Submitted:
05 December 2023
Posted:
06 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
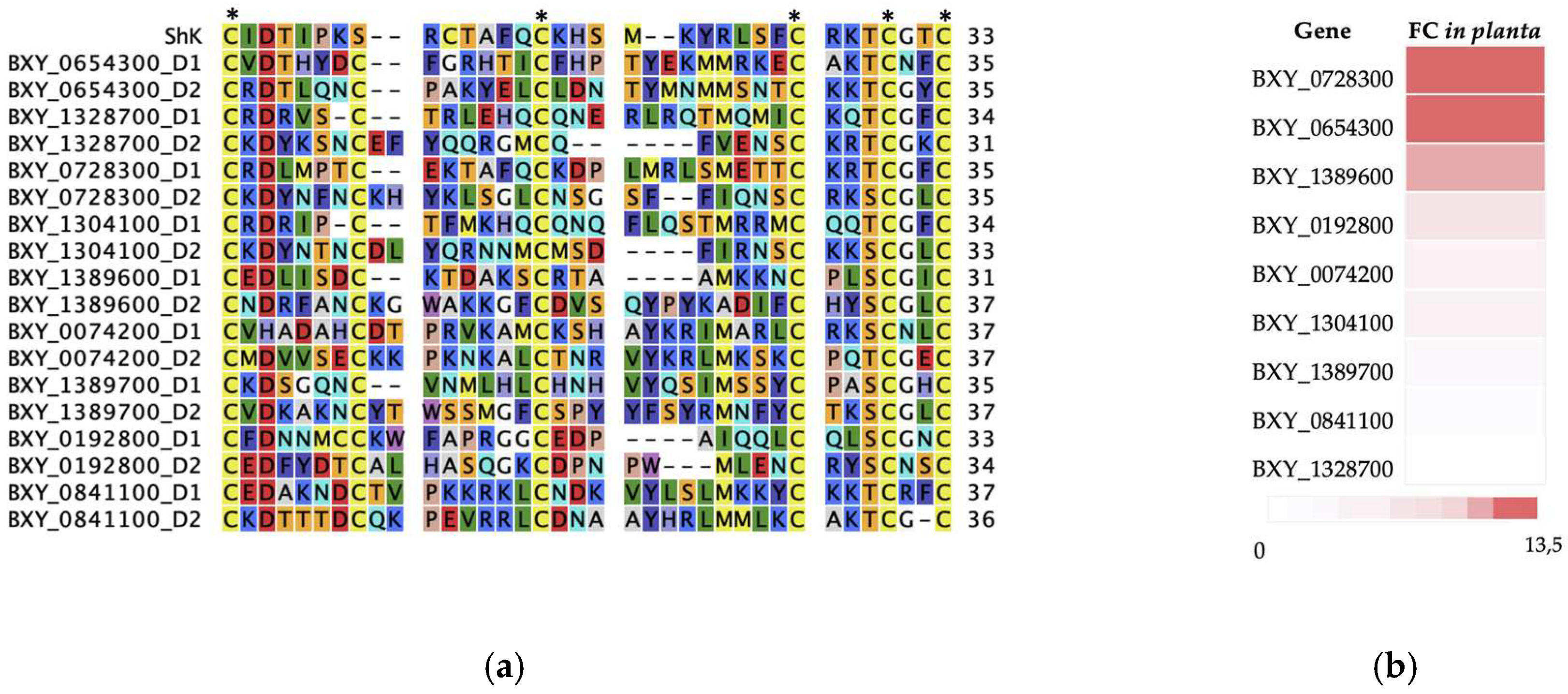
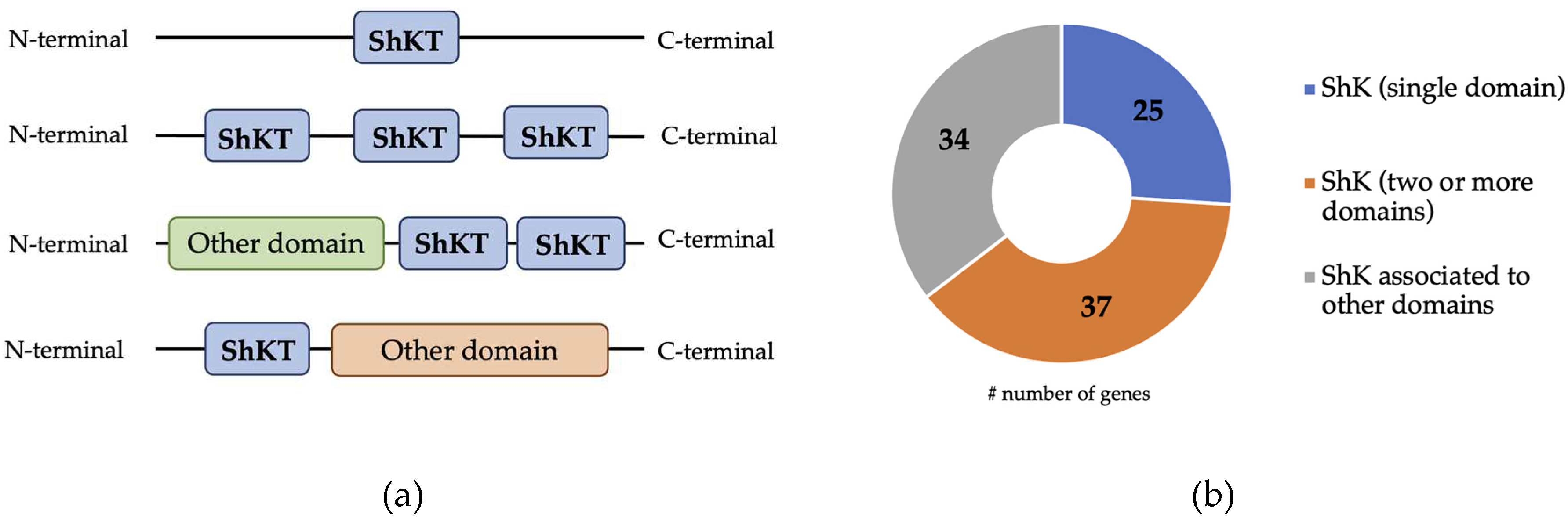
2.1. Characterization of ShK domain-containing protein from B. xylophilus
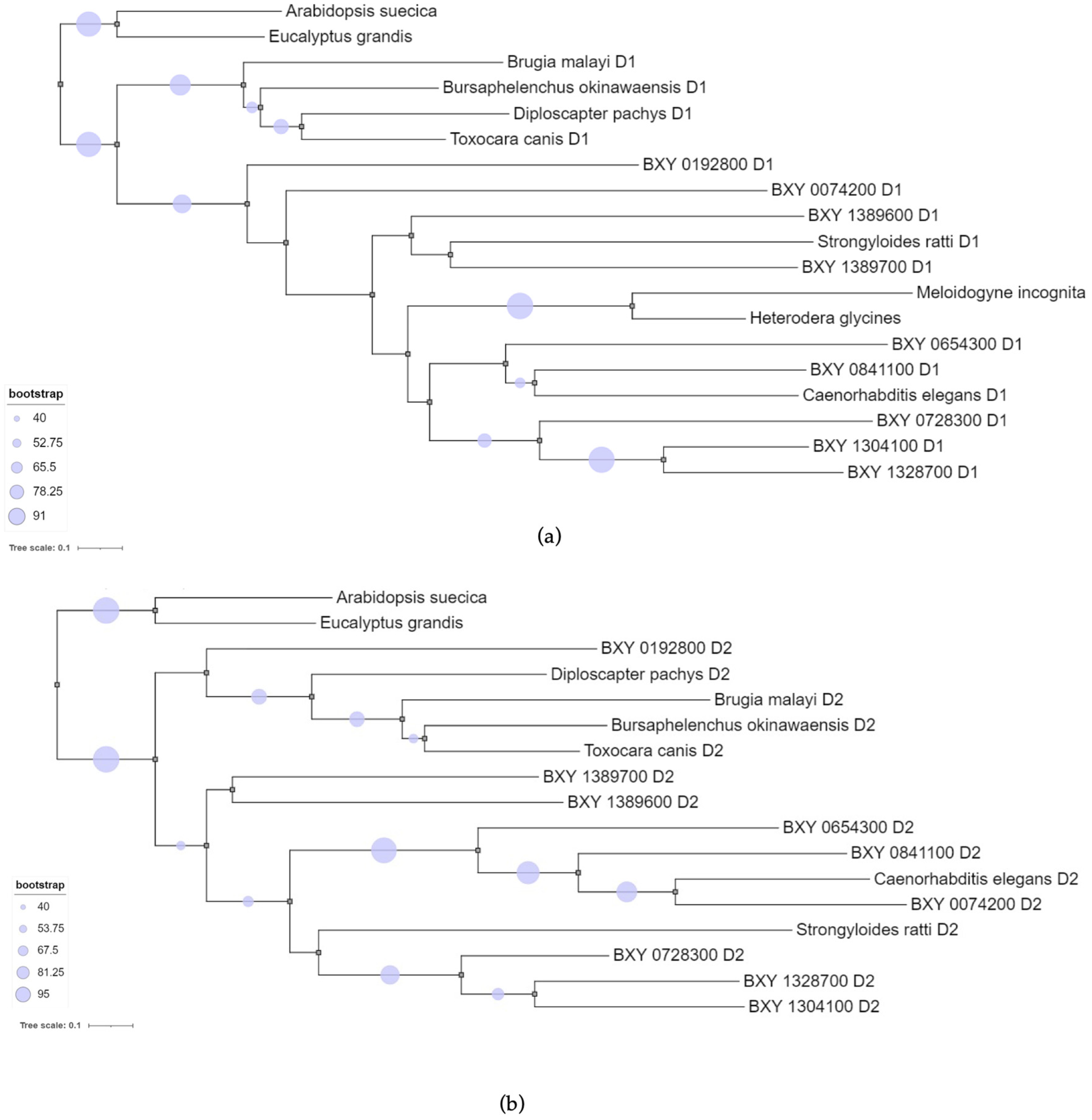
2.2. In silico and phylogenetics analysis of the nine candidate B. xylophilus-specific transcripts
| Gene ID | GL* | IN | TL* | PS** | SP | Sequence similarity ID (NCBI accession number) | BLAST e-value |
|---|---|---|---|---|---|---|---|
| BXY_0192800 | 630 | 2 | 510 | 169 | Yes, 1-17 | [CAD5228099.1] Bursaphelenchus xylophilus, 100% | 4e-123 |
| BXY_0728300 | 502 | 1 | 429 | 142 | Yes, 1-16 | [CAD5230694.1] Bursaphelenchus xylophilus, 100% | 6e-94 |
| BXY_1389600 | 534 | 1 | 492 | 163 | Yes, 1-21 | [CAD5234029.1] Bursaphelenchus xylophilus, 100% | 1e-111 |
| BXY_0074200 | 447 | 0 | 447 | 148 | Yes, 1-22 | [CAD5228719.1] Bursaphelenchus xylophilus, 100% | 3e-105 |
| BXY_0841100 | 1170 | 2 | 669 | 229 | Yes, 1-17 | [CAD5208706.1] Bursaphelenchus xylophilus, 100% | 8e-157 |
| BXY_1304100 | 922 | 1 | 423 | 140 | Yes, 1-16 | [CAD5233070.1] Bursaphelenchus xylophilus, 100% | 2e-98 |
| BXY_1328700 | 673 | 1 | 444 | 147 | Yes, 1-23 | [CAD5233069.1] Bursaphelenchus xylophilus, 100% | 3e-99 |
| BXY_0654300 | 3250 | 4 | 1926 | 641 | Yes, 1-20 | [CAD5235767.1] Bursaphelenchus xylophilus, 100% | <0 |
| BXY_1389700 | 3240 | 2 | 2625 | 874 | Yes, 1-21 | [CAD5234030.1] Bursaphelenchus xylophilus, 100% | <0 |
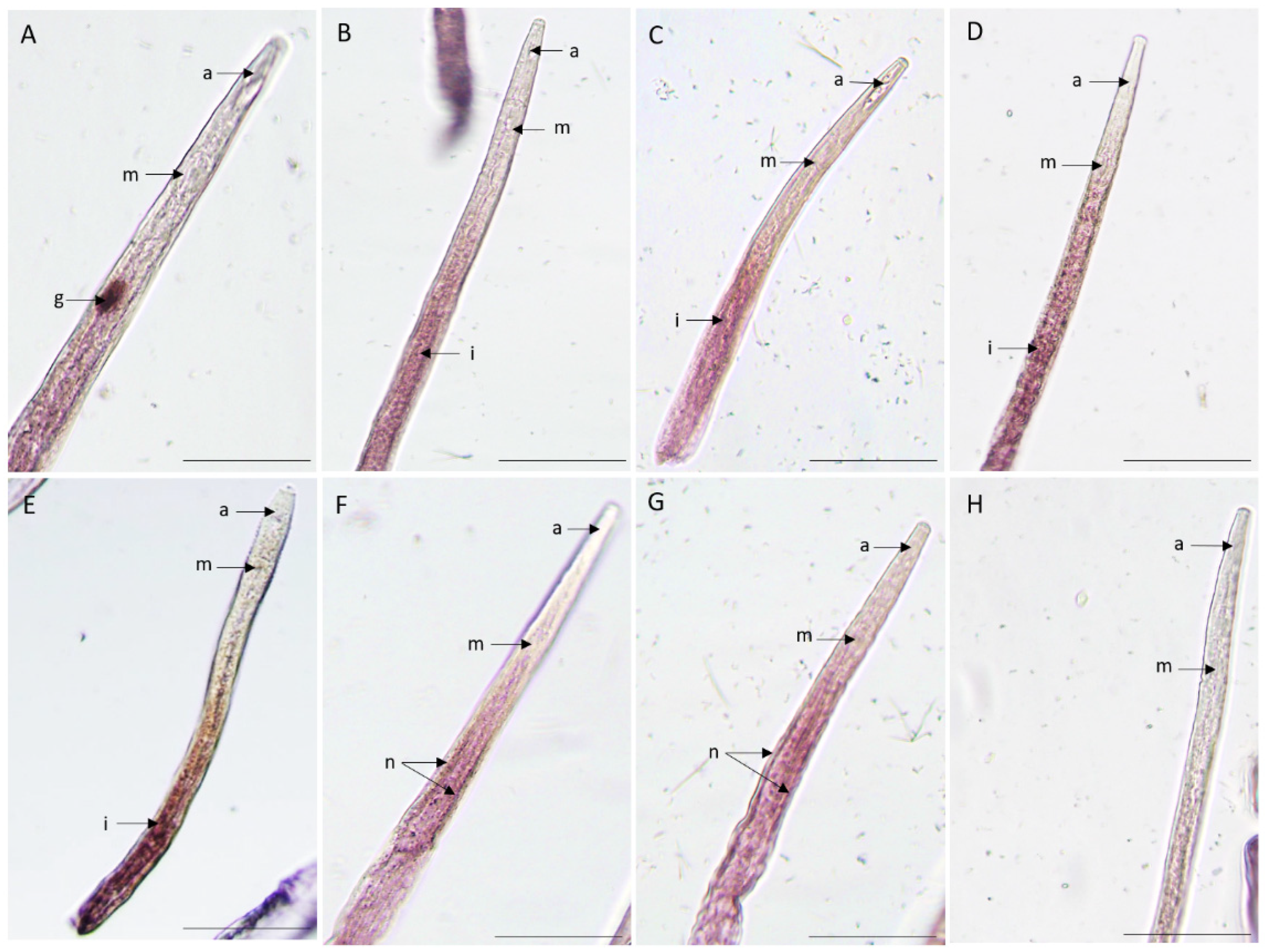
2.3. Spatial expression of the candidate ShK domain-containing proteins
2.4. High gene expression of B. xylophilus ShK domain-containing proteins under oxidative stress
3. Discussion
4. Materials and Methods
4.1. Biological material
4.2. In silico and phylogenetics analysis of B. xylophilus ShK domain-containing protein sequences
4.3. Validation of the candidate genes
4.4. Oxidative stress assay
4.5. RNA extraction and gene relative expression profile
4.6. In situ hybridization assay
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Boyd, I.L; Freer-Smith, P. H.; Gilligan, C. A.; Godfray, H.C.J. The Consequence of Tree Pests and Diseases for Ecosystem Services. Science 2013, 342, 1235773. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J; Jones, M. G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, E.J.; Wesemael, W.M.L.; Perry, R.N. Top 10 plant-parasitic nematodes in molecular plant pathology. Molecular plant pathology, 2013, 14, 946–961. [Google Scholar] [CrossRef]
- EPPO Bulletin. 2023. PM 7/4 (4) Bursaphelenchus xylophilus. [CrossRef]
- Mamiya, Y. Pathology of the pine wilt disease caused by Bursaphelenchus xylophilus. Annual review of Phytopathology 1983, 21, 201–220. [Google Scholar] [CrossRef]
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Robertson, L.; Cobacho Arcos, S.; Escuer, M.; Santiago Merino, R.; Esparrago, G.; Abelleira, A.; Navas, A. Incidence of the pinewood nematode Bursaphelenchus xylophlius Steiner & Buhrer, 1934 (Nickle, 1970) in Spain. Nematology 2011, 13, 6–755. [Google Scholar] [CrossRef]
- Vicente, C.S.L.; Espada, M.; Vieira, P.; Mota, M. Pine wilt disease: a threat to European forestry. Eur. J. Plant Pathol. 2012, 133, 89–99. [Google Scholar] [CrossRef]
- Vieira, P.; Gleason, C. Plant-parasitic nematode effectors - insights into their diversity and new tools for their identification. Curr Opin Plant Biol. 2019, 50, 37–43. [Google Scholar] [CrossRef]
- Espada, M.; Silva, A.C.; Eves-van den Akker, S.; Cock, P.J.A.; Mota, M.; Jones, J.T. Identification and characterization of parasitism genes from the pinewood nematode Bursaphelenchus xylophilus reveals a multi-layered detoxification strategy. Molecular Plant Pathology 2016, 17, 286–295. [Google Scholar] [CrossRef]
- Tsai, I.J.; Tanaka, R.; Kanzaki, N.; Akiba, M.; Yokoi, T.; Espada, M.; Jones, J.T.; Kikuchi, T. Transcriptional and morphological changes in the transition from mycetophagous to phytophagous phase in the plant-parasitic nematode Bursaphelenchus xylophilus. Molecular Plant Pathology 2016, 17, 77–83. [Google Scholar] [CrossRef]
- Rangaraju, S.; Khoo, K.; Feng, Z.P.; Crossley, G.; Nugent, D.; Khaytin, I.; Chi, V.; Pham, C.; Calabresi, P.; Pennington, M.; Norton, R.; Chandy, G. Potassium Channel Modulation by A Toxin Domain in Matrix Metalloprotease 23. The Journal of biological chemistry 2009, 285, 12, 9124–9136. [Google Scholar] [CrossRef]
- Castañeda, O.; Sotolongo, V.; Amor, A.M.; Stöcklin, R.; Anderson, A.J.; Harvey, A.L.; Engström, A.; Wernstedt, C.; Karlsson, E. ; Characterization of a potassium channel toxin from the Caribbean Sea anemone Stichodactyla helianthus. Toxicon 1995, 33, 5–603. [Google Scholar] [CrossRef]
- Cotton, J.; Crest, M.; Bouet, F.; Alessandri, N.; Gola, M.; Forest, E.; Karlsson, E.; Castañeda, O.; Harvey, A.L.; Vita, C.; Ménez, A. Eur. J. Biochem. 1997, 244, 192–202. [CrossRef]
- Tudor, J.E.; Pallaghy, P.K.; Pennington, M.W.; Norton, R.S. Solution structure of ShK toxin, a novel potassium channel inhibitor from a sea anemone. Nat. Struct. Biol. 1996, 3, 317–320. [Google Scholar] [CrossRef]
- Loukas, A.; Hintz, M.; Linder, D.; Mullin, N.P.; Parkinson, J.; Tetteh, K.K.; Maizels, R.M. A family of secreted mucins from the parasitic nematode Toxocara canis bears diverse mucin domains but shares similar flanking six-cysteine repeat motifs. J. Biol. Chem. 2000, 275, 39600–39607. [Google Scholar] [CrossRef]
- Prentis, P.J.; Pavasovic, A.; Norton, R.S. Sea Anemones: Quiet Achievers in the Field of Peptide Toxins. Toxins 2018, 10, 30. [Google Scholar] [CrossRef]
- von Reumont, B.M.; Campbell, L.I.; Richter, S.; Hering, L.; Sykes, D.; Hetmank, J.; Jenner, R.A.; Bleidorn, C. A Polychaete's powerful punch: venom gland transcriptomics of Glycera reveals a complex cocktail of toxin homologs. Genome Biol Evol. 2014, 6, 9–2406. [Google Scholar] [CrossRef]
- Verdes, A.; Simpson, D.; Holford, M. Are Fireworms Venomous? Evidence for the Convergent Evolution of Toxin Homologs in Three Species of Fireworms (Annelida, Amphinomidae). Genome Biol. Evol. 2018, 10, 249–268. [Google Scholar] [CrossRef]
- Gerdol, M.; Cervelli, M.; Mariottini, P.; Oliverio, M.; Dutertre, S.; Modica, M.V. A Recurrent Motif: Diversity and Evolution of ShKT Domain Containing Proteins in the Vampire Snail Cumia reticulata. Toxins 2019, 11, 106. [Google Scholar] [CrossRef]
- Suh, J.; Hutter, H. A survey of putative secreted and transmembrane proteins encoded in the C. elegans genome. BMC Genomics 2012, 13, 333. [Google Scholar] [CrossRef]
- Lima, A.K.; Dhillon, H.; Dillman, A.R. ShK-Domain-Containing Protein from a Parasitic Nematode Modulates Drosophila melanogaster Immunity. Pathogens 2022, 11, 1094. [Google Scholar] [CrossRef]
- Frias, J.; Toubarro, D.; Bjerga, G.E.K.; Puntervoll, P.; Vicente, J.B.; Reis, R.L.; Simões, N. A ShK-like Domain from Steinernema carpocapsae with Bioinsecticidal Potential. Toxins 2022, 14, 754. [Google Scholar] [CrossRef]
- Vieira, P.; Myers, R.Y.; Pellegrin, C.; Wram, C.; Hesse, C.; Maier, T.; Shao, J.; Koutsovoulos, G.D.; Zasada, I.; Matsumoto, T.; Danchin, E.; Baum, T.J.; Eves-van den Akker, S.; Nemchinov, L.G. Targeted transcriptomics reveals signatures of large-scale independent origins and concerted regulation of effector genes in Radopholus similis. PLoS Pathog. 2021, 17, 11–e1010036. [Google Scholar] [CrossRef]
- Vieira, P.; Shao, J.; Vijayapalani, P.; Maier, T.; Pellegrin, C.; Eves-van den Akker, S.; Baum, T.J.; Nemchinov, L. A new esophageal gland transcriptome reveals signatures of large scale de novo effector birth in the root lesion nematode Pratylenchus penetrans. BMC Genomics 2020, 21, 738. [Google Scholar] [CrossRef]
- Kumar, A.; Fitoussi, N.; Sanadhya, P.; Sichov, N.; Bucki, P.; Bornstein, M.; Belausuv, E.; Miyara, B. S. Two Candidate Meloidogyne javanica Effector Genes, MjShKT and MjPUT3: A Functional Investigation of Their Roles in Regulating Nematode Parasitism. Molecular Plant-Microbe Interactions 2023, 36, 2–79. [Google Scholar] [CrossRef]
- Habash, S.S.; Radakovic, Z.S.; Vankova, R.; Siddique, S.; Dobrev, P.; Gleason, C.; Grundler, F.M.W.; Elashry, A. Heterodera schachtii Tyrosinase-like protein - a novel nematode effector modulating plant hormone homeostasis. Sci Rep. 2017, 7, 6874. [Google Scholar] [CrossRef]
- Cantacessi, C.; Mitreva, M.; Campbell, B.E.; Hall, R.S.; Young, N.D.; Jex, A.R.; Ranganathan, S.; Gasser, R.B. First transcriptomic analysis of the economically important parasitic nematode, Trichostrongylus colubriformis, using a next-generation sequencing approach. Infection, Genetics and Evolution 2010, 10, 8–1199. [Google Scholar] [CrossRef]
- Cahalan, M.D.; Chandy, K.G. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009, 231, 1–59. [Google Scholar] [CrossRef]
- Beeton, C.; Pennington, M.W.; Norton, R.S. ; Analogs of the Sea Anemone Potassium Channel Blocker ShK for the Treatment of Autoimmune Diseases. Inflamm Allergy Drug Targets 2011, 10, 5, 5–321. [Google Scholar] [CrossRef]
- Chhabra, S.; Chang, S.C.; Nguyen, H.M.; Huq, R.; Tanner, M.R.; Londono, L.M.; Estrada, R.; Dhawan, V.; Chauhan, S.; Upadhyay, S.K.; Gindin, M.; Hotez, P.J.; Valenzuela, J.G.; Mohanty, B.; Swarbrick, J.D.; Wulff, H.; Iadonato, S.P.; Gutman, G.A.; Beeton, C.; Pennington, M.W.; Norton, R.S.; Chandy, K.G. Kv1.3 channel-blocking immunomodulatory peptides from parasitic worms: Implications for autoimmune diseases. FASEB J. 2014, 28, 3952–3964. [Google Scholar] [CrossRef]
- Dreyer, I.; Uozumi, N. Potassium channels in plant cells. The FEBS Journal 2011, 278, 4293–4303. [Google Scholar] [CrossRef]
- Joshi, I. , Kumar, A., Singh, A.K. et al. Development of nematode resistance in Arabidopsis by HD-RNAi-mediated silencing of the effector gene Mi-msp2. Sci Rep. 2019, 9, 17404. [Google Scholar] [CrossRef]
- Kikuchi, T.; Cotton, J.A.; Dalzell, J.J.; Hasegawa, K.; Kanzaki, N.; McVeigh, P.; Takanashi, T.; Tsai, I.J.; Assefa, S.A.; Cock, P.J.A.; Otto, T.D.; Hunt, M.; Reid, A.J.; Sanchez-Flores, A.; Tsuchihara, K.; Yokoi, T.; Larsson, M. C.; Miwa, J.; Maule, A.G.; Sahashi, N.; Jones, J.T.; Berriman, M. Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. PLoS Pathog. 2011, 7:e1002219. [CrossRef]
- Tanaka, S.E.; Dayi, M.; Maeda, Y.; Tsai, I.J.; Tanaka, R.; Bligh, M.; Takeuchi-Kaneko, Y.; Fukuda, K.; Kanzaki, N.; Kikuchi, T. Stage-specific transcriptome of Bursaphelenchus xylophilus reveals temporal regulation of effector genes and roles of the dauer-like stages in the lifecycle. Sci Rep. 2019, 9, 6080. [Google Scholar] [CrossRef]
- Vicente, C.S.L.; Ikuyo, Y.; Shinya, R.; Mota, M.; Hasegawa, K. Catalases Induction in High Virulence Pinewood Nematode Bursaphelenchus xylophilus under Hydrogen Peroxide-Induced Stress. PLoS ONE 2015, 10, 4: e0123839. [CrossRef]
- McNeilly, T.N.; Frew, D.; Burgess, S.T.G.; Wright, H.; Bartley, D.J.; Bartley, Y.; Nisbet, A.J. Niche-specifc gene expression in a parasitic nematode; increased expression of immunomodulators in Teladorsagia circumcincta larvae derived from host mucosa. Sci Rep. 2017, 7, 7214. [Google Scholar] [CrossRef]
- Espada, M.; Eves-van den Akker, S.; Maier, T.; Paramasivan, V.; Baum, T.J; Mota, M.; Jones J.T. STATAWAARS: a promoter motif associated with spatial expression in the major effector-producing tissues of the plant-parasitic nematode Bursaphelenchus xylophilus. BMC Genomics 2018, 19:553. [CrossRef]
- Columbus-Shenkar, Y.Y.; Sachkova, M.Y.; Macrander, J.; Fridrich, A.; Modepalli, V.; Reitzel, A.M.; Sunagar, K.; Moran, Yehu, M. Dynamics of Venom Composition Across a Complex Life Cycle. Elife 2018, 7:e35014. [CrossRef]
- Schafer, W. Nematode nervous systems. Current Biology 2016, 26, 937–980. [Google Scholar] [CrossRef]
- Espada, M.; Jones, J.T.; Mota, M. Characterization of glutathione S-transferases from the pine wood nematode, Bursaphelenchus xylophilus. Nematology 2016, 18, 697–709. [Google Scholar] [CrossRef]
- Evans, A.A.F. Mass culture of mycophagous nematodes. J. Nematol. 1970, 2, 99–100. [Google Scholar]
- Southey, J.F. Laboratory Methods for Work with Plant and Soil Nematodes 1986, London: Ministry of Agriculture Fisheries and Food, HMSO.
- McGinnis S, Madden TL. BLAST: at the core of a powerful and diverse set of sequence analysis tools. Nucleic Acids Res. 2004, 32, 20–25. [Google Scholar] [CrossRef]
- Boratyn, G.M.; Camacho, C.; Cooper, P.S.; Coulouris, G.; Fong, A.; Ma, N.; Madden, T.L.; Matten, W.T.; McGinnis, S.D.; Merezhuk, Y.; Raytselis, Y.; Sayers, E.W.; Tao, T.; Ye, J.; Zaretskaya, I. BLAST: a more efficient report with usability improvements. Nucleic Acids Res. 2013, 41, 29–33. [Google Scholar] [CrossRef]
- Blum, M.; Chang, H.; Chuguransky, S.; Grego, T.; Kandasaamy, S.; Mitchell, A.; Nuka, G.; Paysan-Lafosse, T.; Qureshi, M.; Raj, S.; Richardson, L.; Salazar, G.A.; Williams, L.; Bork, P.; Bridge, A.; Gough, J.; Haft, D.H.; Letunic, I.; Marchler-Bauer, A.; Mi, H.; Natale, D.A.; Necci, M.; Orengo, C.A.; Pandurangan, A.P.; Rivoire, C.; Sigrist, C.J.A.; Sillitoe, I.; Thanki, N.; Thomas, P.D.; Tosatto, S.C.E.; Wu, C.H.; Bateman, A.; Finn, R.D. The InterPro protein families and domains database: 20 years on. Nucleic Acids Research 2020, 49, 344–354. [Google Scholar] [CrossRef]
- Teufel, F.; Almagro Armenteros, J.J. , Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation, Nucleic Acids Research 2021, 49, 1, 293–296. [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symposium Series 1999, 41, 95–98. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—new capabilities and interfaces. Nucleic Acids Research 2012, 40. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- de Boer, J.M.; Yan, Y.; Smant, G.; Davis, E.L.; Baum, T.J. In-situ hybridization to messenger RNA in Heterodera glycines. J Nemat. 1998, 30, 309–12. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





