1. Introduction
Rich protein foods, including fish, shrimp, meat and dairy products, are important sources of protein, fat-soluble vitamins, micronutrients and special flavor. Thus, rich protein foods have a high demand and are crucial for maintaining human health. However, along the supply chain, oxidative reactions and microbial infections lead to deterioration of flavor, texture, and color of rich protein foods. At the same time, ketones, aldehydes, and aromatic amines produced by oxidation of lipids and proteins are potentially toxic. And food contamination caused by pathogenic microorganisms can easily lead to serious foodborne illness [
1]. It is difficult for consumers to evaluate these changes in most cases. Meanwhile, misjudgments on food quality and safety can lead to food waste [
2,
3]. In order to mitigate and monitor these potential risks, appropriate packaging is needed.
Traditional plastic packaging has excellent mechanical, barrier and thermal properties, mitigating the impact of the external environment on food. However, plastics are difficult to be biodegraded and recycled, and mainly treated by incineration and landfill, which are easy to pollute water, soil, air and cause severe environmental problems. As a result, natural materials such as polysaccharides, protein and lipids, which are biodegradable, environmentally friendly and readily available. In addition, they have a good film forming ability [
4,
5]. However, the functionality of single-component film is restricted. For example, pure starch film has poor water resistance and tensile strength, but good barrier properties [
6]. Similarly, gelatin film possesses good physical properties, but lacks antioxidant and antibacterial ability [
7]. Studies have shown that the addition of natural active ingredients, in particular anthocyanin extracts, could enhance the functional of biodegradable films. Thereby expanding its application in rich protein foods preservation and spoilage monitoring [
8,
9,
10].
Anthocyanins, as a natural pigment, is safe, non-toxic and rich in resources, and is commonly used as food colorants, additives in pharmaceuticals and health products, highly favored by consumers [
11,
12]. Previous studies have shown that anthocyanins have good color responsiveness to pH changes caused by spoilage of rich protein foods. Moreover, the added anthocyanins into biomass materials could improve the UV blocking capacity, reduced oxygen and WVP of film, inhibited the growth of microorganisms in rich protein foods [
1,
13,
14].
In previous reviews, the preparation, function and application of active or intelligent packaging with natural polymers and functional additives including anthocyanins, essential oils and nanocomponents as main components in food preservation have been comprehensively discussed [
15,
16,
17]. Different from previous reviews, this article was to highlight the active and intelligent biodegradable packaging based on anthocyanins for preservation and monitoring rich protein foods. Firstly, the extraction and functional properties of anthocyanins were briefly introduced. Also, the mixing of anthocyanins with different bio-based materials, the improvement of anthocyanins on film function and the on-demand release of anthocyanins by substrates were discussed. Additionally, the preservation and monitoring of active and intelligent film for meat, aquatic products and milk were summarized. Finally, the future research and commercial prospect of active and intelligent packaging were predicted.
2. Extraction, structure and function of anthocyanins
2.1. Extraction of anthocyanins
Solvent extraction usually used to extract anthocyanins, with ethanol, methanol, acetone, water or mixtures of the aforementioned solvents as the medium. In addition, anthocyanins may degrade during extraction, and the stability of anthocyanins can be improved by adding formic acid or hydrochloric acid [
15]. However, alcoholic extracts have poor heat stability and short half-life. In recent years, the use of natural deep eutectic solvent (NADESs) to extract anthocyanins has become a hotspot. NADESs is homogeneous liquid consisting of hydrogen-bond acceptor (HBA) and hydrogen-bond donor (HBD) [
18]. NADESs has non-toxicity, biodegradability, and reusability. Additionally, the hydroxyl or carboxyl groups could form hydrogen bonds with extracts, bring higher extraction yields, enhance stability, and increase antimicrobial and antioxidant ability of anthocyanins. Bi et al. experimented with the extraction of mulberry anthocyanins using 6 different NADESs, and found all of them exhibited higher extraction yields compared to acidified ethanol, with the best results obtained using ChCl/lactic acid [
19]. Jovanović et al. developed a NADES-based extraction process for elderberry anthocyanins. It showed that anthocyanins extracted using ChCl/xylitol NADES exhibited the highest antioxidant and antimicrobial activities, and had better stability [
20]. Moreover, the acidity of the solution can affect the extraction efficiency of anthocyanins. Acid NADESs can improve the extraction efficiency of anthocyanins, by preventing the degradation of non-acylated anthocyanins [
21].
2.2. Chemical structure and color indication mechanism of anthocyanins
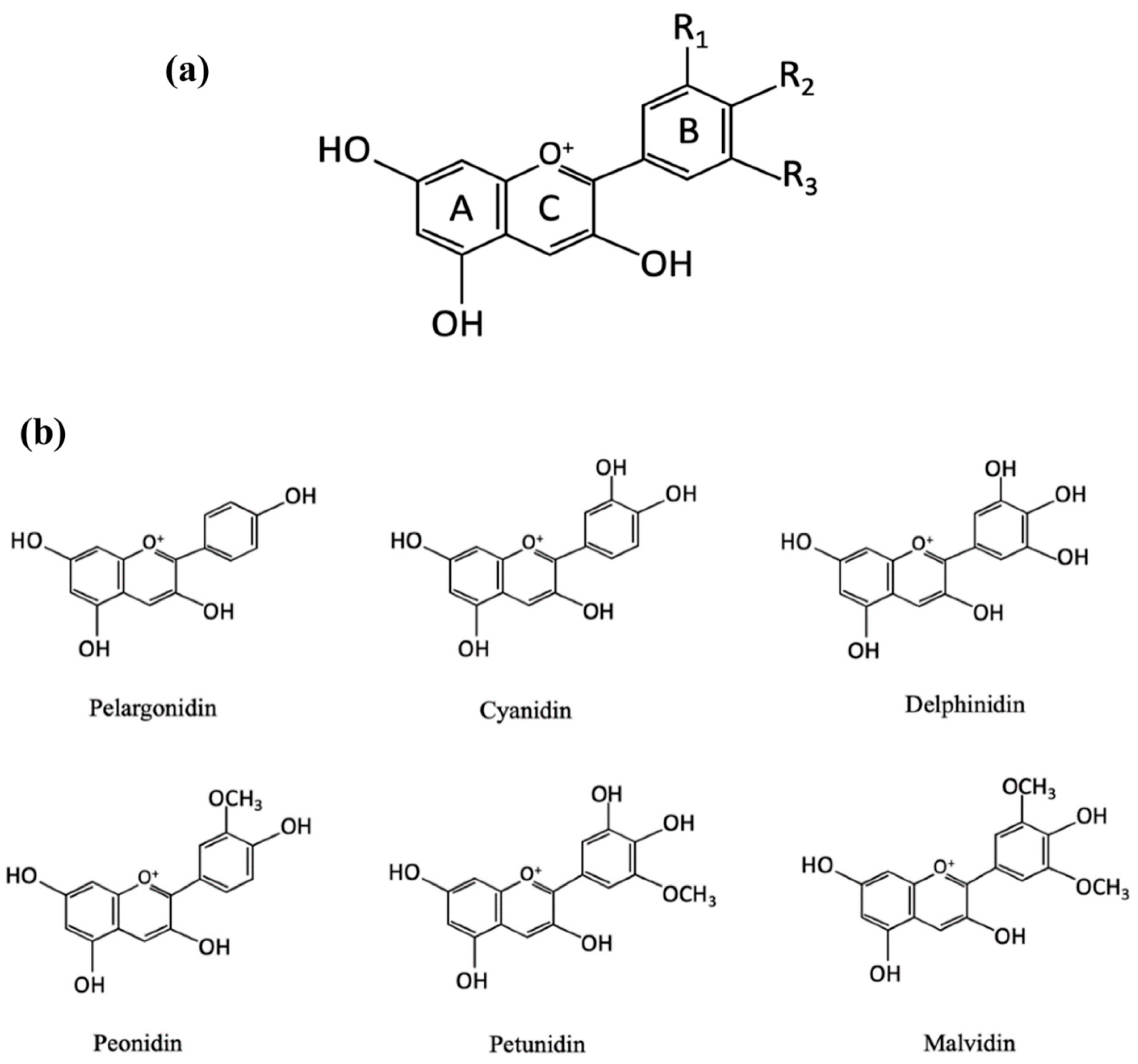
The flowers, leaves, stems and fruits show different colors due to different types and contents of pigments, which are mainly affected by flavonoids, carotene and beet pigments. Anthocyanins is the most important color developing substances in flavonoids, with C
6-C
3-C
6 as the carbon skeleton, and 3,5,7-trihydroxy-2-phenylbenzopyran as the basic structural unit (
Figure 1a) [
15,
22,
23]. Due to the methylation and hydroxylation of different carbon positions in anthocyanins, more than 700 anthocyanins in 27 classes have been discovered [
24]. There are 6 common anthocyanins (
Figure 1b), including pelargonidin, cyanidin, delphinidin, peonidin, petunia and malvacin [
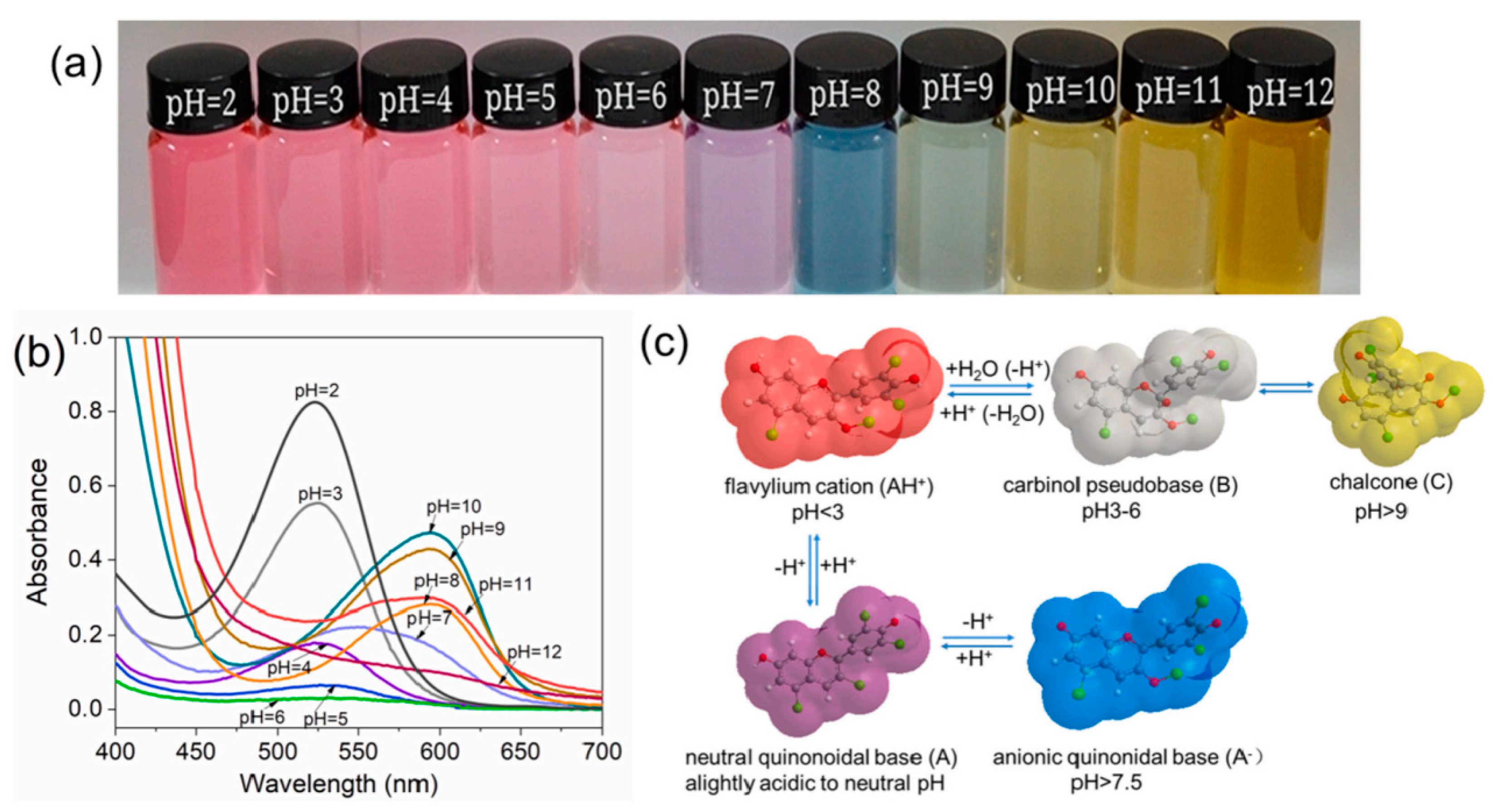
15]. Meanwhile, anthocyanins exhibit highly reactivity to pH values. In H
+ or OH
- environment, the distribution of π electrons in the pigment molecules changes, resulting in changes in the structure of anthocyanins. This results in changes in the absorption and reflection of light by anthocyanins, leading to the display of different colors [
25,
26] (
Figure 2). In addition, the molecular structure of anthocyanins also affects antioxidant and antimicrobial activity [
27,
28]. This will be analyzed in detail in the following sections.
2.3. Antioxidant mechanism of anthocyanins
As summarized in
Table 1, anthocyanins from jambolao skins [
29], black rice bran [
30], shikonin [
31], blueberry [
32] and so on had scavenging effects on ABTS and DPPH. This is attributed to the conjugated structure, hydroxyl functional groups, o-diphenol structure and polar substituents present in the molecular structure of anthocyanins, which endow it with antioxidant activity and inhibitors of oxidative reactions [
33,
34]. Moreover, it has been demonstrated that the anthocyanin glycosides antioxidant was higher than glycosides, monoglycosides was higher than polysaccharides, and anthocyanosides was higher than acylated anthocyanosides [
35]. Typically, anthocyanins have multiple aromatic rings that form a conjugated structure. This conjugated structure gives anthocyanins good electron transfer properties, allowing them to capture and neutralize free radicals. In addition, the o-diphenol structure of anthocyanins enhances their ability to capture free radicals. The o-diphenol structure at the 3' and 4' positions on ring B of anthocyanins forms a more stable o-quinone structure or conjugated semiquinone by two single-electron transfer reactions with ROꞏ. Moreover, the hydroxyl functional group in anthocyanins can provide hydrogen atoms or electrons, thus stabilizing free radicals and reducing the occurrence of oxidation reactions. For example, the 5'-hydroxyl group on ring A of anthocyanins can be easily oxidized, releasing H
+. Which had a strong scavenging effect on ROꞏ. Then, the ROꞏ combined with 3', 5', and 7' hydroxyl groups to form pseudo-semiquinone structures and through keto-enol tautomerization to improve stability [
12]. Unlike other polyphenols, anthocyanins lack an electron in the C ring, forming a secondary oxonium ion, which easily attracts free radical attack.
2.4. Antimicrobial mechanism of anthocyanins
Table 1 summarizes the inhibitory effects of different sources of anthocyanins against common foodborne pathogens such as
S. Enteritidis,
P. fluorescens,
E. coli and
S. aureus [
36,
37,
38,
39]. The molecular structure of anthocyanins contains several hydroxyl functional groups, such as the hydroxyl groups in phenyl rings and aromatic alcohols. These hydroxyl groups react with lipid peroxides on microbial cell membranes, leading to membrane damage and lysis. In addition, the hydroxyl functional groups can undergo ionization and exhibit acid-base properties. Under acidic conditions, anthocyanins molecules, in the form of positively charged ions, bind to negatively charged components (proteins and cell wall polysaccharides) of the bacterial surface. Changes the membrane potential of bacteria and disrupts the cycle of bacterial. Additionally, anthocyanins can interact with the electron transport chains in microbial cells, interfering with enzyme systems and ATP synthesis, thereby affecting microbial metabolism and proliferation. This is attributed to the existence of multiple conjugated aromatic rings in the anthocyanins structure, which gives them electron-transferring ability. In summary, anthocyanin-microbe interactions occur through specific structural interactions between anthocyanins and microorganisms. Thus, structural differences result in different responses of microorganisms to the same anthocyanin. For example, anthocyanins may exhibit excellent inhibitory and bactericidal properties against one type of microorganism, while having no effect on another. Research demonstrated that red onion skin extract had an inhibitory effect on
S. aureus DSM 20,231, but had no inhibitory effect on
E. coli DSM 30083 and
Salmonella DSM 13,772 [
38]. This was part contrary to the founds of Sagar et al. they discovered that onion extracts have antimicrobial activity against Gram- bacteria [
40]. This may be related to the onion species.
3. Active and intelligent films containing anthocyanins
Anthocyanins is susceptible to light, heat, humidity and oxygen. To promote the application of anthocyanins into food package, it is important to compound anthocyanins into suitable matrix. Biomass materials are more sustainably sourced and less environmentally polluting than non-renewable resources. Additionally, biomass materials can effectively disperse anthocyanins and bind them through hydrogen bonding or electrostatic interaction [
5]. Many researches have indicated that the incorporation of anthocyanins causes the film color changes in response to conditions, and improves the film’s mechanical properties, UV blocking ability, antibacterial and antioxidant capacity [
5,
10,
41,
42,
43,
44]. After a preliminary literature search, the substrates obtained directly from natural products polysaccharides (cellulose, chitosan and starch) and protein (gelatin) that have been used to develop active-freshness indicator packaging were summarized (
Table 1).
3.1. Cellulose-based film
Cellulose is the most abundant renewable resource in the world and has a linear and high molecular weight structure. The linear arrangement of cellulose gives it a comparatively ordered crystalline structure, providing cellulose film with good mechanical strength and stability. However, cellulose is highly crystalline in its natural state and only soluble in a few organic solvents, which makes it less susceptible to film formation [
16,
45]. Cellulose molecules are abundance of hydroxyl group, and could form strong intermolecular and intramolecular hydrogen bonds [
46]. Compared to natural cellulose, the cellulose derivatives such as cellulose acetate [
26], methylcellulose [
29], hydroxypropyl methylcellulose [
47] possess good film-forming, high modulus, and strong barrier properties, making them favored for active-freshness indicator packaging. However, the hydroxyl group of cellulose is replaced by methoxy group, resulting in high solubility of methylcellulose. It was found that jambolao extracts (50%) added could enhance the water resistance of the methylcellulose film, which was attribute to the interaction of jambolao extracts with methylcellulose via intermolecular hydrogen bonds. In addition, the extracts could improve the mechanical property of the methylcellulose film, while decrease the water vapor permeability [
29]. Moreover, anthocyanins as pH color index, endows cellulose derivatives film to indicate food freshness [
47,
48]. You et al. fabricated carboxymethyl cellulose/konjac glucomannan composite film incorporated blackcurrant anthocyanins (BCA). Composite film was red at pH 2-3, light pink at pH 4-8 and yellow-green at pH 9-13, respectively. In addition, composite film inhibited both
E. coli and
S. aureus [
9].
3.2. Chitosan-based film
Chitosan, derived from deacetylated chitin, has obvious inhibitory effect on bacteria and fungi [
4,
13,
49,
50]. The positively charged -NH
3+ group in chitosan molecules can adsorb onto negatively charged bacteria, disrupting the integrity of cell wall, increasing membrane permeability and causing leakage of cellular contents. Inside the cell, chitosan can adsorb and bind to proteins and nucleic acids, influencing the normal physiological functions of microorganisms, inhibiting their growth and reproduction. Moreover, a large number of amino and hydroxyl groups exist on the molecular chain of chitosan, and can selectively bind metal ions (such as Mg
2 + and Ca
2 +) on the outer membrane of bacteria, inhibiting the production of bacterial toxins [
10]. Furthermore, chitosan exhibits excellent film forming properties and is made into highly transparent, non-toxic, and edible film. However, the mechanical properties, water resistance and gas barrier of pure chitosan film is deficient [
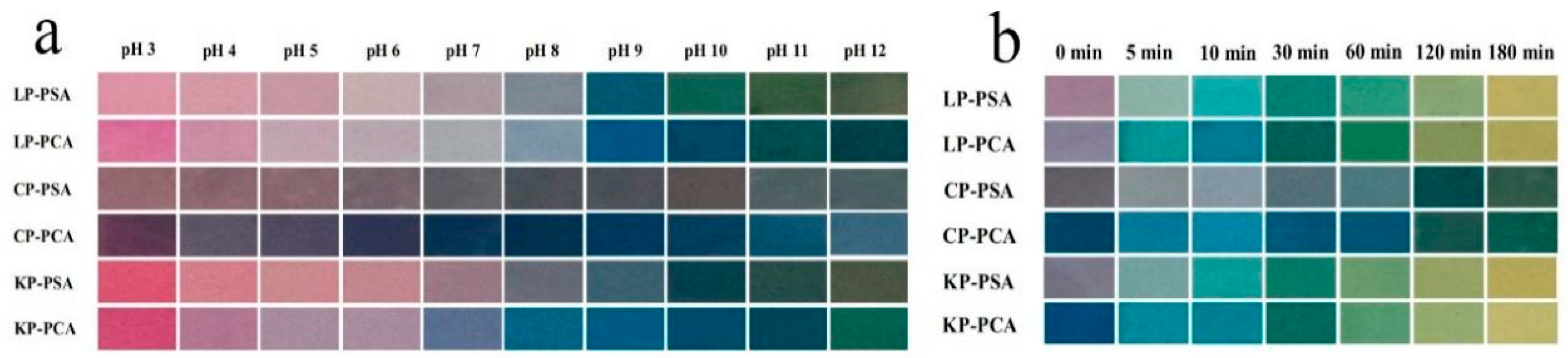
51]. Incorporation of anthocyanins into chitosan film improves its mechanical properties and air barrier capacity. This is mainly due to the interaction of the hydroxyl groups of anthocyanins with the hydroxyl and amino groups of chitosan. Yong et al. developed an active/intelligent film by added purple cabbage anthocyanins (PCA) and purple sweet potato anthocyanins (PSA) into chitosan/polyvinyl alcohol (CP), κ-carrageenan/polyvinyl alcohol (KP) and locust bean gum/polyvinyl alcohol (LP) matrices. The results indicated that the incorporation of PSA and PCA improved film homogeneity, light barrier, antioxidant, pH and ammonia sensitivity through electrostatic interactions and hydrogen bonding between anthocyanins and the matrix. Due to the pH-sensitivity of PSA and PCA, films showed obvious color changes (
Figure 3a) under pH value from 3-12. In addition, the color changes of PSA and PCA films in the presence of ammonia were significant (
Figure 3b) [
8]. Similarly, Li et al. found the pure chitosan film showed some antioxidant activity, and the mulberry anthocyanins added film significantly improved the DPPH free base scavenging ability [
52].
Moreover, chitosan could control the release of anthocyanins, achieving the goal of extending shelf life of rich protein foods and immediate indication the edibility endpoints [
36,
52]. Wang et al. fabricated a chitosan/esterified chitin film loaded with eggplant peel (EE) derived anthocyanins. They found that the release rate and cumulative release of EE in different food simulation systems were different. CS has a well solubility in acetic acid, its structure is more stretched, thus allowing rapid release of EE in 3% acetic acid. Meanwhile, the release rate of EE decreased sequentially in 50% ethanol, 10% ethanol and distilled water, which may be due to the better solubility of EE in alcoholic solutions. In addition, attributed to the electrostatic interactions and hydrogen bonds between CS and EE, the release of total anthocyanins in all films is incomplete, which favors a sustained release effect [
53].
3.3. Starch-based film
Starch has become the most promising material for the production of biodegradable polymers with low cost and good film forming [
54,
55,
56]. The formulation of starch film is critical and determines the barrier and mechanical properties. Adding anthocyanin extracts to formulations could prevents film cracking and brittleness, increasing flexibility and ductility, making starch film with rich color variation via pH changes. Meanwhile, starch could encapsulate anthocyanins effectively through electrostatic interactions, hydrogen bonding and hydration, and enhances the stability of anthocyanins, preventing it from precipitating or dissolving [
57,
58]. Additionally, the branching structure of starch molecules has more voids and adsorption sites, which would enhance the encapsulation of anthocyanins. The release of anthocyanins from film can be controlled by adjusting the shape and size of the starch molecule. Besides, the crystalline form of the starch can affect the release behavior of anthocyanins. Compared to crystalline regions, amorphous regions are looser and favor anthocyanins diffusion [
59,
60]. Zhang et al. compared the release of shikonin from starch and agar-based films in 50% ethanol and water. The results showed that the release of shikonin was very rapid in the first 30 minutes, and due to its alcohol solubility, the release of shikonin in 50% ethanol was faster. In addition, the release of shikonin from agar-based film was faster due to the higher swelling rate of agar than that of the starch [
31]. Furthermore, molecular interactions between starch and anthocyanins affect its conformation, which explains the color change of anthocyanins may not match the anthocyanins film even at the same pH value [
6,
61,
62,
63].
Figure 3.
(a) Color changes of different polysaccharide/PVA films with PSA or PCA after being immersed in buffer solutions (pH 3-12) for 1 min. (b) Color changes of different polysaccharide/PVA films with PSA or PCA after being exposed to ammonia (1 mol/L) at 20 ℃ for 5-180 min [
8].
Figure 3.
(a) Color changes of different polysaccharide/PVA films with PSA or PCA after being immersed in buffer solutions (pH 3-12) for 1 min. (b) Color changes of different polysaccharide/PVA films with PSA or PCA after being exposed to ammonia (1 mol/L) at 20 ℃ for 5-180 min [
8].
3.4. Gelatin-based film
Gelatin is arranged by proline, hydroxyproline and glycine repeating units [
16]. The gelatin molecular contains a number of hydroxyl groups, which enables it to form colloidal particles in aqueous solution, and the cross-linking effect could form a stable, flexible and barrier property gelatin film [
7,
30,
64]. The formation of intermolecular hydrogen bonds between anthocyanins and gelatin significantly increased the tensile strength, WVP and UV-visible barrier and antioxidant capacity of gelatin film [
65]. Moreover, the addition of naturally anthocyanins would enhance pH-color response of the gelatin film, which could be used to maintain food quality and monitor food freshness [
23,
65,
66]. For instance, a novel pH-sensitive and active indicator incorporated with
Coleus scutellarioides anthocyanin extracts (CSAE) was fabricated for fish conservation by Hematian et al. Compared with gelatin film, film containing CSAE had good EAB and UV light barrier capacity, but lower TS. The CSAE film in acidic pH was purple, and green in alkaline pH [
66].
3.5. Film forming methods
For the preparation of active and intelligent biodegradable packaging, casting method is the most commonly used, which is simple and convenient. However, starch and gelatin that need to be heated to higher temperatures to form film, and anthocyanins is easily denatured at high temperatures, which affect the color indication, antibacterial and antioxidant effects of anthocyanins [
31,
62]. Electrostatic spinning can continuously prepare sub-micron or nano-sized ultrafine film without high temperature and pressure. Moreover, the film formed by electrospinning has unique pore structure, large specific surface area and easily modified surface, which provide significant advantages in stimulus sensing and release control of anthocyanins [
7,
26]. In addition, three-dimensional (3D) printing can quickly and accurately print composite labels with specified shapes, avoiding shape and size errors and each film can be loaded with the same amounts of anthocyanins [
52]. Li et al. found that 3D printing made anthocyanins located in the right place of the indicator film, which could reduce the over-oxidation of anthocyanins and did not affect its antimicrobial, antioxidant and color response [
52].
Table 1.
Preparations of active and intelligent film containing anthocyanins.
Table 1.
Preparations of active and intelligent film containing anthocyanins.
| Substrates |
Extracts |
Methods |
Effects of anthocyanins |
Reference |
| carrageenan |
jaboticaba peels |
casting |
improves the opacity property, UV-vis light barrier, against E. coli, scavenging DPPH |
[14] |
| methylcellulose |
jambolao skins |
casting |
scavenging ABTS and DPPH, increase mechanical and barrier properties |
[29] |
| gelatin, oxidized chitin nanocrystals |
black rice bran |
casting |
UV–vis light barrier, scavenging ABTS, DPPH and FRAP |
[30] |
| potato starch |
onion |
casting |
against Staphylococcus aureus DSM 20,231, Salmonella bongori DSM 13,772 and
Escherichia coli DSM 30083, scavenging DPPH |
[38] |
| potato starch |
purple corn cob |
casting |
improve light barrier, against Escherichia coli
|
[62] |
| quercetin-loaded chitosan, agar, sodium alginate |
purple sweet potato |
casting |
UV blocking, water vapor barrier |
[67] |
| starch, agar |
shikonin |
casting |
UV-light barrier, mechanical strength, scavenging DPPH and ABTS, against Listeria monocytogenes
|
[31] |
| chitosan |
black rice bran |
casting |
UV–vis light barrier, sensitive and rapid response to pH/NH3, scavenging DPPH, reduce spoilage bacteria |
[36] |
| alginate, carboxymethyl chitosan |
purple cauliflower |
casting |
improved mechanical strength, reduced the swelling degree, improved the sensitivity of the colorimetric response |
[10] |
| hydroxypropyl methylcellulose |
epigallocatechin-3-gallate |
casting |
enhanced mechanical strength, superior water vapor barrier, UV protection, detect bacterial growth, kill bacteria on-demand |
[47] |
| zein |
blueberry |
casting |
scavenging DPPH and ABTS, against Escherichia coli and Staphylococcus aureus
|
[32] |
| chitosan, cassava starch |
mulberry anthocyanin |
casting |
reduced oxygen and water vapor transmittance, scavenging DPPH, against E. coli and S. aureus
|
[52] |
| gelatin, carrageenan |
shikonin |
casting |
UV blocking, against E. coli and L. monocytogenes |
[64] |
| cellulose nanofiber |
Brassica oleracea |
casting |
UV blocking, improved the physicochemical, scavenging DPPH and ABTS |
[48] |
| gelatin |
Alizarin |
casting |
rapid response to pH/NH3, light barrier, hydrophobicity, scavenging ABTS, against E. coli and S. aureus |
[23] |
| cellulose acetate |
Perilla frutescens |
electrospinning |
scavenging DPPH, enhanced hydrophobicity, against E. coli and S. aureus, |
[26] |
| locust bean gum, polyvinyl alcohol, chitosan, κ-carrageenan |
purple sweetpotato, purple cabbage |
casting |
improved light barrier, scavenging DPPH, ammonia sensitivity |
[8] |
| potato starch |
blueberry |
casting |
improved mechanical, ammonia responsive |
[63] |
| gelatin, zein |
blueberry |
electrospinning |
Fe 2+ enhances the color response of anthocyanins |
[7] |
| gelatin |
Coleus scutellarioides
|
casting |
increased film flexibility, decreased tensile strength and UV-vis light transmittance |
[66] |
| gelatin |
haskap berries |
casting |
increased water vapor, UV-vis light barrier and tensile strength, scavenging DPPH |
[65] |
4. Application in rich protein foods
4.1. Fresh meat
Fresh meat is prone to lipid peroxidation, microbial contamination during processing, transportation, storage and consumption. It would result in color, odor and pH changes that impact the acceptable nature of the meat product [
68]. For these reasons, fresh meat has a very short shelf life (3-5 days) at 4 °C [
69]. Active and intelligent film containing anthocyanins could delay meat spoilage and indicate the freshness of meat [
70]. For example, Hao et al. prepared a CS-OEO-BRBA film use chitosan embedded with black rice bran anthocyanin (BRBA) and oregano essential oil (OEO). CS-OEO-BRBA film could improve the quality indexes of sensory and color of pork, slowed down the rise of pH and TVB-N value at 4°C (
Figure 4). The inclusion of BRBA and OEO reduced the abundance of spoilage bacteria associated with resistance, pathogenicity, and biofilm formation in pork and delayed the emergence of odor volatiles. Moreover, the CS-OEO-BRBA film turned to bottle green on day 12 (red color at the beginning), indicating that the pork has lost its commercial value [
36]. Wang et al. developed an active and intelligent film to monitor the freshness of pork. According to the Chinese Standard GB 2707–2016, the limit of TVB-N for pork should be < 15 mg/100 g. The TVB-N value (15.16 ± 1.15 mg/100 g) of the pork slightly exceeded the standard limit on the first day, which indicated that the pork was not fit for consumption, but the change of appearance pork was difficult to be observed with naked eye. However, the color of the film had changed from blue to navy blue. On the second day, the color of the film changed to green and the TVB-N value of the pork had increased to 24.32 ± 1.02 mg/100 g, which indicated the pork had been severely spoiled [
53].
Moreover, the antioxidant and antibacterial of anthocyanins can also extend the meat shelf life. The addition of
Amaranthus leaf extract (ALE) significantly delayed the growth of total bacterial count (TBC) and
Staphylococcus aureus during chicken preservation kept at chilled.
Staphylococcus aureus and TBC increased to 3.88 log cfu/g and 6.53 log cfu/g on day 3 in the control group, while the ALE film packaged group increased to 2.91 log cfu/g and 6.00 log cfu/g after 12 days. At the same time, the film changed from red to yellow when the chicken goes from fresh to rotten [
71]. Liu et al. showed that add butterfly bean anthocyanins could extend the freshness of beef stored at 4 ℃ for 2 days. When the film color changed from purple, blue to blue-green, it indicated that the beef has changed from fresh to sub-fresh and corrupt [
72].
4.2. Aquatic products
Aquatic products are popular among consumers, but they are susceptible to microbial infestation and enzymatic reactions during storage after inactivation, triggering biochemical reactions that lead to spoilage. It’s not only causes the loss of the eating quality and nutritional value of aquatic products, but also brings food safety problems [
67]. Bacterial-induced spoilage of aquatic products produces basic compounds resulting from the degradation of proteins, such as trimethylamine and dimethylamine, and the consequent pH increase is considered to be an important indicator of deterioration in quality [
43]. Changes in pH value can alter the molecular structure or conformation of the anthocyanins within the active and intelligent film, inducing a change in the film color to determine the quality of the aquatic products [
66,
68]. In conclusion, the color of the film is highly correlated with the sensory quality, lipid peroxide, colony count, volatile salt nitrogen, and pH value of the food.
Ezati et al. observed that the controlled release of shikonin from complex films exhibited strong antioxidant (ABTS and DPPH). In addition, the film supplemented with shikonin had obvious inhibitory effect on
Listeria monocytogenes. After 12 h, the growth rate of
Lactobacillus monocytogenes was 3-fold lower in the shikonin-added films compared to the pure film. Particularly, the films showed quick color changes when exposed to ammonia vapor and different pH values. The films show a characteristic color change from reddish-pink to bluish-purple when used for shrimp packaging, indicating the onset of shrimp spoilage [
31]. Wu et al. also found that after fresh shrimp was stored at 25 ℃ for 24 hours, the
Clitoria ternatea extracts added film changed from blue to blue-green, indicating that the shrimp changed from fresh to rotten [
73]. Moreover, Kanatt developed an
Amaranthus leaf extract added film, which could effectively reduce the total bacterial count of fish stored at 4 ℃, inhibit the growth of
Staphylococcus aureus and oxidative rancidity. Increased the shelf life of fish from 3 days to 12 days. When the film color changed from red to yellow, it means the edible end of the fish [
71].
4.3. Milk
Milk, as a nutritious and comprehensive food, contains high quality proteins, oligosaccharides, fats and vitamins. It is also highly susceptible to the growth of microorganisms, carbohydrate fermentation, fatty acid failure and protein denaturation, and thus spoiling nutrition. A lot of milk is wasted due to spoilage during distribution and consumption. Milk stored in supermarkets or at home has to be checked for freshness before consumption. Currently, the commonly used methods to monitor milk freshness are nuclear magnetic resonance, near-infrared spectroscopy, and mid-infrared. Which need expensive equipment and tedious operations [
7]. In this regard, active and intelligent packaging has emerged to provide convenience, real-time monitoring food safety and quality and reduction food waste. Carrageenan/gelatin-based film containing shikonin exhibited terrific against
L. monocytogenes and
E. coli. The film showed pH-dependent color variation, red at pH 2-7, purple at pH 9, blue at pH 10-12. After three months, the film still exhibited good color stability. The film changed from purple to reddish-pink when it immersing in fresh, under decaying, and spoiled milk for 10 min. At the same time, the RCS index, pH and the degree of spoilage of milk corresponded to each other [
64]. Also, Gao et al. prepared indicator film for monitoring milk freshness by incorporating gelatin, blueberry anthocyanins, and Fe
2+ into a corn protein matrix using the electrostatic spinning method. The change of film color was perceptible visually, from purple-black (fresh milk), royal purple (spoiled milk) to purple-red (spoiled milk). At the same time, the color parameters of the film (
L*,
a*, R, G and B) were highly correlated with the pH of the milk during storage [
7]. Moreover, the freshness monitor could reflect by digitized color information via intelligent phone [
67].
5. Summary and future prospects
Compositing anthocyanins can improve the physicochemical properties of the biological matrices, endow the films with antimicrobial and antioxidant capabilities, and have a high potential for developing active and intelligent packaging in rich protein foods. Meanwhile, rich protein foods contain an abundance of fat, the rancidity of lipids would produce undesirable flavors and promote food spoilage. However, fewer studies have been conducted on the inhibition of lipid oxidation and specific spoilage bacteria in rich protein foods. Much of the research on slow release of anthocyanins was stuck in simulated conditions, but the situation inside the actual pouch is much more complex and more worth to research. Therefore, future research should focus on the above issues. The simplified preparation and industrialized production of the active and intelligent film will provide protection for the rapid development of fresh food in electricity suppliers.
Funding
This work was supported by National Natural Science Foundation of China (grant NO. 32072270), Yunnan Fundamental Research Projects (grant NO. 202301AS070014), and Yunnan Department of Science and Technology Innovation Guidance and Technology Enterprise Cultivation Plan (grant NO.202204BP090031).
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aloui, H.; Deshmukh, A.R.; Khomlaem, C.; Kim, B.S. Novel composite films based on sodium alginate and gallnut extract with enhanced antioxidant, antimicrobial, barrier and mechanical properties. Food Hydrocoll. 2021, 113, 1-11. [CrossRef]
- Müller, P.; Schmid, M. Intelligent packaging in the food sector: a brief overview. Foods. 2019, 8 (1), 1-12. [CrossRef]
- Dodero, A.; Escher, A.; Bertucci, S.; Castellano, M.; Lova, P. Intelligent packaging for real-time monitoring of food-quality: current and future developments. Appl Sci-Basel. 2021; 11, 8, 3532. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Fernandez, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: impact on food quality and effect of innovative processing technologies. Compr Rev Food Sci F. 2021, 20(2), 1333–1380. [Google Scholar] [CrossRef] [PubMed]
- Novais, C.; Molina, A.K.; Abreu, R.M.V.; Santo-Buelga, C.; Ferreira, I.C.F.R.; Pereira, C.; Barros, L. Natural food colorants and preservatives: a review, a demand, and a challenge. J Agr Food Chem. 2022, 70(9), 2789–2805. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.F.; Lu, W.W.; Qin, Y.Y.; Cheng, G.G.; Yuan, M.L.; Li, L. An intelligent pH indicator film based on cassava starch/polyvinyl alcohol incorporating anthocyanin extracts for monitoring pork freshness. J Food Process Pres. 2021, 45(10), e15822. [Google Scholar] [CrossRef]
- Gao, R.C.; Hu, H.L.; Shi, T.; Bao, Y.L.; Sun, Q.C.; Wang, L.; Ren, Y.H.; Jin, W.G.; Yuan, L. Incorporation of gelatin and Fe increases the pH-sensitivity of zein-anthocyanin complex films used for milk spoilage detection. Curr Res Food Sci. 2022, 5, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.; Liu, J.; Kan, J.; Liu, J. Active/intelligent packaging films developed by immobilizing anthocyanins from purple sweetpotato and purple cabbage in locust bean gum, chitosan and κ-carrageenan-based matrices. Int J Biol Macromol. 2022, 211, 238–248. [Google Scholar] [CrossRef]
- You, P.; Wang, L.; Zhou, N.; Yang, Y.; Pang, J. A pH-intelligent response fish packaging film: konjac glucomannan/carboxymethyl cellulose/blackcurrant anthocyanin antibacterial composite film. Int J Biol Macromol. 2022, 204, 386–396. [Google Scholar] [CrossRef]
- Huang, H.L.; Tsai, I.L.; Lin, C.; Hang, Y.H.; Ho, Y.C.; Tsai, M.L.; Mi, F.L. Intelligent films of marine polysaccharides and purple cauliflower extract for food packaging and spoilage monitoring. Carbohyd Polym. 2023, 299, 120133. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Bose, I.; Goksen, G.; Roy, S. Himalayan sources of anthocyanins and its multifunctional applications: a review. Foods. 2023, 12(11), 2203. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: antioxidant activity and health effects-A review. J Funct Foods. 2015, 18, 820–897. [Google Scholar] [CrossRef]
- AlMohammed, H.I.; Khalaf, A.K.; Albalawi, A.E.; Alanazi, A.D.; Baharvand, P.; Moghaddam, A.; Mahmoudvand, H. Chitosan-based nanomaterials as valuable sources of anti-leishmanial agents: a systematic review. Nanomaterials-Basel. 2021, 11(3), 689. [Google Scholar] [CrossRef]
- Avila, L.B.; Barreto, E.R.C.; Moraes, C.C.; Morais, M.M.; Rosa, G.S.D. Promising new material for food packaging: an active and intelligent carrageenan film with natural jaboticaba additive. Foods 2022, 11(6), 792. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Ghasemlou, M.; Ariffin, F.; Singh, Z.; Al-Hassan, A.A. Natural anthocyanins: sources, extraction, characterization, and suitability for smart packaging. Food Packaging Shelf. 2022, 33, 100872. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Rambabu, K.; Banat, F.; Mittal, V. Natural antioxidants-based edible active food packaging: an overview of current advancements. Food Biosci. 2021, 43, 101251. [Google Scholar] [CrossRef]
- Wu, C.; Jiang, H.; Zhao, J.; Humayun, M.; Wu, S.; Wang, C.; Zhi, Z.; Pang, J. A novel strategy to formulate edible active-intelligent packaging films for achieving dynamic visualization of product freshness. Food Hydrocolloid. 2022, 133, 107998. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.H.; Chi, X.W.; Zhang, R.; Lu, Y.H.; Wang, Z.Y.; Dong, Q.; Ding, C.X.; Yang, R.L.; Jiang, L. Highly efficient extraction of mulberry anthocyanins in deep eutectic solvents: insights of degradation kinetics and stability evaluation. Innov Food Sci Emerg. 2020, 66, 102512. [Google Scholar] [CrossRef]
- Jovanovic, M.S.; Krgovic, N.; Zivkovic, J.; Stevic, T.; Zdunic, G.; Bigovic, D.; Savikin, K. Ultrasound-assisted natural deep eutectic solvents extraction of bilberry anthocyanins: optimization, bioactivities, and storage stability. Plants-Basel, 2022; 11, 20, 2680. [Google Scholar] [CrossRef]
- Jovanovic, M.S.; Krgovic, N.; Radan, M.; Cujic-Nikolic, N.; Mudric, J.; Lazarevic, Z.; Savikin, K. Natural deep eutectic solvents combined with cyclodextrins: a novel strategy for chokeberry anthocyanins extraction. Food Chem. 2023, 405, 134816. [Google Scholar] [CrossRef] [PubMed]
- Myint, K.Z.; Yu, Q.; Qing, J.; Zhu, S.; Shen, J.; Xia, Y. Botanic antimicrobial agents, rrrrtheir antioxidant properties, application and safety issue. Food Packaging Shelf. Aa 2022, 34, 100924. [Google Scholar] [CrossRef]
- Wang, J.; Sun, X.; Zhang, H.; Dong, M.; Li, L.; Zhangsun, H.; Wang, L. Dual-functional intelligent gelatin based packaging film for maintaining and monitoring the shrimp freshness. Food Hydrocolloid. 2022, 124, 107258. [Google Scholar] [CrossRef]
- Adaku, C.; Skaar, I.; Berland, H.; Byamukama, R.; Jordheim, M.; Andersen, O.M. , Anthocyanins from mauve flowers of Erlangea tomentosa (Bothriocline longipes) based on erlangidin-The first reported natural anthocyanidin with C-ring methoxylation. Phytochem Lett. 2019, 29, 225–230. [Google Scholar] [CrossRef]
- Duan, M.X.; Yu, S.; Sun, J.S.; Jiang, H.X.; Zhao, J.B.; Tong, C.L.; Hu, Y.Q.; Pang, J.; Wu, C.H. Development and characterization of electrospun nanofibers based on pullulan/chitin nanofibers containing curcumin and anthocyanins for active-intelligent food packaging. Int J Biol Macromol. 2021, 187, 332–340. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, H.; Qi, D.; Xia, L.; Li, L.; Li, X.; Jiang, S. Multifunctional colorimetric cellulose acetate membrane incorporated with Perilla frutescens (L.) Britt. anthocyanins and chamomile essential oil. Carbohyd Polym. 2022; 278, 118914. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems-An overview. Food Res Int. 2011, 44(2), 499–509. [Google Scholar] [CrossRef]
- Sun, X.H.; Zhou, T.T.; Wei, C.H.; Lan, W.Q.; Zhao, Y.; Pan, Y.J.; Wu, V.C.H. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control. 2018, 94, 155–161. [Google Scholar] [CrossRef]
- da Silva Filipini, G.; Romani, V.P.; Guimarães Martins, V. Biodegradable and active-intelligent films based on methylcellulose and jambolão (Syzygium cumini) skins extract for food packaging. Food Hydrocolloid. 2020, 109, 106139. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Y.; Bai, Y.; Yuan, C.; Wu, C.; Hu, Y. Intelligent gelatin/oxidized chitin nanocrystals nanocomposite films containing black rice bran anthocyanins for fish freshness monitorings. Int J Biol Macromol. 2020, 155, 1296–1306. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.W. Starch and agar-based color-indicator films integrated with shikonin for smart packaging application of shrimp. ACS Food Science & Technology. 2021; 1, 10, 1963–1969. [Google Scholar] [CrossRef]
- Kong, J.; Ge, X.; Sun, Y.; Mao, M.; Yu, H.; Chu, R.; Wang, Y. Multi-functional pH- aaa sensitive active and intelligent packaging based on highly cross-linked zein for the aaa monitoring of pork freshness. Food Chem. 2023, 404, 134754. [Google Scholar] [CrossRef]
- Moura-Alves, M.; Esteves, A.; Ciríaco, M.; Silva, J. A.; Saraiva, C. Antimicrobial and antioxidant edible films and coatings in the shelf-life improvement of chicken meat. Foods. 2023, 12(12), 2308. [Google Scholar] [CrossRef] [PubMed]
- Amic, D.; Davidovic-Amic, D.; Beslo, D.; Rastija, V.; Lucic, B.; Trinajstic, N. SAR aaa and QSAR of the antioxidant activity of flavonoids. Curr Med Chem. 2007; 14, 7, aaa 827–845. [Google Scholar]
- Jing, P.; Bomser, J.A.; Schwartzt, S.J.; He, J.; Magnuson, B.A.; Giusti, M.M. Structure-function relationships of anthocyanins from various anthocyanin-rich extracts on the inhibition of colon cancer cell growth. J Agr Food Chem. 2008, 56(20), 9391–9398. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Kang, J.; Guo, X.; Sun, M.; Li, H.; Bai, H.; Cui, H.; Shi, L. pH-responsive aaa chitosan-based film containing oregano essential oil and black rice bran aaaaanthocyanin for preserving pork and monitoring freshness. Food Chem. 2023, 403, aaaa134393. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Acharya, B.; Korber, D.R. Multilayer photonic films based on interlocked chiral-nematic cellulose nanocrystals in starch/chitosan. Carbohyd Polym. 2022, 275, 118709. [Google Scholar] [CrossRef]
- Boccalon, E.; Viscusi, G.; Lamberti, E.; Fancello, F.; Zara, S.; Sassi, P.; Marinozzi, M.; Nocchetti, M.; Gorrasi, G. Composite films containing red onion skin extract as intelligent pH indicators for food packaging. Appl Surf Sci. 2022, 593, 153319. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, H.; Li, L.; Wang, Q.; Jiang, S.; Chen, M.; Li, X.; Shaotong, J. pH-responsive antibacterial film based polyvinyl alcohol/poly (acrylic acid) incorporated with aminoethyl-phloretin and application to pork preservation. Food Res Int. 2021, 147, 110532. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S. Antimicrobial assessment of polyphenolic extracts from onion (Allium cepa L.) skin of fifteen cultivars by sonication-assisted extraction method. Heliyon, 2020; 6, 11, e05478. [Google Scholar] [CrossRef]
- Becerril, R.; Nerín, C.; Silva, F. Bring some colour to your package: freshness indicators based on anthocyanin extracts. Trends Food Sci Tech. 2021, 111, 495–505. [Google Scholar] [CrossRef]
- Koosha, M.; Hamedi, S. Intelligent chitosan/PVA nanocomposite films containing aaa black carrot anthocyanin and bentonite nanoclays with improved mechanical, aa aaa thermal and antibacterial properties. Prog Org Coat. 2019, 127, 338–347. [Google Scholar] [CrossRef]
- Li, T.; Wang, D.; Ren, L.; Mei, J.; Xu, Y.; Li, J. Preparation of pH-sensitive polylactic acid-naringin coaxial electrospun fiber membranes for maintaining and monitoring salmon freshness. Int J Biol Macromol. 2021, 188, 708–718. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, J.; Zhou, H.; Zhou, S.; Lv, Y.; Cheng, Y.; Tao, Y.; Lu, J.; Wang, H. Biodegradable intelligent film for food preservation and real-time visual detection of food freshness. Food Hydrocolloid. 2022, 129, 107665. [Google Scholar] [CrossRef]
- Gu, R.; Yun, H.; Chen, L.F.; Wang, Q.; Huang, X.J. Regenerated cellulose films with amino-terminated hyperbranched polyamic anchored nanosilver for active food packaging. Acs Appl Bio Mater. 2020, 3(1), 602–610. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, protein and lipid-based natural edible films in food packaging: a review. Carbohyd Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.W.; Lu, H.T.; Ho, Y.C.; Lu, K.Y.; Wang, P.; Mi, F.L. A smart and active film with tunable drug release and color change abilities for detection and inhibition of bacterial growth. Mat Sci Eng C-Mater. 2021; 118, 111396. [Google Scholar] [CrossRef]
- Wagh, R.V.; Khan, A.; Priyadarshi, R.; Ezati, P.; Rhim, J.W. Cellulose nanofiber-based multifunctional films integrated with carbon dots and anthocyanins from Brassica oleracea for active and intelligent food packaging applications. Int J Biol Macromol. 2023, 233, 123567. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.; Ayoub, G.M.; Malaeb, L. Antibacterial activity of chitosan nano-composites and carbon nanotubes: a review. Sci Total Environ. 2019, 668, 566–576. [Google Scholar] [CrossRef]
- Zheng, H.; Deng, W.; Yu, L.; Shi, Y.; Deng, Y.; Wang, D.; Zhong, Y. Chitosan coatings with different degrees of deacetylation regulate the postharvest quality of sweet cherry through internal metabolism. Int J Biol Macromol. 2024, 254, 127419. [Google Scholar] [CrossRef]
- Wang, J. L.; Zhuang, S. T. Chitosan-based materials: preparation, modification and aaaapplication. J Clean Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Zhou, Y.; Li, R.; Jiang, Y.; Alomgir Hossen, M.; Dai, J.; Qin, W.; Liu, Y. Facile fabrication of sandwich-like anthocyanin/chitosan/lemongrass essential oil films via 3D printing for intelligent evaluation of pork freshness. Food Chem. 2022, 370, 131082. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xie, C.; Tang, H.; Hao, W.; Wu, J.; Sun, Y.; Sun, J.; Liu, Y.; Jiang, L. Development, characterization and application of intelligent/active packaging of chitosan/chitin nanofibers films containing eggplant anthocyanins. Food Hydrocolloid. 2023, 139, 108496. [Google Scholar] [CrossRef]
- Luchese, C.L.; Benelli, P.; Spada, J.C.; Tessaro, I.C. Impact of the starch source on aaathe physicochemical properties and biodegradability of different starch-based films. aaaJ Appl Polym Sci. 2018, 135(33), 46564. [Google Scholar] [CrossRef]
- Matheus, J.R.V.; de Farias, P.M.; Satoriva, J.M.; de Andrade, C.J.; Fai, A.E.C. Cassava starch films for food packaging: Trends over the last decade and future research. Int J Biol Macromol. 2023, 225, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Nizam, N.H.M.; Rawi, N.F.M.; Ramle, S.F.M.; Abd Aziz, A.; Abdullah, C.K.; Rashedi, A.; Kassim, M.H.M. Physical, thermal, mechanical, antimicrobial and physicochemical properties of starch based film containing aloe vera: a review. J Mater Res Technol. 2021, 15, 1572–1589. [Google Scholar] [CrossRef]
- Sun, C.; Wei, Z.H.; Xue, C.H.; Yang, L. Development, application and future trends aaaof starch-based delivery systems for nutraceuticals: a review. Carbohyd Polym. aaa 2023, 308, 120675. [Google Scholar] [CrossRef]
- Kanha, N.; Osiriphun, S.; Rakariyatham, K.; Klangpetch, W.; Laokuldilok, T. On-package indicator films based on natural pigments and polysaccharides for monitoring food quality: a review. J Sci Food Agr. 2022, 102(15)), 6804–6823. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, D.; Wei, L.F.; Zhu, W.J.; Yan, X.Q.; Zhou, R.; Din, Z.U.; Ding, W.P.; Ma, T.Z.; Cai, J. Structural and mechanistic insights into starch microgel/anthocyanin complex assembly and controlled release performance. Int J Biol Macromol. 2022, 213, 718–727. [Google Scholar] [CrossRef]
- Jiang, M.H.; Zhang, Y. Biopolymer-based encapsulation of anthocyanins as reinforced natural colorants for food applications. J Agr Food Res. 2023, 11, 100488. [Google Scholar] [CrossRef]
- Li, J.; Zhu, B.F.; Yu, H.D.; Yuan, M.L.; Chen, H.Y.; Qin, Y.Y. Application of pH-indicating film containing blue corn anthocyanins on corn starch/polyvinyl alcohol as substrate for preservation of tilapia. J Food Meas Charact. 2022, 16(6), 4416–4424. [Google Scholar] [CrossRef]
- Chavez-Marquez, E.; Bernedo, M.S.B.; de Jara, E.M.; Quequezana-Bedregal, M. J.; Gutierrez-Oppe, E.E.; Pessôa Filho, P.D.A. Development of intelligent and active potato starch films based on purple corn cob extract and molle essential oil. Int J Biol Macromol. 2023, 242, 125080. [Google Scholar] [CrossRef]
- Bao, Y.; Cui, H.; Tian, J.; Ding, Y.; Tian, Q.; Zhang, W.; Wang, M.; Zang, Z.; Sun, X.; Li, D.; Si, X.; Li, B. Novel pH sensitivity and colorimetry-enhanced anthocyanin indicator films by chondroitin sulfate co-pigmentation for shrimp freshness monitoring. Food Control. 2022, 131, 108441. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of gelatin/carrageenan-based color-indicator film integrated with shikonin and propolis for smart food packaging applications. ACS Applied Bio Materials. 2020, 4(1), 770–779. [Google Scholar] [CrossRef]
- Liu, J.; Yong, H.M.; Liu, Y.P.; Qin, Y.; Kan, J.; Liu, J. Preparation and characterization of active and intelligent films based on fish gelatin and haskap berries (Lonicera caerulea L.) extract. Food Packaging Shelf. 2019; 22, 100417. [Google Scholar] [CrossRef]
- Hematian, F.; Baghaei, H.; Nafchi, A.M.; Bolandi, M. Preparation and characterization of an intelligent film based on fish gelatin and anthocyanin to monitor the freshness of rainbow trout fish fillet. Food Sci Nutr. 2023, 11(1), 379–389. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Zhang, Y.; Lu, D.; Gao, W.; Zhao, Q.; Shi, X. Multifunctional intelligent film integrated with purple sweet potato anthocyanin and quercetin-loaded chitosan nanoparticles for monitoring and maintaining freshness of shrimp. Food Packaging Shelf. 2023, 35, 101022. [Google Scholar] [CrossRef]
- Bekhit, A.E.A.; Giteru, S.G.; Holman, B.W.B.; Hopkins, D.L. Total volatile basic nitrogen and trimethylamine in muscle foods: Potential formation pathways and effects on human health. Compr Rev Food Sci F. 2021, 20(4), 3620–3666. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Langroodi, A.M.; Molaei, R.; Mahmoudian, A. Chitosan-starch film containing pomegranate peel extract and essential oil can prolong the shelf life of beef. Meat Sci 2020, 163, 108073. [Google Scholar] [CrossRef]
- Firouz, M.S.; Mohi-Alden, K.; Omid, M. A critical review on intelligent and active packaging in the food industry: Research and development. Food Res Int. 2021, 141, 110113. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of active/intelligent food packaging film containing leaf aaaextract for shelf life extension of chicken/fish during chilled storage. Food aaaPackaging Shelf. 2020, 24, 100506. [Google Scholar] [CrossRef]
- Liu, X.X.; Song, X.S.; Gou, D.J.; Li, H.L.; Jiang, L.; Yuan, M.L.; Yuan, M.W. A polylactide based multifunctional hydrophobic film for tracking evaluation and maintaining beef freshness by an electrospinning technique. Food Chem. 2023, 428, 136784. [Google Scholar] [CrossRef]
- Wu, L.T.; Tsai, I.L.; Ho, Y.C.; Hang, Y.H.; Lin, C.; Tsai, M.L.; Mi, F.L. Active and intelligent gellan gum-based packaging films for controlling anthocyanins release and monitoring food freshness. Carbohyd Polym. 2021, 254, 117410. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).