Submitted:
06 December 2023
Posted:
07 December 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
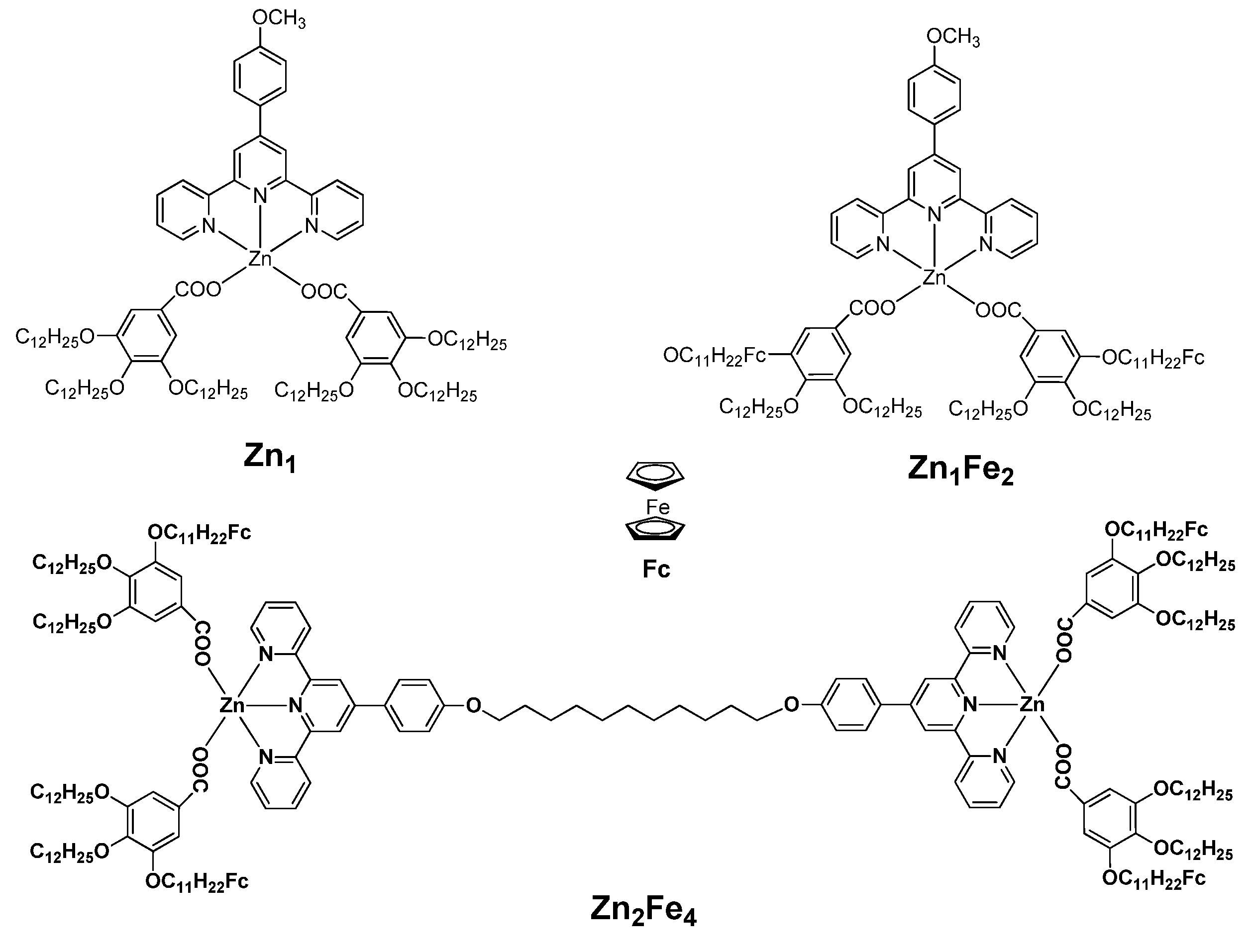
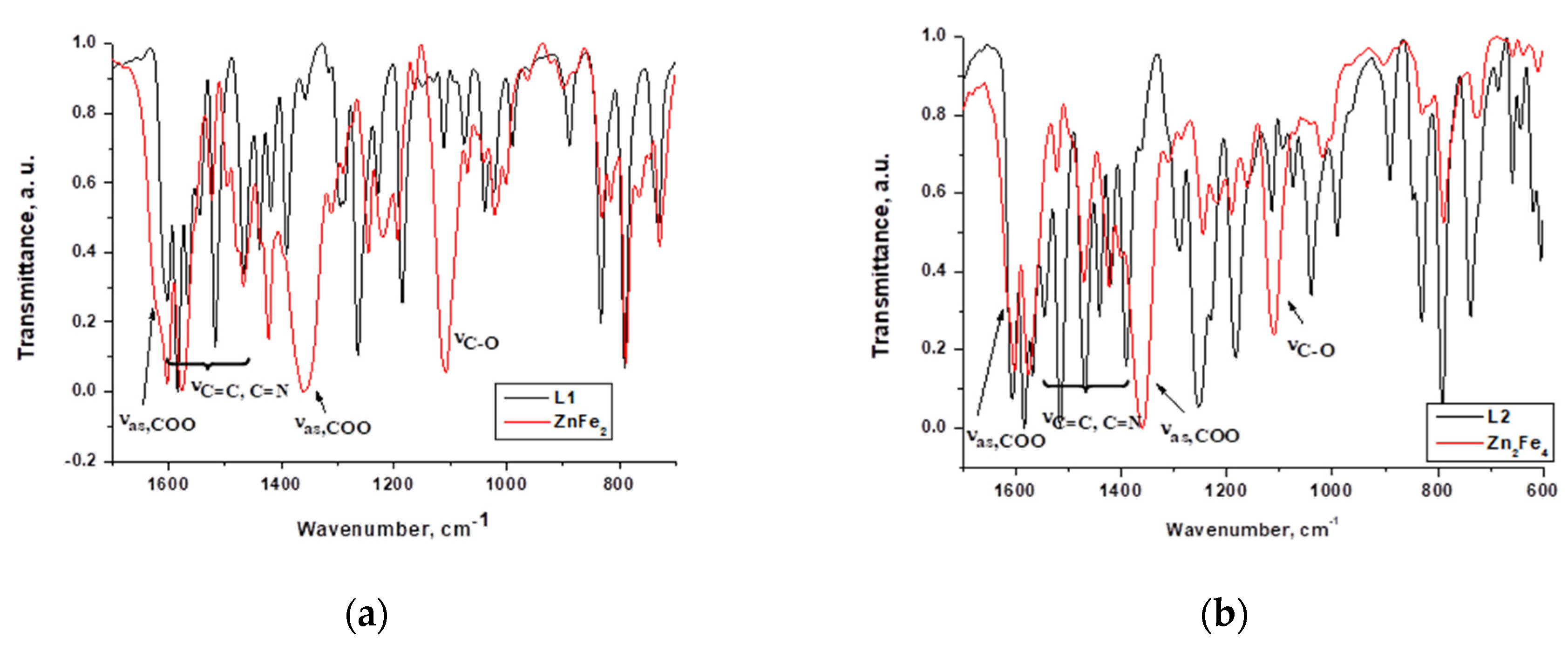
2.1. Synthesis and structural characterisation
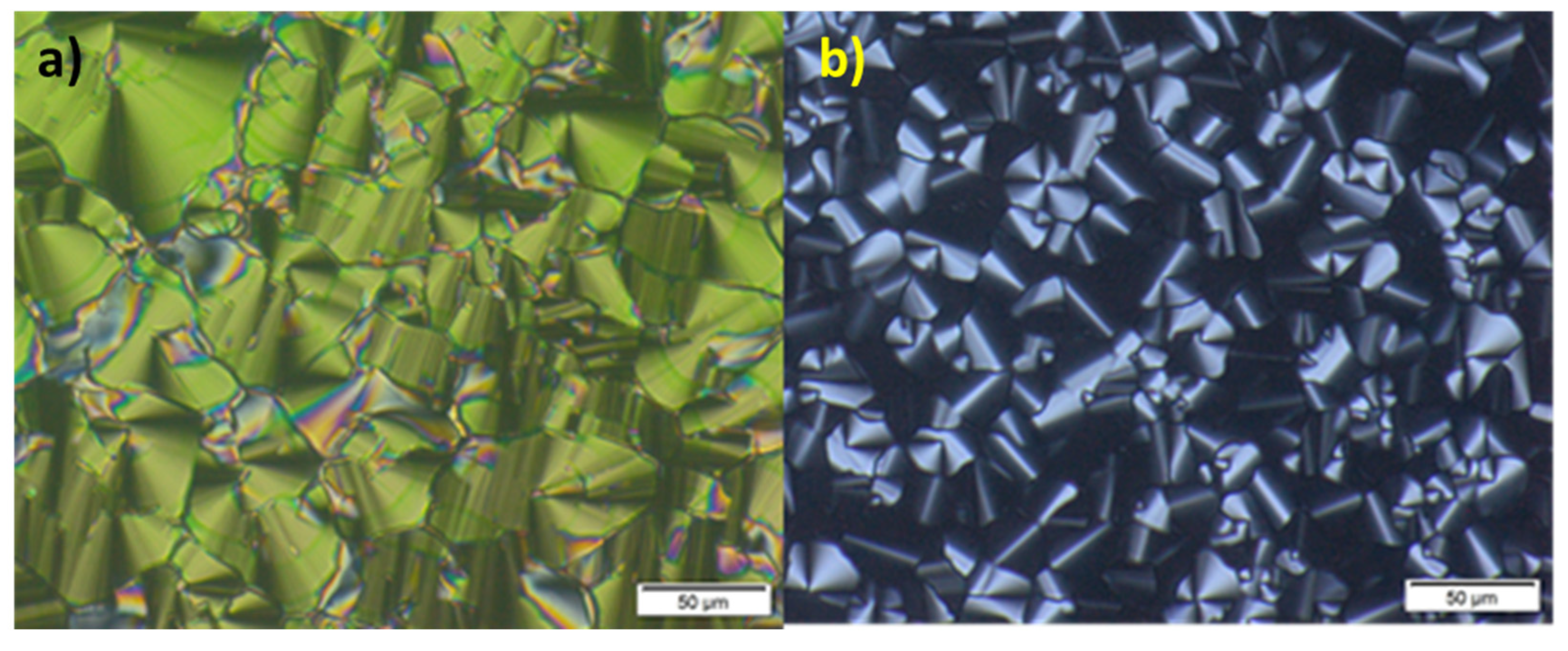
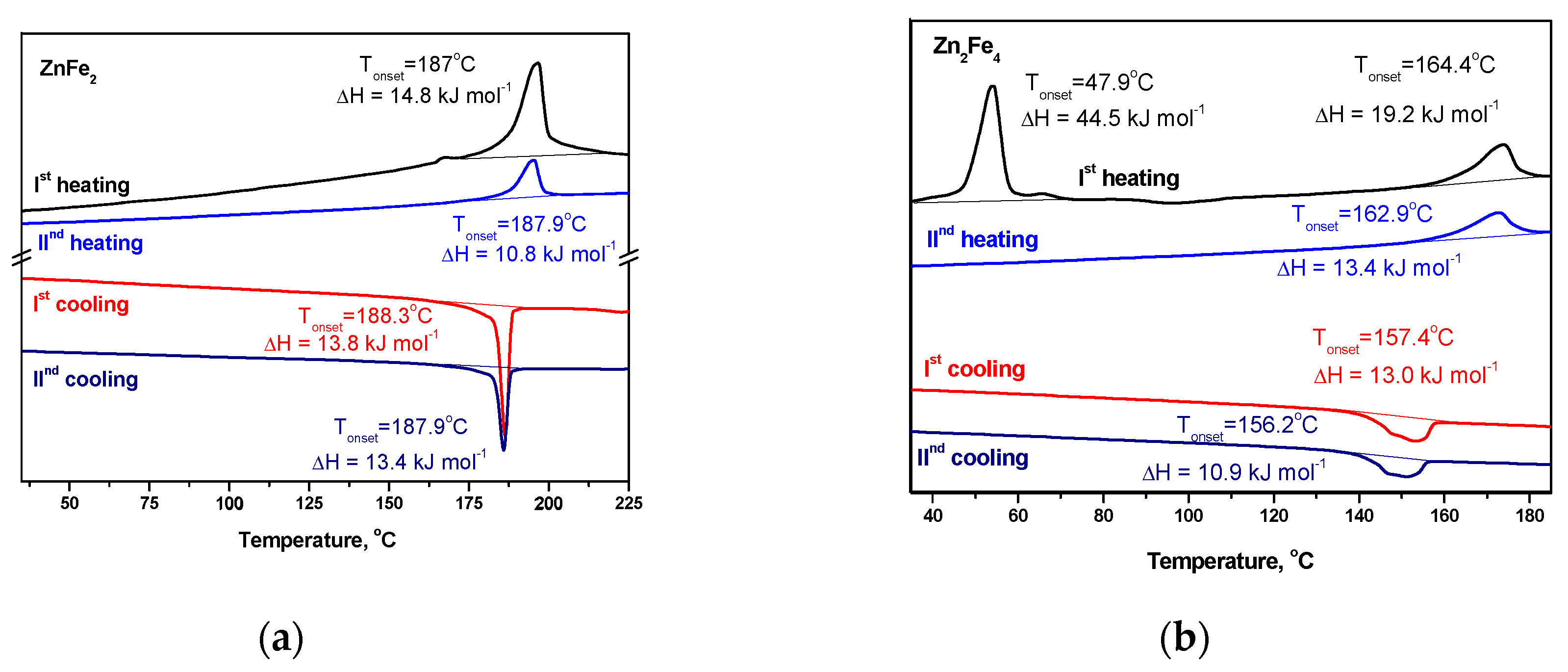
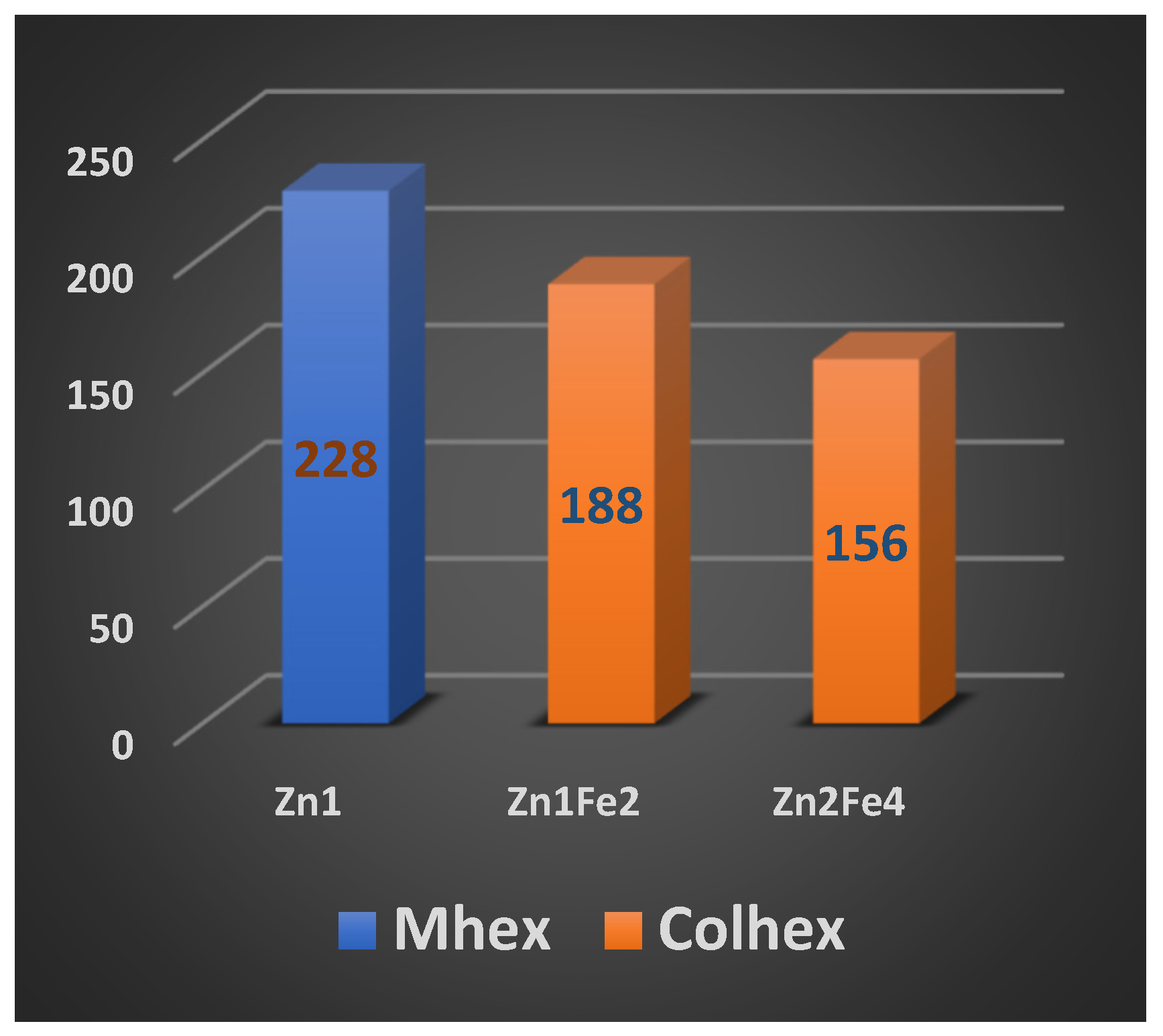
2.2. Mesomorphic properties
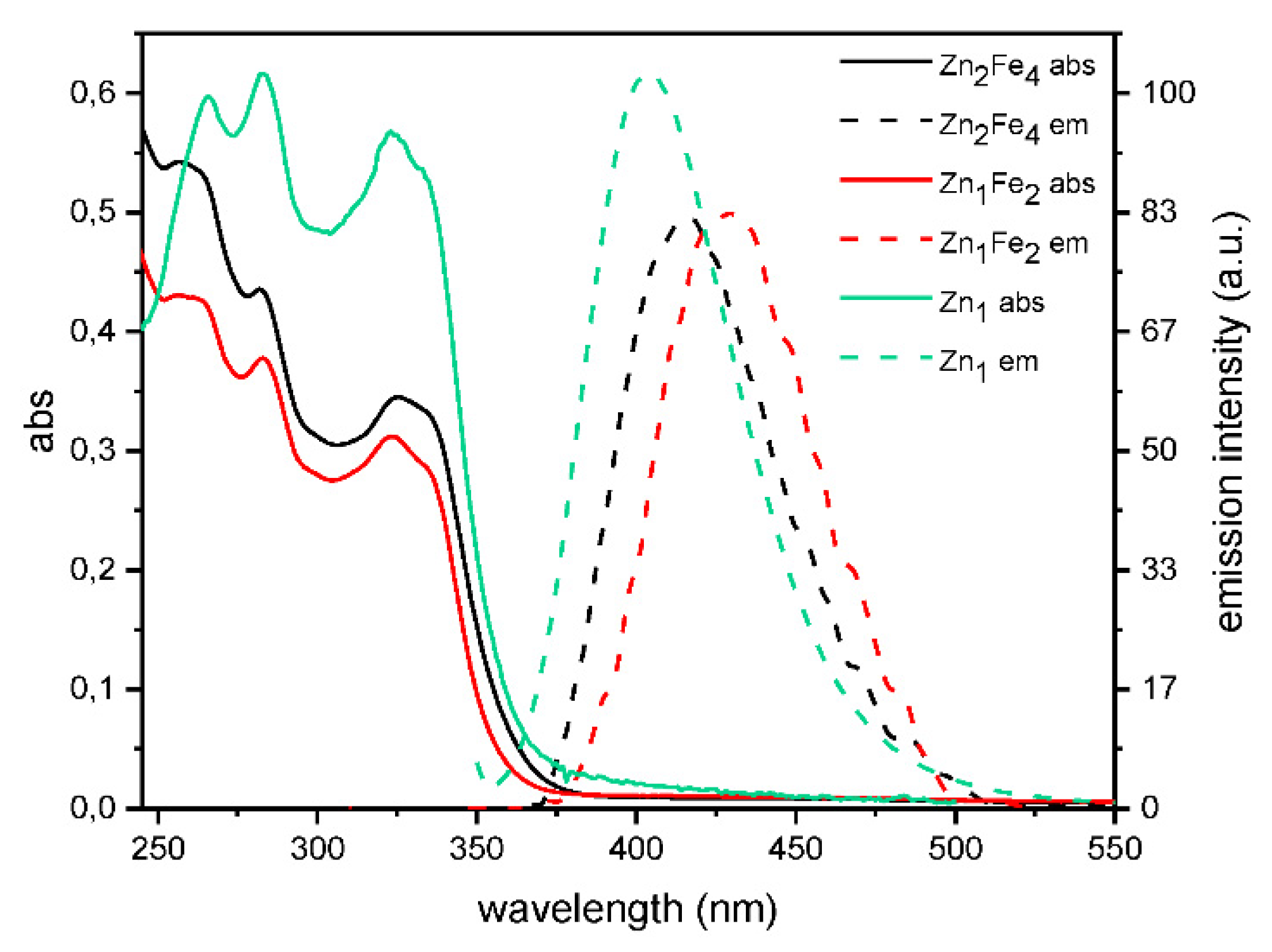
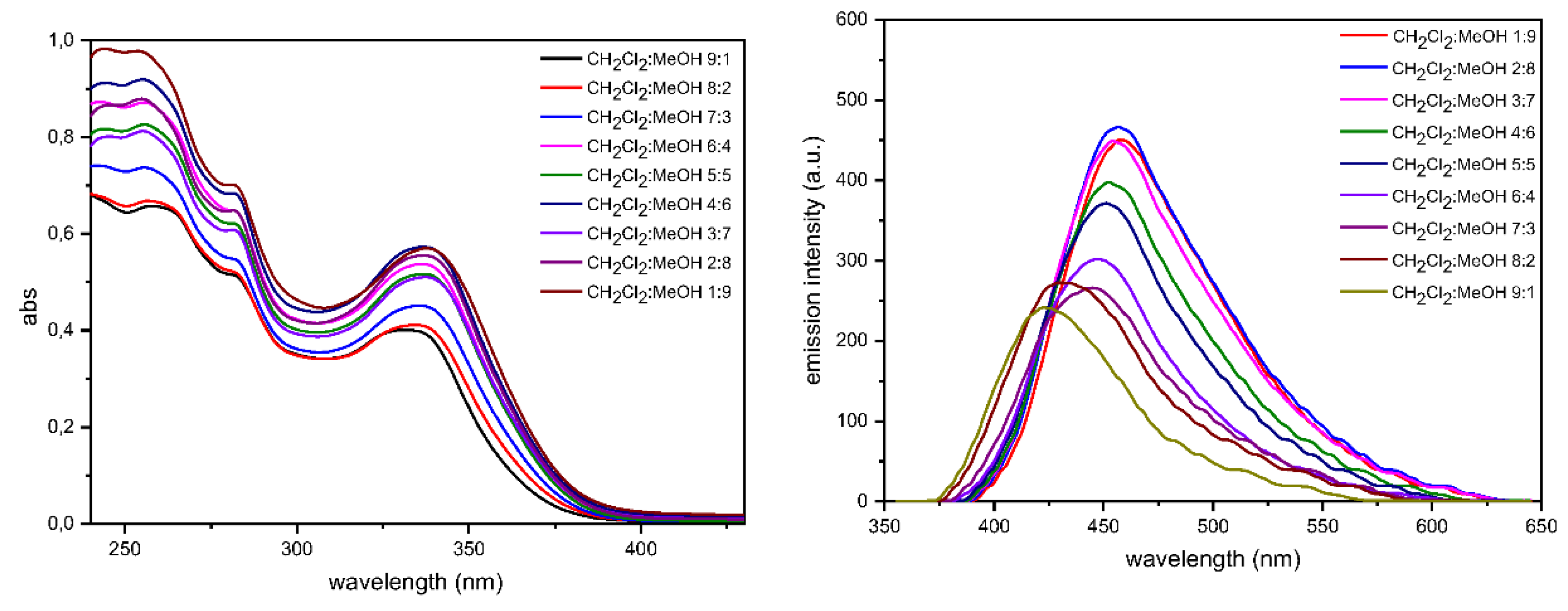
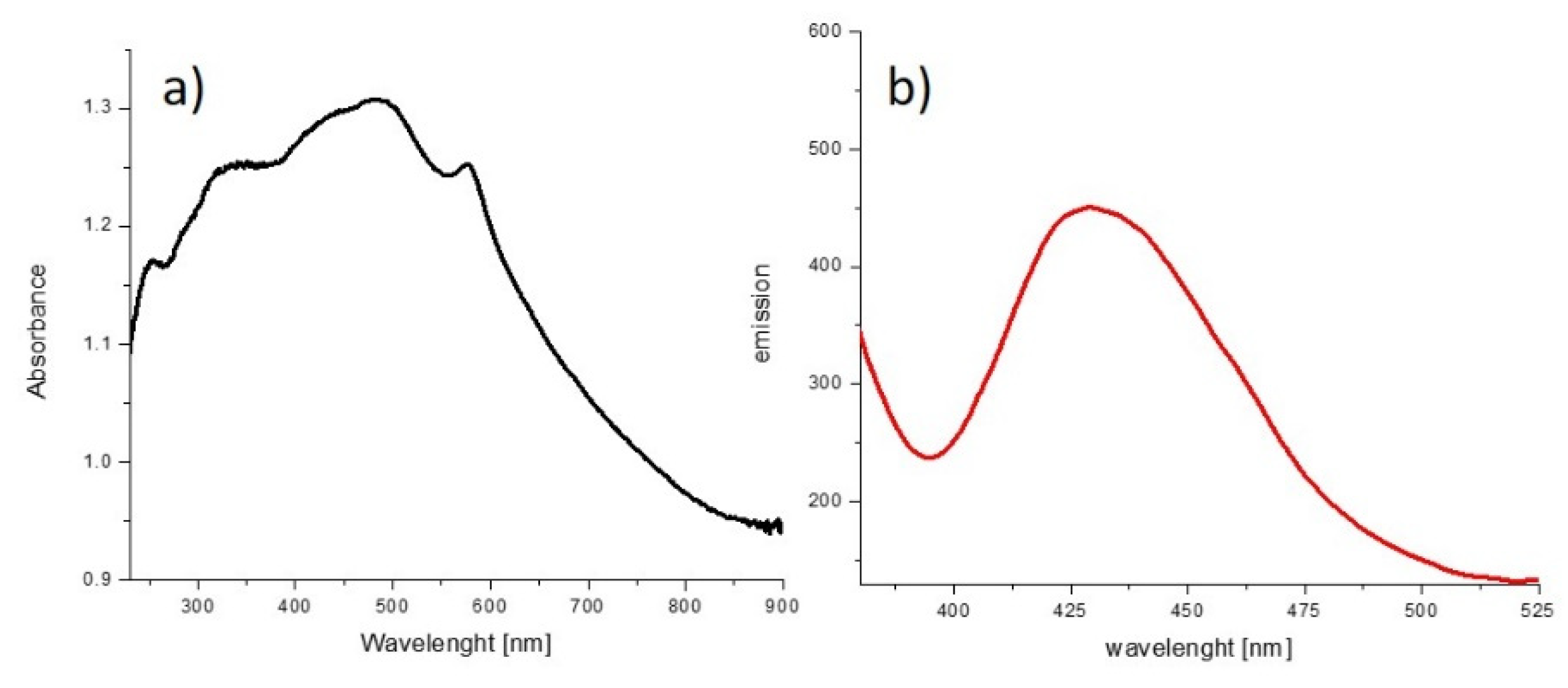
2.3. Photophysics
3. Discussion
4. Materials and Methods
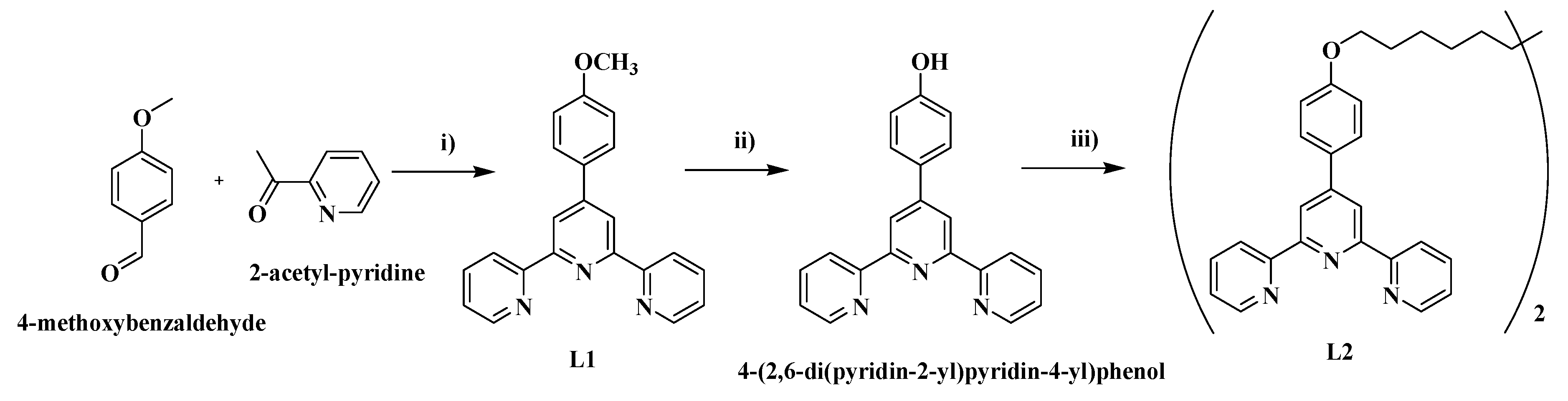
4.1. Synthesis of ligands
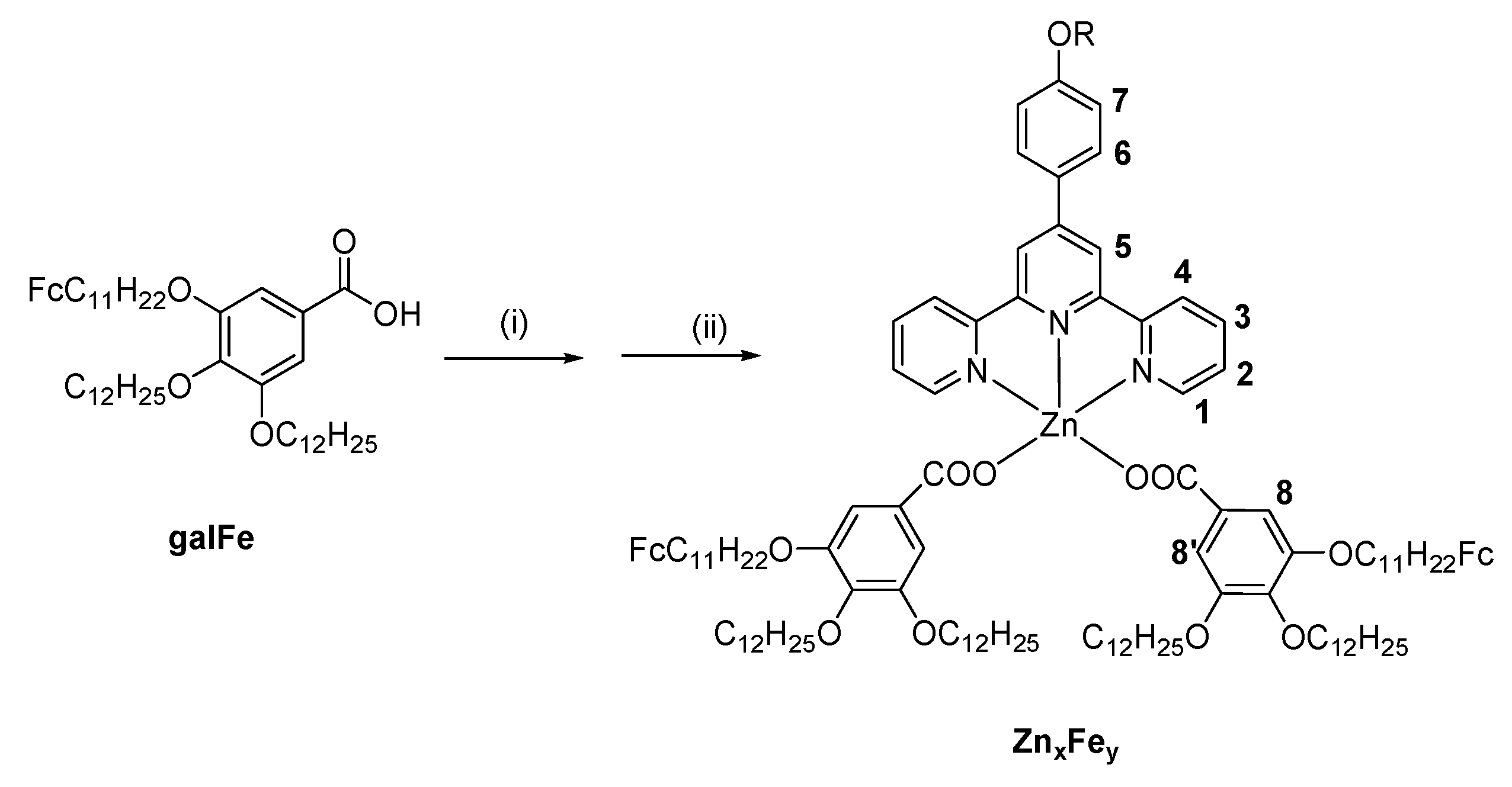
4.2. Synthesis of complexes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bizzarri, C.; Spuling, E.; Knoll, D.M.; Volz, D.; Bräse, S. Sustainable metal complexes for organic light-emitting diodes (OLEDs). Coord. Chem. Rev. 2018, 373, 49–82. [Google Scholar] [CrossRef]
- Zhang, Q.-C.; Xiao, H.; Zhang, X.; Xu, L.-J.; Chen, Z.-N. Luminescent oligonuclear metal complexes and the use in organic light-emitting diodes. Coord. Chem. Rev. 2019, 378, 121–133. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. TADF: Enabling luminescent copper(I) coordination compounds for light-emitting electrochemical cells. J. Mater. Chem. C 2022, 10, 4456–4482. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.; Haynes, C.J.E. Supramolecular Coordination Complexes as Optical Biosensors. ChemPlusChem 2021, 86, 418–433. [Google Scholar] [CrossRef] [PubMed]
- Pobłocki, K.; Drzeżdżon, J.; Kostrzewa, T.; Jacewicz, D. Coordination Complexes as a New Generation Photosensitizer for Photodynamic Anticancer Therapy. Int. J. Mol. Sci. 2021, 22, 8052. [Google Scholar] [CrossRef] [PubMed]
- Consiglio, G.; Oliveri, I.P.; Failla, S.; Di Bella, S. On the Aggregation and Sensing Properties of Zinc(II) Schiff Base Complexes of Salen-Type Ligands. Molecules 2019, 24, 2514. [Google Scholar] [CrossRef] [PubMed]
- Cuerva, C.; Cano, M.; Lodeiro, C. Advanced Functional Luminescent Metallomesogens: The Key Role of the Metal Center. Chem. Rev. 2021, 121, 12966–13010. [Google Scholar] [CrossRef] [PubMed]
- Diana, R.; Caruso, U.; Panunzi, B. Stimuli-Responsive Zinc (II) Coordination Polymers: A Novel Platform for Supramolecular Chromic Smart Tools. Polymers 2021, 13, 3712. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kato, M. Stimuli-Responsive Luminescent Copper(I) Complexes for Intelligent Emissive Devices. Chem. Letters 2017, 46, 154–162. [Google Scholar] [CrossRef]
- Li, K.; Chen, Y.; Wang, J.; Yang, C. Diverse emission properties of transition metal complexes beyond exclusive single phosphorescence and their wide applications. Coord. Chem. Rev. 2021, 433, 213755. [Google Scholar] [CrossRef]
- Szerb, E.I.; Crispini, A.; Aiello, I.; La Deda, M. in Springer Handbook of Inorganic Photochemistry, Bahnemann D.W., Patrocinio A.O.T. Eds. Part XII: Inorganic Materials for Optoelectronics, 62: Liquid Crystals, Section editor: Eli Zysman-Colman. 2022, pp. 1811–1848.
- De Sio, L.; Ricciardi, L.; Serak, S.; La Deda, M.; Tabiryan, N.; Umeton, C. Photo-sensitive liquid crystals for optically controlled diffraction gratings. J. Mater. Chem. 2012, 22, 6669–6673. [Google Scholar] [CrossRef]
- Panicker, R.R.; Sivaramakrishna, A. Remarkably flexible 2,2’:6’,2”-terpyridines and their group 8-10 transition metal complexes - Chemistry and applications. Coord. Chem. Rev. 2022, 459, 214426. [Google Scholar] [CrossRef]
- Saleh, D.A.; Rana, U.; Higuchi, M.; Sosnik, A. Luminescent amphiphilic nanogels by terpyridine-Zn(II) complexation of polymeric micelles. Mater. Today Chem. 2020, 18, 100359. [Google Scholar] [CrossRef]
- Yan, Y.; Yin, G.-Q.; Khalife, S.; He, Z.-H.; Xu, C.; Li, X. Self-assembly of emissive metallocycles with tetraphenylethylene, BODIPY and terpyridine in one system. Supramol. Chem. 2019, 31, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.D.; Dwivedi, B.K.; Paitandi, R.P.; Kumar, Y.; Pandey, D.S. Effect of substituents on photophysical and aggregation behaviour in quinoline based bis-terpyridine Zn(II) complexes. Inorganica Chim. Acta 2019, 487, 24–30. [Google Scholar] [CrossRef]
- Li, M.; Jiang, S.; Zhang, Z.; Hao, X.-Q.; Jiang, X.; Yu, H.; Wang, P.; Xu, B.; Wang, M.; Tian, W. Tetraphenylethylene-Based Emissive Supramolecular Metallacages Assembled by Terpyridine Ligands. CCS Chem. 2020, 2, 337–348. [Google Scholar] [CrossRef]
- Jung, S.H.; Kwon, K.-Y.; Jung, J.H. A turn-on fluorogenic Zn(II) chemoprobe based on a terpyridine derivative with aggregation-induced emission (AIE) effects through nanofiber aggregation into spherical aggregates. Chem. Commun. 2015, 51, 952–955. [Google Scholar] [CrossRef]
- Popa, E.; Andelescu, A.A.; Ilies, S.; Visan, A.; Cretu, C.; Scarpelli, F.; Crispini, A.; Manea, F.; Szerb, E.I. Hetero-Bimetallic Ferrocene-Containing Zinc(II)-Terpyridyl-Based Metallomesogen: Structural and Electrochemical Characterization. Materials 2023, 16, 1946. [Google Scholar] [CrossRef] [PubMed]
- Andelescu, A.A.; Ilies (b. Motoc), S.; Cretu, C.; Popa, E.; Marinescu, S.; Heinrich, B.; Manea, F.; Negrea, S.; Donnio, B.; Szerb, E.I. Pentacoordinated Liquid Crystalline Zn(II) Complex Organized in Smectic Mesophase: Synthesis, Structural and Electrochemical Properties. Appl. Sci. 2022, 12, 8306. [Google Scholar] [CrossRef]
- La Deda, M.; Di Maio, G.; Candreva, A.; Heinrich, B.; Andelescu, A.A.; Popa, E.; Voirin, E.; Badea, V.; Amati, M.; Costisor, O.; Donnio, B.; Szerb, E.I. Very intense polarized emission in self-assembled room temperature metallomesogens based on Zn(II) coordination complexes: an experimental and computational study. J. Mater. Chem. C 2022, 10, 115–125. [Google Scholar] [CrossRef]
- Lakshmanana, R.; Shivaprakash, N.C.; Sindhu, S. Switching from sky blue to deep green fluorescent Zn(II) complexes for OLEDs applications. J. Lumin. 2018, 196, 136–145. [Google Scholar] [CrossRef]
- Winter, A.; Friebe, C.; Chiper, M.; Schubert, U.S.; Presselt, M.; Dietzek, B.; Schmitt, M.; Popp, J. Synthesis, Characterization, and Electro-Optical Properties of ZnII Complexes withπ-Conjugated Terpyridine Ligands. ChemPhysChem 2009, 10, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.D.; Singh, R.S.; Paitandi, R.P.; Dwivedi, B.K.; Maiti, B.; Pandey, D.S. Solvent Dependent Self-Assembly and AIE in Zn(II) Complexes Containing Phenothiazine Based Terpyridine Ligand and Its Efficacy in Pyrophosphate Sensing. J. Phys. Chem. C 2018, 122, 5178–5187. [Google Scholar] [CrossRef]
- Kaushik, R.; Ghosh, A.; Jose, D.A. Simple terpyridine based Cu(II)/Zn(II) complexes for the selective fluorescent detection of H2S in aqueous medium. J. Lumin. 2016, 171, 112–117. [Google Scholar] [CrossRef]
- Das, D.; Sarkar, P.; Kumar, A.H.U.; Sutradhar, S.; Kotakonda, M.; Lokanath, N.K.; Ghosh, B.N. Nanomolar pyrophosphate detection in water using a zinc-terpyridine receptor and its applications in antiproliferative and antioxidant activity. J. Photochem. Photobiol. A 2023, 441, 114726. [Google Scholar] [CrossRef]
- Velugula, K.; Chinta, J.P. Silver nanoparticles ensemble with Zn(II) complex of terpyridine as a highly sensitive colorimetric assay for the detection of Arginine. Biosens. Bioelectron. 2017, 87, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.B.; Ni, S.T. Nanomolar pyrophosphate detection and nucleus staining in living cells with simple terpyridine-Zn(II) complexes. Sci. Rep. 2016, 6, 26477. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.W.; Zhang, Q.Q.; Huang, C.Z.; Li, Y.F. Luminescent Zn(II)-terpyridine metal-organic gel for visual recognition of anions. RSC Adv. 2015, 5, 2857–2860. [Google Scholar] [CrossRef]
- Shen, Y.; Shao, T.; Fang, B.; Du, W.; Zhang, M.Z.; Liu, J.J.; Liu, T.Y.; Tian, X.H.; Zhang, Q.; Wang, A.D.; Yang, J.X.; Wu, J.Y.; Tian, Y.P. Visualization mitochondrial DNA in living cells under superresolution microscopy using thiophene-based terpyridine Zn(II) complexes. Chem. Commun. 2018, 54, 11288–11291. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.W.; Hsu, H.C.; Chan, H.Y.; Fang, J.M. A Terpyridine Zinc Complex for Selective Detection of Lipid Pyrophosphates: A Model System for Monitoring Bacterial O- and N-Transglycosylations. J. Org. Chem. 2020, 85, 12747–12753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Qiu, Q.X.; Xiang, Q.; Ren, S.M. Two Zn(II)-organic frameworks based on “V”-shaped terpyridine ligand and dicarboxylic ligands: Fascinating architectures and efficient luminescent aqueous-phase dual-responsive detection. J. Solid State Chem. 2021, 294, 121849. [Google Scholar] [CrossRef]
- Negrea, S.; Andelescu, A.A.; Ilies (b. Motoc), S.; Cretu, C.; Cseh, L.; Rastei, M.; Donnio, B.; Szerb, E.I.; Manea, F. Design of Nanostructured Hybrid Electrodes Based on a Liquid Crystalline Zn(II) Coordination Complex-Carbon Nanotubes Composition for the Specific Electrochemical Sensing of Uric Acid. Nanomaterials 2022, 12, 4215. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhu, J.; Zhang, Y.; Xiao, N.; Guo, Z. DNA binding property, nuclease activity and cytotoxicity of Zn(II) complexes of terpyridine derivatives. Biometals 2009, 22, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Cao, Y.; Li, Q.; Guedes da Silva, M.F.C.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Synthesis, characterization, solid-state photo-luminescence and anti-tumor activity of zinc(II) 4-phenyl-terpyridine compounds. J. Inorg. Biochem. 2010, 104, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Busto, N.; Carrión, M.C.; Montanaro, S.; de Greñu, B.D.; Biver, T.; Jalón, F.A.; Manzano, B.R.; García, B. Targeting G-Quadruplex structures with Zn(II) terpyridine derivatives: a SAR study. Dalton Trans. 2020, 49, 13372–13385. [Google Scholar] [CrossRef] [PubMed]
- Gao, E.J.; Feng, Y.H.; Su, J.Q.; Meng, B.; Jia, B.; Qi, Z.Z.; Peng, T.T.; Zhu, M.C. Synthesis, characterization, DNA binding, apoptosis and molecular docking of three Mn(II), Zn(II) and Cu(II) complexes with terpyridine-based carboxylic acid. Appl. Organomet. Chem. 2018, 32, e4164. [Google Scholar] [CrossRef]
- Wang, H.; Cai, F.Z.; Feng, D.X.; Zhou, L.; Li, D.; Wei, Y.; Feng, Z.J.; Zhang, J.; He, J.; Wu, Y.J. Synthesis, crystal structure, photophysical property and bioimaging application of a series of Zn(II) terpyridine complexes. J. Mol. Struct. 2019, 1194, 157–162. [Google Scholar] [CrossRef]
- Kong, C.C.; Peng, M.H.; Shen, H.; Wang, Y.M.; Zhang, Q.; Wang, H.; Zhang, J.; Zhou, H.P.; Yang, J.X.; Wu, J.Y.; Tian, Y.P. A novel D-A type terpyridine-based carbazole Zn(II) complex with enhanced twophoton absorption and its bioimaging application. Dyes Pigm. 2015, 120, 328–334. [Google Scholar] [CrossRef]
- Du, W.; Pan, D.Y.; Xiang, P.; Xiong, C.Y.; Zhang, M.Z.; Zhang, Q.; Tian, Y.P.; Zhang, Z.P.; Chen, B.; Luo, K.; Gong, Q.Y.; Tian, X.H. Terpyridine Zn(II) Complexes with Azide Units for Visualization of Histone Deacetylation in Living Cells under STED Nanoscopy. ACS Sensors 2021, 6, 3978–3984. [Google Scholar] [CrossRef] [PubMed]
- Kröhnke, F. Synthesis 1976, 1-24.
- Chen, F.; Tian, Y.-K.; Chen, Y. Chem. Asian J. 2018, 13, 3169–3172. [CrossRef] [PubMed]
- Andelescu, A.-A.; Heinrich, B.; Spirache, M.A.; Voirin, E.; La Deda, M.; Di Maio, G.; Szerb, E.I.; Donnio, B.; Costisor, O. Playing with PtII and ZnII Coordination to Obtain Luminescent Metallomesogens. Chem. Eur. J. 2020, 26, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. (1999). Principles of Fluorescence Spectroscopy. Springer, Boston, MA.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




