1. Introduction
Influenza remains to be a substantial burden on modern public healthcare system. According to the World Health Organization (WHO), up to 10% of adult population and 30% of children are infected every year. Influenza, especially in risk groups, can develop severe forms, which require hospitalization, and eventually leads to death cases [
1,
2,
3,
4]. Meanwhile influenza viruses retain a high pandemic potential, in particular, avian influenza viruses, as well as swine and of swine origin [
5,
6,
7,
8]. Currently, the main approach to prevent influenza is vaccination with inactivated or subunit vaccines administered intramuscularly [
9].
Prior to 2012, seasonal trivalent influenza vaccines included two strains of influenza A virus (A(H1N1), A(H3N2)) and one of the strains of influenza type B (either B1 (Yamagata-like) or B2 (Victoria-like)) [
10]. It should be noted that the selected strains did not always correspond to the seasonal one, moreover, sometimes both strains of the subtype B circulated simultaneously during the epidemical season [
11]. These situations caused a significant decrease in the effectiveness of vaccination during such epidemical seasons when the vaccine strain of influenza B virus did not correspond to the circulating strain and could not provide full protection against the wild-type virus strain line [
12,
13]. In such cases, the effectiveness of trivalent vaccines can be reduced by 20-25% [
14,
15,
16,
17].
These types of vaccines may not be effective against drifted and heterologous strains, because they were not included in the formula. To increase the effectiveness of influenza prevention, tetravalent vaccines were developed to target both lines of influenza B with addition of various adjuvants [
19,
20].
Among one of the most promising adjuvants for vaccines are nanoparticles (NPs) [
21,
22,
23,
24]. Their main advantage is that they are effectively absorbed by antigen-presenting cells [
25]. As a result, an antigen bound to the NPs will be deliberately absorbed by macrophages leading to an increased immune response. Among the spectrum of NPs, the most interesting are adjuvants based on birch bark triterpenoids, in particular, NPs derived from natural pentacyclic triterpene substance – betulin (adjuvant, BET) [
26,
27,
28].
In the present study, we investigated the safety and immunogenicity of a candidate tetravalent influenza vaccine containing corpuscular adjuvant derived from natural betulin. We further examined the immunogenicity compared to the vaccines available on the Russian market.
2. Materials and Methods
2.1. Manufacturing of subunit influenza vaccine containing corpuscular adjuvant derived from natural betulin TetraFluBet (TFB)
The technology of TetraFluBet manufacturing was described previously in patent [
1].
One dose (0.5 ml) of TetraFluBet vaccine containing 200 µg of natural betulin-based corpuscular adjuvant, 5 µg of influenza A (H1N1) virus, 5 µg of influenza A (H3N2) virus, 5 µg of influenza B (Yamagata-like) virus and 5 µg of influenza B (Victoria-like) virus in PBS buffer solution pH 7.3.
2.2. Animals
Female BALB/c mice (12-14 g), guinea pigs (250-340 g), male and female mongrel rats (140-220 g) originally obtained from Andreevka Animal Center (Andreevka, Russian Federation) were used in the study. Animals were fed
ad libitum. All animals were housed according to the Directive 2010/63/EU and the Ethical Guidelines for the Use of Animals in Research [
29,
30].
2.3. Chronic Toxicity
Chronic toxicity was assessed in guinea pigs and mongrel rats. Sexually mature mongrel rats were administered 8 ul of vaccine daily during 10 days (80 ul in total), 3.2 ul of adjuvant daily during 10 days (32 ul in total), and placebo. Sexually mature guinea pigs were administered 13 ul of vaccine daily during 10 days (130 ul in total), 5.2 ul of adjuvant daily during 10 days (52 ul in total), and placebo (0.9% NaCl). The volume of injection was 0.5 ml i.m. Treated and control groups of animals were monitored for 24 days after injection.
Mortality, weight, behavior, appearance, clinical symptoms of intoxication were registered on daily basis. The animals of treated and control groups were sacrificed after 11, 17 and 24 days of the experiment. Necropsy, full body examination, histopathological studies (immunohistochemistry, IHC) were performed after sacrifice.
2.3.1. Evaluation of the body weight
All animals were weighted prior to the administration of the preparation, 24 h after administration of the preparation, thereafter weekly for 2 weeks.
2.3.2. Clinical pathology
The animals in all groups were sacrificed after 11, 17 and 24 days of the experiment. At the time of sacrifice, the brain, heart, kidneys, liver, lungs, adrenal glands, thymus, spleen, lymph nodes (mesenteric and inguinal) and genitals (ovaries and testicles) of the animals of the treated group and control were extracted and weighed. Paired organs were weighed together.
2.3.3. Hematoxylin and Eosin Staining
Extracted organs on days 11, 17, 24 were immediately fixed in 10% neutral buffered formalin for 24 h, followed by embedding in paraffin, cut sections were stained with hematoxylin and eosin (H&E) [
31].
2.3.4. Hematological and biochemical parameters of blood
Hematological and biochemical parameters of blood were assessed by taking blood samples from the tail vein (mongrel rats) or heart (guinea pigs) prior injection after 11, 17 and 24 days.
1 ml of blood was collected in test tubes with a solution of sodium citrate. The numbers of erythrocytes, leukocytes, leukocyte formula, hemoglobin were analyzed. Blood smears were stained according to Romanovsky-Giemsa protocol.
Biochemical parameters (glucose, total protein, creatinine, urea, bilirubin, AST, ALT, cholesterol) were assayed using diagnostic kits (Vital Development Corporation, JSC, RF).
2.4. Protectivity study
Protectivity of TFB vaccine was assessed upon double i.m. administration with 1 or ½ human dose in the lethal influenza model of infection of Balb/c mice with influenza subtypes A/California/7/09 (H1N1)рdm09 (5 and 10 LD50), В/ Phuket/3073/13 (Yamagata lineage) (5 and 10 LD50), adapted to mice for intranasal administration with 50 ul.
Criteria for protectivity evaluation in mouse strains:
Animals were monitored for 14 days after infection. Deaths in treated and control groups were registered daily at the same time during this time period. Body weight was measured daily in each group.
Mortality (M) and Index of Protection (IP) were calculated using obtained data of death rates (formulas №1 and №2)
N1 – death rates during 14 days; N2 – total number of infected animals per group.
M1 – mortality rate in treated group; M2 – mortality rate in control group.
2.4.1. Mouse Lethal Dose (LD50) and Infection Dose (ID)
Titration of virus to determine one mouse Lethal Dose (1LD50) for female Balb/c mice 6-8 weeks old were used. 10-fold serial dilutions of virus from 10
-2 to 10
-5 for influenza subtypes A/California/7/09 H1N1pdm09 and from undiluted to 10
-2 for B/Phuket/3073/13AM with PBS. Animals were infected intranasally under light anesthesia with 50 ul of each dilution (5 mice per dilution). Animals were monitored daily during 14 days post infection, including registration of deaths. LD50 and ID were determined by Reed–Muench method [
15].
2.5. Immunogenicity study
Study was conducted using female Balb/c mice. Tetravalent inactivated subunit adjuvanted influenza vaccine Grippol® QUADRIVALENT (NPO Petrovax Pharm, LLC) and trivalent inactivated split influenza vaccine Flu-M (FSUE SPbSRIVS FMBA of Russia), containing influenza subtypes А and subtype В of Victoria lineage, were used as comparison group (both are approved for the market in the Russian Federation). Animals were immunized twice i.m. in 2-week interval. Group 1 - TFB, Group 2 and 3 - Flu-M and Grippol® QUADRIVALENT, respectively, were immunized by human dose (0.5 ml), Group 4 (Control) was administered 0.9% NaCl (0.5 ml). Immunogenicity in mice was assessed by:
antibody titers in hemagglutination inhibition (HI) assay against subtypes A/Michigan/45/2015(H1N1); A/Singapore/INFIMN-16-/0019/2016(H3N2); B/Colorado/06/2017 (Victoria lineage); B/Phuket/3073/2013 (Yamagata lineage); day 14, day 28;
antibody titers in microneutralization assay against subtypes A/Michigan/45/2015(H1N1); A/Singapore/INFIMN-16-/0019/2016(H3N2); B/Colorado/06/2017 (Victoria lineage); B/Phuket/3073/2013 (Yamagata lineage); day 28.
2.6. Hemagglutination inhibition (HI) assay
Determination of antibodies against influenza subtypes A/Michigan/45/2015(H1N1), A/Singapore/INFIMN-16-/0019/2016(H3N2), B/Colorado/06/2017 (Victoria linage), B/Phuket/3073/2013 (Yamagata linage) was performed in individual sera of treated groups in hemagglutination inhibition (HI) assay. 1 volume of animal serum sample was mixed with 3 volumes of Receptor Destroying Enzyme (RDE). The mixture was incubated at 37°С for 18 h, then heat-inactivated at 56 °С for 30 min. Sera were diluted with PBS from 1:10 to 1:1280. 50 ul of antigen influenza standard were added to each dilution. Plates were mixed gently and incubated for 1 h at RT. Each assay included control of spontaneous agglutination of erythrocytes and control to exclude the presence of chicken red blood cells (cRBCs) hemagglutinins. 1% (v/v) of cRBC suspension was used. The readout of the assay was registered after erythrocyte sedimentation in control wells (30-40 min).
2.7. Microneutralization assay using MDCK cells
Determination of neutralizing antibodies against influenza subtypes A/Michigan/45/2015(H1N1), A/Singapore/INFIMN-16-/0019/2016(H3N2), B/Colorado/06/2017 (Victoria linage), B/Phuket/3073/2013 (Yamagata linage) was performed in individual sera of treated and control groups in microneutralization assay using MDCK cell culture.
1 volume of animal serum sample was mixed with 3 volumes of RDE. The mixture was incubated at 37°С for 18 h, then heat-inactivated at 56 °С for 30 min. Each sample was assayed individually.
2-fold serial dilutions starting from 1:20 in 200 ul of AMEM medium were prepared. 200 ul of virus dilution were added to each sera dilution. 100 (cytopathic dose) CPD50 of virus dilution was used, containing 4 µg/mL TPCK-trypsin in AMEM medium. Samples were mixed gently and incubated for 1 h at RT. 200 ul of mixture were added to the rinsed monolayers in the wells of plates in duplicates. Each microtiter plate included control samples:
Plates were incubated in 5% СО2 at 37 °С for 72 h. 50 ul of cell culture from each well were transferred in U-well microtiter plates. 50 ul of 1% (v/v) cRBCs were added to each well. The highest dilution of virus that achieved complete hemagglutination was considered the titration end point for the specific virus. Geometric mean titer (GMT) lower than <1:20 was quantified as 1:10.
2.8. Ethics Statement
The animal experimental protocols used in this study were approved by the Ethics Committee of the I.I. Mechnikov Research Institute for Vaccines and Sera (protocol No. 6, April 2, 2018). Animals were maintained in accordance with the Directive of the European Parliament and of the Council 2010/63/ EU dated 22 September 2010 on the protection of animals used for scientific purposes and the Sanitary Rules for the design and maintenance of experimental biological clinics in the Russian Federation (1045-73).
2.9. Statistical analysis
Statistical analyses were performed using GraphPad Prism (Version 5.0, La Jolla, CA, USA). Geometric mean titer, mean, SEM were calculated for quantitative data. GMT, body weight comparison was performed using one-way ANOVA, and Dunn post-hoc test with adjustment for multiple comparisons of non-normally distributed continuous data between groups. Groups of data were compared between tested groups and days. Normal distribution and homogeneous variance were tested for all the variables. Throughout this article, asterisks denote significant differences at *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
3. Results
3.1. Chronic toxicity assay
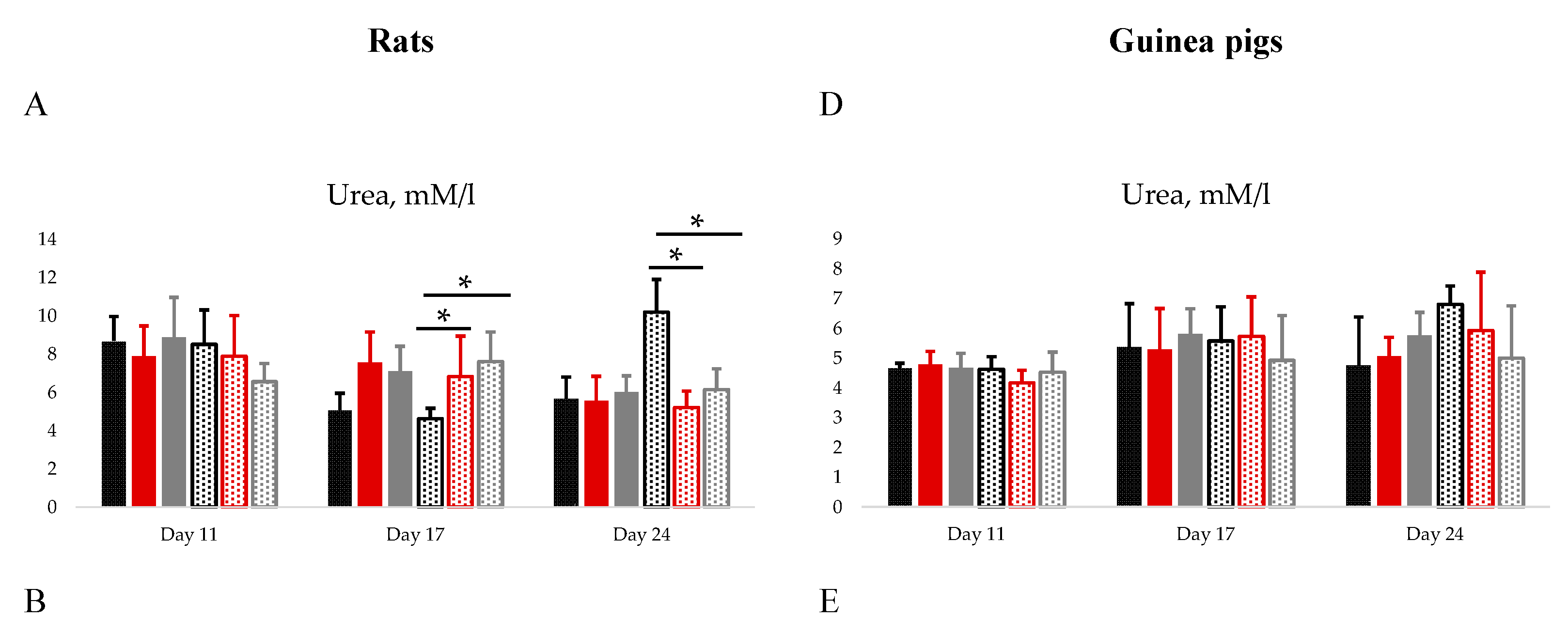
Chronic toxicity study demonstrated no alterations in behavior, fur condition, motor activity. Weight loss, decreased appetite, mortality were not elicited during the assay upon vaccine administration. Organs of treated animals were examined macroscopically and microscopically by IHC (Figures S1.1–S1.72).
On day 11
th AST (
Figure 1B) and monocyte (
Table S1) levels were significantly higher in TetraFluBet treated female rats. On day 17
th and 24
th the level of urea in female group of rats treated with adjuvant and TetraFluBet demonstrated significant differences compared to placebo group (
Figure 1A). Cholesterol (
Figure 1C) and hemoglobin (
Table S1) decreased by 27%
Adj/74%
TFB and 11%
Adj /17%
TFB in female rats treated with adjuvant and TetraFluBet, respectively, on day 17
th. On day 24
th erythrocytes, leukocytes, hemoglobin, lymphocytes, neutrophils, eosinophils, monocytes (
Table S1), glucose, total protein, creatinine, ALT, bilirubin (
Figure S1A–E) were within physiological reference ranges (
Table S3) and did not show any differences.
On day 11
th the number of erythrocytes (
Table S2) for female guinea pigs were significantly elevated by 2 times compared to placebo.
Administration of both vaccine and adjuvant did not elicit pathological, including pronounced dystrophic and necrobiotic, changes in animal organs. Hematological (
Tables S1 and S2) and biochemical blood parameters (glucose, total protein, creatinine, urea, bilirubin, AST, ALT, cholesterol) of animals (
Figure 1,
Figure S2) did not reveal significant differences before and after injection of vaccine and adjuvant, all values were within the physiological limits for corresponding species (rats, guinea pigs).
3.2. Protectivity study
Maximum body weight changes and mortality of treated and control animals after infection by influenza subtype A/California/7/09(H1N1)рdm09 with
5LD50 are presented in
Table 1. Control group of mice lost 20.9% of body weight. Mice immunized by TetraFluBet and comparison vaccine lost 3.1/7.9% and 6.1% of body weight, respectively. Significant differences (р<0.0001) in weight loss were observed between control group and immunized groups (TetraFluBet both doses, comparison vaccine) upon infection by 5LD50 of influenza subtype A/California/7/09 (H1N1)рdm09.
1 and ½ vaccination doses of TetraFluBet provided 100% and 90% of protectivity, respectively, and showed significant difference compared to control group (р<0.0001) (
Table 1). Comparison vaccine Grippol® QUADRIVALENT also provided 100% of protectivity in animals. Index of protection (IP) against
5LD50 of influenza subtype A/California/7/09(H1N1)рdm09 for all the vaccines in 1 dose was 100%.
Maximum body weight changes and mortality of treated and control animals after infection by influenza subtype A/California/7/09(H1N1)рdm09 with
10LD50 are presented in
Table 1. Control group of mice lost 18.1% of body weight. Mice immunized by TetraFluBet lost 5.3%/5.8% of body weight. Significant differences (р<0.001) in weight loss were observed between control group and immunized groups (TetraFluBet both doses, comparison vaccine) upon infection by 10LD50 of influenza subtype A/California/7/09(H1N1)рdm09.
1 and ½ vaccination doses of TetraFluBet provided 100% of protectivity, respectively, and showed significant difference from control group (р<0.0001) (
Table 1). Comparison vaccine Grippol® QUADRIVALENT also provided 100% of protectivity in animals. IP against infection with 10LD50 of influenza subtype A/California/7/09(H1N1)рdm09 for all vaccines in 1 dose was 100%.
Maximum body weight changes and mortality of treated and control animals after infection by influenza subtype B/Phuket/3073/13AM with 5LD50 and 10LD50 are presented in
Table 2. Control group of mice lost 18.3% and 22.0% of body weight, respectively. Mice immunized by 1 and ½ doses of TetraFluBet and Grippol® QUADRIVALENT lost from 17% to 21.8% of body weight. Significant differences (р<0.05) in weight loss were observed between control group and immunized group (TetraFluBet ½ dose) upon infection by 5LD50 of influenza subtype B/Phuket/3073/13AM.
IP against 5LD50 of influenza subtype B/Phuket/3073/13AM for TetraFluBet in 1 and ½ dose, Grippol® QUADRIVALENT was 75%, 87.5, and 62.5%, respectively
1 and ½ vaccination dose of TetraFluBet provided 60-80% of protectivity, and showed significant difference from control group (р<0.04) (
Table 2). IP against infection with 10LD50 of influenza subtype B/Phuket/3073/13AM for TetraFluBet was 60-80%.
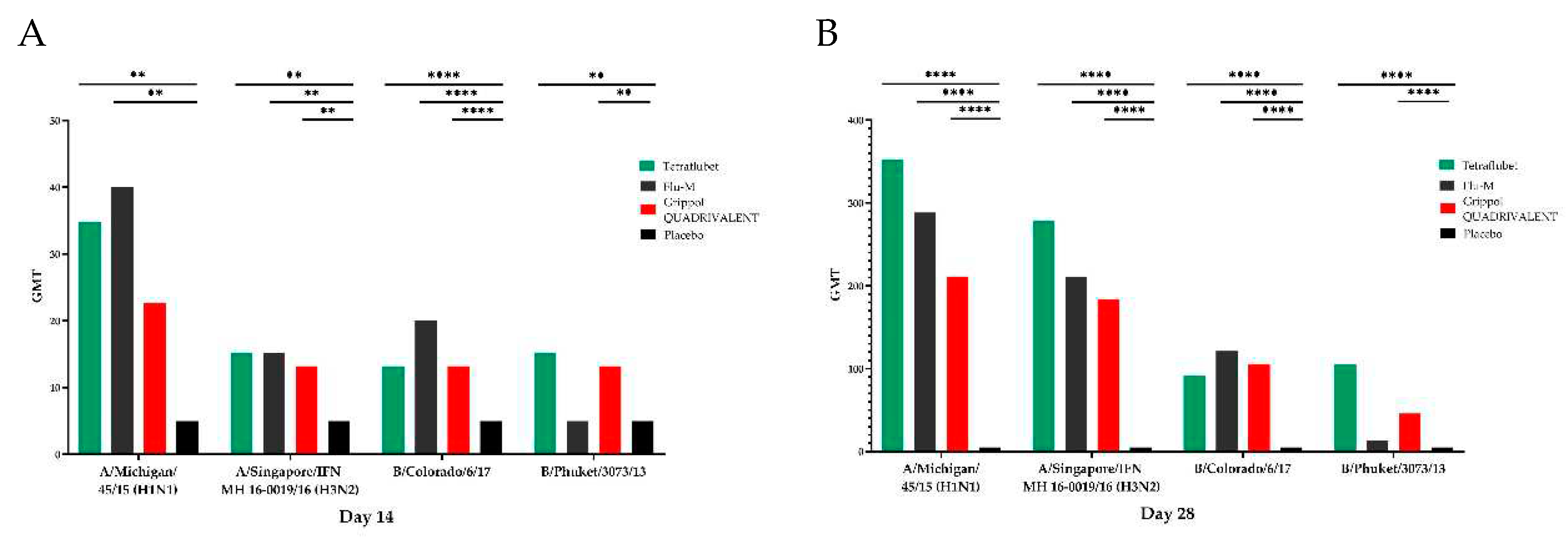
3.3. Immunogenicity
HI assay GMTs in response to influenza subtype A/ Michigan /45/15(H1N1) are presented at
Figure 2. At day 14
th of the study GMT of TetraFluBet group was 34.8 and increased to 352.2 by day 28
th. No significant differences were observed between GMTs of group immunized with 1 vaccine dose of TetraFluBet on day 14
th and day 28
th of the study (
Figure 2A,B) and GMTs of comparison groups (Flu-M: GMT
day14 = 40, GMT
day 28 = 288.4; Grippol® QUADRIVALENT: GMT
day14 = 22.7, GMT
day 28 = 211.1). GMTs of all immunized groups were significantly higher than control placebo group (day 14
th р<0.01; day 28
th р<0.0001).
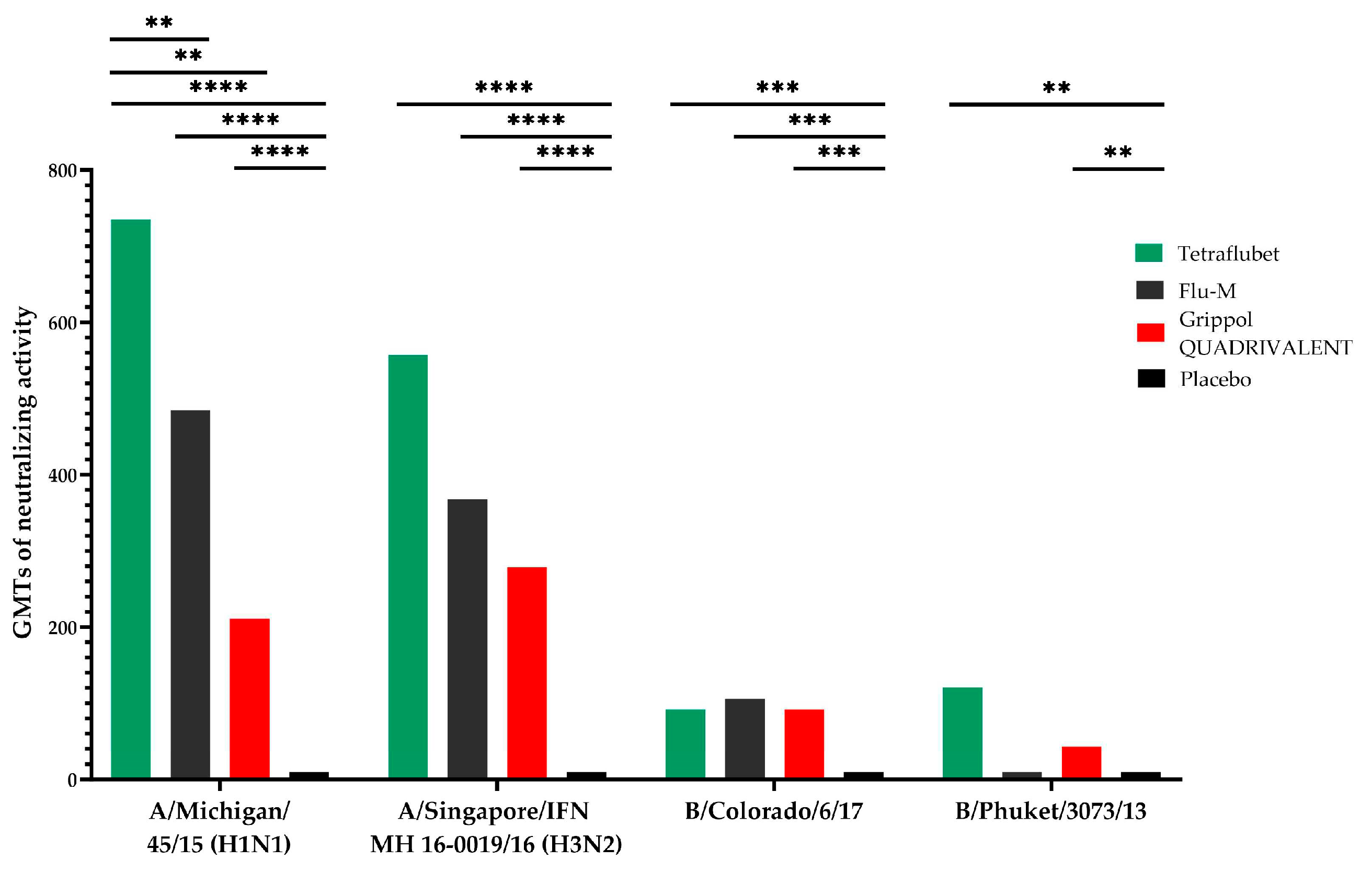
Microneutralization assay GMTs in response to influenza subtype A/ Michigan /45/15(H1N1) are presented at
Figure 3. GMTs of neutralizing activity in response to A/ Michigan /45/15(H1N1) was significantly (р<0.0001) increased by 3.5-4 times in TetraFluBet group in comparison to Grippol® QUADRIVALENT. GMTs of neutralizing antibodies of all immunized groups were significantly higher than control placebo group (р<0.0001).
HI assay GMTs in response to influenza subtype A/Singapore/INFIMN-16-/0019/2016 (H3N2) and B/Colorado/6/17 are presented at
Figure 2. No significant differences were detected between treated animal groups. GMTs were higher in comparison to placebo control (р<0.01).
The same results were obtained for microneutralization assay GMTs in response to influenza subtype А/Singapore/IFN MH 16-0019/16 (H3N2) and B/Colorado/6/17 (
Figure 3). GMTs of neutralizing antibodies of all immunized groups were significantly higher than control placebo group (р<0.0001 and р<0.0004, respectively) without any differences between treated animal groups.
HI assay GMTs in response to influenza subtype B/ Phuket /3073/2013 are presented at
Figure 2. By day 14
th and day 28
th of the study GMTs in groups immunized with TetraFluBet and Grippol® QUADRIVALENT were significantly increased in comparison to Flu-M group and placebo. Since Flu-M vaccine is trivalent, and does not include influenza subtype B/ Phuket /3073/2013. However, after 2
nd immunization GMT of Flu-M group (13.2) was higher than in placebo group.
Microneutralization assay GMTs in response to influenza subtype B/Phuket/3073/13 on day 28
th are presented at
Figure 3. GMTs of neutralizing antibodies of groups immunized with TetraFluBet and Grippol® QUADRIVALENT were significantly increased in comparison to placebo control (p≤0.01) and Flu-M group. GMT of TetraFluBet group was increased by 3 times in comparison to group immunized Grippol® QUADRIVALENT with (р<0.0001).
4. Discussion
Chronic toxicity study did not reveal any side effects of candidate TetraFluBet vaccine. No toxic effects on hematological and biochemical blood parameters were revealed in rats’ and guinea pigs’ sera. All statistically significant variations of values were in physiological reference ranges and proved the absence of pathological deviations.
Immunogenicity study of candidate TetraFluBet vaccine in comparison with commercial vaccines in murine vaccination model showed that titers of specific antibodies against all 4 influenza subtypes had no statistically significant differences after both 1st and 2nd immunizations. TetraFluBet demonstrated the same immunogenicity potential as commercially available products, and with regards to influenza subtype B GMTs of neutralization antibodies were higher than in comparison vaccine.
Results of specific immunity assessment by HI and microneutralization assays after vaccination with TetraFluBet confirmed the formation of a high antibody levels against all 4 virus strains, included in the TetraFluBet formulation. Animals vaccinated with comparison vaccines also showed high antibody levels against virus strains, included in their contents. Whereas neutralizing antibody activity in response to subtypes A/Michigan/45/15(H1N1) and B/Phuket/3073/13 of animals, immunized with 1 dose of TetraFluBet was significantly increased in comparison to commercial vaccines. TetraFluBet GMT response in both assays was in a dose-dependent manner.
1 vaccination dose of TetraFluBet and comparison vaccines provided 100% protection of treated with 5 and 10LD50 of A/California/7/09(H1N1)рdm09 experimental animals. We noted a consistent trend for a higher protectivity of 1 and ½ vaccination doses of TetraFluBet after infection by subtype B/Phuket/3073/13 than in comparison vaccines, however, the result was not statistically significant.
Preclinical immunogenicity and protectivity study of betulin-adjuvanted tetravalent influenza vaccine confirmed specific activity with respect to all influenza virus subtypes included in the formulation, showed the same immunogenicity as the commercially available vaccines, thus it can be recommended for clinical studies applicable for commercial vaccines (single i.m. injection with vaccine containing actual seasonal influenza strains).
Supplementary Materials
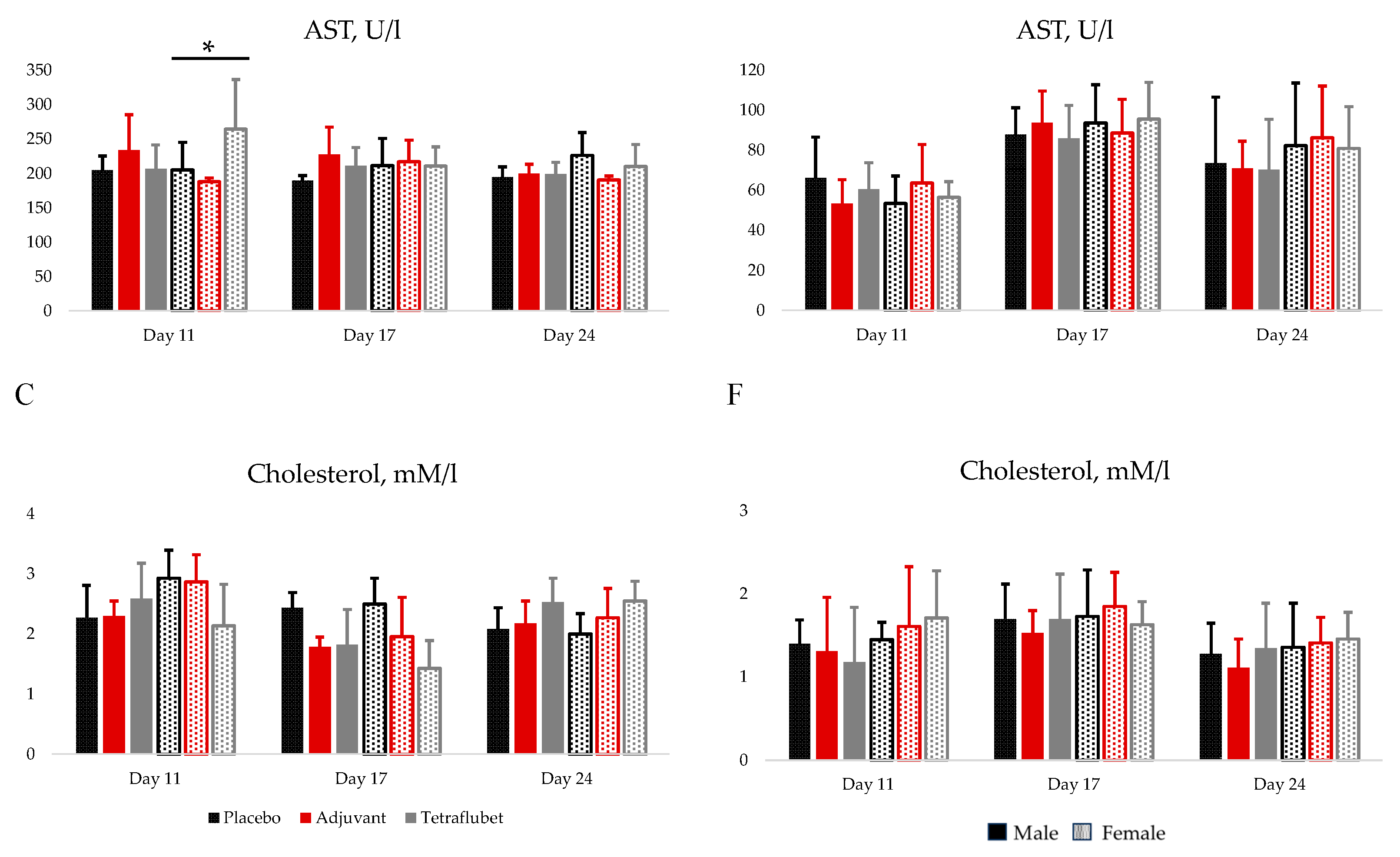
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Figure S1.1–S1.72 Chronic Toxicity (IHC) in Rats and Guinea Pigs. Table S1 Hematological blood parameters and the leukocyte formula of rats (n=210). Mean±SE. Table S2 Hematological blood parameters and the leukocyte formula of guinea pigs (n=100). Mean±SE. Table S3 Physiological range of values of hematological and biochemical blood parameters and the leukocyte formula of rats and guinea pigs. [Mean-2SD; Mean+2SD]. Figure S2 Biochemical blood parameters (Glucose, total protein, ALT, bilirubin, cholesterol) of rats (A, B, C, D, E) and guinea pigs (F, G, H, I, J) on days 11, 17 and 24. Data are depicted as Mean±2SD.
Author Contributions
Conceptualization, I.K. and A.I.; methodology, investigation, A.N., M.S., E.R.-R., N.M, A.B., O.B.; writing—original draft preparation, I.T. and A.K.; writing—review and editing, I.T. and A.K.; visualization, I.T.; project administration, I.K.
Funding
30% of this research was supported by Ministry of Science and Higher Education of the Russian Federation “Pharma-2020”.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of the I.I. Mechnikov Research Institute for Vaccines and Sera (protocol No. 6, April 2, 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Maria Sukhova for preparation graphic data and Taras Ivanishin for administrative support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Krasilnikov I.V., Ivanov A.V., Belyakova O.V., Nikolaeva A.M., Pogodin P.I. Method for producing tetravalent subunit influenza vaccine. RU 2740751 C1; 2021.
- Krasilnikov IV, Kudriavtsev AV, Vakhrusheva AV, Frolova ME, Ivanov AV, Stukova MA, Romanovskaya-Romanko EA, Vasilyev KA, Mushenkova NV, Isaev AA. Design and Immunological Properties of the Novel Subunit Virus-like Vaccine against SARS-CoV-2. Vaccines, 2022;10(1):69. [CrossRef]
- Krasilnikov IV, Kudriavtsev AV, Vakhrusheva AV, Frolova ME, Ivanov AV, Stukova MA, Romanovskaya-Romanko EA, Vasilyev KA, Mushenkova NV, Isaev AA. Design and Immunological Properties of the Novel Subunit Virus-like Vaccine against SARS-CoV-2. Vaccines, 2022;10(1):69. [CrossRef]
- Karpova, L. S., et al. Analysis of the 2016 influenza epidemic and the 2009 pandemic based on materials from two WHO National Centers in the Russian Federation. Epidemiology and vaccine prevention, 2016;15.4 (89): 4-12.
- Karpova, L. S., et al. Analysis of the 2016 influenza epidemic and the 2009 pandemic based on materials from two WHO National Centers in the Russian Federation. Epidemiology and vaccine prevention, 2016;15.4 (89): 4-12. [CrossRef]
- Freidl, Gudrun S., et al. Influenza at the animal–human interface: a review of the literature for virological evidence of human infection with swine or avian influenza viruses other than A (H5N1). Eurosurveillance, 2014;19.18:20793. [CrossRef]
- Kida, Hiroshi, et al. Potential for transmission of avian influenza viruses to pigs. Journal of general virology, 1994; 75.9: 2183-2188. [CrossRef]
- Thacker, Eileen, and Bruce Janke. Swine influenza virus: zoonotic potential and vaccination strategies for the control of avian and swine influenzas. The Journal of infectious diseases, 2008; 197.Supplement_1: S19-S24. [CrossRef]
- Antigenic and genetic characteristics of influenza A(H5N1) and influenza A(H9N2) viruses and candidate vaccine viruses developed for potential use in human vaccines. Global Alert and Response (GAR), 2010.
- Grohskopf, Lisa A., et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)--United States, 2012-13 influenza season, 2012.
- Meeting of the WHO working group on polymerase chain reaction protocols for detecting subtype influenza A viruses. Weekly Epidemiological Record (WER), 2010; 85 (46): 453–460.
- Goldstein, Edward, et al. Predicting the epidemic sizes of influenza A/H1N1, A/H3N2, and B: a statistical method. PLoS medicine, 2011;8.7: e1001051. [CrossRef]
- Caini, Saverio, et al. Epidemiological and virological characteristics of influenza B: results of the Global Influenza B Study. Influenza and other respiratory viruses, 2015;9: 3-12. [CrossRef]
- Wright, P.F. & Neumann, G. & Kawaoka, Y. Orthomyxoviruses, Fields virology, 2013; 1186-1243.
- Bandell, Allyn R., and Eric Simoes. Live attenuated influenza vaccine tetravalent: a clinical review. Expert Review of Vaccines, 2015;14.7: 963-973. [CrossRef]
- Christopher S. Ambrose, Myron J. Levin. The rationale for quadrivalent influenza vaccines. Human Vaccines & Immunotherapeutics, 2012; 8:1, 81–88. [CrossRef]
- Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine. 2010;28 Suppl 4:D45-53. [CrossRef]
- Joyce H S You 1, Wai-Kit Ming, Paul K S Chan. Cost-effectiveness of quadrivalent influenza vaccine in Hong Kong - A decision analysis. Hum Vaccin Immunother 2015;11(3):564-71. [CrossRef]
- Shichinohe, S.;Watanabe, T. Advances in Adjuvanted Influenza. Vaccines. Vaccines. 2023;11,1391. [CrossRef]
- Krasilnikov, I. & Ivanov, A. & Nikolaeva, A. & Belyakova, O. & Shevchenko, E. & Mikhailova, N. & Leneva, I. & Zverev, Vitaly. Preclinical study of immunogenicity of adjuvanted quadrivalent subunit influenza vaccine. Journal of microbiology, epidemiology and immunobiology 2022; 99. 300-308.
- Alfagih IM, Aldosari B, AlQuadeib B, Almurshedi A, Alfagih MM. Nanoparticles as Adjuvants and Nanodelivery Systems for mRNA-Based Vaccines. Pharmaceutics 2020;13(1):45. [CrossRef]
- Mody, Karishma T., et al. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale, 2013;5.12:5167-5179.
- Zhao, Liang, et al. Nanoparticle vaccines. Vaccine, 2014;32.3: 327-337. [CrossRef]
- Smith, Josiah D., Logan D. Morton, and Bret D. Ulery. Nanoparticles as synthetic vaccines. Current opinion in biotechnology, 2015;34: 217-224. [CrossRef]
- Joyce H S You 1, Wai-Kit Ming, Paul K S Chan. Cost-effectiveness of quadrivalent influenza vaccine in Hong Kong - A decision analysis. Hum Vaccin Immunother 2015;11(3):564-71. [CrossRef]
- Cichewicz R.H., Kouzi S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev. 2004; V. 24(1)90–114. [CrossRef]
- Chen, Yingying, et al. Facial synthesis and bioevaluation of well-defined OEGylated betulinic acid-cyclodextrin conjugates for inhibition of influenza infection. Molecules 2022;27.4:1163. [CrossRef]
- Rios, Jose Luis, and Salvador Manez. New pharmacological opportunities for betulinic acid. Planta medica 2018 84.01: 8-19. [CrossRef]
- Directive 2010/63/EU Of the European parlament and of the council of 22 September 2010.
- Smith JA, van den Broek FAR, Canto Martorell J, Hackbarth H, Ruksenas O, Zeller W. Principles and practice in ethical review of animal experiments across Europe: Summary of the report of the FELASA Working Group on Ethical Evaluation of Animal Experiments. Lab Anim 2007; 41:143-160. [CrossRef]
- Cardiff, Robert D., Claramae H. Miller, and Robert J. Munn. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc 2014;2014.6: 655-658. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).