Submitted:
05 December 2023
Posted:
06 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. SPTFE membrane
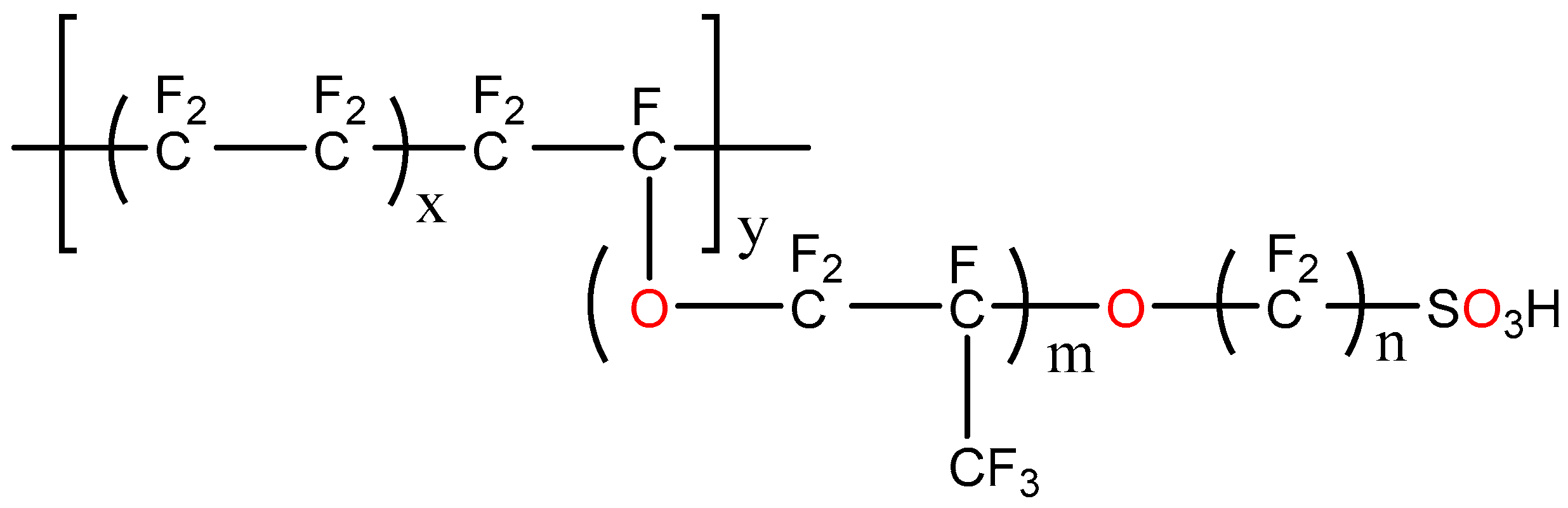
2.2. P-H20 synthesis
2.3. Mechanical properties
2.4. Ion-exchange capacity and water uptake
2.5. Conductivity and diffusion permeability measurememnt
2.6. Current-voltage curves and specific permselectivity measurement
3. Results and discussion
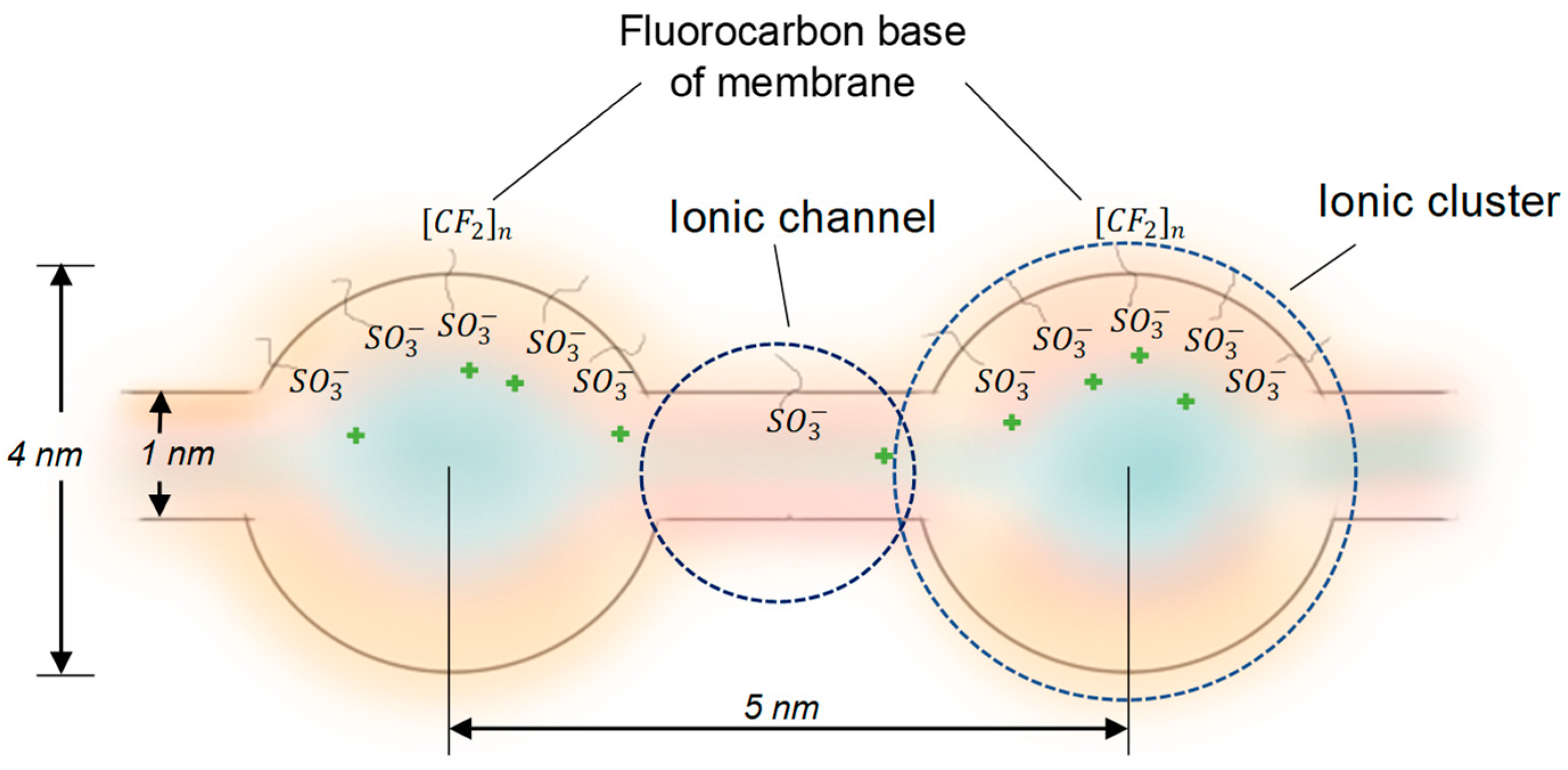
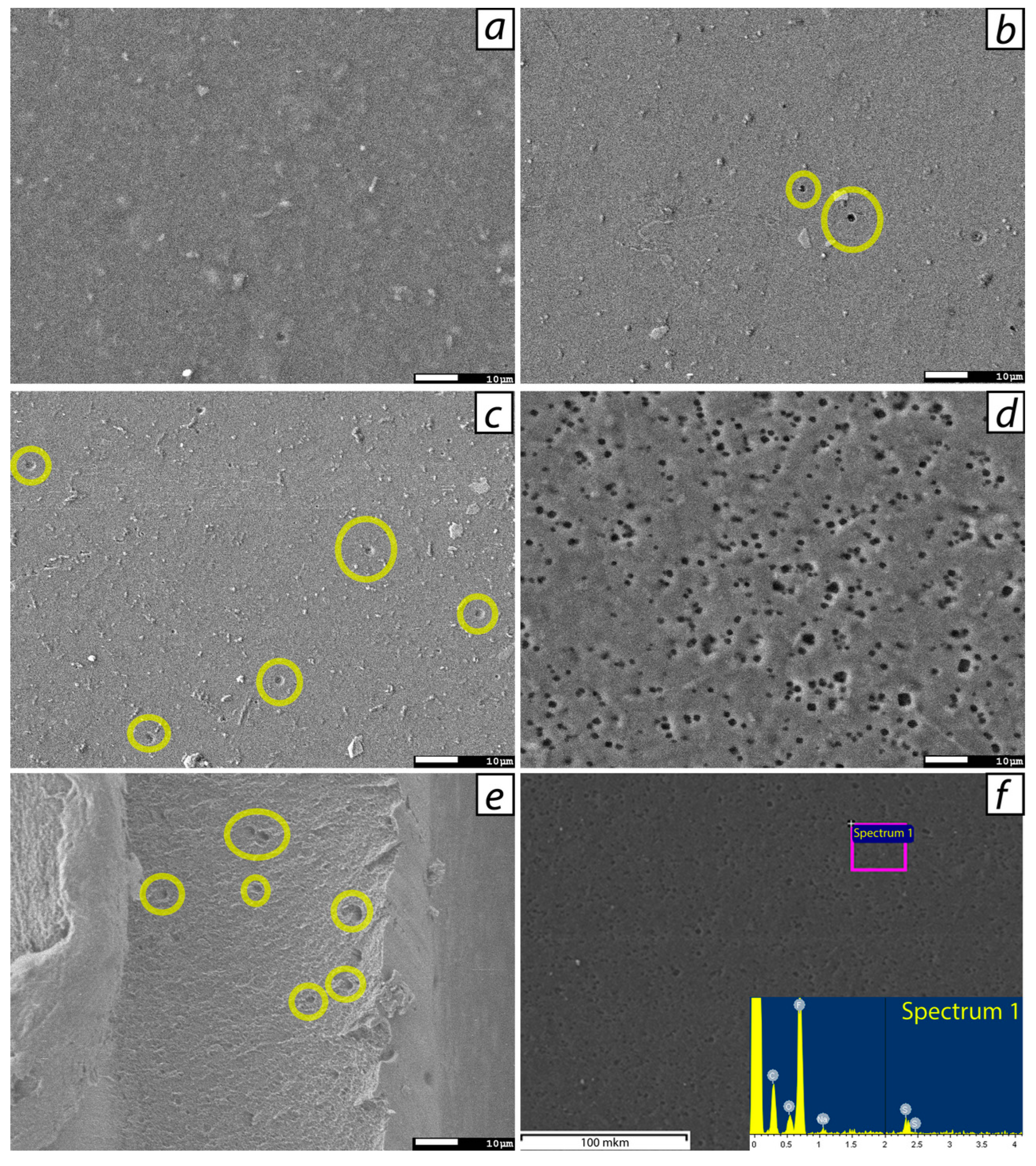
3.1. Dopant distribution, mechanical and physico-chemical properties
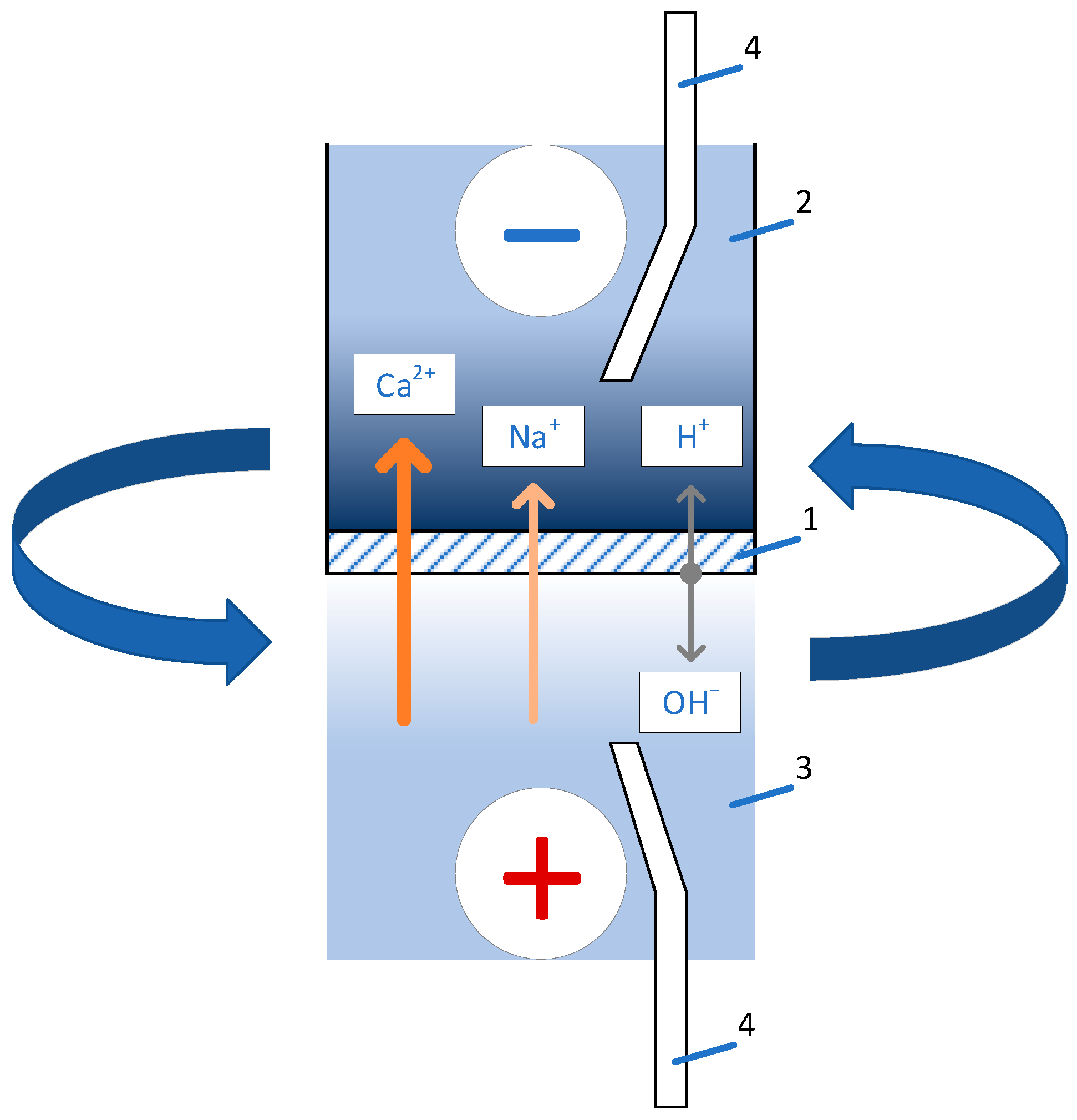
3.2. Current-voltage curves and mechanisms of ion transport
3.3. Prospects for application
3.3.1. Monovalent/divalent selectivity
3.3.2. Bipolar membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Memb. Sci. 2018, 555, 429–454. [CrossRef]
- Helfferich, F.G. Ion Exchange; McGraw Hill Book Company, Inc.: New York, 1962; ISBN 0-486-68784-8.
- Zhang, Y.; Paepen, S.; Pinoy, L.; Meesschaert, B.; Van Der Bruggen, B. Selectrodialysis: Fractionation of divalent ions from monovalent ions in a novel electrodialysis stack. Sep. Purif. Technol. 2012, 88, 191–201. [CrossRef]
- Galama, A.H.; Daubaras, G.; Burheim, O.S.; Rijnaarts, H.H.M.; Post, J.W. Fractioning electrodialysis: a current induced ion exchange process. Electrochim. Acta 2014, 136, 257–265. [CrossRef]
- Ge, L.; Wu, B.; Li, Q.; Wang, Y.; Yu, D.; Wu, L.; Pan, J.; Miao, J.; Xu, T. Electrodialysis with nanofiltration membrane (EDNF) for high-efficiency cations fractionation. J. Memb. Sci. 2016, 498, 192–200. [CrossRef]
- Khoiruddin; Ariono, D.; Subagjo; Wenten, I.G.; Ariono, D.; Gede Wenten, I.; Khoiruddin; Ariono, D.; Subagjo; Wenten, I.G.; et al. Surface modification of ion-exchange membranes: Methods, characteristics, and performance. J. Appl. Polym. Sci. 2017, 134, 1–13. [CrossRef]
- Sata, T. Studies on anion exchange membranes having permselectivity for specific anions in electrodialysis — effect of hydrophilicity of anion exchange membranes on permselectivity of anions. J. Memb. Sci. 2000, 167, 1–31. [CrossRef]
- Sata, T.T.; Sata, T.T.; Yang, W. Studies on cation-exchange membranes having permselectivity between cations in electrodialysis. J. Memb. Sci. 2002, 206, 31–60. [CrossRef]
- Wang, M.; Gao, C. The State-of-The-Art of the Cation Exchange Membrane Having Monovalent Ion Selectivity - A Patent Review. Recent Patents Chem. Eng. 2011, 4, 132–140. [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Memb. Sci. 2017, 522, 267–291. [CrossRef]
- Ge, L.; Wu, B.; Yu, D.; Mondal, A.N.; Hou, L.; Afsar, N.U.; Li, Q.; Xu, T.T.; Miao, J.; Xu, T.T. Monovalent cation perm-selective membranes (MCPMs): New developments and perspectives. Chinese J. Chem. Eng. 2017, 25, 1606–1615. [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science (80-. ). 2017, 356, 1138–1148. [CrossRef]
- Chen, Q.B.; Ren, H.; Tian, Z.; Sun, L.; Wang, J. Conversion and pre-concentration of SWRO reject brine into high solubility liquid salts (HSLS) by using electrodialysis metathesis. Sep. Purif. Technol. 2019, 213, 587–598. [CrossRef]
- Shaposhnik, V.A.; Kesore, K. An early history of electrodialysis with permselective membranes. J. Memb. Sci. 1997, 136, 35–39. [CrossRef]
- Culcasi, A.; Gurreri, L.; Zaffora, A.; Cosenza, A.; Tamburini, A.; Cipollina, A.; Micale, G. Ionic shortcut currents via manifolds in reverse electrodialysis stacks. Desalination 2020, 485, 114450. [CrossRef]
- Bazinet, L.; Ippersiel, D.; Montpetit, D.; Mahdavi, B.; Amiot, J.; Lamarche, F. Effect of membrane permselectivity on the fouling of cationic membranes during skim milk electroacidification. J. Memb. Sci. 2000, 174, 97–110. [CrossRef]
- Zabolotsky, V.I.; Achoh, A.R.; Lebedev, K.A.; Melnikov, S.S. Permselectivity of bilayered ion-exchange membranes in ternary electrolyte. J. Memb. Sci. 2020, 608, 118152. [CrossRef]
- White, N.; Misovich, M.; Alemayehu, E.; Yaroshchuk, A.; Bruening, M.L. Highly selective separations of multivalent and monovalent cations in electrodialysis through Nafion membranes coated with polyelectrolyte multilayers. Polym. (United Kingdom) 2015, 103, 1–8. [CrossRef]
- Zhao, Y.; Zhu, J.; Ding, J.; Van der Bruggen, B.; Shen, J.; Gao, C. Electric-pulse layer-by-layer assembled of anion exchange membrane with enhanced monovalent selectivity. J. Memb. Sci. 2018, 548, 81–90. [CrossRef]
- Melnikov, S.; Bondarev, D.; Nosova, E.; Melnikova, E.; Zabolotskiy, V. Water Splitting and Transport of Ions in Electromembrane System with Bilayer Ion-Exchange Membrane. Membranes (Basel). 2020, 10, 346. [CrossRef]
- Abdu, S.; Wessling, M.; Martí-Calatayud, M.-C.; Wong, J.E.; García-Gabaldón, M.; Wessling, M. Layer-by-Layer Modification of Cation Exchange Membranes Controls Ion Selectivity and Water Splitting. ACS Appl. Mater. Interfaces 2014, 6, 1843–1854. [CrossRef]
- White, N.; Misovich, M.; Yaroshchuk, A.; L. Bruening, M.; Bruening, M.L. Coating of Nafion membranes with polyelectrolyte multilayers to achieve high monovalent/divalent cation electrodialysis selectivities. ACS Appl. Mater. Interfaces 2015, 7, 6620–6628. [CrossRef]
- Golubenko, D. V; Karavanova, Y.A.; Melnikov, S.S.; Achoh, A.R.; Pourcelly, G.; Yaroslavtsev, A.B. An approach to increase the permselectivity and mono-valent ion selectivity of cation-exchange membranes by introduction of amorphous zirconium phosphate nanoparticles. J. Memb. Sci. 2018, 563, 777–784. [CrossRef]
- Siekierka, A.; Calahan, D.L.; Kujawski, W.; Dumée, L.F. Ultra-selective chelating membranes for recycling of cobalt from lithium-ion spent battery effluents by electrodialysis. Desalination 2023, 556. [CrossRef]
- Falina, I. V; Zabolotsky, V.I.; Demina, O.A.; Sheldeshov, N.V. Capillary model of free solvent electroosmotic transfer in ion-exchange membranes: Verification and application. J. Memb. Sci. 2019, 573, 520–527. [CrossRef]
- Porozhnyy, M.V.; Shkirskaya, S.A.; Butylskii, D.Y.; Dotsenko, V.V.; Safronova, E.Y.; Yaroslavtsev, A.B.; Deabate, S.; Huguet, P.; Nikonenko, V.V. Physicochemical and electrochemical characterization of Nafion-type membranes with embedded silica nanoparticles: Effect of functionalization. Electrochim. Acta 2021, 370, 137689. [CrossRef]
- Patnaik, P.; Hossain, S.M.; Pal, S.; Sarkar, S.; Sharma, R.; Chatterjee, U. Controlling the sub-nano ion channels by crosslinking in PVDF-based anion exchange membrane for enhanced mono/bivalent anion permselectivity and acid reclamation. J. Memb. Sci. 2023, 688, 122105. [CrossRef]
- Zabolotsky, V.; Utin, S.; Bespalov, A.; Strelkov, V. Modification of asymmetric bipolar membranes by functionalized hyperbranched polymers and their investigation during pH correction of diluted electrolytes solutions by electrodialysis. J. Memb. Sci. 2015, 494, 188–195. [CrossRef]
- Melnikov, S.; Shkirskaya, S. Transport properties of bilayer and multilayer surface-modified ion-exchange membranes. J. Memb. Sci. 2019, 590, 117272. [CrossRef]
- Achoh, A.R.; Zabolotsky, V.I.; Lebedev, K.A.; Sharafan, M. V.; Yaroslavtsev, A.B. Electrochemical Properties and Selectivity of Bilayer Ion-Exchange Membranes in Ternary Solutions of Strong Electrolytes. Membr. Membr. Technol. 2021, 3, 52–71. [CrossRef]
- Bondarev, D.; Melnikov, S.; Zabolotskiy, V. New homogeneous and bilayer anion-exchange membranes based on N,N-diallyl-N,N-dimethylammonium chloride and ethyl methacrylate copolymer. J. Memb. Sci. 2023, 675, 121510. [CrossRef]
- Hsu, W.Y.; Gierke, T.D. Ion transport and clustering in nafion perfluorinated membranes. J. Memb. Sci. 1983, 13, 307–326. [CrossRef]
- Li, Y.S.; Zhao, T.S.; Yang, W.W. Measurements of water uptake and transport properties in anion-exchange membranes. Int. J. Hydrogen Energy 2010, 35, 5656–5665. [CrossRef]
- Karpenko, L. V.; Demina, O.A.; Dvorkina, G.A.; Parshikov, S.B.; Larchet, C.; Auclair, B.; Berezina, N.P. Comparative study of methods used for the determination of electroconductivity of ion-exchange membranes. Russ. J. Electrochem. 2001, 37, 287–293. [CrossRef]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of ion-exchange membrane materials: properties vs structure. Adv. Colloid Interface Sci. 2008, 139, 3–28. [CrossRef]
- Falina, I.; Loza, N.; Loza, S.; Titskaya, E.; Romanyuk, N. Permselectivity of cation exchange membranes modified by polyaniline. Membranes (Basel). 2021, 11. [CrossRef]
- Zabolotsky, V.I.; Nikonenko, V.V. Effect of structural membrane inhomogeneity on transport properties. J. Memb. Sci. 1993, 79, 181–198. [CrossRef]
- Sharafan, M. V; Zabolotsky, V.I. Study of electric mass transfer peculiarities in electromembrane systems by the rotating membrane disk method. Desalination 2014, 343, 194–197. [CrossRef]
- Zabolotskii, V.I.; Sharafan, M. V; Sheldeshov, N.V.; Lovtsov, E.G. Electric mass transport of sodium chloride through cation-exchange membrane MK-40 in dilute sodium chloride solutions: A rotating membrane disk study. Russ. J. Electrochem. 2008, 44, 141–146. [CrossRef]
- Zabolotskii, V.I.; Bugakov, V. V.; Sharafan, M. V.; Chermit, R.K. Transfer of electrolyte ions and water dissociation in anion-exchange membranes under intense current conditions. Russ. J. Electrochem. 2012, 48, 650–659. [CrossRef]
- Golubenko, D. V.; Manin, A.D.; Wang, Y.; Xu, T.; Yaroslavtsev, A.B. The way to increase the monovalent ion selectivity of FujiFilm® anion-exchange membranes by cerium phosphate modification for electrodialysis desalination. Desalination 2022, 531. [CrossRef]
- Zhang, Z.; Qiao, C.; Zhang, J.; Zhang, W.; Yin, J.; Wu, Z. Synthesis of Unimolecular Micelles with Incorporated Hyperbranched Boltorn H30 Polyester modified with Hyperbranched Helical Poly(phenyl isocyanide) Chains and their Enantioselective Crystallization Performance. Macromol. Rapid Commun. 2017, 38. [CrossRef]
- Yaroslavtsev, A.B.; Karavanova, Y.A.; Safronova, E.Y. Ionic conductivity of hybrid membranes. Pet. Chem. 2011, 51, 473–479. [CrossRef]
- Chintapalli, M.; Timachova, K.; Olson, K.R.; Mecham, S.J.; Devaux, D.; Desimone, J.M.; Balsara, N.P. Relationship between Conductivity, Ion Diffusion, and Transference Number in Perfluoropolyether Electrolytes. Macromolecules 2016, 49, 3508–3515. [CrossRef]
- Kovalenko, A. V.; Nikonenko, V. V.; Chubyr, N.O.; Urtenov, M.K. Mathematical modeling of electrodialysis of a dilute solution with accounting for water dissociation-recombination reactions. Desalination 2023, 550, 116398. [CrossRef]
- Mikhaylin, S.; Nikonenko, V.; Pismenskaya, N.; Pourcelly, G.; Choi, S.; Kwon, H.J.; Han, J.; Bazinet, L. How physico-chemical and surface properties of cation-exchange membrane affect membrane scaling and electroconvective vortices: Influence on performance of electrodialysis with pulsed electric field. Desalination 2016, 393, 102–114. [CrossRef]
- Zabolotskiy, V.I.; But, A.Y.; Vasil’eva, V.I.; Akberova, E.M.; Melnikov, S.S. Ion transport and electrochemical stability of strongly basic anion-exchange membranes under high current electrodialysis conditions. J. Memb. Sci. 2017, 526, 60–72. [CrossRef]
- Dukhin, S.S. Electrokinetic phenomena of the second kind and their applications. Adv. Colloid Interface Sci. 1991, 35, 173–196. [CrossRef]
- Nikonenko, V. V.; Mareev, S.A.; Pis’menskaya, N.D.; Uzdenova, A.M.; Kovalenko, A. V.; Urtenov, M.K.; Pourcelly, G. Effect of electroconvection and its use in intensifying the mass transfer in electrodialysis (Review). Russ. J. Electrochem. 2017, 53, 1122–1144. [CrossRef]
- Akberova, E.M.; Vasil’eva, V.I.; Zabolotsky, V.I.; Novak, L. Effect of the sulfocation-exchanger dispersity on the surface morphology, microrelief of heterogeneous membranes and development of electroconvection in intense current modes. J. Memb. Sci. 2018, 566, 317–328. [CrossRef]
- Rubinstein, I. Electroconvection at an electrically inhomogeneous permselective interface. Phys. Fluids A 1991, 3, 2301–2309. [CrossRef]
- Simons, R. Strong electric field effects on proton transfer between membrane bound amines and water. Nature 1979, 280, 824–826.
- Zabolotskii, V.I.; Shel, N. V; Gnusin, N.P. Dissociation of Water Molecules in Systems with Ion-exchange Membranes. Russ. Chem. Rev. 1988, 57, 801–808.
- Mishchuk, N.A.; Takhistov, P. V. Electroosmosis of the second kind. Colloids Surfaces A Physicochem. Eng. Asp. 1995, 95, 119–131. [CrossRef]
- Balster, J.; Yildirim, M.H.; Stamatialis, D.F.; Ibanez, R.; Lammertink, R.G.H.; Jordan, V.; Wessling, M. Morphology and microtopology of cation-exchange polymers and the origin of the overlimiting current. J. Phys. Chem. B 2007, 111, 2152–2165. [CrossRef]
- Nikonenko, V.V.; Kovalenko, A. V.; Urtenov, M.K.; Pismenskaya, N.D.; Han, J.; Sistat, P.; Pourcelly, G. Desalination at overlimiting currents: State-of-the-art and perspectives. Desalination 2014, 342, 85–106. [CrossRef]
- Aritomi, T.; van den Boomgaard, T.; Strathmann, H. Current-voltage curve of a bipolar membrane at high current density. Desalination 1996, 104, 13–18. [CrossRef]
- Balster, J.; Stamatialis, D.F.; Wessling, M. Electro-catalytic membrane reactors and the development of bipolar membrane technology. Chem. Eng. Process. Process Intensif. 2004, 43, 1115–1127. [CrossRef]
- Zabolotskii, V.I.; Nikonenko, V. V.; Korzhenko, N.M.; Seidov, R.R.; Urtenov, M.K. Mass transfer of salt ions in an electromembrane system with violated electroneutrality in the diffusion layer: The effect of a heterolytic dissociation of water. Russ. J. Electrochem. 2002, 38, 810–818. [CrossRef]
- Melnikov, S.S.; Nosova, E.N.; Melnikova, E.D.; Zabolotsky, V.I. Reactive separation of inorganic and organic ions in electrodialysis with bilayer membranes. Sep. Purif. Technol. 2021, 268, 118561. [CrossRef]
- Sharafan, M. V.; Zabolotskii, V.I.; Bugakov, V. V. Electric mass transport through homogeneous and surface-modified heterogeneous ion-exchange membranes at a rotating membrane disk. Russ. J. Electrochem. 2009, 45, 1162–1169. [CrossRef]
- Belashova, E.D.; Melnik, N.; Pismenskaya, N.D.; Shevtsova, K.; Nebavsky, A.; Lebedev, K.A.; Nikonenko, V.V. Overlimiting mass transfer through cation-exchange membranes modified by Nafion film and carbon nanotubes. Electrochim. Acta 2012, 59, 412–423. [CrossRef]
- Gil, V.; Porozhnyy, M.; Rybalkina, O.; Butylskii, D.; Pismenskaya, N. The development of electroconvection at the surface of a heterogeneous cation-exchange membrane modified with perfluorosulfonic acid polymer film containing titanium oxide. Membranes (Basel). 2020, 10, 1–21. [CrossRef]
- Zyryanova, S.; Mareev, S.; Gil, V.; Korzhova, E.; Pismenskaya, N.; Sarapulova, V.; Rybalkina, O.; Boyko, E.; Larchet, C.; Dammak, L.; et al. How electrical heterogeneity parameters of ion-exchange membrane surface affect the mass transfer and water splitting rate in electrodialysis. Int. J. Mol. Sci. 2020, 21. [CrossRef]
- Choi, J.-H.H.; Lee, H.-J.J.; Moon, S.-H.H. Effects of electrolytes on the transport phenomena in a cation-exchange membrane. J. Colloid Interface Sci. 2001, 238, 188–195. [CrossRef]
- Titorova, V.D.; Moroz, I.A.; Mareev, S.A.; Pismenskaya, N.D.; Sabbatovskii, K.G.; Wang, Y.; Xu, T.; Nikonenko, V. V. How bulk and surface properties of sulfonated cation-exchange membranes response to their exposure to electric current during electrodialysis of a Ca2+ containing solution. J. Memb. Sci. 2022, 644, 120149. [CrossRef]
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar membranes: A review on principles, latest developments, and applications. J. Memb. Sci. 2021, 617, 118538. [CrossRef]
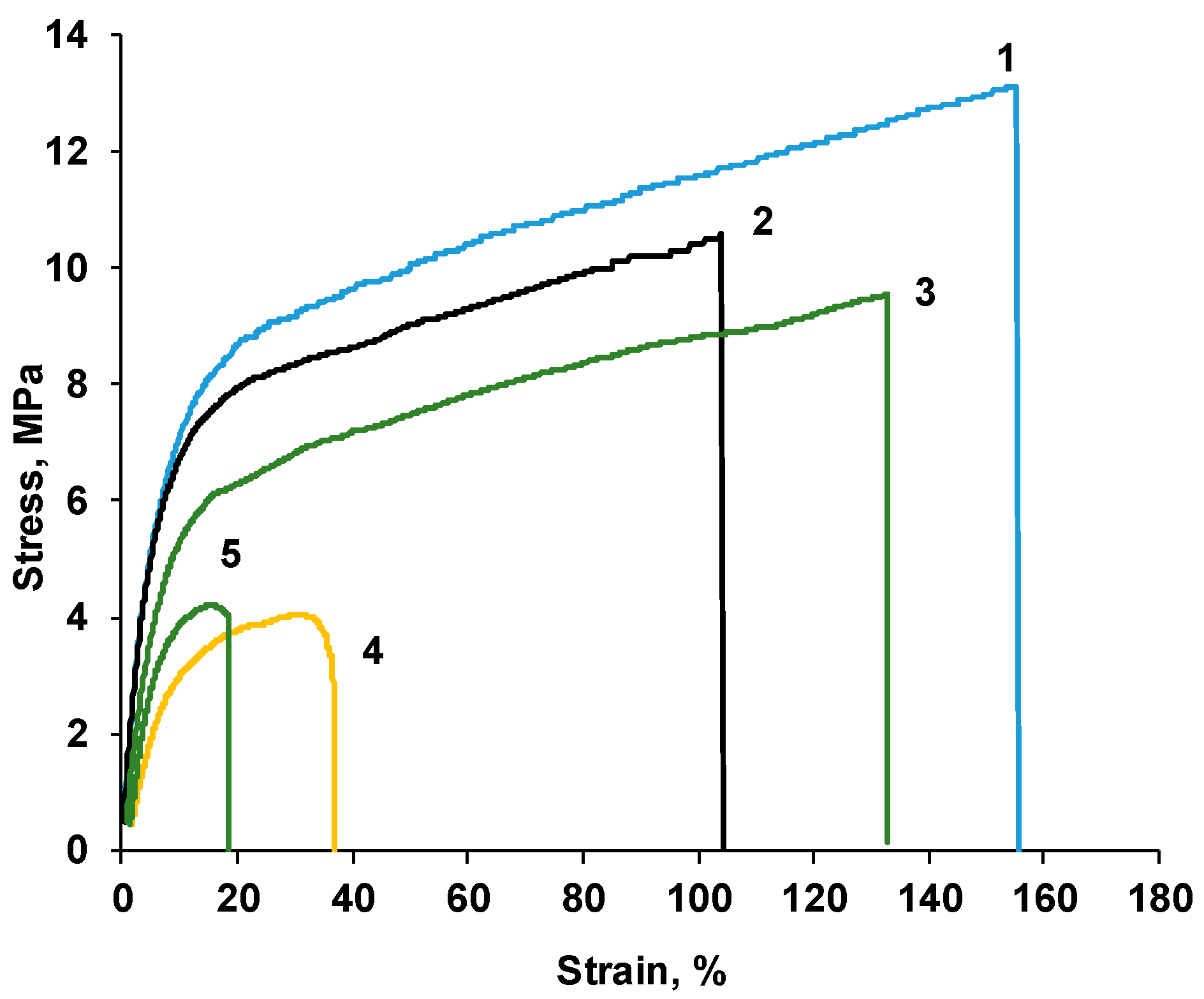
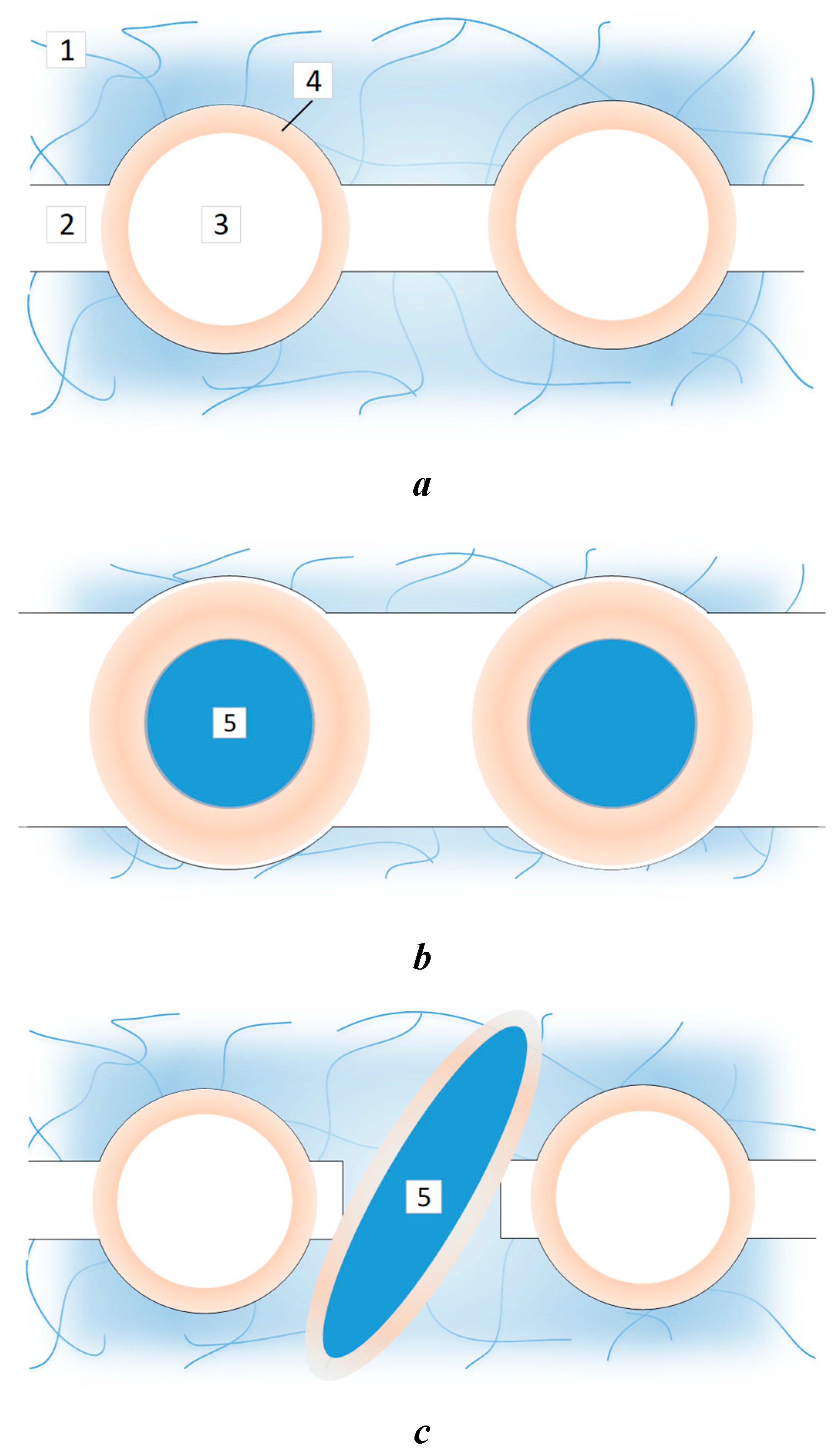
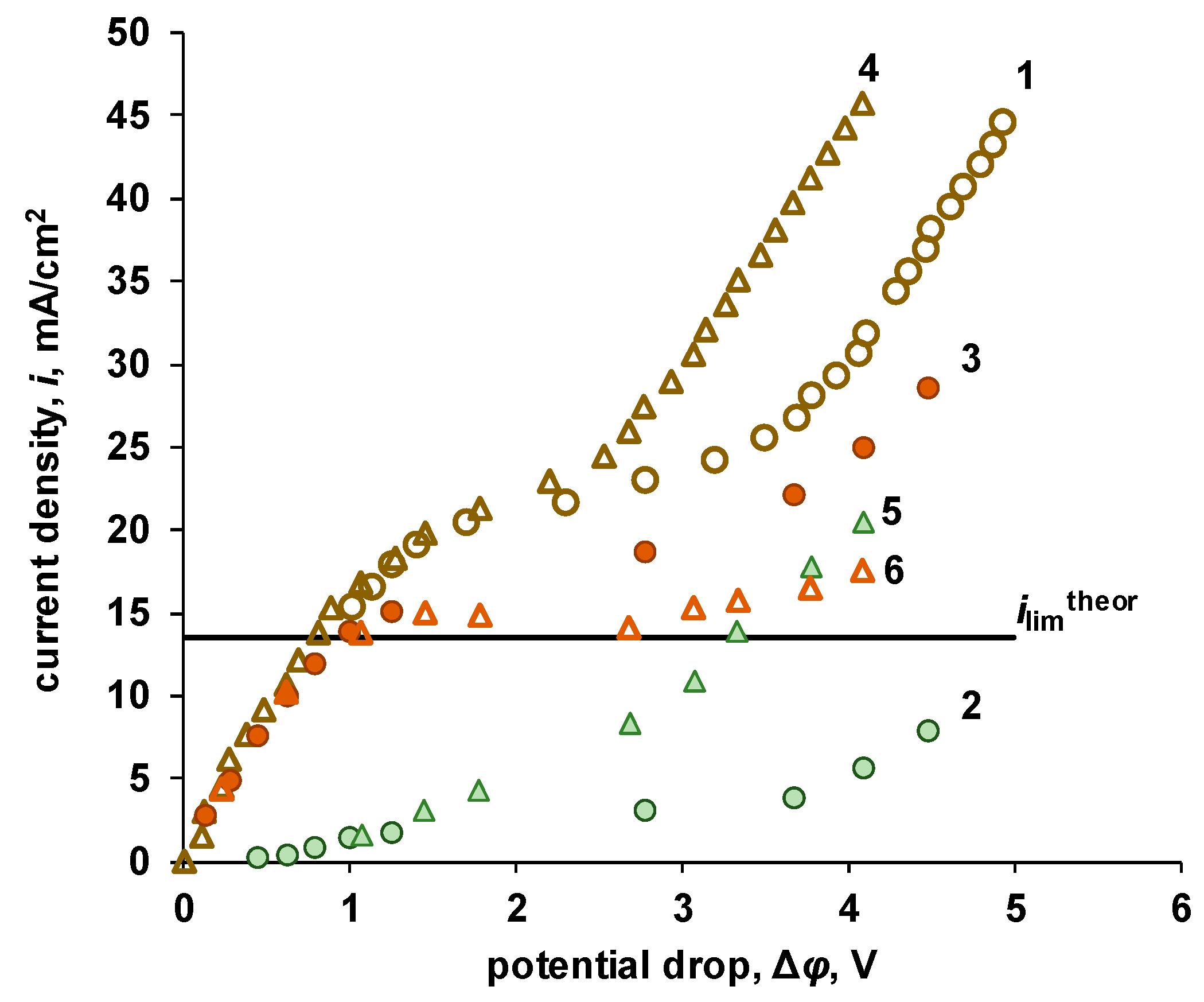
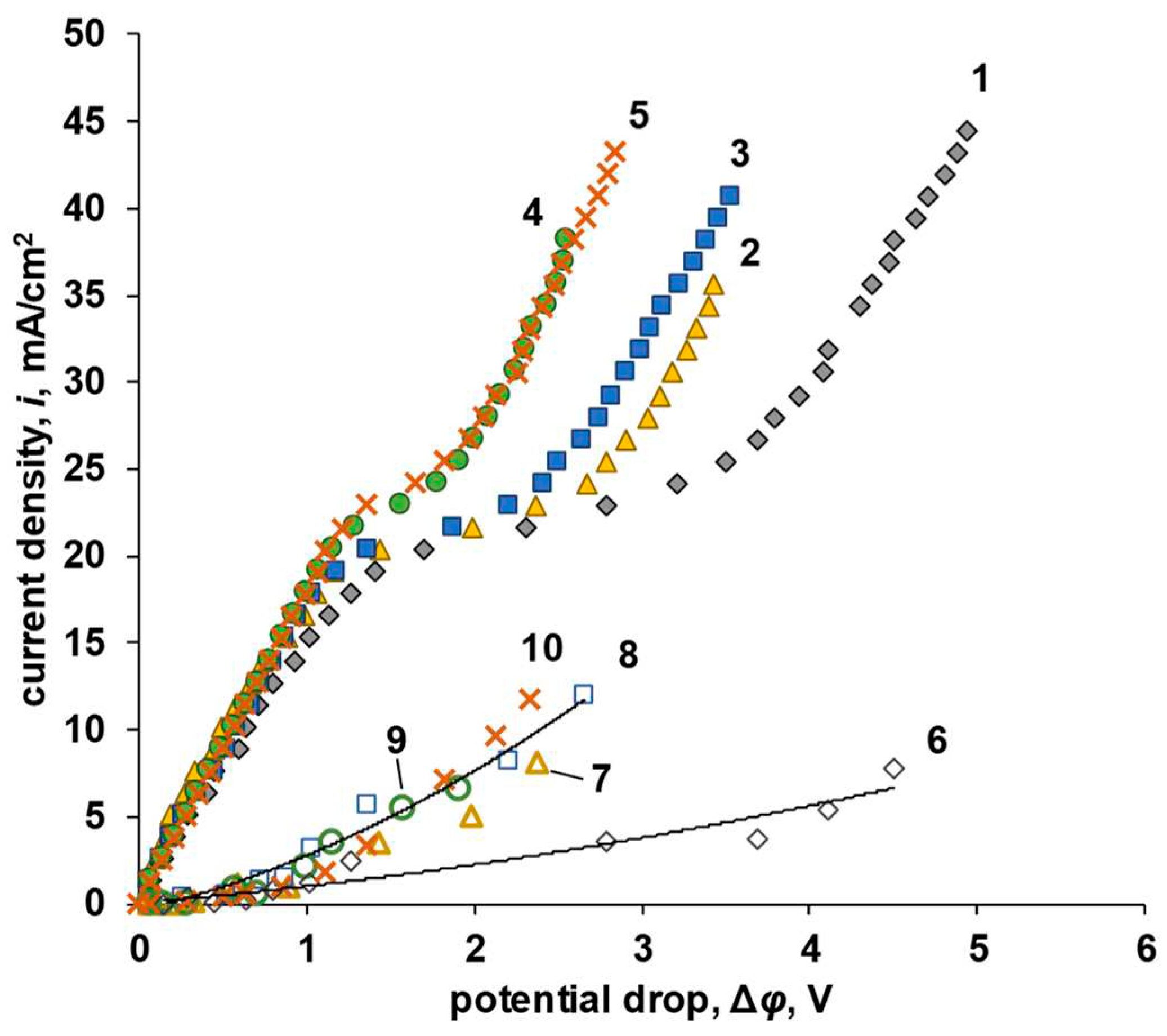
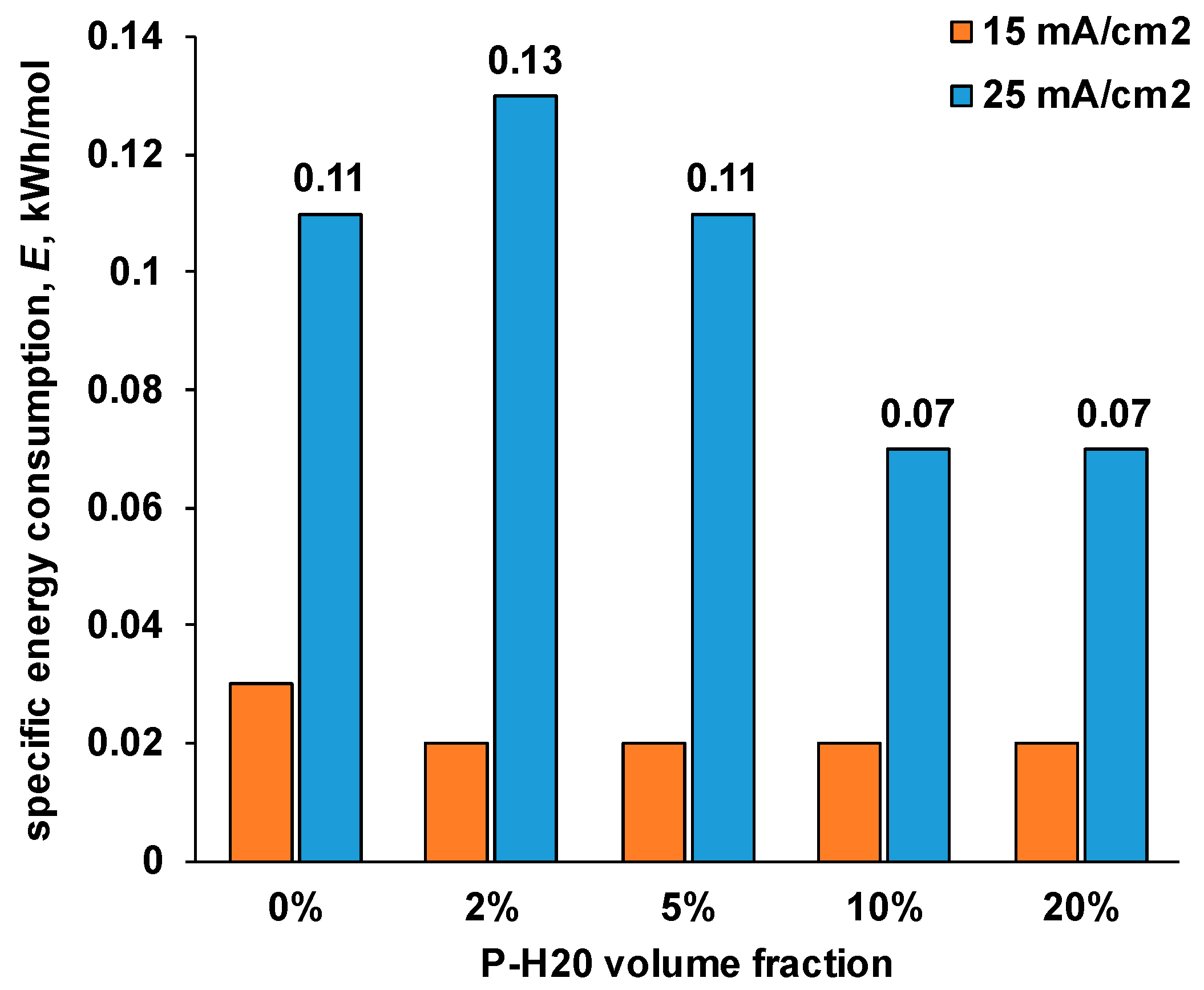
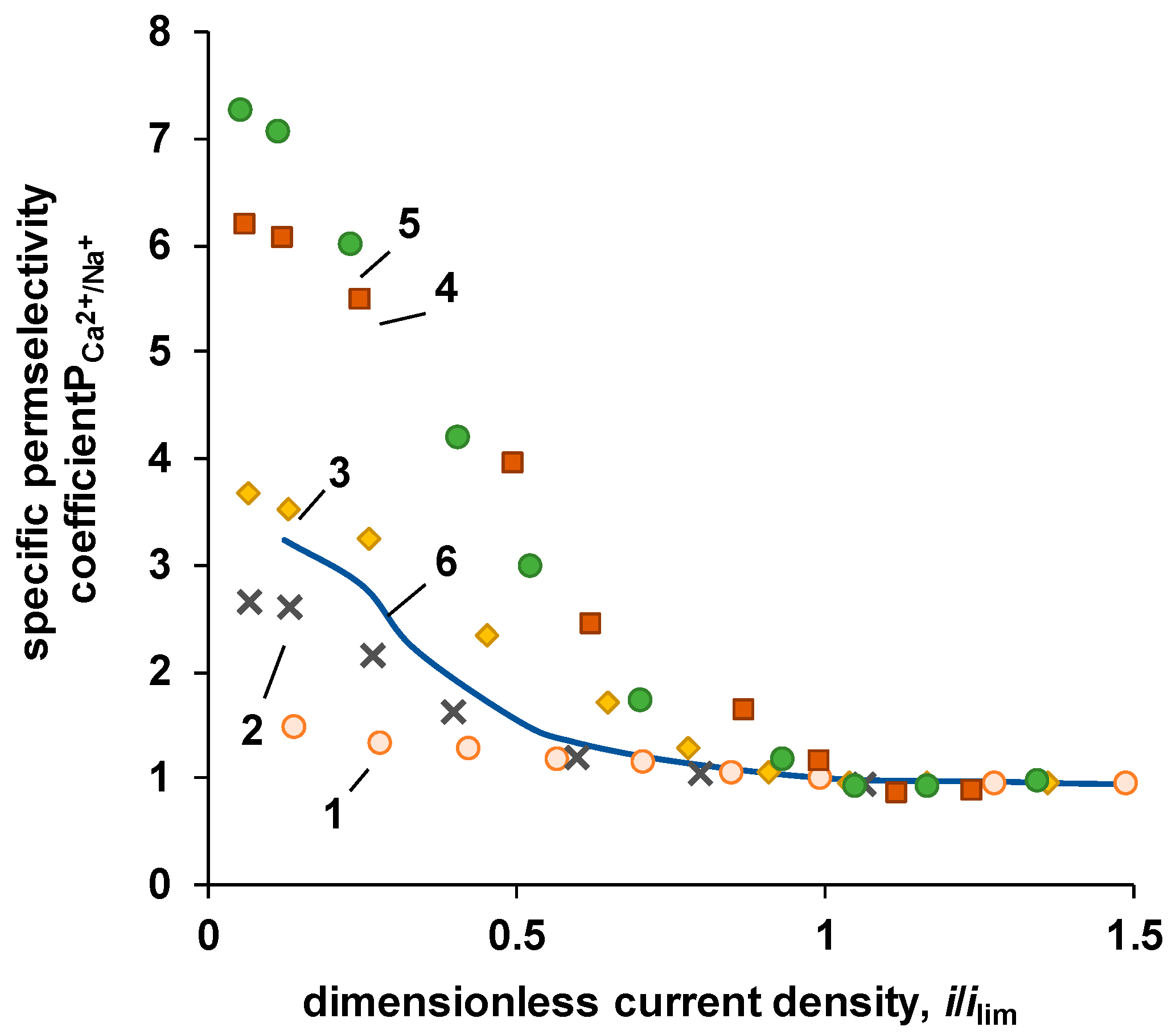
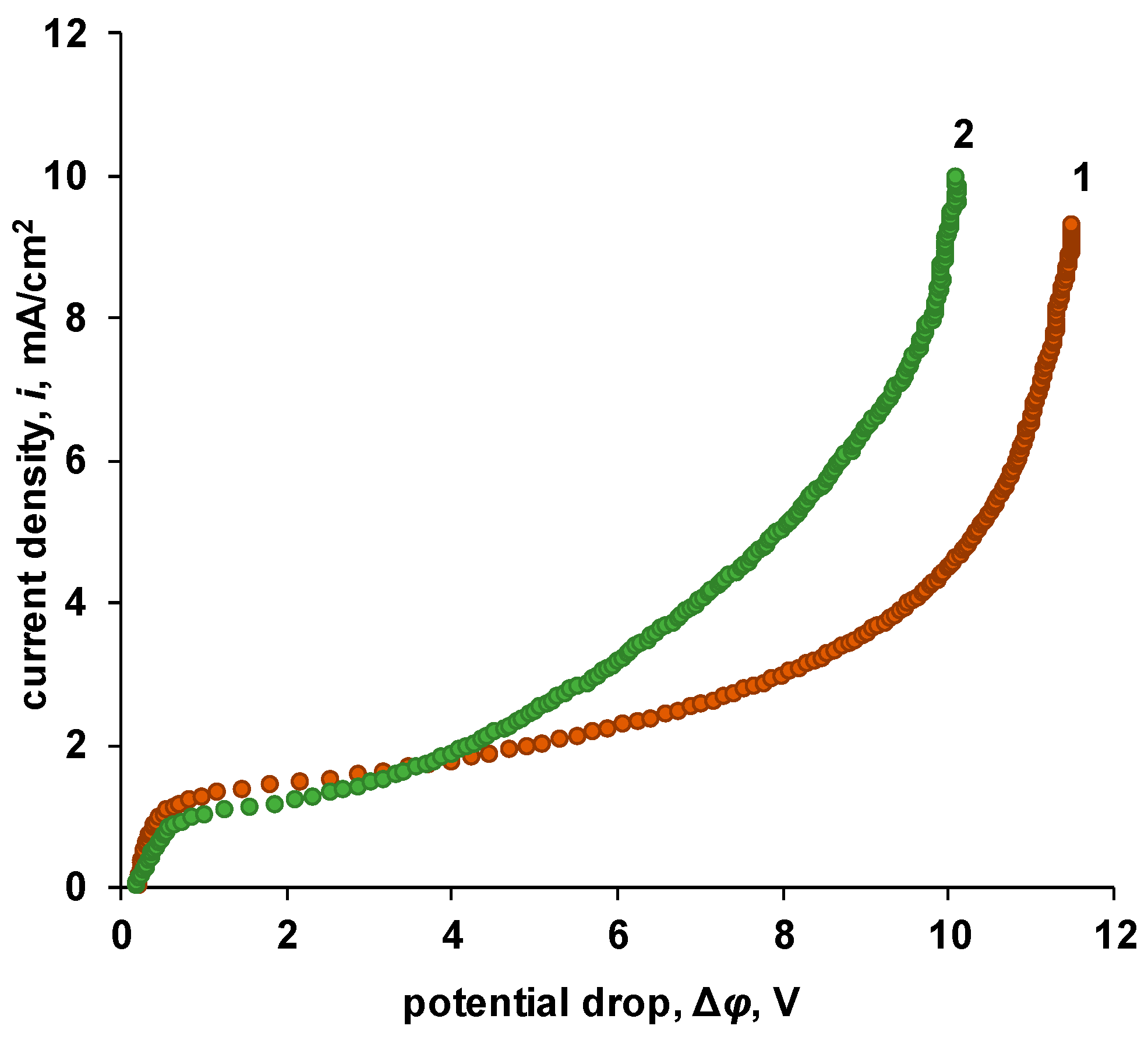
| P-H20 content | Stress, MPa | Strain at rupture, % | Young's modulus, MPa | Yield strength, MPa |
|---|---|---|---|---|
| 0% | 12.0±1.1 | 118±44 | 159±12 | 8.0±0.3 |
| 2% | 10.4±0.7 | 112±7 | 142±8 | 7.2±0.3 |
| 5% | 11.0±1.3 | 138±10 | 116±8 | 6.5±0.6 |
| 10% | 3.4±1.4 | 22±9 | 52±22 | 2.8±1.1 |
| 20% | 5.3±1.0 | 23±12 | 110±31 | 3.7±0.7 |
| Volume fraction of P-H20, % | 0 | 2 | 5 | 10 | 20 |
|---|---|---|---|---|---|
| Ion-exchange capacity, mmol/gwet | 0.83±0.05 | 0.81±0.05 | 0.79±0.05 | 0.72±0.05 | 0.66±0.05 |
| Water uptake, % | 15.6±0.3 | 15.4±0.3 | 14.7±0.3 | 12.5±0.3 | 10.3±0.3 |
| Gel conductivity, kiso, mS/cm | 9.7 | 5.3 | 4.2 | 3.7 | 2.8 |
| Intergel fraction, f2 | 0.02 | 0.06 | 0.07 | 0.07 | 0.07 |
| Integral diffusion permeability in 1 M NaCl, Pm, 106·cm2/s |
| Membrane | SPTFE | SPTFE+P-H20 |
|---|---|---|
| Volume fraction of P-H20, % | 0 | 10 |
| Limiting current density, mA/cm2 | 18 | 17 |
| Potential drop Δφ = 1 V | ||
| Total current, mA/cm2 | 14.8 | 16.4 |
| Salt ions current, mA/cm2 | 13.6 | 14.6 |
| Water-splitting products current, mA/cm2 | 1.3 | 1.8 |
| Non-eqilibrium electroconvection current, mA/cm2 | - | - |
| Exaltation current, mA/cm2 | 0.2 | 0.3 |
| Potential drop Δφ = 2 V | ||
| Total current, mA/cm2 | 21.3 | 21.7 |
| Salt ions current, mA/cm2 | 19.1 | 16.9 |
| Water-splitting products current, mA/cm2 | 2.2 | 4.9 |
| Non-eqilibrium electroconvection current, mA/cm2 | 1.8 | - |
| Exaltation current, mA/cm2 | 0.3 | 0.7 |
| Potential drop Δφ = 4 V | ||
| Total current, mA/cm2 | 30.4 | 44.5 |
| Salt ions current, mA/cm2 | 25.3 | 24.8 |
| Water-splitting products current, mA/cm2 | 5.1 | 19.8 |
| Non-eqilibrium electroconvection current, mA/cm2 | 7.6 | 4.9 |
| Exaltation current, mA/cm2 | 0.7 | 2.8 |
| Membrane | SPTFE | SPTFE+P-H20 | |||
|---|---|---|---|---|---|
| Volume fraction of P-H20, % | 0 | 2 | 5 | 10 | 20 |
| Limiting current density, mA/cm2 | 18 | 18.5 | 19 | 21 | 22 |
| Total current, mA/cm2 | 21.3 | 16.4 | 21.8 | 21.9 | 26.7 |
| Salt ions current, mA/cm2 | 19.1 | 14.6 | 16.1 | 14.3 | 19.2 |
| Water-splitting products current, mA/cm2 | 2.2 | 1.8 | 5.7 | 7.6 | 7.5 |
| Non-eqilibrium electroconvection current, mA/cm2 | 1.8 | - | - | - | - |
| Exaltation current, mA/cm2 | 0.3 | 0.3 | 1.5 | 2.0 | 2.0 |
| Membrane | SPTFE | SPTFE+P-H20 | |||
|---|---|---|---|---|---|
| Volume fraction of P-H20, % | 0 | 2 | 5 | 10 | 20 |
| Potential drop, V | 3.4 | 2.8 | 2.4 | 1.9 | 1.8 |
| Salt ions current, mA/cm2 | 21.5 | 14.8 | 14.3 | 18.7 | 18.6 |
| Water-splitting products current, mA/cm2 | 3.4 | 10.6 | 10.1 | 6.8 | 6.6 |
| Non-eqilibrium electroconvection current, mA/cm2 | 2.7 | - | - | - | - |
| Exaltation current, mA/cm2 | 0.9 | 2.8 | 2.7 | 1.8 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





