Submitted:
05 December 2023
Posted:
06 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- The combination of fine soda-lime glass powders with NaAlO4 aqueous solutions has a great potential for the production of components based on stable Na-alumino-silicate gels;
- The glass-solution interaction led to semi-crystalline gels, comprising hydrosodalite and LTA zeolite; the formation of AlO4 units (stabilized by alkali ions) was confirmed by means of NMR analysis;
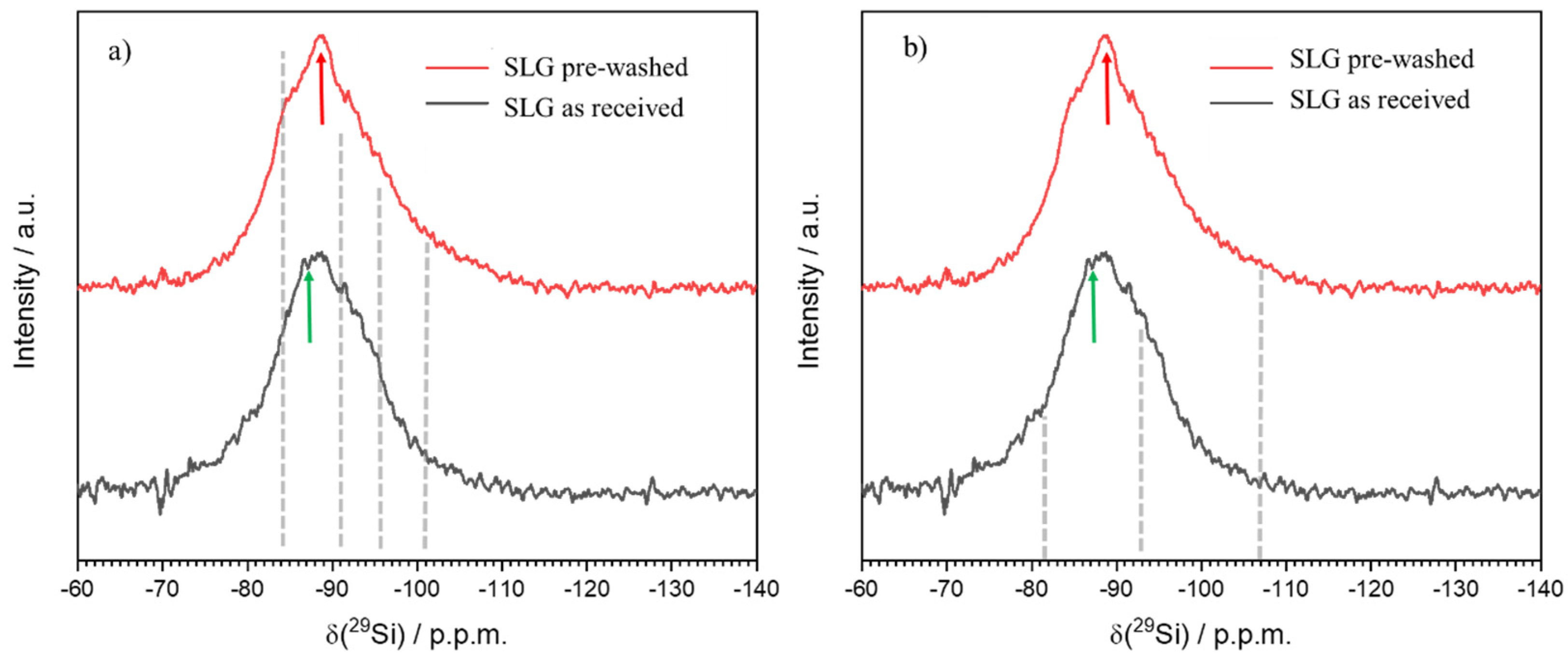
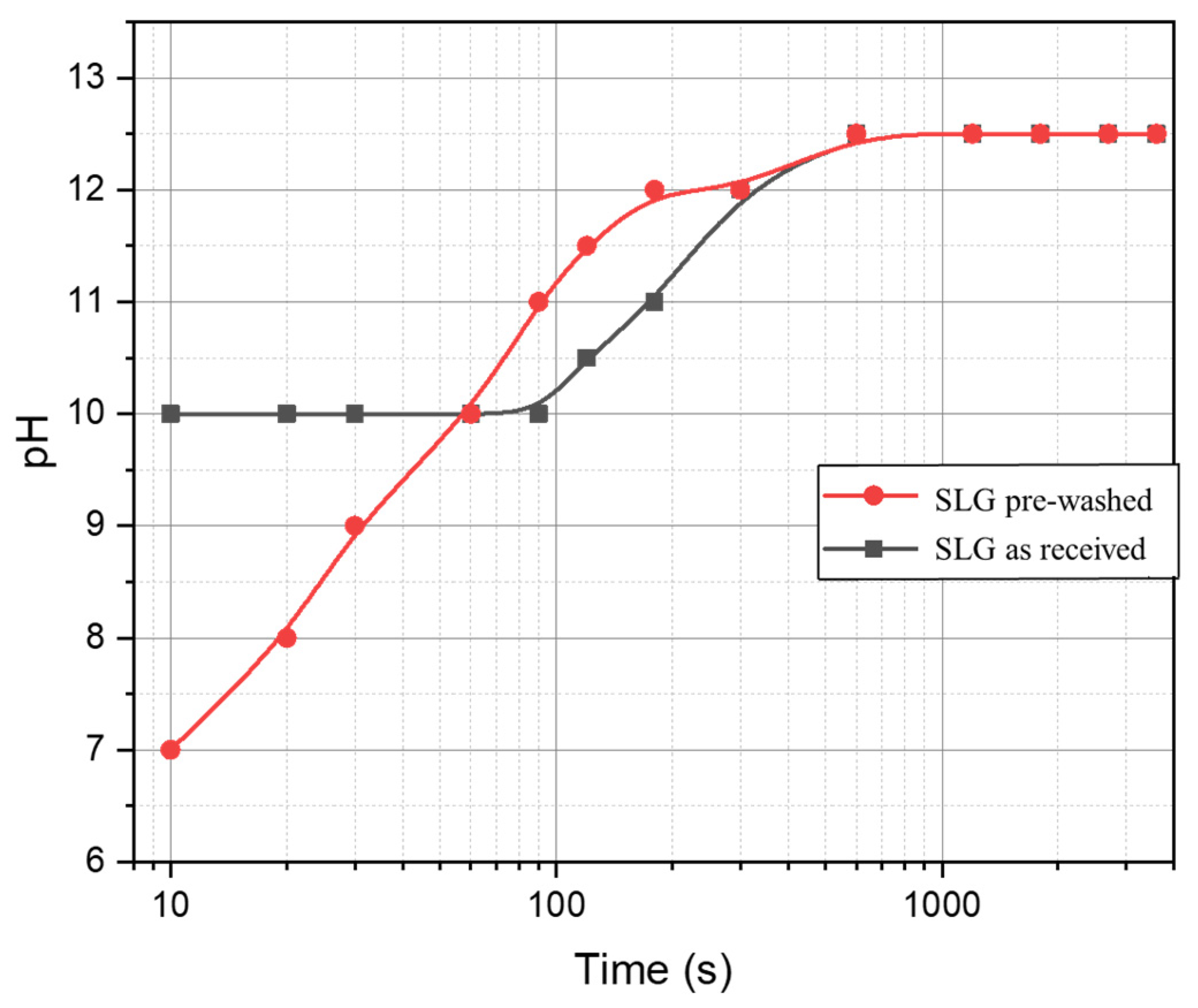
- The process can be tuned by subjecting the SLG glass powders to an acid washing pre-treatment; (surface) de-alkalinized powders led to gels with enhanced content of LTA zeolite, less prone to alkali release;
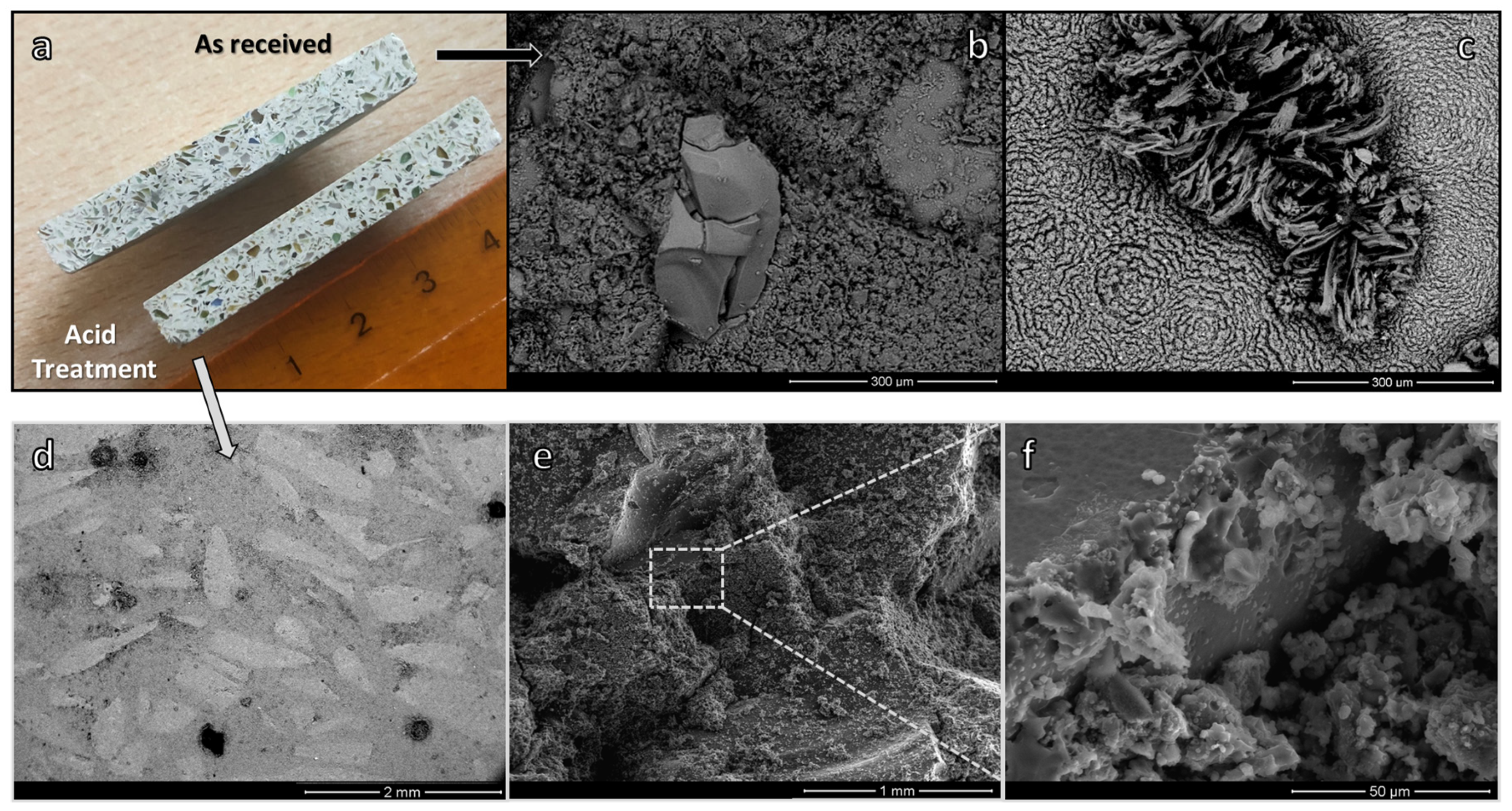
- The sustainability of the process may be significantly enhanced by using fine soda-lime glass powders with NaAlO4 aqueous solutions for the development of a binder phase connecting coarse glass granules, leading to components with pleasant aesthetic appearance; furthermore, Na-carbonate efflorescence was not present when using a binder containing acid-washed soda-lime glass powders.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Silva, R.V.; De Brito, J.; Lye, C.Q.; Dhir, R-K. The role of glass waste in the production of ceramic-based products and other applications: A review. J. Clean. Prod. 2017, 167, 346–364. [Google Scholar] [CrossRef]
- Ramteke, D.D.; Hujova, M.; Kraxner, J.; Galusek, D.; Rincon Romero, A.; Bernardo, E. Up-cycling of ‘unrecyclable ’ glasses in glass -based foams by weak alkali- activation, gel casting and low-temperature sintering. J. Clean. Prod. 2021, 278, 123985. [Google Scholar] [CrossRef]
- Kim, K.; Kim, K.; Hwang, J. Characterization of ceramic tiles containing LCD waste glass. Ceram. Int. 2016, 42, 7626–31. [Google Scholar] [CrossRef]
- Rincon Romero, A.; Giacomello, G.; Pasetto, M.; Bernardo, E. Novel ‘inorganic gel casting’ process for the manufacturing of glass foams. J. Eur. Ceram. Soc. 2017, 37, 2227–34. [Google Scholar] [CrossRef]
- Rincon Romero, A.; Marangoni, M.; Cetin, S.; Bernardo, E. Recycling of inorganic waste in monolithic and cellular glass-based materials for structural and functional applications. J. Chem. Technol. Biotechnol. 2016, 91, 1946–61. [Google Scholar] [CrossRef]
- Taveri, G.; Tousek, J.; Bernardo, E.; Toniolo, N.; Boccaccini, A.R.; Dlouhy, I. Proving the role of boron in the structure of fly-ash / borosilicate glass based geopolymers. Mater. Lett. 2017, 200, 105–8. [Google Scholar] [CrossRef]
- Waguchi, M.; Kato, T.; Imamura, Y.; Yoshida, N.; Aoki, S. Challenge to improve glass melting and fining process. Ceram. Silikaty 2008, 52, 218–24. [Google Scholar]
- Idir, R.; Cyr, M.; Tagnit-Hamou, A. Pozzolanic properties of fine and coarse color-mixed glass cullet. Cem. Concr. Compos. 2011, 33, 19–29. [Google Scholar] [CrossRef]
- Ashby, M.F. Materials and Environment. Buttersworth-Heinemann, Oxford, UK, 2013.
- Fletcher, R.A.; Mackenzie, K.J.D.; Nicholson, C.L.; Shimada, S. The composition range of aluminosilicate geopolymers. J. Eur. Ceram. Soc. 2015, 25, 1471–7. [Google Scholar] [CrossRef]
- Strozi, M.; Colombo, P.; Raymundo, M. Geopolymer foams by gelcasting. Ceram. Int. 2014, 40, 5723–30. [Google Scholar] [CrossRef]
- He, J.; Zhang, J.; Yu, Y.; Zhang, G. The strength and microstructure of two geopolymers derived from metakaolin and red mud-fly ash admixture: A comparative study. Constr. Build. Mater. 2012, 30, 80–91. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: why, how, and what ? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Bai, C.; Li, H.; Bernardo, E.; Colombo, P. Waste-to-resource Preparation of Glass-containing Foams from Geopolymers Waste-to-resource preparation of glass-containing foams from geopolymers. Ceram. Int. 2019, 45, 7196–202. [Google Scholar] [CrossRef]
- Toniolo, N.; Boccaccini, A.R. Fly ash-based geopolymers containing added silicate waste. A review. Ceram. Int. 2017, 43, 14545–51. [Google Scholar] [CrossRef]
- Rincón Romero, A.; Toniolo, N.; Boccaccini, A.R.; Bernardo, E. Glass-Ceramic Foams from ‘Weak Alkali Activation’ and Gel-Casting of Waste Glass/Fly Ash Mixtures. Materials 2019, 12, 588. [Google Scholar] [CrossRef] [PubMed]
- Hajjaji, W.; Andrejkovičová, S.; Zanelli, C.; Alshaaer, M.; Dondi, M.; Labrincha, J.A.; Rocha, F. Composition and technological properties of geopolymers based on metakaolin and red mud, Mat. & Des. 2013, 52, 648–54. [Google Scholar]
- Rincón Romero, A.; Elsayed, H.; Bernardo, E. Highly porous mullite ceramics from engineered alkali activated suspensions. J. Am. Ceram. Soc. 2017, 101, 1036–41. [Google Scholar] [CrossRef]
- Sturm, P.; Greiser, S.; Gluth, G.J.G.; Jäger, C.; Brouwers, H.J.H. Degree of reaction and phase content of silica-based one-part geopolymers investigated using chemical and NMR spectroscopic methods. J. Mater. Sci. 2015, 50, 6768–78. [Google Scholar] [CrossRef]
- Greiser, S.; Sturm, P.; Gluth, G.J.G.; Hunger, H.; Jäger, C. Differentiation of the solid-state NMR signals of gel, zeolite phases and water species in geopolymer-zeolite composites. Ceram. Int. 2017, 43, 2202–8. [Google Scholar] [CrossRef]
- Phair, J.W.; Van Deventer, J.S.J. Characterization of Fly-Ash-Based Geopolymeric Binders Activated with Sodium Aluminate. Ind. Eng. Chem. Res. 2002, 41, 4242–51. [Google Scholar] [CrossRef]
- Brew, D.R.M.; Mackenzie, K.J.D. Geopolymer synthesis using silica fume and sodium aluminate. J. Mater. Sci. 2007, 42, 3990–3. [Google Scholar] [CrossRef]
- Greiser, S.; Gluth, G.J.G.; Sturm, P.; Jäger, C. 29Si{27Al}, 27Al{29Si} and 27Al{1H} double-resonance NMR spectroscopy study of cementitious sodium aluminosilicate gels (geopolymers) and gel-zeolite composites. RSC Adv. 2018, 70, 40164–71. [Google Scholar] [CrossRef]
- Singh, P.S.; Trigg, M.; Burgar, I.; Bastow, T. Geopolymer formation processes at room temperature studied. Mat. Sci. Eng. A 2005, 396, 392–402. [Google Scholar] [CrossRef]
- Katada, N.; Nakata, S.; Kato, S.; Kanehashi, K.; Saito, K.; Niwa, M. Detection of active sites for paraffin cracking on USY zeolite by Al MQMAS NMR operated at high magnetic field 16 T. J. Mol. Cat. A: Chem. 2005, 236, 239–45. [Google Scholar] [CrossRef]
- Walkley, B.; Rees, G.J.; Nicolas, R.S.; Van Deventer, J.S.J.; Hanna, J.V.; Provis, J.L. New Structural Model of Hydrous Sodium Aluminosilicate Gels and the Role of Charge-Balancing Extra-Framework Al. J. Phys. Chem. C 2018, 122, 5673–85. [Google Scholar] [CrossRef]
- Herisson, J.; Guéguen-Minerbe, M.; van Hullebusch, E.D.; Chaussadent, T. Influence of the binder on the behaviour of mortars exposed to H2S in sewer networks: a long-term durability study. Mater. Struct. 2016, 50, 8. [Google Scholar] [CrossRef]
- Herisson, J.; Guéguen-Minerbe, M.; van Hullebusch, E.D. Chaussadent, Behaviour of different cementitious material formulations in sewer networks. Water Sci. Technol. 2014, 69, 1502–8. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2017, 103, 21–34. [Google Scholar] [CrossRef]
- Hemmings, R.; Berry, E. On the glass in coal fly ashes: recent advances. Mater. Res. Soc. Symp. Proc. 1988, 113, 3–38. [Google Scholar] [CrossRef]
- Bunker, B.C.; Headley, T.J.; Douglas, S.C. Gel Structures in Leached Alkali Silicate Glass. Mater. Res. Soc. Symp. Proc. 1984, 32, 41. [Google Scholar] [CrossRef]
- Simion, A.; Vasilescu, M.; Filip, C.; Todea, M.; Muresan-Pop, M.; Simon, S. Structural characterization of interfaces in silica core-alumina shell microspheres by solid-state NMR spectroscopy, Sol. St. Nucl. Mag. Res. 2022, 117, 101773. [Google Scholar] [CrossRef] [PubMed]
- Man, P.P.; Peltre, M.J.; Barthomeuf, D. Nuclear Magnetic Resonance Study of the Dealumination of an Amorphous Silica-Alumina Catalyst. J. Chem. Soc., Faraday Trans., 1990, 86, 1599–602. [Google Scholar] [CrossRef]
- Price, L.; Leung, K.; Sartbaeva, A. Local and Average Structural Changes in Zeolite A upon Ion Exchange. Magnetochem. 2017, 3, 42. [Google Scholar] [CrossRef]
- Günther, C.; Richter, H.; Voigt, I.; Michaelis, A.; Tzscheutschler, H.; Krause-Rehberg, R.; Serra, J.M. Synthesis and characterization of a sulfur containing hydroxy sodalite without sulfur radicals. Micropor. Mesopor. Mater. 2015, 214, 1–7. [Google Scholar] [CrossRef]
- Jones, A.R.; Winter, R.; Greaves, G.N.; Smith, I.H. MAS NMR study of soda-lime-silicate glasses with variable degree of polymerization. J. Non-Cryst. Sol. 2001, 295, 87–92. [Google Scholar] [CrossRef]
- Delair, D.; Prud’homme, E.; Peyratout, C.; Smith, A.; Michaud, P.; Eloy, L.; Joussein, E.; Rossignol, S. Durability of inorganic foam in solution: The role of alkali elements in the geopolymer network. Corros. Sci. 2012, 59, 213–21. [Google Scholar] [CrossRef]
- Ansys CES (Cambridge Engineering Selector) Granta EduPack, 2022.
- Ashby, M.F. Materials selection in mechanical design, vol. 86, no. 10. Elsevier Ltd., 1994.
- Al-Zboon, K.; Tahat, M.; Abu-Hamatteh, Z.S.H.; Al-Harahsheh, M.S. Recycling of stone cutting sludge in formulations of bricks and terrazzo tiles. Waste Manag. Res. 2010, 28, 568–574. [Google Scholar] [CrossRef]
- Najafi Kani, E.; Allahverdi, A.; Provis, J.L. Efflorescence control in geopolymer binders based on natural pozzolan. Cem. Concr. Compos. 2012, 34, 25–33. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.L.; Reid, A. Efflorescence: a critical challenge for geopolymer applications? in ‘Institute of Australia’s Biennial National Conference (Concrete 2013): Understanding Concrete’.
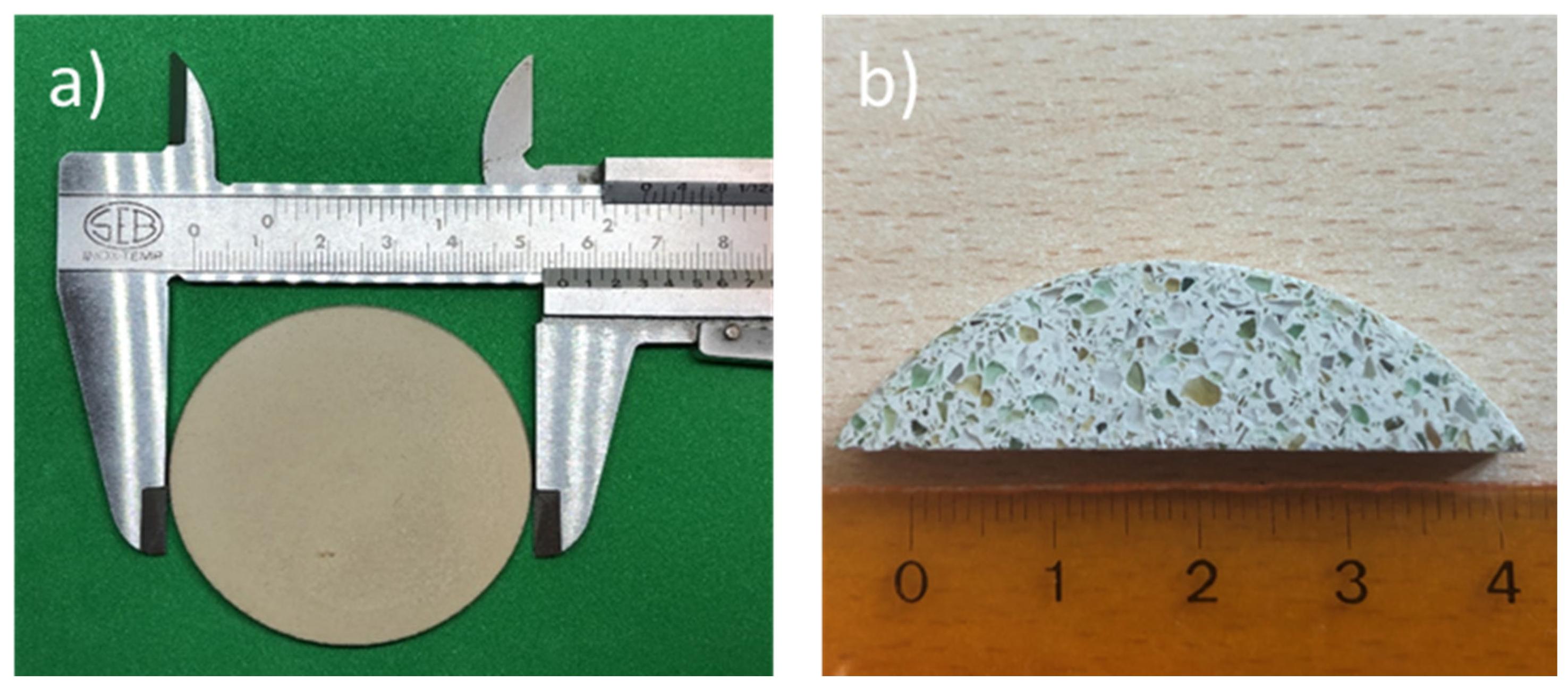
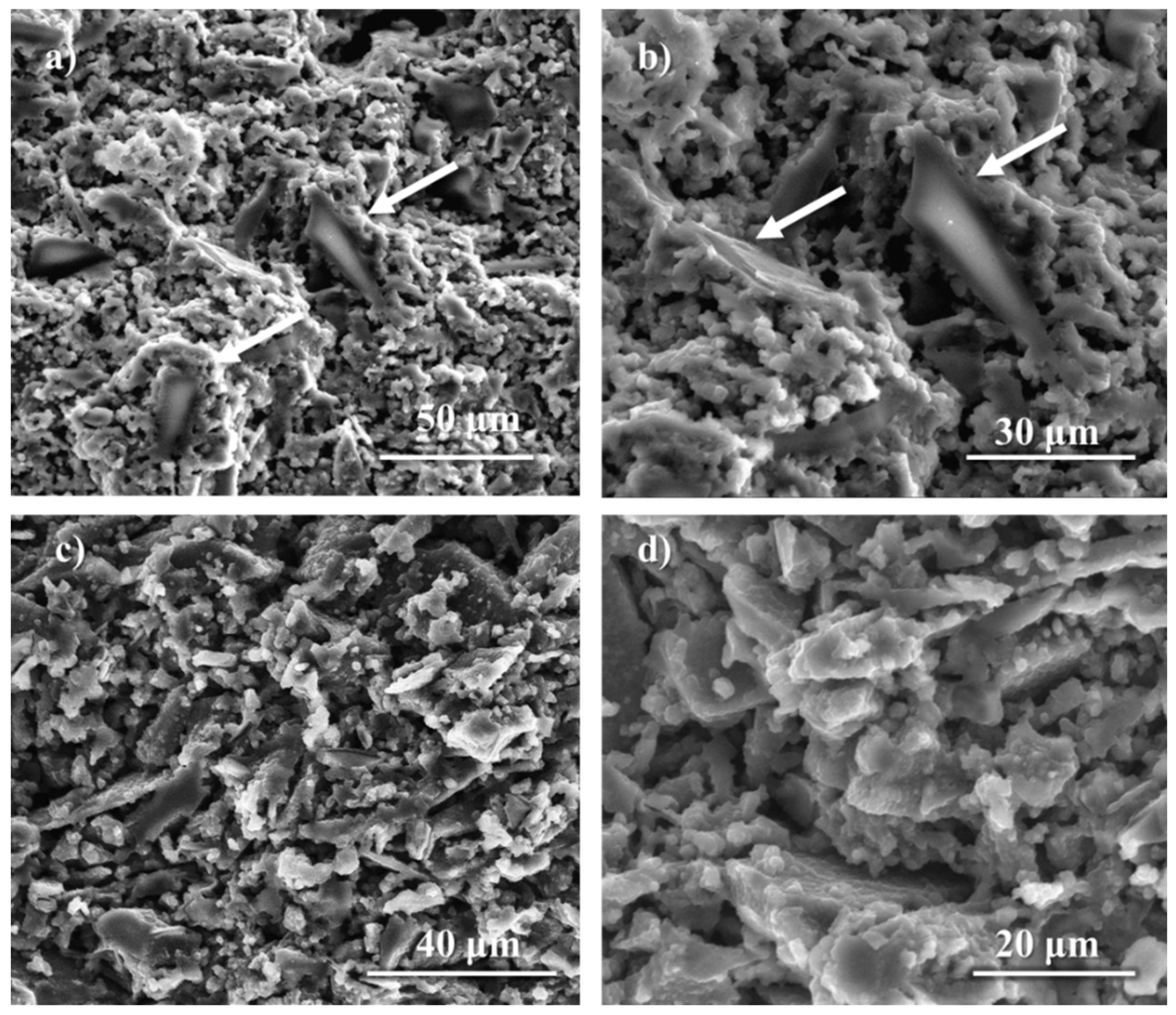
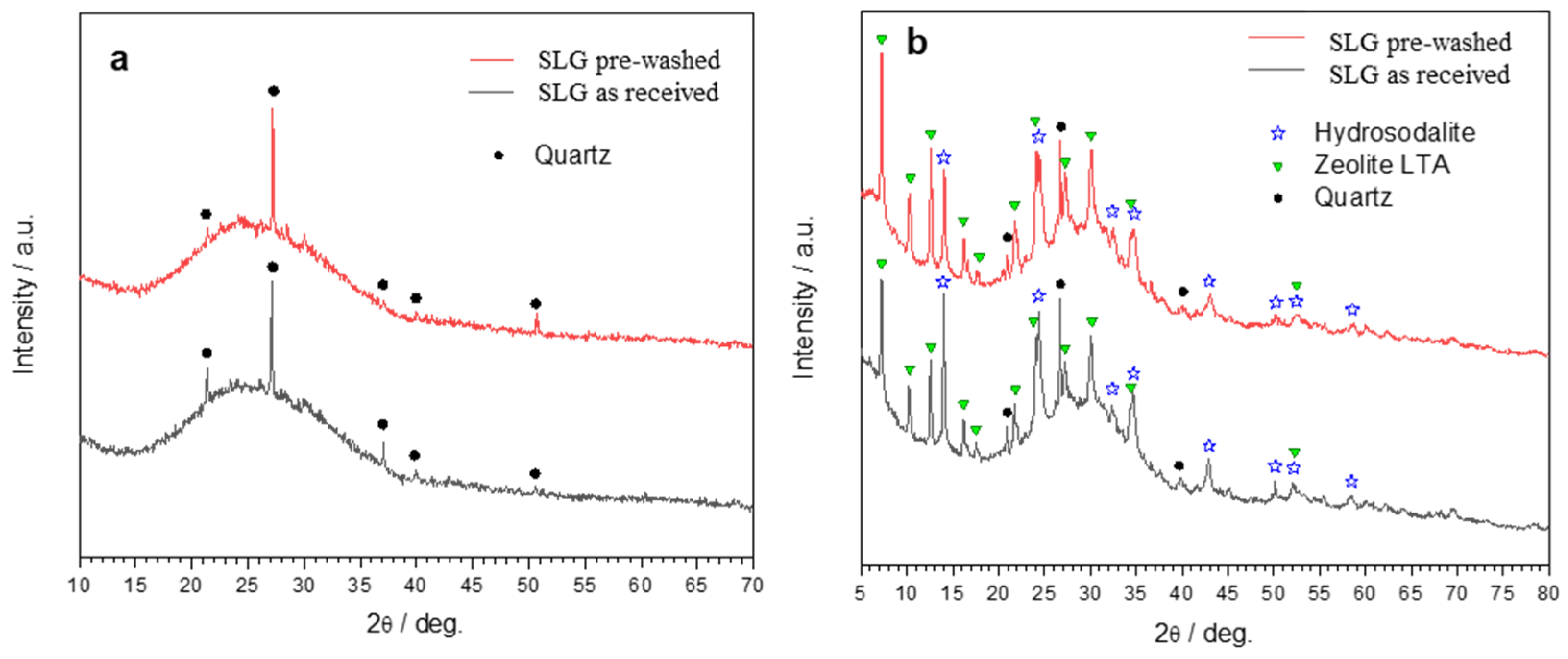
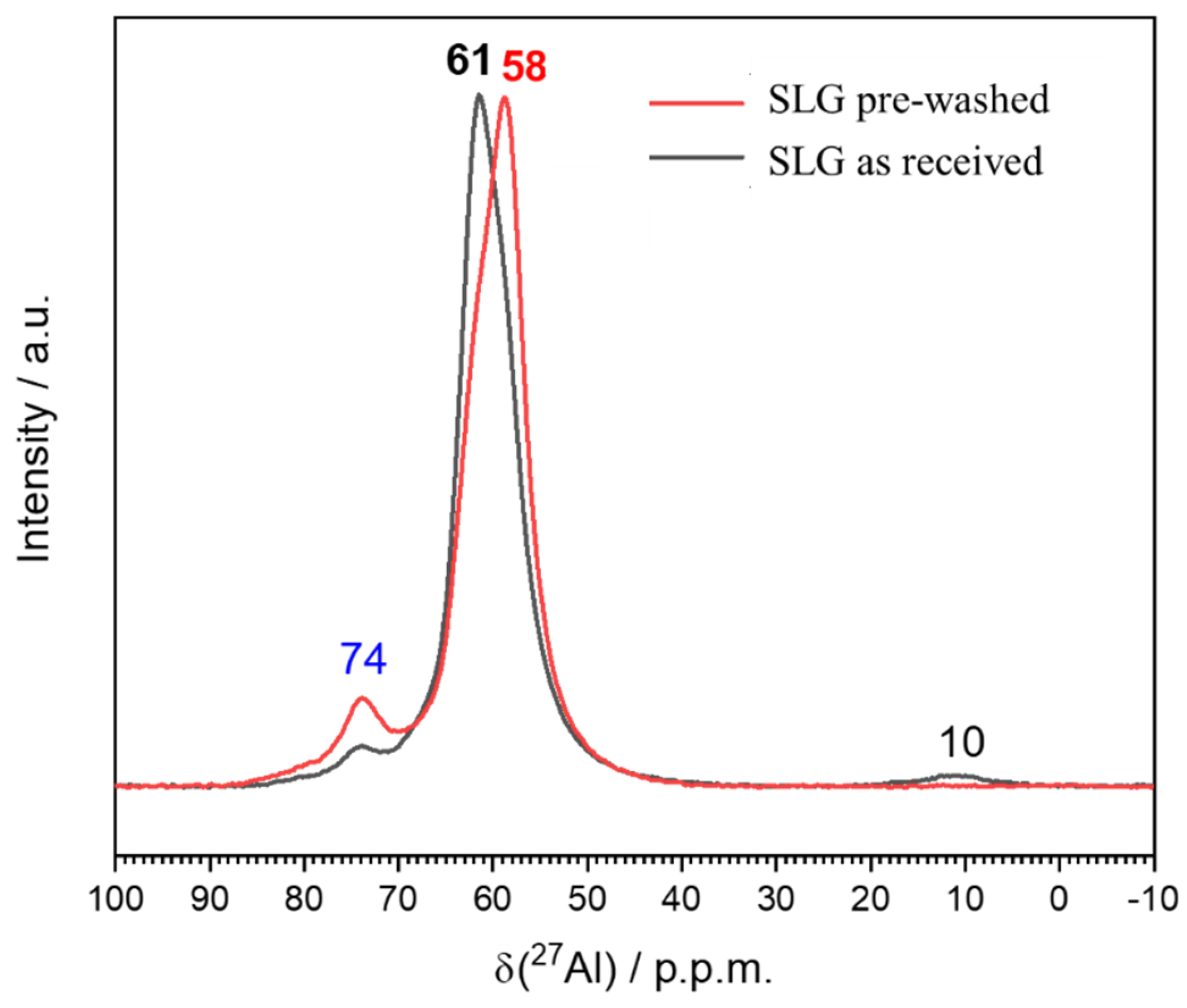
| Glass-based geopolymer | Normal concrete | Lightweight concrete | Marble | Granite | ||
| SLS as rec. | washed SLG | |||||
| Density, ρ (g/cm3) | 1.61 ± 0.03 | 1.58 ± 0.03 | 2.2 - 2.6 | 1.4 - 2 | 2.7 - 2.9 | 2.6 - 3.2 |
| Total porosity (vol%) | 36.4 | 43.0 | ||||
| Closed porosity (vol%) | 15 | 16.8 | ||||
| Open porosity (vol%) | 21.4 | 26.2 | ||||
| Elastic modulus, E (GPa) | 11.1 ± 0.6 | 7.6 ± 0.4 | 15 - 25 | 11 - 21 | 50 - 70 | 50 - 70 |
| I=E1/3/ρ (GPa1/3⋅cm3/g) | 1.4 | 1.5 | 1.1 | 1.5 | 1.4 | 1.3 |
| Bending strength, σb (MPa) | 19.9 ± 0.6 | 13.2 ± 1.8 | 1.7 – 2.4 | 3 - 17 | 6 - 10 | 8 - 23 |
| I’=σb1/2/ρ (MPa1/2⋅cm3/g) | 2.8 | 2.3 | 0.6 | 1.6 | 1 | 1.3 |
| Crushing strength, σc (MPa) | 29.4 ± 6.6 | 22.0 ± 6.9 | 13 - 30 | 11 - 28 | 55 - 105 | 110 - 255 |
| I’’=σc/ρ (MPa /g) | 18.2 | 13.9 | 8.4 | 10.6 | 27.3 | 57.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





