Submitted:
04 December 2023
Posted:
07 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
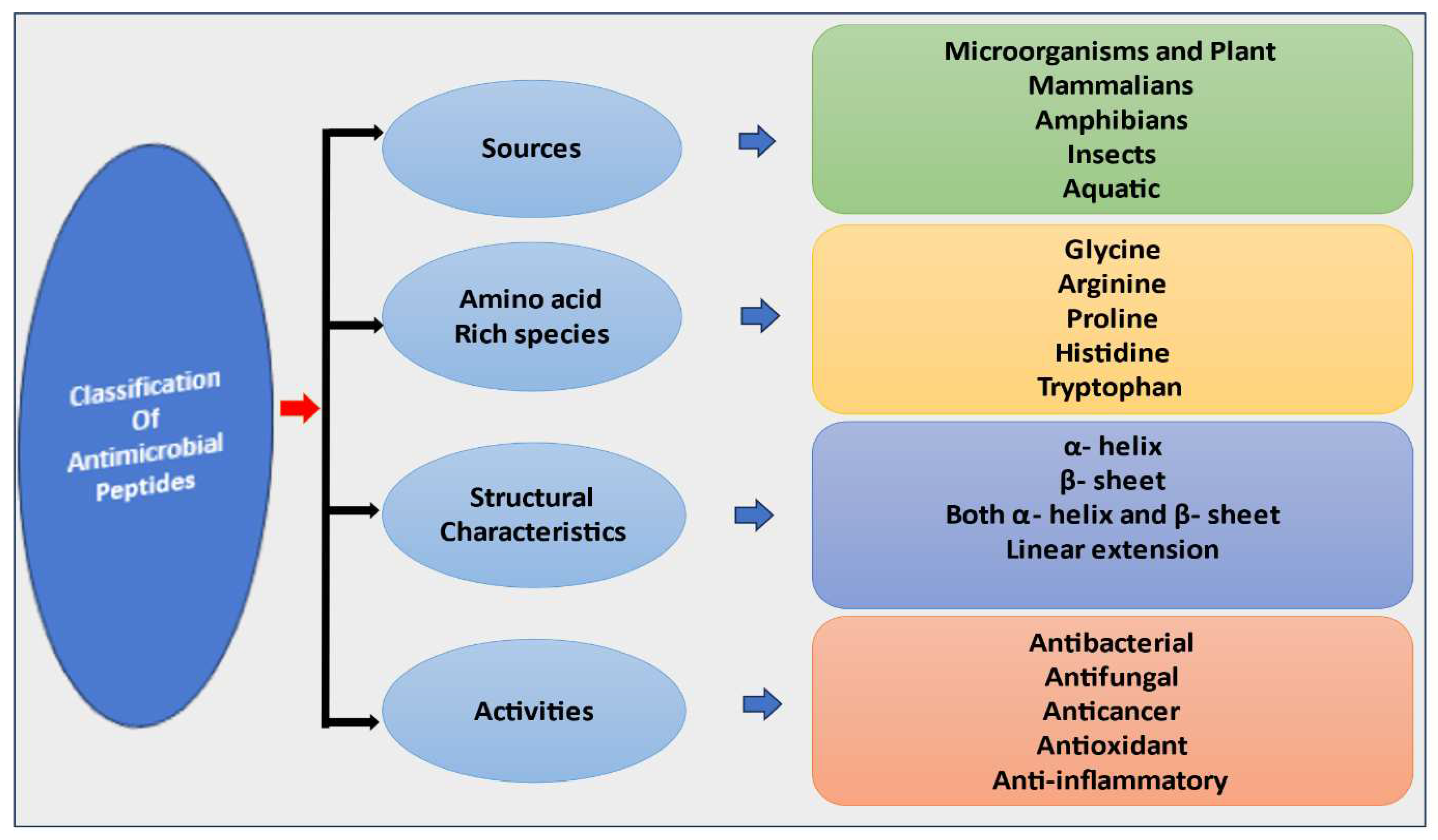
2. Classification Antimicrobial peptides (AMPs)
2.1. Classification of AMPs Based on Sources
2.1.1. Antimicrobial Peptides Derived from Mammals
2.1.2. Antimicrobial Peptides Derived from Amphibians
2.1.3. Antimicrobial Peptides Derived from Insects
2.1.4. Antimicrobial Peptides Derived from Microorganisms
2.2. Classification of AMPs Based on Activity
2.2.1. Antibacterial Peptides
2.2.2. Antifungal Peptides
2.2.3. Antiviral Peptides
2.2.4. Anti-parasitic Peptides
2.2.5. Anticancer Peptides
2.3. Classification of AMPs Based on Amino Acid Rich Species
2.4. Classification of AMPs Based on Antimicrobial Peptide Structure
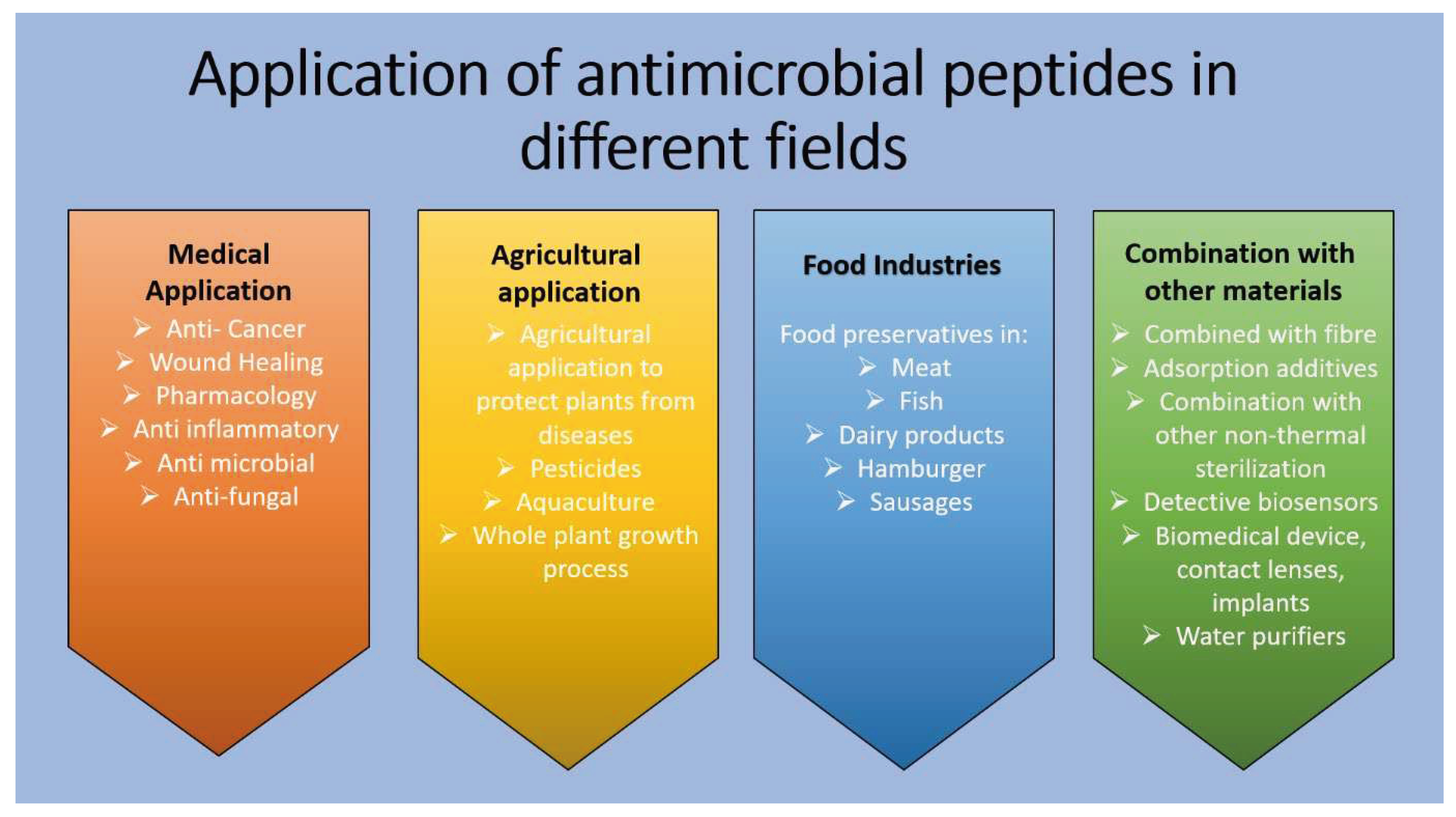
3. Application of Antimicrobial Peptides in Different Fields
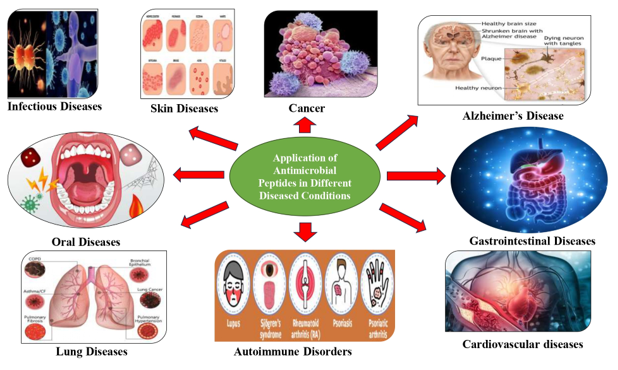
4. Application of Antimicrobial Peptides in Different Diseased Conditions
4.1. Role of AMPs in Infectious Diseases
4.2. Role of AMPs in Skin Diseases
4.3. Role of AMPs in Oral Diseases
4.4. Role of AMPs in Lung Diseases
4.5. Role of AMPs in Gastrointestinal Diseases
4.6. Role of AMPs in Autoimmune Disorders
4.7. Role of AMPs in Alzheimer’s Disease
4.8. Role of AMPs in Cardiovascular diseases
4.9. Role of AMPs in Cancer
| Disease state | Peptides | Expression levels and potential functions | References |
|---|---|---|---|
| Skin inflammatory diseases | |||
| Psoriasis | LL-37, defensins | Overexpressed, absence of S. aureus | [142] |
| Atopic dermatitis | LL-37, defensins | Downregulated, presence of S. aureus | [142] |
| Lupus, erythrematous, and contact dermatitis | LL-37 | Increased | [143] |
| Acne vulgaris | MX-594 AN | Inhibits P. acne | [144] |
| Granulysin | Kills P. acne, anti-inflammatory action | [145] | |
| Respiratory diseases | |||
| Cystic fibrosis | LL-37, β-defensins |
Reduced antimicrobial activity due to salt accumulation | [146] |
| Periodontal disease | Defensins | Reduced in saliva of patients with oral candidiasis | [147] |
| LL-37 | Absent in patients with congenital neutropenia | [148] | |
| Histatin 5 | Protects periodontium from bacterial infection and prevents biofilm formation | [149,150] | |
| Inflammatory bowel disease | |||
| Crohn’s disease | HD5 and HD6 | Deficient expression in Paneth cells | [151] |
| HD5 and HD6 | Reduced in CD patients with Nod2 mutation | [151] | |
| LL-37 | Expression is altered | [151] | |
| Ulcerative colitis | HD5, 6; hBD2–4 | Upregulated in patients with UC | [152] |
| Cancer Magainin | II Toxic | effect against canc er cell lines melanoma, breast and lung cancer, lymphoma, and leukemia | [153,154] |
| Insect cecropins | Lyse tumour cells | [155] | |
| Bovine lactoferrin | Inhibits lung and liver metastasis of murine melanomas and lymphomas and cytotoxic toward neuroblastoma cells | [156,157] | |
| Atherosclerosis | Defensins | Involved in lipoprotein metabolism, exhibit anti-fibrolytic activity and regulate angiogenesis | [158,159,160] |
| LL-37 | Increased expression in human lesions | [161] | |
| Inflammatory articular joints | hBD-3, LL-37 | Upregulated in osteoarthritis | [162] |
5. Clinical development of antimicrobial peptides
6. Conclusion and Future perspectives
Author Contributions
Conflicts of Interest
References
- Lehrer, R.I.; Ganz, T. Antimicrobial peptides in mammalian and insect host defence. Current Opinion in Immunology 1999, 11, 23–27. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial peptides: classification, design, application and research progress in multiple fields. Frontiers in microbiology 2020, 11, 2559. [Google Scholar] [CrossRef] [PubMed]
- Malkoski, M.; Dashper, S.G.; O'Brien-Simpson, N.M.; Talbo, G.H.; Macris, M.; Cross, K.J.; Reynolds, E.C. Kappacin, a novel antibacterial peptide from bovine milk. Antimicrobial agents and chemotherapy 2001, 45, 2309–2315. [Google Scholar] [CrossRef]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F.; Rassner, G. Dermcidin: a novel human antibiotic peptide secreted by sweat glands. Nature immunology 2001, 2, 1133–1137. [Google Scholar] [CrossRef]
- Bulet, P.; Stocklin, R.; Menin, L. Antimicrobial peptides from invertebrates to vertebrates. Immunological Reviews 2004, 198, 169–184. [Google Scholar] [CrossRef]
- Wang, G. Human antimicrobial peptides and proteins. Pharmaceuticals 2014, 7, 545–594. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins and host defense. Science 1999, 286, 420–421. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef]
- Lai, A.C.; Chen, F.Z. Comparison of a new Eulerian model with a modified Lagrangian approach for particle distribution and deposition indoors. Atmospheric Environment. 2007, 41, 5249–5256. [Google Scholar] [CrossRef]
- McKelvey, J.A.; Yang, M.; Jiang, Y.; Zhang, S. Salmonella enterica serovar enteritidis antimicrobial peptide resistance genes aid in defense against chicken innate immunity, fecal shedding, and egg deposition. Infection and Immunity 2014, 82, 5185–5202. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, C.; Zhang, X.; Zhang, M.Z.; Rottinghaus, G.E.; Zhang, S. Structure-function analysis of Avian beta-defensin-6 and beta-defensin- 12: role of charge and disulfide bridges. BMC Microbiol 2016, 16, 210. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, C.; Zhang, M.Z.; Zhang, S. Beta-defensin derived cationic antimicrobial peptides with potent killing activity against gram negative and gram positive bacteria. BMC Microbiology 2018, 18, 54. [Google Scholar] [CrossRef]
- Wimley, W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chemical Biology 2010, 5, 905–917. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, C.; Zhang, M.Z.; Zhang, S. (2017). Novel synthetic analogues of avian beta-defensin-12: the role of charge, hydrophobicity, and disulfide bridges in biological functions. BMC Microbiology 2017, 17, 43. [Google Scholar] [CrossRef]
- Yang, D.; Biragyn, A.; Kwak, L.W.; Oppenheim, J.J. Mammalian defensins in immunity: more than just microbicidal. Trends in Immunololgy 2002, 23, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Zaiou, M. Multifunctional antimicrobial peptides: therapeutic targets in several human diseases. Journal of Molecular Medicines 2007, 85, 317–329. [Google Scholar] [CrossRef]
- Yang, M. Avian β-Defensins as Antimicrobial and Immunomodulatory Agents 2020. Available online at: https://hdl.handle.net/10355/68953.
- Zhang, C.; Yang, M.; Ericsson, A.C. Antimicrobial peptides: potential application in liver cancer. Frontiers in Microbiology 2019, 10, 1257. [Google Scholar] [CrossRef]
- Shai, Y. Mode of action of membrane active antimicrobial peptides. Peptide Science 2002, 66, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Vasil, A.I.; Hale, J.D.; Hancock, R.W.E.; Vasil, M.L.; Hodges, R.S. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic α-helical cationic antimicrobial peptides. Journal of Peptide Science 2002, 90, 369–383. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, J.; Chen, Y. Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein Cell 2010, 1, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Kim, H.S.; Kim, S.C. Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions, Biochemical Biophysical Research Communication 1998, 244, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, K.; Dang, W.; Chen, R.; Xie, J.; Zhang, B. Two hits are better than one: membrane-active and DNA binding-related double-action mechanism of NK-18, a novel antimicrobial peptide derived from mammalian NK-Lysin. Antimicrobial Agents and Chemotherapy 2013, 57, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Hwang, J.S.; Lee, D.G. Antibacterial action of lactoferricin B like peptide against Escherichia coli: reactive oxygen species-induced apoptosis-like death. Journal of applied microbiology 2020, 129, 287–295. [Google Scholar] [CrossRef]
- Ramos, R.; Silva, J.P.; Rodrigues, A.C.; Costa, R.; Guardão, L.; Schmitt, F. Wound healing activity of the human antimicrobial peptide LL37. Peptides 2011, 32, 1469–1476. [Google Scholar] [CrossRef]
- Mangoni, M.L.; McDermott, A.M.; Zasloff, M. Antimicrobial peptides and wound healing: biological and therapeutic considerations. Experimental Dermatology 2016, 25, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, O.E. Antimicrobial peptides in cutaneous wound healing. Antimicrobial Peptides 2016, 1–15. [Google Scholar] [CrossRef]
- Pfalzgraff, A.; Brandenburg, K.; Weindl, G. Antimicrobial peptides and their therapeutic potential for bacterial skin infections and wounds. Frontiers in Pharmacology 2018, 9, 281. [Google Scholar] [CrossRef]
- Majewski, K.; Kozłowska, E.; Żelechowska, E.P.; Brzezińska-Błaszczyk, A. Serum concentrations of antimicrobial peptide cathelicidin LL-37 in patients with bacterial lung infections. Central Europian Journal of Immunology 2018, 43, 453–457. [Google Scholar] [CrossRef]
- Griffith, G.L.; Kasus-Jacobi, A.; Pereira, H.A. Bioactive antimicrobial peptides as therapeutics for corneal wounds and infections. Adv Wound Care (New Rochelle) 2017, 6, 175–190. [Google Scholar] [CrossRef]
- Rivas-Santiago, B.; Schwander, S.K.; Sarabia, C.; Diamond, G.; Klein-Patel, M.E.; Hernandez-Pando, R.; Ellner, J.J.; Sada, E. Human {beta}-defensin 2 is expressed and associated with Mycobacterium tuberculosis during infection of human alveolar epithelial cells. Infection and Immunity 2005, 73, 4505–4511. [Google Scholar] [CrossRef]
- Bormann, N.; Koliszak, A.; Kasper, S.; Schoen, L.; Hilpert, K.; Volkmer, R. A short artificial antimicrobial peptide shows potential to prevent or treat bone infections. International Journal of Scientific Reports 2017, 7, 1506. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Bellm, L.A.; Epstein, J.B.; Sonis, S.T.; Symonds, R.P. Antimicrobial therapy to prevent or treat oral mucositis. The Lancet Infectious Diseases 2003, 3, 405–412. [Google Scholar] [CrossRef]
- Deslouches, B.; Di, Y.P. Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications. OncoTargets and Therapy 2017, 8, 46635. [Google Scholar] [CrossRef] [PubMed]
- Sibel Akalın, A. Dairy-derived antimicrobial peptides: action mechanisms,pharmaceutical uses and production proposals. Trends in Food Science and Technology 2014, 36, 79–95. [Google Scholar] [CrossRef]
- Reddy, K.V.R.; Yedery, R.D.; Aranha, C. Antimicrobial peptides:premises and promises. International Journal of Antimicrobial Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Gsehwandtner, M.; Zhong, S.; Tschachler, A.; Miltz, V.; Karner, S.; Elbe-Burger, A. Fetal human keratinocytes produce large amounts of antimicrobial peptides: involvement of histone-methylation process. Journal of Investigative Dermatology 2014, 134, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- Field, C.J. The immunological components of human milk and their effect on immune development in infants. Journal of Nutrition 2005, 135, 1–4. [Google Scholar] [CrossRef]
- Wang, J.; Chou, S.; Xu, L.; Zhu, X.; Dong, N.; Shan, A. High specific selectivity and membrane-active mechanism of the synthetic centrosymmetric α-helical peptides with Gly-Gly pairs. Scientific Reports 2015, 5, 15963. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A. The role of amphibian antimicrobial peptides in protection of amphibians from pathogens linked to global amphibian declines. Biochimica et Biophysica Acta Biomembranes 2009, 1788, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Conlon, J.M.; Mechkarska, M. Host-defence peptides with therapeutic potential from skin secretions of frogs from the family pipidae. Pharmaceuticals 2014, 7, 58–77. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, Y.; Wang, X.; Liang, J.; Zhang, C.; Zhang, K. The first antimicrobial peptide from sea amphibian. Molecular Immunology 2008, 45, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Vilcinskas, A. Evolutionary plasticity of insect immunity. Journal of Insect Physiology 2013, 59, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Sahu, R.K.; Dey, T.; Lahkar, M.D.; Manna, P.; Kalita, J. Beneficial role of insect derived bioactive components against inflammation and its associated complications (colitis and arthritis) and cancer. Chemico-Biological Interactions 2019, 313, 108824. [Google Scholar] [CrossRef] [PubMed]
- Shelomi, M.; Jacobs, C.; Vilcinskas, A.; Vogel, H. The unique antimicrobial peptide repertoire of stick insects. Dev. Compar. Immunol. 2020, 103, 103471. [Google Scholar] [CrossRef]
- Zahedifard, F.; Lee, H.; No, J.H.; Salimi, M.; Seyed, N.; Asoodeh, A. Comparative study of different forms of jellein antimicrobial peptide on leishmania parasite. Experimental Parasitology 2020, 209, 107823. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; de la Fuente-Nuez, C.; Ou, R.W.; Torres, M.D.T.; Pande, S.G.; Sinskey, A.J. Yeast based synthetic biology platform for antimicrobial peptide production. ACS Synthetic Biology 2018, 896–902. [Google Scholar] [CrossRef]
- Parachin, N.S.; Mulder, K.C.; Viana, A.A.B.; Dias, S.C.; Franco, O.L. Expression systems for heterologous production of antimicrobial peptides. Peptides 2002, 38, 446–456. [Google Scholar] [CrossRef]
- Tang, S.-S.; Prodhan, Z.H.; Biswas, S.K.; Le, C.-F.; Sekaran, S.D. Antimicrobial peptides from different plant sources: isolation, characterisation, and purification. Phytochemistry 2018, 154, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Oh, R.; Lee, M.J.; Kim, Y.-O.; Nam, B.-H.; Kong, H.J.; Kim, J.-W. Myticusin-beta, antimicrobial peptide from the marine bivalve. Mytilus coruscus. Fish and Shellfish Immunology 2020, 99, 342–352. [Google Scholar] [CrossRef]
- Huang, H.N.; Rajanbabu, V.; Pan, C.Y.; Chan, Y.L.; Wu, C.J.; Chen, J.Y. A cancer vaccine based on the marine antimicrobial peptide pardaxin (GE33) for control of bladder associated tumors. Biomaterials 2013, 34, 10151–10159. [Google Scholar] [CrossRef]
- Li, C.; Zhu, C.; Ren, B.; Yin, X.; Shim, S.H.; Gao, Y. Two optimized antimicrobial peptides with therapeutic potential for clinical antibiotic resistant Staphylococcus aureus. European journal of medicinal chemistry 2019, 183, 111686. [Google Scholar] [CrossRef] [PubMed]
- Madanchi, H.; Shoushtari, M.; Kashani, H.H.; and Sardari, S. Antimicrobial peptides of the vaginal innate immunity and their role in the fight against sexually transmitted diseases. New Microbes New Infections 2020, 34, 100627. [Google Scholar] [CrossRef] [PubMed]
- Muhialdin, B.J.; Algboory, H.L.; Kadum, H.; Mohammed, N.K.; Saari, N.; Hassan, Z. Antifungal activity determination for the peptides generated by Lactobacillus plantarum TE10 against Aspergillus flavus in maize seeds. Food Control 2020, 109, 106898. [Google Scholar] [CrossRef]
- Jung, Y.; Kong, B.; Moon, S.; Yu, S.H.; Chung, J.; Ban, C. Envelope-deforming antiviral peptide derived from influenza virus M2 protein. Biochemical and Biophysical Research Communications 2019, 517, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Mahlapuu, M.; Hakansson, J.; Ringstad, L.; Bjorn, C. Antimicrobial peptides: an emerging category of therapeutic agents. Frontiers in Cellular and Infection Microbiology 2016, 6, 194. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, K.; She, R.; Ma, W.; Peng, F.; Jin, H. Swine intestine antimicrobial peptides inhibit infectious bronchitis virus infectivity in chick embryos. Poultry Science 2010, 89, 464–469. [Google Scholar] [CrossRef]
- Huang, H.N.; Pan, C.Y.; Chen, J.Y. Grouper (Epinephelus coioides) antimicrobial peptide epinecidin-1 exhibits antiviral activity against foot-and-mouth disease virus in vitro. Peptides 2018, 106, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Wohlford-Lenane, C.L.; Meyerholz, D.K.; Perlman, S.; Zhou, H.; Tran, D.; Selsted, M.E. Rhesus theta-defensin prevents death in a mouse model of severe acute respiratory syndrome coronavirus pulmonary disease. Journal of Virology 2009, 83, 11385–11390. [Google Scholar] [CrossRef]
- Marimuthu, S.K.; Nagarajan, K.; Perumal, S.K.; Palanisamy, S.; Subbiah, L. (2019). Insilico alpha-helical structural recognition of temporin antimicrobial peptides and its interactions with middle-east respiratory syndrome corona virus. International Journal of Peptide Research and Therapeutics 2019, 26, 1473–1483. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Saugar, J.M.; Dellisanti, M.; Barra, D.; Simmaco, M.; Rivas, L. Temporins, small antimicrobial peptides with leishmanicidal activity. Journal of Biological Chemistry 2005, 280, 984–990. [Google Scholar] [CrossRef]
- Rhaiem, R.B.; Houimel, M. Targeting Leishmania major parasite with peptides derived from a combinatorial phage display library. Acta Tropica 2016, 159, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Abbassi, F.; Raja, Z.; Oury, B.; Gazanion, E.; Piesse, C.; Sereno, D. Antibacterial and leishmanicidal activities of temporin-SHd, a 17-residue long membrane-damaging peptide. Biochimie 2013, 95, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Neshani, A.; Zare, H.; Akbari, E.M.R.; Khaledi, A.; and Ghazvini, K. Epinecidin-1, a highly potent marine antimicrobial peptide with anticancer and immunomodulatory activities. BMC Pharmacology and Toxicology 2019, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Jiang, W.; Cao, S.; Zhao, P.; Liu, J.; Dong, H. In vitro leishmanicidal activity of antimicrobial peptide KDEL against Leishmania tarentolae. Acta Biochimica et Biophysica Sinica 2019, 51, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Gao, Y.; Qi, Y.; Chen, L.; Ma, Y.; Li, Y. Peptide-based cancer therapy: opportunity and challenge. Cancer Letters 2014, 351, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wong, S.W.; Ge, L.; Shaw, C.; Siu, S.W.I.; and Kwok, H.F. In vitro and MD simulation study to explore physicochemical parameters for antibacterial peptide to become potent anticancer peptide. Molecular Theory Oncolytics 2020, 16, 7–19. [Google Scholar] [CrossRef]
- Arias, M.; Haney, E.F.; Hilchie, A.L.; Corcoran, J.A.; Hyndman, M.E.; Hancock, R.E.W. Selective anticancer activity of synthetic peptides derived from the host defence peptide tritrpticin. Biochimica et Biophysica Acta - Biomembranes 2020, 1862, 183228. [Google Scholar] [CrossRef] [PubMed]
- Seefeldt, A.C.; Nguyen, F.; Antunes, S.; Pérébaskine, N.; Graf, M.; Arenz, S. The proline-rich antimicrobial peptide Onc112 inhibits translation by blocking and destabilizing the initiation complex. Nature Structural and Molecular Biology 2015, 22, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Wang, C.; Zhang, T.; Zhang, L.; Xue, C.; Feng, X. Bioactivity and bactericidal mechanism of histidine rich β-hairpin peptide against gram negative bacteria. The International Journal of Molecular Sciences 2019, 20, 3954. [Google Scholar] [CrossRef]
- Wang, D.; Shen, Y.; Ma, J.; Hancock, R.E.W.; Haapasalo, M. Antibiofilm effect of denantiomeric peptide alone and combined with edta in vitro. Journal of Endodontics 2017, 43, 1862–1867. [Google Scholar] [CrossRef]
- Lei, J.; Sun, I.; Huang, S.; Zhu, C.; Li, P.; He, J. The antimicrobial peptides and their potential clinical applications. American journal of translational research 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Koehbach, J.; Craik, D.I. The vast structural diversity of antimicrobial peptides. Trends in Pharmacological Sciences 2019, 40, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Mazurkiewicz-Pisarek, A.; Baran, J.; Ciach, T. Antimicrobial Peptides: Challenging Journey to the Pharmaceutical, Biomedical, and Cosmeceutical Use. International Journal of Molecular Sciences 2023, 24, 9031. [Google Scholar] [CrossRef]
- Teixeira, V.; Feio, M.J.; Bastos, M. Role of lipids in the interaction of antimicrobial peptides with membranes. Progress in Lipid Research 2012, 51, 149–177. [Google Scholar] [CrossRef] [PubMed]
- Subbalakshmi, C.; Sitaram, N. FEMS Microbiology Letters 1998, 160, 91. [CrossRef]
- Barriere, S.L. Bacterial resistance to beta-lactams, and its prevention with combination antimicrobial therapy. Pharmacotherapy 1992, 12, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O.; Silvestri, C.; Ghiselli, R.; Orlando, F.; Riva, A.; Mocchegiani, F.; Chiodi, L.; Castelletti, S.; Gabrielli, E.; Saba, V.; et al. Protective effects of the combination of alpha-helical antimicrobial peptides and rifampicin in three rat models of Pseudomonas aeruginosa infection. The Journal of Antimicrobial Chemotherapy 2008, 62, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Jeong, N.; Kim, J.Y.; Park, S.C.; Lee, J.K.; Gopal, R.; Yoo, S.; Son, B.K.; Hahm, J.S.; Park, Y.; Hahm, K.S. Antibiotic and synergistic effect of Leu-Lys rich peptide against antibiotic resistant microorganisms isolated from patients with cholelithiasis. Biochemical and Biophysical Research Communications 2010, 399, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Porat, Y.; Marynka, K.; Tam, A.; Steinberg, D.; Mor, A. Acyl-substituted dermaseptin S4 derivatives with improved bactericidal properties, including on oral microflora. Antimicrobial Agents and Chemotheapy 2005, 50, 4153–4160. [Google Scholar] [CrossRef]
- Willcox, M.D.; Hume, E.B.; Aliwarga, Y.; Kumar, N.; Cole, N. A novel cationic-peptide coating for the prevention of microbial colonization on contact lenses. Journal Applied Microbiology 2008, 105, 1817–1825. [Google Scholar] [CrossRef]
- Cirioni, O.; Giacometti, A.; Ghiselli, R.; Kamysz, W.; Orlando, F.; Mocchegiani, F.; Silvestri, C.; Licci, A.; Chiodi, L.; Lukasiak, J.; Saba, V.; Scalise, G. Citropin 1.1-treated central venous catheters improve the efficacy of hydrophobic antibiotics in the treatment of experimental staphylococcal catheter-related infection. Peptides 2006, 27, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, M.; Miyazaki, T.; Makau, J.N.; Mizuta, S.; Tanaka, Y.; Ishikawa, T.; Makimura, K.; Hirayama, T.; Takazono, T.; Saijo, T. Scientific Reports, 2020; 10, 17745. [CrossRef]
- Ali, R.S.; Falconer, A.; Ikram, M. Expression of the peptide antibiotics human beta defensin-1 and human beta defensin-2 in normal human skin. Journal of Investigative Dermatology. 2001, 117, 106–111. [Google Scholar] [CrossRef]
- Rieg, S.; Garbe, C.; Sauer, B. Dermcidin is constitutively produced by eccrine sweat glands and is not induced in epidermal cells under inflammatory skin conditions. British Journal of Dermatology 2004, 151, 534–539. [Google Scholar] [CrossRef]
- Harder, J.; Dressel, S.; Wittersheim, M. Enhanced expression and secretion of antimicrobial peptides in atopic dermatitis and after superfiial skin injury. Journal of Investigative Dermatology 2010, 130, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- White, S.H.; Wimley, W.C.; Selsted, M.E. Structure, function, and membrane integration of defensins. Current Opinion in Structural Biology 1995, 5, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, R.I.; Ganz, T. Defensins of vertebrate animals. Current Opinion in Immunology 2002, 14, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Selsted, M.E.; Szklarek, D. Defensins. Natural peptide antibiotics of human neutrophils. Journal of Clinical Investigation 1985, 76, 1427–1435. [Google Scholar] [CrossRef]
- Ferris, L.K.; Mburu, Y.K.; Mathers, A.R. Human beta defensin 3 induces maturation of human Langerhans cell like dendritic cells: an antimicrobial peptide that functions as an endogenous adjuvant. Journal of Investigative Dermatology 2013, 133, 460–468. [Google Scholar] [CrossRef]
- Rieg, S.; Steffn, H.; Seeber, S. Deficiency of dermcidin derived antimicrobial peptides in sweat of patients with atopic dermatitis correlates with an impaired innate defense of human skin in vivo. Journal of Immunology 2005, 174, 8003–8010. [Google Scholar] [CrossRef]
- Zhang, J.; Dyer, K.D.; Rosenberg, H.F. Human RNase 7: a new cationic ribonuclease of the RNase A superfamily. Nucleic Acids Research 2003, 31, 602–607. [Google Scholar] [CrossRef]
- Koczulla, R.; von Degenfeld, G.; Kupatt, C. An angiogenicrole for the human peptide antibiotic LL-37/hCAP-18. Journal of Clinical Investigation 2003, 111, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Alowami, S.; Qing, G.; Emberley, E. Psoriasin (S100A7) expression is altered during skin tumorigenesis. BMC Dermatol 2003, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Jinquan, T.; Vorum, H.; Larsen, C.G. Psoriasin: a novel chemotactic protein. Journal of Investigative Dermatology 1996, 107, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Abtin, A.; Eckhart, L.; Gläser, R. The antimicrobial heterodimer S100A8/S100A9 (calprotectin) is upregulated by bacterial flgellin in human epidermal keratinocytes. Journal of Investigative Dermatology 2010, 130, 2423–2430. [Google Scholar] [CrossRef] [PubMed]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. (Dental caries). Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gomes, B.P.; Pinheiro, E.T.; Jacinto, R.C.; Zaia, A.A.; Ferraz, C.C.; Souza-Filho, F.J. Microbial analysis of canals of root-filled teeth with periapical lesions using polymerase chain reaction. The Journal of Endodontics 2008, 34, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Paris, S.; Wolgin, M.; Kielbassa, A.M.; Pries, A.; Zakrzewicz, A. Gene expression of human beta-defensins in healthy and inflamed human dental pulps. Journal of Endodontics 2009, 35, 520–523. [Google Scholar] [CrossRef]
- Lee, S.H.; Baek, D.H. Antibacterial and neutralizing effect of human beta-defensins on Enterococcus faecalis and Enterococcus faecalis lipoteichoic acid. The Journal of Endodontics 2012, 38, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Krzysciak, W.; Jurczak, A.; Piatkowski, J.; Koscielniak, D.; Gregorczyk-Maga, I.; Kolodziej, I.; Papiez, M.A.; Olczak-Kowalczyk, D. Effect of histatin-5 and lysozyme on the ability of Streptococcus mutans to form biofilms in in vitro conditions. Postępy Higieny i Medycyny Doświadczalnej 2015, 69, 1056–1066. [Google Scholar]
- Guo, Y.J.; Zhang, B.; Feng, X.S.; Ren, H.X.; Xu, J.R. Human cathelicidin ll-37 enhance the antibiofilm effect of egcg on Streptococcus mutans. BMC Oral Health 2016, 16, 101. [Google Scholar] [CrossRef]
- Kreling, P.F.; Aida, K.L.; Massunari, L.; Caiaffa, K.S.; Percinoto, C.; Bedran, T.B.; Spolidorio, D.M.; Abuna, G.F.; Cilli, E.M.; Duque, C. Cytotoxicity and the effect of cationic peptide fragments against cariogenic bacteria under planktonic and biofilm conditions. Biofouling 2016, 32, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.B.; Kim, A.R.; Kum, K.Y.; Yun, C.H.; Han, S.H. The synthetic human beta-defensin-3 c15 peptide exhibits antimicrobial activity against Streptococcus mutans, both alone and in combination with dental disinfectants. Journal of Microbiology 2017, 55, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Winfred, S.B.; Meiyazagan, G.; Panda, J.J.; Nagendrababu, V.; Deivanayagam, K.; Chauhan, V.S.; Venkatraman, G. Antimicrobial activity of cationic peptides in endodontic procedures. Europian Journal of Dentistry 2014, 8, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.G.C.; Xavier, P.D.; Cantuaria, A.P.C.; Porcino, R.A.; Almeida, J.A.; Franco, O.L.; Rezende, T.M.B. Host defense peptide idr-1002 associated with ciprofloxacin as a new antimicrobial and immunomodulatory strategy for dental pulp revascularization therapy. Microbial Pathogens 2020, 104634. [Google Scholar] [CrossRef] [PubMed]
- Ouhara, K.; Komatsuzawa, H.; Yamada, S.; Shiba, H.; Fujiwara, T.; Ohara, M.; Sayama, K.; Hashimoto, K.; Kurihara, H.; Sugai, M. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, {beta}-defensins and ll37, produced by human epithelial cells. Journal of Antimicroial Chemotherapy 2005, 55, 888–896. [Google Scholar] [CrossRef]
- Into, T.; Inomata, M.; Shibata, K.; Murakami, Y. Effect of the antimicrobial peptide ll-37 on toll-like receptors 2-, 3- and 4-triggered expression of il-6, il-8 and cxcl10 in human gingival fibroblasts. Cell Immunol 2010, 264, 104–109. [Google Scholar] [CrossRef]
- Brogden, K.A.; Nordholm, G.; Ackermann, M. Antimicrobial activity of cathelicidins bmap28, smap28, smap29, and pmap23 against Pasteurella multocida is more broad-spectrum than host species specific. Veterinary microbiology 2007, 119, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Brice, D.C.; Toth, Z.; Diamond, G. LL-37 disrupts the kaposi's sarcoma-associated herpesvirus envelope and inhibits infection in oral epithelial cells. Antiviral Research 2018, 158, 25–33. [Google Scholar] [CrossRef]
- Winter, J.; Pantelis, A.; Reich, R.; Martini, M.; Kraus, D.; Jepsen, S.; Allam, J.P.; Novak, N.; Wenghoefer, M. Human beta-defensin-1, -2, and -3 exhibit opposite effects on oral squamous cell carcinoma cell proliferation. Cancer Investigation 2011, 29, 196–201. [Google Scholar] [CrossRef]
- Goldman, M.J.; Anderson, G.M.; Stolzenberg, E.D.; Kari, U.P.; Zasloff, M.; Wilson, J.M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997, 88, 553–560. [Google Scholar] [CrossRef]
- Duits, L.A.; Ravensbergen, B.; Rademaker, M.; Hiemstra, P.S.; Nibbering, P.H. Expression of beta-defensin 1 and 2 mRNA by human monocytes, macrophages and dendritic cells. Journal of Immunological Sciences 2002, 106, 517–525. [Google Scholar] [CrossRef]
- Hess, C.; Herr, C.; Beisswenger, C.; Zakharkina, T.; Schmid, R.M.; Bals, R. Myeloid RelA regulates pulmonary host defense networks. European Respiratory Journal 2010, 35, 343–352. [Google Scholar] [CrossRef]
- Scharf, S.; Vardarova, K.; Lang, F.; Schmeck, B.; Opitz, B.; Flieger, A.; Heuner, K.; Hippenstiel, S.; Suttorp, N.; N’Guessan, P.D. Legionella pneumophila induces human beta defensin-3 in pulmonary cells. Respiratory Research 2010, 11, 93. [Google Scholar] [CrossRef]
- Rivas-Santiago, B.; Serrano, C.J.; Enciso-Moreno, J.A. Susceptibility to infectious diseases based on antimicrobial peptide production. Infection and Immunity 2009, 77, 4690–4695. [Google Scholar] [CrossRef]
- Merkel, D.; Rist, W.; Seither, P.; Weith, A.; Lenter, M.C. Proteomic study of human bronchoalveolar lavage fluids from smokers with chronic obstructive pulmonary disease by combining surface-enhanced laser desorption/ionization-mass spectrometry profiling with mass spectrometric protein identification. Proteomics 2005, 5, 2972–2980. [Google Scholar] [CrossRef]
- Felgentreff, K.; Beisswenger, C.; Griese, M.; Gulder, T.; Bringmann, G.; Bals, R. The antimicrobial peptide cathelicidin interacts with airway mucus. Peptides 2006, 27, 3100–3106. [Google Scholar] [CrossRef]
- Hase, K.; Murakami, M.; Iimura, M. Expression of LL-37 by human gastric epithelial cells as a potential host defense mechanism against Helicobacter pylori. Gastroenterology 2003, 125, 1613–1625. [Google Scholar] [CrossRef]
- Grubman, A.; Kaparakis, M.; Viala, J. The innate immune molecule, NOD1, regulates direct killing of Helicobacter pylori by antimicrobial peptides. Cellular Microbiology 2010, 12, 626–639. [Google Scholar] [CrossRef]
- Kotarsky, K.; Sitnik, K.M.; Stenstad, H. A novel role for constitutively expressed epithelial-derived chemokines as antibacterial peptides in the intestinal mucosa. Mucosal immunology 2010, 3, 40–48. [Google Scholar] [CrossRef]
- Harada, K.; Ohba, K.; Ozaki, S. Peptide antibiotic human beta-defensin-1 and -2 contribute to antimicrobial defense of the intrahepatic biliary tree. Hepatology, 2004; 40, 925–925. [Google Scholar] [CrossRef]
- Wehkamp, J.; Chu, H.; Shen, B. Paneth cell antimicrobial peptides: topographical distribution and quantification in human gastrointestinal tissues. FEBS Letters 2006, 580, 5344–5350. [Google Scholar] [CrossRef]
- Bevins, C.L.; Nita, H.; Salzman, N.H.; Ghosh, D.; Huttner, K.M. Human defensin-5 (HD-5) transgenic mice: paneth cell expression and protection from lethal Salmonella typhimurium infection. World Journal of Gastroenterology 2002, 122, 169. [Google Scholar]
- Kaser, A.; Lee, A.-H.; Franke, A. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008, 134, 743–756. [Google Scholar] [CrossRef]
- Zilbauer, M.; Dorrell, N.; Boughan, P.K. Intestinal innate immunity to Campylobacter jejuni results in induction of bactericidal human beta-defensins 2 and 3. Infection and Immunity 2005, 73, 7281–7289. [Google Scholar] [CrossRef]
- Limura, M.; Gallo, R.L.; Hase, K.; Miyamoto, Y.; Eckmann, L.; Kagnoff, M.F. Cathelicidin mediates innate intestinal defense against colonization with epithelial adherent bacterial pathogens. Journal of Immunology 2005, 174, 4901–4907. [Google Scholar] [CrossRef]
- Fahlgren, A.; Hammarstrom, S.; Danielsson, A.; Hammarstrom, M.L. Increased expression of antimicrobial peptides and lysozyme in colonic epithelial cells of patients with ulcerative colitis. Clinical and Experimental Immunology 2003, 131, 90–101. [Google Scholar] [CrossRef]
- Kanyshkova, T.G.; Buneva, V.N.; Nevinsky, G.A. Lactoferrin and its biological functions. Biochemistry (Mosc) 2001, 66, 1–7. [Google Scholar] [CrossRef]
- Collins, H.L. Withholding iron as a cellular defence mechanism--friend or foe? The European Journal of Immunology 2008, 38, 1803–1806. [Google Scholar] [CrossRef]
- Van Bambeke, F. Glycopeptides and glycodepsipeptides in clinical development: a comparativereview of their antibacterial spectrum, pharmacokinetics and clinical efficacy. Current Opinion in Investigational Drugs 2006, 7, 740–749. [Google Scholar]
- Lande, R.; Botti, E.; Jandus, C.; Dojcinovic, D.; Fanelli, G.; Conrad, C. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nature Communications 2014, 5, 5621. [Google Scholar] [CrossRef]
- Itzhaki, R.F.; Wozniak, M.A.; Appelt, D.M.; Balin, B.J. Infiltration of the brain by pathogens causes Alzheimer’s disease. Neurobiol Aging 2004, 25, 619–627. [Google Scholar] [CrossRef]
- Carter, C. Alzheimer’s disease: APP, gamma secretase, APOE, CLU, CR1, PICALM, ABCA7, BIN1, CD2AP, CD33, EPHA1, and MS4A2, and their relationships with Herpes simplex, C. pneumoniae, other suspect pathogens, and the immune system. International Journal of Alzheimer's Disease Research 2011, 1–34. [Google Scholar] [CrossRef]
- Sparks, S.P.; Steffen, M.J.; Smith, C.; Jicha, G.; Ebersole, J.L.; Abner, E. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers and Dementia 2012, 8, 196–203. [Google Scholar] [CrossRef]
- Kourie, J.I.; Shorthouse, A.A. Properties of cytotoxic peptide-formed ion channels. American Journal of Physiology-Cell Physiology 2000, 278, C1063–1087. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, K.; Schluesener, H. Antimicrobial peptides in the brain. Archivum Immunologiae et Therapiae Experimentalis 2010, 58, 365–377. [Google Scholar] [CrossRef]
- Kagan, B.L.; Jang, H.; Capone, R.; Teran Arce, F.; Ramachandran, S.; Lal, R. Antimicrobial properties of amyloid peptides. Molecular Pharmaceutics 2011, 9, 708–717. [Google Scholar] [CrossRef]
- Edfeldt, K.; Agerberth, B.; Rottenberg, M.E.; Gudmundsson, G.H.; Wang, X.B.; Mandal, K.; Xu, Q.; Yan, Z.Q. Involvement of the Antimicrobial Peptide LL-37 in Human Atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 2006, 26, 1551–1557. [Google Scholar] [CrossRef]
- Baxter, A.A.; Lay, F.T.; Poon, I.K.; Kvansakul, M.; Hulett, M.D. Tumor cell membranetargeting cationic antimicrobial peptides: novel insights into mechanisms of action and therapeutic prospects. Cellular and Molecular Life Sciences 2017, 74, 3809–3825. [Google Scholar] [CrossRef]
- Tsai, T.L.; Li, A.C.; Chen, Y.C.; Liao, Y.S.; Lin, T.H. Antimicrobial peptide m2163 or m2386 identified from Lactobacillus casei ATCC 334 can trigger apoptosis in the human colorectal cancer cell line SW480. Tumor Biology 2015, 36, 3775–3789. [Google Scholar] [CrossRef]
- Ong, P.Y.; Ohtake, T.; Brandt, C.; Strickland, I.; Boguniewicz, M.; Ganz, T.; Gallo, R.L.; Leung, D.Y. Endogenous Antimicrobial Peptides and Skin Infections in Atopic Dermatitis. New England Journal of Medicine 2002, 347, 1151–1160. [Google Scholar] [CrossRef]
- Frohm, M.; Agerberth, B.; Ahangari, G.; Ståhle-Bäckdahl, M.; Lidén, S.; Wigzell, H.; Gudmundsson, G.H. The Expression of the Gene Coding for the Antibacterial Peptide LL-37 Is Induced in Human Keratinocytes during Inflammatory Disorders. Journal of Biological Chemistry 1997, 272, 15258–15263. [Google Scholar] [CrossRef]
- Friedland, H.D.; Sharp, D.D.; Robinson, J.R. Double-blind, randomized, vehicle-controlled study to asses the safety and efficacy of MBI 594AN in the treatment of acne vulgaris. Abstracts of the 61st annual meeting of American Academy of Dermatology 2003, 61, 22. [Google Scholar]
- McInturff, J.E.; Wang, S.J.; Machleidt, T.; Richard, L.T.; Oren, A.; Hertz, C.J.; Krutzik, S.R.; Hart, S.; Zeh, K.; Anderson, D.H.; Gallo, R.L.; Modlin, R.L.; Kim, J. Granulysin-Derived Peptides Demonstrate Antimicrobial and Anti-Inflammatory Effects Against Propionibacterium acnes. Journal of Investigative Dermatology 2005, 125, 256–263. [Google Scholar] [CrossRef]
- Boman, H.G. Innate immunity and the normal microflora. Immunological Reviews 2000, 173, 5–16. [Google Scholar] [CrossRef]
- Tanida, T.; Okamoto, T.; Okamoto, A.; Wang, H.; Hamada, T.; Ueta, E.; Osaki, T. Decreased excretion of antimicrobial proteins and peptides in saliva of patients with oral candidiasis. Journal of Oral Pathology Medicine 2003, 32, 586–594. [Google Scholar] [CrossRef]
- Pütsep, K.; Carlsson, G.; Boman, H.G.; Andersson, M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet 2002, 360, 1144–1149. [Google Scholar] [CrossRef]
- Gusman, H.; Travis, J.; Helmerhorst, E.J.; Potempa, J.; Troxler, R.F.; Oppenheim, F.G. Salivary Histatin 5 Is an Inhibitor of Both Host and Bacterial Enzymes Implicated in Periodontal Disease. Infection and Immunity 2001, 69, 1402–1408. [Google Scholar] [CrossRef]
- Yoshinari, M.; Kato, T.; Matsuzaka, K.; Hayakawa, T.; Inoue, T.; Oda, Y.; Okuda, K.; Shimono, M. Adsorption behavior of antimicrobial peptide histatin 5 on PMMA. Journal of Biomedical Materials Research Part B: Applied Biomaterials 2005, 77B, 47–54. [Google Scholar] [CrossRef]
- Wehkamp, J.; Salzman, N.H.; Porter, E.; Nuding, S.; Weichenthal, M.; Petras, R.E.; Shen, B.; Schaeffeler, E.; Schwab, M.; Linzmeier, R.; Feathers, R.W.; Chu, H.; Lima, H.; Fellermann, K.; Ganz, T.; Stange, E.F.; Bevins, C.L. Reduced Paneth cell α-defensins in ileal Crohn’s disease. Proceedings of the National Academy of Sciences 2005, 102, 18129–18134. [Google Scholar] [CrossRef]
- Fahlgren, A.; Hammarström, S.; Danielsson; Hammarström, M. L. β-Defensin-3 and -4 in intestinal epithelial cells display increased mRNA expression in ulcerative colitis. Clinical and Experimental Immunology 2004, 137, 379–385. [Google Scholar] [CrossRef]
- Baker, M.A.; Maloy, W.L.; Zasloff, M.; Jacob, L.S. Anticancer efficacy of magainin2 and analogue peptides. Cancer Research and Treatment 1993, 53, 3052–3057. [Google Scholar]
- Sloballe, P.W.; Lee Maloy, W.; Myrga, M.L.; Jacob, L.S.; Herlyn, M. Experimental local therapy of human melanoma with lytic magainin peptides. International Journal of Cancer 1995, 60, 280–284. [Google Scholar] [CrossRef]
- Moore, A.J.; Devine, D.A.; Bibby, M.C. Preliminary experimental anticancer activity of cecropins. Peptide Research 1994, 7, 265–269. [Google Scholar]
- Yoo, Y.; Watanabe, S.; Watanabe, R.; Hata, K.; Shimazaki, K.; Azuma, I. Bovine Lactoferrin and Lactoferricin, a Peptide Derived from Bovine Lactoferrin, Inhibit Tumor Metastasis in Mice. Japanese Journal of Cancer Research 1997, 88, 184–190. [Google Scholar] [CrossRef]
- Eliassen, L.T.; Berge, G.; Leknessund, A.; Wikman, M.; Lindin, I.; Løkke, C.; Ponthan, F.; Johnsen, J.I.; Sveinbjørnsson, B.; Kogner, P.; Flægstad, T.; Rekdal, Y. The antimicrobial peptide, lactoferricin B, is cytotoxic to neuroblastoma cells in vitro and inhibits xenograft growth in vivo. International Journal of Cancer 2006, 119, 493–500. [Google Scholar] [CrossRef]
- Higazi, A.A.R.; Lavi, E.; Bdeir, K.; Ulrich, A.M.; Jamieson, D.G.; Rader, D.J.; Usher, D.C.; Kane, W.; Ganz, T.; Cines, D.B. Defensin Stimulates the Binding of Lipoprotein (a) to Human Vascular Endothelial and Smooth Muscle Cells. Blood 1997, 89, 4290–4298. [Google Scholar] [CrossRef]
- Higazi, A.A.; Nassar, T.; Ganz, T.; Rader, D.J.; Udassin, R.; Bdeir, K.; Hiss, E.; Sachais, B.S.; Williams, K.J.; Leitersdorf, E.; Cines, D.B. The alpha-defensins stimulate proteoglycan-dependent catabolism of low-density lipoprotein by vascular cells: a new class of inflammatory apolipoprotein and a possible contributor to atherogenesis. Blood, 2000; 96, 1393–1398. [Google Scholar] [CrossRef]
- Chavakis, T.; Cines, D.B.; Rhee, J.S.; Liang, O.D.; Schubert, U.; Hammes, H.P.; Higazi, A.A.R.; Nawroth, P.P.; Preissner, K.T.; Bdeir, K. Regulation of neovascularization by human neutrophil peptides (α-defensins): a link between inflammation and angiogenesis. Federation of American Societies for Experimental Biology 2004, 18, 1306–1308. [Google Scholar] [CrossRef]
- Edfelt, K.; Agerberth, B.; Rottenberg, M.E.; Gudmundsson, G.H.; Wang, X.B.; Mandal, K. Involvement of the antimicrobial peptide LL-37 in human atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology 2006, 26, 1551–1557. [Google Scholar] [CrossRef]
- Varoga, D.; Pufe, T.; Mentlein, R.; Kohrs, S.; Grohmann, S.; Tillmann, B.; Hassenpflug, J.; Paulsen, F. Expression and regulation of antimicrobial peptides in articular joints. Annals of Anatomy - Anatomischer Anzeiger 2005, 187, 499–508. [Google Scholar] [CrossRef]
- Mygind, P.H.; Fischer, R.L.; Schnorr, K.M.; Hansen, M.T.; Sönksen, C.P.; Ludvigsen, S.; Raventós, D.; Buskov, S.; Christensen, B.; De Maria, L.; Taboureau, O.; Yaver, D.; Elvig-Jørgensen, S.G.; Sørensen, M.V.; Christensen, B.E.; Kjærulff, S.; Frimodt-Moller, N.; Lehrer, R.I.; Zasloff, M.; Kristensen, H.H. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature 2005, 437, 975–980. [Google Scholar] [CrossRef]
- Paquette, D.W.; Simpson, D.M.; Friden, P.; Braman, V.; Williams, R.C. Safety and clinical effects of topical histatin gels in humans with experimental gingivitis. Journal of Clinical Periodontology 2002, 29, 1051–1058. [Google Scholar] [CrossRef]
- Review Editor, B. Micrologix Licenses Anti-Infective to Fujisawa. Pharma Deals Review 2002, (26). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





