Submitted:
05 December 2023
Posted:
06 December 2023
You are already at the latest version
Abstract

Keywords:
1. Introduction
2. Materials and Methods
2.1. Plant Samples
2.2. Analytical Procedures
2.2.1. Profiles of Phenolic Compounds
2.2.2. Determination of Arbutin and Hydroquinone Content
2.2.3. Determination of Antioxidant Activity
2.2.4. Analysis of Total Phenolic Compounds (TPC)
2.3. Statistical Analysis
2.4. Chemicals and Reagents
3. Results and Discussion
5. Conclusions
Author Contributions
Funding

Conflicts of Interest
References
- Raudone, L.; Vilkickyte, G.; Pitkauskaite, L.; Raudonis, R.; Vainoriene, R.; Motiekaityte, V. Antioxidant Activities of Vaccinium vitis-idaea L. Leaves within Cultivars and Their Phenolic Compounds. Molecules 2019, 24, 844. [CrossRef]
- Ștefănescu, B.-E.; Călinoiu, L.F.; Ranga, F.; Fetea, F.; Mocan, A.; Vodnar, D.C.; Crișan, G. Chemical Composition and Biological Activities of the Nord-West Romanian Wild Bilberry (Vaccinium myrtillus L.) and Lingonberry (Vaccinium vitis-idaea L.) Leaves. Antioxidants. 2020, 9, 495. [CrossRef]
- Sugier, P.; Sęczyk, Ł.; Sugier, D.; Krawczyk, R.; Wójcik, M.; Czarnecka, J.; Okoń, S.; Plak, A. Chemical Characteristics and Antioxidant Activity of Arctostaphylos uva-ursi L. Spreng. at the Southern Border of the Geographical Range of the Species in Europe. Molecules. 2021, 26, 7692. [CrossRef]
- Gramza-Michałowska, A.; Sidor, A.; Kulczyński, B. Berries as a potential anti-influenza factor – A review. J. Funct. Foods. 2017, 37, 116–137. [CrossRef]
- EMEA/HMPC/246816/2005 https://www.ema.europa.eu/documents/scientific-guideline/guideline-good-agricultural-collection-practice-gacp-starting-materials-herbal-origin_en.pdf; https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition.
- Tarapatskyy, M.; Kapusta, I.; Gumienna, A.; Puchalski, C. Assessment of the Bioactive Compounds in White and Red Wines Enriched with a Primula veris L.. Molecules 2019, 24, 4074. [CrossRef]
- Krzepiłko, A.; Prażak, R.; Święciło, A. Chemical Composition, Antioxidant and Antimicrobial Activity of Raspberry, Blackberry and Raspberry-Blackberry Hybrid Leaf Buds. Molecules. 2021, 10, 26,2, 327. [CrossRef]
- Pires, E.O.Jr.; Di Gioia, F.; Rouphael, Y.; Ferreira, I.C.F.R.; Caleja, C.; Barros, L.; Petropoulos, S.A. The Compositional Aspects of Edible Flowers as an Emerging Horticultural Product. Molecules 2021, 26, 6940. [CrossRef]
- Egebjerg, M.M.; Olesen, P.T.; Eriksen, F.D.; Ravn-Haren, G.; Bredsdorff L, Pilegaard K. Are wild and cultivated flowers served in restaurants or sold by local producers in Denmark safe for the consumer? Food Chem Toxicol. 2018, 120, 129–142. [CrossRef]
- Socaci, S. A.; Fărcaş, A. C.; Dulf, F. V.; Pop, O. L.; Diaconeasa, Z. M.; Fogarasi, M. Health-promoting activities and bioavailability of bioactive compounds from functional foods. Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress, 2022. 17–31. [CrossRef]
- Tarapatskyy, M.; Gumienna, A.; Sowa, P.; Kapusta, I.; Puchalski, C. Bioactive Phenolic Compounds from Primula veris L.: Influence of the Extraction Conditions and Purification. Molecules, 2021, 26, 4, 997. [CrossRef]
- Ferlemi, A.-V.; Makri, O.E.; Mermigki, P.G.; Lamari, F.N.; Georgakopoulos, C.D. Quercetin glycosides and chlorogenic acid in highbush blueberry leaf decoction prevent cataractogenesis in vivo and in vitro: Investigation of the effect on calpains, antioxidant and metal chelating properties. Exp Eye Res. 2016, 145, 258–268. [CrossRef]
- Pervin, M.; Hasnat, M. A.; Lim, B. O. Antibacterial and antioxidant activities of Vaccinium corymbosum L. leaf extract. Asian Pac. J. Trop. Dis. 2013, 3, 444–453. [CrossRef]
- Han, H.-S.; Song, K.B.. Noni (Morinda citrifolia) fruit polysaccharide films containing blueberry (Vaccinium corymbosum) leaf extract as an antioxidant packaging material. Food Hydrocolloids. 2021, 112, 106372. [CrossRef]
- Miles, B.; Wilkerson, M.. The dark side of hydroquinone for skin lightening: 3-fold increased risk of skin cancer - a cohort study. J Invest Dermatol. 2022, 20. Conference abstract LB918. [CrossRef]
- Tarapatskyy, M.; Zaguła, G.; Bajcar, M.; Puchalski, C.; Saletnik, B. Magnetic Field Extraction Techniques in Preparing High-Quality Tea Infusions. Appl. Sci. 2018, 8, 1876. [CrossRef]
- Piechowiak, T.; Sowa, P.; Tarapatskyy, M.; Balawejder,M. The Role of Mitochondrial Energy Metabolism in Shaping the Quality of Highbush Blueberry Fruit During Storage in Ozone-Enriched Atmosphere. Food Bioprocess Technol. 2021, 14, 1973–1982. [CrossRef]
- European Pharmacopoeia 7th, European Directorate for the Quality of Medicines & Health Care, Strasbourg (2010).
- Sowa P., Marcinčáková D., Miłek M., Sidor E., Legáth J., Dżugan M.. Analysis of Cytotoxicity of Selected Asteraceae Plant Extracts in Real Time, Their Antioxidant Properties and Polyphenolic Profile. Molecules. 2020, 25 (23), 5517. [CrossRef]
- Stratil, P.; Kubáˇn, V.; Fojtová, J. Comparison of the Phenolic Content and Total Antioxidant Activity in Wines as Determined by Spectrophotometric Methods. Czech. J. Food Sci. 2008, 26, 242–253. [CrossRef]
- Wang) Hao-Nan, W.; Zheng, S.; Qing, L.; Xiao-Ying, H.; Yan, C., Da-Hui, L.; Hong-Zhi, D.U. Isochlorogenic acid (ICGA): natural medicine with potentials in pharmaceutical developments. Chin. J. Nat. 2020, 18(11): 860–871. [CrossRef]
- Lee, K.H.; Do, H.-K.; Kim, D.-Y.; Kim W. Impact of chlorogenic acid on modulation of significant genes in dermal fibroblasts and epidermal keratinocytes. Biochem. Biophys. Res. Commun. 2021, 583, 22–28. [CrossRef]
- Gupta, A.; Atanasov, A. G. ; Li, Y.; Kumar, N., Bishayee, A. Chlorogenic acid for cancer prevention and therapy: Current status on efficacy and mechanisms of action. Pharmacol Res. 2022, 186, 106505. [CrossRef]
- Chang, Y.; Huang, K.; Yang, F.; Gao, Y.; Zhang, Y.; Li, S.; Liu, B.; Guo, S. Metabolites of chlorogenic acid and its isomers: Metabolic pathways and activities for ameliorating myocardial hypertrophy. J. Funct. Foods. 2022, 96, 105216. [CrossRef]
- Wang, L. J.; Wu, J.; Wang, H. X.; Li, S. S.; Zheng, X.C.; Du, H.; Wang, L. S. Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J. Funct. Foods, 2015, 16, 295–304. [CrossRef]
- Jie, L.; Shao-Ping, W.; Yu-Qi, W.; Lei, S.; Ze-Kun, Z., Fan, D.; Hao-Ran, L.; Jia-Yu, Z.; Yu-Qing, M. Comparative metabolism study on chlorogenic acid, cryptochlorogenic acid and neochlorogenic acid using UHPLC-Q-TOF MS coupled with network pharmacology. Chin J Nat Med Chinese. 2021, 19(3), 212-224. [CrossRef]
- Wang, D.; Wang Y.; Zhang Z.; Qiu S.; Yuan, Y.; Song, G.; Li, L.; Yuan, T.; Gong, J. Degradation, isomerization and stabilization of three dicaffeoylquinic acids under ultrasonic treatment at different pH. Ultrason Sonochem. 2023, 95, 106401. [CrossRef]
- Boots A.W.; Haenen G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585 (2–3), 325-337. [CrossRef]
- Ulusoy, H.G.; Sanlier, N. A minireview of quercetin: from its metabolism to possible mechanisms of its biological activities. Critical Reviews in Food Science and Nutrition 2020, 60, 19, 3290–3303. [CrossRef]
- Kandemir, K.; Tomas, M.; McClements, D. J.; Capanoglu, E. Recent advances on the improvement of quercetin bioavailability. Trends Food Sci Technol. 2022, 119, 192–200. [CrossRef]
- Wu, H.; Chai, Z.; Hutabarat, R.P.; Zeng, Q.; Niu, L.; Li, D.; Yu, H.; Huang, W. Blueberry leaves from 73 different cultivars in southeastern China as nutraceutical supplements rich in antioxidants. Food Res Int. 2019, 122, 548–560. [CrossRef]
- Riihinen, K.; Jaakola, L.; Kärenlampi, S.; Hohtola, A. Organ-specific distribution of phenolic compounds in bilberry (Vaccinium myrtillus) and ‘northblue’ blueberry (Vaccinium corymbosum x V. angustifolium). Food Chem. 2008, 110, 156–160. [CrossRef]
- Tenutaa, M.C.; Malfa, G.A.; Bonesi, M.; Acquaviva, R.; Loizzo, M.R.; Dugay, A.; Bouzidi, C.; Tomasello, B.; Tundis, R.; Deguin, B. LC-ESI-QTOF-MS profiling protective effects on oxidative damage and inhibitory activity of enzymes linked to type 2 diabetes and nitric oxide production of Vaccinium corymbosum L. (Ericaceae) extracts. J Berry Res. 2020, 10, 603–622. [CrossRef]
- European Pharmacopoeia 7.8 https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiOkI2S4-OCAxWmcfEDHTADDjwQFnoECBEQAQ&url=https%3A%2F%2Ffile.wuxuwang.com%2Fyaopinbz%2FEP7%2FEP7.8_01__17.pdf&usg=AOvVaw01KfyDlVHvi_5TyYsPQYGK&opi=89978449.
- Farmakopea Polska, XII (2020) Vol. III, p. 4716–4717.
- Kim, T.J.; Park, Y.J.; Park, S.U.; Ha, S.-H., Kim, J.K. Determination and quantification of arbutin in plants using stable isotope dilution liquid chromatography– mass spectrometry. Appl. Biol. Chem. 2018, 61, 523–530. [CrossRef]
- Yavorska, N.Y.; Vorobets, N.M.; Salyha, Y.T.; Vishchur, O.I. Preliminary comparative phytochemical screening and antioxidant activity of varieties Vaccinium corymbosum L. (Ericaceae) shoot’ extracts. The Animal Biology. 2020, 22, 4, 3–8. [CrossRef]
- Dyrektywa 2000/6/EC Directive 2000/6 - Twenty-fourth Commission Directive 2000/6/EC adapting to technical progress Annexes II, III, VI and VII to Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products.
- Piljac-Žegarac J.; Belščak, A.; Piljac A. Antioxidant Capacity and Polyphenolic Content of Blueberry (Vaccinium corymbosum L.) Leaf Infusions. J Med Food. 2009, 12,3, 608–614. [CrossRef]
- de Arriba S.G., Naser, B.; Nolte K.-U.. Risk Assessment of Free Hydroquinone Derived from Arctostaphylos Uva-ursi folium Herbal Preparations. Risk assessment of free hydroquinone derived from Arctostaphylos Uva-ursi folium herbal preparations. Int J Toxicol. 2013, 32, 6, 442–453. [CrossRef]
- Routray, W.; Orsat, V. MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Industrial Crops and Products. 2014 58, 36–45. [CrossRef]
- Tarapatskyy, M.; Gumienna, A.; Sowa, P.; Kapusta, I.; Puchalski, C. Bioactive Phenolic Compounds from Primula veris L.: Influence of the Extraction Conditions and Purification. Molecules 2021, 26, 997. [CrossRef]
- Tarapatskyy, M.; Sowa, P.; Zaguła, G.; Dżugan, M.; Puchalski, C. Assessment of the Botanical Origin of Polish Honeys Based on Physicochemical Properties and Bioactive Components with Chemometric Analysis. Molecules. 2021, 26, 4801. [CrossRef]
- Deisinger, P.J.; Hill, T.S.; English, J.C. Human exposure to naturally occurring HQ. J. Toxicol. Environ. Health 1996. 47,101–116. [CrossRef]
- O’Donoghue, J.L.; Beevers, C.; Buard, A. Hydroquinone: assessment of genotoxic potential in the in vivo alkaline comet assay. Toxicology Reports. 2021, 8, 206–214. [CrossRef]
- European Medicines Agency 2018 European Medicines Agency. (2018). European Union herbal monograph on Arctostaphylos uva-ursi (L.) Spreng., folium EMA/HMPC/750269/2016.
- Istek, N.; Gurbuz, O. Investigation of the impact of blueberries on metabolic factors influencing health. J. Funct. Foods 2017, 38, 298–307. [CrossRef]
- Martini, D.; Marino, M.; Venturi, S.; Tucci, M.; Klimis-Zacas, D.; Riso, P.; Porrini, M.; Del, Bo, C. Blueberries and their bioactives in the modulation of oxidative stress, inflammation and cardio/vascular function markers: a systematic review of human intervention studies. J Nutr Biochem. 2023, 111, 109154. [CrossRef]
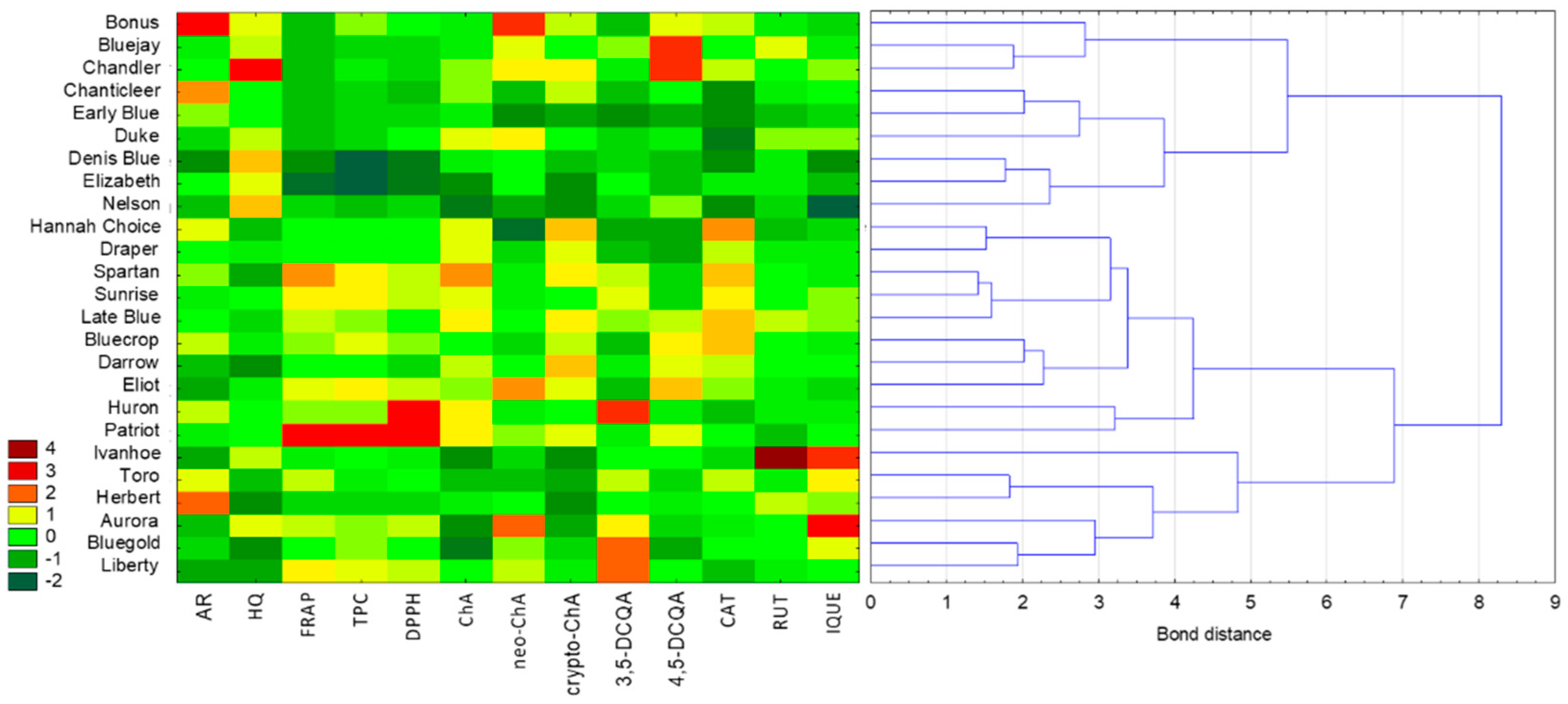
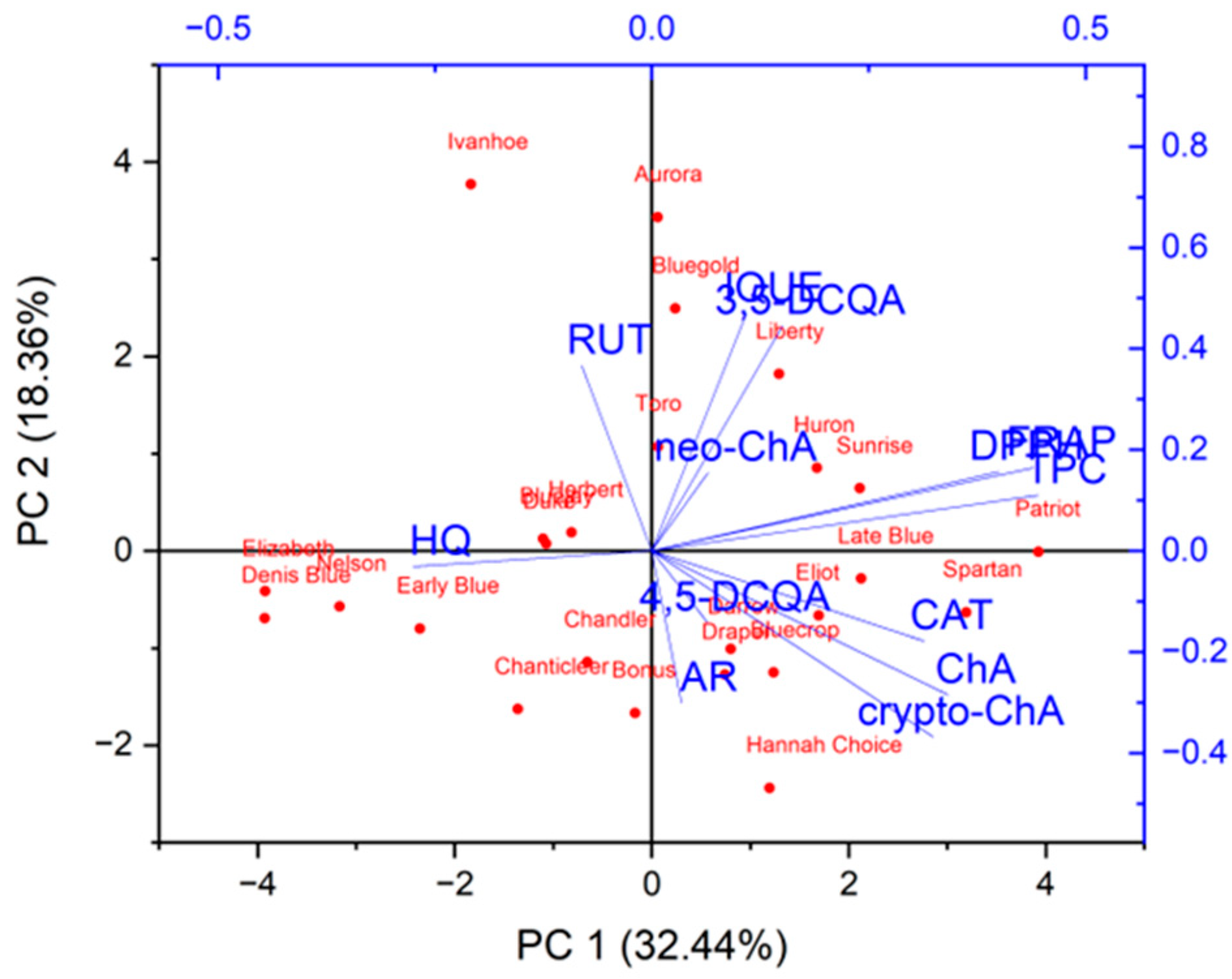
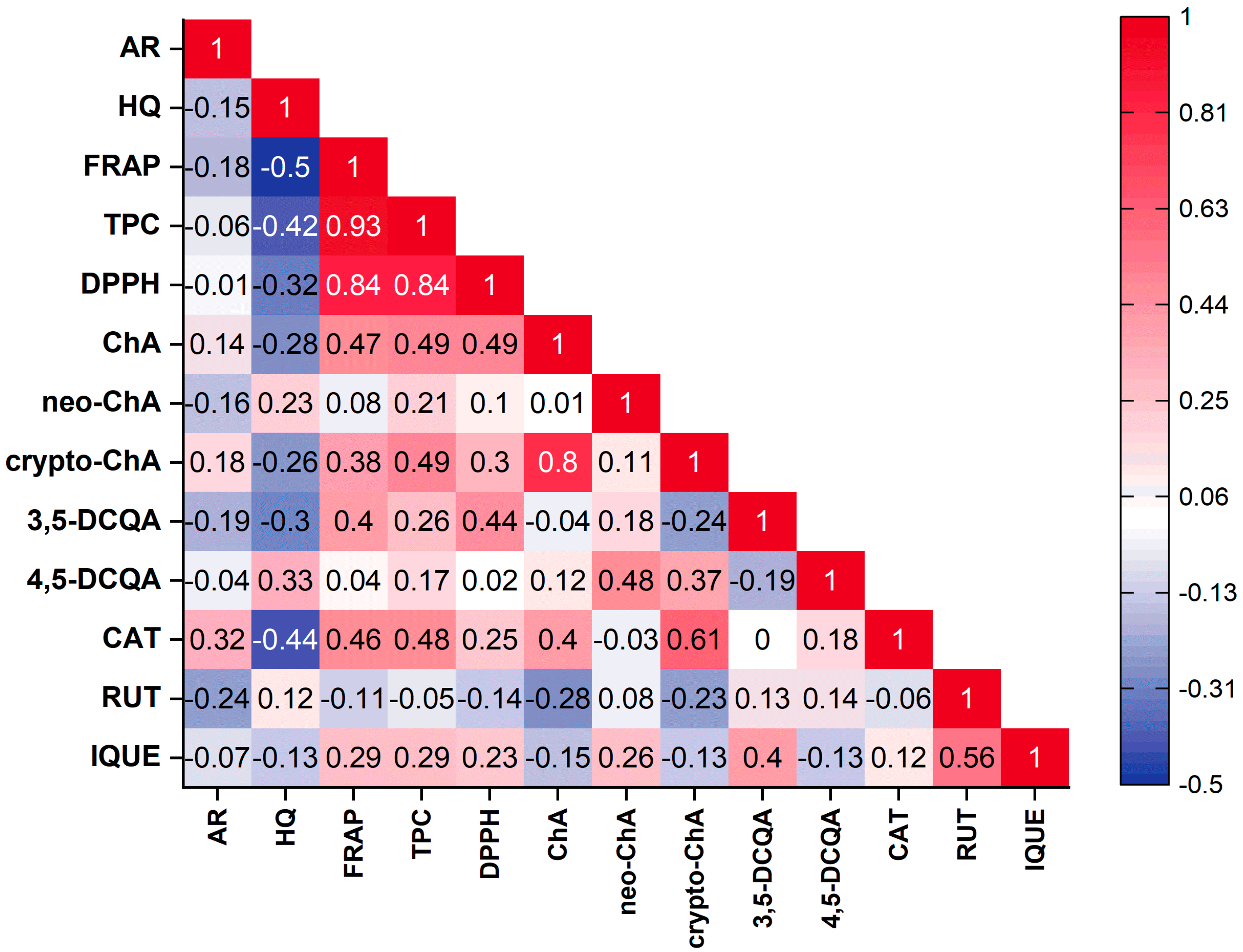
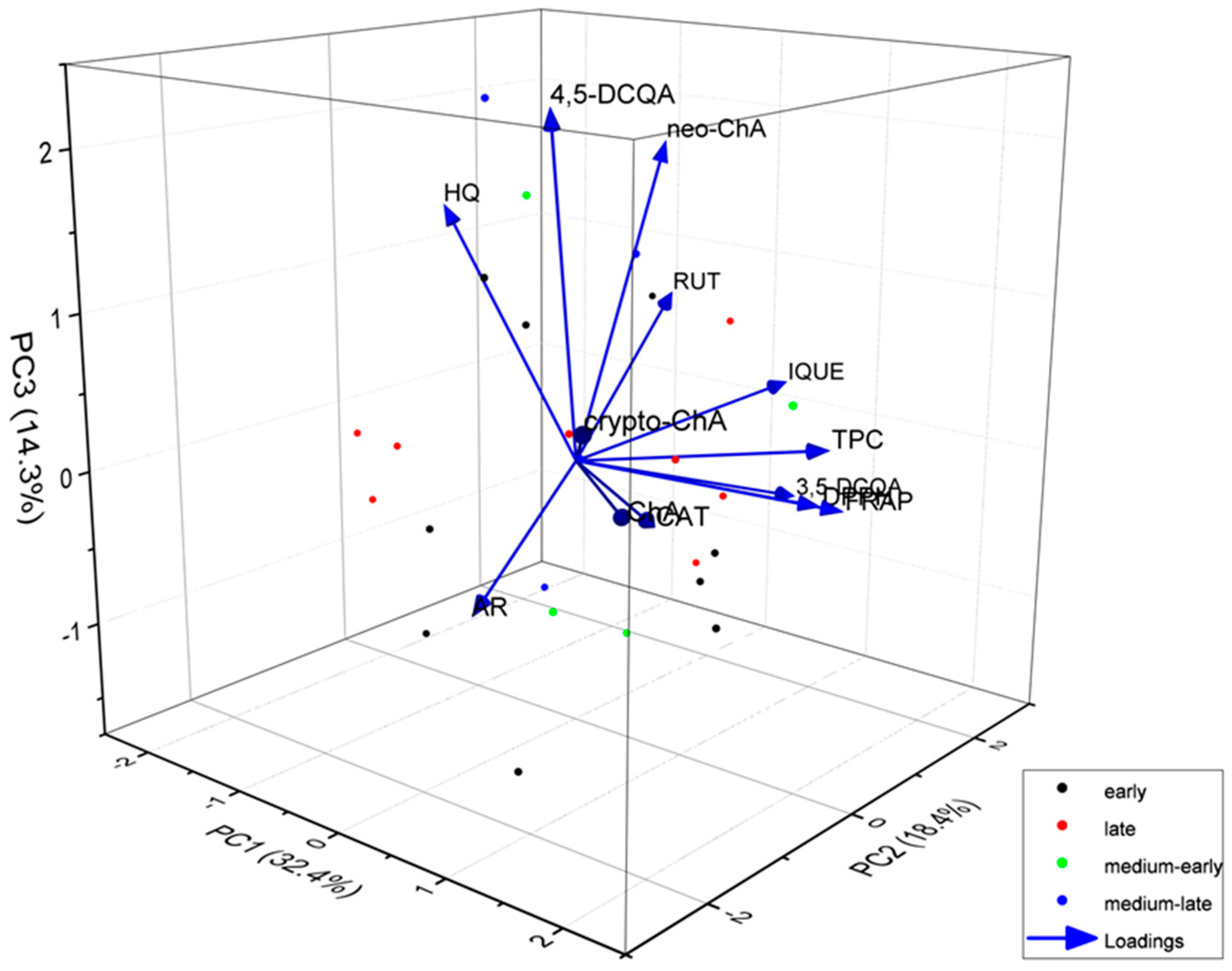
| Variety names | Fruit harvest time |
| Bonus, Chanticleer, Duke, Early Blue, Hannah Choice, Huron, Ivanhoe, Spartan, Sunrise | early |
| Blue Jay, Draper, Patriot, Toro | mid to early |
| Blue Crop, Chandler, Eliott, Herbert | mid to late |
| Aurora, Blue Gold, Darrow, Denis Blue, Elizabeth, Late Blue, Liberty, Nelson | late |
| Variety | Chlorogenic acid | Neochlorogenic acid | Cryptochlorogenic acid | 3,5-dicaffeoylquinic acid | 4,5-dicaffeoylquinic acid |
| Aurora | 34.90 ± 1.21a | 8.43 ± 0.28 | 2.20 ± 0.18a | 6.21 ± 0.05 | 1.47 ± 0.04a |
| Bluecrop | 41.65 ± 0.21b | 2.94 ± 0.05a | 3.69 ± 0.13b | 2.46 ± 0.03b | 5.72 ± 0.01 |
| Bluegold | 33.39 ± 0.30c | 5.26 ± 0.32b | 2.75 ± 0.06c | 7.63 ± 0.03 | <LOQ |
| Bluejay | 40.53 ± 0.09b | 6.41 ± 0.03c | 3.17 ± 0.38d | 4.89 ± 0.04 | 7.79 ± 0.01b |
| Bonus | 41.02 ± 0.29b | 9.03 ± 0.21 | 3.65 ± 0.10b | 2.03 ± 0.04c | 5.04 ± 0.02 |
| Chandler | 45.88 ± 0.30d | 6.66 ± 0.11c | 4.00 ± 0.07e | 3.23 ± 0.09 | 7.83 ± 0.06b |
| Chanticleer | 44.88 ± 0.16e | 2.43 ± 0.13d | 3.72 ± 0.04b | 2.27 ± 0.01d | 2.72 ± 0.08 |
| Darrow | 46.67 ± 0.40f | 4.58 ± 0.00e | 4.31 ± 0.24f | 3.01 ± 0.03e | 4.65 ± 0.03 |
| Denis Blue | 39.99 ± 0.04 | 3.84 ± 0.03f | 2.38 ± 0.13a | 2.57 ± 0.04b | 1.01 ± 0.01c |
| Draper | 48.60 ± 0.13g | 3.02 ± 0.38a | 3.91 ± 0.04b,e,g | 2.25 ± 0.11d | <LOQ |
| Duke | 48.23 ± 0.38g,h | 7.05 ± 0.08 | 3.37 ± 0.06d | 2.48 ± 0.04b | 2.03 ± 0.07d |
| Early Blue | 40.04 ± 0.20b | 1.17± 0.01 | 2.16 ± 0.12a | 1.35 ± 0.07 | <LOQ |
| Eliot | 45.86 ± 0.00d | 7.80 ± 0.03 | 3.94 ± 0.02b,e,g | 2.00 ± 0.01c | 6.20 ± 0.01 |
| Elizabeth | 33.84 ± 0.24c,i | 4.00 ± 0.04f | 1.99 ± 0.08a | 3.76 ± 0.06 | 0.99 ± 0.01c |
| Hannah Choice | 47.63 ± 0.00h | <LOQ | 4.30 ± 0.03f | 1.74 ± 0.04 | <LOQ |
| Herbert | 40.95 ± 0.42b | 4.33 ± 0.09e,g | 2.14 ± 0.15a | 4.43 ± 0.14 | 2.00 ± 0.04d |
| Huron | 49.98±0.09f | 3.50 ± 0.11h | 3.03 ± 0.04d | 8.32 ± 0.14 | 1.71 ± 0.05 |
| Ivanhoe | 34.21±0.04a,i | 2.93 ± 0.13a | 2.08 ± 0.02a | 3.90 ± 0.06 | 2.46 ± 0.01e |
| Late Blue | 50.52±0.35j | 4.16 ± 0.08f,g | 4.12 ± 0.01e,f,g | 5.00 ± 0.06 | 4.10 ± 0.06 |
| Liberty | 44.44±0.21e | 5.82 ± 0.08 | 2.79 ± 0.06c | 7.97 ± 0.02 | 2.50 ± 0.01e |
| Nelson | 32.37±0.23 | 1.73 ± 0.04 | 1.99 ± 0.01a | 2.57 ± 0.01b | 3.87 ± 0.04 |
| Patriot | 50.26±0.15j | 5.09 ± 0.04b | 3.84 ± 0.06b,e,g | 3.05 ± 0.01e | 4.77 ± 0.03 |
| Spartan | 52.76±0.33 | 3.68 ± 0.21f,h | 4.14 ± 0.06e,f,g | 5.27 ± 0.01f | 1.39 ± 0.00a |
| Sunrise | 47.79±0.04h | 3.67 ±0.06f,h | 3.33 ± 0.11d | 5.99 ± 0.05 | 1.24 ± 0.12f |
| Toro | 36.99±0.06 | 2.42 ±0.04d | 2.34 ± 0.07a | 5.26 ± 0.08f | 1.20 ± 0.01f |
| Variety | Catechin | Rutin | Isoquercetin |
| Aurora | 2.43 ± 0.28a | 6.79 ± 0.18a | 28.40 ± 0.17 |
| Bluecrop | 3.94 ± 0.16b | 6.09 ± 0.08 | 13.57 ± 0.02a |
| Bluegold | 2.48 ± 0.09a | 6.91 ± 0.13a | 19.89 ± 0.09 |
| Bluejay | 2.63 ± 0.11a,c | 10.55 ± 0.03 | 14.20 ± 0.13b |
| Bonus | 3.15 ± 0.08d | 4.52 ± 0.13b | 13.13 ± 0.08c |
| Chandler | 3.24 ± 0.19d | 7.08 ± 0.04a | 17.02 ± 0.06d |
| Chanticleer | 1.40 ± 0.12e | 4.04 ± 0.06c | 15.40 ± 0.08e |
| Darrow | 3.21 ± 0.06d | 5.51 ± 0.01d | 15.53 ± 0.32e |
| Denis Blue | 1.41 ± 0.05e | 3.86 ± 0.09c,e | 8.75 ± 0.07 |
| Draper | 3.19 ± 0.00d | 4.71 ± 0.07b | 14.20 ± 0.04b |
| Duke | 1.35 ± 0.03e | 8.07 ± 0.06 | 17.25 ± 0.00d |
| Early Blue | 1.53 ± 0.00e | 2.06 ± 0.05f | 13.18 ± 0.02c |
| Eliot | 2.92 ± 0.32d | 4.49 ± 0.02b | 12.06 ± 0.05 |
| Elizabeth | 2.40 ± 0.05c | 4.16 ± 0.04c | 11.43 ± 0.07 |
| Hannah Choice | 4.15 ± 0.06b | 2.38 ± 0.05 | 13.00 ± 0.05c |
| Herbert | 2.90 ± 0.18c,d | 8.71 ± 0.12 | 17.07 ± 0.05d |
| Huron | 1.95 ± 0.05f | 4.08 ± 0.01c | 13.80 ± 0.22a |
| Ivanhoe | 2.06 ± 0.15f | 27.19 ± 0.59 | 26.24 ± 0.02 |
| Late Blue | 3.91 ± 0.01b | 9.06 ± 0.08 | 17.85 ± 0.02 |
| Liberty | 1.91 ± 0.03f | 4.67 ± 0.03b | 15.01 ± 0.00 |
| Nelson | 1.46 ± 0.16e | 3.61 ± 0.04e | 5.13 ± 0.04 |
| Patriot | 2.88 ± 0.13c,d | 2.06 ± 0.09f | 16.51 ± 0.29 |
| Spartan | 3.95 ± 0.04b | 5.14 ± 0.01g | 14.00 ± 0.02b |
| Sunrise | 3.60 ± 0.14 | 5.25 ± 0.13d,g | 17.34 ± 0.09d |
| Toro | 3.19 ± 0.02d | 4.27 ± 0.01b,c | 21.57 ± 0.55 |
| Variety | Arbutin | Hydroquinone | ||
| mg/g d.w. | % | mg/g d.w. | % | |
| Aurora | 24.30±1.87a | 2.43 | 0.62±0.01a | 0.06 |
| Bluecrop | 34.21±1.56b | 3.42 | 0.42±0.03b | 0.04 |
| Bluegold | 25.41±0.84a | 2.54 | 0.24±0.02c | 0.02 |
| Bluejoy | 27.01±1.39a | 2.70 | 0.59±0.06a | 0.06 |
| Bonus | 44.24±3.84 | 4.42 | 0.61±0.04a | 0.06 |
| Chandler | 30.18±1.61c | 3.02 | 0.88±0.02 | 0.09 |
| Chanticleer | 40.01±1.23d | 4.00 | 0.52±0.02d | 0.05 |
| Darrow | 24.05±1.96a | 2.40 | 0.26±0.02c,e | 0.03 |
| Denis Blue | 19.94±1.63e | 1.99 | 0.73±0.05f | 0.07 |
| Draper | 30.27±1.07c | 3.03 | 0.40±0.06b,g | 0.04 |
| Duke | 24.97±1.39a | 2.50 | 0.59±0.05a | 0.06 |
| Early Blue | 31.51±1.15c | 3.15 | 0.51±0.02d | 0.05 |
| Eliot | 21.16±1.23e | 2.12 | 0.41±0.01b,g | 0.04 |
| Elizabeth | 30.38±1.71c | 3.04 | 0.62±0.04a | 0.06 |
| Hannah Choise | 35.69±1.63b | 3.57 | 0.31±0.03e,h | 0.03 |
| Herbert | 41.64±1.11d | 4.16 | 0.26±0.01c,e | 0.03 |
| Huron | 32.70±0.75b,c | 3.27 | 0.52±0.02d | 0.05 |
| Ivanhoe | 21.14±0.28e | 2.11 | 0.60±0.03a | 0.06 |
| Late Blue | 30.14±1.05c | 3.01 | 0.36±0.01e,g,h | 0.04 |
| Liberty | 21.15±0.97e | 2.11 | 0.27±0.02c | 0.03 |
| Nelson | 23.31±1.06a | 2.33 | 0.71±0.03f | 0.07 |
| Patriot | 26.52±1.32a | 2.65 | 0.50±0.03d,i | 0.05 |
| Spartan | 31.12±0.48c | 3.11 | 0.28±0.01c,e | 0.03 |
| Sunrise | 26.65±1.44a | 2.66 | 0.45±0.01b,g,i | 0.04 |
| Toro | 34.51±1.89b | 3.45 | 0.34±0.02g,h | 0.03 |
| Variety |
FRAP μmol Trolox/g s.m. |
TPC mg GAE/g s.m. |
DPPH μmol Trolox/g s.m. |
| Aurora | 793.85±10.88a | 121.44±2.04a | 792.78±4.05a |
| Bluecrop | 758.08±16.86b | 129.01±1.02a,b | 751.21±16.22b |
| Bluegold | 718.08±14.69 | 118.74±5.35a,b,c | 730.43±5.12b,c |
| Bluejay | 549.23±1.09c | 89.73±1.53d | 573.47±24.33d |
| Bonus | 552.69±15.77c,d | 114.95±12.23a,e | 665.21±24.33e |
| Chandler | 537.31±2.72c | 94.77±1.05d,f | 579.21±4.05d |
| Chanticleer | 521.15±13.62e | 87.93±1.10d | 556.98±15.24d |
| Darrow | 661.54±17.41f | 101.62±1.15f,g | 606.44±4.05f |
| Denis Blue | 433.08±8.70 | 49.19±0.76h | 400.74±19.26g |
| Draper | 685. 00±2.72g | 114.05±1.78a,g | 681.69±3.04e |
| Duke | 541.15±20.13c,e | 90.45±12.74d | 661.62±1.01e |
| Early Blue | 513.85±4.35e | 87.39±9.94d | 599.99±23.31d,f |
| Eliot | 840.77±10.88h | 136.22±5.13b,h | 798.65±2.21a |
| Elizabeth | 350.77±6.53 | 48.11±0.76h | 403.65±8.83g |
| Hannah Choice | 676.54±4.92f,g | 107.75±4.59c,e,g,i | 715.93±24.29c,f |
| Herbert | 573.85±11.97d,i | 88.11±0.25d | 608.96±16.56 |
| Huron | 763.08±8.71b,j | 116.04±1.08a,e,i | 1144.47±12.14h |
| Ivanhoe | 628.85±7.07 | 106.49±7.39e,g,i | 642.52±4.42e |
| Late Blue | 809.62±13.6a | 118.21±2.55a,b,e,i | 736.98±3.31b,c |
| Liberty | 853.85±10.88h | 129.91±0.76a,b | 790.84±8.83a |
| Nelson | 583.08±2.18i | 83.60±3.57d | 574.61±7.73d |
| Patriot | 1086.15±8.7 | 177.31±3.57 | 1124.17±3.31h |
| Spartan | 938.46±1.09 | 141.98±2.57h | 809.58±6.62a |
| Sunrise | 885.77±7.07 | 138.56±5.35b,h | 814.26±4.42a |
| Toro | 783.46±5.98a,j | 98.23±4.84d,g,i | 711.22±24.29c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





