]
- Xu; Cui; Zhang; Jialan; Gao; Mengxiang; Zeng; Dongjie Effect of low-frequency magnetic field on formation of pigments of Monascus purpureus. Zeitschrift Fur Lebensmittel Untersuchung Und Forschung A 2015.
- Masood, S.; Saleem, I.; Smith, D.; Chu, W.K. Growth Pattern of Magnetic Field Treated Bacteria 2018.
- Falone, S.; Grossi, M.R.; Cinque, B.; Barbara D’Angelo; Tettamanti, E. ; Cimini, A.; Ilio, C.D.; Amicarelli, F. Fifty hertz extremely low-frequency electromagnetic field causes changes in redox and differentiative status in neuroblastoma cells. International Journal of Biochemistry & Cell Biology 2007, 39, 2093–2106. [Google Scholar] [CrossRef]
- Mermall; V Unconventional Myosins in Cell Movement, Membrane Traffic, and Signal Transduction. Science 1998, 279, 527–533. [CrossRef] [PubMed]
- Klotz; J; L CELL BIOLOGY SYMPOSIUM: Membrane trafficking and signal transduction. J. Anim. Sci. 2017, 95, 2183–2184. [CrossRef]
- Zablotskii, V.; Polyakova, T.; Dejneka, A. Modulation of the Cell Membrane Potential and Intracellular Protein Transport by High Magnetic Fields. Bioelectromagnetics 2020. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Noda, Y.; Eckert, V.; Traber, M.G.; Packer, L. The phorbol 12-myristate 13- acetate (PMA)-induced oxidative burst in rat peritoneal neutrophils is increased by a 0.1 mT (60 Hz) magnetic field. FEBS Letters 1995, 376, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Dansen, T.B.; Wirtz, K.W.A. The Peroxisome in Oxidative Stress. IUBMB Life 2001. [Google Scholar] [CrossRef] [PubMed]
- Bonekamp, N.A.; Vlkl, A.; Fahimi, H.D.; Schrader, M. Reactive oxygen species and peroxisomes: Struggling for balance. Biofactors 2009, 35, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Nanou, K.; Roukas, T.; Papadakis, E. Oxidative stress and morphological changes in Blakeslea trispora induced by enhanced aeration during carotene production in a bubble column reactor. Biochem. Eng. J. 2011, 54, 172–177. [Google Scholar] [CrossRef]
- Wang, H.B.; Luo, J.; Huang, X.Y.; Lu, M.B.; Yu, L.J. Oxidative stress response of Blakeslea trispora induced by HO during β-carotene biosynthesis. Journal of Industrial Microbiology & Biotechnology 2014, 41, 555–561. [Google Scholar] [CrossRef]
- Qin, S.; Yin, H.; Yang, C.; Xie, C. A magnetic protein biocompass. 2015.
- Zhang, Y.; Navarro, E.; Cánovas-Márquez, J.T.; Almagro, L.; Chen, H.; Chen, Y.Q.; Zhang, H.; Torres-Martínez, S.; Chen, W.; Garre, V. A new regulatory mechanism controlling carotenogenesis in the fungus Mucor circinelloides as a target to generate β-carotene over-producing strains by genetic engineering. Microbial Cell Factories 2016, 15, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Long, X.; Ye, J.; Zhao, D.; Zhang, S.J.; Sciences, S.O.L.; University, T.; Medicine, S.O.; Brain, I.G.I.F.; Sciences, T.P.C.F.L. Magnetogenetics: remote non-invasive magnetic activation of neuronal activity with a magnetoreceptor. Sci. Bull. 2015. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.N.; Li, X.Y.; Zhang, X.Y.; Ma, H.L. Effect of low-intensity magnetic field on the growth and metabolite of Grifola frondosa in submerged fermentation and its possible mechanisms. Food Res. Int. 2022, 159. [Google Scholar] [CrossRef] [PubMed]
- Voychuk, S.I.; Gromozova, E.N.; Lytvyn, P.M.; Podgorsky, V.S. Changes of Surface Properties of Yeast Cell Wall Under Exposure of Electromagnetic Field (40.68 MHz) and Action of Nystatin. Environmentalist 2005, 25, 139–144. [Google Scholar] [CrossRef]
- Weaver, J.C. Electroporation: a general phenomenon for manipulating cells and tissues. Journal of Cellular Biochemistry 1993, 51, 426–435. [Google Scholar] [CrossRef]
- María, D. Quiles-Rosillo; Rosa M. Ruiz-Vázquez; Santiago Torres-Martínez; Garre, V. Light induction of the carotenoid biosynthesis pathway in Blakeslea trispora. Fungal Genetics & Biology 2005, 42, 141–153. [Google Scholar] [CrossRef]
- Liao, Q.; Liu, Y.; Zhang, J.; Li, L.; Gao, M. A low-frequency magnetic Field regulates Monascus pigments synthesis via reactive oxygen species in M. purpureus. Process Biochem. 2019, 86, 16–24. [Google Scholar] [CrossRef]
- Ikehara, T.; Yamaguchi, H.; Hosokawa, K.; Miyamoto, H.; Aizawa, K. Effects of ELF magnetic field on membrane protein structure of living HeLa cells studied by Fourier transform infrared spectroscopy. Bioelectromagnetics 2010, 24. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.N.; Rubin, A.B. Theoretical Concepts in Magnetobiology after 40 Years of Research. Cells 2022, 11, 274. [Google Scholar] [CrossRef] [PubMed]

| Gene name | Gene code | Forward primer sequence(5’-3’) | Reverse primer sequence(5’-3’) |
| carRA | gene_8655 | CATCTCGTCGTTGGTTCA | AAGCATAGGCAATAACACAAG |
| carB | gene_5071 | GGCACAGATATAACTTGA | TTATTCTTATTGGCTTCCT |
| sod | gene_5118 | ATCACTACAATCCTACTG | ACCATACTTCTTCCAATA |
| cat | gene_6144 | CTATGCTACCAGAGATATG | CCAGACCTTAGTTACATC |
| iscA | gene_10317 | CTGCTGCCAACTCGTTAA | CTGCTGGTGTCAGTGTAAG |
| elo3 | gene_7519 | TGGTCATCAAGAAGAAGA | GTCAAGTTCAGGATAATAGG |
| tef1 | GGTAAGTCTACCACCACTGGTCACT | CAAGAGGAGGGTAGTCAGTGTAAGC |
| Name | Gene ID | Description | Fold change of RNA-Seq | 2⊿⊿Ctof RT-qPCR |
|---|---|---|---|---|
| carRA | gene_8655 | phytoene synthase | 1.12 | 2.15 |
| carB | gene_5071 | Phytoene desaturase | 2.23 | 1.47 |
| sod | gene_5118 | Superoxide dismutase | 2.42 | 2.15 |
| cat | gene_6144 | catalase | 8.18 | 7.80 |
| iscA | gene_10317 | iron-sulfur cluster assembly protein | 1.27 | 1.08 |
| elo3 | gene_7519 | ELO family | 4.26 | 3.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




