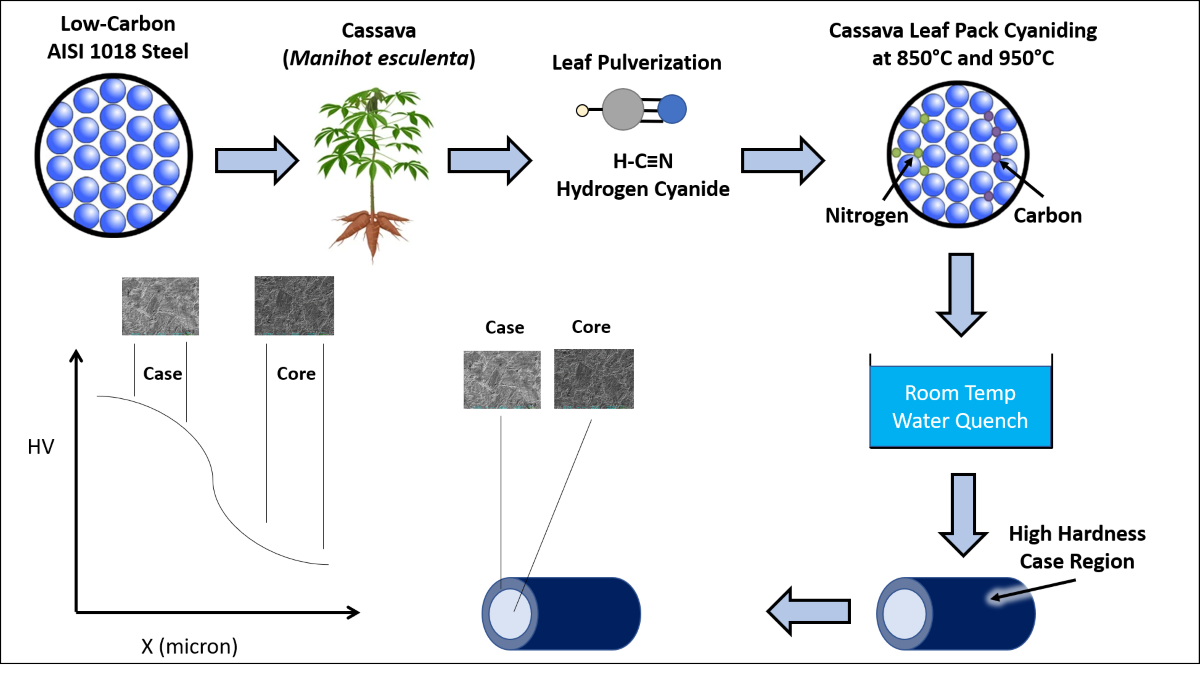
1. Introduction
Many developing countries have an acute shortage of spare parts and components for maintenance work, especially in the automobile industry. With the high local exchange rates, the importation of such spare parts in these countries has become virtually unfeasible [1].
Gear teeth, camshafts, and some other automobile components need good surface hardness to resist wear in combination with considerable interior toughness to withstand impact. These components are generally made by surface or case hardening of mild steel. Case hardening is an immensely broad area of study, spanning many modes of processing. Case hardening of steel is a vastly utilized surface treatment process. It relies on the diffusion of carbon and/or nitrogen into the surface of steel specimens followed by suitable heat-treatment. The process is conducted by putting the steel in a carbon-rich and/or nitrogen-rich atmosphere at elevated temperatures. This results in the interstitial diffusion of the carbon and/or nitrogen into the steel surface so that the steel surface may be hardened thereby increasing the material surface hardness and wear resistance. A major drawback of conventional case hardening is the potential hazardous nature of the process, particularly the use of sodium cyanide salts.
To address some of the disadvantages of conventional case hardening, alternative methods utilizing organic biomass have been considered. In some cases, coffee grinds, sea shells, and cow bones have been shown to successfully case harden mild steel [
2,
3,
4]. Pack-cyaniding of mild steel using the cyanide product from the cassava leaf has been previously investigated [
5]. These greener processes, as additional options for enhancing mechanical properties of materials, has been demonstrated by previous studies through quantification of surface microhardness and carbon concentration profiles [
6,
7,
8].
The objective of this work is to assess the major parameters which influence the pack-cyaniding effectiveness of cassava leaf with respect to holding time, processing temperature, and medium/environment. A common practice in pack-cyaniding is to add an energizer or catalyst to the pulverized cassava leaves - typically barium carbonate (BaCO3). In this study, the effect of adding such energizer or catalyst is also addressed.
1.1. Decomposition of Cassava
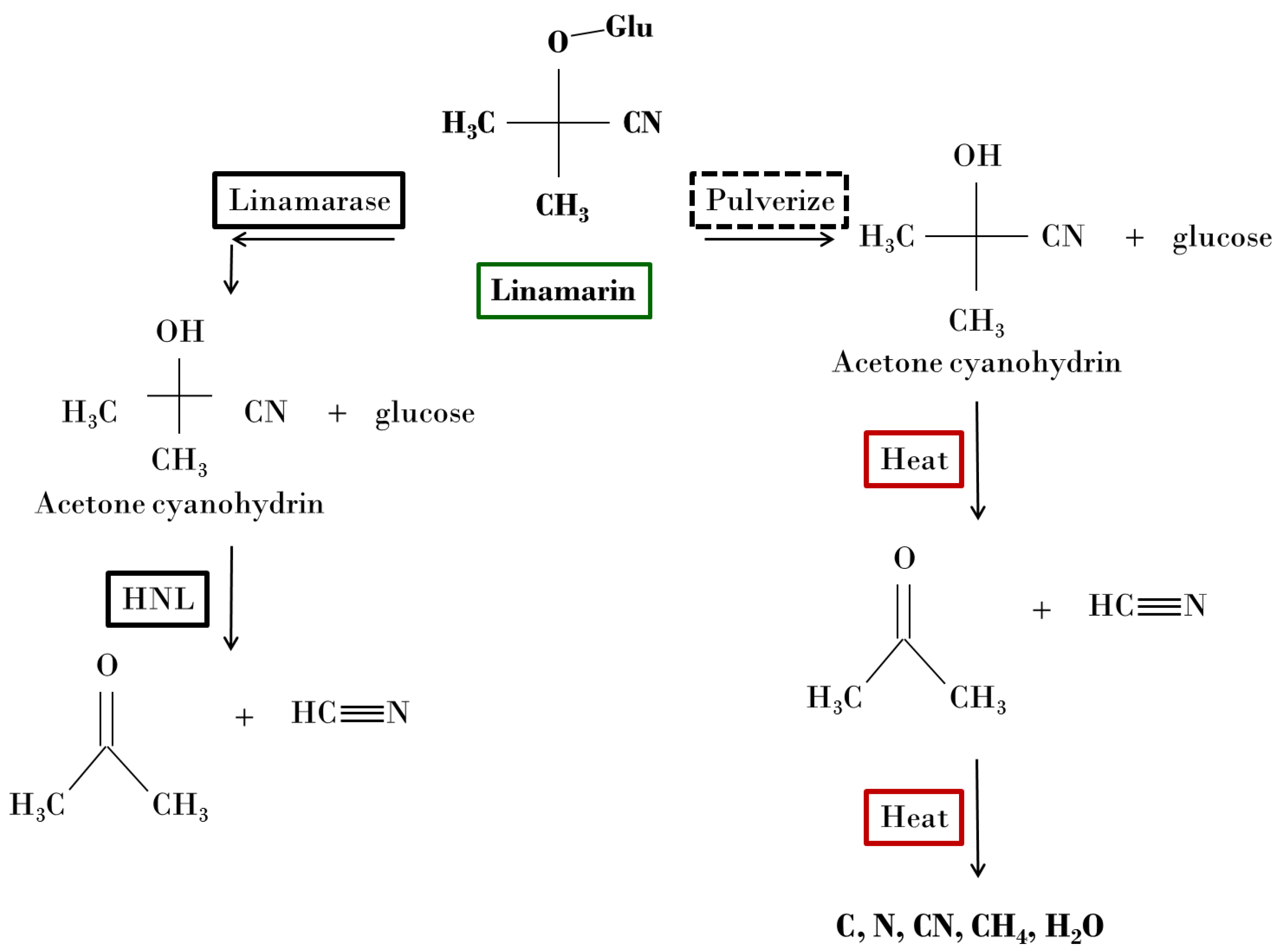
Cassava (
Manihot esculenta) is an important food and source of starch to at least 500 million people in tropical and subtropical regions [
9]. Upon tissue disruption of cassava leaf, the release of cyanogenic glucosides (CNGs) is activated [
10]. This acts as a defense mechanism for the plant against herbivore attack [
11]. Linamarin, the dominant type of CNG, are brought into contact with the enzyme linamarase when cassava leaf is subjected to chewing or grinding, to produce the compound acetone cyanohydrin [
12]. Acetone cyanohydrin is then enzymatically broken down by hydroxynitrile lyase (HNL) [
13]. Finally, the HNL degrades to produce acetone, and hydrogen cyanide, along with additional chemical by-products as outlined in
Figure 1. The hydrogen cyanide (HCN) produced becomes the active ingredient for the pack-cyaniding and case-hardening of steel.
As a substitution for the enzymatic decomposition of cassava, elevated temperature can act as a decomposing mechanism in the production of acetone and hydrogen cyanide from cassava. This is similar to the tissue disruption that occurs during herbivore attack. The biological breakdown is replaced by a thermochemical breakdown. In the case of the cassava leaf case hardening process, heat acts as a substitute to the linamarase enzyme. High temperatures (850˚C-950˚C) further reduce acetone and hydrogen cyanide into carbon, nitrogen, methane, and water vapor (
Figure 1). These thermally produced constituents will be explored for pack-cyaniding of AISI 1018 steel.
2. Experimental
Low-carbon AISI 1018 was selected for this study and the chemical composition is given in
Table 1. The steel was machined into cube-shaped pieces of approximately 12 mm length on each side.
Fresh cassava leaves were cut, dehydrated, then pulverized using a mortar and pestle. Pulverization produced an average powder particle size of 0.6 mm. In order to determine if the pulverized cassava leaf had any effect on the properties of the steel, three processing conditions were used as shown in
Table 2. Process 1, which was used as the control, involved heat treatment of the steel in air (with no cassava leaf present). This baseline experiment provided a means to assess the influence of processing media/environment. Process 2 involved heat treatment of the steel in contact with pulverized cassava leaf. Process 3 was carried out in the presence of pulverized cassava leaf with BaCO
3 as energizer (CBC mixture). The ratio of pulverized cassava leaf to energizer was 4:1 by weight.
For processing, samples were packed into a mild steel metal boat. The metal boat lid was sealed using fire clay and placed into a Gallenkomp Muffle Furnace (SXL-1008) at temperatures of 850˚C and 950˚C, for time periods 1 hour and 5 hours (
Table 2).
The Fe-C phase diagram provides important temperature-composition information. When the ferrite-pearlite structure transforms to the austenite phase, this is designated as the A3 transformation temperature. For AISI 1018 steel, the A3 transformation temperature occurs at approximately 870˚C. It is important to recognize that above 870˚C, the AISI 1018 steel completely transforms to austenite phase. Therefore, all samples processed at 950°C had an austenite microstructure during heat treatment. Once removed from the furnace, the steel samples were immediately quenched in room temperature water.
The pack-cyanided samples were prepared for microhardness testing and metallographic examination by cold-mounting and surface grinding to the fineness of P-4000 silicon carbide paper. Polishing was done using a Buehler Vibromet 2 vibratory polisher with 0.25μm OP-S solution for 4 hours. Samples were immersed in a 3% nital etchant, then sprayed with deionized water and air dried. A Tukon 2100 Vickers microhardness tester was used with a 300-gram applied load and a dwell time of 10 seconds to obtain near-surface microhardness profile measurements. This was analyzed with respect to distance/depth from the outer surface. Sample microstructure was examined utilizing the Zeiss 1540 EsB dual beam field emission Scanning Electron Microscope. Energy Dispersive X-ray Spectroscopy (EDS) was performed using the same system. Thermodynamic calculation software was used as a speculative tool to identify phases present in AISI 1018. Computations were performed using the TCFE8 database, which included steel and iron alloys.
3. Results and Discussion
3.1. Microhardness profiles
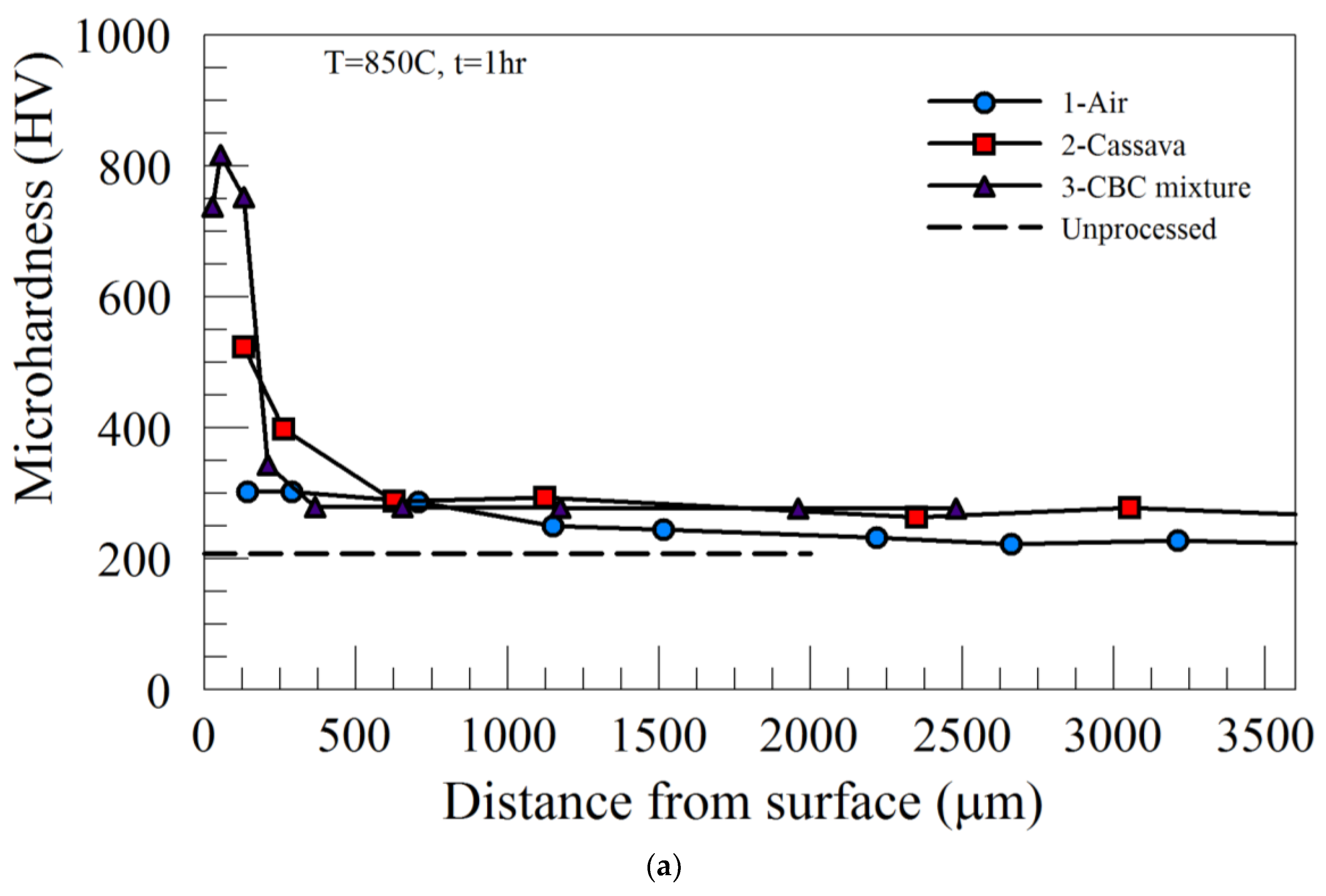
In order to establish a baseline control for the study, low-carbon AISI 1018 steel was heat treated in air at 850˚C and 950˚C and quenched in room temperature water (Process 1). Using Process 1 for any combination of the various experimental conditions (
Table 2), there was no significant dissimilarity in the cross-section microhardness of the steel. For Process 1, average microhardness values of AISI 1018 steel were 243.6 ± 3.3 HV and 272.1± 8.1 HV for 850˚C and 950˚C, respectively. A comparison of the microhardness of all samples subjected to Process 1 heat treatment and the as-received material is also shown in
Figure 2. In addition to being uniform across the thickness, the microhardness profiles of Process 1 materials were very similar to that of the as-received material. This clearly shows that the steel used in this study does not exhibit any form of hardenability without assistance from the medium/environment.
The influence of processing medium/environment on the microhardness profile for heat treatments carried out at 850˚C can be seen in
Figure 2a,b. In contrast to Process 1, the microhardness profiles of both Process 2 and Process 3 are characterized by three regions: high microhardness surface regions (case); a transition region where the microhardness decreased gradually, then finally an unaffected, softer interior region (core). Employing Process 2 for the holding time of 1 hour produced a microhardness value of approximately 534.2 ± 28.8 HV and 245.1± 45.4 HV in the case and core regions, respectively. However, when Process 3 was utilized with the same 1-hour time condition, the case microhardness increased to 852 ± 63.2 HV, while the core remained low at 256.9 ± 50.5 HV. The depth of the case regions for either condition was less than about 600 μm. Increasing the heat treatment time to 5 hours increased the case microhardness marginally, but significantly increased the depth of the case region to more than 700 μm for both Process 2 and Process 3. It is evident that Process 3, with the addition of BaCO
3 energizer to the pulverized cassava leaf (CBC mixture) resulted in higher microhardness in the case region.
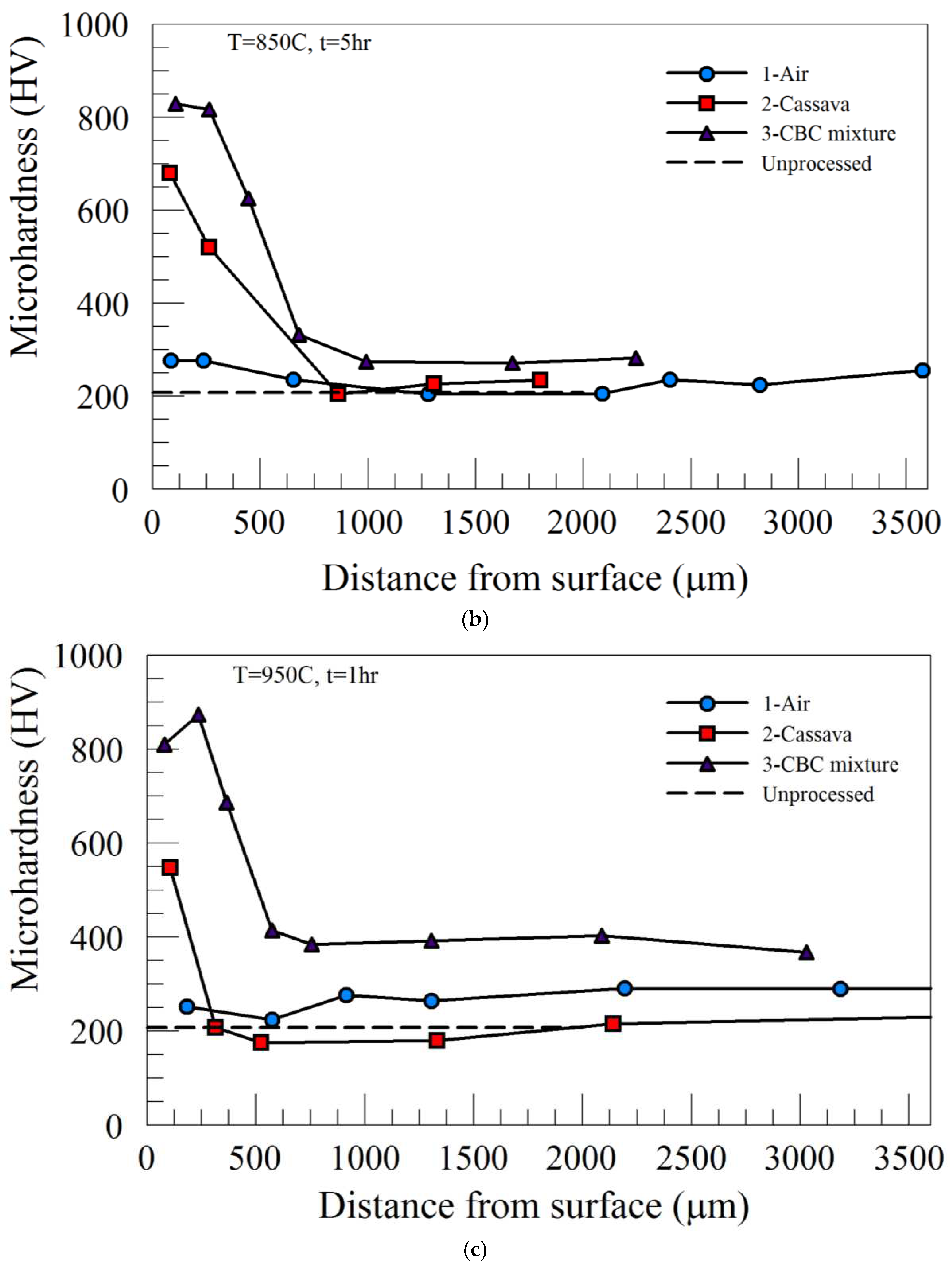
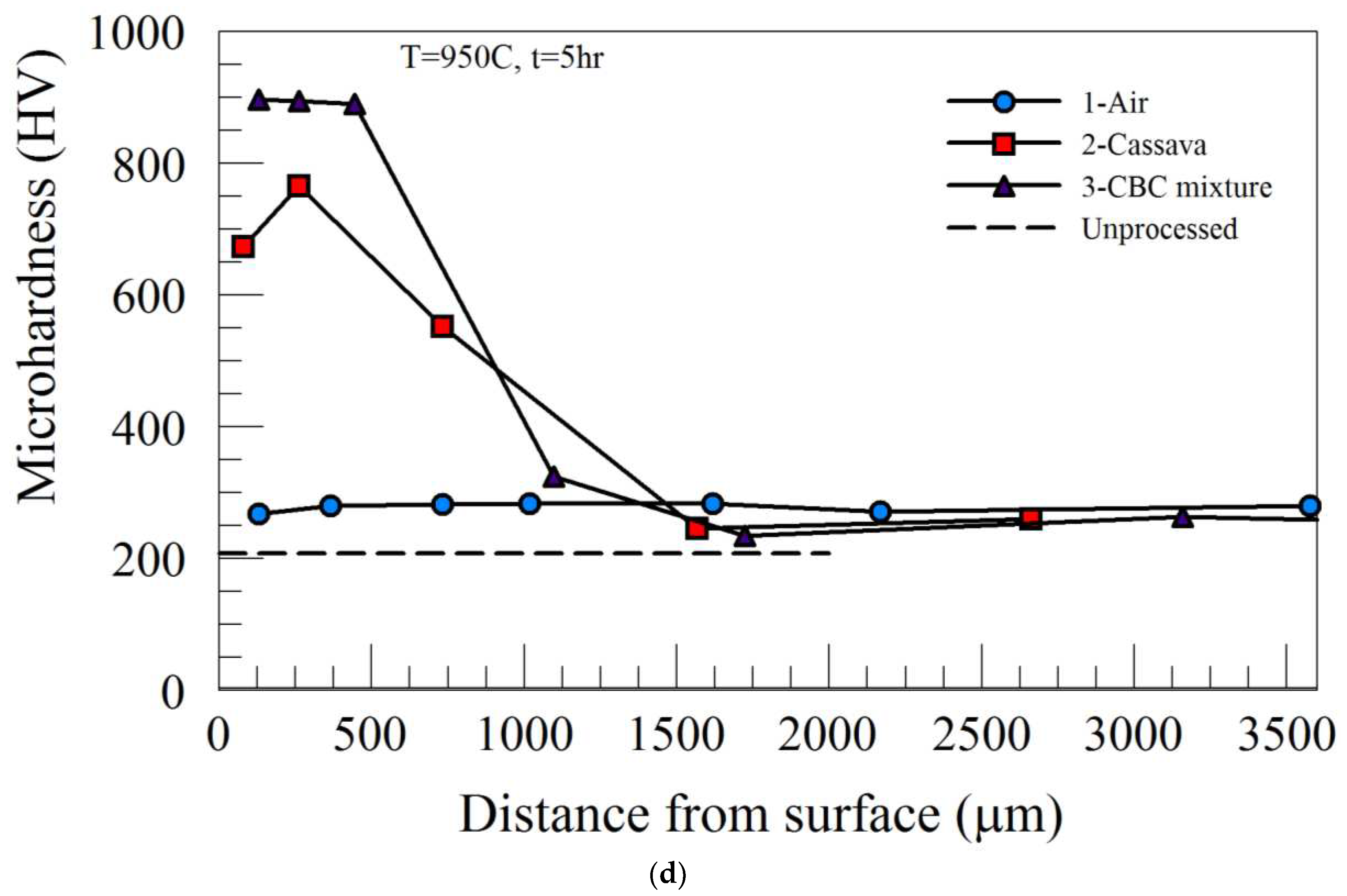
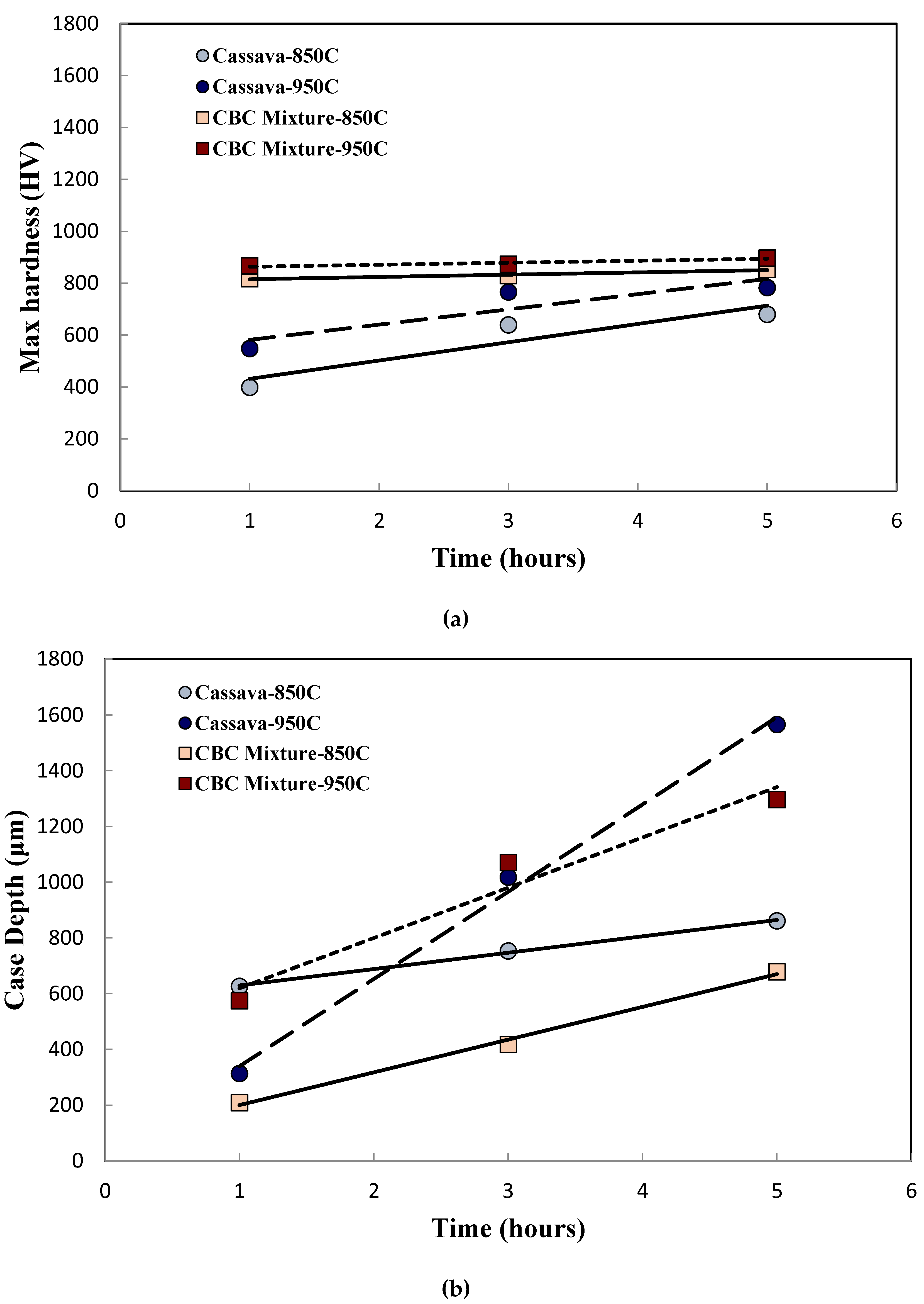
Increasing the processing temperature to 950˚C, (temperature at which complete austenitic steel transformation occurs), produced higher peak microhardness in the case region for both Process 2 and Process 3. However, the microhardness profiles across the specimen thickness and the values of the core microhardness were similar to those of 850˚C. The summary of the peak microhardness and the case depth as a function of processing time for Process 2 and Process 3 are provided in
Figure 3a and
Figure 3b, respectively. While the peak microhardness of Process 2 increased with processing time, there was little or no effect of heat treatment time on the peak microhardness of materials heat-treated using Process 3 (see
Figure 3a). Furthermore, Process 3 (CBC mixture) carried out at 950˚C produced the highest microhardness value (~900 HV), which was about the same regardless of either holding time used in this study.
The experimental parameters of holding time, temperature, and medium/environment were assessed to understand level of effect on the steel sample hardness. For Process 2, the difference in peak microhardness (Δh
max) between 950˚C and 850˚C was ~103 HV, while that for Process 3 was ~45 HV. From
Figure 3b, it can be seen that the case depths resulting from Process 2 and Process 3 increased with heat treatment time. While the magnitude and rate of increase in case depth were more pronounced for processing carried out at 950˚C, those of 850˚C were at a slower rate and gradual. This suggests that the case depth was more dependent on the heat treatment temperature than the processing medium/environment.
3.2. Microstructure
As noted, the A
3 transformation temperature occurs when ferrite-pearlite structure transforms to austenite phase, which is ~ 870˚C for AISI 1018 steel. Therefore, the choice of 850˚C and 950˚C provides an assessment of the effect of processing below and above the A
3 transformation temperature, respectively. The microstructure of AISI 1018 is a combination of ferrite and austenite at 850˚C, while at 950˚C the steel specimen is completely transformed to austenite (γ-iron) [
14]. It is important to recognize that the maximum solubility of carbon in steel at 850˚C and 950˚C is approximately 0.25 wt% and 1.4 wt%, respectively. While a substantial amount of interstitial solute atoms (particularly carbon) will diffuse from the atmosphere into the steel matrix when processed at 950˚C, the converse is true at 850˚C.
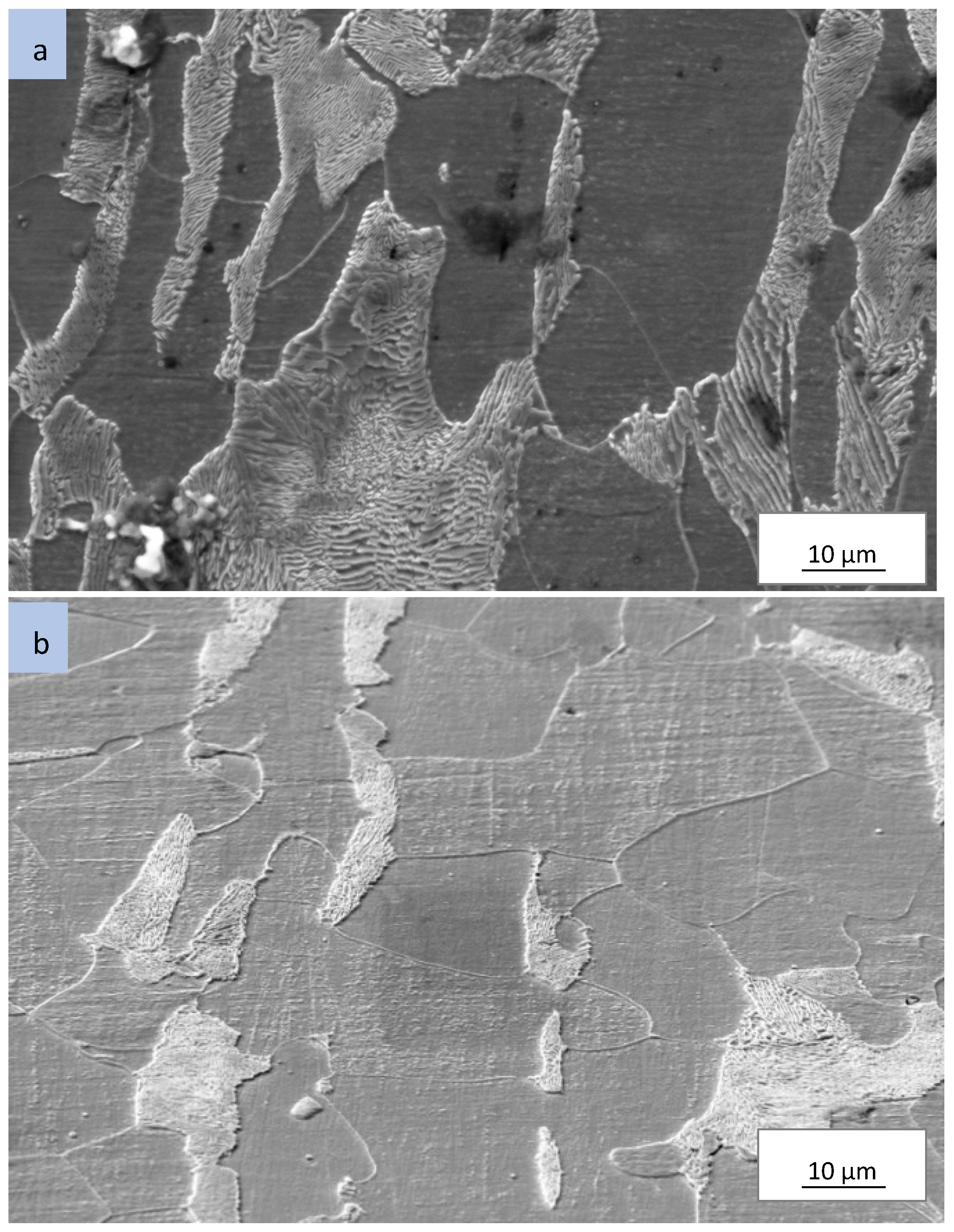
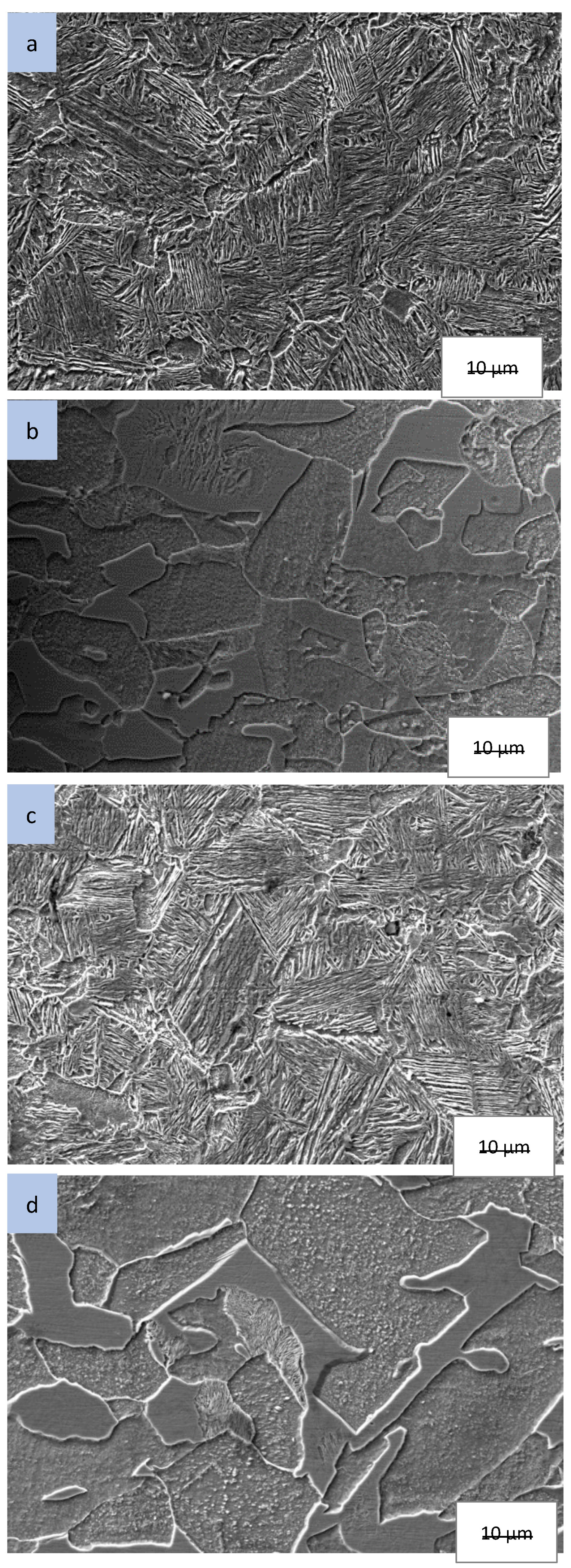
Scanning electron micrographs of as-received and processed AISI 1018 are presented in
Figure 4 and
Figure 5. The as-received microstructure in
Figure 4a is characterized by ferrite and pearlite structure. Upon processing in air only medium (process 1), microstructure characteristics exhibited no significant change as shown in
Figure 4b. Specimens processed in pulverized cassava leaf (process 2-cassava) resulted in the formation of martensite near the surface (case region), shown in
Figure 5a. The microstructure of the core region of the same sample is characterized by ferrite and pearlite (
Figure 5b). Samples processed in pulverized cassava leaf + BaCO
3 (Process 3) resulted in similar microstructure as that of pulverized cassava leaf (Process 2).
The microstructure results correlate well with the microhardness data for all processes. It is evident that the by ferrite and pearlite microstructure was responsible for the low microhardness values present in the core region for all processing conditions. In contrast, the martensite microstructure within the case region produced high microhardness. As martensite is present for Process 2 and Process 3 samples, martensitic transformation is the hardening mechanism.
It has been discussed that the addition of BaCO
3 to CBC mixture as a source of nascent carbon resulted in an increase in maximum hardening. However, processing temperature had a greater effect on the maximum hardness. This can possibly be attributed to the high temperature required for the breakdown of barium carbonate. At approximately 1260 K (~986˚C) begins the onset of the decomposition reaction of barium carbonate: BaCO
3 (solid) = BaO (solid) + CO
2 (gas) [
14]. The CO
2 gas and its derivatives increase the carbon potential of the atmosphere making it greater than the carbon potential at the surface of the specimen. Experimental temperatures did not exceed 950˚C, therefore complete decomposition of barium carbonate was not yet achieved.
It has been established from other studies that microhardness can be used to assess the amount of solute present in the steel matrix. In the absence of precipitation, the microhardness gradient can only be caused by the concentration gradient within the microstructure. Therefore, one can use the microhardness value as a means of assessing the carbon concentration of the material.
3.3. Diffusion of Pulverized Cassava Leaf Media in Steel
The atmosphere at the steel sample surface during Process 2 and Process 3 is rich in carbon and thus presents a concentration gradient for carbon at the surface versus the interior. The carbon concentration gradient can be approximated using Fick’s second law, whose governing equations for the one-dimensional case are:
where C is concentration, D is the diffusion coefficient dependent on temperature but independent of species composition, t is time, and x is distance from material surface. It is assumed the concentration of carbon in the case-hardened steel is proportional to its relative microhardness such that:
where h
s is the maximum microhardness near the contact surface, h
0 is the lowest microhardness of the untreated steel (230 HV), h is the microhardness at any distance x from the contact surface, and let H be defined as the relative microhardness. This assumption is based on literature which demonstrates that through examination of carbon diffusion in steel, carbon content can be replaced by microhardness [
16,
17].
Correlation between concentration and microhardness can yield a simple analytical model to express the effect properties of steel encountered during cassava leaf case hardening. On linearizing Eq. (3) with respect to x
2, a plot of ln(H) versus x
2 for each processing temperature of the experiment is obtained. Diffusion coefficients were calculated from the slopes of these lines and presented in
Table 3. The effect of temperature on diffusivity is apparent. Increasing temperature is the most effective parameter in improving surface hardness and diffusivity growth. In the work of Grube and Gay, plasma carburization was conducted on AISI 1018 at 1050˚C—yielding a diffusivity of 1.6
10
-10 m
2/sec [
18]. This is within 10% difference in comparison to the obtained diffusivity rates of this present work.
From
Figure 3, pulverized cassava leaf as the processing medium (Process 2) produced an increase in surface hardness (
Figure 3a) with a generally large case depth (
Figure 3b). Treatment in the CBC mixture (Process 3) produced a markedly higher microhardness than that of Process 2 (
Figure 3a). CBC Mixture (Process 3) also yielded a slightly thinner case depth, particularly at 850˚C (
Figure 3b).
Table 3 uncovered that CBC mixture (Process 3) rendered a significantly reduced diffusion coefficient in comparison to pulverized cassava leaf (Process 2). The addition of barium carbonate increased the carbon available for diffusion in Process 3; however, it reduced the rate of diffusion by up to a factor of ten.
Development of the case layer can be described using Fick’s second law with the fixed boundary condition, where distance of penetration is proportional to the square root of time, expressed by:
where
is case layer thickness,
is a constant,
is treatment time,
is the diffusion coefficient in case layer, and
is related to
.
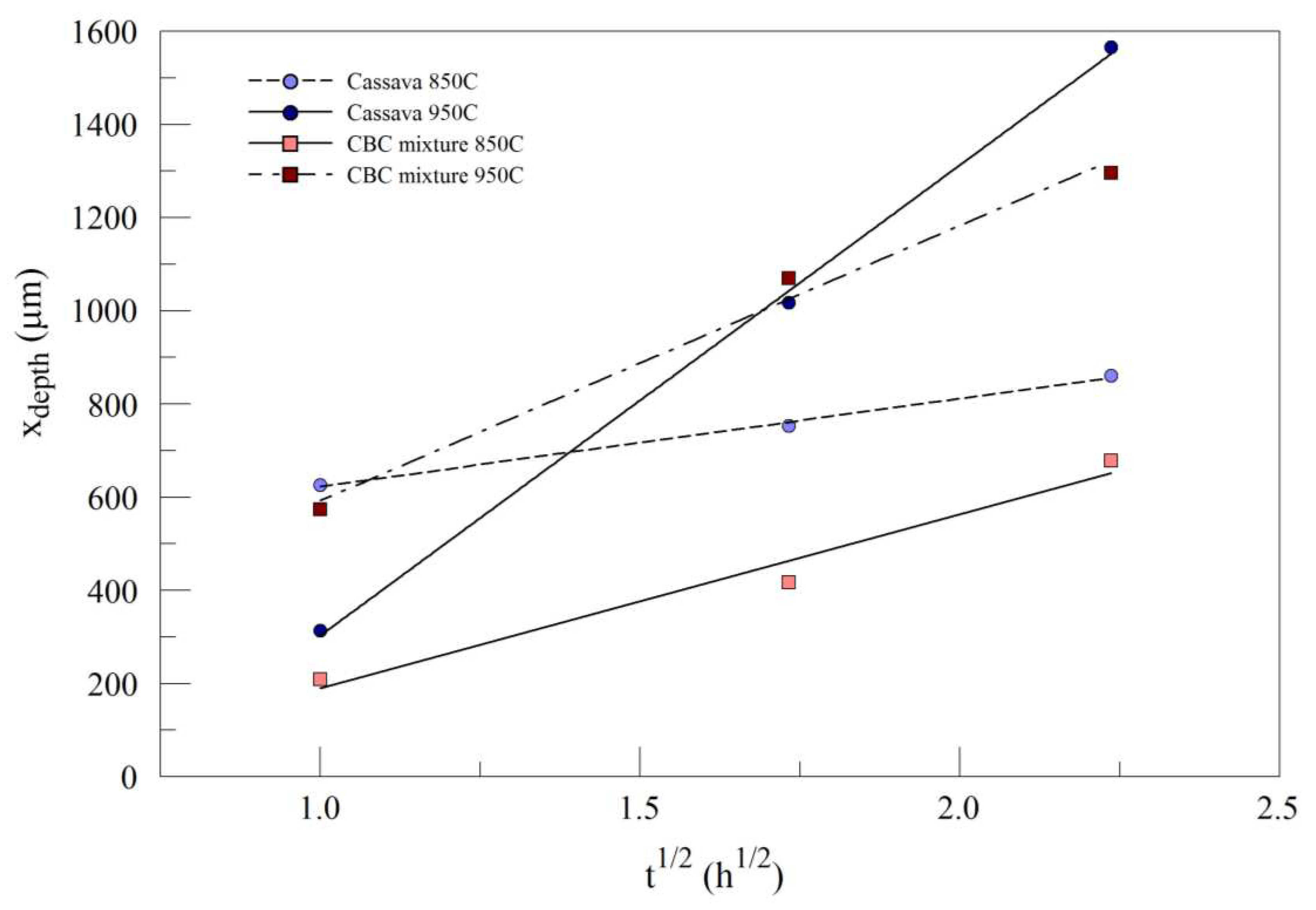
Figure 7 displays the case depth (x
depth) as a function of the square root of time (
) for AISI 1018 processed in pulverized cassava leaf (Process 2) and CBC mixture (Process 3) at 850
oC and 950
oC. As treatment time increases, case depth is increased. Growth kinetics of the case layer can be described by:
where
is in μm, and
is in hours. From Eqs. (5)-(8) it is apparent the K value increases with temperature, which is fitting as the diffusion coefficient is also increasing.
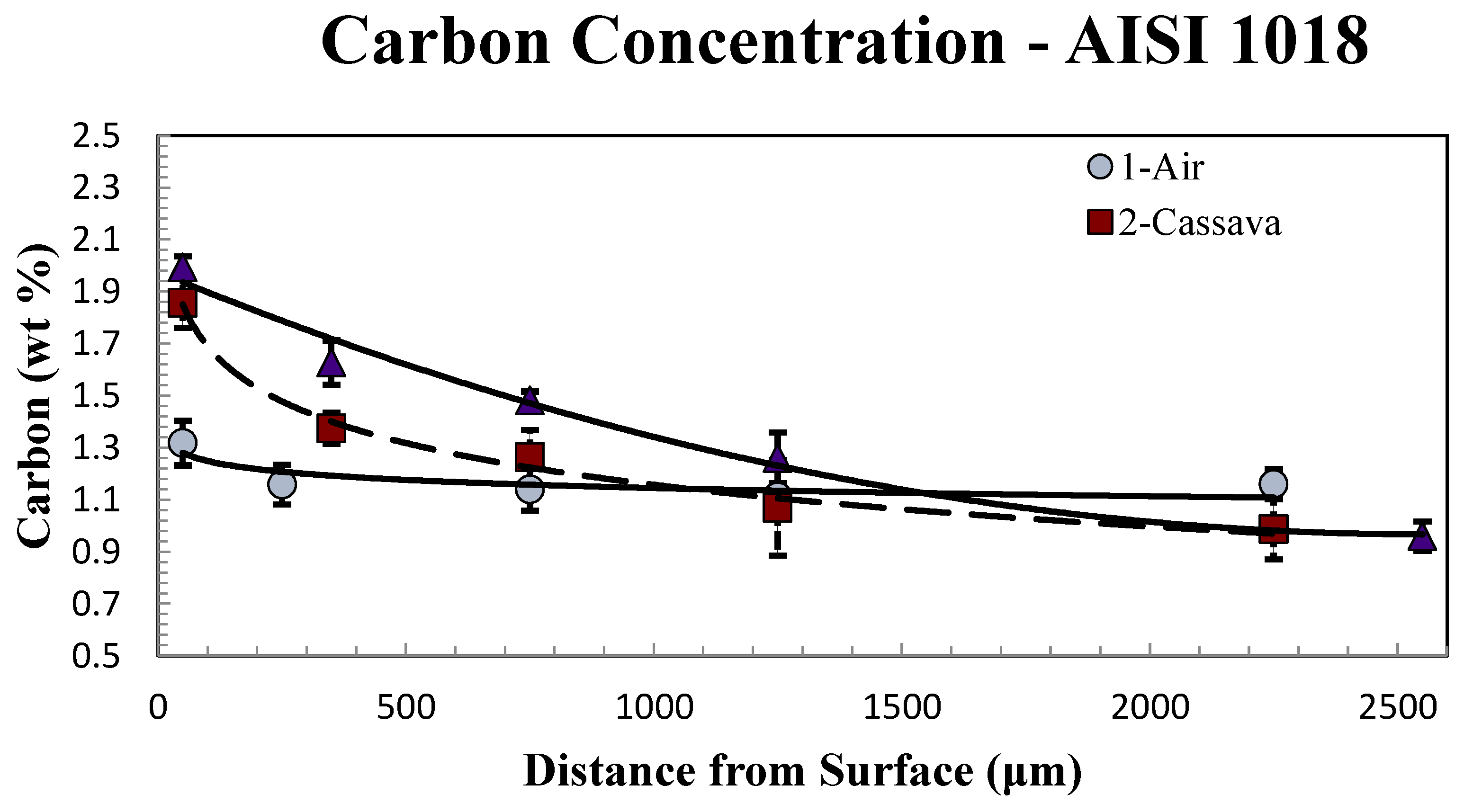
Figure 6.
Carbon concentration with respect to distance from surface of AISI 1018 processed in various media, at treatment temperature 950˚C for 5 hours.
Figure 6.
Carbon concentration with respect to distance from surface of AISI 1018 processed in various media, at treatment temperature 950˚C for 5 hours.
Figure 7.
Case depth () as a function of the square root of time () for AISI 1018 processed in pulverized cassava leaf and CBC mixture, at 850˚C and 950˚C.
Figure 7.
Case depth () as a function of the square root of time () for AISI 1018 processed in pulverized cassava leaf and CBC mixture, at 850˚C and 950˚C.
4. Conclusions
There was little or no change in the microhardness profile of AISI 1018 steel that was heat-treated in an air (Process 1). However, a significant difference in hardness between the surface region and the interior was observed when processed in either pulverized cassava leaf (Process 2) or the mixture of pulverized cassava leaf and BaCO3 (Process 3). This confirms that the environment/medium containing cassava leaves resulted in case hardening.
For the same temperature and time, the Process 3 environment/medium produced higher surface microhardness values when compared to treatment using the Process 2 environment/medium. The average difference in peak microhardness (Δhmax) between Process 3 and Process 2 was above 100HV for all samples.
In Process 3, the addition of barium carbonate (BaCO3) produced the highest peak microhardness values (hmax), and resulted in faster attainment of maximum case hardness in comparison to Process 2.
When AISI 1018 steel completed Process 2 and Process 3, the case depth was found to be lower when holding temperature was below the A3 transformation temperature.
The microstructure of AISI 1018 after Process 2 and Process 3 was characterized by martensite in the case region, while the core region remained a combination of ferrite and pearlite microstructure.
For the same time and temperature, the environment/medium of Process 3 produced a lower rate of diffusion in comparison to Process 2. This was displayed by a shorter distance of penetration in Process 3. Process 2 produced the longest case depth of 1566 μm at 950˚C for 5 hrs.
The cassava leaf used in processing environment/medium increased the amount of carbon solute for case hardening of AISI 1018.
Author Contributions
Conceptualization, P.N.K., A.R.A., E.E.K. and R.G.; Methodology, P.N.K., A.R.A. and R.G.; Validation, R.G., P.N.K. and E.E.K.; Formal Analysis, P.N.K., E.E.K., A.R.A., D.C. and R.G.; Investigation, R.G.; Resources, P.N.K. and A.R.A.; Data Curation, R.G.; Writing – Original Draft Preparation, R.G.; Writing – Review & Editing, P.N.K., E.E.K. and D.C.; Visualization, R.G.; Supervision, P.N.K., A.R.A. and E.E.K.; Project Administration, P.N.K.
Funding
The author R.G. sincerely thanks Florida Agricultural and Mechanical University for financially supporting her graduate doctoral program. The National High Magnetic Field Laboratory (NHMFL) in Tallahassee, Florida, USA provided the scanning electron microscopy SEM facility. For the work conducted in Nigeria, the experimental facility was provided by Prototype Engineering Development Institute (PEDI), a financially supported institute of National Agency for Science and Engineering Infrastructure (NASENI).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgements
Renee Gordon gratefully acknowledges financial support for this research by the Fulbright U.S. Student Program, which is sponsored by the U.S. Department of State. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Fulbright Program and the Government of the United States.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- A.R. Adetunji, "Metallographic Studies of Pack Cyanided Mild Steel Using Cassava Leaves," Materials and Manufacturing Processes, vol. 23, p. 385–390, 2008. [CrossRef]
- G. D. Oliver and K. Cooke, "An example of the infusion of endogenous technology in engineering curricula: the case hardening of mild steel using local Jamaica blue mountain coffee shells," in World Transactions on Engineering and Technology Education, Kingston, Jamaica, 2005.
- P. A. Ihom, "Case hardening of mild steel using cowbone as energiser," African Journal of Engineering Research, vol. 1, no. 4, pp. 97-101, 2013.
- L. Akanji, O. S. Fatoba and A. S. Aasa, "The Influence of Particle Size and Soaking Time on Surface Hardness of Carburized AISI 1018 Steel," British Journal of Applied Science & Technology, vol. 7, no. 1, pp. 37-44, 2015. [CrossRef]
- J. Ibironke, A. Falaiye, T. V. Ojumu, E. A. Odo and O. O. Adewoye, "Case-Depth Studies of Pack Cyaniding of Mild Steel Using Cassava Leaves," Materials and Manufacturing Processes, vol. 19, no. 5, pp. 899-905, 2004. [CrossRef]
- Y. Sun, "Kinetics of low temperature plasma carburizing of austenitic stainless steels," Journal of Materials Processing Technology, vol. 168, pp. 189-194, 2005. [CrossRef]
- C. Wang and A. Mandelis, "Case depth determination in heat-treated industrial steel products using photothermal radiometric interferometric phase minima," NDT&E International, vol. 40, pp. 158-167, 2007. [CrossRef]
- A. Katsamas and G. Haidemenopoulos, "Surface hardening of low-alloy 15CrNi6 steel by CO2 laser beam," Surface and Coatings Technology, vol. 115, pp. 249-255, 1999. [CrossRef]
- J. Cock, Cassava: New Potential for a Neglected Crop, Boulder, CO, USA: Westview Press, 1985. [CrossRef]
- K. Jørgensen, A. V. Morant, M. Morant, N. B. Jensen, C. E. Olsen, R. Kannangara, M. S. Motawia, B. L. Møller and S. Bak, "Biosynthesis of the Cyanogenic Glucosides Linamarin and Lotaustralin in Cassava: Isolation, Biochemical Characterization, and Expression Pattern of CYP71E7, the Oxime-Metabolizing Cytochrome P450 Enzyme," Plant Physiology, vol. 155, pp. 282-292, 2011. [CrossRef]
- M. Zagrobelnya, S. Bak, A. V. Rasmussena, B. Jørgensen, C. M. Naumann and B. L. Møller, "Cyanogenic glucosides and plant–insect interactions," Phytochemistry, vol. 65, pp. 293-306, 2004. [CrossRef]
- N. Narayanan, U. Ihemere, C. Ellery and R. T. Sayre, "Overexpression of Hydroxynitrile Lyase in Cassava Roots Elevates Protein and Free Amino Acids while Reducing Residual Cyanogen Levels," PLoS ONE, vol. 6, no. 7, e21996, pp. 1-11, July 2011. [CrossRef]
- D. Siritunga, D. Arias-Garzon, W. White and R. T. Sayre, "Over-expression of hydroxynitrile lyase in transgenic cassava roots accelerates cyanogenesis and food detoxification," Plant Biotechnology Journal, no. 2, pp. 37-43, 2004. [CrossRef]
- S.-J. Lee, D. K. Matlock and C. J. Van Tyne, "Carbon diffusivity in multi-component austenite," Scripta Materialia, vol. 64, p. 805–808, 2011. [CrossRef]
- S. S. Arvanitidis, "A Study of the Thermal Decomposition of BaCO3," Metallurgical and Materials Transactions B, vol. 27B, pp. 409-416, June 1996. [CrossRef]
- A. Sugianto, M. Narazaki, M. Kogawara, A. Shirayori, S.-Y. Kim and S. Kubota, "Numerical simulation and experimental verification of carburizing-quenching process of SCr420H steel helical gear," Journal of Materials Processing Technology, vol. 209, pp. 3597-3609, 2009. [CrossRef]
- Y. Sarıkaya and M. Onal, "High temperature carburizing of a stainless steel with uranium carbide," Journal of Alloys and Compounds journal, vol. 542, p. 253–256, 2012. [CrossRef]
- W. L. Grube and J. G. Gay, "High-rate carburizing in a glow- discharge methane plasma," Metallurgical Transactions A, vol. 9, no. 10, p. 1421–1429, 1978. [CrossRef]
- D. Siritunga and R. T. Sayre, "Generation of cyanogen-free transgenic cassava," Planta, no. 217, pp. 367-373, 2003. [CrossRef]
- L. Samuels, Light Microscopy of Carbon Steels, Materials Park, OH: ASM International, The Materials Information Society, 1999.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).