Introduction
Hidradenitis suppurativa and Langerhans cell histiocytosis are rare disorders that can reveal similar clinical manifestations, making differential diagnosis difficult. A definitive diagnosis requires biopsy and histological confirmation in suspicious cases. The complex pathophysiology of these diseases has still not been understood, therefore the treatment is still a challenge for physicians.
Hidradenitis suppurativa (HS), also known as acne inversa, is a chronic autoimmune, non-contagious, recurrent inflammatory disease affecting the skin and localized in anatomical regions containing the apocrine glands. This disease was first described in 1839 by the French anatomist and surgeon Alfred-Armand-Louis-Marie Velpeau and was known as 'Velpeau's disease'. In 1854 it was named 'Verneuil's disease' after the surgeon who described the association between hidradenitis suppurativa or acnee inversa and sweat glands. (1)
The disease has also been described by many different other names: by Politzer, in 1894 as hidradenitis destruens suppurativa, by Unna as spiradenitis suppurativa disseminata, Barthelemy used the terms 'acnitis' or 'folliclis' to distinguish regional diseases, Bronson and Fordyee described acne varioliformis of the extremities, Tenneson gave the name granulonme innomine, etc. (2), (3)
It is seen with predilection in the intertriginous areas of the body, predominantly in the axillary region, rarely requiring a surgical procedure. However perineal, perianal, and gluteal HS usually necessitates some form of surgical treatment. Minor lesions, like comedones, and painfully inflammatory nodules can also be encountered on the face and in the abdominal region, and these lesions can be resolved with wide local excision followed by primary wound closure. The clinical appearance is defined by the presence of dolorous subcutaneous nodules that may rupture, leading to deep dermal abscesses, which are constantly extending and worsening, forming tunneling sinus tracts and scarring. These lesions are accompanied by symptoms such as burning pruritus, local warmth, hyperhidrosis, pain, and suppuration with discharge through multiple openings. Abscesses may heal, producing skin fibrosis with dermal contractures, but in most cases, the lesions without treatment are progressing and worsening. (4)
The diagnosis of hidradenitis suppurativa is based on clinical findings of characteristic HS lesions, which are predominantly limited to the intertriginous areas, and the disease course is typically characterized by recurrent flares, therefor biopsy is rarely required but may help in the definitive diagnosis of unusual case presentations. Early lesions of hidradenitis suppurativa can imitate other skin conditions, which results in delayed diagnosis and difficulty in assessing the severity of the disease. (4), (5)
CT scans and ultrasound are important diagnostic tools, especially for deep-seated lesions. This disease is highly debilitating for sufferers, both physically and psychologically, leading to social isolation, failed relationships, and depression.
Two types of staging are commonly used for hidradenitis suppurativa by Hurley and Sartorius. The most common hidradenitis suppurativa classification system is the Hurley Staging System, which is very simple, based on the presence and extent of scarring and sinus tracts, and can be divided into three stages:
| Stage I |
characterized by an inflammatory nodule or abscess formation, single or multiple isolated abscesses without sinus tract formation or scarring |
| Stage II |
single or multiple lesions widely separated by normal skin, characterized by recurrent abscesses and nodules, with one or more sinus tracts and scarring; |
| Stage III |
characterized by diffuse boils with multiple interconnected sinus tracts, scarring, and abscesses involving the entire area without intervening with normal skin |
Sartorius's staging system is a more dynamic and detailed staging process than Hurley's. The system is based on the anatomical region involved, the number and type of lesions and the presence of sinus tracts, the maximum distance between the 2 lesions, and whether the lesions are clearly separated by normal, intact skin. (4), (6), (7)
The pathogenesis of HS is only partially understood. This chronic skin disease can be caused by genetic factors such as genetic "weak pores" that easily rupture, and microbiological, endocrine, environmental, and dietary factors that also increase insulin levels, which also lead to follicular blockage of the folliculopilosebaceous unit. (8) This mechanism is followed by follicular rupture of the sebofollicular canal, leading to perifollicular lymphohistiocytic inflammation. (9), (10), (11) A genetic factor has been identified in one-third of patients with autosomal dominant inheritance. (12)
Langerhans cell histiocytosis (histiocytosis X) is a very rare systemic disease manifested by clonal cell proliferation of histiocytic-dendritic nature, with pseudonodular manifestation that deeply infiltrates the hypodermis and ulcerates the epidermis on the surface. Langerhans cells are dendritic, antigen-presenting cells localized in the suprabasal regions of the squamous epithelium of the skin and mucous membranes, which help regulate the immune system. They are normally found throughout the body, particularly in the skin, lymph nodes, spleen, lungs, liver, and bone marrow, but they can be found in the urothelium, bronchial and oesophageal epithelium, and sometimes in the anal canal. These cells form in the bone marrow from a stem cell common to the mononuclear phagocytic system. The specific marker of this stem cell is CD34. (13). Clinically, the manifestations are very diverse, there are spontaneously resolving unifocal forms, but also severe forms with multi- visceral involvement with a poor vital prognosis. The most common presentation of LCH is the single-system disease. The multifocal form particularly affects the organs and tissues that have Langerhans cell deposits. The most important unfavorable prognostic factor for survival is the functional impairment of the affected organs. (14) Perianal cutaneous Langerhans cell histiocytosis is one of its rarest manifestations. (15)
The etiology of the disease has still not been understood, but the manifestation of the disease may be due to the presence of an oncogene or a viral involvement (human herpesvirus type 6), due to a somatic mutation in a gene that would cause clonal proliferation of Langerhans cells or activation of Langerhans cells by cytokines.
To establish the diagnosis of certainty, it is necessary for the pathological examination of the tissue obtained by biopsy of skin or bone lesions or, more rarely, histiocytes highlighted in the myelogram. To confirm the diagnosis of certainty it is necessary at least one of the criteria: the presence of CD1a antigen revealed by immunohistochemical techniques or Birbeck granule evidence by electron microscopy.
In case of multiorgan involvement, it is necessary to examine the spinal cord aspirate (myelogram) with identification of CD1a positive cells: if there is cytopenia on the peripheral blood smear; collection of broncho-alveolar lavage; thoracic computer tomography and brain MRI (nuclear magnetic resonance) investigation in case of pituitary involvement. (16), (17), (18)
In this report, we present our experience with extensive gluteal, perineal, and perianal hidradenitis suppurativa and Langerhans cell histiocytosis cases, including our treatment methods and outcomes.
Case 1:
The first patient is a 46-year-old male patient with a previous history of hidradenitis suppurativa who presented persistent multiple abscesses involving the pelvic region associated with symptoms of suppuration and pain. He was diagnosed 10 years ago, and since then, he has undergone different dermatological therapies, including antibiotics, minor surgical procedures, such as incision, drainage or limited local excision, and primary closure, without any results.
Past medical history included diabetes mellitus type 2, treated with oral agents, whereas history of heart disease: grade III arterial hypertension, previous acute myocardial infarction treated with percutaneous transluminal angioplasty, chronic cardiac insufficiency, hypercholesterolemia, and heavy smoking history with more than 12-15 cigarettes per day.
Clinical examination revealed multiple draining sinus tracts and abscesses in the perineal, inguinal, inferior abdominal, and bilateral axillary regions. Blood tests and wound secretion cultures were ordered, and antibiotic management with clindamycin was initiated. Insulin therapy was started for his diabetes control. Skin cultures showed Streptococcus spp. and Acinetobacter baumannii, therefore, Vancomycin and Gentamycin were added to his antibiotic regimen.
Figure 1.
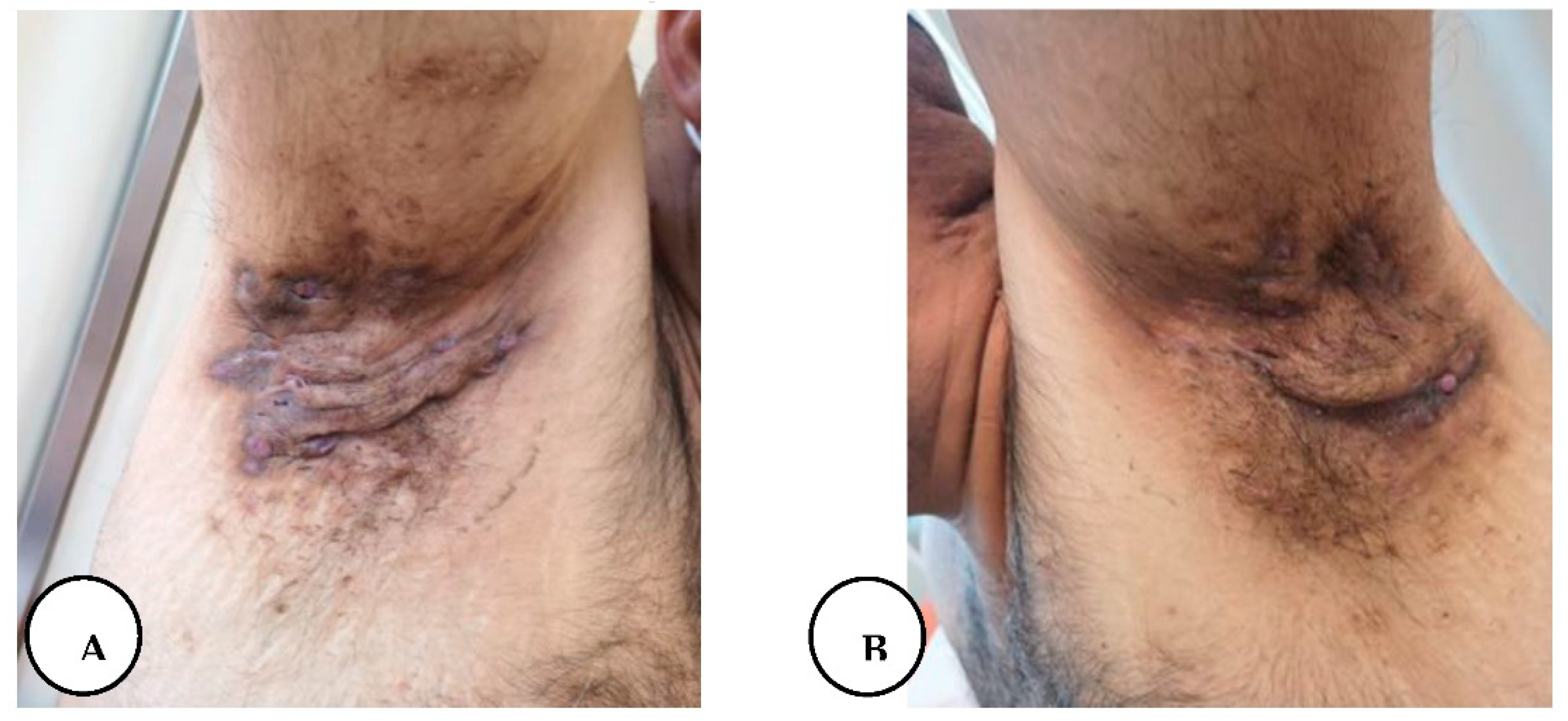
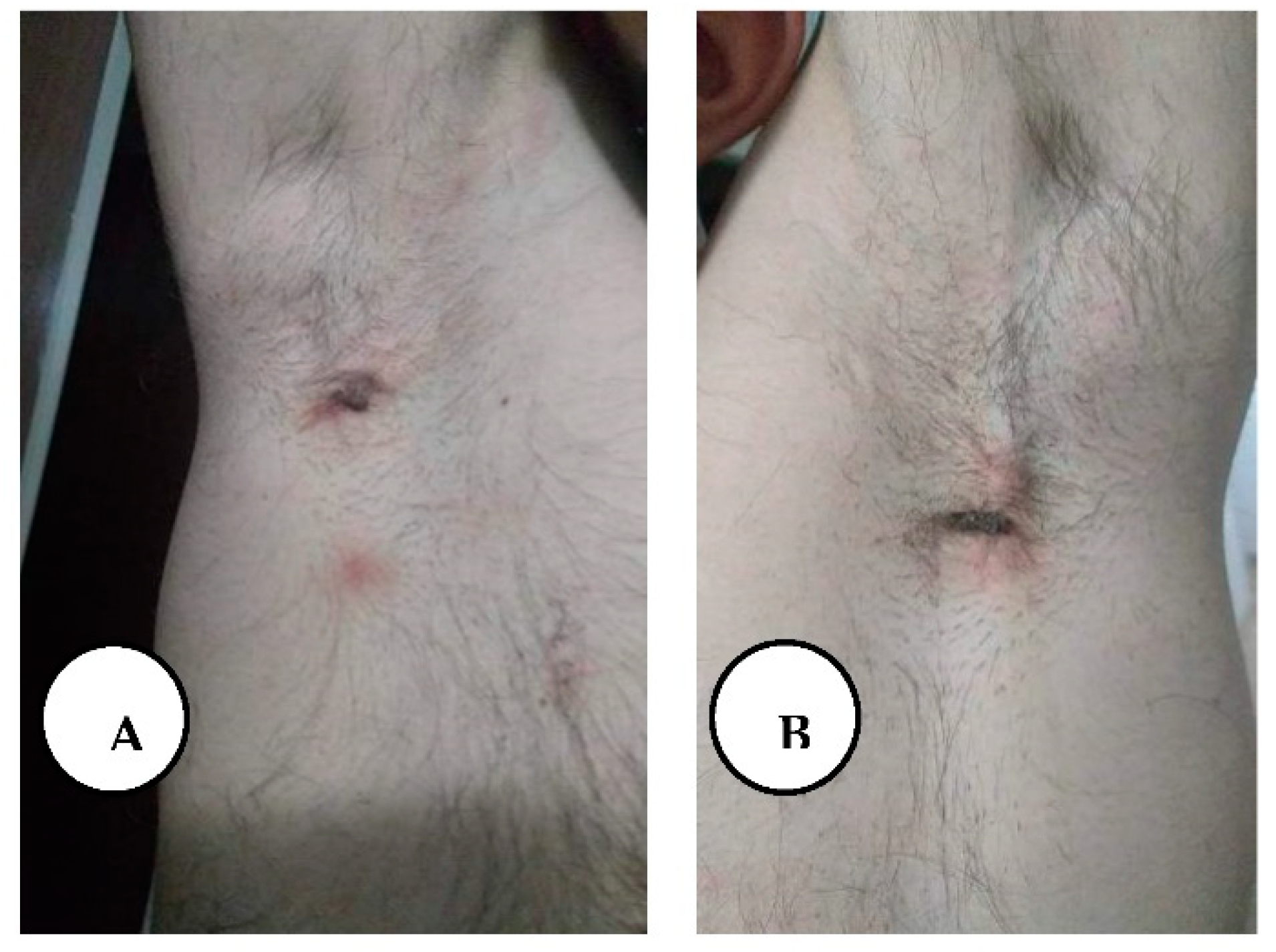
A, B. Multiple draining sinus tracts and abscesses in the bilateral axillary region.
Figure 1.
A, B. Multiple draining sinus tracts and abscesses in the bilateral axillary region.
Figure 2.
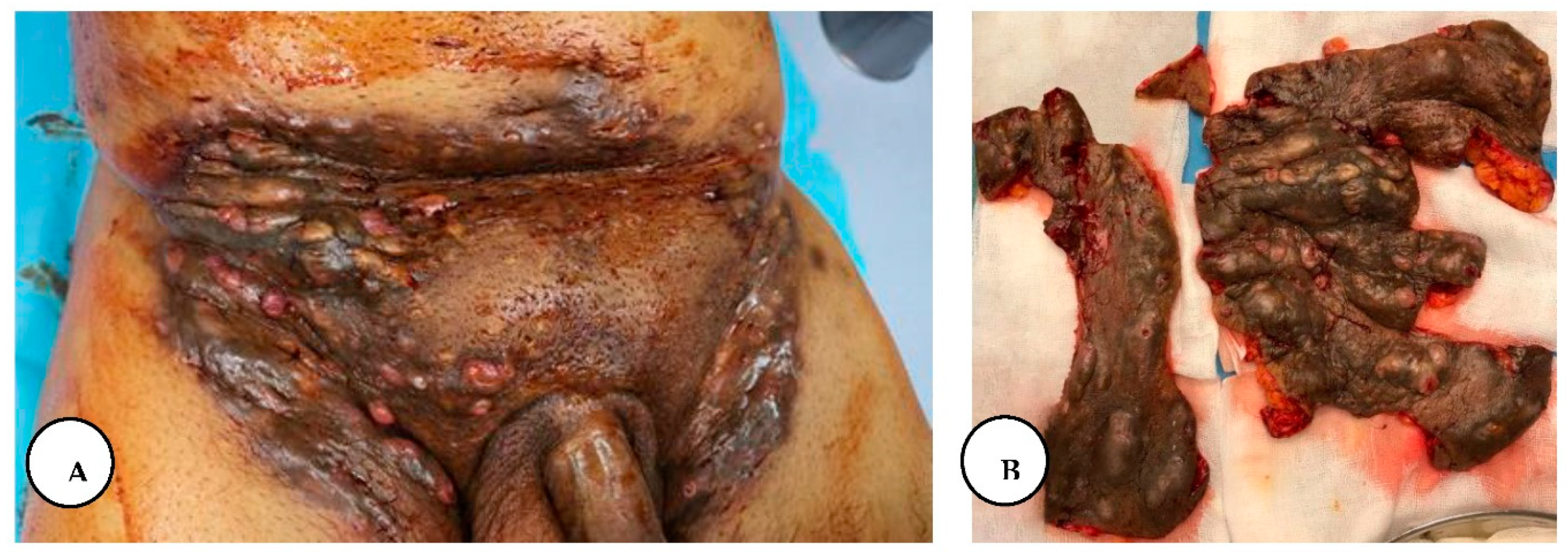
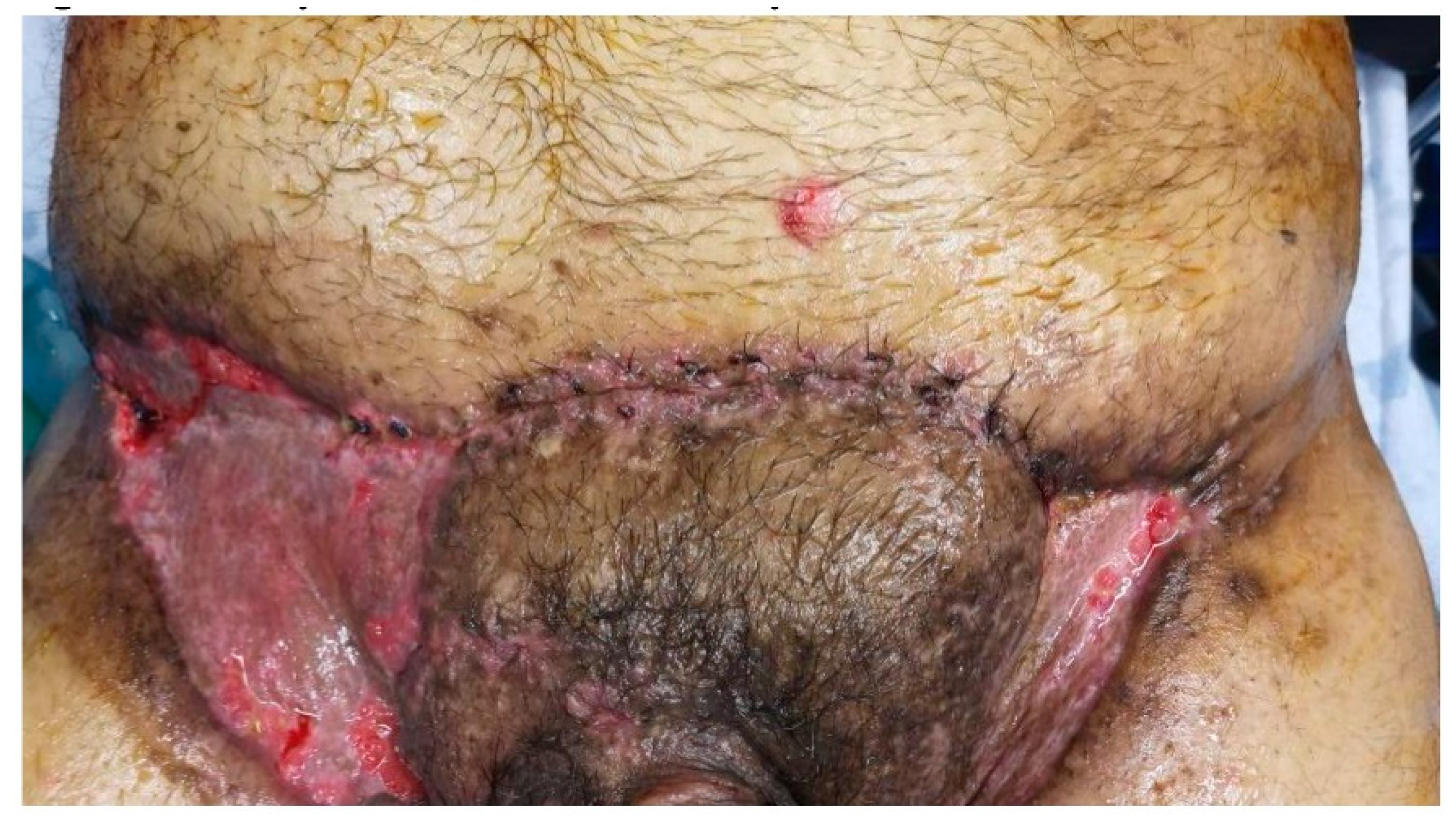
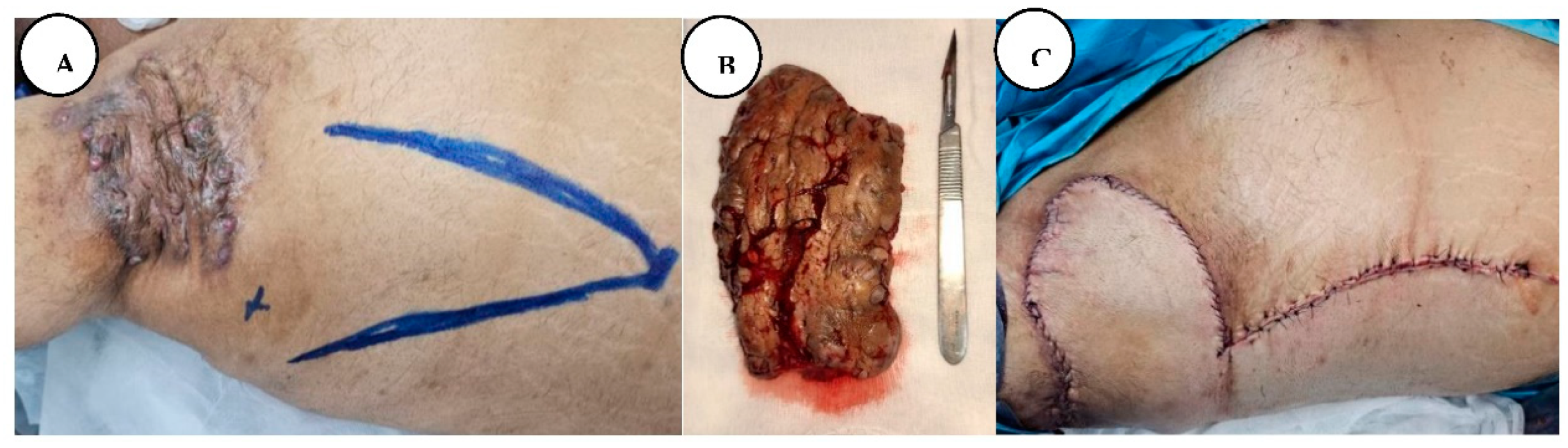
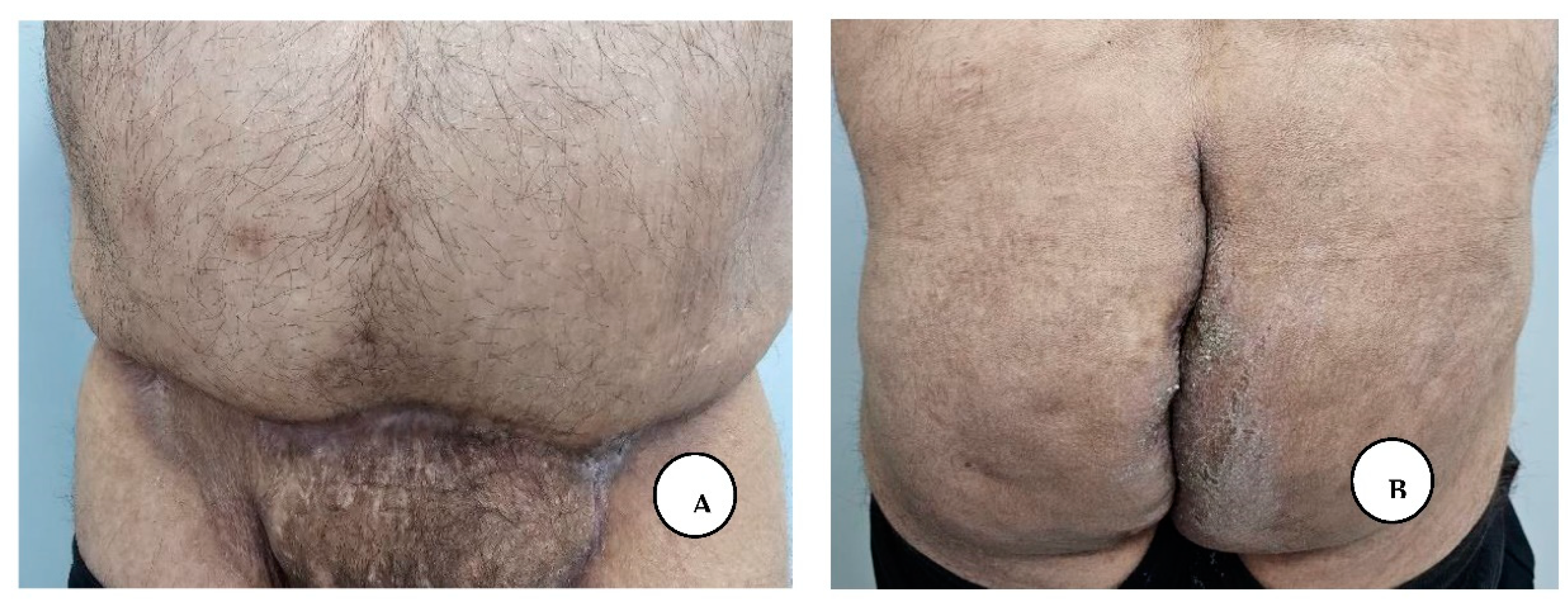
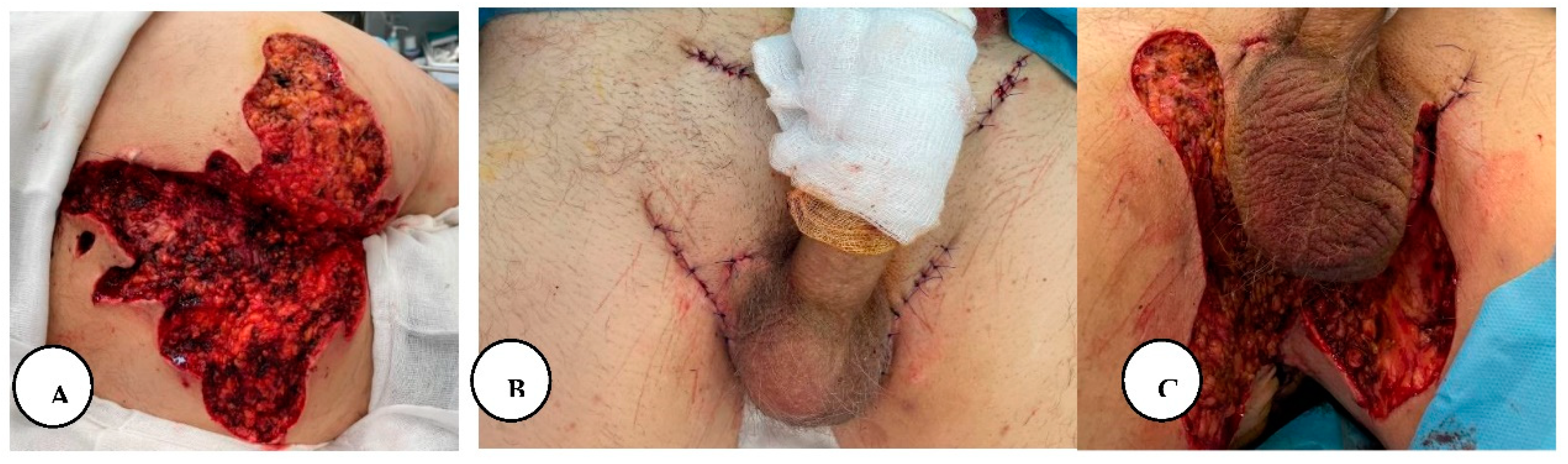
A. Multiple painful nodules, draining sinus tracts and confluent abscesses in the inguinal, perineal, and inferior abdominal regions, and diffuse boils with multiple interconnected sinus tracts, scarring, and abscesses involving the perianal region, B. The excised specimen from the lower abdominal, inguinal, and perianal regions.
Figure 2.
A. Multiple painful nodules, draining sinus tracts and confluent abscesses in the inguinal, perineal, and inferior abdominal regions, and diffuse boils with multiple interconnected sinus tracts, scarring, and abscesses involving the perianal region, B. The excised specimen from the lower abdominal, inguinal, and perianal regions.
Family history shows that his sister and brother also suffer from this disease with similar localization.
Figure 3.
A, B. Painful inflammatory nodules and draining abscesses localized in the bilateral axillary regions of the patient's sister.
Figure 3.
A, B. Painful inflammatory nodules and draining abscesses localized in the bilateral axillary regions of the patient's sister.
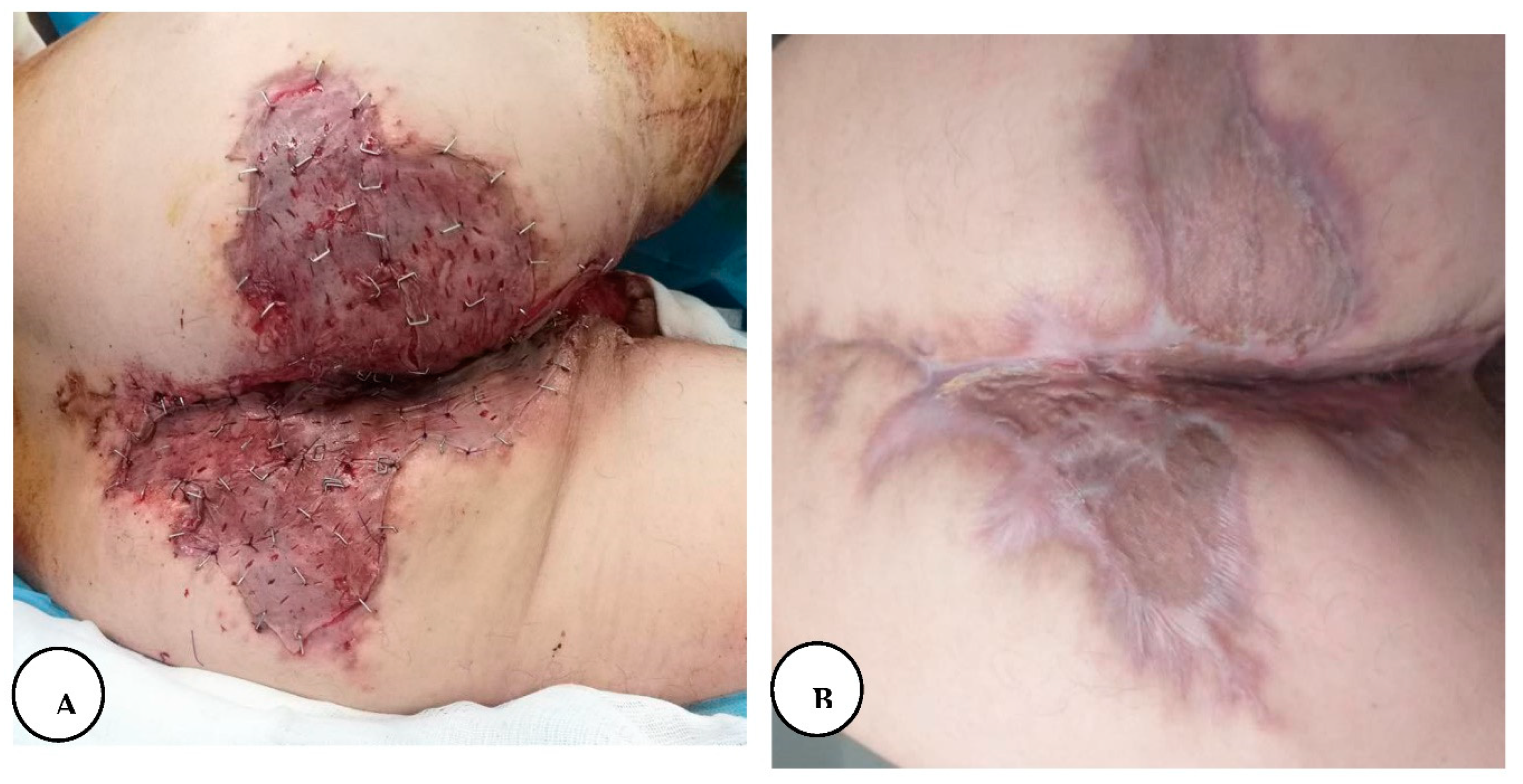
On the fourth day of hospitalization, he was taken into surgery for wide local excision of the affected tissues from the inguinal, inferior abdominal, and perineal regions. The operative technique was based on wide local excision of the entire diseased skin and subcutaneous fatty tissue down to the muscular fascia, with the protection of the external anal sphincter in the anogenital region.
Figure 4.
A. Immediate postoperative appearance, after wide resection of the affected regions in the inferior abdominal and inguinal regions, B. Postoperative result in the perineal and perianal regions.
Figure 4.
A. Immediate postoperative appearance, after wide resection of the affected regions in the inferior abdominal and inguinal regions, B. Postoperative result in the perineal and perianal regions.
Haemostasis was achieved, and we performed primary closure in the inferior abdominal region, and the other region the wound was covered with silver-containing dressings and connected to a negative pressure system. The procedure was done without any complications and post-operative analgesia was achieved with intravenous painkillers.
The negative pressure system was changed on the sixth and ninth days, after that every third day post-operatively, without any complications or significant findings.
The patient continued to make a stable recovery over the next few days. He was taken to the operating room again on day 33, for a secondary closure of the recurrent wound dehiscence of the inferior abdominal region.
Figure 5.
60th day postoperatively.
Figure 5.
60th day postoperatively.
The surgical defect of the inguinal area was reconstructed with a split-thickness skin graft harvested from the medial aspect of the contralateral thigh region and connected again to a negative pressure wound therapy (NPWT), 9 days later we also reconstructed the surgical defect of the perineal and gluteal regions with a split-thickness skin graft and applied negative pressure therapy.
On the fourth postoperative day, the NPWT dressing was removed and he was discharged from the hospital on the sixty-third day of his hospitalization, without any symptoms.
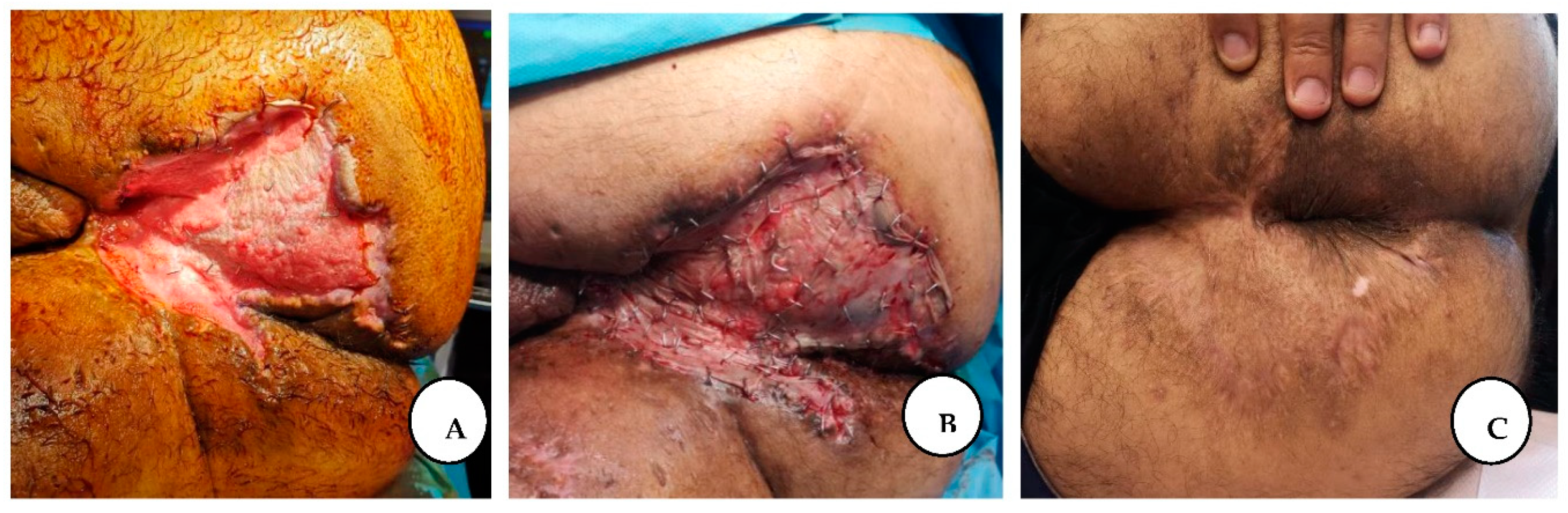
Figure 6.
A. The appearance of the perianal region after NPWT dressing; B. Immediate post-application of split-thickness skin graft; C. The final result after complete healing.
Figure 6.
A. The appearance of the perianal region after NPWT dressing; B. Immediate post-application of split-thickness skin graft; C. The final result after complete healing.
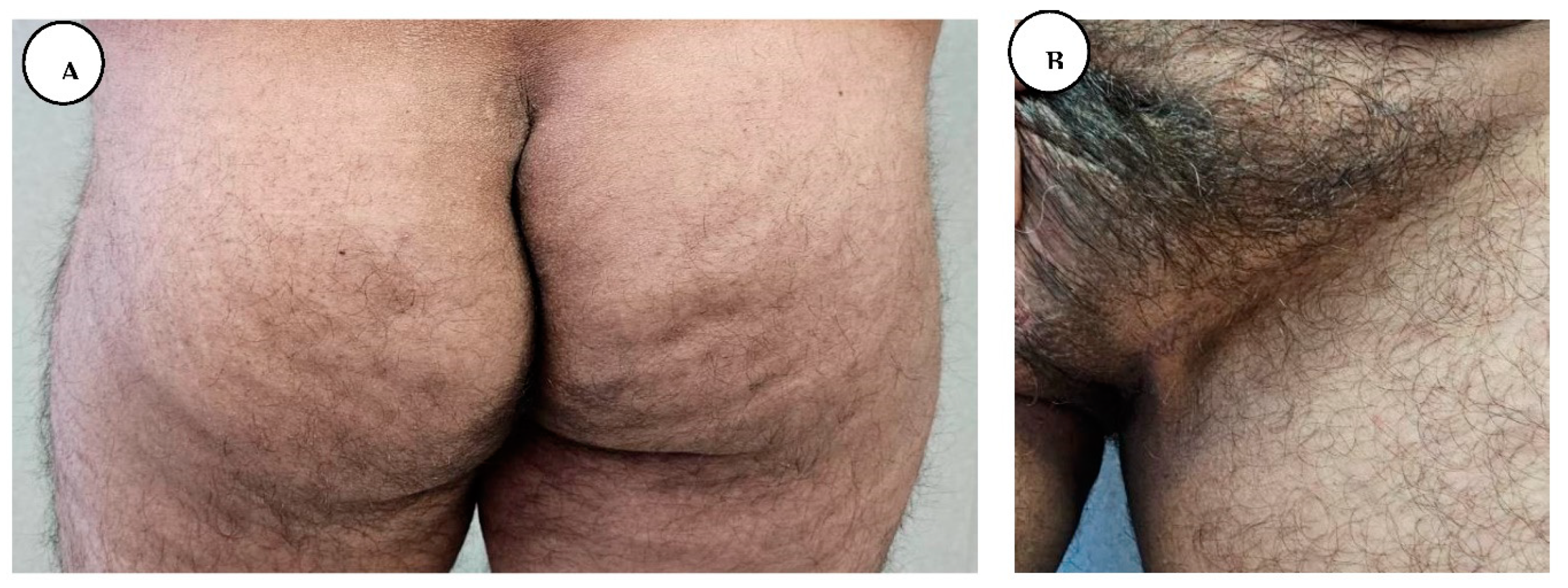
Postoperative follow-up after 2 years revealed local cutaneous recurrence in the gluteal region with multiple isolated inflammatory nodules without sinus tract formation.
Figure 7.
A, B. Gluteal and lower abdominal recurrence after 2 years.
Figure 7.
A, B. Gluteal and lower abdominal recurrence after 2 years.
The patient presented again 2.5 years later for further surgical management of the bilateral axillary region.
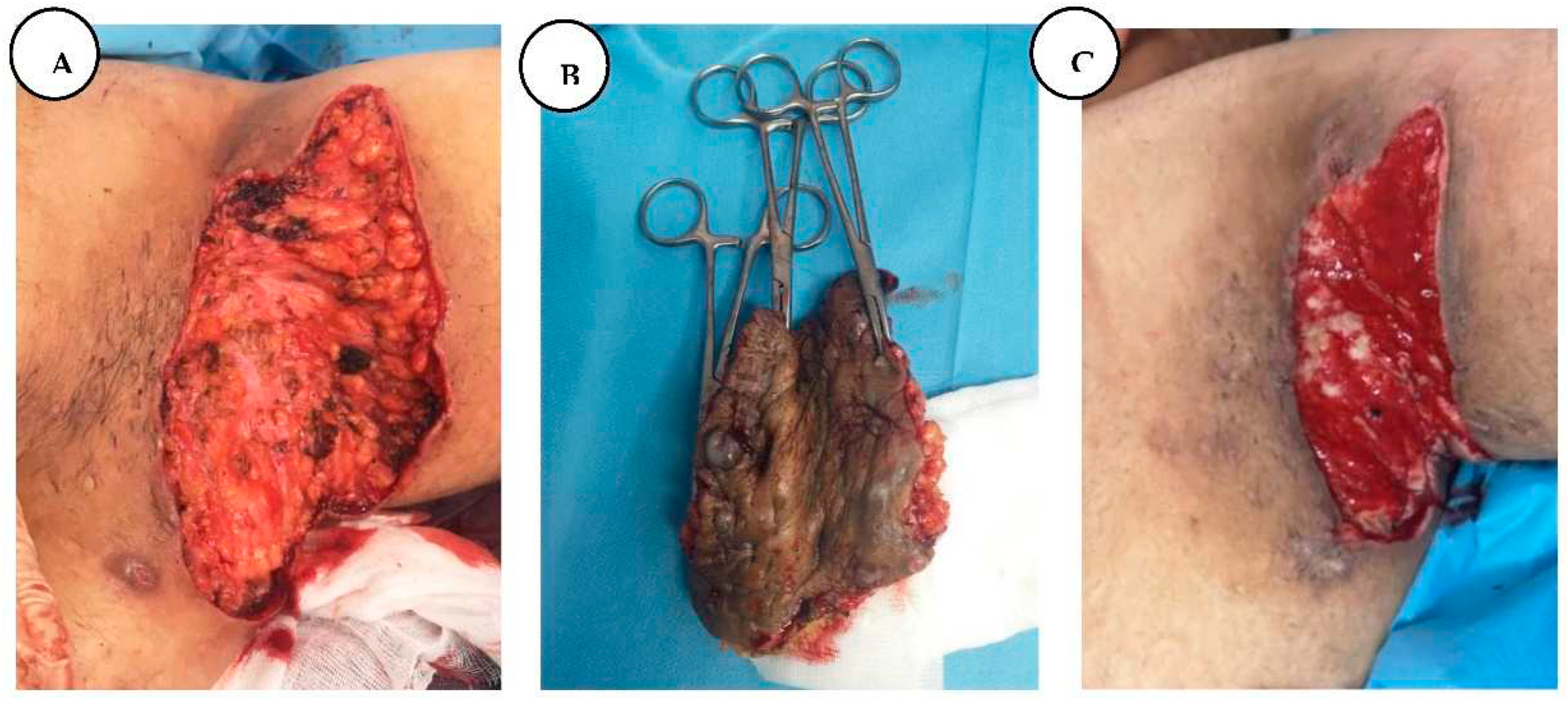
Compared to the 2.5 years earlier finding, the lesions in the bilateral axillary regions had worsened, making the patient's daily life more difficult, forming additional sinus tracts, with marked purulent discharge and unbearable pain. Physical examination of the bilateral axillary region revealed stage III hidradenitis suppurativa with multiple interconnected nodules and ulcerated, malodorous sinus tracts with purulent discharge.
We performed wide local excision of bilateral axillary lesions of hidradenitis suppurativa with margins well beyond the clinical borders of activity. We covered the defect of the right axillary region with a lateral thoracic flap and applied aspiration drainage. The drain was removed after the output was less than 25- 30 ml/ day.
We also excised the recurrent solitary lesions from the gluteal and lower abdominal regions, and the abdominal defect was closed by direct primary closure with subcuticular and interrupted vertical mattress sutures, and the perineal region was covered with split-thickness skin graft.
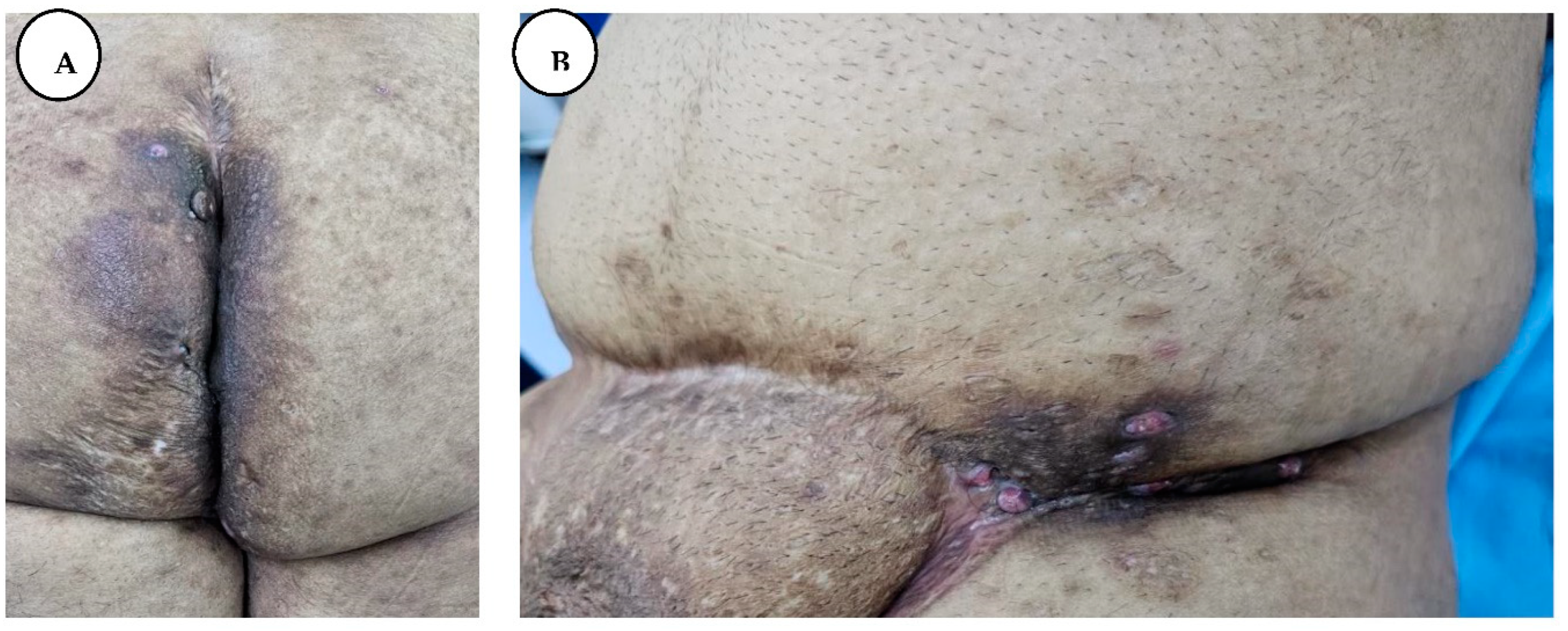
Figure 8.
The appearance of the perianal region healed after split-thickness skin graft coverage.
Figure 8.
The appearance of the perianal region healed after split-thickness skin graft coverage.
Figure 9.
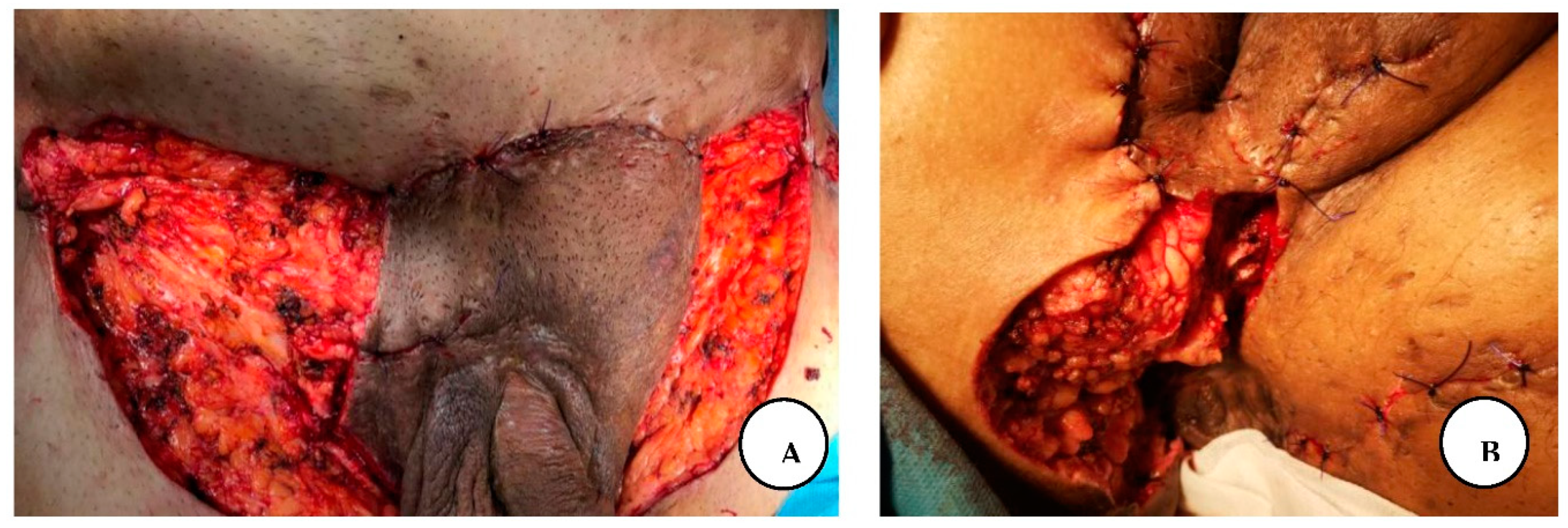
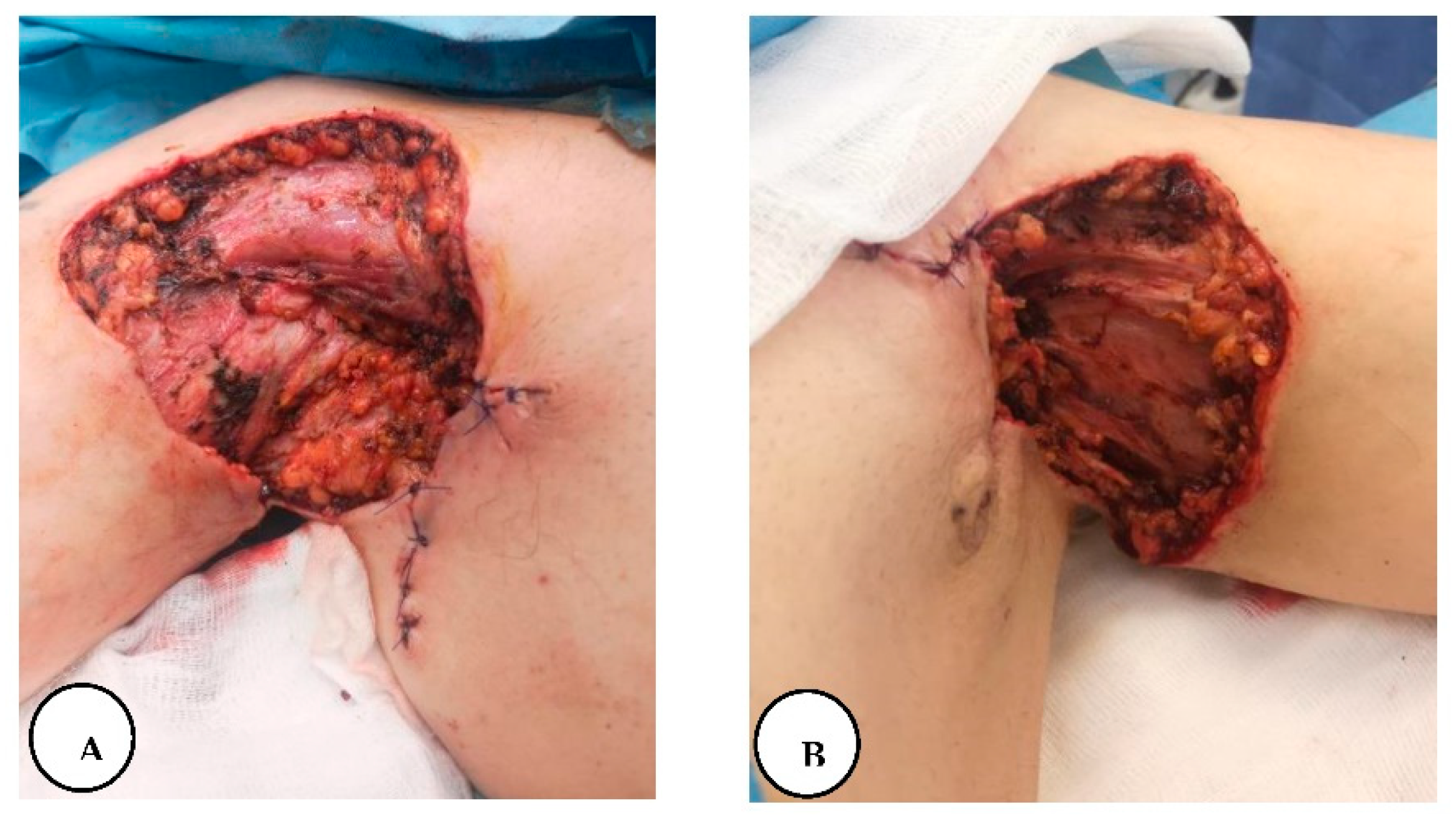
A. Stage III right axillary hidradenitis suppurativa with preoperative flap markings, B. The excised axillary specimen, C. Immediate postoperative presentation with reconstruction of the defect with a lateral thoracic flap.
Figure 9.
A. Stage III right axillary hidradenitis suppurativa with preoperative flap markings, B. The excised axillary specimen, C. Immediate postoperative presentation with reconstruction of the defect with a lateral thoracic flap.
In the left axillary region, we performed an extensive local excision of hidradenitis suppurativa lesions and covered the defect with negative pressure wound therapy for two weeks to achieve effective local treatment, pus drainage and promote local treatment efficacy. After a negative bacteriological result, we covered the remaining defect with a transpositional Limberg-type local flap in combination with negative pressure wound therapy, without the insertion of a drainage tube underneath the flap. A non-adherent silver-containing barrier dressing (Atrauman Ag) and an S-shape foam dressing were applied along the suture line of the Limberg flap to prevent wound dehiscence and facilitate faster wound healing. The NPWT machine was set with the tubing and suction connector in the axillary area.
Figure 10.
A. After wide local excision of the left axillary lesions, B. The excised specimen, C. The local appearance after negative pressure wound therapy.
Figure 10.
A. After wide local excision of the left axillary lesions, B. The excised specimen, C. The local appearance after negative pressure wound therapy.
Figure 11.
A. Stage III axillary HS, B. Left axillary region reconstruction with lateral thoracic Limberg flap, and preoperative flap markings, C. immediate result after reconstruction, D. S-shape foam dressing was applied along the suture line of the Limberg flap to prevent wound dehiscence and to facilitate faster wound healing.
Figure 11.
A. Stage III axillary HS, B. Left axillary region reconstruction with lateral thoracic Limberg flap, and preoperative flap markings, C. immediate result after reconstruction, D. S-shape foam dressing was applied along the suture line of the Limberg flap to prevent wound dehiscence and to facilitate faster wound healing.
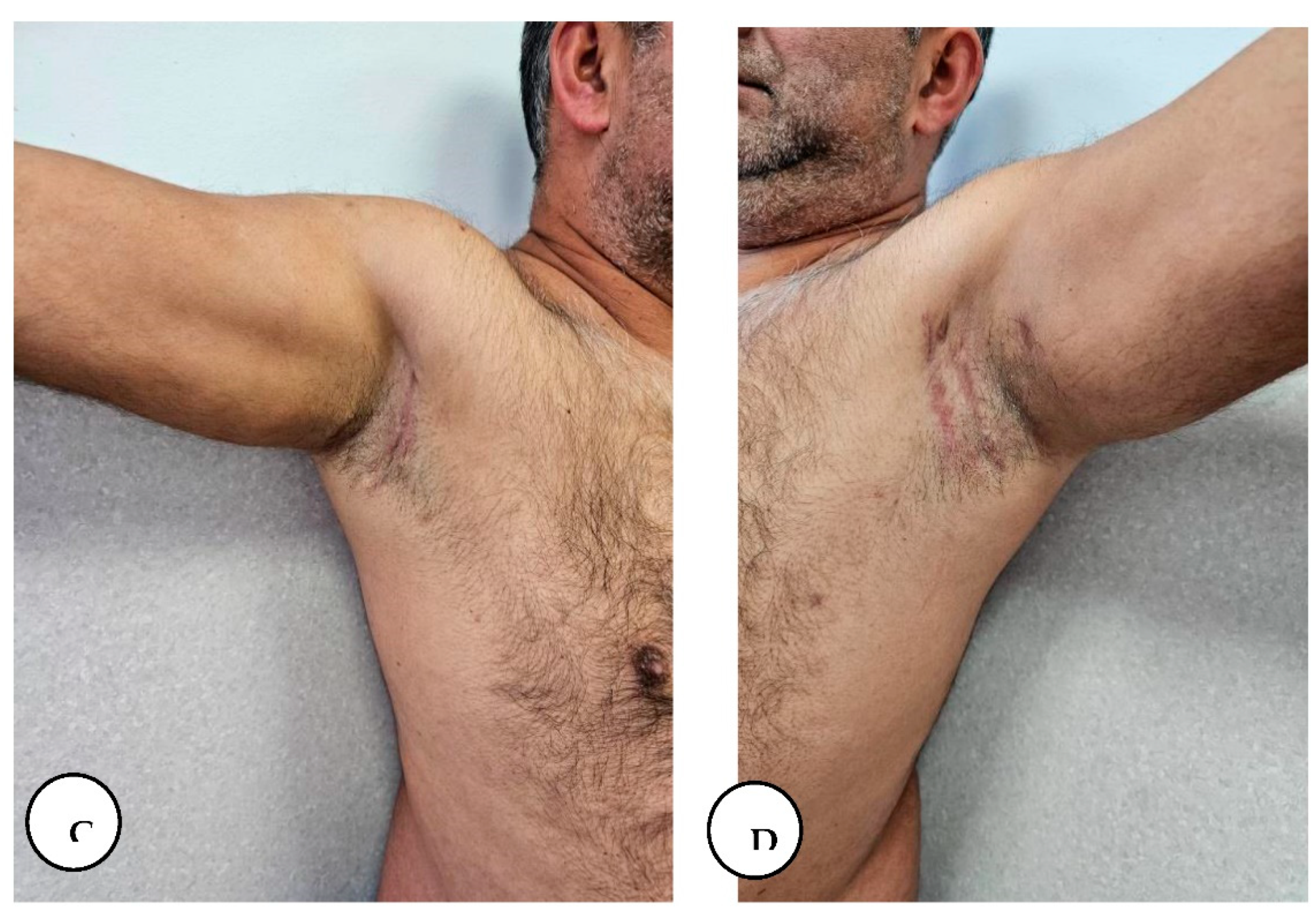
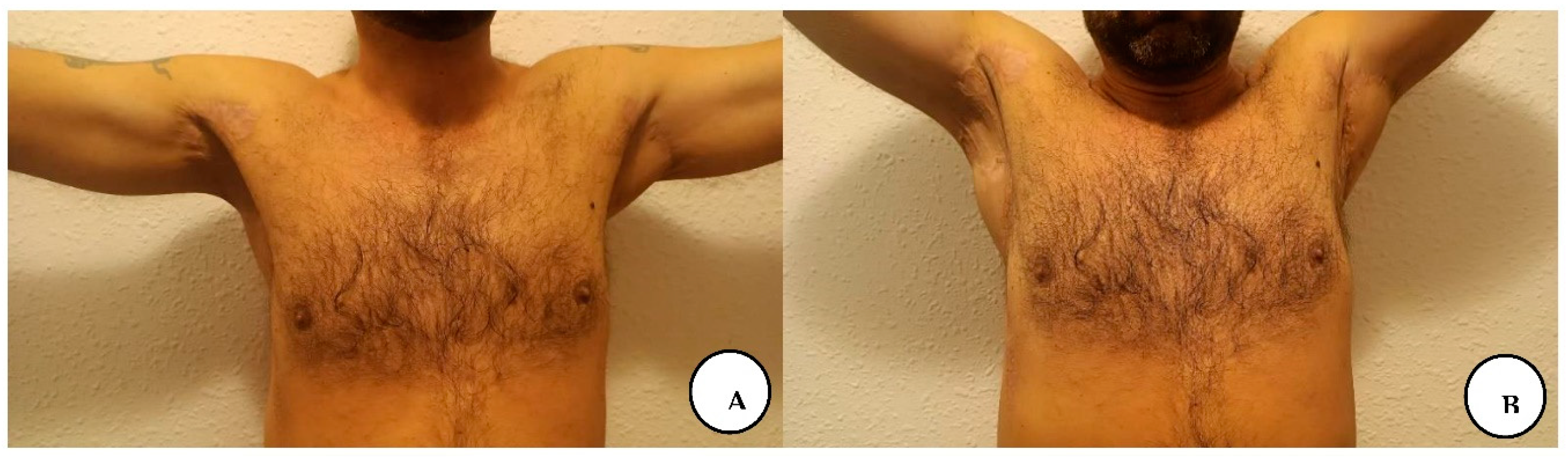
The patient presented for postoperative follow-up after 6 months with a mature scar in the axillary regions, gluteal, and lower abdominal region with partially regained tensile strength of the original skin, with progressive recovery of shoulder range of motion, with no axillary contractures, and without local recurrence in all regions.
Figure 12.
A, B. 6-month follow-up with gradual restoration of shoulder range of motion.
Figure 12.
A, B. 6-month follow-up with gradual restoration of shoulder range of motion.
Figure 13.
A, B. Follow-up at 6 months with a mature scar and with no local recurrence in the lower abdominal and gluteal region.
Figure 13.
A, B. Follow-up at 6 months with a mature scar and with no local recurrence in the lower abdominal and gluteal region.
Case 2:
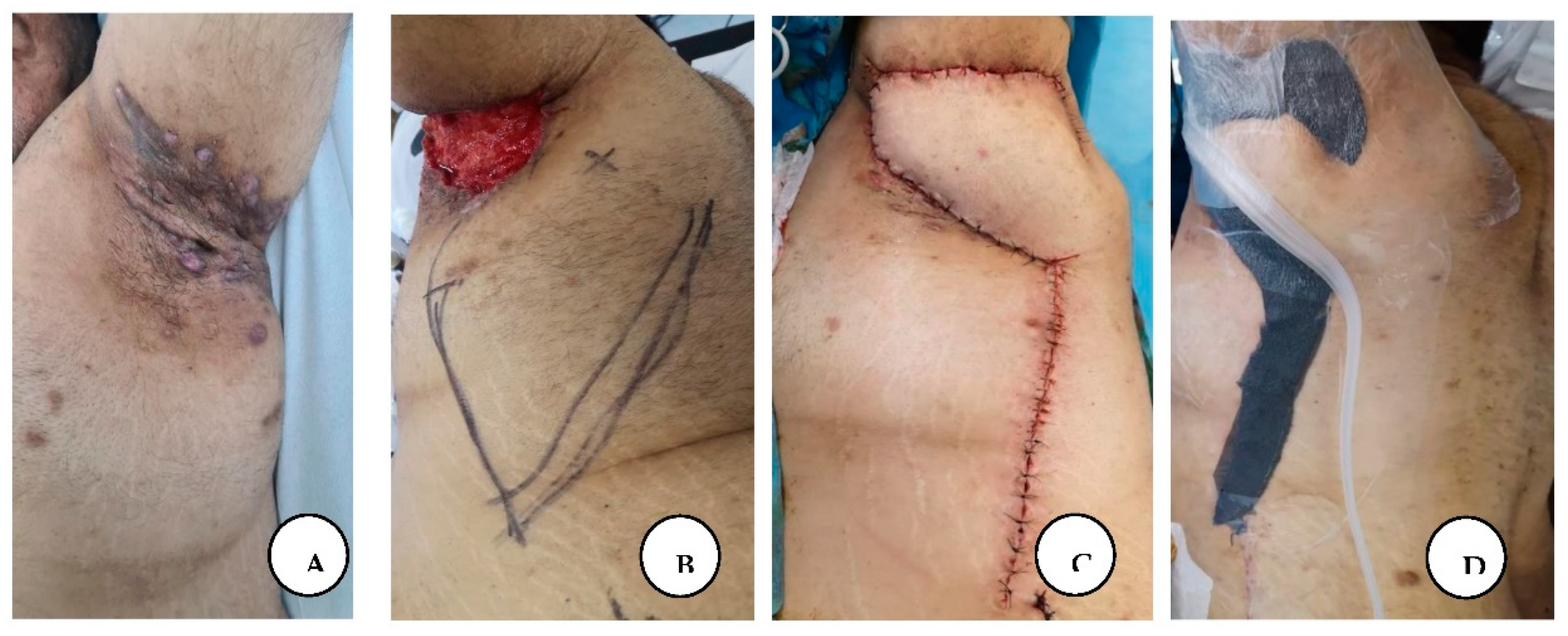
The first patient's brother came to our clinic for surgical treatment after his brother had recovered. A 44-year-old male patient with stage I hidradenitis suppurativa in the axillary region, characterized by painful inflammatory nodules and multiple isolated abscesses without sinus tracts formation, and stage III hidradenitis suppurativa in the gluteal and inguinal region, characterized by multiple interconnected sinus tracts, scarring, and abscesses, without intervening with normal skin. This was the first time the patient had consulted a doctor with these symptoms and had not previously received medical treatment.
The patient's medical history revealed grade II arterial hypertension controlled by medication, and he is a chronic smoker, smoking an average of 10 cigarettes per day for 10 years.
Figure 14.
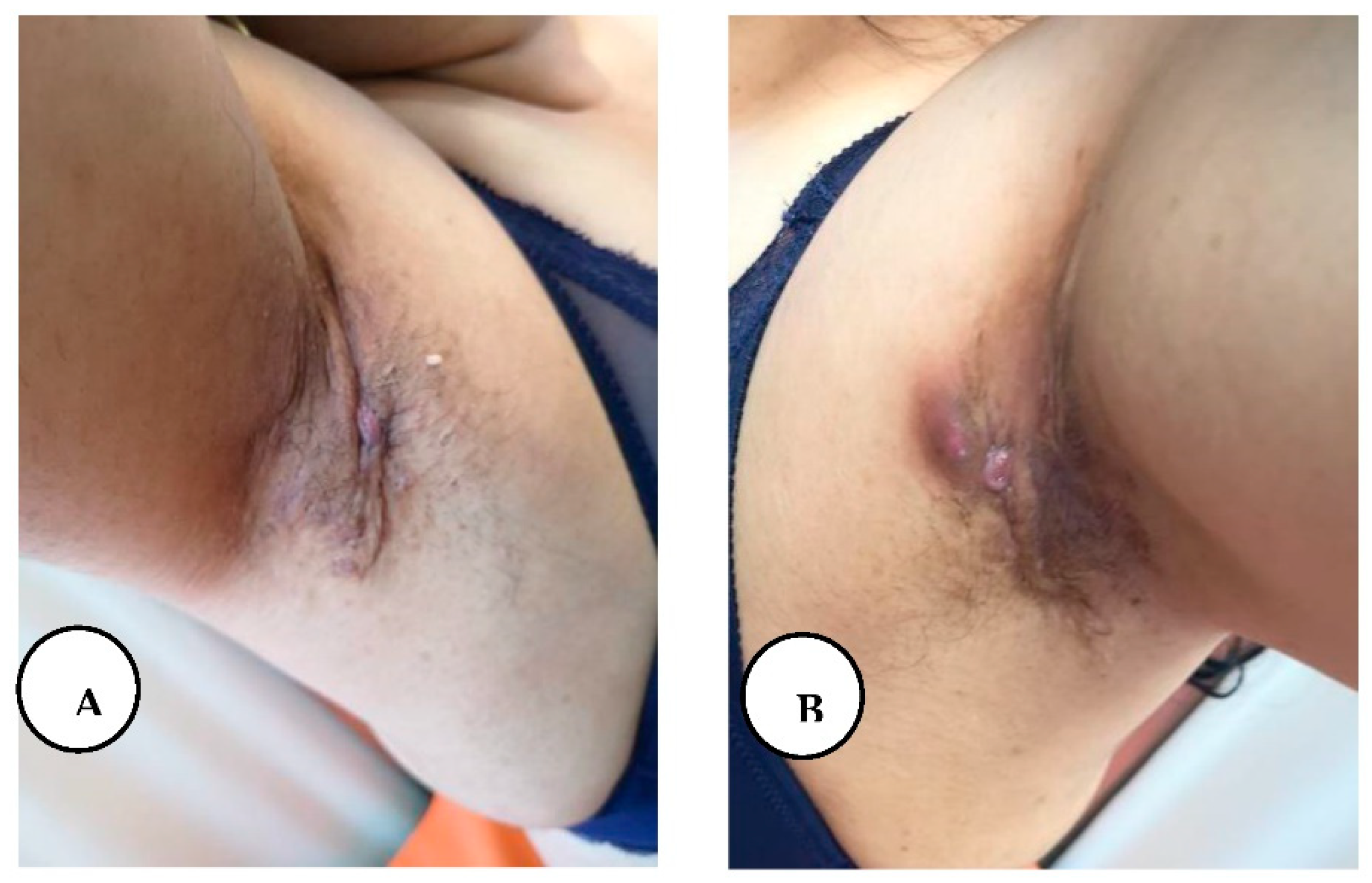
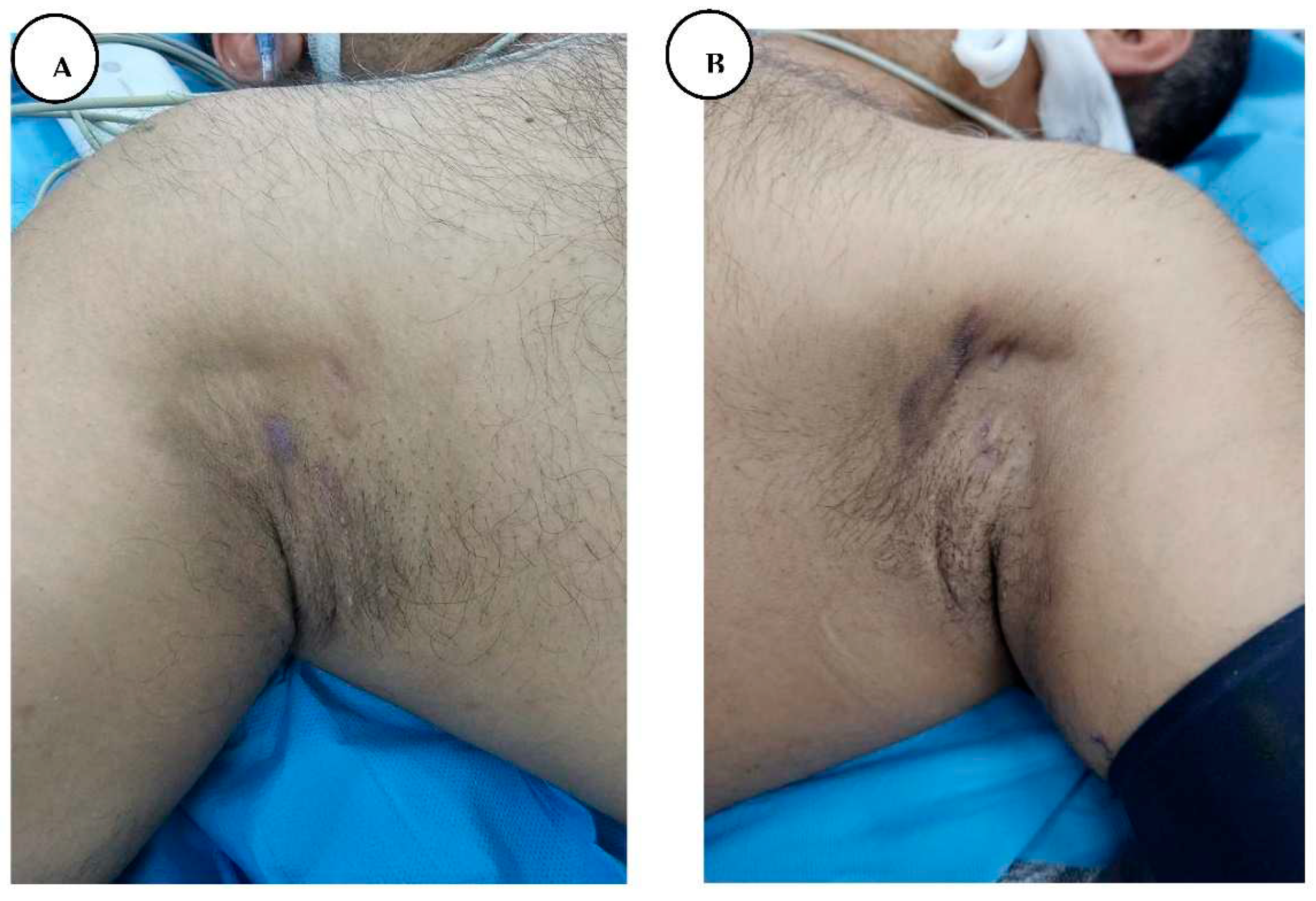
A, B. Stage I hidradenitis suppurativa in the axillary region with inflammatory nodules and multiple isolated abscesses formation without sinus tracts.
Figure 14.
A, B. Stage I hidradenitis suppurativa in the axillary region with inflammatory nodules and multiple isolated abscesses formation without sinus tracts.
Figure 15.
A, B. Stage III hidradenitis suppurativa in the gluteal and inguinal region.
Figure 15.
A, B. Stage III hidradenitis suppurativa in the gluteal and inguinal region.
We performed a wide local excision of the axillary solitary lesions at an appropriate marginal distance from the lesions, thereby removing the affected apocrine glands, and then we closed the defect primarily intention.
At the same time, we performed excision of the gluteal and perianal lesions, with preservation of a skin bridge between the anus and scrotum, protecting the external anal sphincter to maintain its normal function. These lesions were very deep in the subcuticular layer with abundant exteriorization of purulent secretions. For local infection and faster wound healing, we used intrasite dressings impregnated with rifampicin until the wound was completely healed.
Figure 16.
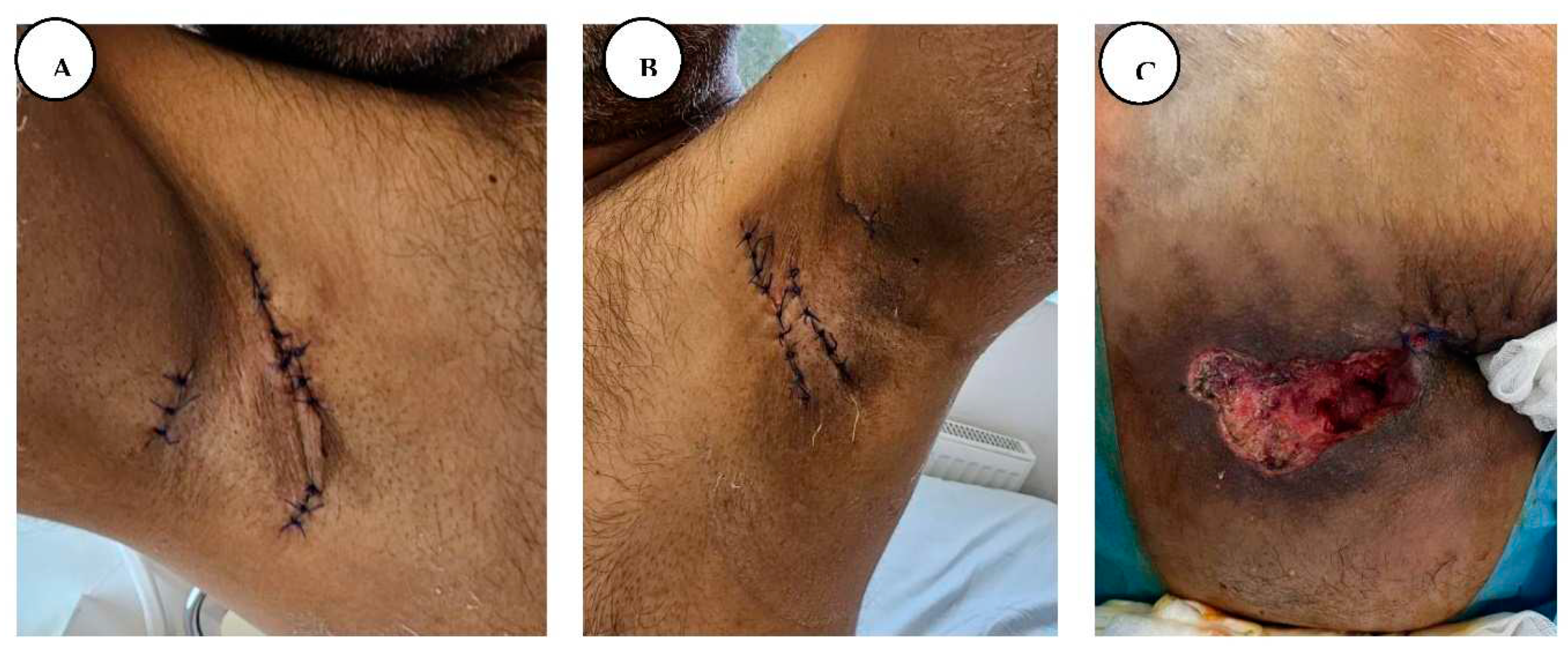
A, B. Immediate postoperative appearance, after wide local excision of the axillary lesions, and primary closure, C. Post-excisional appearance of the intergluteal cleft and anogenital regions.
Figure 16.
A, B. Immediate postoperative appearance, after wide local excision of the axillary lesions, and primary closure, C. Post-excisional appearance of the intergluteal cleft and anogenital regions.
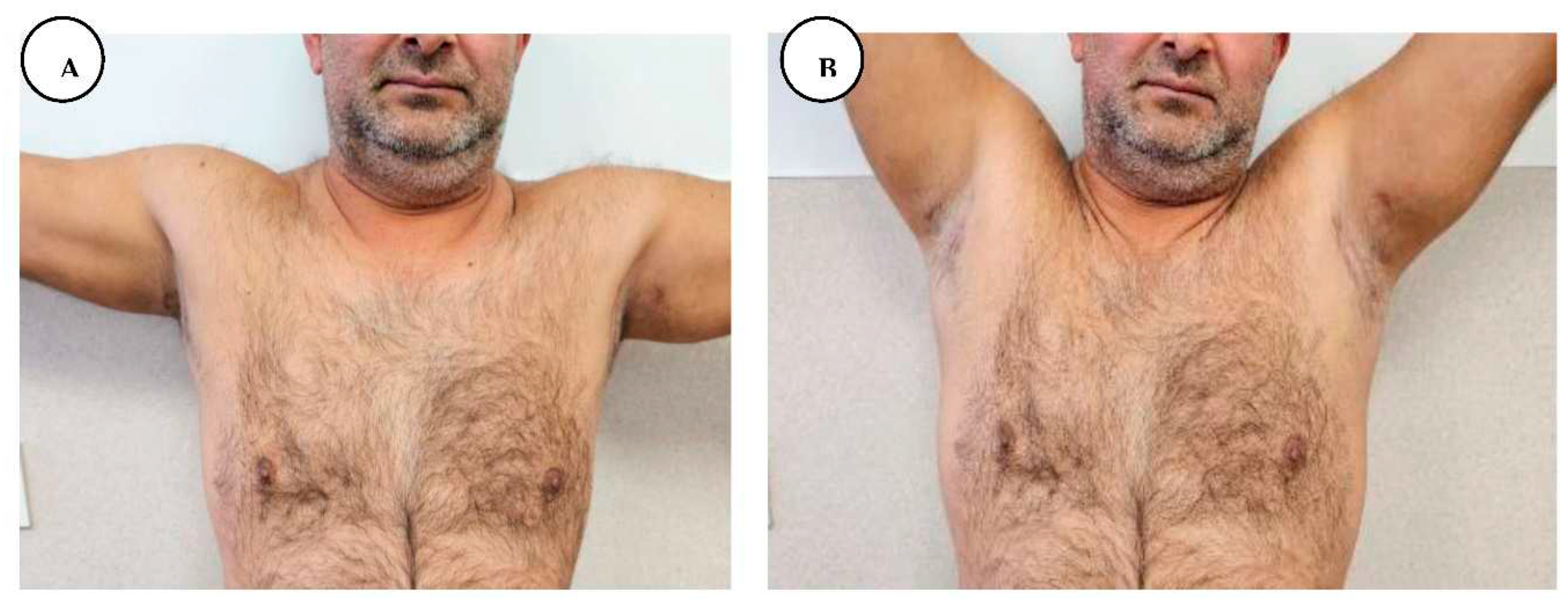
The patient was discharged from the hospital on the 18th postoperative day, with completely healed postoperative wounds, and without any symptoms, with the recommendation of passive and active physiotherapy of the shoulder joint until full range of motion was regained.
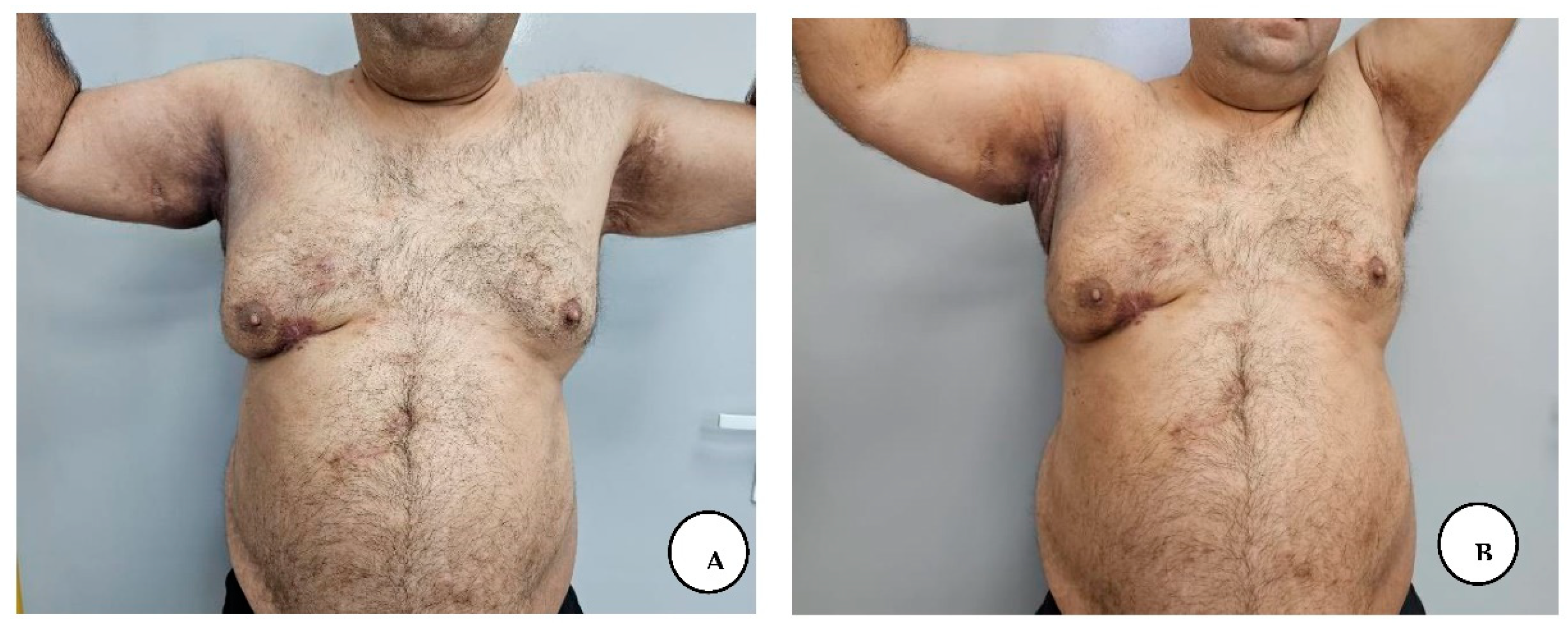
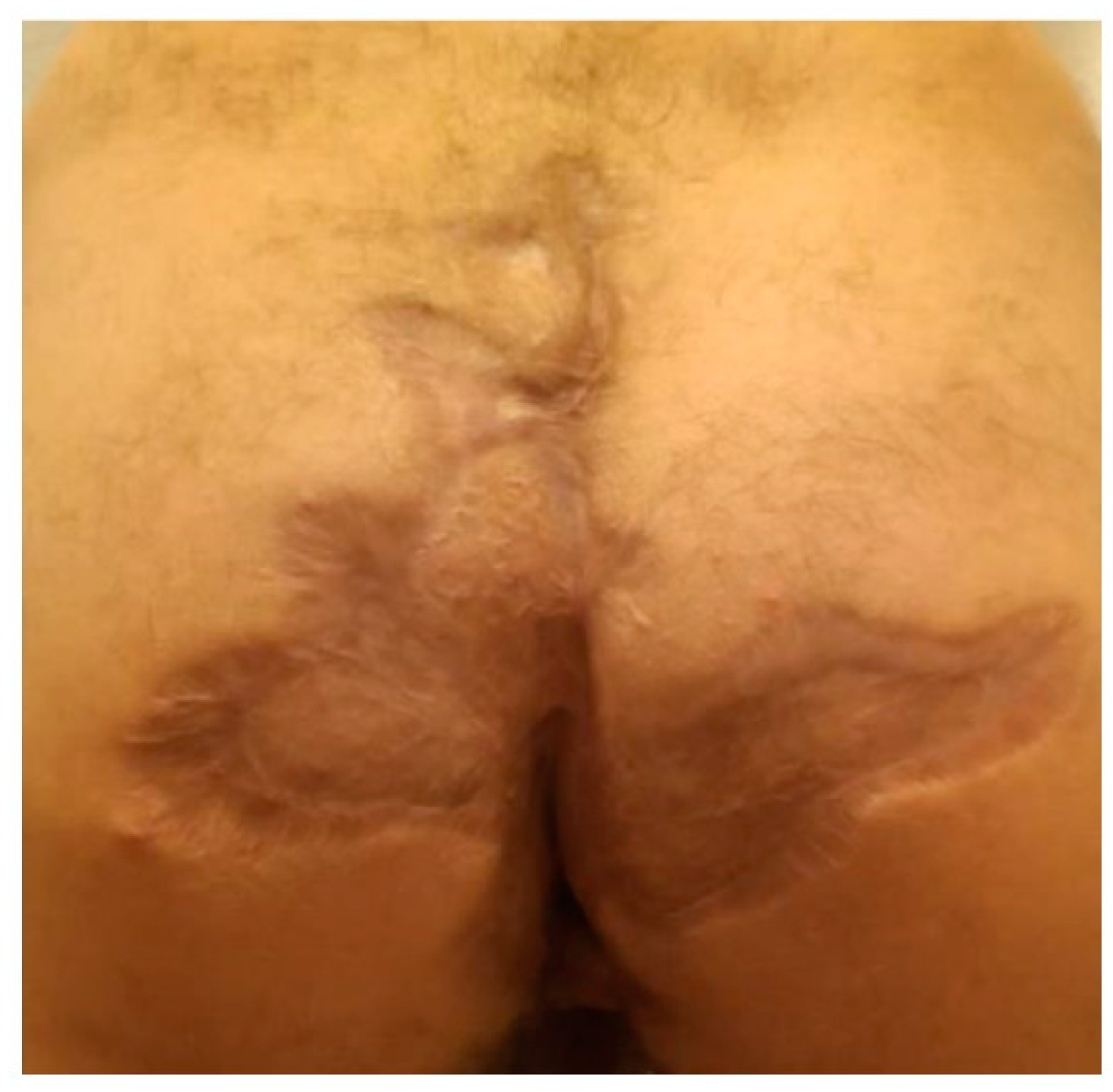
The patient presented for postoperative follow-up after 6 months, with good wound healing, with a mature pink scar in the axillary region, without axillary contractures, and with regained shoulder full range of motion. He presented mature scarring in the anogenital and inguinal regions with normal anal sphincter function and without any symptoms in all regions.
Figure 17.
A, B, C, D. Postoperative follow-up after 6 months with regained shoulder full range of motion.
Figure 17.
A, B, C, D. Postoperative follow-up after 6 months with regained shoulder full range of motion.
Figure 18.
A, B. Postoperative follow-up after 6 months without any symptoms in the gluteal and inguinal regions.
Figure 18.
A, B. Postoperative follow-up after 6 months without any symptoms in the gluteal and inguinal regions.
Case 3:
A 42-year-old male patient with severe symptoms of hidradenitis suppurativa, a similar case to the first patient, with extensive gluteal, anogenital, and axillary involvement. The patient has a strong smoking history, smoking an average of 20 cigarettes (one pack) per day. He had been diagnosed with hidradenitis suppurativa 15 years earlier, and since then he has undergone different dermatological therapies, including variant types of antibiotic combinations, antibiotics with broad antibacterial spectrum, antibiotics as local agents, isotretinoin capsules, treatment with Stelara injections for 6 months, that is a combination with immunomodulators and corticosteroids and he has tried treatment with a TNF blocker medicine, Humira (adalimumab) for 1.5 year period, that can lower the ability of patients immune system to fight infections. (20) He has also tried biological therapy with Amgevita, which uses the active substance adalimumab, a recombinant human immunoglobulin (IgG1) to reduce inflammation. (21)
Against all these treatments the lesions slowly progressed and continued to spread with worsening symptoms, accompanied by local suppuration, burning pruritus, hyperhidrosis, pain, unpleasant smell, and purulent discharge, causing the patient to feel constantly anxious and social isolation, that’s why he presented to our clinic for surgical treatment.
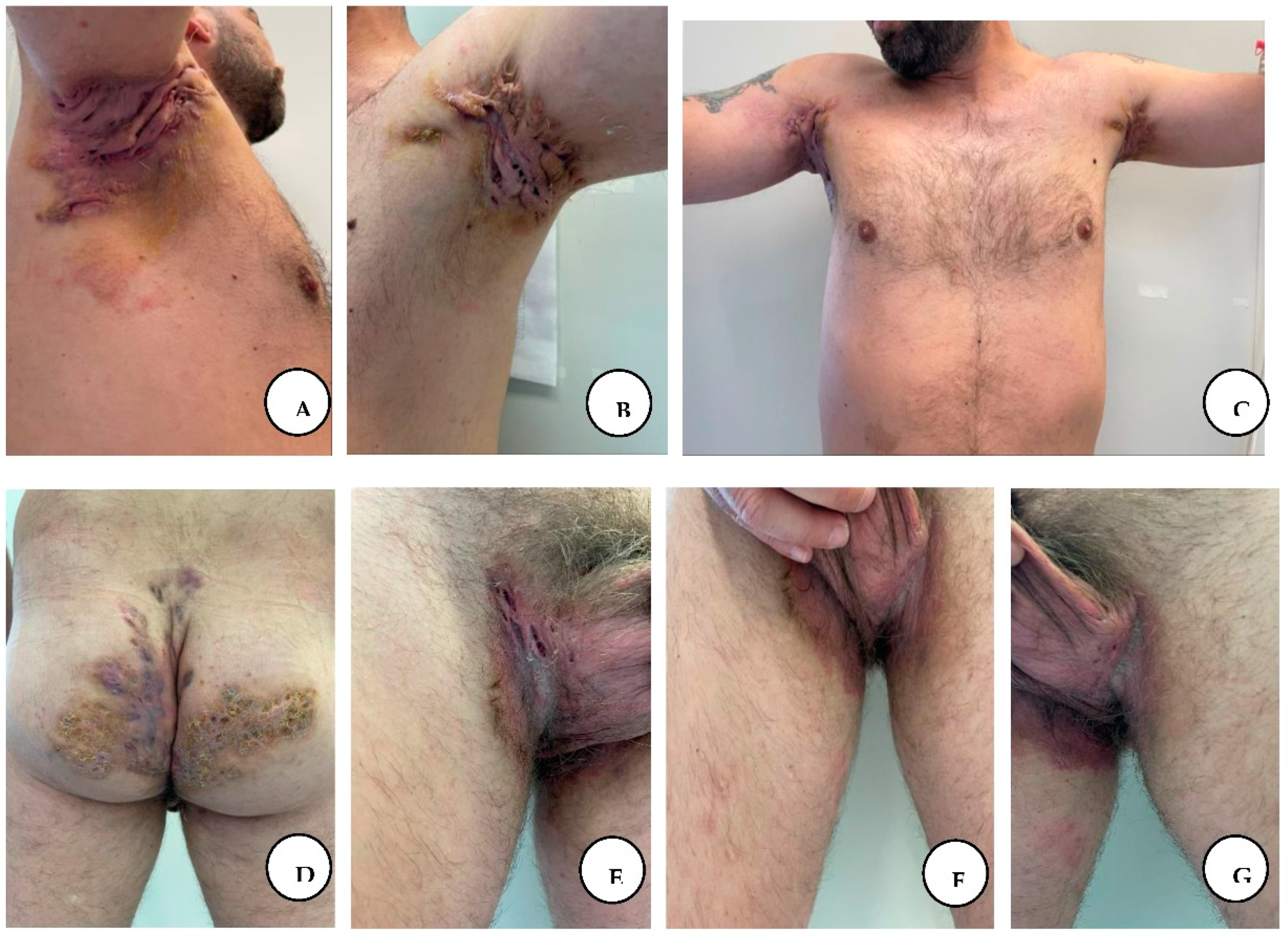
On physical examination of his bilateral axillary regions, he had multiple inflammatory nodules and draining sinus tracts coalescing into cord-like bluish abscesses, associated with suppuration, intense pain, scars, and skin retraction, the range of motion at both axillary regions was impaired, with the right side having a narrower range of motion than the left. The bilateral inguinal, gluteal, and perianal regions revealed multiple conflated nodules and scars forming a confluent painful purple-red structure with multiple abscesses, ulceration, and suppuration. Based on the clinical findings and past medical history, a diagnosis of hidradenitis suppurativa stage III was established.
Figure 19.
A, B, C, D, E, F, G. Local appearance with multiple draining sinus tracts, cord-like bluish, and abscesses in the bilateral axillary region, inguinal, perineal, and perianal areas.
Figure 19.
A, B, C, D, E, F, G. Local appearance with multiple draining sinus tracts, cord-like bluish, and abscesses in the bilateral axillary region, inguinal, perineal, and perianal areas.
His 12-year-old son also suffers from this disease with axillary involvement.
Figure 20.
A, B. Painful inflammatory nodules in the bilateral axillary region of the patient's 12-year-old son.
Figure 20.
A, B. Painful inflammatory nodules in the bilateral axillary region of the patient's 12-year-old son.
First week, we performed radical surgical excision of the affected skin tissue with adequate free margins in the gluteal and anogenital regions. The wounds of the inguinal region were closed by primary intention and we covered the remaining defect with silver-containing dressing.
Figure 21.
A. Post-excisional appearance of the gluteal and anogenital regions; B. Post-excision of inguinal hidradenitis suppurativa; C. Primary closure of bilateral inguinal regions. In the second week, the bilateral axillary lesions were removed, and the defect was covered with negative pressure wound therapy, and after 4 days of negative pressure dressings, we covered the defect of the left axillary region with a Limberg flap, which is created as a rhomboid-shaped transpositional flap based on random circulation from the lateral thoracic region, and 3 days later the right axillary defect was covered in a similar manner.
Figure 21.
A. Post-excisional appearance of the gluteal and anogenital regions; B. Post-excision of inguinal hidradenitis suppurativa; C. Primary closure of bilateral inguinal regions. In the second week, the bilateral axillary lesions were removed, and the defect was covered with negative pressure wound therapy, and after 4 days of negative pressure dressings, we covered the defect of the left axillary region with a Limberg flap, which is created as a rhomboid-shaped transpositional flap based on random circulation from the lateral thoracic region, and 3 days later the right axillary defect was covered in a similar manner.
Figure 22.
A, B. After radical excision of stage III HS from the bilateral axillary regions and marginal approximation sutures.
Figure 22.
A, B. After radical excision of stage III HS from the bilateral axillary regions and marginal approximation sutures.
Figure 23.
A, B, C. Fourth post-operative day, covering the defect with a lateral thoracic Limberg flap.
Figure 23.
A, B, C. Fourth post-operative day, covering the defect with a lateral thoracic Limberg flap.
Figure 24.
A. After 8 days of silver-containing dressing we covered the defect with a split-thickness skin graft. B. Six months after complete healing of intergluteal cleft.
Figure 24.
A. After 8 days of silver-containing dressing we covered the defect with a split-thickness skin graft. B. Six months after complete healing of intergluteal cleft.
The patient was discharged from the hospital on the 47th day of hospitalization with 95% healed lesions, without any symptoms.
Figure 25.
A, B. Postoperatively follow-up after one and a half years. The patient regained full range of motion in the axillary regions, good wound healing, without axillary contractures, and improved shoulder excursion.
Figure 25.
A, B. Postoperatively follow-up after one and a half years. The patient regained full range of motion in the axillary regions, good wound healing, without axillary contractures, and improved shoulder excursion.
Figure 26.
Postoperative follow-up after one and a half years presenting mature scarring in the anogenital region with normal anal sphincter function without any symptoms.
Figure 26.
Postoperative follow-up after one and a half years presenting mature scarring in the anogenital region with normal anal sphincter function without any symptoms.
Case 4:
A 47-year-old male patient suffering from Langerhans cell histiocytosis involving bone, presented to our department with painful a ulcero-vegetative purulent mass in the perianal region. He had been diagnosed 2 years earlier, when a thoracotomy was performed for resection of a 7th costal tumor, and the histological findings show Langerhans cell histiocytosis.
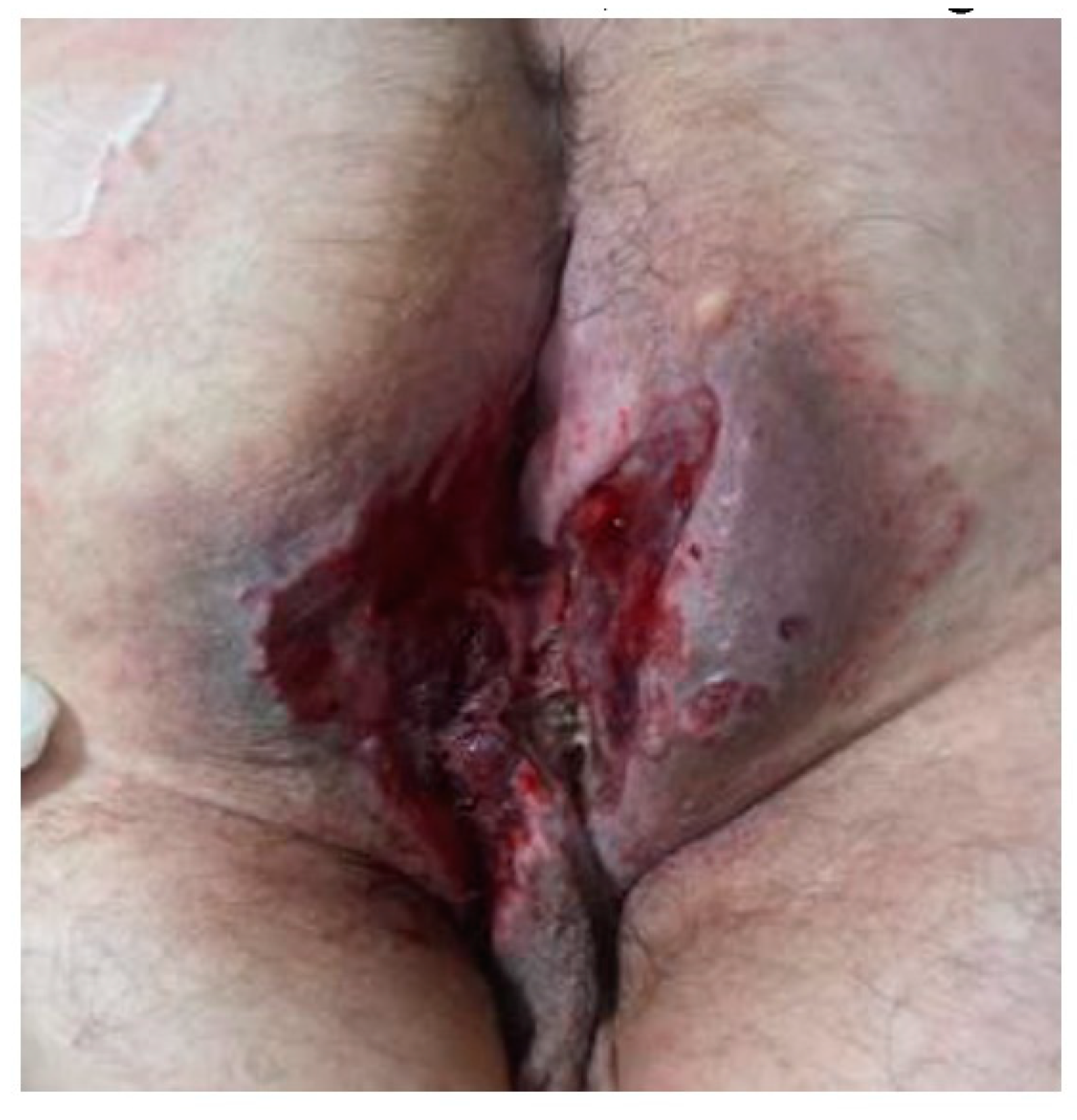
Past medical history included diabetes treated with oral agents. The patient had been diagnosed with anal abscesses and operated in 2019 at the general surgery department. Upon progression of disease with no relief of symptoms and occurrence of perianal lesions, the patient was re-operated in 2021 and the pathological findings revealed the diagnosis of LCH, therefore a temporary colostomy was performed to facilitate further surgical interventions.bClinical examination revealed a painful ulcero-vegetative purulent mass, nodules with sinus tract formation, and scarring with local erythema and tenderness in the perianal region.
Figure 25.
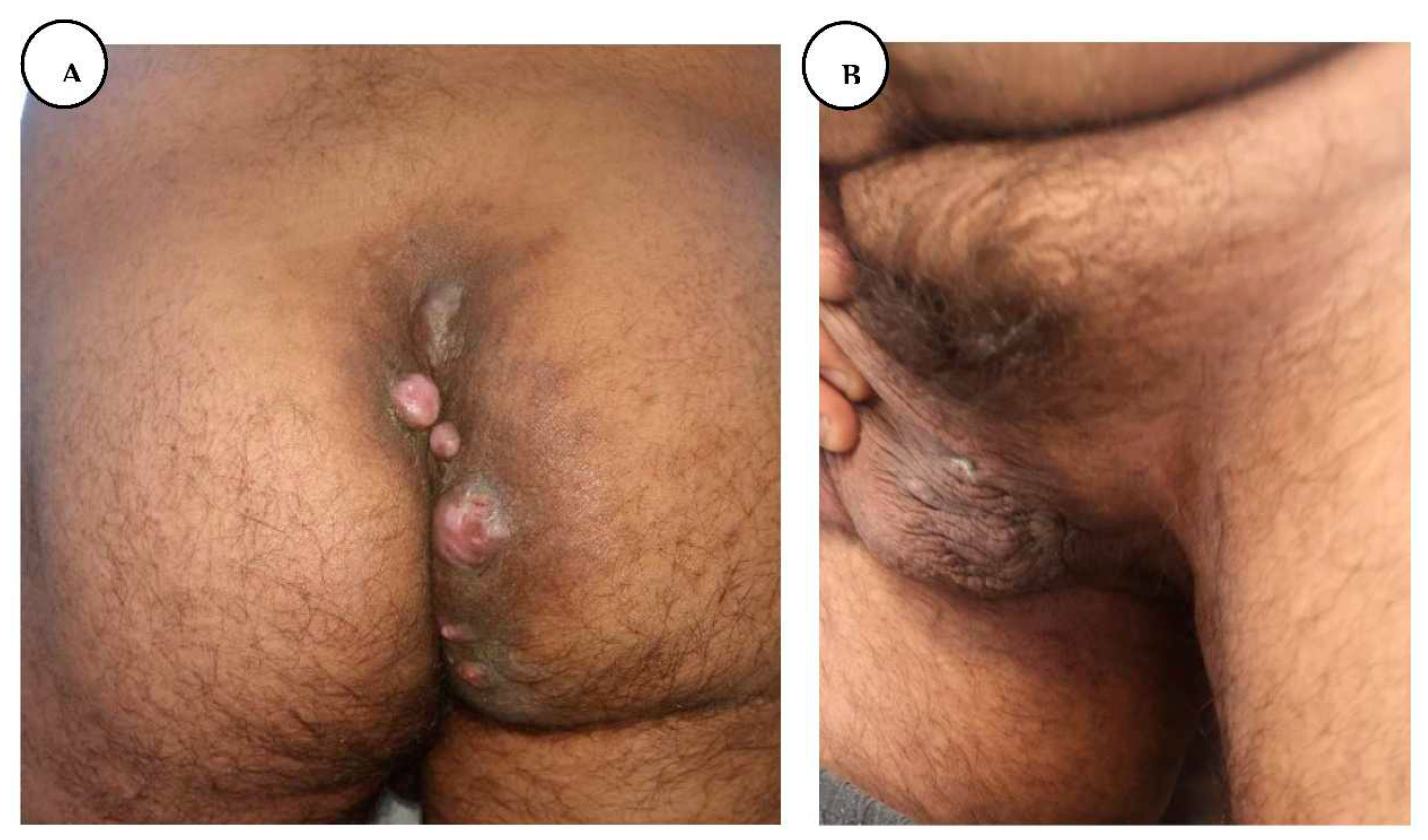
Local appearance before surgery, presenting ulcero-vegetative purulent mass in the perianal region.
Figure 25.
Local appearance before surgery, presenting ulcero-vegetative purulent mass in the perianal region.
At our department, blood tests and wound secretion cultures were ordered. Evaluation of blood tests revealed leukocytosis with mild neutrophilia, chronic mild anemia, and reactive thrombocytosis, therefore we requested a hematological consultation. The hematologist indicated bone scintigraphy, endocrinological consultation, medullary aspirations, and osteomedullary biopsy.
The patient also presented thirst, polyuria, and polydipsia, and after hospital discharge, he was forwarded for endocrinological assessment.
The bone scintigraphy suggested a space-occupying tumoral formation in the left mandibular body, moderate osteogenic reaction in the ribs 6 and 8, and polyarticular inflammatory changes. We also performed a total body computer tomography (CT scan), that identified an intense inhomogeneous liver, with the presence of multiple hypodense lesions, which raises the suspicion of secondary determination.
Surgical treatment consisted of intraoperative color marking of sinus tracts with methylene blue to define the operative region, radical block excision of the affected lesions from the perianal region to the appearance of macroscopic normal subcutaneous tissue, and similarly to the previous patient, we covered the defect with silver-containing dressings and connected to a negative pressure wound therapy.
The dressing was changed every seven days until granular tissue was obtained. On the 32nd day, split-thickness skin grafts were used to cover the defect, and connected again to a negative pressure system, six days later the dressing was changed, and four days later the bandage was removed and the final result was satisfactory both functionally and aesthetically. The patient was discharged from the hospital on the 42nd day of his hospitalization and the temporary colostomy was removed 2 weeks later.
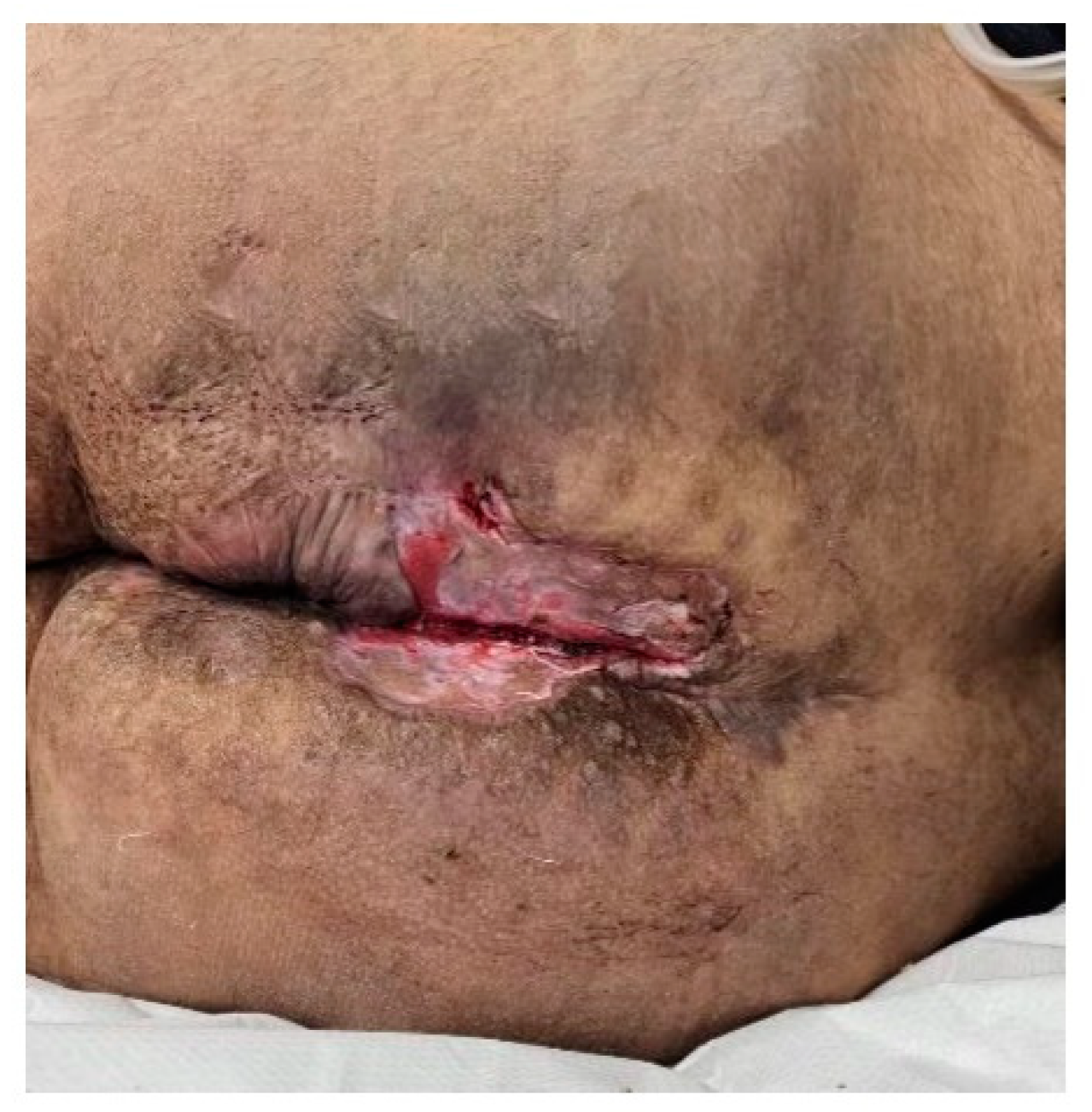
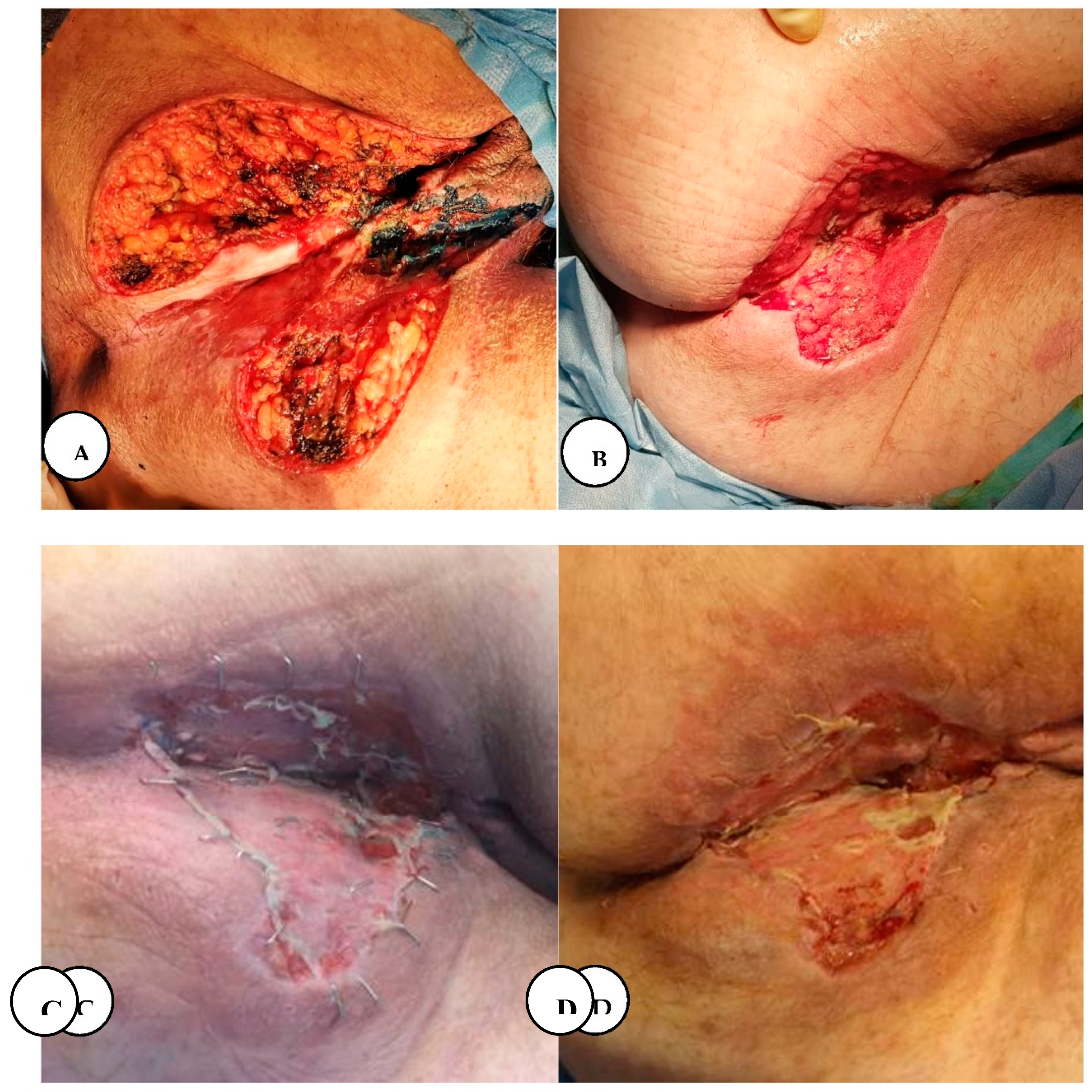
Figure 26.
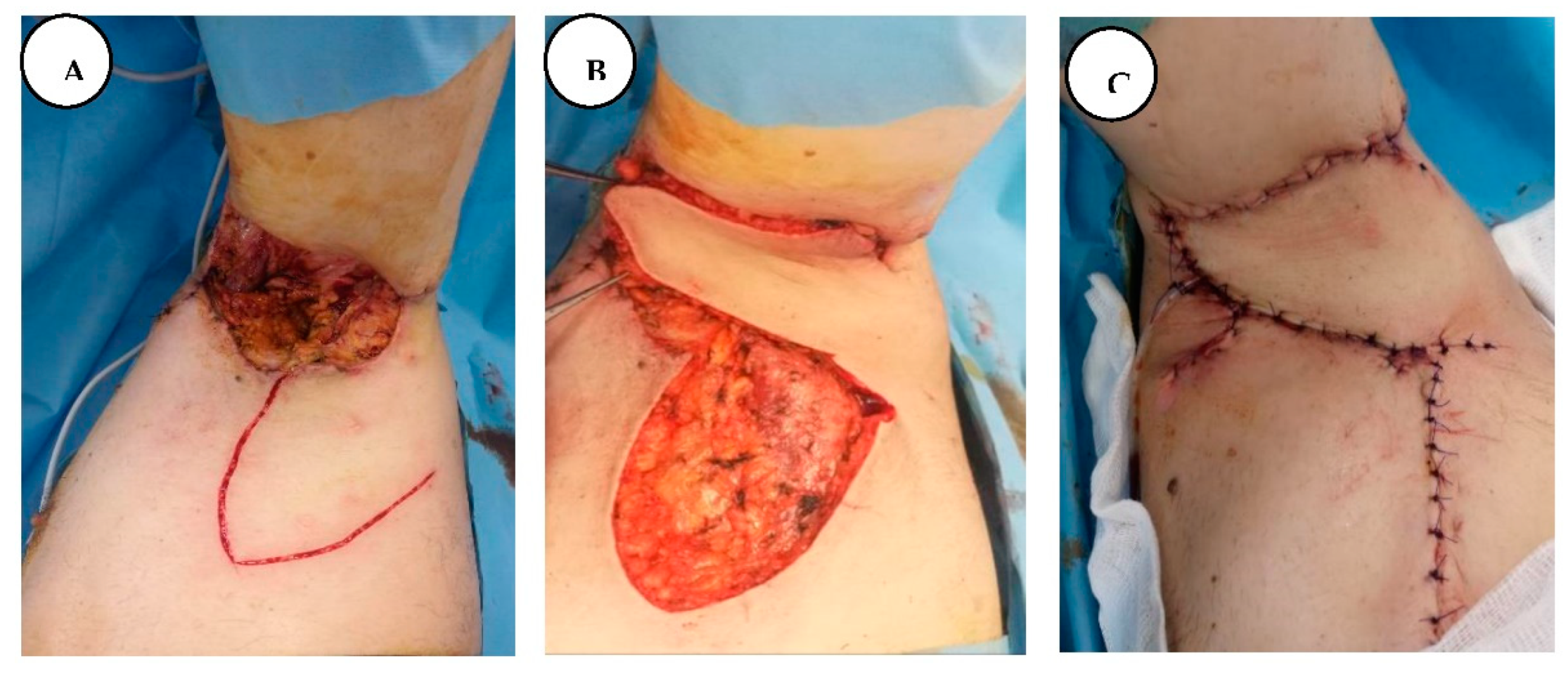
A. Immediate postoperative appearance after wide local excision of the intergluteal clef and perianal lesions, B. Local aspect after negative pressure wound therapy. C. Fourth postoperative day post-application of split-thickness skin graft, D. Local appearance on the 20th postoperative day.
Figure 26.
A. Immediate postoperative appearance after wide local excision of the intergluteal clef and perianal lesions, B. Local aspect after negative pressure wound therapy. C. Fourth postoperative day post-application of split-thickness skin graft, D. Local appearance on the 20th postoperative day.
The case presented above is a multisystemic variant of the disease called Hand-Schuller-Christian Syndrome: because it has multiple bone lesions, including localization in the left mandibular body, suspected diabetes insipidus, histological and immunohistochemical aspects with diffuse positivity for CD68 and S100 protein. (19) After a follow-up period of 6 months no recurrence has been observed.