Submitted:
05 December 2023
Posted:
06 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
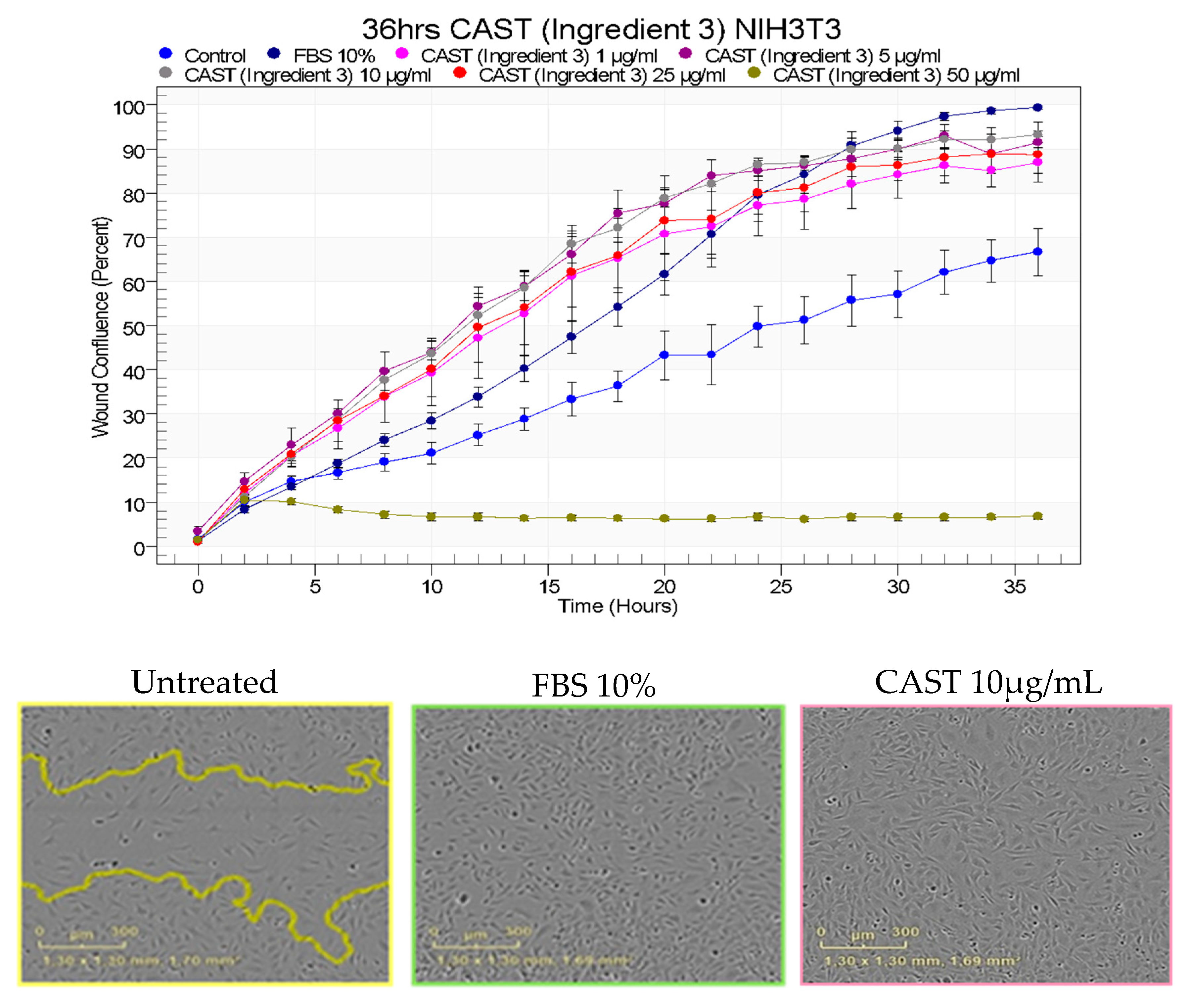
2.1. Wound healing on fibroblast
2.2. Transcriptomic analysis on fibroblast
2.3. Collagen network analysis on skin explant
2.4. Elastin immunofluorescence on skin explant
2.5. Statistical analysis
3. Results
3.1. Centella asiatica effect on fibroblast’s migration
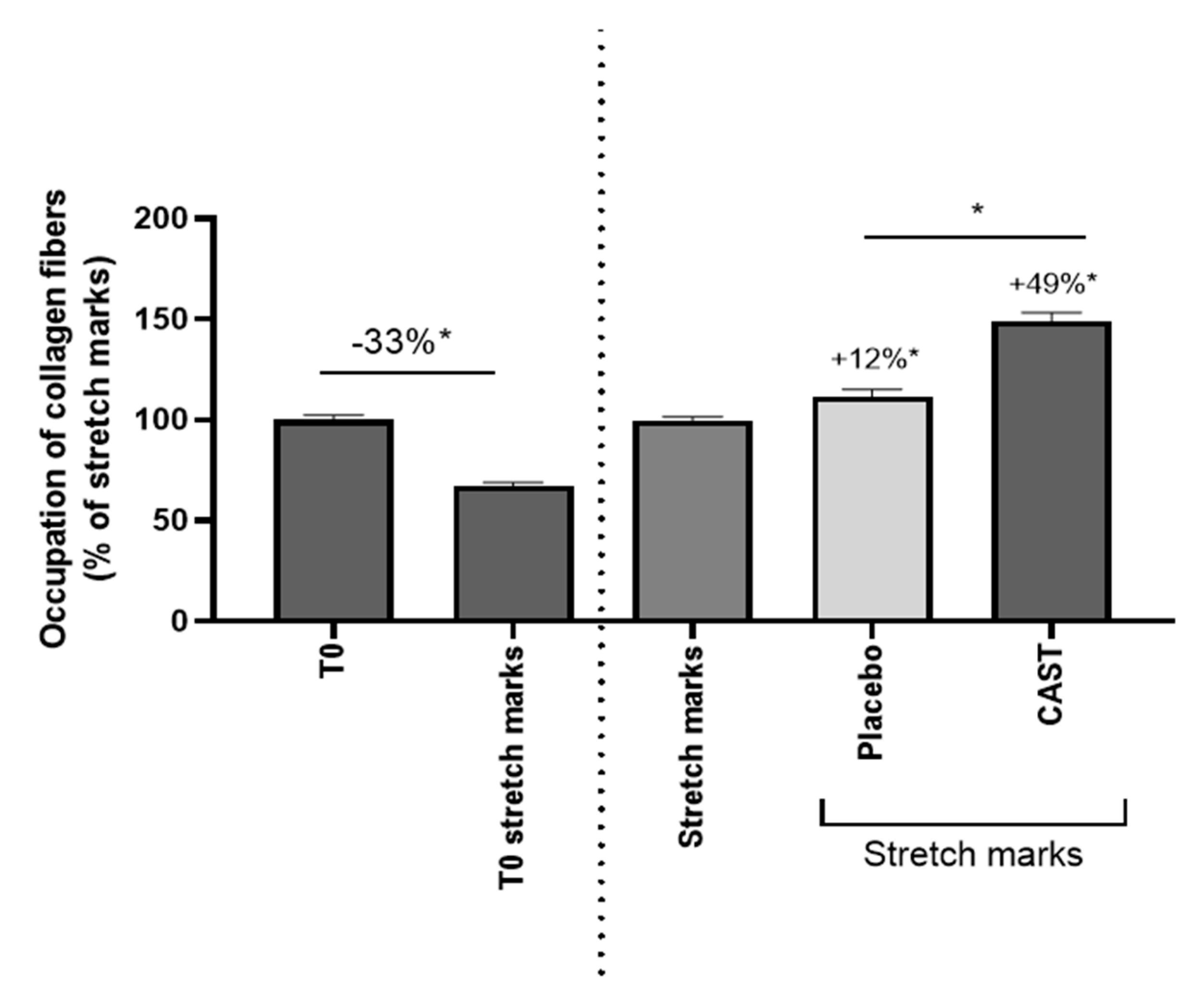
3.2. Anti-fibrotic effect of Centella asiatica
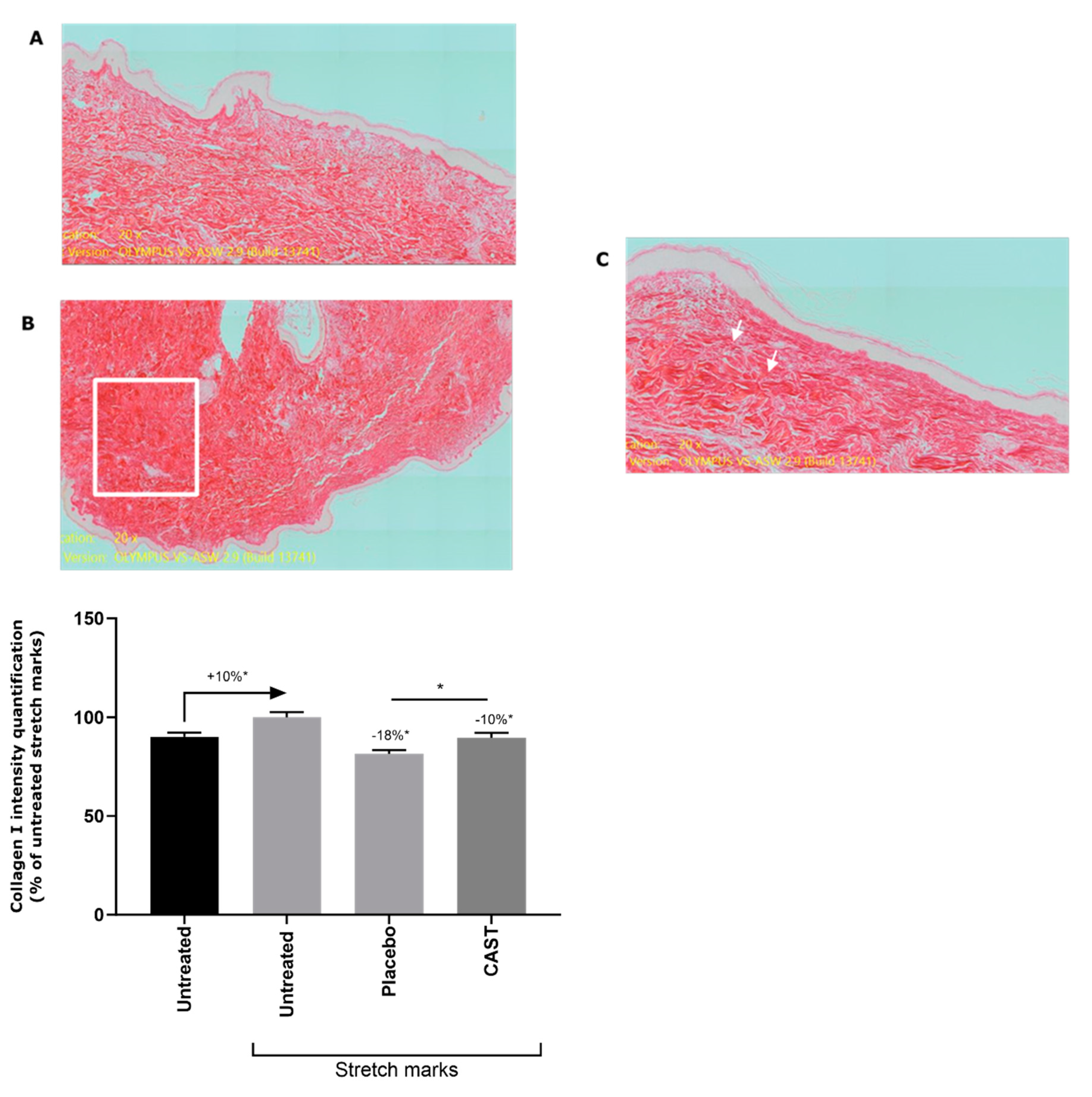
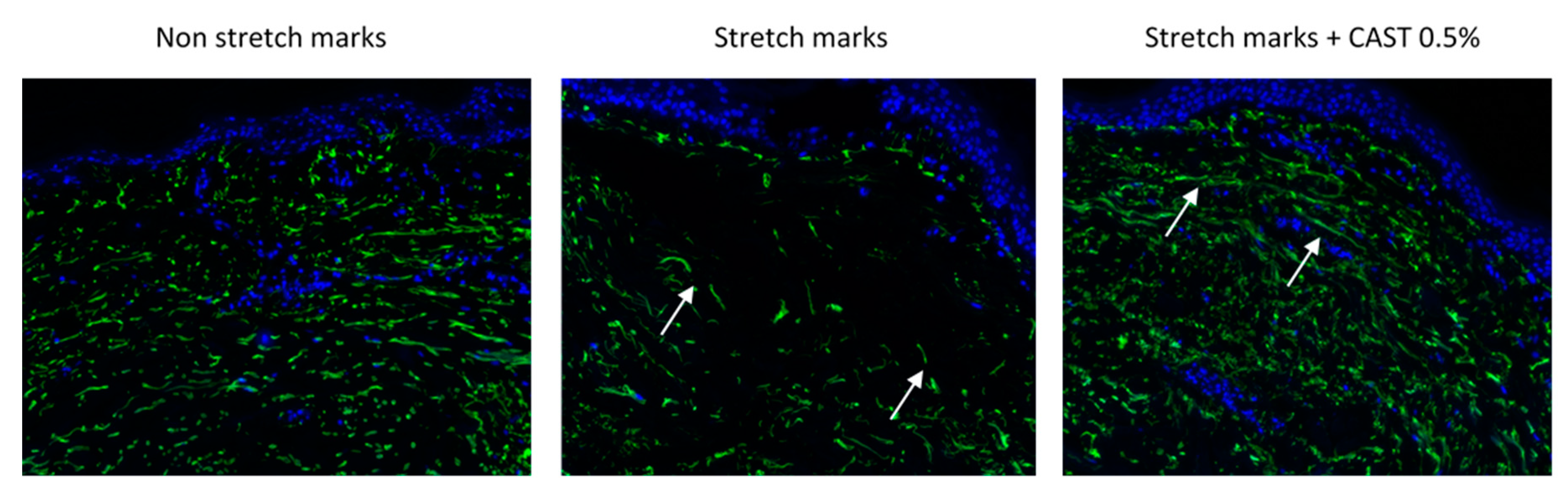
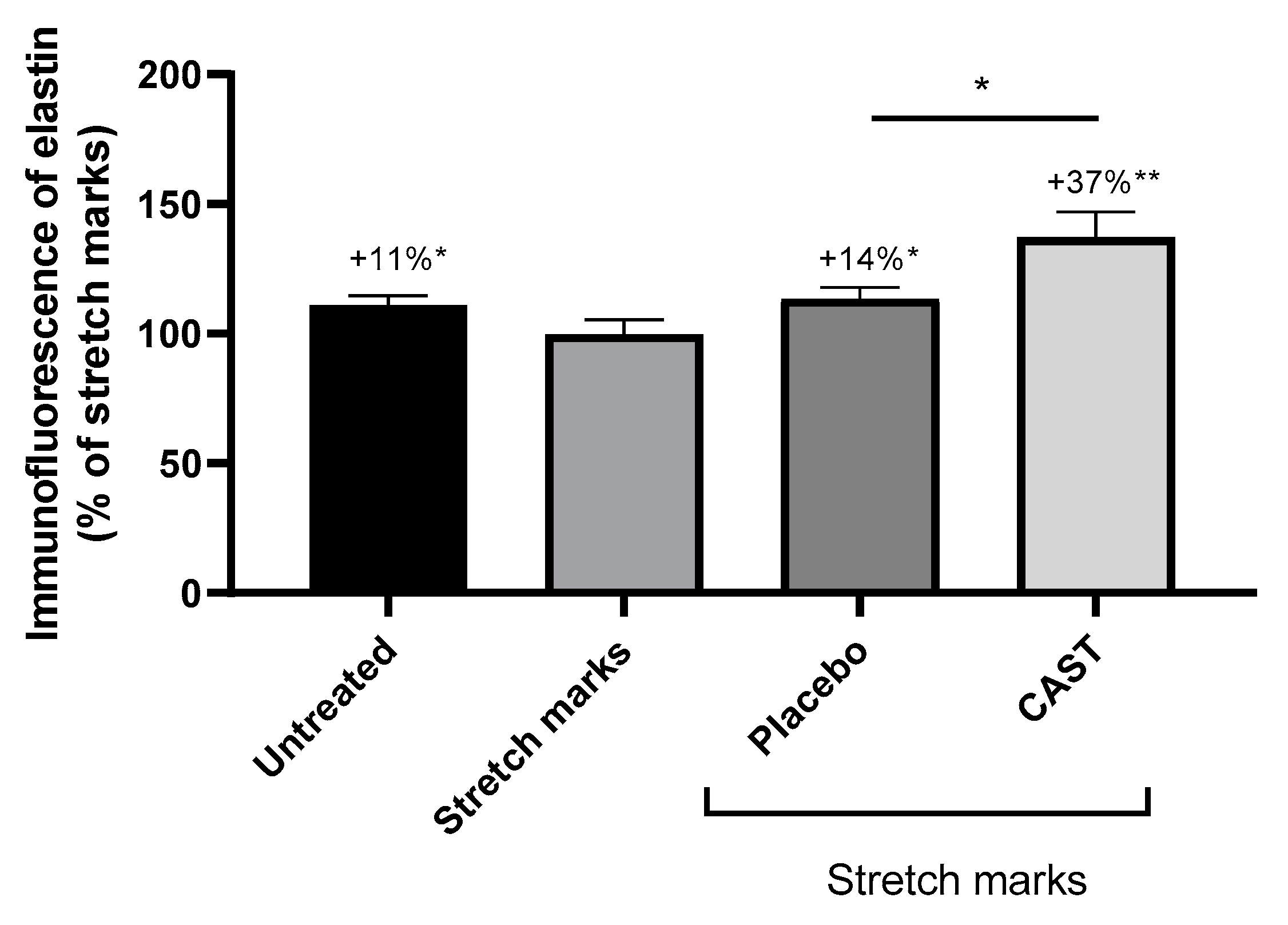
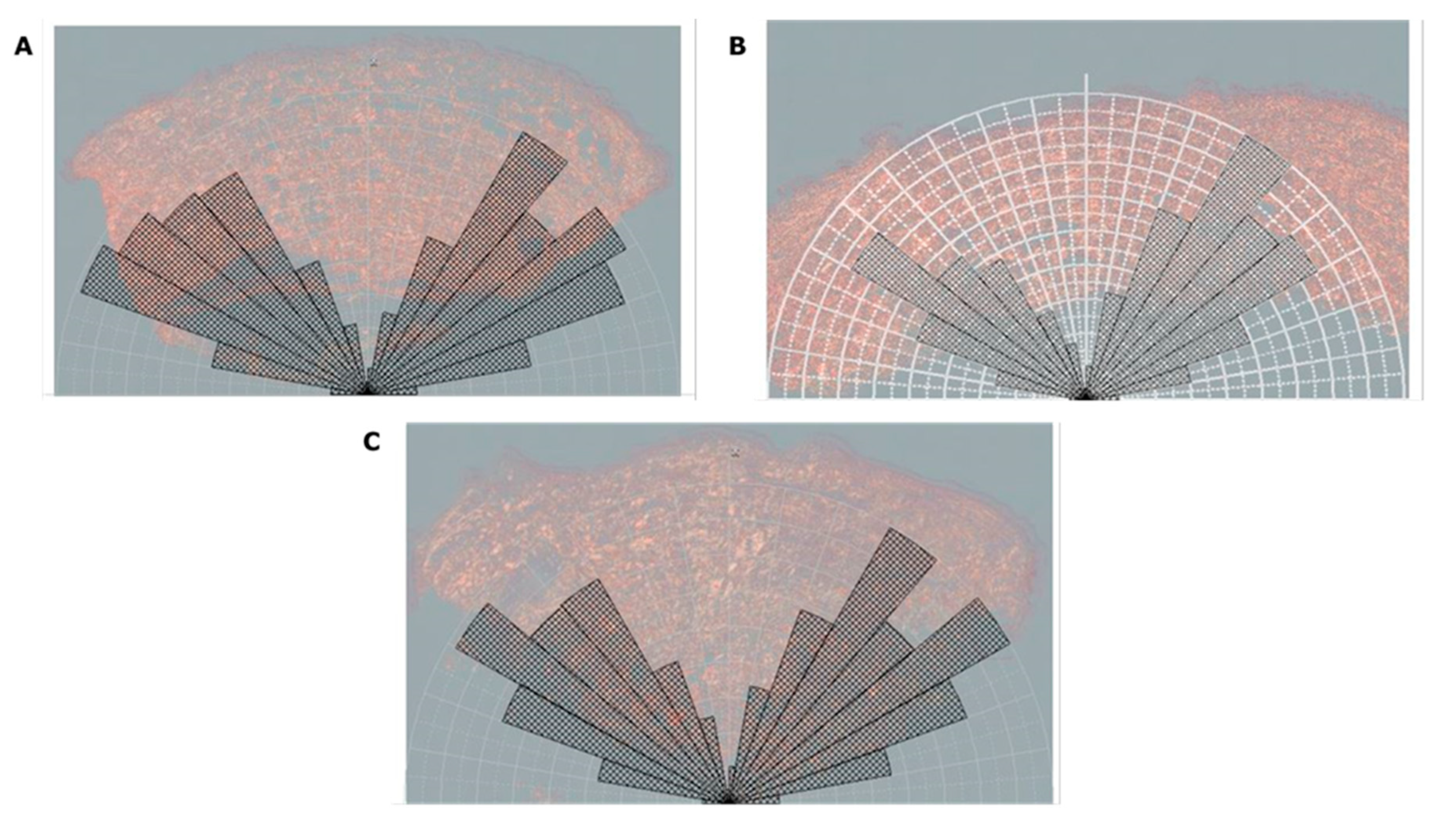
3.2. Centella asiatica improves skin elasticity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ud-Din, S., McGeorge, D. & Bayat, A. Topical management of striae distensae (stretch marks): prevention and therapy of striae rubrae and albae. J Eur Acad Dermatol Venereol 2016, 30, 211–222. [PubMed]
- Borrelli, M. R., Griffin, M., Ngaage, L. M., Longaker, M. T. & Lorenz, H.P. Striae Distensae: Scars without Wounds. Plastic & Reconstructive Surgery 2021, 148, 77–87.
- García Hernández, J. Á., Madera González, D., Padilla Castillo, M. & Figueras Falcón, T. Use of a specific anti-stretch mark cream for preventing or reducing the severity of striae gra-vidarum. Randomized, double-blind, controlled trial. Int J Cosmet Sci 2013, 35, 233–237. [PubMed]
- Veronese, S.; et al. The pathology under stretch marks? An elastosonography study. J of Cosmetic Dermatology 2022, 21, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Oakley, A. M. & Patel, B. C. Stretch Marks. in StatPearls (StatPearls Publishing, 2023).
- Lokhande, A. & Mysore, V. Striae distensae treatment review and update. Indian Dermatol Online J 2019, 10, 380. [PubMed]
- Liu, L., Ma, H. & Li, Y. Interventions for the treatment of stretch marks: a systematic review. Cutis 2014, 94, 66–72. [PubMed]
- Draelos, Z. D.; et al. Evaluation of an onion extract, Centella asiatica, and hyaluronic acid cream in the appearance of striae rubra. Skinmed 2010, 8, 80–86. [Google Scholar] [PubMed]
- Rawlings, A. V., Bielfeldt, S. & Lombard, K. J. A review of the effects of moisturizers on the appearance of scars and striae. Int J Cosmet Sci 2012, 34, 519–524. [PubMed]
- Farahnik, B., Park, K., Kroumpouzos, G. & Murase, J. Striae gravidarum: Risk factors, prevention, and management. International Journal of Women’s Dermatology 2017, 3, 77–85. [PubMed]
- Young, G.; Jewell, D. Creams for preventing stretch marks in pregnancy. In Cochrane Database of Systematic Reviews (ed. The Cochrane Collaboration) CD000066 (John Wiley & Sons, Ltd, 1996). [CrossRef]
- Sun, B. Therapeutic Potential of Centella asiatica and Its Triterpenes: A Review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef] [PubMed]
- Bylka, W., Znajdek-Awiżeń, P., Studzińska-Sroka, E. & Brzezińska, M. Centella asiatica in cosmetology. pdia 2013, 1, 46–49.
- Korgavkar, K.; Wang, F. Stretch marks during pregnancy: a review of topical preventi-on. Br J Dermatol 2015, 172, 606–615. [Google Scholar] [PubMed]
- Togni, S.; et al. Postpartum stretch marks treated with Centella asiatica cream: report of efficacy from a pilot registry study. Esperienze Dermatol 20, (2018).
- Maquart, F.X.; et al. Triterpenes from Centella asiatica stimulate extracellular matrix accumulation in rat experimental wounds. Eur J Dermatol 1999, 9, 289–296. [Google Scholar] [PubMed]
- Sh Ahmed, A. et al. Pharmacological properties of Centella asiatica hydrogel in accele-rating wound healing in rabbits. BMC Complement Altern Med 2019, 19, 213.
- Dong, S.-H., Liu, Y.-W., Wei, F., Tan, H.-Z. & Han, Z.-D. Asiatic acid ameliorates pulmo-nary fibrosis induced by bleomycin (BLM) via suppressing pro-fibrotic and inflammatory signaling pathways. Biomedicine & Pharmacotherapy 2017, 89, 1297–1309.
- Wang, F.; et al. Severe disruption and disorganization of dermal collagen fibrils in early striae gravidarum. Br J Dermatol 2018, 178, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T. M., Lowe, L., Brown, M. D., Sullivan, M. J. & Nelson, B. R. Histology and Physiology of Tissue Expansion. The Journal of Dermatologic Surgery and Oncology 1993, 19, 1074–1078. [PubMed]
- Wang, F.; et al. Marked disruption and aberrant regulation of elastic fibres in early stri-ae gravidarum. Br J Dermatol 2015, 173, 1420–1430. [Google Scholar] [CrossRef] [PubMed]
- Basile, F. V., Basile, A.V. & Basile, A.R. Striae Distensae After Breast Augmentation. Aesth Plast Surg 2012, 36, 894–900.
| Genes | Fold change Stretch marks versus un-stretch marked |
Fold change CAST versus stretch marks |
|
|---|---|---|---|
| Fibrosis | CTGF (CCN2) | x1,6* | X0.44** |
| PTK2 (FAK) | x1.7# | X0.63** | |
| Extra Cellular Matrix | MMP1 | x9.7* | x0.40** |
| MMP7 | x13,3*** | x0.67* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





