1. Introduction
Our earth’s climate is changing primarily due to anthropogenic global warming, which is attributed to the burning of Fossil Fuels. It will continue to change at that pace or even more quickly until we take corrective measures [1]. Fortunately, we have started acknowledging the negative impacts of climate change on our environment [2]. As such, the major world powers and the scientific community have focused on renewable energy to curb fossil fuels, a primary culprit of climate change. Since renewable energies are intermittent energy sources, they require energy storage devices to maintain a steady supply. Recently, electric vehicles (EVs) have become increasingly popular as they are not an active source of carbon emissions and depend on an electric grid or solar array at an invariable or reduced cost. As a result, there has been a great interest in developing efficient electrochemical energy storage (EES) devices.
Among EES technologies, rechargeable batteries (RBs) and supercapacitors (SCs) are the two most desired candidates for powering a range of electrical and electronic devices [3,4,5,6,7,8,9,10]. The RBs operate on Faradaic processes, whereas the underlying mechanism of the SCs varies as non-Faradaic at the electrical double layer capacitors (EDLCs), Faradaic at the surface of the electrodes in the pseudocapacitors (PCs), and a combination of both non-Faradaic and Faradaic in the hybrid SCs (HSCs) [3]. The EDLCs offer high power density but low energy density. The HSCs take advantage of the Faradaic process without compromising their capacitive nature. Unlike batteries, supercapacitors provide high power density and numerous charge-discharge cycles; however, they lag batteries in energy density. To take advantage of the merits of both RBs and SCs, researchers have focused on merging the two technologies into a single device known as “supercapattery” (=
supercapacitor + ba
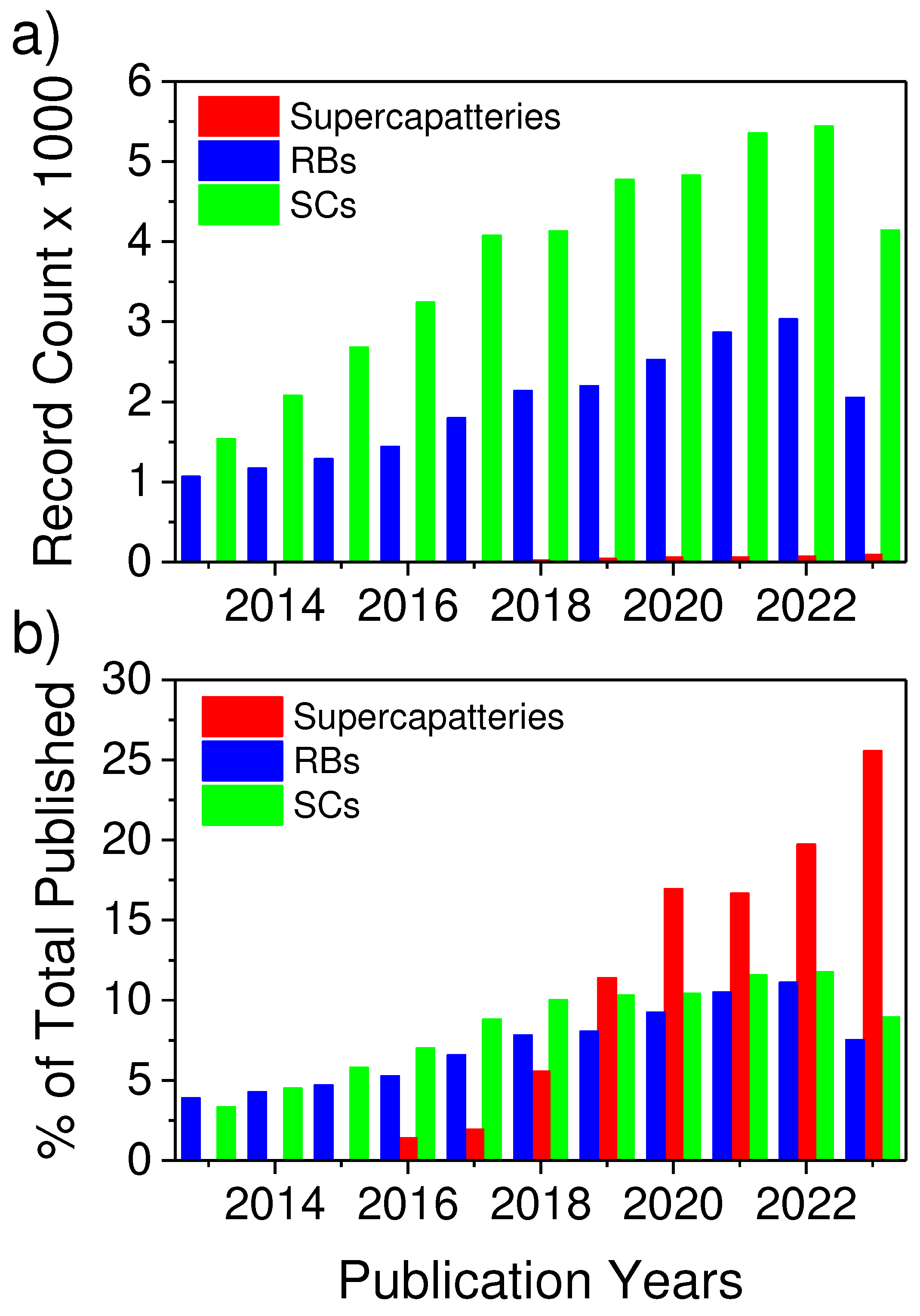
ttery) [11,12,13,14,15], a generic term used to identify a unique category of energy storage devices that offer high energy density like an RB without compromising the ability to deliver high power density and large cyclability of EDLC. Though supercapatteries are relatively new compared to RBs and SCs, supercapatteries have received significant attention, as evidenced by the exponential growth of related publications over the past ten years (
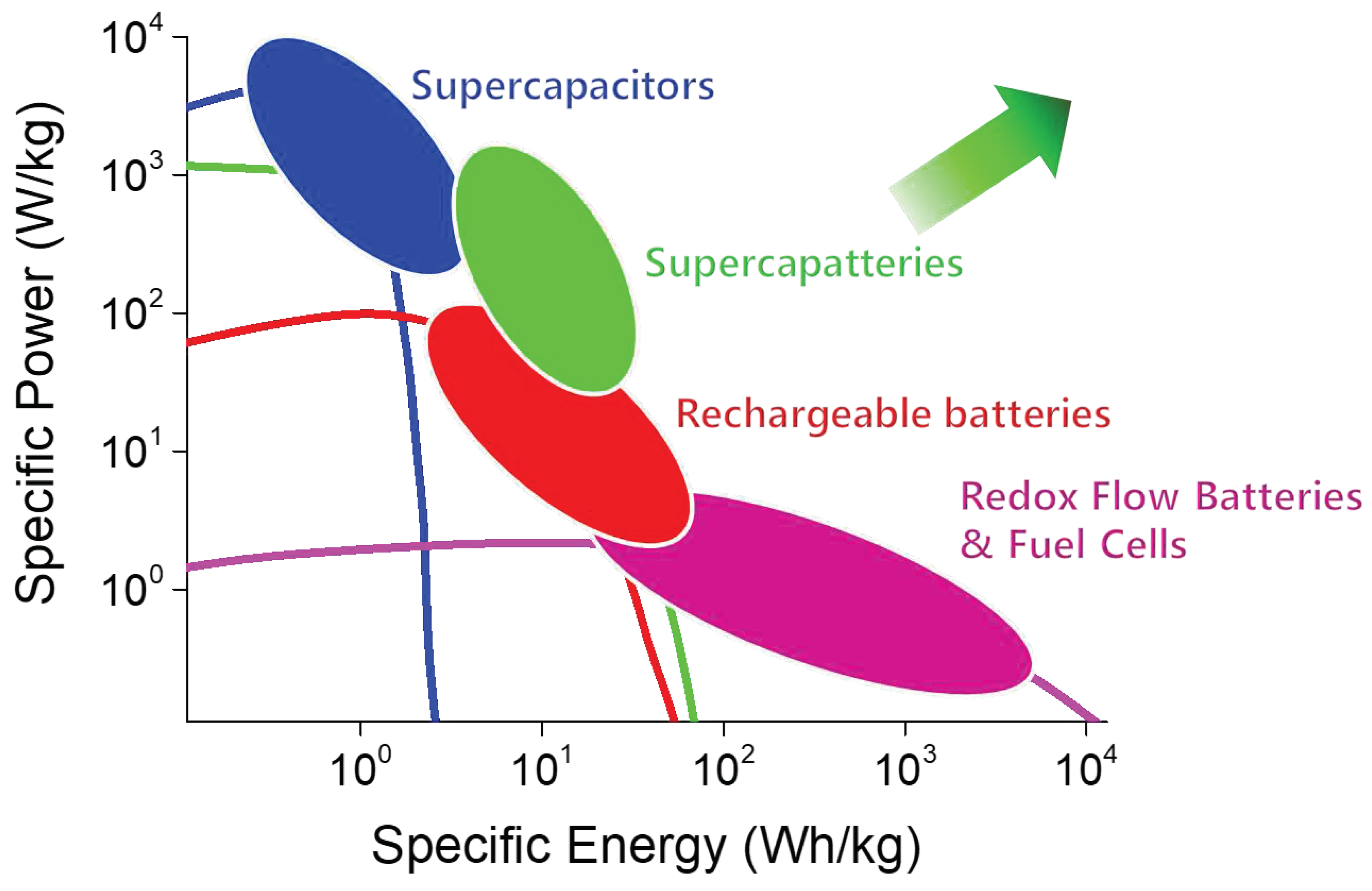
Figure 1). The advantage of supercapatteries over other EES is evident in the Ragone plots in
Figure 2, which compare the power and energy densities of several typical EES systems. However, it is often difficult to distinguish between supercapatteries and other hybrid EES devices due to their overlapping properties.
Here, we review selected articles on supercapatteries encompassing characteristics of RBs and SCs with high-energy and high-power densities, respectively. The review discusses different types of electrochemical energy storage devices in terms of mechanisms and materials to form a supercapattery. The properties and the design strategies of supercapatteries, along with their electrochemical characterization, are also discussed. The final section summarizes the review with a perspective of supercapatteries in the future.
2. Some Important Definitions and Parameters of EES
2.1. Capacitance and Charge of an Electrode
The capacitance (
C) of a dielectric capacitor depends on the area (
A) of the conducting electrodes and the distance (
d) between the electrodes as
where
and
are the relative and vacuum permittivity, respectively. According to Eq. (1), the capacitance of the EDLCs increases as
A increases and
d decreases. The behavior of an electrode/electrolyte interface is analogous to that of a capacitor. At a voltage (
V) across the capacitor with a capacitance of
C, the total amount of charge (
Q) stored is [16]
When two capacitors with capacitance
C1 and
C2 are connected in series, their total capacitance (
CT) is expressed as
If
C1 and
C2 are the same, that is,
, then Eq. (3) becomes.
However, if the two capacitors are different and
C1 is much smaller than
C2 (
), then
and Eq. (3) becomes
That is, the total capacitance of a series combination of two capacitors with different capacitances will be dominated by the capacitor with a smaller capacitance.
Moreover, differentiating Eq. (2) with respect to time (
t) gives Eq. (7).
Since
and considering
C does not change with time, Eq. (7) can be written as [17]
which can be written for
as
2.2. Galvanostatic charge-discharge (GCD)
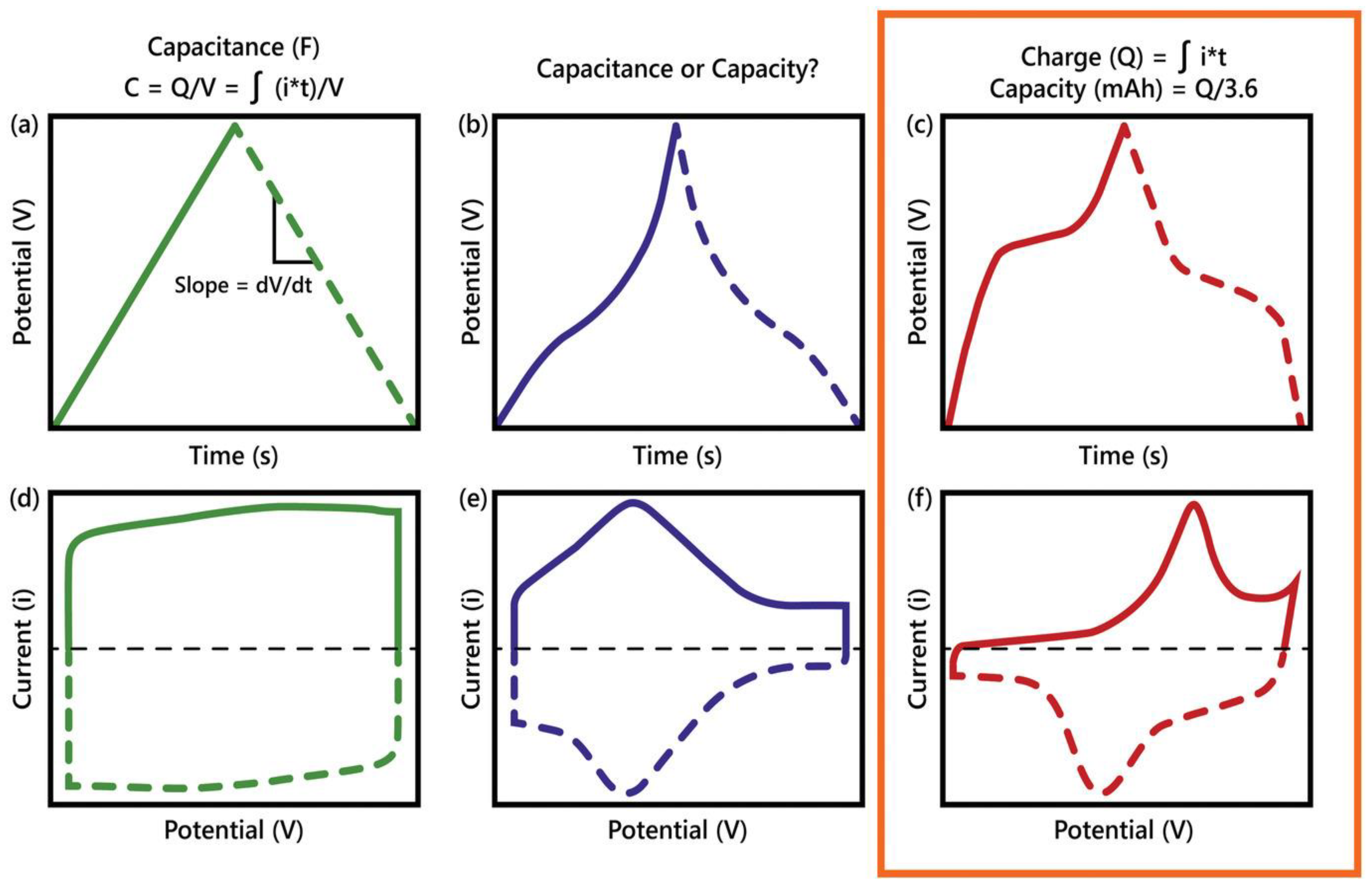
In the Galvanic technique, voltage response is measured by applying a constant current within a potential window bounded by initial and final voltages. Galvanostatic charge-discharge (GCD) is one of the most effective techniques to evaluate the capacity and capacitance of RBs and SCs at a constant current. According to Eq. (7), during the charging process of a capacitor at a constant current, the voltage increases at a constant rate. Conversely, during the discharging process, the voltage decreases at a constant rate. This results in a triangular curve in GCD, as depicted in
Figure 3a [18]. Thus, one can calculate the capacitance of an electrode material from the discharge current (
) and the slope (
) of the discharging part of the triangular GCD curve [19].
2.3. Cyclic Voltammetry (CV)
Cyclic voltammetry (CV) is one of the most popular electrochemical techniques for investigating new electrochemical systems or materials. In CV measurement, a linear potential sweep or ramp is applied where the potential (
V, V) is changed from an initial value
Vi (V) as [20]
Therefore, the current flowing through a capacitor is in a linear relationship with
but independent of
V. For a constant
C, Eq. (8) gives the rectangular
i-V plots as shown in
Figure 3d. However, CV plots for pseudocapacitive and faradaic electrodes are not linear, as shown in
Figure 3e-f. Analysis of the CV profile or voltammogram can provide several useful information, including the cyclability of the process, the total capacitance, the optimum potential window, the electrochemical kinetics of electrodes, and an ability to distinguish the capacitive and diffusion-limited charge storage mechanisms by altering the sweep rate [18]. The capacitance of the electrodes under study can be estimated by integrating the CV curves according to Eq. (10) [19].
where
is the discharge current, i.e., the current below the X axis,
is the scan rate, and
is the operating discharge potential range.
2.4. Electrochemical impedance spectroscopy (EIS)
Electrochemical impedance spectroscopy (EIS) is a powerful method for characterizing the electrical properties of materials and their interfaces. In EIS, a small amplitude modulated voltage
is applied over a wide range of frequencies (
) and the corresponding currents
are recorded, or vice versa. The resultant impedance
of the system is calculated as [21]
The impedance is often represented by the real part
and imaginary part
as a complex number.
A detailed account of electrochemical impedance and complex capacitance to interpret electrochemical capacitors was given by Itagaki et al.[22]. Like complex impedance, complex capacitance can also be expressed as
The relationship between Eq. (12) and Eq. (13) is defined by , where and .
Figure 5 shows typical EIS spectra in complex plane distinguishing different charge storage mechanisms [23].
2.5. Energy Density
The electric energy stored in SCs, i.e., the energy density (
E, Wh/kg), can be evaluated by integrating the GCD curves. For EDLCs and PCs with linear GCD curves, the integration turns into the calculation of triangle areas, as shown in
Figure 4d; therefore, the energy density can be calculated by [19]
However, in the case of HSC with a nonlinear GCD curve, as shown in
Figure 4e, the energy density cannot be calculated simply by using Eq. (14) due to the nonlinear change of
. In this case, energy density should be calculated following Eq. (15).
Eq. (15) considers all discharge times () in hour (h), and discharge voltages () for the calculation after the initial IR drop. In Eq. (15), is the time after the initial IR drop, is the moment that the discharge is finished, and is the constant current applied to the supercapacitor.
2.6. Power density
The power density (
P, W/kg) values of SCs can be calculated according to Eq. (16)[24]
where
is the discharge time in hour (
).
2.7. Notation for Electrodes of EES
Several terms have often been used interchangeably in EES to denote electrodes like positrode for the positive electrode and negatrode for the negative electrode, since G. Z. Chen proposed those terms in 2015 to avoid any confusion among the newcomers to the EES community [17]. It is important to note that the terms cathode or anode are not always suitable for EES as EDLCs are non-Faradaic and capacitive.
3. Electrochemical Energy Storage (EES) Devices
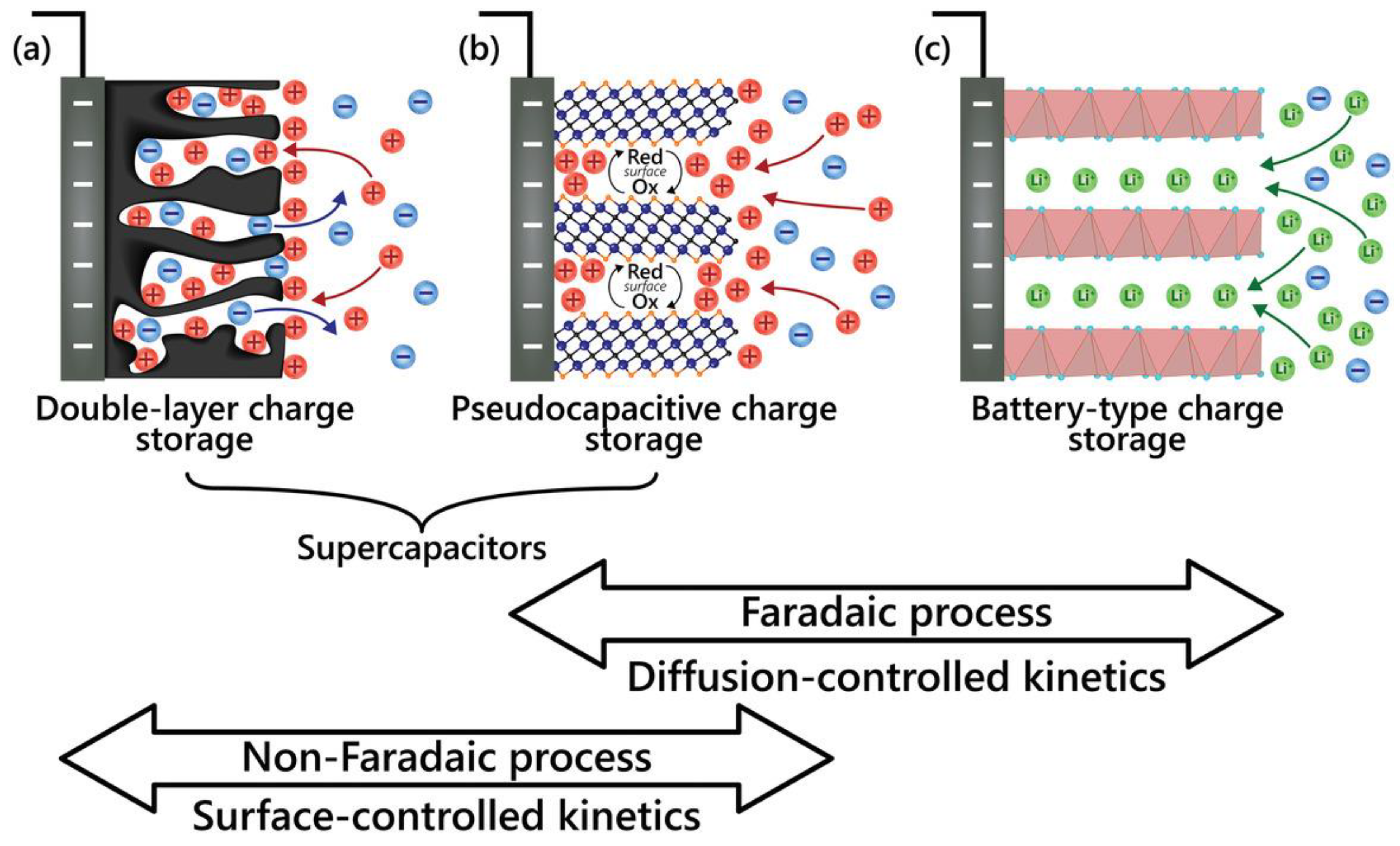
Batteries and capacitors are the two types of energy storage devices relevant to EES devices [25]. Essentially, batteries are non-rechargeable (primary cell) and rechargeable (RBs, secondary cell). On the other hand, capacitors are of three types – non-electrolytic, electrolytic, and electrochemical or supercapacitors (SCs). The EES devices comprise RBs, SCs, and their derivatives. The EES devices are different in that these devices store energy following different storage mechanisms – non-Faradaic (surface-controlled kinetics) and Faradaic (diffusion-controlled kinetics) – that depend on the materials (electrode and electrolyte) and how those materials are used in the device [8,23].
Figure 6 illustrates three charge storage mechanisms and how those differentiate SCs from RBs. In a non-Faradaic process, the charge accumulation occurs electrostatically by opposite charges residing on two interfaces separated by a vacuum (non-electrolytic) or a molecular dielectric (electrolytic). In contrast, in a Faradaic process, a redox reaction achieves the same, causing chemical or oxidation state changes in the electroactive materials [3,25]. The Faradaic process can be capacitive (pseudocapacitive), as in PCs, and non-capacitive, as in batteries [18]. Before diving into supercapatteries, understanding three main EES – EDLCs, PCs, and RBs – is essential, as shown in
Figure 6. The charge storage mechanisms in those devices can be well understood by their electrochemical signature in cyclic voltammetry (CV) and galvanostatic charge/discharge (GCD) profiles (
Figure 3). Mathis et al. outlined a set of guidelines for interpreting the performance of EES systems [23]. An EDLC material will show a linear voltage versus time response (a triangular-shaped GCD profile) during constant current charging/discharging (
Figure 3a) and a rectangular CV profile or cyclic voltammogram (
Figure 3d). In this case, the amount of charge stored depends linearly on potential, and the capacitance of the material can be easily calculated and reported for the EDLC. On the other hand, an RB material will show plateaus in the GCD profile (
Figure 3c) and separated oxidative and reductive peaks in the CV profile (
Figure 3f). Unlike the case of charge storage at EDLC electrodes, charge storage by RB-type electrodes follows a nonlinear relationship with the applied potential. In the case of pseudocapacitive materials, the GCD profile (
Figure 3b) is symmetric but non-linear, and the CV response (
Figure 3e) does not separate the oxidative and reductive peaks.
3.1. Supercapacitors (SCs)
Depending on the storage mechanism, SCs can be classified mainly into three categories: EDLCs, PCs, and a combination of the two as HSCs [17,26,27,28] or Asymmetric SCs (ASCs)[18,19,29], where HSCs are the subset of ASCs. Another way of differentiating ASCs from HSCs is that ASCs are configured by combining the electrode materials of EDLCs and PCs. In contrast, HSCs combine the electrode materials of EDLCs and RBs [30].
3.1.1. Electrical Double Layer Capacitors (EDLCs)
Like conventional dielectric capacitors, EDLCs store energy electrostatically, forming an electrical double layer (EDL) at the electrode/electrolyte interfaces, where the applied voltage polarizes the electrolyte that acts as a dielectric (
Figure 7) [16,17,31,32]. The process is purely non-Faradaic and physical in nature. Thus, Eq. (1) applies to EDLCs, and the charging-discharging mechanism of EDLCs is very fast and reversible. Also, EDLCs primarily utilize carbonaceous materials like activated carbon (AC), graphene, carbon nanotubes (CNTs), carbon-aerogel (CA), carbide-derived carbon (CDC), carbon fibers, etc. [33] The capacitance of EDLCs mostly depends on the pore size of the electrode materials. Because of the porous electrodes with large surface areas that allow the formation of compact double layers with atomic range separation between electronic and ionic charges at the electrode surface, EDLCs show greater capacitance than conventional dielectric capacitors [31,33].
3.1.2. Pseudocapacitors (PCs)
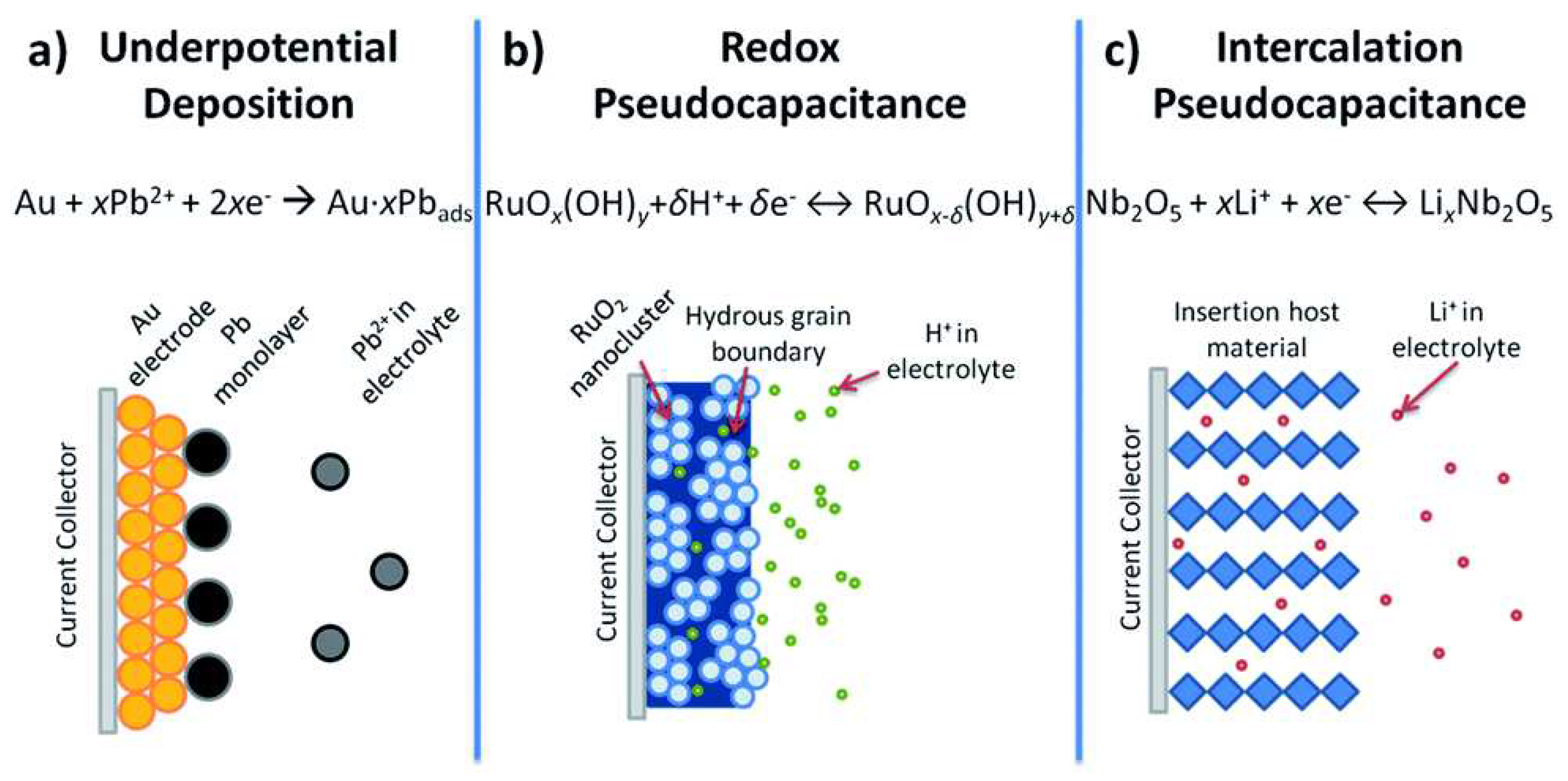
In PCs, energy is stored via a sequence of fast reversible processes, which are Faradaic in nature, at the surface or near-surface of the electrode materials [8,34,35,36]. Conway identified three different Faradaic mechanisms, including underpotential deposition, electrosorption, and intercalation, that cause pseudocapacitance, as shown in
Figure 8 [34,37]. In underpotential deposition, metal ions form an adsorbed monolayer at the surface of a different metal well above their redox potential. An example of such an underpotential deposition is the deposition of lead (Pb) on the surface of a gold (Au) electrode (
Figure 8a) [38]. Redox pseudocapacitance occurs when ions are electrochemically adsorbed onto the surface or near the surface of a material following a Faradaic charge-transfer process (
Figure 8b). Intercalation pseudocapacitance occurs when ions intercalate into the layers of a redox-active material in a Faradaic charge-transfer process without changing the crystallographic phase (
Figure 8c) [34].
The above three Faradaic processes occur due to different physical processes involving various types of materials that result in similar electrochemical signatures owing to the relationship between potential and the extent of charge developed from adsorption/desorption processes at the electrode/electrolyte interface [34]:
where
E is the potential,
R is the ideal gas constant, T is the temperature,
n is the number of electrons,
F is the Faraday constant, and
X is the extent of fractional coverage of the surface or inner structure. From Eq. (16), a capacitance (
C) may be defined in regions where the plot of
E vs.
X is linear:
where
m is the molecular weight of the active material. The capacitance,
C, is not always constant since the plot of
E vs.
X is not entirely linear as in a capacitor, and so it is termed pseudocapacitance [34].
There are detailed reviews on PCs and related pseudocapacitive materials [33,35,36,39]. Electrode materials that are used in PCs are transition-metal oxides (TMOs) such as IrO2, RuO2, Fe3O4, MnO2, NiO, V2O5, Co3O4 etc.; transition-metal sulfides (TMSs) such as MoS2, WS2, FeS2; and conducting polymers (CPs) such as polyaniline (PANI), polythiophene, polypyrrole (PPy), polyvinyl alcohol (PVA), poly (3,4-ethylene dioxythiophene) (PEDOT), polyacetylene, poly (4-styrene sulfonate) (PSS), poly-phenylene-vinylene (PPV), etc. [33,40]. Recently, many nanomaterials have been introduced to RBs that showed fast redox kinetics comparable to pseudocapacitive materials due to very short ionic diffusion length and high surface area of the nanosized materials. As a result, pseudocapacitive and battery materials are becoming increasingly indistinguishable [36]. According to Brousse et al., some materials are described as “pseudocapacitive” materials even though their electrochemical signature is analogous to that of a “battery material,” as commonly observed for Ni(OH)2 in KOH electrolyte. In contrast, true pseudocapacitive electrode materials such as MnO2 display electrochemical behavior typical of that observed for a capacitive carbon electrode [41]. Faradaic electrodes exhibit electrochemical behavior distinct from that of pseudocapacitive electrodes. So, they proposed that the term “pseudocapacitive” must be only used to describe electrode materials (e.g., MnO2) that display an electrochemical behavior typical of that observed for a capacitive carbon electrode in a mild aqueous electrolyte to avoid any confusion between battery materials and pseudocapacitive materials.
3.1.3. Hybrid supercapacitors (HSCs)
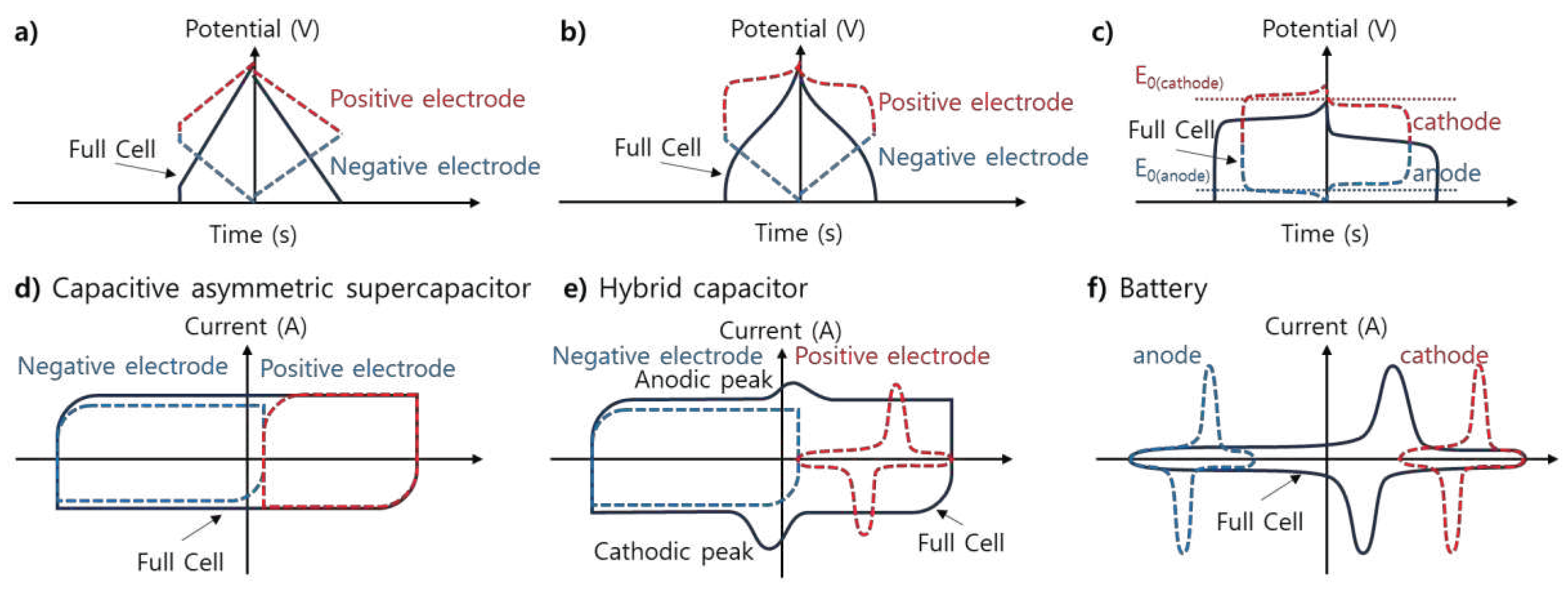
Hybrid SCs (HSCs) combine the best of EDLCs with the best of PCs or RBs into a single device in different combinations of electrode materials and storage mechanisms (non-Faradaic and Faradaic) (
Figure 9) [18,19,42]. Hybridizing different electrode materials into a single electrode or fabricating a hybrid cell configuration consisting of Faradaic and non-Faradaic electrodes has become an obvious strategy for developing high-energy and high-power HSCs [8]. Thus, asymmetry in HSCs may arise from electrode materials and storage mechanisms. These devices take advantage of the fast kinetics of EDLC materials and the improved energy storage performance of pseudocapacitive or battery electrode materials [36]. Schematic illustrations of electrochemical profiles (CV and GCD curves) of a capacitive asymmetric supercapacitor (different non-Faradaic materials) and a hybrid capacitor (non-Faradaic and Faradaic materials) are shown in
Figure 4a,d, and
Figure 4b,e, respectively.
3.2. Rechargeable batteries (RBs)
Like most electrochemical devices, RBs are composed of two electrodes – a cathode and an anode – separated by an electrolyte [4,43,44,45]. In RBs, electrical energy is converted and stored electrochemically within the bulk of the electrodes through reversible chemical reactions at the electrode/electrolyte interfaces during the charging and discharging processes. There are various kinds of RBs; among them, lithium (Li)-ion batteries (LiBs), a type of metal-ion battery, are the most commercially successful RB technology [4,43].
The emergence of LiBs, a Nobel prize-winning and one of the most popular EES technologies in the nineties, has revolutionized consumer electronics and EVs [4,7,46,47]. Typically, LiBs comprise five key components – anode, cathode, electrolyte, separator, and current collectors – as shown in
Figure 10 [4]. Generally, copper (Cu) and aluminum (Al) foils are used as current collectors at the anode and cathode. The negative electrode (anode) is made of carbonaceous materials (e.g., graphite), whereas Li-based metal oxides (e.g., LiCoO
2) are used in the positive electrode (cathode). Other materials used in the anode are germanium-based materials, transition metal chalcogenides, silicon, and metallic oxides [48]. The two electrodes are separated by a separator, typically a porous polyolefin film soaked in a non-aqueous solution of a lithium salt (e.g., LiPF
6 in ethylene carbonate, ethyl methyl carbonate, or diethyl carbonate) [4,43]. In LiBs, the reversible chemical reaction occurs in two ways: displacement and insertion in the electrodes [4]. During the charging cycle, the positive electrolyte ions (Li
+) are deintercalated (displaced) from the cathode and intercalated (inserted) into the anode. The reverse process occurs during the discharging cycle, where the positive ions transport from the anode to the cathode, and electrons travel from the anode to the cathode via an external load and thereby complete the circuit (
Figure 11a). This displacement/insertion process involves reversible Faradaic processes that can be identified as well-separated oxidative and reductive peaks in the CV profile (
Figure 11b) and asymmetric curves in the GCD profile (
Figure 11c, d). Emerging RBs based on abundant alkali, alkaline earth, and transition metals – sodium (Na), potassium (K), magnesium (Mg), calcium (Ca), zinc (Zn), and aluminum (Al) – are promising alternatives to LiBs [6,45].
Figure 4c,f illustrates the CV and GCD profiles of an RB.
3.3. Supercapatteries
Supercapatteries are hybrid EES devices that combine the advantages of SCs and RBs, such as high energy density, high power density, and long cycle life. This EES hybrid design involves a combination of an SC electrode with an RB electrode, such as the so-called Li-ion capacitor [49,50,51], Na-ion capacitors [50,52], and other hybrid EES devices [14]. Supercapatteries can exhibit capacitive performances like conventional capacitors, including CV and linear GCD profiles. Thus, the fundamentals of conventional capacitors can also be applied to supercapatteries in which capacitance (
C) is the ratio of the change of stored charge (
) to the variation in applied voltage (
ΔV) as the voltage of a capacitor is swept at a constant voltage scan rate (
) in CV. Because current (
i) flowing through a capacitor is proportional to
, this proportionality is also equal to
C, as described in Eq. (18)[14].
which is essentially the same as Eq. (7).
Figure 4b,e show the schematic of electrochemical profiles, including CV and GCD curves of typical supercapatteries. Another term often used along with supercapattery is “supercabattery,” which performs more like an RB but with higher power capability and/or longer charge-discharge durability [53,54,55,56].
Table 1 shows how different combinations of electrode materials with varying mechanisms of storage give rise to supercapatteries [17].
3.3.1. Electrode and Electrolyte Materials of Supercapatteries
Different supercapatteries can be fabricated utilizing electrodes with capacitive, pseudocapacitive, or battery-type materials. To comprehensively discuss these hybrid devices, Liu and Chen constructed several hypothetical supercapatteries to illustrate their performance by using corresponding GCD plots one by one (
Figure 12a–d), and these hypothetical devices were confirmed by using relevant experimental data from the literature (
Figure 12e–h)[14].
Table 2 summarizes different supercapatteries based on different electrodes and electrolytes, their energy, and power density. At the negatrode, mostly activated carbon of different sources was used in asymmetric battery//EDLC and pseudocapacitive//EDLC type supercapatteries. On the other hand, different metal iodide (BiOI-Bi
9I
2)[57], metal oxides (β-NiMoO
4[58], Phosphate ion-functionalized NiO (P-NiO)[59], Zn
0.5CoO
0.5Mn(PO
4)
2[60], Co
3(PO
4)
2)[61], Co
0.5Ni
0.5WO
4[62], etc.), metal hydroxides (Co–Ni LDH [63]), metal sulfides (FeCoCuS
2[64], Co
0.125Cu
0.375Mn
0.500S [65], etc.), composites (Co-MOF-PAN [66]I, Sr
3P
2-PANI [67], MWCNT-NiMnPO
4[68], graphitic carbon nitride (g-C
3N
4)-BiVO
4[69], Zn-Carbon cloths,[70], etc. ), etc. were used for positrode.
In the electrolyte, mostly, aqueous KOH of different concentrations has been used in supercapatteries [57,71,72,73,74]. Other electrolytes used are LiPF6[70], K2SO4[75], H2SO4[76], KBr [75], NaClO4[70], PVA-KOH [61], KI/VOSO4[76], etc. Electrolytes with additional redox species have been investigated in supercapatteries with significantly enhanced energy capacity [27,53,77]. Also, EDLC materials with redox electrolytes showed enhanced performance [78].
3.3.2. Performance and Experimental Evaluation of Supercapatteries
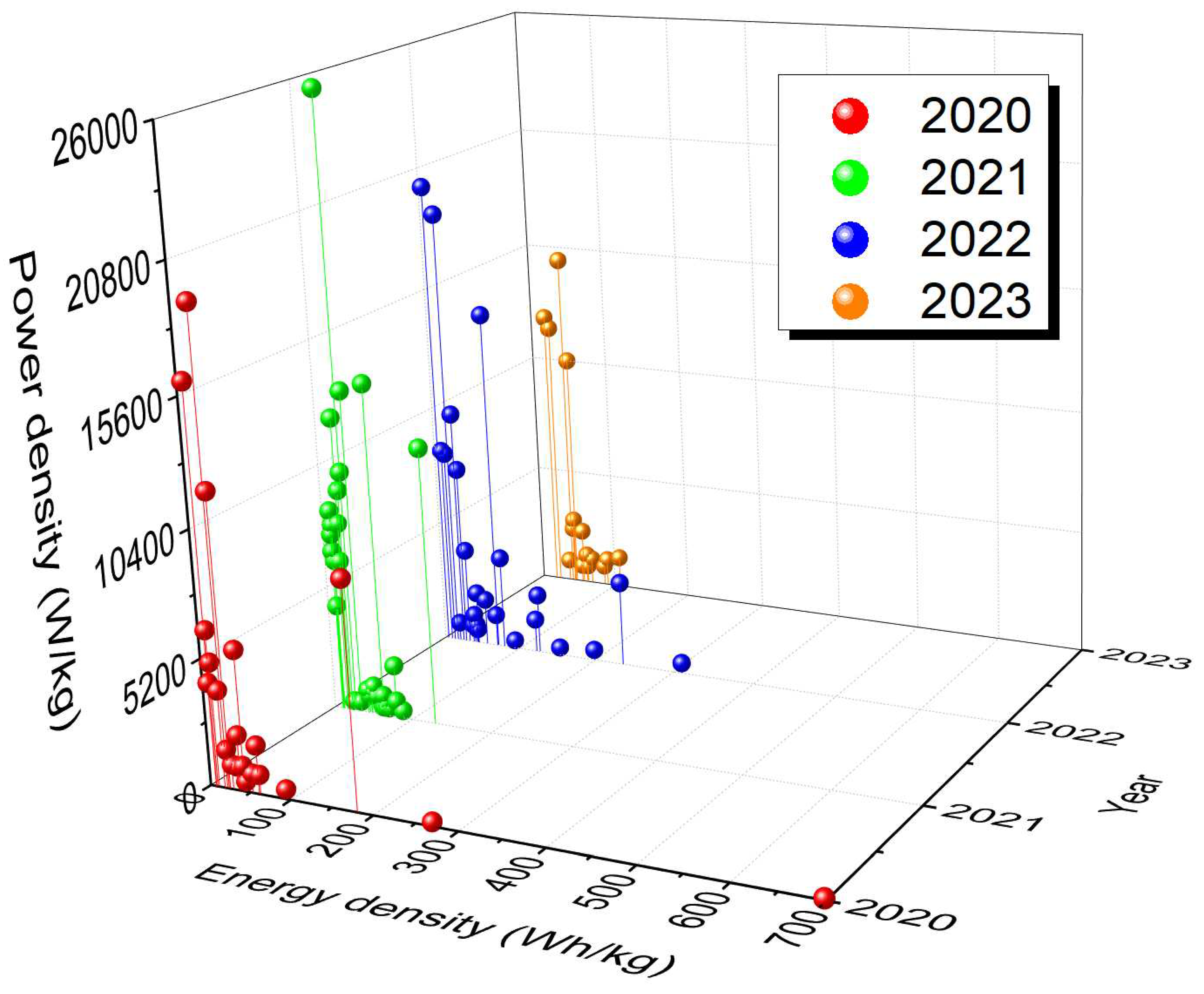
Figure 13 shows Ragone plots of supercapatteries reported in published articles during the last four years, from 2020 to 2023. The corresponding data is summarized in
Table 2. Primarily, the reported supercapatteries performed either like an EDLC or an RB. However, Devi et al. reported a Na-ion supercapattery with outstanding specific energy of 236 W h kg
−1 at a higher specific power of 3630 W kg
−1 with appreciable retention of over 95% even at the 10,000th cycle [70]. It is important to note that capacitance can be used only when there is a linear relationship between charge and voltage, and the capacitance value should be a single constant value in the chosen potential window; any deviation from this behavior requires that integration be used to calculate the charge being stored or delivered. Also, capacity instead of capacitance should be measured for RBs. As Chen pointed out, many authors have ignored the critical differences between SCs and RBs as they applied the concept of pseudocapacitance to some new battery-type materials [17]. As a result, deceptively high specific capacitance values have been claimed, and the high specific capacitance was also used in calculating specific energy.
3.4. Classification of EES Devices
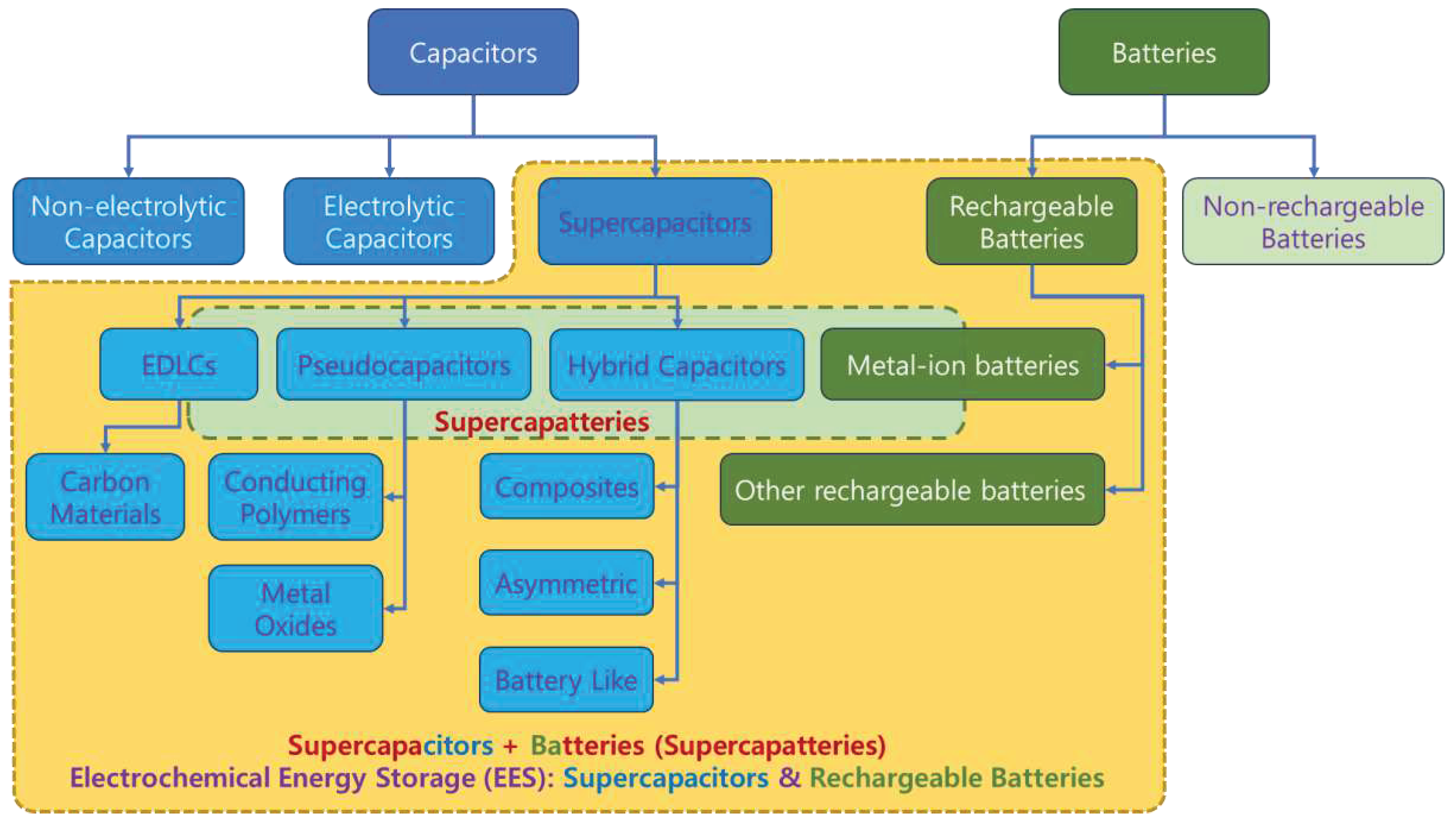
From the above discussion, it is evident that RBs and SCs belong to EES, as shown in
Figure 14, where EDLCs and PCs belong to SCs. However, placing HSCs and supercapatteries in the classification tree is inconsistent throughout the literature [8,11,17,19,26,27,28]. Even ASCs were placed parallel to HSCs or in place of HSCs, making HSCs a subclass of ASCs [19]. Initially, HSCs appeared to be defined as hybrids of EDLCs and PCs, whereas supercapatteries were considered as hybrids of EDLCs and RBs. Guan et al. proposed defining and differentiating storage mechanisms in EES devices according to
Figure 9 without explicitly differentiating ASCs and HSCs [18]. Considering all possible combinations of electrode materials and storage mechanisms, EES devices were classified vividly in a tabulated form (
Table 1)[17], where the combination results in nine different devices depending on electrode materials and storage mechanisms.
4. Summary and perspective
All supercapatteries are hybrid EES devices, but not all hybrid EES devices are supercapatteries. Supercapatteries are envisioned as a technology that will mutually complement the drawbacks of RBs and SCs. Supercapatteries are EES devices that can integrate the benefits of RBs and SCs using all three charge storage mechanisms: non-Faradaic capacitive storage (EDL capacitive storage), capacitive Faradaic storage (pseudocapacitive storage) and non-capacitive Faradaic storage (rechargeable battery-type storage or Nernstian charge storage). Moreover, supercapatteries can be made of EDLCs, non-faradaic capacitive, using a redox electrolyte. In summary, supercapatteries have gained increasing attention in the EES field, as seen in the growing number of publications using the term “supercapattery”. The development of supercapattery materials can benefit from the advances in battery and supercapacitor materials. Nanotechnologies and engineering will play a more significant role in advancing micro supercapatteries in powering IoTs. Like LiBs, supercapatteries are on the verge of revolutionizing the whole ecosystem of energy storage and related industries, including renewable energy, portable electronics, and the EV industry.
Acknowledgments
This work was supported by the Brain Pool Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (RS-2023-00222798 and RS-2022-00156291).
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- A.J. Gabric, The Climate Change Crisis: A Review of Its Causes and Possible Responses, Atmosphere (Basel). 14 (2023) 1081. [CrossRef]
- K. Abbass, M.Z. Qasim, H. Song, M. Murshed, H. Mahmood, I. Younis, A review of the global climate change impacts, adaptation, and sustainable mitigation measures, Environmental Science and Pollution Research. 29 (2022) 42539–42559. [CrossRef]
- A. Dutta, S. Mitra, M. Basak, T. Banerjee, A comprehensive review on batteries and supercapacitors: Development and challenges since their inception, Energy Storage. 5 (2023). [CrossRef]
- J.B. Goodenough, K.-S. Park, The Li-Ion Rechargeable Battery: A Perspective, J Am Chem Soc. 135 (2013) 1167–1176. [CrossRef]
- H.S. Choi, C.R. Park, Theoretical guidelines to designing high performance energy storage device based on hybridization of lithium-ion battery and supercapacitor, J Power Sources. 259 (2014) 1–14. [CrossRef]
- M.S. Kiai, O. Eroglu, N. Aslfattahi, Metal-Ion Batteries: Achievements, Challenges, and Prospects, Crystals (Basel). 13 (2023). [CrossRef]
- J. Verma, D. Kumar, Metal-ion batteries for electric vehicles: current state of the technology, issues and future perspectives, Nanoscale Adv. 3 (2021) 3384–3394. [CrossRef]
- D.P. Chatterjee, A.K. Nandi, A review on the recent advances in hybrid supercapacitors, J Mater Chem A Mater. 9 (2021) 15880–15918. [CrossRef]
- D. Gao, Z. Luo, C. Liu, S. Fan, A survey of hybrid energy devices based on supercapacitors, Green Energy and Environment. 8 (2023) 972–988. [CrossRef]
- A. Yu, V. Chabot, J. Zhang, ELECTROCHEMICAL SUPERCAPACITORS FOR ENERGY STORAGE AND DELIVERY FUNDAMENTALS AND APPLICATIONS, 2013.
- M.Z. Iqbal, M.M. Faisal, S.R. Ali, Integration of supercapacitors and batteries towards high-performance hybrid energy storage devices, Int J Energy Res. 45 (2021) 1449–1479. [CrossRef]
- L. Xia, B. Tang, J. Wei, Z. Zhou, Recent Advances in Alkali Metal-Ion Hybrid Supercapacitors, Batter Supercaps. 4 (2021) 1108–1121. [CrossRef]
- D. Majumdar, M. Mandal, S.K. Bhattacharya, Journey from supercapacitors to supercapatteries: recent advancements in electrochemical energy storage systems, Emergent Mater. 3 (2020) 347–367. [CrossRef]
- L. Yu, G.Z. Chen, Supercapatteries as High-Performance Electrochemical Energy Storage Devices, Electrochemical Energy Reviews. 3 (2020) 271–285. [CrossRef]
- M. Prajapati, V. Singh, M. V. Jacob, C. Ravi Kant, Recent advancement in metal-organic frameworks and composites for high-performance supercapatteries, Renewable and Sustainable Energy Reviews. 183 (2023). [CrossRef]
- A.J. Bard, L.R. Faulkner, H.S. White, Electrochemical methods: fundamentals and applications, John Wiley & Sons, 2022.
- G.Z. Chen, Supercapacitor and supercapattery as emerging electrochemical energy stores, International Materials Reviews. 62 (2017) 173–202. [CrossRef]
- L. Guan, L. Yu, G.Z. Chen, Capacitive and non-capacitive faradaic charge storage, Electrochim Acta. 206 (2016) 464–478. [CrossRef]
- Y. Shao, M.F. El-Kady, J. Sun, Y. Li, Q. Zhang, M. Zhu, H. Wang, B. Dunn, R.B. Kaner, Design and Mechanisms of Asymmetric Supercapacitors, Chem Rev. 118 (2018) 9233–9280. [CrossRef]
- S. Sarker, H.W. Seo, Y.-K. Jin, K.-S. Lee, M. Lee, D.M. Kim, On the Hysteresis of Current Density-Voltage Curves of Dye-sensitized Solar Cells, Electrochim Acta. 182 (2015) 493–499. [CrossRef]
- S. Sarker, A.J.S. Ahammad, H.W. Seo, D.M. Kim, Electrochemical Impedance Spectra of Dye-Sensitized Solar Cells: Fundamentals and Spreadsheet Calculation, International Journal of Photoenergy. 2014 (2014) 1–17. [CrossRef]
- M. ITAGAKI, S. SUZUKI, I. SHITANDA, K. WATANABE, Electrochemical Impedance and Complex Capacitance to Interpret Electrochemical Capacitor, Electrochemistry. 75 (2007) 649–655. [CrossRef]
- T.S. Mathis, N. Kurra, X. Wang, D. Pinto, P. Simon, Y. Gogotsi, Energy Storage Data Reporting in Perspective—Guidelines for Interpreting the Performance of Electrochemical Energy Storage Systems, Adv Energy Mater. 9 (2019). [CrossRef]
- K. Kannadasan, V. Sankar Devi, S. Archana, P. Thomas, P. Elumalai, Deconvolution of capacitive and diffusive charge/lithium storage in lyophilized NiCo2S4-NiCo2O4 composite for supercapattery and lithium-ion battery, New Journal of Chemistry. 47 (2023) 13963–13978. [CrossRef]
- M. Winter, R.J. Brodd, What Are Batteries, Fuel Cells, and Supercapacitors?, Chem Rev. 104 (2004) 4245–4270. [CrossRef]
- L. Yu, G.Z. Chen, Redox electrode materials for supercapatteries, J Power Sources. 326 (2016) 604–612. [CrossRef]
- B. Akinwolemiwa, G.Z. Chen, Fundamental consideration for electrochemical engineering of supercapattery, J Braz Chem Soc. 29 (2018) 960–972. [CrossRef]
- S. Dsoke, K. Pfeifer, Z. Zhao, The role of nanomaterials for supercapacitors and hybrid devices, in: Frontiers of Nanoscience, Elsevier Ltd., 2021: pp. 99–136. [CrossRef]
- B. Akinwolemiwa, C. Wei, G.Z. Chen, Mechanisms and Designs of Asymmetrical Electrochemical Capacitors, Electrochim Acta. 247 (2017) 344–357. [CrossRef]
- J. Zhao, A.F. Burke, Review on supercapacitors: Technologies and performance evaluation, Journal of Energy Chemistry. 59 (2021) 276–291. [CrossRef]
- P. Sinha, K.K. Kar, Introduction to Supercapacitors, in: K.K. Kar (Ed.), Handbook of Nanocomposite Supercapacitor Materials II, Springer, 2020: pp. 1–28. [CrossRef]
- M.Z. Iqbal, U. Aziz, Supercapattery: Merging of battery-supercapacitor electrodes for hybrid energy storage devices, J Energy Storage. 46 (2022). [CrossRef]
- Poonam, K. Sharma, A. Arora, S.K. Tripathi, Review of supercapacitors: Materials and devices, J Energy Storage. 21 (2019) 801–825. [CrossRef]
- V. Augustyn, P. Simon, B. Dunn, Pseudocapacitive oxide materials for high-rate electrochemical energy storage, Energy Environ Sci. 7 (2014) 1597. [CrossRef]
- Y. Liu, S.P. Jiang, Z. Shao, Intercalation pseudocapacitance in electrochemical energy storage: recent advances in fundamental understanding and materials development, Mater Today Adv. 7 (2020) 100072. [CrossRef]
- Y. Jiang, J. Liu, Definitions of Pseudocapacitive Materials: A Brief Review, Energy and Environmental Materials. 2 (2019) 30–37. [CrossRef]
- B.E. Conway, Two-dimensional and quasi-two-dimensional isotherms for Li intercalation and upd processes at surfaces, Electrochim Acta. 38 (1993) 1249–1258. [CrossRef]
- E. Herrero, L.J. Buller, H.D. Abruña, Underpotential Deposition at Single Crystal Surfaces of Au, Pt, Ag and Other Materials, Chem Rev. 101 (2001) 1897–1930. [CrossRef]
- H.W. Park, K.C. Roh, Recent advances in and perspectives on pseudocapacitive materials for Supercapacitors–A review, J Power Sources. 557 (2023) 232558. [CrossRef]
- R. Barik, P.P. Ingole, Challenges and prospects of metal sulfide materials for supercapacitors, Curr Opin Electrochem. 21 (2020) 327–334. [CrossRef]
- T. Brousse, D. Bélanger, J.W. Long, To Be or Not To Be Pseudocapacitive?, J Electrochem Soc. 162 (2015) A5185–A5189. [CrossRef]
- P. Lamba, P. Singh, P. Singh, P. Singh, Bharti, A. Kumar, M. Gupta, Y. Kumar, Recent advancements in supercapacitors based on different electrode materials: Classifications, synthesis methods and comparative performance, J Energy Storage. 48 (2022). [CrossRef]
- A.G. Olabi, Q. Abbas, P.A. Shinde, M.A. Abdelkareem, Rechargeable batteries: Technological advancement, challenges, current and emerging applications, Energy. 266 (2023) 126408. [CrossRef]
- S.A. Khan, S. Ali, K. Saeed, M. Usman, I. Khan, Advanced cathode materials and efficient electrolytes for rechargeable batteries: Practical challenges and future perspectives, J Mater Chem A Mater. 7 (2019) 10159–10173. [CrossRef]
- Q. Liu, H. Wang, C. Jiang, Y. Tang, Multi-ion strategies towards emerging rechargeable batteries with high performance, Energy Storage Mater. 23 (2019) 566–586. [CrossRef]
- B. Halford, Lithium-ion battery pioneers nab 2019 Nobel Prize in Chemistry, (2019). https://cen.acs.org/people/nobel-prize/Li-ion-batteries-win-2019-Nobel-Prize-in-Chemistry/97/web/2019/10 (accessed November 25, 2023).
- W. Chen, J. Liang, Z. Yang, G. Li, A Review of Lithium-Ion Battery for Electric Vehicle Applications and Beyond, Energy Procedia. 158 (2019) 4363–4368. [CrossRef]
- P.U. Nzereogu, A.D. Omah, F.I. Ezema, E.I. Iwuoha, A.C. Nwanya, Anode materials for lithium-ion batteries: A review, Applied Surface Science Advances. 9 (2022) 100233. [CrossRef]
- S.-W. WOO, K. DOKKO, H. NAKANO, K. KANAMURA, Bimodal Porous Carbon as a Negative Electrode Material for Lithium-Ion Capacitors, Electrochemistry. 75 (2007) 635–640. [CrossRef]
- H. Wang, C. Zhu, D. Chao, Q. Yan, H.J. Fan, Nonaqueous Hybrid Lithium-Ion and Sodium-Ion Capacitors, Advanced Materials. 29 (2017). [CrossRef]
- Z.-K. Chen, J.-W. Lang, L.-Y. Liu, L.-B. Kong, Preparation of a NbN/graphene nanocomposite by solution impregnation and its application in high-performance Li-ion hybrid capacitors, RSC Adv. 7 (2017) 19967–19975. [CrossRef]
- M. Arnaiz, J.L. Gómez-Cámer, J. Ajuria, F. Bonilla, B. Acebedo, M. Jáuregui, E. Goikolea, M. Galceran, T. Rojo, High Performance Titanium Antimonide TiSb 2 Alloy for Na-Ion Batteries and Capacitors, Chemistry of Materials. 30 (2018) 8155–8163. [CrossRef]
- B. Akinwolemiwa, C. Peng, G.Z. Chen, Redox Electrolytes in Supercapacitors, J Electrochem Soc. 162 (2015) A5054–A5059. [CrossRef]
- W. Shimizu, S. Makino, K. Takahashi, N. Imanishi, W. Sugimoto, Development of a 4.2 V aqueous hybrid electrochemical capacitor based on MnO2 positive and protected Li negative electrodes, J Power Sources. 241 (2013) 572–577. [CrossRef]
- S. Makino, Y. Shinohara, T. Ban, W. Shimizu, K. Takahashi, N. Imanishi, W. Sugimoto, 4 V class aqueous hybrid electrochemical capacitor with battery-like capacity, RSC Adv. 2 (2012) 12144. [CrossRef]
- D. Hu, C. Peng, G.Z. Chen, Electrodeposition of Nonconducting Polymers: Roles of Carbon Nanotubes in the Process and Products, ACS Nano. 4 (2010) 4274–4282. [CrossRef]
- S. Park, N.M. Shinde, P. V. Shinde, D. Lee, J.M. Yun, K.H. Kim, Chemically grown bismuth-oxy-iodide (BiOI/Bi9I2) nanostructure for high performance battery-type supercapacitor electrodes, Dalton Transactions. 49 (2020) 774–780. [CrossRef]
- N. Padmanathan, H. Shao, K.M. Razeeb, Honeycomb micro/nano-architecture of stable β-NiMoO4 electrode/catalyst for sustainable energy storage and conversion devices, Int J Hydrogen Energy. 45 (2020) 30911–30923. [CrossRef]
- M. Kang, H. Zhou, B. Qin, N. Zhao, B. Lv, Ultrathin nanosheet-assembled, phosphate ion-functionalized NiO microspheres as efficient supercapacitor materials, ACS Appl Energy Mater. 3 (2020) 9980–9988. [CrossRef]
- M.Z. Iqbal, J. Khan, H.T.A. Awan, M. Alzaid, A.M. Afzal, S. Aftab, Cobalt-manganese-zinc ternary phosphate for high performance supercapattery devices, Dalton Transactions. 49 (2020) 16715–16727. [CrossRef]
- T. Yan, H. Feng, X. Ma, L. Han, L. Zhang, S. Cao, Regulating the electrochemical behaviours of a hierarchically structured Co3(PO4)2/Ni-Co-O for a high-performance all-solid-state supercapacitor, Dalton Transactions. 49 (2020) 10621–10630. [CrossRef]
- B. Huang, H. Wang, S. Liang, H. Qin, Y. Li, Z. Luo, C. Zhao, L. Xie, L. Chen, Two-dimensional porous cobalt–nickel tungstate thin sheets for high performance supercapattery, Energy Storage Mater. 32 (2020) 105–114. [CrossRef]
- P. Thondaiman, C.J. Raj, R. Velayutham, A.D. Savariraj, R. Manikandan, V. Cristobal, B.C. Kim, Engineering redox active sites enriched 3D-on-2D bimetallic double layered hydroxide electrode for supercapatteries, Mater Today Energy. 30 (2022). [CrossRef]
- M. Amiri, S.E. Moosavifard, S.S. Hosseiny Davarani, M. Shamsipur, Novel Rugby-Ball-like FeCoCuS2Triple-Shelled Hollow Nanostructures with Enhanced Performance for Supercapattery, Energy and Fuels. 35 (2021) 15108–15117. [CrossRef]
- M. Alzaid, M.Z. Iqbal, J. Khan, S. Alam, N.M.A. Hadia, W.S. Mohamed, Drive towards Sonochemically Synthesized Ternary Metal Sulfide for High-Energy Supercapattery, Energy Technology. 9 (2021). [CrossRef]
- M.Z. Iqbal, M.M. Faisal, S.R.A. Meshal Alzaid, A facile approach to investigate the charge storage mechanism of MOF/PANI based supercapattery devices, Solid State Ion. 354 (2020). [CrossRef]
- M.Z. Iqbal, M.M. Faisal, S.R. Ali, A.M. Afzal, M.R. Abdul Karim, M.A. Kamran, T. Alharbi, Strontium phosphide-polyaniline composites for high performance supercapattery devices, Ceram Int. 46 (2020) 10203–10214. [CrossRef]
- V. Sharmila, R. Packiaraj, N. Nallamuthu, M. Parthibavarman, Fabrication of MWCNTs wrapped nickel manganese phosphate asymmetric capacitor as a supercapattery electrode for energy storage applications, Inorg Chem Commun. 121 (2020). [CrossRef]
- C. Murugan, K. Subramani, R. Subash, M. Sathish, A. Pandikumar, High-performance high-voltage symmetric supercapattery based on a graphitic carbon nitride/bismuth vanadate nanocomposite, Energy and Fuels. 34 (2020) 16858–16869. [CrossRef]
- V. Sankar Devi, K. Kannadasan, P.C. Sharafudeen, P. Elumalai, Performance of sodium-ion supercapattery using LaMnO3 and rGO in non-aqueous electrolyte, New Journal of Chemistry. 46 (2022) 15130–15144. [CrossRef]
- L. Gurusamy, L. Karuppasamy, S. Anandan, N. Liu, G.J. Lee, C.H. Liu, J.J. Wu, Enhanced performance of charge storage supercapattery by dominant oxygen deficiency in crystal defects of 2-D MoO3-x nanoplates, Appl Surf Sci. 541 (2021). [CrossRef]
- M.P. Harikrishnan, A. Chandra Bose, Binder-free synthesis of cerium nickel oxide for supercapattery devices, Int J Energy Res. 46 (2022) 21826–21840. [CrossRef]
- A.M. Kale, R. Velayutham, A.D. Savariraj, M. Demir, B.C. Kim, Unravelling the influence of interfacial tailoring in metal-organic framework-derived ultrathin sheets of Co2P/Cu3P for high-performance hybrid supercapacitor, Materials Today Sustainability. 21 (2023). [CrossRef]
- A.T. Sivagurunathan, T. Kavinkumar, S. Seenivasan, Y. Kwon, D.-H. Kim, Enhancing high-performance supercapattery electrodes: harnessing structural and compositional synergies via phosphorus doping on bimetallic boride for rapid charging , J Mater Chem A Mater. (2023). [CrossRef]
- F. Yu, C. Zhang, F. Wang, Y. Gu, P. Zhang, E.R. Waclawik, A. Du, K. Ostrikov, H. Wang, A zinc bromine “supercapattery” system combining triple functions of capacitive, pseudocapacitive and battery-type charge storage, Mater Horiz. 7 (2020) 495–503. [CrossRef]
- T.A. Raja, P. Vickraman, Role of dual redox additives KI/VOSO4 in manganese ammonium phosphate at graphene quantum dots for supercapattery, Int J Energy Res. 46 (2022) 9097–9113. [CrossRef]
- J. Lee, P. Srimuk, S. Fleischmann, X. Su, T.A. Hatton, V. Presser, Redox-electrolytes for non-flow electrochemical energy storage: A critical review and best practice, Prog Mater Sci. 101 (2019) 46–89. [CrossRef]
- B. Akinwolemiwa, C. Wei, Q. Yang, L. Yu, L. Xia, D. Hu, C. Peng, G.Z. Chen, Optimal Utilization of Combined Double Layer and Nernstian Charging of Activated Carbon Electrodes in Aqueous Halide Supercapattery through Capacitance Unequalization, J Electrochem Soc. 165 (2018) A4067–A4076. [CrossRef]
- M.P. Harikrishnan, A.C. Bose, Porous CeNiO3 with an enhanced electrochemical performance and prolonged cycle life (>50 000 cycles) via a lemon-assisted sol-gel autocombustion method, New Journal of Chemistry. 46 (2022) 15118–15129. [CrossRef]
- Z.H. Ren, Z.R. Zhang, L.J. Ma, C.Y. Luo, J. Dai, Q.Y. Zhu, Oxidatively Doped Tetrathiafulvalene-Based Metal-Organic Frameworks for High Specific Energy of Supercapatteries, ACS Appl Mater Interfaces. 15 (2023) 6621–6630. [CrossRef]
- S.R. Ali, M.M. Faisal, S.L. Loredo, S.K. Gadi, K.C. Sanal, Anomalous electrochemical performance of binary silver–strontium phosphate-based electrode material in supercapattery, Ceram Int. 49 (2023) 18311–18321. [CrossRef]
- H.H. Hegazy, A.M. Afzal, E.R. Shaaban, M. Waqas Iqbal, S. Muhammad, A.A. Alahmari, Synthesis of MXene and design the high-performance energy harvesting devices with multifunctional applications, Ceram Int. 49 (2023) 1710–1719. [CrossRef]
- M.Z. Iqbal, U. Aziz, N. Amjad, S. Aftab, S.M. Wabaidur, Porous activated carbon and highly redox active transition metal sulfide by employing multi-synthesis approaches for battery-supercapacitor applications, Diam Relat Mater. 136 (2023). [CrossRef]
- S.K. Babu, B. Gunasekaran, Ultrathin α-Ni(OH)2 nanosheets coated on MOF-derived Fe2O3 nanorods as a potential electrode for solid-state hybrid supercapattery device, Electrochim Acta. 447 (2023). [CrossRef]
- M. Ali, A.M. Afzal, M.W. Iqbal, A. Ur Rehman, S.M. Wabaidur, E.A. Al-Ammar, S. Mumtaz, E.H. Choi, Synthesis and analysis of the impact of rGO on the structural and electrochemical performance of CoMnS for high-performance energy storage device, FlatChem. 40 (2023). [CrossRef]
- A. Zaka, M.W. Iqbal, A.M. Afzal, H. Hassan, H. Rafique, S.M. Wabaidur, A.M. Tawfeek, E. Elahi, A bimetallic Fe-Mg MOF with a dual role as an electrode in asymmetric supercapacitors and an efficient electrocatalyst for hydrogen evolution reaction (HER), RSC Adv. 13 (2023) 26528–26543. [CrossRef]
- S.R. Ali, M.M. Faisal, S. Pushpan, N.P. Aguilar, K.K. Singh, A. Cerdán-Pasarán, M.M.A. Rodríguez, E.M. Sánchez, A.T. Castro, S.L. Loredo, K.C. Sanal, Mesoporous silver-cobalt-phosphate nanostructures synthesized via hydrothermal and solid-state reaction for supercapattery devices, Int J Energy Res. 46 (2022) 23757–23774. [CrossRef]
- M.Z. Iqbal, U. Aziz, M.W. Khan, S. Siddique, M. Alzaid, Strategies to enhance the electrochemical performance of strontium-based electrode materials for battery-supercapacitor applications, Journal of Electroanalytical Chemistry. 924 (2022). [CrossRef]
- M.R. Khawar, N.A. Shad, S. Hussain, Y. Javed, M.M. Sajid, A. Jilani, M. Faheem, A. Asghar, Cerium oxide nanosheets-based tertiary composites (CeO2/ZnO/ZnWO4) for supercapattery application and evaluation of faradic & non-faradic capacitive distribution by using Donn’s model, J Energy Storage. 55 (2022). [CrossRef]
- M.Z. Iqbal, M.W. Khan, M. Shaheen, S. Siddique, S. Aftab, M. Alzaid, M.J. Iqbal, Evaluation of d-block metal sulfides as electrode materials for battery-supercapacitor energy storage devices, J Energy Storage. 55 (2022). [CrossRef]
- S. Alam, M.Z. Iqbal, N. Amjad, R. Ali, M. Alzaid, Magnetron sputtered tungsten di-sulfide: An efficient battery grade electrode for supercapattery devices, J Energy Storage. 46 (2022). [CrossRef]
- Z.Y. Hong, L.C. Chen, Y.C.M. Li, H.L. Hsu, C.M. Huang, Response Surface Methodology Optimization in High-Performance Solid-State Supercapattery Cells Using NiCo2S4–Graphene Hybrids, Molecules. 27 (2022). [CrossRef]
- Y.Y. How, F. Bibi, A. Numan, R. Walvekar, P. Jagadish, M. Khalid, J. Iqbal, N.M. Mubarak, Fabrication of binary metal phosphate-based binder-free electrode for new generation energy storage device, Surf Coat Technol. 429 (2022). [CrossRef]
- M. Roman, S.S.A. Gillani, S. Farid, M. Shakil, R. Ahmad, M.Z. Iqbal, M.M. Faisal, I. Ahmed, S. Alam, Exalted redox frameworks of Cu-MOF/polyaniline/RGO based composite electrodes by integrating silver nanoparticles as a catalytic agent for superior energy featured supercapatteries, Electrochim Acta. 400 (2021). [CrossRef]
- M.Z. Iqbal, J. Khan, Optimization of cobalt-manganese binary sulfide for high performance supercapattery devices, Electrochim Acta. 368 (2021). [CrossRef]
- M.K. Raihana, N. Padmanathan, V. Eswaramoorthi, D. McNulty, J. Sahadevan, P. Mohanapriya, S.E. Muthu, Reduced graphene oxide/VSB-5 composite micro/nanorod electrode for high energy density supercapattery, Electrochim Acta. 391 (2021). [CrossRef]
- M.Z. Iqbal, J. Khan, S. Alam, R. Ali, M.J. Iqbal, A.M. Afzal, S. Aftab, Enhanced electrochemical performance of battery-grade cobalt phosphate via magnetron sputtered copper interfacial layer for potential supercapattery applications, Int J Energy Res. 45 (2021) 18658–18669. [CrossRef]
- S. Alam, M.Z. Iqbal, J. Khan, Green synthesis of nickel-manganese/polyaniline-based ternary composites for high-performance supercapattery devices, Int J Energy Res. 45 (2021) 11109–11122. [CrossRef]
- M.Z. Iqbal, J. Khan, S. Siddique, A.M. Afzal, S. Aftab, Optimizing electrochemical performance of sonochemically and hydrothermally synthesized cobalt phosphate for supercapattery devices, Int J Hydrogen Energy. 46 (2021) 15807–15819. [CrossRef]
- K. Nasrin, K. Subramani, M. Karnan, M. Sathish, MnCo2S4 – MXene: A novel hybrid electrode material for high performance long-life asymmetric supercapattery, J Colloid Interface Sci. 600 (2021) 264–277. [CrossRef]
- M.Z. Iqbal, S. Alam, J. Khan, R. Ali, A. Muhammad Afzal, M. Alzaid, S. Aftab, Synergestic effect of magnetron sputtered silver nano-islands and Co3(PO4)2 for high performance supercapattery devices, Journal of Electroanalytical Chemistry. 898 (2021). [CrossRef]
- M. Alzaid, M.Z. Iqbal, S. Alam, N. Almoisheer, A.M. Afzal, S. Aftab, Binary composites of nickel-manganese phosphates for supercapattery devices, J Energy Storage. 33 (2021). [CrossRef]
- M.Z. Iqbal, J. Khan, A. Gul, S. Siddique, M. Alzaid, M. Saleem, M.J. Iqbal, Copper doped cobalt-manganese phosphate ternary composites for high-performance supercapattery devices, J Energy Storage. 35 (2021). [CrossRef]
- T.S. Renani, S.M. Khoshfetrat, J. Arjomandi, H. Shi, S. Khazalpour, Fabrication and design of new redox active azure A/3D graphene aerogel and conductive trypan blue-nickel MOF nanosheet array electrodes for an asymmetric supercapattery, J Mater Chem A Mater. 9 (2021) 12853–12869. [CrossRef]
- T. Kavinkumar, S. Seenivasan, A.T. Sivagurunathan, D.H. Kim, Supercapattery driven electrolyzer both empowered by the same superb electrocatalyst, J Mater Chem A Mater. 9 (2021) 21750–21759. [CrossRef]
- Y. Li, Z. Luo, S. Liang, H. Qin, X. Zhao, L. Chen, H. Wang, S. Chen, Two-dimensional porous zinc cobalt sulfide nanosheet arrays with superior electrochemical performance for supercapatteries, J Mater Sci Technol. 89 (2021) 199–208. [CrossRef]
- M. Alzaid, M.Z. Iqbal, S. Siddique, N.M.A. Hadia, Exploring the electrochemical performance of copper-doped cobalt-manganese phosphates for potential supercapattery applications, RSC Adv. 11 (2021) 28042–28051. [CrossRef]
- J.J. Zhou, K. Li, W. Wang, Y. Lei, J. Wu, C. Zhou, P. Zhang, J. Liu, Z. Hua, L. Chen, L. Han, Boosting Specific Capacity for Supercapattery by in Situ Formation of Amorphous Ni-Co-Borate on MOF-Derived Ni-Co-LDH Nanosheet Array, ACS Appl Energy Mater. 3 (2020) 12046–12053. [CrossRef]
- J. Aftab, S. Mehmood, A. Ali, I. Ahmad, M.F. Bhopal, M.Z.U. Rehman, M.Z.U. Shah, A.U. Shah, M. Wang, M.F. Khan, A.S. Bhatti, Synergetic electrochemical performance of tungsten oxide/tungsten disulfide/MWCNTs for high-performance aqueous asymmetric supercapattery devices, J Alloys Compd. 965 (2023). [CrossRef]
- M. Fawad Khan, M. Ali Marwat, Abdullah, S. Shaheen Shah, M.R. Abdul Karim, M. Abdul Aziz, Z. Ud Din, Saad Ali, K. Muhammad Adam, Novel MoS2-sputtered NiCoMg MOFs for high-performance hybrid supercapacitor applications, Sep Purif Technol. 310 (2023). [CrossRef]
- W. Shehzad, M.R.A. Karim, M.Z. Iqbal, N. Shahzad, A. Ali, Sono-chemical assisted synthesis of carbon nanotubes-nickel phosphate nanocomposites with excellent energy density and cyclic stability for supercapattery applications, J Energy Storage. 54 (2022). [CrossRef]
- L.G. Ghanem, M.M. Taha, M. Salama, N.K. Allam, Binder-free Mn-V-Sn oxyhydroxide decorated with metallic Sn as an earth-abundant supercapattery electrode for intensified energy storage, Sustain Energy Fuels. (2022). [CrossRef]
- M. Moradi, M. Mousavi, M. Pooriraj, M. Babamoradi, S. Hajati, Enhanced pseudocapacitive performance of two-dimensional Zn-metal organic framework through a post-synthetic amine functionalization, Thin Solid Films. 749 (2022). [CrossRef]
- M.Z. Iqbal, J. Khan, A.M. Afzal, S. Aftab, Exploring the synergetic electrochemical performance of cobalt sulfide/cobalt phosphate composites for supercapattery devices with high-energy and rate capability, Electrochim Acta. 384 (2021). [CrossRef]
- H. Wu, Z. Qiu, Fe3O4@N-porous carbon nano rice/rGO sheet as positive electrode material for a high performance supercapattery, J Alloys Compd. 879 (2021). [CrossRef]
- G. Liu, J. Xie, Y. Sun, P. Zhang, X. Li, L. Zheng, L. Hao, G. Shanmin, Constructing 3D honeycomb-like CoMn2O4 nanoarchitecture on nitrogen-doped graphene coating Ni foam as flexible battery-type electrodes for advanced supercapattery, Int J Hydrogen Energy. 46 (2021) 36314–36322. [CrossRef]
Figure 1.
a) Number of articles and b) percent of total number of articles published on supercapatteries, rechargeable batteries (RBs), and supercapacitors (SCs) over the last ten years (from 2013 to 2023).
Figure 1.
a) Number of articles and b) percent of total number of articles published on supercapatteries, rechargeable batteries (RBs), and supercapacitors (SCs) over the last ten years (from 2013 to 2023).
Figure 2.
Ragone plot of different energy storage devices showing relative energy and power density for supercapacitors, rechargeable batteries, redox flow batteries, fuel cells, and supercapatteries [15].
Figure 2.
Ragone plot of different energy storage devices showing relative energy and power density for supercapacitors, rechargeable batteries, redox flow batteries, fuel cells, and supercapatteries [15].
Figure 3.
Archetypal electrical output behavior of three main types of electrodes, including a,d) electrical double layer, b, e) pseudocapacitive, and c,f) battery type. a–c) Schematic of galvanostatic charge-discharge (GCD) profiles showing linear and nonlinear responses with time and d–f) corresponding cyclic voltammetry (CV) profiles [23].
Figure 3.
Archetypal electrical output behavior of three main types of electrodes, including a,d) electrical double layer, b, e) pseudocapacitive, and c,f) battery type. a–c) Schematic of galvanostatic charge-discharge (GCD) profiles showing linear and nonlinear responses with time and d–f) corresponding cyclic voltammetry (CV) profiles [23].
Figure 4.
Schematic illustration of the typical GCD (up) and CV (bottom) curves depicting characteristics of (a and d) a capacitive asymmetric supercapacitor, (b and e) a hybrid capacitor, and (c and f) a battery [53].
Figure 4.
Schematic illustration of the typical GCD (up) and CV (bottom) curves depicting characteristics of (a and d) a capacitive asymmetric supercapacitor, (b and e) a hybrid capacitor, and (c and f) a battery [53].
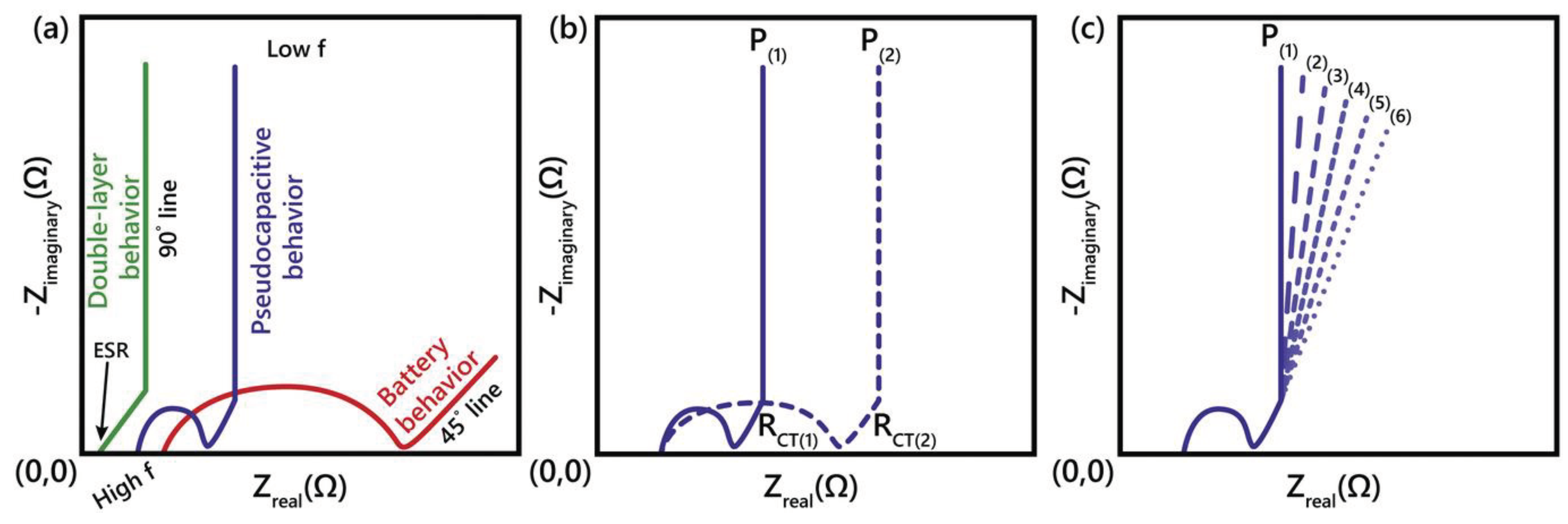
Figure 5.
a) Typical Nyquist plot representations for an EDLC (green curve), pseudocapacitive materials (blue) and battery (red). ESR, 45° and 90° lines0020are marked, b) Example spectra of confirming real charge transfer resistance (RCT) by doing EIS at two different potentials (solid and dotted blue curves), in contrast to c) where interfacial impedance would lead to constant RCT at all potentials. Pseudocapacitive materials will show minimal diffusion-limited behavior (meaning no diffusion limitations relative to batteries)[23].
Figure 5.
a) Typical Nyquist plot representations for an EDLC (green curve), pseudocapacitive materials (blue) and battery (red). ESR, 45° and 90° lines0020are marked, b) Example spectra of confirming real charge transfer resistance (RCT) by doing EIS at two different potentials (solid and dotted blue curves), in contrast to c) where interfacial impedance would lead to constant RCT at all potentials. Pseudocapacitive materials will show minimal diffusion-limited behavior (meaning no diffusion limitations relative to batteries)[23].
Figure 6.
Illustration of the electrode processes occurring at a) electrical double layer capacitive, b) pseudocapacitive, and c) Faradaic electrodes [23].
Figure 6.
Illustration of the electrode processes occurring at a) electrical double layer capacitive, b) pseudocapacitive, and c) Faradaic electrodes [23].
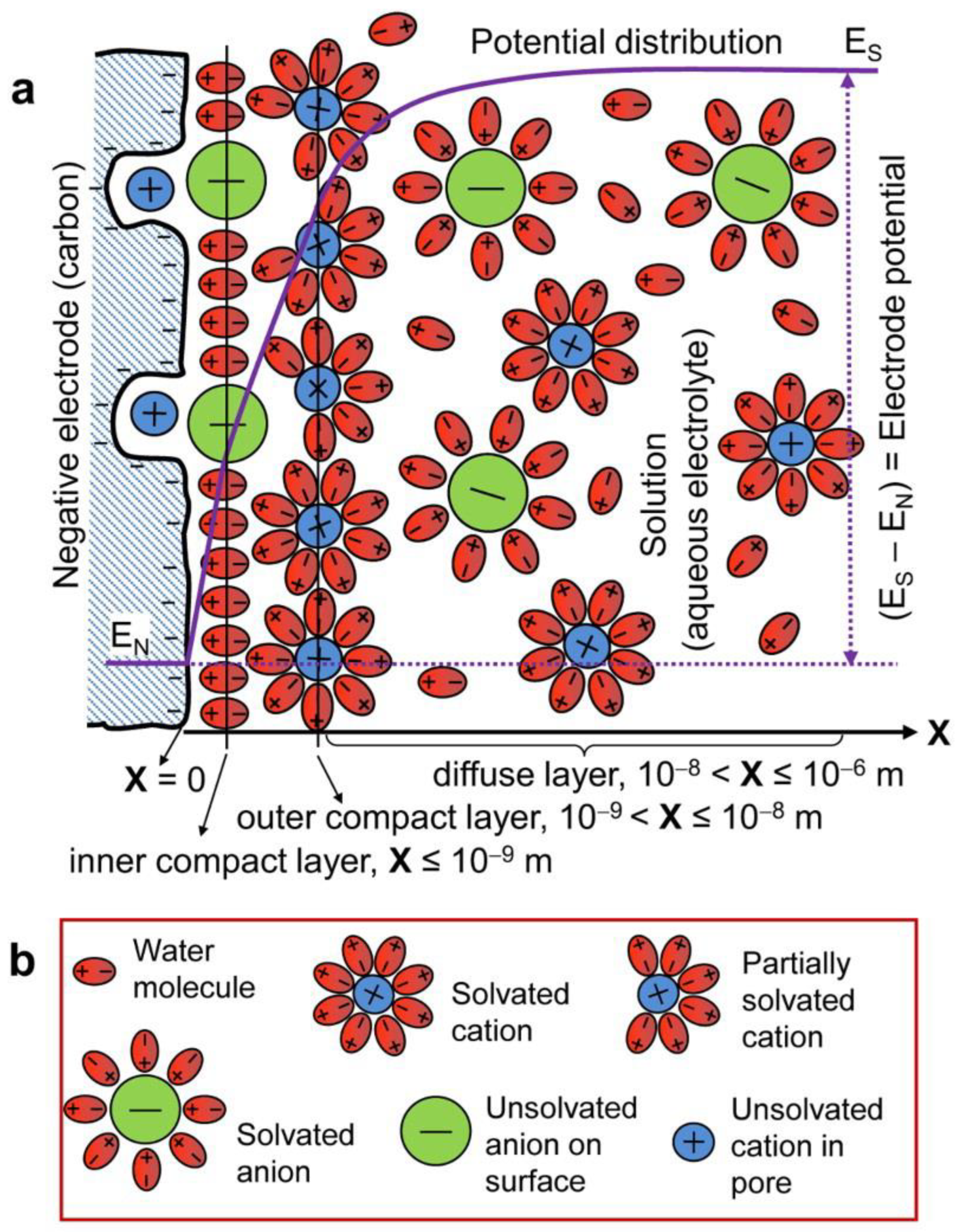
Figure 7.
Schematic representations of a) the EDL structure (cross-section) of the interface between a porous carbon negative electrode and an aqueous electrolyte; b) explanations of symbols in a) [16,17].
Figure 7.
Schematic representations of a) the EDL structure (cross-section) of the interface between a porous carbon negative electrode and an aqueous electrolyte; b) explanations of symbols in a) [16,17].
Figure 8.
Different types of reversible redox mechanisms that cause pseudocapacitance: a) underpotential deposition, b) redox pseudocapacitance, and c) intercalation pseudocapacitance [34].
Figure 8.
Different types of reversible redox mechanisms that cause pseudocapacitance: a) underpotential deposition, b) redox pseudocapacitance, and c) intercalation pseudocapacitance [34].
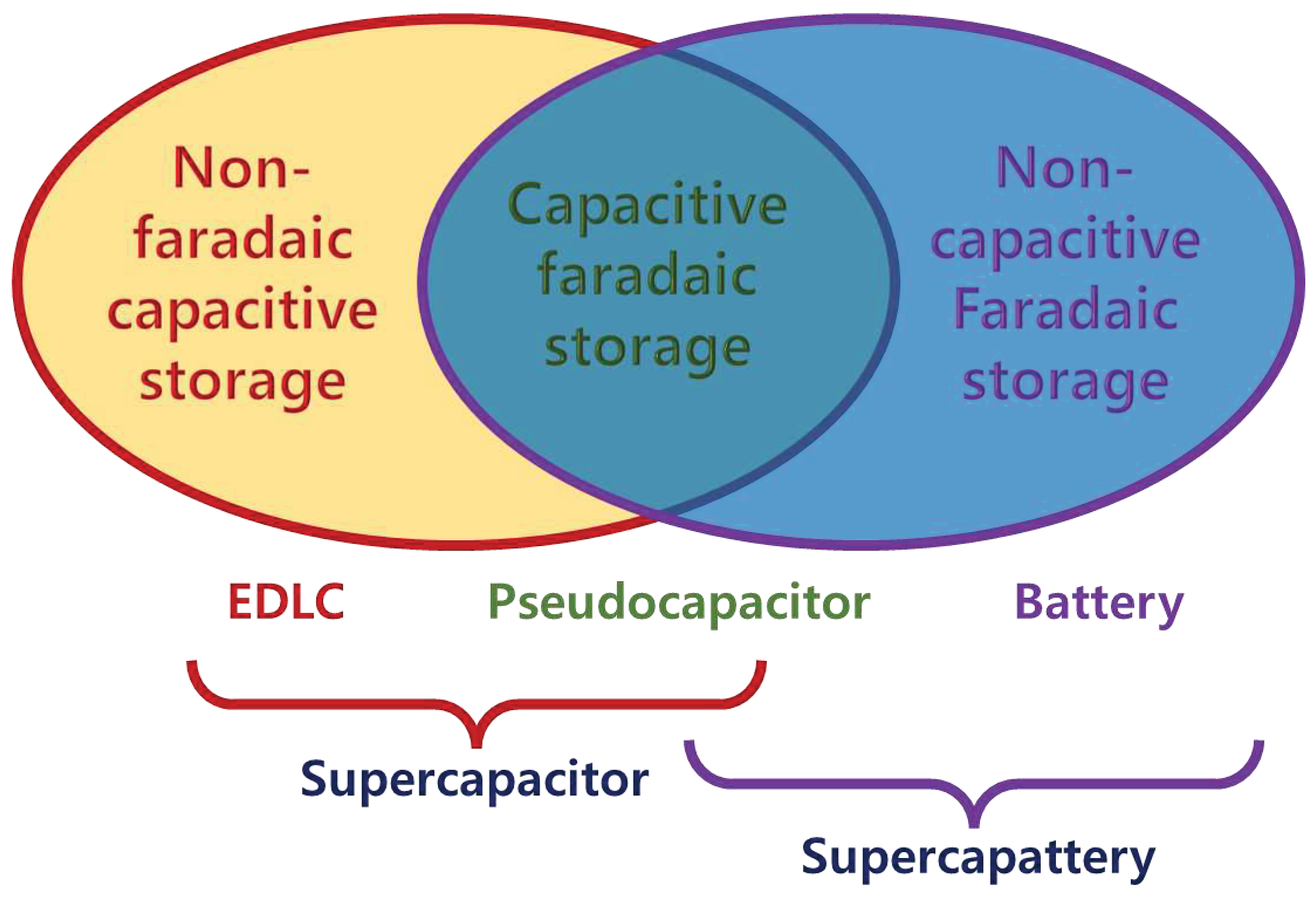
Figure 9.
Schematic correlation between EDL capacitor, pseudocapacitor, battery, and supercapattery according to charge storage processes [18].
Figure 9.
Schematic correlation between EDL capacitor, pseudocapacitor, battery, and supercapattery according to charge storage processes [18].
Figure 10.
Schematic illustration of the first Li-ion battery (LiCoO2/Li+ electrolyte/graphite) [4].
Figure 10.
Schematic illustration of the first Li-ion battery (LiCoO2/Li+ electrolyte/graphite) [4].
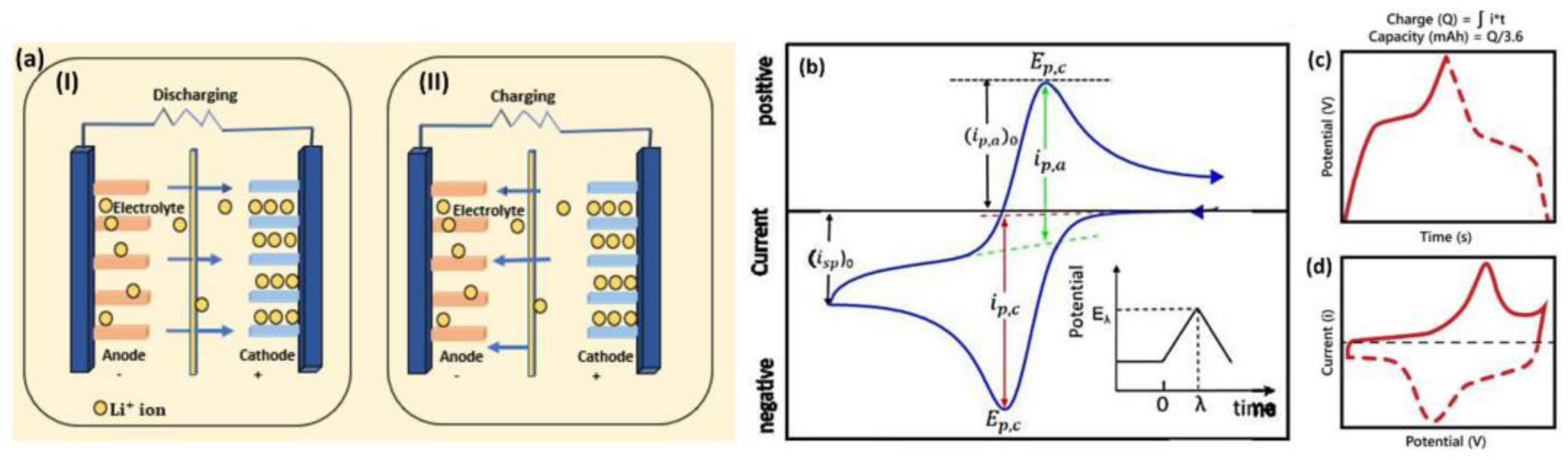
Figure 11.
Schematic diagram of a) charge storage mechanism: (i) charging and (ii) discharging, b) cyclic voltammogram (CV), and (c, d) galvanostatic charge-discharge (GCD) curves of LiBs [15].
Figure 11.
Schematic diagram of a) charge storage mechanism: (i) charging and (ii) discharging, b) cyclic voltammogram (CV), and (c, d) galvanostatic charge-discharge (GCD) curves of LiBs [15].
Figure 12.
Calculated GCD plots of positrodes (blue lines), negatrodes (black lines) and relevant cells (red dash lines): a) hypothetical pseudocapacitor with an Act-C negatrode and a pseudocapacitive positrode and a hypothetical supercapattery with a negatrode of Li metal or lithiated carbon and b) an Act-C positrode or c) a pseudocapacitive positrode. d) A hypothetical supercapattery with a typical battery-type negatrode and a pseudocapacitive positrode. e) Experimental demonstration of (a), (−) Act-C | KCl | PPy-CNT (+). f) Experimental demonstration of (b), (−) Li | IL + LiClO4 | Act-C (+). g) Experimental demonstration of (c), (−) Li | PEO-LiTFSI | LTAP | 1.0 M LiCl aq. | MnO2 (+). h) Experimental demonstration of (d), (−) C-MnOy-CNT | LiPF6| MnOx-CNT (+) [14].
Figure 12.
Calculated GCD plots of positrodes (blue lines), negatrodes (black lines) and relevant cells (red dash lines): a) hypothetical pseudocapacitor with an Act-C negatrode and a pseudocapacitive positrode and a hypothetical supercapattery with a negatrode of Li metal or lithiated carbon and b) an Act-C positrode or c) a pseudocapacitive positrode. d) A hypothetical supercapattery with a typical battery-type negatrode and a pseudocapacitive positrode. e) Experimental demonstration of (a), (−) Act-C | KCl | PPy-CNT (+). f) Experimental demonstration of (b), (−) Li | IL + LiClO4 | Act-C (+). g) Experimental demonstration of (c), (−) Li | PEO-LiTFSI | LTAP | 1.0 M LiCl aq. | MnO2 (+). h) Experimental demonstration of (d), (−) C-MnOy-CNT | LiPF6| MnOx-CNT (+) [14].
Figure 13.
Ragone plots of different supercapatteries reported over the last four years (20020-2023) showing relative energy and power density.
Figure 13.
Ragone plots of different supercapatteries reported over the last four years (20020-2023) showing relative energy and power density.
Figure 14.
Classification of various electrochemical energy storage (EES) systems based on storage mechanisms [8]. EES and supercapatteries are highlighted in yellow and green rectangles bordered with dashed lines [42].
Figure 14.
Classification of various electrochemical energy storage (EES) systems based on storage mechanisms [8]. EES and supercapatteries are highlighted in yellow and green rectangles bordered with dashed lines [42].
Table 1.
Ways of pairing the same or different electrode materials into supercapacitor, battery, supercapattery or supercabattery [17.].
Table 1.
Ways of pairing the same or different electrode materials into supercapacitor, battery, supercapattery or supercabattery [17.].
| Device |
|
Supercapattery |
Battery |
| Supercapacitor |
Hybrid |
Supercabattery |
| EDLC |
Pseudocapacitor |
| Electrode Material |
NFCS |
NFCS |
NFCS |
CFS |
CFS |
NFCS |
CFS |
NCFS |
NCFS |
| 1+1 |
1+2 |
1+3 |
1+1 |
1+2 |
1+3 |
1+1 |
1+1 |
1+2 |
| NFCS |
NFCS |
CFS |
CFS |
CFS |
NCFS |
NCFS |
NCFS |
NCFS |
Table 2.
Performance of supercapatteries with different configurations. The data was collected from articles published during the last four years (2020-2023).
Table 2.
Performance of supercapatteries with different configurations. The data was collected from articles published during the last four years (2020-2023).
| Device configuration |
Electrolyte |
Electrode
Type |
Energy density
(Wh/kg) |
Power density
(W/kg) |
Publication
Year |
Reference |
| CeNiO3/Ni foam (symmetric) |
6M KOH |
(+)RB//RB(-) |
38.70
28.41 |
774.81
7750 |
2022 |
[72] |
| CeNiO3/Ni foam (symmetric) |
3M KOH |
(+)RB//RB(-) |
43.45 |
800 |
2022 |
[79] |
| BiOI-Bi9I2/Ni foam (symmetric) |
6M KOH |
(+)RB//RB(-) |
38.2 |
2280.4 |
2020 |
[57] |
|
β-NiMoO4/Ni foam (symmetric)
|
3M KOH |
(+)RB//RB(-) |
35.8
21.3 |
981.56
19282.4 |
2020 |
[58] |
| Ni(py-TTF-py)(BPDC)/Ni foam//AC/NI foam |
6M KOH |
(+)RB//ED(-) |
90.3
47.2 |
1180
10400 |
2023 |
[80] |
| AgSr-Phosphate/Ni foam//CNT/Ni foam |
1M KOH |
(+)RB//ED(-) |
55.02
42.37 |
741.54
9075 |
2023 |
[81] |
| Mxene/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
68.8 |
1120 |
2023 |
[82] |
| NiS/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
61.76 |
1275 |
2023 |
[83] |
|
Fe2O3-α-Ni(OH)2/Ni foam//AC/Ni foam
|
1M KOH |
(+)RB//ED(-) |
44.51 |
2465 |
2023 |
[84] |
| CoMnS-rGO/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
45.6 |
2880 |
2023 |
[85] |
| CoCuP/Ni foam//O, N, S-AC/Ni foam |
6M KOH |
(+)RB//ED(-) |
37.3
18.4 |
915
12308.8 |
2023 |
[73] |
| Fe-Mg MOF/Ni foam//AC/Ni foam |
KOH |
(+)RB//ED(-) |
57 |
2393 |
2023 |
[86] |
| Ag2Co3(PO6)2/Ni foam//CNT/Ni foam |
1M KOH |
(+)RB//ED(-) |
40.92
33.26 |
1237.5
4125 |
2022 |
[87] |
| SrS/Ni foam//AC/Ni foam |
KOH |
(+)RB//ED(-) |
44.39
12.9 |
595
8400 |
2022 |
[88] |
| CeO2-ZnO-ZnWO4-AC/Ni foam//AC/Ni foam |
2M KOH |
(+)RB//ED(-) |
56.92 |
2000 |
2022 |
[89] |
| MnS/Ni foam//AC/Ni foam |
KOH |
(+)RB//ED(-) |
127.5 |
2550 |
2022 |
[90] |
| WS/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
45.2 |
608 |
2022 |
[91] |
| NiCo LDH/Ni foam//AC/Ni foam |
6M KOH |
(+)RB//ED(-) |
20.5
9.01 |
774.5
8522.7 |
2022 |
[63] |
| NiCo2S4-graphene/Ni foam//AC-graphene/Ni foam |
4M KOH +
carboxymethyl cellulose |
(+)RB//ED(-) |
80 |
4000 |
2022 |
[92] |
| LaMnO3/carbon cloth//rGO/carbon cloth |
1M KOH |
(+)RB//ED(-) |
154
72 |
324
14700 |
2022 |
[70] |
| LaMnO3/carbon cloth//rGO/carbon cloth |
1M NaClO4
|
(+)RB//ED(-) |
236 |
3630 |
2022 |
[70] |
| LaMnO3/carbon cloth//rGO/carbon cloth |
2M LiPF6
|
(+)RB//ED(-) |
123 |
1430 |
2022 |
[70] |
| NiFe-Phosphate/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
45.6 |
2250 |
2022 |
[93] |
| 2D MoO3-S4-C/Ni foam//rGO/Ni foam |
6M KOH |
(+)RB//ED(-) |
129.6 |
11600 |
2021 |
[71] |
| Cu-MOF-PANI-rGO-Ag/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
52
27.2 |
1192
10200 |
2021 |
[94] |
| Co0.5Mn0.5S/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
61.34
9.92 |
850
8500 |
2021 |
[95] |
| VSB-5-rGO/Ni foam//AC/Ni foam |
1M NaOH |
(+)RB//ED(-) |
80.5 |
2216.5 |
2021 |
[96] |
| FeCoCuS2/Ni foam//AC/Ni foam |
3M KOH |
(+)RB//ED(-) |
48.2
27.2 |
820.1
25700.2 |
2021 |
[64] |
| Co0.125Cu0.375Mn0.500S/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
88.71
20 |
320
8000 |
2021 |
[65] |
| Co3(PO4)2/Cu/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
62.6
11.8 |
425
7924 |
2021 |
[97] |
| NiMn(PO4)2-PANI/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
71.3 |
340 |
2021 |
[98] |
| Co3(PO4)2/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
34.8
10.0 |
425
6800 |
2021 |
[99] |
| MnCo2S4-Mxene/Ni foam//AC/Ni foam |
3M KOH |
(+)RB//ED(-) |
25.6
12.44 |
400
6400 |
2021 |
[100] |
| Co3(PO4)2/Ag/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
65.8 |
510 |
2021 |
[101] |
| Ni0.75Mn0.25(PO4)2/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
64.2 |
340 |
2021 |
[102] |
| Co0.125Cu0.375Mn0.500(PO4)2/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
56
16.88 |
800
6420 |
2021 |
[103] |
| Trypan blue-Ni-MOF/Ni foam//Azure A-graphene aerogel/Ni foam |
3M KOH |
(+)RB//ED(-) |
66.55
11.11 |
349
4450 |
2021 |
[104] |
| NiO@CuCo2O4/MoNi/Ni foam//AC/Ni foam |
2M KOH |
(+)RB//ED(-) |
80.6
63.8 |
692.8
14000 |
2021 |
[105] |
| Zn0.5Co0.5S/Ni foam//AC/Ni foam |
2M KOH |
(+)RB//ED(-) |
49
23 |
957
9413 |
2021 |
[106] |
| Co0.5Cu0.5Mn(PO4)2/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
55
19 |
800
6400 |
2021 |
[107] |
| Ni-Co-Bi double hydroxide/Ni-Co-B/Ni foam//AC/Ni foam |
2M KOH |
(+)RB//ED(-) |
62.8 |
800 |
2020 |
[108] |
| Phosphate ion-functionalized NiO (P-NiO)/Ni foam//AC/Ni foam |
3M KOH |
(+)RB//ED(-) |
53.4
24.7 |
800
12000 |
2020 |
[59] |
| Sr3P2-PANI/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
28.9
10.95 |
1020
5100 |
2020 |
[67] |
| Zn0.5CoO0.5Mn(PO4)2/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
45.45
6.86 |
425
4250 |
2020 |
[60] |
| Co3(PO4)2/Ni-Co-O/Ni foam//Fe2P/graphene hydrogel/Ni foam |
PVA-KOH |
(+)RB//ED(-) |
95
18 |
400
4000 |
2020 |
[61] |
| Co0.5Ni0.5WO4/Ni foam//AC/Ni foam |
2M KOH |
(+)RB//ED(-) |
42.2 |
1047.7 |
2020 |
[62] |
| Co-MOF-PANI/Ni foam//AC/Ni foam |
1M KOH |
(+)RB//ED(-) |
23.11
8.906 |
1600
6400 |
2020 |
[66] |
| WO3-WS2-MWCNT/Ni foam//AC/Ni foam |
3M KOH |
(+)PC//ED(-) |
86
24 |
848
11828 |
2023 |
[109] |
| Ni-Co-Mg MOF/MoS2/Ni foam//AC/Ni foam |
1M KOH |
(+)PC//ED(-) |
107.32 |
1350 |
2023 |
[110] |
| NH4MnPO4@Graphene QD/Graphite//rGO/Graphite |
3M H2SO4
3M H2SO4 + 0.025M (KI/VOSO4) |
(+)PC//ED(-) |
199
311 |
450
450 |
2022 |
[76] |
| Ni3(PO4)2-MWCNTs/Ni foam//AC/Ni foam |
|
(+)PC//ED(-) |
94.4
24.82 |
340
10200 |
2022 |
[111] |
| Mn-V-Sn oxyhydroxide/Ni foam//N-carbon/Ni foam |
1M KOH |
(+)PC//ED(-) |
70.6
17.1 |
1372.4
18861.3 |
2022 |
[112] |
| NH4OH-ZIF/Ni foam//GO/Ni foam |
6M KOH |
(+)PC//ED(-) |
4.16 |
20000 |
2022 |
[113] |
| CoS-Co3(PO4)2/Ni foam//AC/Ni foam |
1M KOH |
(+)PC//ED(-) |
34.68
63.93 |
13600
850 |
2021 |
[114] |
| Fe3O4@N-carbon-rGO/Ni foam//rGO/Ni foam |
6M KOH |
(+)PC//ED(-) |
46
10 |
750
7500 |
2021 |
[115] |
| MWCNT-NiMnPO4/Ni foam//AC/Ni foam |
2M KOH |
(+)PC//ED(-) |
698
43 |
78
5780 |
2020 |
[68] |
| P-NiCoB/Ni foam//rGO/Ni foam |
2M KOH |
(+)RB//PC(-) |
63.125
41.56 |
750
15000 |
2023 |
[74] |
| CoMn2O4/N-graphene/Ni foam//N-graphene/Ni foam |
PVA-KOH |
(+)RB//PC(-) |
44.1
20.3 |
992.6
12430 |
2021 |
[116] |
| graphitic carbon nitride (g-C3N4)-BiVO4/Graphite paper (symmetric) |
3.5M KOH |
(+)PC//PC(-) |
61
7.2 |
1996
16200 |
2020 |
[69] |
| Zn-Carbon cloths//S/P doped carbon (S/p-C)/graphite rod |
0.5M K2SO4
1M KBr |
(+)PC//PC(-) |
270
181 |
185
9300 |
2020 |
[75] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).