Submitted:
05 December 2023
Posted:
06 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Epidemiology
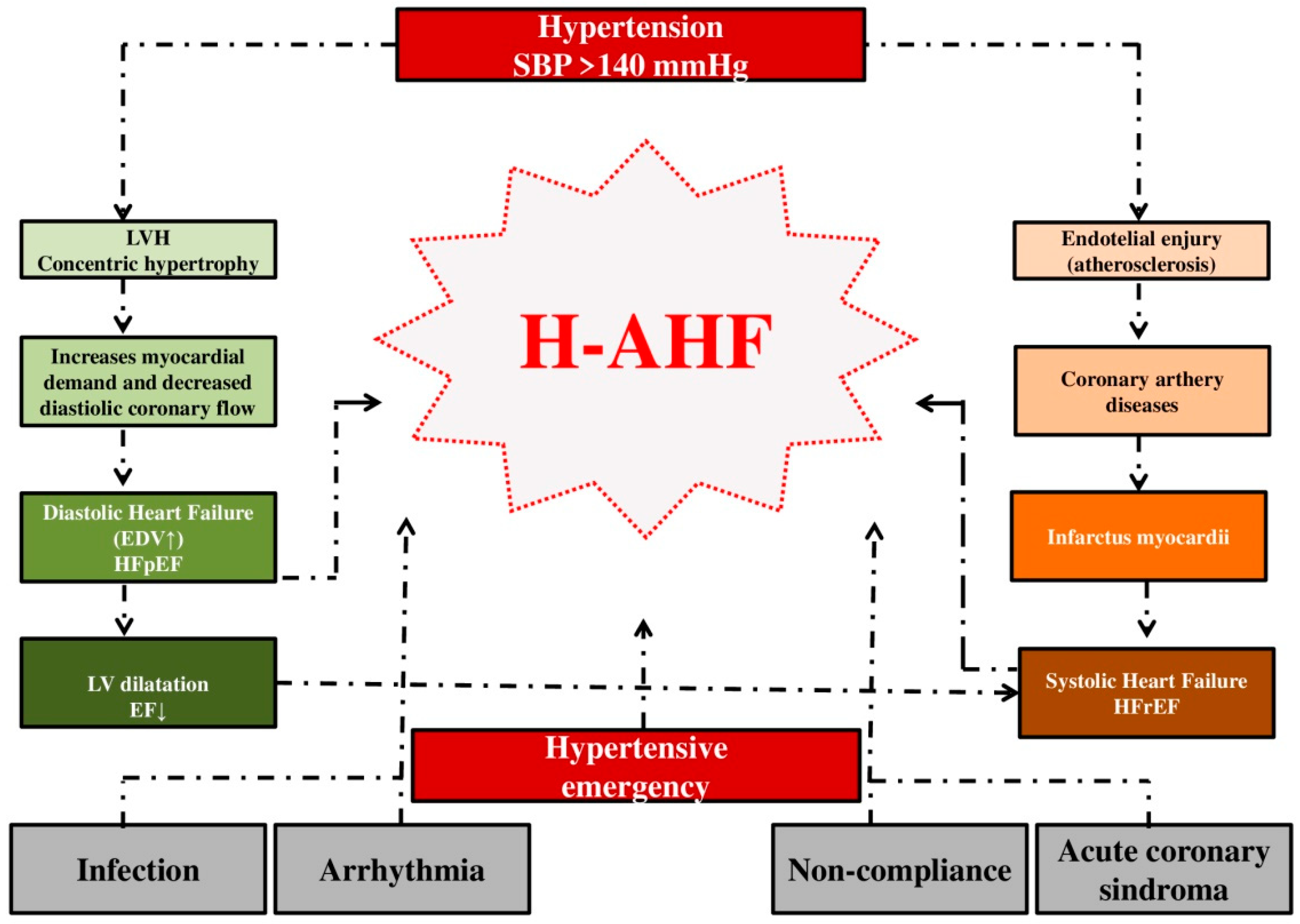
3. Pathophysiological mechanisms of H-AHF
4. Clinical picture of H-AHF
5. Diagnostic modalities
6. Treatment of patients with H-AHF
6.1. Vasodilators
6.1.1. Nitrates
6.1.2. Natriuretic Peptide Vasodilators
6.2. Diuretics
6.3. ACE-inhibitors
6.4. Serelaxin
6.5. Calcium channel blockers
6.6. Urapidil
6.7. Beta blockers
6.8. Respiratory support
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021, 42, 3599–3726. [CrossRef]
- Javaloyes, P.; Miró, Ò.; Gil, V.; Martín-Sánchez, F.J.; Jacob, J.; Herrero, P.; Takagi, K.; Alquézar-Arbé, A.; López Díez, M.P.; Martín, E.; Bibiano, C.; et al. ICA-SEMES Research Group. Clinical phenotypes of acute heart failure based on signs and symptoms of perfusion and congestion at emergency department presentation and their relationship with patient management and outcomes. Eur J Heart Fail. 2019, 21, 1353–1365. [CrossRef]
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.J.; Ponikowski, P.; Poole-Wilson, P.A.; Strömberg, A.; van Veldhuisen, D.J.; Atar, D.; Hoes, A.W.; et al. ESC Committee for Practice Guidelines (CPG). ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008, 10, 933–989. [CrossRef]
- Nohria, A.; Tsang, S.W.; Fang, J.C.; Lewis, E.F.; Jarcho, J.A.; Mudge, G.H.; Stevenson, L.W. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003, 41, 1797–1804. [Google Scholar] [CrossRef]
- McMurray, J.J.; Adamopoulos, S.; Anker, S.D.; Auricchio, A.; Böhm, M.; Dickstein, K.; Falk, V.; Filippatos, G.; Fonseca, C.; Gomez-Sanchez, M.A, et al. ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail, 2012, 14, 803–869. [CrossRef]
- Chioncel, O.; Mebazaa, A.; Harjola, V.P.; Coats, A.J.; Piepoli, M.F.; Crespo-Leiro, M.G.; Laroche, C.; Seferovic, P.M.; Anker, S.D.; Ferrari, R. ESC Heart Failure Long-Term Registry Investigators. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC Heart Failure Long-Term Registry. Eur J Heart Fail. 2017, 19, 1242–1254. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. ESC Scientific Document Group. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016, 37, 2129–2200.
- Kamran, H.; Tang, W.H.W. Medical management of acute heart failure. Fac Rev. 2021, 10, 82. [Google Scholar] [CrossRef]
- Chioncel, O.; Mebazaa, A.; Maggioni, A.P.; Harjola, V.P.; Rosano, G.; Laroche, C.; Piepoli, M.F.; Crespo-Leiro, M.G.; Lainscak, M.; Ponikowski, P.; et al. ESC-EORP-HFA Heart Failure Long-Term Registry Investigators. Acute heart failure congestion and perfusion status - impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur J Heart Fail. 2019, 21, 1338–1352. [CrossRef]
- Nieminen, M.S.; Brutsaert, D.; Dickstein. K.; Drexler, H.; Follath, F.; Harjola, V,P.; Hochadel, M.; Komajda, M.; Lassus, J.; Lopez-Sendon, J.L:, et al. EuroHeart Survey Investigators; Heart Failure Association, European Society of Cardiology. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J. 2006, 27, 2725–2736. [CrossRef]
- Masip, J.; Frank Peacok, W.; Arrigo, M.; Rossello, X.; Platz, E.; Cullen, L.; Mebazaa, A.; Price, S.; Bueno, H.; Di Somma, S.; et al. Acute Heart Failure Study Group of the Association for Acute Cardiovascular Care (ACVC) of the European Society of Cardiology. Acute Heart Failure in the 2021 ESC Heart Failure Guidelines: a scientific statement from the Association for Acute CardioVascular Care (ACVC) of the European Society of Cardiology. Eur Heart J Acute Cardiovasc Care. 2022, 11, 173–185. [CrossRef]
- Lombardi, C.; Peveri, G.; Cani, D.; Latta. F.; Bonelli, A.; Tomasoni, D.; Sbolli, M.; Ravera, A.; Carubelli, V.; Saccani, N.; et al. In-hospital and long-term mortality for acute heart failure: analysis at the time of admission to the emergency department. ESC Heart Fail. 2020, 7, 2650–2661. [CrossRef] [PubMed]
- Follath, F.; Yilmaz, M.B.; Delgado, J.F.; Parissis, J.T.; Porcher, R.; Gayat, E.; Burrows, N.; McLean, A.; Vilas-Boas, F.; Mebazaa, A. Clinical presentation, management and outcomes in the Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF). Intensive Care Med. 2011, 37, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, A.P.; Dahlström, U.; Filippatos, G.; Chioncel, O.; Crespo Leiro, M.; Drozdz, J.; Fruhwald, F.; Gullestad, L.; Logeart, D.; Fabbri, G.; et al. Heart Failure Association of the European Society of Cardiology (HFA). EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013, 15, 808–817. [CrossRef]
- Cleland, J.G.; Swedberg, K.; Follath, F.; Komajda, M.; Cohen-Solal, A.; Aguilar, J.C.; Dietz, R.; Gavazzi, A.; Hobbs, R.; Korewicki, J.; et al. Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology. The EuroHeart Failure survey programme-- a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003, 24, 442–463. [CrossRef]
- Komajda, M.; Follath, F.; Swedberg, K.; Cleland, J.; Aguilar, J.C.; Cohen-Solal, A.; Dietz, R.; Gavazzi, A.; Van Gilst, W.H.; Hobbs, R.; et al. Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology. The EuroHeart Failure Survey programme--a survey on the quality of care among patients with heart failure in Europe. Part 2: treatment. Eur Heart J. 2003, 24, 464–474. [CrossRef]
- O'Connor, C.M.; Abraham, W.T.; Albert, N.M.; Clare, R.; Gattis Stough, W.; Gheorghiade, M.; Greenberg, B.H.; Yancy, C.W.; Young, J.B.; Fonarow, G.C. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). Am Heart J. 2008, 156, 662–673. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.F. Jr.; Fonarow, G.C.; Emerman, C.L.; LeJemtel, T.H.; Costanzo, M.R.; Abraham, W.T.; Berkowitz, R.L.; Galvao, M.; Horton, D.P; ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005, 149, 209–216.
- Berg, D.D.; Bohula, E.A.; van Diepen, S.; Katz, J.N.; Alviar, C.L.; Baird-Zars, V.M.; Barnett, C.F.; Barsness, G.W.; Burke, J.A.; Cremer, P.C.; et al. Epidemiology of Shock in Contemporary Cardiac Intensive Care Units. Circ Cardiovasc Qual Outcomes. 2019, 12, e005618. [Google Scholar] [CrossRef]
- Miró, Ò.; García Sarasola, A.; Fuenzalida, C.; Calderón, S.; Jacob, J.; Aguirre, A.; Wu, D.M.; Rizzi, M.A.; Malchair, P.; Haro, A.; et al. ICA-SEMES Research Group. Departments involved during the first episode of acute heart failure and subsequent emergency department revisits and rehospitalisations: an outlook through the NOVICA cohort. Eur J Heart Fail. 2019, 21, 1231–1244. [CrossRef]
- Kociol, R.D.; Hammill, B.G.; Fonarow, G.C.; Klaskala, W.; Mills, R.M.; Hernandez, A.F.; Curtis, L.H. Generalizability and longitudinal outcomes of a national heart failure clinical registry: Comparison of Acute Decompensated Heart Failure National Registry (ADHERE) and non-ADHERE Medicare beneficiaries. Am Heart J. 2010, 160, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.T.; Fonarow, G.C.; Albert, N.M.; Stough, W.G.; Gheorghiade, M.; Greenberg, B.H.; O'Connor, C.M.; Sun, J.L.; Yancy, C.W.; Young, J.B. OPTIMIZE-HF Investigators and Coordinators. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF). J Am Coll Cardiol. 2008, 52, 347–356. [CrossRef]
- Metra, M.; Mentz, R.J.; Hernandez, A.F.; Heizer, G.M.; Armstrong, P.W.; Clausell, N.; Corbalan, R.; Costanzo, M.R.; Dickstein, K.; Dunlap, M.E.; et al. Geographic Differences in Patients in a Global Acute Heart Failure Clinical Trial (from the ASCEND-HF Trial). Am J Cardiol. 2016, 117, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Fonarow, G.C.; Stough, W.G.; Abraham, W.T.; Albert, N.M.; Gheorghiade, M.; Greenberg, B.H.; O'Connor, C.M.; Sun, J.L.; Yancy, C.W.; Young, J.B. OPTIMIZE-HF Investigators and Hospitals. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007, 50, 768–777. [CrossRef]
- Harrison, N.; Pang, P.; Collins, S.; Levy, P. Blood Pressure Reduction in Hypertensive Acute Heart Failure. Curr Hypertens Rep. 2021, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Al-Lawati, J.A.; Sulaiman, K.J.; Al-Zakwani, I.; Alsheikh-Ali, A.A.; Panduranga, P.; Al-Habib, K.F.; Al-Suwaidi, J.; Al-Mahmeed, W.; Al-Faleh, H.; El-Asfar. A. et al. Systolic Blood Pressure on Admission and Mortality in Patients Hospitalized With Acute Heart Failure: Observations From the Gulf Acute Heart Failure Registry. Angiology 2017, 68, 584–591. [CrossRef]
- Gheorghiade, M.; Abraham, W.T.; Albert, N.M.; Greenberg, B.H.; O'Connor, C.M.; She, L.; Stough, W.G.; Yancy,C.W.; Young, J.B.; Fonarow, G.C. OPTIMIZE-HF Investigators and Coordinators. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006, 296, 2217–2226. [CrossRef]
- Collins, S.P.; Jenkins, C.A.; Harrell, F.E.Jr.; Liu, D.; Miller, K.F.; Lindsell, C.J.; Naftilan, A.J.; McPherson, J.A.; Maron, D.J.; Sawyer, D.B.; et al. Identification of Emergency Department Patients With Acute Heart Failure at Low Risk for 30-Day Adverse Events: The STRATIFY Decision Tool. JACC Heart Fail. 2015, 3, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Rosman, Y.; Kopel, E.; Shlomai, G.; Goldenberg, I.; Grossman, E. The association between admission systolic blood pressure of heart failure patients with preserved systolic function and mortality outcomes. Eur J Intern Med. 2015, 26, 807–812. [Google Scholar] [CrossRef]
- Fonarow, G.C. Epidemiology and risk stratification in acute heart failure. Am Heart J. 2008, 155, 200–207. [Google Scholar] [CrossRef]
- Collins, S.P.; Levy, P.D.; Martindale, J.L.; Dunlap, M.E.; Storrow, A.B.; Pang, P.S.; Albert, N.M.; Felker, G.M.; Fermann, G.J.; Fonarow, G.C.; et al. Clinical and Research Considerations for Patients With Hypertensive Acute Heart Failure: A Consensus Statement from the Society of Academic Emergency Medicine and the Heart Failure Society of America Acute Heart Failure Working Group. J Card Fail. 2016, 22, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Emmens, J.E.; Ter Maaten, J.M.; Matsue, Y.; Figarska, S.M.; Sama, I.E.; Cotter, G.; Cleland, J.G.F.; Davison, B.A.; Felker, G.M.; Givertz, M.M.; et al. Worsening renal function in acute heart failure in the context of diuretic response. Eur J Heart Fail. 2022, 24, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Borovac, J.A.; D'Amario, D.; Bozic, J.; Glavas, D. Sympathetic nervous system activation and heart failure: Current state of evidence and the pathophysiology in the light of novel biomarkers. World J Cardiol. 2020, 12, 373–408. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Martindale, J. Optimizing Hypertensive Acute Heart Failure Management with Afterload Reduction. Curr Hypertens Rep. 2018, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A.; Melenovsky, V.; Russell, S.D.; Kessler, K.; Pacak, K.; Becker, L.C.; Kass, D.A. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006, 114, 2138–2147. [Google Scholar] [CrossRef] [PubMed]
- Farmakis, D.; Parissis, J.; Lekakis, J.; Filippatos, G. Acute heart failure: Epidemiology, risk factors, and prevention. Rev Esp Cardiol (Engl Ed). 2015, 68, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Viau, D.M.; Sala-Mercado, J.A.; Spranger, M.D.; O'Leary, D.S.; Levy, P.D. The pathophysiology of hypertensive acute heart failure. Heart. 2015, 101, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Ooi, H.; Chung, W.; Biolo, A. Arterial stiffness and vascular load in heart failure. Congest Heart Fail. 2008, 14, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Gheorghiade, M.; De Luca, L.; Fonarow, G.C.; Filippatos, G.; Metra, M.; Francis, G.S. Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol. 2005, 96, 11G–17G. [Google Scholar] [CrossRef]
- Levy, P.D.; Bellou, A. Acute Heart Failure Treatment. Curr Emerg Hosp Med Rep. 2013, 1, 10–10.1007/s40138-013-0012-8. [Google Scholar] [CrossRef]
- Oh, G.C.; Cho, H.J. Blood pressure and heart failure. Clin Hypertens. 2020, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, S.F.; Yuzefpolskaya, M.; Allemann, Y.; Messerli, F. Flash pulmonary edema. Prog Cardiovasc Dis. 2009, 52, 249–259. [Google Scholar] [CrossRef] [PubMed]
- López-Rivera, F.; Cintrón Martínez, H.R.; Castillo LaTorre, C.; Rivera González, A.; Rodríguez Vélez, J.G.; Fonseca Ferrer, V.; Méndez Meléndez, O.F.; Vázquez Vargas, E.J.; González Monroig, H.A. Treatment of Hypertensive Cardiogenic Edema with Intravenous High-Dose Nitroglycerin in a Patient Presenting with Signs of Respiratory Failure: A Case Report and Review of the Literature. Am J Case Rep. 2019, 20, 83–90. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022, 24, 4–131. [CrossRef]
- Park, J.J.; Choi, D.J.; Yoon, C.H.; Oh, I.Y.; Lee, J.H.; Ahn, S.; Yoo, B.S.; Kang, S.M.; Kim, J.J.; et al. KorHF Registry. The prognostic value of arterial blood gas analysis in high-risk acute heart failure patients: an analysis of the Korean Heart Failure (KorHF) registry. Eur J Heart Fail. 2015, 17, 601–611. [CrossRef]
- Aubier, M.; Trippenbach, T.; Roussos, C. Respiratory muscle fatigue during cardiogenic shock. J Appl Physiol Respir Environ Exerc Physiol. 1981, 51, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.; Cooper, J.S. Physiology, Carbon Dioxide Transport. [Updated 2023 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532988/.
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; et al. ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018, 39, 3021–3104. [CrossRef]
- Dhadke, S.V.; Dhadke, V.N.; Batra, D.S. Clinical Profile of Hypertensive Emergencies in an Intensive Care Unit. J Assoc Physicians India. 2017, 65, 18–22. [Google Scholar] [PubMed]
- Andrès, E.; Gass, R.; Charloux, A.; Brandt, C.; Hentzler, A. Respiratory sound analysis in the era of evidence-based medicine and the world of medicine 2.0. J Med Life. 2018, 11, 89–106. [Google Scholar]
- Sarkar, M.; Madabhavi, I.; Niranjan, N.; Dogra, M. Auscultation of the respiratory system. Ann Thorac Med. 2015, 10, 158–168. [Google Scholar] [CrossRef]
- Lee, D.S.; Lee, J.S.; Schull, M.J.; Borgundvaag, B.; Edmonds, M.L.; Ivankovic, M.; McLeod, S.L.; Dreyer, J.F.; Sabbah, S.; Levy, P.D.; et al. Prospective Validation of the Emergency Heart Failure Mortality Risk Grade for Acute Heart Failure. Circulation. 2019, 139, 1146–1156. [Google Scholar] [CrossRef]
- Sokolska, J.M.; Sokolski, M.; Zymliński, R.; Biegus, J.; Siwołowski, P.; Nawrocka-Millward, S.; Jankowska, E.A.; Todd, J.; Banasiak, W. Ponikowski P. Patterns of dyspnoea onset in patients with acute heart failure: clinical and prognostic implications. ESC Heart Fail. 2019, 6, 16–26. [CrossRef]
- Thibodeau, J.T.; Drazner, M.H. Reply: The Role of the Clinical Examination in Patients With Heart Failure. JACC Heart Fail. 2018, 6, 971. [Google Scholar] [CrossRef]
- Herring, N.; Paterson, D.J. ECG diagnosis of acute ischaemia and infarction: past, present and future. QJM. 2006, 99, 219–230. [Google Scholar] [CrossRef]
- Harjola, V.P.; Parissis, J.; Bauersachs, J.; Brunner-La Rocca, H.P.; Bueno, H.; Čelutkienė, J.; Chioncel, O.; Coats, A.J.S.; Collins, S.P, et al.; et al. Acute coronary syndromes and acute heart failure: a diagnostic dilemma and high-risk combination. A statement from the Acute Heart Failure Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2020, 22, 1298–1314. [Google Scholar] [CrossRef] [PubMed]
- Arenja, N.; Reichlin, T.; Drexler, B.; Oshima, S.; Denhaerynck, K.; Haaf, P.; Potocki, M.; Breidthardt, T.; Noveanu, M.; et al. Sensitive cardiac troponin in the diagnosis and risk stratification of acute heart failure. J Intern Med. 2012, 271, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Felker, G.M.; Hasselblad, V.; Tang, W.H.; Hernandez, A.F.; Armstrong, P.W.; Fonarow, G.C.; Voors, A.A.; Metra, M.; McMurray, J.J.; Butler, J.; et al. Troponin I in acute decompensated heart failure: insights from the ASCEND-HF study. Eur J Heart Fail. 2012, 14, 1257–1264. [Google Scholar] [CrossRef]
- Peacock, W.F 4th.; De Marco, T.; Fonarow, G.C.; Diercks, D.; Wynne, J.; Apple, F.S.; Wu, A.H; ADHERE Investigators. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008, 358, 2117–2126. [CrossRef]
- You, J.J.; Austin, P.C.; Alter, D.A.; Ko, D.T.; Tu, J.V. Relation between cardiac troponin I and mortality in acute decompensated heart failure. Am Heart J. 2007, 153, 462–470. [Google Scholar] [CrossRef]
- Kim, W.; Kim, B.S.; Kim, H.J.; Lee, J.H.; Shin, J.; Shin, J.H. Clinical implications of cardiac troponin-I in patients with hypertensive crisis visiting the emergency department. Ann Med. 2022, 54, 507–515. [Google Scholar] [CrossRef]
- Weber, M.; Hamm, C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart. 2006, 92, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L.; van Kimmenade, R.; Lainchbury, J.; Bayes-Genis, A.; Ordonez-Llanos, J.; Santalo-Bel, M.; Pinto, Y.M.; Richards, M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006, 27, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Belagavi, A.C.; Rao, M.; Pillai, A.Y.; Srihari, U.S. Correlation between NT proBNP and left ventricular ejection fraction in elderly patients presenting to emergency department with dyspnoea. Indian Heart J. 2012, 64, 302–304. [Google Scholar] [CrossRef]
- Krishnaswamy, P.; Lubien, E.; Clopton, P.; Koon. J.; Kazanegra, R.; Wanner, E.; Gardetto, N.; Garcia, A.; DeMaria, A.; Maisel, A.S. Utility of B-natriuretic peptide levels in identifying patients with left ventricular systolic or diastolic dysfunction. Am J Med. 2001, 111, 274–279. [CrossRef] [PubMed]
- Christ, M.; Mueller, C. Use of natriuretic peptide assay in dyspnea. Dtsch Arztebl Int. 2008, 105, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L. Jr.; Camargo, C.A.; Anwaruddin, S.; Baggish, A.L.; Chen, A.A.; Krauser, D.G.; Tung, R.; Cameron, R.; Nagurney, J.T.; et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005, 95, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.S.; Krishnaswamy, P.; Nowak, R.M.; McCord, J.; Hollander, J.E.; Duc, P.; Omland, T.; Storrow, A.B.; Abraham, W.T.; Wu, A.H, et al. Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002, 347, 161–167. [CrossRef]
- Arrigo, M.; Jessup, M.; Mullens, W.; Reza, N.; Shah, A.M.; Sliwa, K.; Mebazaa, A. Acute heart failure. Nat Rev Dis Primers. 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Dal-Bianco, J.P.; Jaffe, A.S.; Bell, M.R.; Oh, J.K. Cardiac function and brain-type natriuretic peptide in first-time flash pulmonary edema. Mayo Clin Proc. 2008, 83, 289–296. [Google Scholar] [CrossRef]
- Ip, C.; Luk, K.S., Yuen, V.L.C.; Chiang, L.; Chan, C.K.; Ho, K.; Gong, M.; Lee, T.T.L.; Leung, K.S.K.; Roever, L,; Bazoukis, G.; et al; International Health Informatics Study (IHIS) Network. Soluble suppression of tumorigenicity 2 (sST2) for predicting disease severity or mortality outcomes in cardiovascular diseases: A systematic review and meta-analysis. Int J Cardiol Heart Vasc. 2021, 37, 100887. [CrossRef]
- Miró, Ò.; González de la Presa, B.; Herrero-Puente, P.; Fernández Bonifacio, R.; Möckel, M.; Mueller, C.; Casals, G.; Sandalinas, S.; Llorens, P.; Martín-Sánchez, F.J.; et al. The GALA study: relationship between galectin-3 serum levels and short- and long-term outcomes of patients with acute heart failure. Biomarkers. 2017, 22, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Castiglione, V.; Aimo, A.; Vergaro, G.; Saccaro, L.; Passino, C.; Emdin, M. Biomarkers for the diagnosis and management of heart failure. Heart Fail Rev. 2022, 27, 625–643. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cheang, I.; Liao, S.; Wang, K.; Yao, W., Yin, T.; Lu, X.; Zhou, Y.; Zhang, H.; Li, X. Blood Urea Nitrogen to Creatinine Ratio and Long-Term Mortality in Patients with Acute Heart Failure: A Prospective Cohort Study and Meta-Analysis. Cardiorenal Med. 2020, 10, 415–428. [CrossRef] [PubMed]
- Qian, H.; Tang, C.; Yan, G. Predictive value of blood urea nitrogen/creatinine ratio in the long-term prognosis of patients with acute myocardial infarction complicated with acute heart failure. Medicine (Baltimore). 2019, 98, e14845. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.L.; Squire, I.B.; Jones, D.J.L.; Cao, T.H.; Chan, D.C.S.; Sandhu, J.K.; Quinn, P.A.; Davies, J.E.; Struck, J.; Hartmann, O.; et al. GREAT Network. Proenkephalin, Renal Dysfunction, and Prognosis in Patients With Acute Heart Failure: A GREAT Network Study. J Am Coll Cardiol. 2017, 69, 56–69. [CrossRef]
- Price, S.; Platz, E.; Cullen, L.; Tavazzi, G.; Christ, M.; Cowie, M.R.; Maisel, A.S.; Masip, J.; Miro, O.; McMurray, J.J.; et al. Acute Heart Failure Study Group of the European Society of Cardiology Acute Cardiovascular Care Association. Expert consensus document: Echocardiography and lung ultrasonography for the assessment and management of acute heart failure. Nat Rev Cardiol. 2017, 14, 427–440. [CrossRef]
- Milos, R.I.; Bartha, C.; Röhrich, S.; Heidinger, B.H.; Prayer, F.; Beer, L.; Wassipaul, C.; Kifjak, D.; Watzenboeck, M.L.; Pochepnia, S. ; Imaging in patients with acute dyspnea when cardiac or pulmonary origin is suspected. BJR Open. 2023, 5, 20220026. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Simon, B.; Alter, H.J.; Cheung, P. Ability of physicians to diagnose congestive heart failure based on chest X-ray. J Emerg Med. 2011, 40, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Demi, M.; Demi, L. Ultrasound patterns of pulmonary edema. Ann Transl Med. 2019, 7 (Suppl. 1), S16. [Google Scholar] [CrossRef]
- Soldati, G.; Inchingolo, R.; Smargiassi, A.; Sher, S.; Nenna, R.; Inchingolo, C.D.; Valente, S. Ex vivo lung sonography: morphologic-ultrasound relationship. Ultrasound Med Biol. 2012, 38, 1169–1179. [Google Scholar] [CrossRef]
- Soldati, G.; Demi, M. The use of lung ultrasound images for the differential diagnosis of pulmonary and cardiac interstitial pathology. J Ultrasound. 2017, 20, 91–96. [Google Scholar] [CrossRef]
- Copetti, R.; Soldati, G.; Copetti, P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008, 6, 16. [Google Scholar] [CrossRef]
- Garus, M.; Zdanowicz, A.; Fudim, M.; Zymliński, R.; Niewiński, P.; Paleczny, B.; Rosiek-Biegus, M.; Iwanek, G.; Ponikowski, P.; Biegus, J. Clinical determinants and prognostic significance of hypocapnia in acute heart failure. Sci Rep. 2022, 12, 16889. [Google Scholar] [CrossRef]
- Bezati, S.; Velliou, M.; Ventoulis, I.; Simitsis, P.; Parissis, J.; Polyzogopoulou, E. Infection as an under-recognized precipitant of acute heart failure: prognostic and therapeutic implications. Heart Fail Rev. 2023, 28, 893–904. [Google Scholar] [CrossRef]
- Abdin, A.; Anker, S.D.; Butler, J.; Coats, A.J.S.; Kindermann, I.; Lainscak, M.; Lund, L.H.; Metra, M.; Mullens, W.; Rosano, G. 'Time is prognosis' in heart failure: time-to-treatment initiation as a modifiable risk factor. ESC Heart Fail. 2021, 8, 4444–4453. [Google Scholar] [CrossRef]
- Kitai, T.; Tang W.H.W.; Xanthopoulos, A.; Murai, R.; Yamane, T.; Kim, K.; Oishi, S.; Akiyama. E.; Suzuki, S.;Yamamoto, M. Impact of early treatment with intravenous vasodilators and blood pressure reduction in acute heart failure. Open Heart. 2018, 5, e000845. [CrossRef] [PubMed]
- Levy, P.; Compton, S.; Welch, R.; Delgado, G.; Jennett, A.; Penugonda, N.; Dunne, R.; Zalenski, R. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann Emerg Med. 2007, 50, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA 2002, 287, 1531–1540, Erratum in: JAMA 2002, 288(5), 577. [CrossRef]
- Ho, E.C.; Parker, J.D.; Austin, P.C.; Tu, J.V.; Wang, X.; Lee, D.S. Impact of Nitrate Use on Survival in Acute Heart Failure: A Propensity-Matched Analysis. J Am Heart Assoc. 2016, 5, e002531. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Puente, P.; Jacob, J.; Martín-Sánchez, F.J.; Vázquez-Álvarez, J.; Martínez-Camblor, P.; Miró, Ò.; Lucas-Imbernón, F.J.; Martínez-Zapico, A.; Llorens, P. ICA-SEMES group. Influence of Intravenous Nitrate Treatment on Early Mortality Among Patients With Acute Heart Failure. NITRO-EAHFE Study. Rev Esp Cardiol (Engl Ed). 2015, 68, 959–967. [CrossRef]
- Breidthardt, T.; Noveanu, M.; Potocki, M.; Reichlin, T.; Egli, P.; Hartwiger, S.; Socrates, T.; Gayat, E.; Christ, M.; Mebazaa, A.; et al. Impact of a high-dose nitrate strategy on cardiac stress in acute heart failure: a pilot study. J Intern Med. 2010, 267, 322–330. [Google Scholar] [CrossRef]
- Abrams, J. Nitrate tolerance and dependence. A critical assessment. Nouv Presse Med. 1980, 9 (Suppl. 34), 2499–2504. [Google Scholar]
- Liu, J.X.; Uppal, S.; Patel, V. Management of Acute Hypertensive Heart Failure. Heart Fail Clin. 2019, 15, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Strain, W.D. The use of recombinant human B-type natriuretic peptide (nesiritide) in the management of acute decompensated heart failure. Int J Clin Pract. 2004, 58, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Colucci, W.S.; Elkayam, U.; Horton, D.P.; Abraham, W.T.; Bourge, R.C.; Johnson, A.D.; Wagoner, L.E.; Givertz, M.M.; Liang, C.S.; Neibaur, M.; et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N Engl J Med. 2000, 343, 246–253. [CrossRef]
- Elkayam, U.; Akhter, M.W.; Singh, H.; Khan, S.; Usman, A. Comparison of effects on left ventricular filling pressure of intravenous nesiritide and high-dose nitroglycerin in patients with decompensated heart failure. Am J Cardiol. 2004, 93, 237–240. [Google Scholar] [CrossRef]
- O'Connor, C.M.; Starling, R.C.; Hernandez, A.F.; Armstrong, P.W.; Dickstein, K.; Hasselblad, V.; Heizer, G.M.; Komajda, M.; Massie, B.M.; McMurray, J.J.; et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011, 365, 32–43. [Google Scholar] [CrossRef]
- Fu, S.; Yi, S.; Zhu, B.; Wang, L.; Wang, H., Bai, Y.; Ye, P.; Luo, L. Efficacy and safety of a modified dosage regimen of nesiritide in patients older than 75 years with acute heart failure. Aging Clin Exp Res. 2012, 24, 524–529. [CrossRef]
- Pickkers, P.; Dormans, T.P.; Russel, F.G.; Hughes, A.D.; Thien, T.; Schaper, N.; Smits, P. Direct vascular effects of furosemide in humans. Circulation. 1997, 96, 1847–1852. [Google Scholar] [CrossRef]
- Amatruda, J.G.; Scherzer, R.; Rao, V.S.; Ivey-Miranda, J.B.; Shlipak, M.G.; Estrella, M.M.; Testani, JM. Renin-Angiotensin-Aldosterone System Activation and Diuretic Response in Ambulatory Patients With Heart Failure. Kidney Med. 2022, 4, 100465. [Google Scholar] [CrossRef]
- Mullens, W.; Dauw, J.; Martens, P.; Verbrugge, F.H.; Nijst, P.; Meekers, E.; Tartaglia, K.; Chenot, F.; Moubayed, S.; Dierckx, R.; et al. ADVOR Study Group. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. N Engl J Med. 2022, 387, 1185–1195. [CrossRef]
- Trullàs, J.C.; Morales-Rull, J.L.; Casado, J.; Carrera-Izquierdo, M.; Sánchez-Marteles, M.; Conde-Martel, A.; Dávila-Ramos, M.F.; Llácer, P.; Salamanca-Bautista, P.; Pérez-Silvestre, J.; et al. CLOROTIC trial investigators. Combining loop with thiazide diuretics for decompensated heart failure: the CLOROTIC trial. Eur Heart J. 2023, 44, 411–421. [CrossRef]
- Wang, S.Y.; Manyari, D.E.; Scott-Douglas, N.; Smiseth, O.A.; Smith, E.R.; Tyberg, J.V. Splanchnic venous pressure-volume relation during experimental acute ischemic heart failure. Differential effects of hydralazine, enalaprilat, and nitroglycerin. Circulation 1995, 91, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- AlHabeeb, W.; Hayajneh, A. Continuation of Angiotensin Converting Enzyme Inhibitors in Acute Heart Failure. Int J Gen Med. 2021, 14, 2041–2045. [Google Scholar] [CrossRef] [PubMed]
- Mielniczuk, L.; Stevenson, L.W. Angiotensin-converting enzyme inhibitors and angiotensin II type I receptor blockers in the management of congestive heart failure patients: what have we learned from recent clinical trials? Curr Opin Cardiol. 2005, 20, 250–255. [Google Scholar] [CrossRef]
- Ayaz, S.I.; Sharkey, C.M.; Kwiatkowski, G.M.; Wilson, S.S.; John, R.S.; Tolomello, R.; Mahajan, A.; Millis, S.; Levy, P.D. Intravenous enalaprilat for treatment of acute hypertensive heart failure in the emergency department. Int J Emerg Med. 2016, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Miyares, M.A.; Davis, K.A. Serelaxin, a 'breakthrough' investigational intravenous agent for acute heart failure. P T. 2013, 38, 606–611. [Google Scholar]
- Hernandez-Montfort, J.A.; Arora, S.; Slawsky, M.T. Relaxin for treatment of acute heart failure: making the case for treating targeted patient profiles. Curr Heart Fail Rep. 2013, 10, 198–203. [Google Scholar] [CrossRef]
- Dschietzig, T.; Teichman, S.; Unemori, E.; Wood, S.; Boehmer, J.; Richter, C.; Baumann, G.; Stangl, K. First clinical experience with intravenous recombinant human relaxin in compensated heart failure. Ann N Y Acad Sci. 2009, 1160, 387–392. [Google Scholar] [CrossRef]
- Teerlink, J.R.; Cotter, G.; Davison, B.A.; Felker, G.M.; Filippatos, G.; Greenberg, B.H.; Ponikowski, P.; Unemori, E.; Voors, A.A.; Adams, K.F. Jr.; et al. RELAXin in Acute Heart Failure (RELAX-AHF) Investigators. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013, 381, 29–39. [CrossRef]
- Peacock, W.F.; Chandra, A.; Char, D.; Collins, S.; Der Sahakian, G.; Ding, L.; Dunbar, L.; Fermann, G.; Fonarow, G.C.; Garrison, N., et al. Clevidipine in acute heart failure: Results of the A Study of Blood Pressure Control in Acute Heart Failure-A Pilot Study (PRONTO). Am Heart J. 2014, 167, 529–536. [CrossRef]
- Koroki, T.; Abe, T.; Ochiai, H. Nicardipine versus nitroglycerin for hypertensive acute heart failure syndrome: a single-center observational study. J Rural Med. 2022, 17, 33–39. [Google Scholar] [CrossRef]
- Bopp, C.; Auger, C.; Diemunsch, P.; Schini-Kerth, V. The effect of urapidil, an alpha-1 adrenoceptor antagonist and a 5-HT1A agonist, on the vascular tone of the porcine coronary and pulmonary arteries, the rat aorta and the human pulmonary artery. Eur J Pharmacol. 2016, 779, 53–58. [Google Scholar] [CrossRef]
- Yang, W.; Zhou, Y.J.; Fu, Y.; Qin, J.; Qin, S.; Chen, X.M.; Guo, J.C,. Wang, Z.; Zhan, H.; Li, J.; He, J.Y.; et al. Efficacy and Safety of Intravenous Urapidil for Older Hypertensive Patients with Acute Heart Failure: A Multicenter Randomized Controlled Trial. Yonsei Med J. 2017, 58, 105–113. [CrossRef]
- Shi, J.; Li, Y.; Xing, C.; Peng, P.; Shi, H.; Ding, H.; Zheng, P., Ning, G.; Feng, S. Urapidil, compared to nitroglycerin, has better clinical safety in the treatment of hypertensive patients with acute heart failure: a meta-analysis. Drug Des Devel Ther. 2018, 13, 161–172. [CrossRef]
- Jondeau, G.; Neuder, Y.; Eicher, J.C.; Jourdain, P.; Fauveau, E.; Galinier, M.; Jegou, A.; Bauer, F.; Trochu, J.N.; Bouzamondo, A. B-CONVINCED Investigators. B-CONVINCED: Beta-blocker CONtinuation Vs. INterruption in patients with Congestive heart failure hospitalizED for a decompensation episode. Eur Heart J. 2009, 30, 2186–2192. [CrossRef]
- Jondeau, G.; Milleron, O. ; Beta-Blockers in Acute Heart Failure: Do They Cause Harm? JACC Heart Fail. 2015, 3, 654–656. [Google Scholar] [CrossRef]
- Koracevic, G.; Micic, S.; Stojanovic, M.; Tomasevic, M.; Kostic, T.; Velickovic Radovanovic, R.; Lovic, D.; Djordjevic, D.; Randjelovic, M.; Koracevic, M. Beta blocker rebound phenomenon is important, but we do not know its definition, incidence or optimal prevention strategies. Hypertens Res. 2020, 43, 591–596. [Google Scholar] [CrossRef]
- Prins, K.W.; Neill, J.M.; Tyler, J.O.; Eckman, P.M.; Duval, S. Effects of Beta-Blocker Withdrawal in Acute Decompensated Heart Failure: A Systematic Review and Meta-Analysis. JACC Heart Fail. 2015, 3, 647–653. [Google Scholar] [CrossRef]
- Mebazaa, A.; Tolppanen, H.; Mueller, C.; Lassus, J.; DiSomma, S.; Baksyte, G.; Cecconi, M.; Choi, D.J.; Cohen Solal, A.; Christ, M. Acute heart failure and cardiogenic shock: a multidisciplinary practical guidance. Intensive Care Med. 2016, 42, 147–163. [Google Scholar] [CrossRef]
- Masip, J.; Roque, M.; Sánchez, B.; Fernández, R.; Subirana, M.; Expósito, J.A. Noninvasive ventilation in acute cardiogenic pulmonary edema: systematic review and meta-analysis. JAMA. 2005, 294, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.L.; Zhao, Y.T.; Liu, Q.H.; Fu, C.J.; Sun, F.; Ma, Y.L.; Chen, Y.W.; He, Q.Y. Meta-analysis: Noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010, 152, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Vital, F.M.; Ladeira, M.T.; Atallah, A.N. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev. 2013, 5, CD005351. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




