1. Introduction
Heart failure (HF) is a chronic disease that results from the inability of the heart to perform its functions properly. It is a major cause of morbidity and mortality worldwide, and left ventricular (LV) diastolic dysfunction plays a leading role in this clinical context [
1,
2,
3,
4].
The importance of early assessment of LV diastolic function in the prevention of adverse outcomes has been discussed in several studies [
1,
2,
3,
4]. The realization that approximately half of individuals with signs and symptoms of heart failure do not have systolic dysfunction but diastolic dysfunction has highlighted the need for assertive and timely diagnosis of this clinical condition [
5,
6]. LV diastolic dysfunction is usually defined as abnormal left ventricular relaxation, which can be assessed and quantified using echocardiographic measurements and algorithms included in the American Society of Echocardiography/European Association of Cardiovascular Imaging (ASE/EACVI) guidelines [
7,
8].
Obesity is also a major cause of morbidity and mortality worldwide and is an independent risk factor for the development of heart disease. The increase and abnormal distribution of visceral fat in the myocardium and arteries has a strong correlation with cardiovascular disease [
9,
10]. Pericardial Fat (PF) is a type of fat that accumulates in the pericardium. Conversely, Epicardial Fat (EF) is a fat that accumulates between the visceral pericardium and the myocardium. When the accumulation of PF is excessive, it may compress the myocardium, resulting in reduced distensibility and cardiac remodeling. This can lead to diastolic dysfunction [
10]. Individuals with a high body mass index and obesity are therefore more likely to develop diastolic dysfunction [
11,
12]. Given the pathophysiology of these conditions, there may be an association between abdominal circumference, PF, EF, and LV diastolic dysfunction. Several studies have been carried out to assess this relationship [
1,
2,
13,
14].
Currently, the most effective and easy way to diagnose diastolic dysfunction is by transthoracic echocardiography using two-dimensional (2D) and Doppler echocardiography. For the assessment of diastolic function, the ASE/EACVI [
8] has developed two diagnostic algorithms based on echocardiographic parameters [
8].
A recent study conducted by De Wit-Verheggen VHW et al (2020) used magnetic resonance imaging to evaluate PF in healthy individuals. The participants were split into two groups depending on their PF levels. These groups were then compared based on their diastolic function which was evaluated using transthoracic echocardiography. The study discovered notable changes in certain parameters, such as E/e', E/A, septal e', and lateral e', which may be associated with LV relaxation and filling. However, the study also found a surprising decrease in the left atrium volume (LAV) instead of the expected increase in the presence of diastolic dysfunction. The authors propose that this may be due to a limitation in the increase of left atrium (LA) caused by excessive PF. They concluded that the presence of PF alters some echocardiographic parameters of diastolic dysfunction, but further research is required to fully understand this condition [
1].
Ma W et al (2021) conducted a study to evaluate the EF thickness and LV diastolic function in healthy subjects using transthoracic echocardiography. The study included two fat measurements: one taken at the length of the aortic root line perpendicular to the right ventricular free wall (EFT1), and one taken at the maximum fat thickness perpendicular to the right ventricular free wall (EFT2), both measured in end-diastole. The results showed that patients with higher EFT1 had an increase in E/e', and E/A and a decrease in septal e' and lateral e', indicating a correlation between PF and diastolic dysfunction. Furthermore, an independent negative correlation was observed between patients with higher EFT2 and a decrease in mean e', suggesting a greater correlation between maximal thickness measured perpendicular to the right ventricular free wall and diastolic dysfunction. The authors concluded that more attention should be paid to this type of fat [
2].
Despite the significant interest in the subject, few studies have been conducted to determine which echocardiographic parameters related to diastolic dysfunction change in the presence of increased PF and EF. The studies that have been conducted have shown significant variance in their findings [
15]. Therefore, this study becomes more important to standardize the findings.
This study aims to establish a correlation between abdominal circumference and PF and EF and the latter with LV diastolic performance so that, in the future, it will be possible to predict the development of diastolic dysfunction and consequent diastolic heart failure based on the measurement of abdominal circumference and PF and EF. These measurements are often underestimated and ignored in transthoracic echocardiography. In this study, we used routine transthoracic echocardiography to measure all echocardiographic parameters associated with diastolic function and then related them with the thickness of PF and EF, and the latter with the abdominal circumference [
1,
2,
3,
4].
2. Materials and Methods
2.1. Study Design
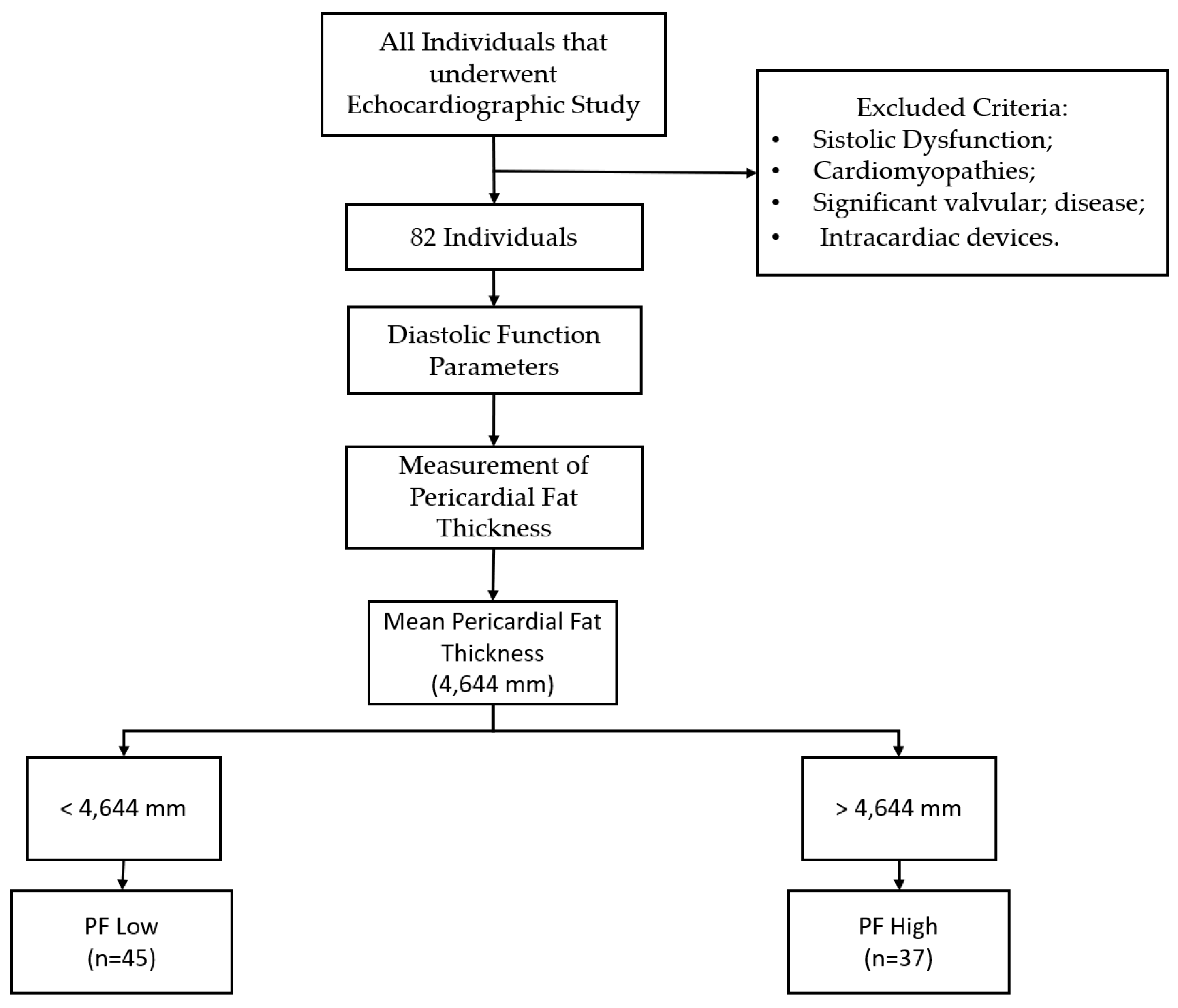
This study is cross-sectional, observational, prospective, and quantitative, with a non-probabilistic sample by rational choice, therefore including all healthy individuals who underwent an echocardiographic study for convenience in the echocardiography laboratory of the cardiology service of a local health unit in the central region of Portugal between August and December of 2022. All individuals with systolic dysfunction, cardiomyopathies, significant valvular pathology, or those with intracardiac devices were excluded from the study. Considering the above criteria, a sample of 82 individuals was collected.
2.2. Protocol
The data collection procedure was carried out in two distinct phases: the first phase involved the measurement of abdominal circumference with a tape measure and the second phase involved transthoracic echocardiography using a Toshiba® model Xario device with a 2.4 - 4.5 MHz frequency transducer.
The abdominal circumference was measured around the abdomen just above the navel. To standardize the method, obtain more accurate measurements, and reduce bias, this measurement was always performed by the same professional [
16,
17,
18].
A transthoracic echocardiogram was then performed according to the latest ASE/EACVI guidelines to measure PF and assess its functionality [
19]. PF and EF were measured through the parasternal long-axis window in the region of the free wall of the right ventricle, both in end-systole and using the average of three consecutive beats [
20].
The remaining echocardiographic measurements were indexed to the individual's surface area, so the left ventricular ejection fraction (LVEF) was determined using the biplanar Simpson method, and end-diastolic diameter and LV mass were determined using the M mode. The E wave, the E wave deceleration time (DT), and the E/A ratio were measured using pulsed Doppler with the cursor perpendicular to the mitral annulus and the sample positioned at the end of the mitral leaflets. LAV was quantified using the biplanar method. To measure septal e' and lateral e', two techniques were used simultaneously, namely pulsed Doppler and tissue Doppler, with the cursor positioned at the intersection of the interventricular septum and mitral annulus and the intersection of the LV lateral wall and mitral annulus, respectively. In this way, it was also possible to obtain the E/e' ratio. Continuous Doppler was used to measure tricuspid regurgitant jet velocity (TRJV) by aligning the cursor with the tricuspid regurgitant jet and recording its maximum velocity. The diagnosis of diastolic dysfunction was made using the ASE/EACVI guidelines' algorithm for individuals with preserved ejection fraction. To confirm this diagnosis, at least three of the four echocardiographic parameters needed to be positive: LAV, E/e' ratio, lateral e' or septal e', and TRJV [
8].
After collecting all variables, the sample was divided into two groups based on their mean PF thickness. This was done for better comparison and easy interpretation as it simplifies complex data, making it more accessible for understanding. The threshold we used was 4.644 mm, which represents the real value of our sample, making it more distributable. The minimum thickness observed was 1.4 mm, while the maximum was 11.6 mm.
Figure 1.
Sample division into two groups based on mean PF thickness.
Figure 1.
Sample division into two groups based on mean PF thickness.
2.3. Study Variables
To achieve the study's objective, we gathered various quantitative echocardiographic variables, including the diameter of the PF and EF, LAV, LVEF, E/A ratio, E/e' ratio, E-wave velocity, TRJV, DT, septal e', lateral e', LV mass, and LV end-diastolic diameter. Sample elements, such as sex, race, age, weight, height, BMI, and waist circumference were also collected to help characterize the data.
2.4. Statistical Analysis
To assess the normality of the sample distribution, we used the Kolmogorov-Smirnov normality test. We conducted the Student’s t-test to compare the two groups of PF with quantitative echocardiographic variables, including E wave, DT, septal e', lateral e', E/e' ratio, LAV, Ejection fraction, Mass of LV and LV diameter. Additionally, we used the Mann-Whitney test to compare the E/A ratio and TRJV. The Spearman correlation test was used to investigate the correlations between abdominal circumference and PF and EF, as well as between echocardiographic parameters of diastolic function and both fats under study. To assess the independent association of PF and echocardiographic parameters of diastolic dysfunction, a multivariable linear regression analysis was performed with either septal e’, or LAV, or TRJV, or LVEF, or LV diameter, or LV mass as the dependent variable. These models were adjusted for sex, age and BMI. To better understand the relationship between the prevalence of diastolic function diagnosis and the two groups of PF, we performed a Chi-square test.
For the statistical treatment of qualitative variables, a descriptive analysis was carried out using relative (%), absolute (n) frequencies, and measures of central tendency (mean) and dispersion (standard deviation).
The data obtained from the sample was analyzed and processed using IBM SPSS Statistics® (Statistical Package for the Social Sciences) version 27, a statistical analysis software. A p-value of 0.05 or less was considered statistically significant for all tests.
2.5. Ethics
The research work began after obtaining clearance from the Ethics Committee of the Cardiology Service where the study was conducted, with approval number 98/2022. This study has strictly adhered to ethical principles and ensured the confidentiality of all data, results, and interpretations. Personal data was collected only when necessary for the study, and all information gathered was treated as confidential. The data collected was solely used for academic purposes within the context of the research.
The research team declares that it has no conflicts of interest and is committed to respecting the principles expressed in the Declaration of Helsinki. This research does not have any profit or commercial purposes.
3. Results
The study sample consists of 82 Caucasian individuals, corresponding to 39 female individuals (48%) and 43 male individuals (52%). After analyzing the age distribution of all individuals, it was discovered that their ages ranged from 20 to 78 years, with an average age of 58 ± 12,8 years. The majority of individuals, n=29 (35.4%), were found to be in the age group of 60 to 69 years old (
Table 1).
Regarding the physical characteristics of the individuals, their average weight was 79.56±25.8 kg, (45 kg - 175 kg). Their average height was 164.85±9.50 cm (147 - 198 cm). The average BMI was 29.17±8.49 kg/m² (18.29 - 68.68 kg/m²). Among the BMI classes, overweight was the most common, accounting for 36.6% of the sample (n=30) In addition the sample had an average abdominal circumference of 94.8 cm ± 13.3 cm (64 - 125 cm) (
Table 1).
3.1. Abdominal Circumference and Pericardial and Epicardial Fat
Out of a total of 82 individuals, 45 belong to the PF Low group, which accounts for 54.9% of the total. The remaining 37 individuals belong to the PF High group, which accounts for 45.1% of the total. On average, the PF Low group had a PF thickness of 3.1 ± 0.9 mm, (1.4 - 4.5 mm). The same group also had an average EF thickness of 3.6 ± 1.4 mm, (1,5 - 7.1 mm). The PF High group had an average PF thickness of 6.5 ± 1.8 mm (4.7 - 11.6 mm) and an average EF thickness of 7.1 mm ± 2.6 mm, (2,1 - 13.1 mm) (
Table 2).
Individuals belonging to the PF High group had an average abdominal circumference value of 101.07cm, while those belonging to the PF Low group had an average value of 89.62cm (p <0.0001) (
Table 1).
3.2. Association between Abdominal Circumference and Age Group
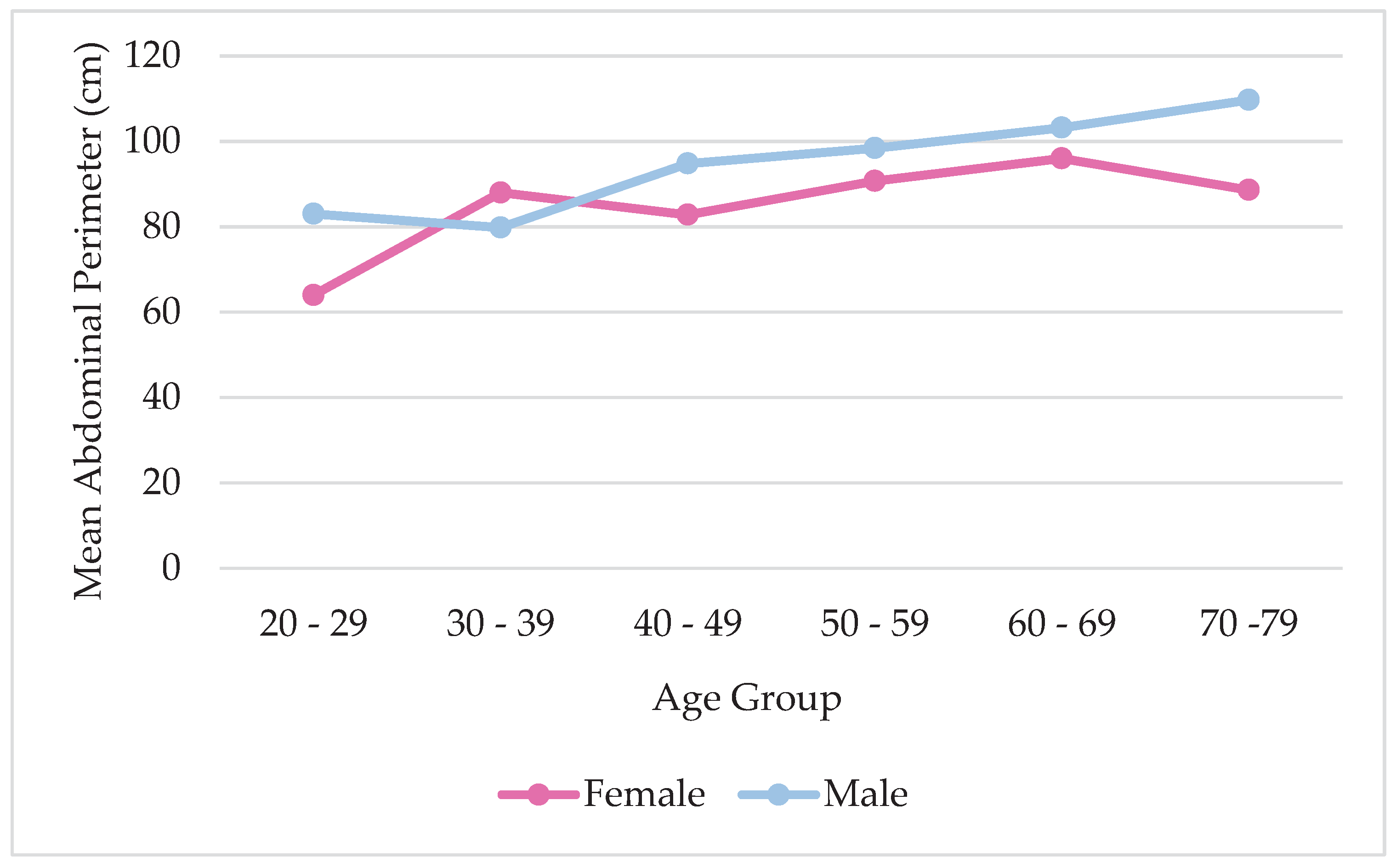
An analysis was conducted to better understand the relationship between abdominal circumference and age for each sex.
Graph 1 displays the distribution of average abdominal circumference by age group and sex. As it is observed, males have a higher mean abdominal perimeter than females and it increases with the increase of age (p<0,0001). A slight difference in the age group of 30-39 where the female has a higher mean abdominal circumference, but not statistically significant.
Graph 1.
Distribution of Abdominal Perimeter by Age.
Graph 1.
Distribution of Abdominal Perimeter by Age.
3.3. Assessment of echocardiographic variables and PF
According to
Table 3, individuals in PF High had higher DT values (0.21 ms) than those in PF Low (0.18 ms), this difference was statistically significant (p=0.013). The same trend was observed in the E/e' ratio, where individuals with high levels of PF had a higher mean value (8.48) compared to those with low levels (6.40) (p<0.0001).
The high PF group had a decrease in the E/A ratio (0.94), while the low PF group had a higher E/A ratio of 1.10, indicating a statistically significant difference (p=0.003).
The study found that individuals in the PF High group had lower mean values of septal e' (6.64 cm/s) and lateral e' (10.12 cm/s) compared to those in the PF Low (9.58 cm/s and 13.23 cm/s). This difference was statistically significant (p<0.0001 and p<0.0001).
Based on the observations made, it was found that individuals categorized as PF High had a higher LAV, with an average of 41.33 ml/m², compared to the PF Low group, whose average was 32.8 ml/m². This difference was statistically significant. Similarly, TRJV was higher in the PF High group compared to the PF Low group (2.65 vs. 2.32 cm/s, p < 0.0001).
There were statistically significant differences in LV diameter and LV mass between the two groups (p=0.014 and p=0.003). The group with high levels of PF had an average LV diameter and LV mass of 54.89 mm and 183.57 g, respectively. On the other hand, the group with low levels of PF had an average LV diameter of 52.07mm and an LV mass of 149.64 g.
In terms of LVEF, it was found that the group with high levels of PF had slightly lower mean values compared to the group with low levels of PF. However, this difference was not statistically significant (p=0.093). A similar trend was observed for the echocardiographic parameter E, where the mean value was higher in the group with low levels of PF compared to the group with high levels of PF (p=0.359).
3.4. Association between Abdominal Circumference and PF and EF Thickness
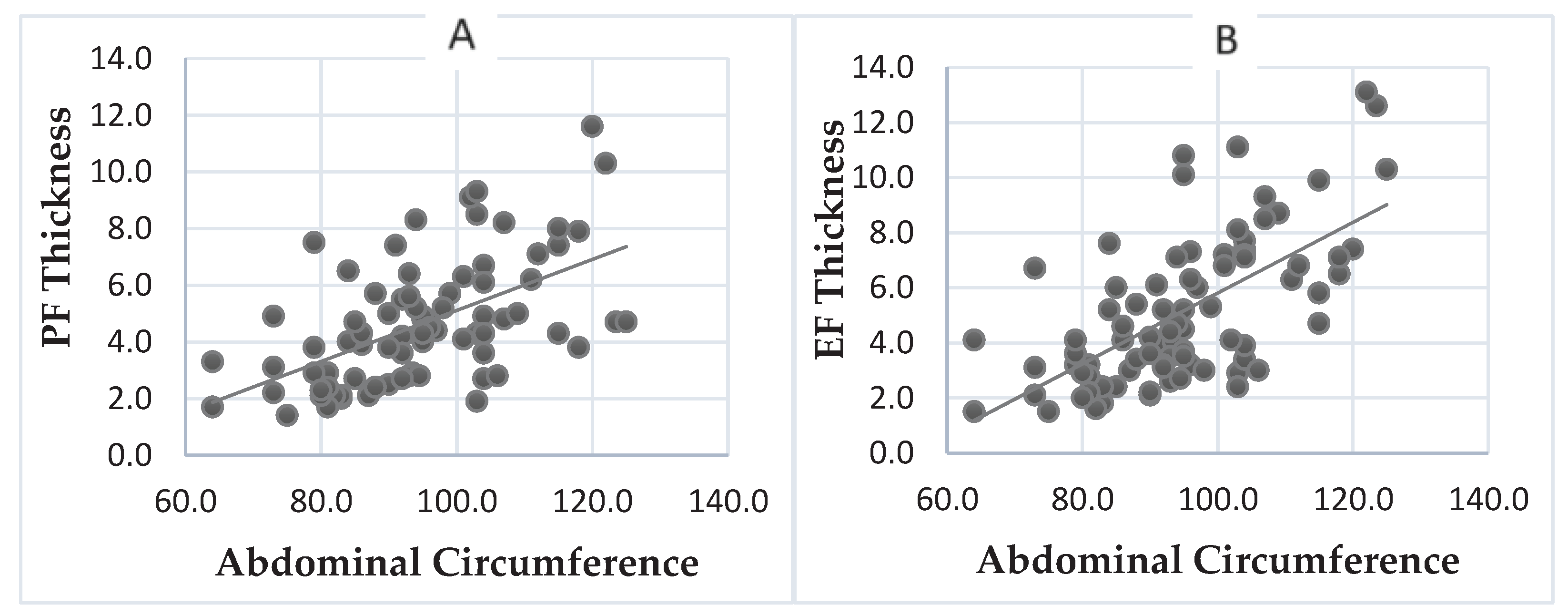
The Spearman correlation test was also used to understand better the association between abdominal circumference and PF and EF.
In
Graph 2, we can see a clear correlation between the abdominal circumference and the amount of fat around the heart. This is shown through a scatter plot, where the abdominal circumference is the independent variable (x) and PF and EF are the dependent variables (y) in Image A and B, respectively. The blue dots on the graph represent the measurements of the abdominal circumference and fat around the heart for each individual. By using the straight line, we can observe that as the abdominal circumference increases, both PF and EF also increase. These changes are statistically significant (p<0.0001).
Graph 2.
Association between Abdominal Perimeter and Pericardial (A) and Epicardial (B) Fat.
Graph 2.
Association between Abdominal Perimeter and Pericardial (A) and Epicardial (B) Fat.
3.5. Association of echocardiographic variables with Pericardial Fat vs. Epicardial Fat
In
Table 4, we present the characterization of the echocardiographic variables that were studied, including the E/A ratio, E, DT, TRJV, LV diameter, septal e', lateral e', E/e' ratio, LAV, LVEF, and LV mass. These variables were analyzed concerning the PF and EF of all individuals. To better understand this association, the Spearman correlation test was used.
It has been observed that an increase in PF leads to a significant increase in the variables DT (p=0,045), E/e' (p<0,0001), LAV (p<0,0001), LV mass (p=0,001), TRJV (p<0,0001), and LV diameter (p=0,041), indicating a positive correlation between PF and these variables. Conversely, a decrease in E/A (p=0,001), e' septal (p<0,0001), and e' lateral (p<0,0001) has also been observed, indicating a negative correlation between these variables and PF (
Table 4). An increase in EF showed a positive correlation with variables such as E/e', LAV, LV mass, TRJV, and LV diameter. On the other hand, it showed a negative correlation with E/A, e' septal, and e' lateral.
3.6. Association between Pericardial Fat and Echocardiographic Variables, Multivariable Adjustment of Co-factors
Given the sensibility of the diastolic parameter and the wide range of ages, a multivariable linear regression analysis was conducted to examine the relationship between PF and the echocardiographic variables in the study while controlling for the effects of sex, age and BMI. After adjusting for these variables, all six parameters (septal e’, LAV, TRJV, LVEF, LV diameter, and LV mass) were found to be statistically significant as depicted in
Table 5.
3.7. Assessment between Pericardial Fat and the diagnosis of Diastolic Function
In
Table 6, the relationship between the diagnosis of diastolic function and two groups of PF is demonstrated. To analyze this relationship in a better way, the Chi-square test was utilized. The table shows the number of individuals diagnosed with normal, undetermined diastolic function, and diastolic dysfunction. It also highlights the significance that exists between both groups.
4. Discussion
The measurement of PF is often overlooked during routine transthoracic echocardiography, as it is not a usual evaluation. However, PF thickness is a crucial predictor of numerous adverse cardiovascular outcomes and should not be ignored. It is important to note that it can indicate cardiovascular risk and should be carefully evaluated by clinicians.
4.1. Sample Characterization
The participants in this study were evenly split between genders and across PF groups. The majority of participants were older, with the most common age range being 60-69. This is likely due to the increased need for healthcare in this age group. Additionally, most participants were overweight, which is a common characteristic of the population in the central region of Portugal. These findings align with data from the 2018 Central Region of Portugal health profile, which showed a high prevalence of overweight individuals in the area [
21].
4.2. Association of Abdominal Circumference with Age in Male and Female
The study also analyzed waist circumference, which was found to be greater in participants with higher levels of PF and EF. Results showed that abdominal circumference increased with age in both sexes, with males having higher index values. This is in line with previous research by Jennifer L Kuk et al. (2005), which suggests that metabolic changes that occur with age may explain this trend. The study also noted that hormonal differences between genders contribute to differences in abdominal circumference, which justifies the need for different cut-offs for males and females [
22,
23].
4.3. Relationship between Pericardial Fat and Abdominal Circumference
As per the literature and the results obtained, a significant increase in abdominal circumference was observed in individuals belonging to the PF High group. Amir Mahabadi et al. (2009) explain these findings by the decrease in the production of adiponectin, which is a stabilizing hormone that inhibits NF-kB, in the presence of an increase in visceral fat. Due to this decrease, the activation of NF-kB increases, which leads to the production of TNF-a, causing more local inflammation and molecular aggregation. As a result, there is an increase in fat around the heart with an increase in visceral fat [
24].
Several studies have found a positive correlation between abdominal circumference and PF and EF. This correlation confirms that waist circumference can be a predictive factor for increased fat around the heart [
25,
26].
4.4. Assessment of Echocardiographic Variables and Pericardial Fat
To investigate the relationship between PF and echocardiographic parameters, two groups were compared based on their PF and echocardiographic variables. The results showed that individuals with greater PF (PF High) had significantly lower mean values of septal e', lateral e', and the E/A ratio while showing an increase in the mean values of E/e' ratio, LAV, LV mass, TRJV, and LV diameter. These findings are consistent with a similar study by Vera H. W. de Wit Verheggen et al. (2020) that also separated their sample into two PF groups, and found that higher PF thickness influences some of the parameters of LV diastolic function, leading to a decrease in the mean values of septal e', lateral e', and the E/A ratio, as well as an increase in the E/e' ratio, LAV, LV mass, and LV diameter [
1,
11].
The E/e' ratio, septal e', and lateral e' are echocardiographic parameters that indicate the speed of left ventricular (LV) relaxation, which decreases due to some compressive condition affecting normal functioning, thus reducing its speed [
11,
27]. It is credible to assume that deregulation in the production of anti-inflammatory and anti-atherogenic cells, deficiency in the mediating enzyme of the renin-angiotensin-aldosterone system, and the migration of fibrosis-inducing cells are the mechanisms behind the development of atherosclerosis and consequent myocardial dysfunction. This suggests that the fat surrounding the heart could potentially trigger cardiovascular diseases. The compression of EF and PF on the myocardium has a significant impact on ventricular relaxation, leading to cardiac remodeling [
28].
The E/A ratio and DT are two crucial parameters used to measure ventricular filling. When PF increases, both of these parameters tend to decrease and increase, respectively. Several studies by Vera H. W. de Wit Verheggen et al. (2020) [
1] and Iacobellis et al. (2007) [
29] reaffirm these results. According to Jin Seok Kim et al. (2021) [
30], the E/A ratio decreases due to excess fat, which exerts a compressive mechanical force on the LV, affecting its normal relaxation. This leads to an increase in pressure within the LV at the start of diastole, which affects the filling of the LV, causing slower passive filling (E wave) and faster active filling (A wave). This, in turn, increases the volume retained in the LA, leading to atrial dilatation. The increase in pressure also prolongs passive filling, increasing DT [
30,
31]. The infiltration of EF in the auricular wall not only predisposes the cavity to enlargement but also to the development of atrial fibrillation [
32].
As a result of the increased difficulty that the right ventricle faces in overcoming the increase in pulmonary pressures during systole, the condition also affects the TRJV. As LV diastolic dysfunction worsens, the tricuspid reflux velocity increases progressively, making it a good marker of the hemodynamic impact of deficient left ventricular relaxation [
33].
According to the study, individuals in the PF High group showed higher mean values of LV mass and LV diameter. This finding is consistent with another investigation published in 2021. The study explains that this relationship is due to the predisposition of these individuals to have greater blood volume because of the higher metabolic demand they face. This, in turn, leads to greater cardiac output [
34]. The increase in ventricular mass occurs as a result of the need to compensate for underlying hemodynamic changes, an increase in LV volume due to an increase in afterload, and as an adaptive mechanism in the face of changes in diastolic function. These changes are characteristic of individuals with high abdominal circumference [
35].
The study also tested the relationship between systolic function and PF. Although LVEF showed lower mean values among individuals with higher PF, the relationship was not statistically significant between groups. This is unlike the findings of Gijs Van Woerden et al. (2018) [
36] who concluded that there is a statistically significant correlation between PF and LVEF. The author explains that while there is a decrease in cardiac output as a result of deficient ventricular filling [
36,
37], fat produces high amounts of pro-inflammatory cytokines due to its contact with the cardiac surface. This results in damage to the myocardial cells. Additionally, fat promotes the abundance of free fatty acids, which, when integrated into the myocardial cell, promote toxic metabolism, leading to cell apoptosis (lipotoxicity) [
38,
39]. One possible explanation for the difference in results found in various studies could be that the values of PF thickness in the sample were lower than those found in other studies.
The relationship between fat and systolic ventricular function has been studied over time. Recently, a study using speckle tracking (transthoracic echocardiogram modality) tested the correlation between fat and systolic ventricular function in individuals with preserved left ventricular function. The results revealed a positive correlation between the two [
40,
41].
4.5. Association of Echocardiographic Variables with Pericardial Fat vs. Epicardial Fat
The study aimed to examine the relationship between PF and EF and echocardiographic variables. The analysis showed that PF had a strong correlation with nine echocardiographic variables, including DT, E/e', LAV, LV Mass, TRJV, and LV Diam, which increased significantly, and E/A, septal e', and lateral e', which decreased significantly. Similarly, EF was found to have a statistically significant correlation with eight echocardiographic variables, including E/e', LAV, LV Mass, TRJV, and LV Diam, which increased significantly, and E/A, septal e', and lateral e', which decreased significantly. These findings suggest that PF has a greater impact on echocardiographic variables related to diastolic function, while EF has a greater influence on left ventricular systolic function.
The results of this study are consistent with the findings of Vera H. W. de Wit Verheggen et al. (2020)(1), who explained that PF exerts compressive mechanisms on ventricular relaxation due to its location. In contrast, Banafsheh Arshi et al. (2023) stated that EF, which is closer to the myocardium, affects systolic function due to the pro-inflammatory cytokines it releases in the myocardium, in addition to its effect on diastolic function. While LVEF was not statistically significant in this study, its values decreased substantially when correlated with EF, compared to when correlated with PF [
41].
4.6. Association between Pericardial Fat and Echocardiographic Variables, Multivariable Adjustment of Co-factors
We found that PF is significantly associated with several diastolic function parameters, including septal e', LAV, TRJV, LVEF, LV diameter, and LV mass, even after adjusting for sex, age, and BMI. These associations suggest that PF has an impact on these diastolic function parameters, despite the wide range of ages in the study. As noted by Okura H. et al. (2009) [
42], the diastolic function can be sensitive to other comorbidities, such as age and sex. Vera H. W. de Wit Verheggen et al. (2020) [
1] also found significant associations between PF and septal e', lateral e', LAV, TRJV, and E/e' after adjusting for sex, age, and BMI. However, they did not find any significant correlations with LVEF, LV diameter, or LV mass. These variations in results highlight the differences that exist between studies [
1,
42].
4.7. Assessment between PF and the diagnosis of Diastolic.Dysfunction
The study found a correlation between PF and the diagnosis of diastolic function. The PF High group had a significantly higher percentage (45.9%) of individuals diagnosed with diastolic dysfunction compared to the PF Low group (6.7%). The majority of the PF Low group (93.3%) had a normal or undetermined diastolic function.
4.8. Limitations
The investigation was limited by insufficient information on participant history, risk factors, and medication use, as well as a lack of age differentiation, which could impact results.
5. Conclusions
Individuals with higher levels of PF tend to have a larger abdominal circumference, indicating poorer LV diastolic performance. Monitoring the abdominal circumference can thus help identify a higher concentration of fat around the heart (PF and EF), which in turn can be an early warning sign of possible changes in diastole. The good news is that measuring the abdominal circumference is a simple task for clinicians, and transthoracic echocardiography is the most accurate way to assess the amount of fat surrounding the heart. By adopting this practice, healthcare providers can help prevent the development and progression of diastolic heart failure. This not only has a direct impact on the future quality of life of these individuals but also reduces the burden on healthcare systems. Based on the research findings, it can be concluded that PF has a greater impact on LV diastolic function compared to EF. However, it is important to consider both types of fat as a risk factor for heart failure. Although the study found some evidence regarding the LVEF, further research is required to explore the impact of EF on systolic function.
References
- De Wit-Verheggen VHW, Altintas S, Spee RJM, Mihl C, Van Kuijk SMJ, Wildberger JE, et al. Pericardial fat and its influence on cardiac diastolic function. Cardiovasc Diabetol 2020, 19.
- Ma W, Zhang B, Yang Y, Qi L, Zhou J, Li M, et al. Association of epicardial fat thickness with left ventricular diastolic function parameters in a community population. BMC Cardiovasc Disord 2021, 21.
- Kitterer D, Latus J, Henes J, Birkmeier S, Backes M, Braun N, et al. Impact of long-term steroid therapy on epicardial and pericardial fat deposition: A cardiac MRI study. Cardiovasc Diabetol 2015, 14.
- Tekin I, Edem E. Association of epicardial fat tissue with coronary artery disease and left ventricle diastolic function indicators. Medical Science Monitor 2018, 24, 6367–6374. [CrossRef]
- Kenchaiah S, Ding J, Carr JJ, Allison MA, Budoff MJ, Tracy RP, et al. Pericardial Fat and the Risk of Heart Failure. J Am Coll Cardiol 2021, 77, 2638–2652.
- Larsen BA, Laughlin GA, Saad SD, Barrett-Connor E, Allison MA, Wassel CL. Pericardial fat is associated with all-cause mortality but not incident CVD: The Rancho Bernardo Study. Atherosclerosis 2015, 239, 470–475. [CrossRef] [PubMed]
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging 2015, 16, 233–271.
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography 2016, 29, 277–314.
- Okura K, Maeno K, Okura S, Takemori H, Toya D, Tanaka N, et al. Pericardial fat volume is an independent risk factor for the severity of coronary artery disease in patients with preserved ejection fraction. J Cardiol 2015, 65, 37–41. [CrossRef] [PubMed]
- Hirata Y, Yamada H, Sata M. Epicardial fat and pericardial fat surrounding the heart have different characteristics. Circulation Journal. Japanese Circulation Society 2018, 82, 2475–2476. [CrossRef] [PubMed]
- Son JW, Sung JK, Lee JW, Youn YJ, Ahn MS, Ahn SG, et al. Abdominal obesity and structure and function of the heart in healthy male Koreans the ARIRANG study. Medicine . (United States) 2016, 95.
- Fontes-Carvalho R, Gonçalves A, Severo M, Lourenço P, Rocha Gonçalves F, Bettencourt P, et al. Direct, inflammation-mediated and blood-pressure-mediated effects of total and abdominal adiposity on diastolic function: EPIPorto study. Int J Cardiol 2015, 191, 64–70. [CrossRef] [PubMed]
- Vural M, Talu A, Sahin D, Elalmis OU, Durmaz HA, UyanIk S, et al. Evaluation of the relationship between epicardial fat volume and left ventricular diastolic dysfunction. Jpn J Radiol 2014, 32, 331–339. [CrossRef] [PubMed]
- Konishi M, Sugiyama S, Sugamura K, Nozaki T, Matsubara J, Akiyama E, et al. Accumulation of pericardial fat correlates with left ventricular diastolic dysfunction in patients with normal ejection fraction. J Cardiol 2012, 59, 344–351. [CrossRef]
- Nerlekar N, Muthalaly RG, Wong N, Thakur U, Wong DTL, Brown AJ, et al. Association of volumetric epicardial adipose tissue quantification and cardiac structure and function. Vol. 7, Journal of the American Heart Association. American Heart Association Inc.; 2018.
- World Health Organization. Regional Office for Europe. WHO European Regional Obesity: Report 2022.
- Wharton S, Lau DCW, Vallis M, Sharma AM, Biertho L, Campbell-Scherer D, et al. Obesity in adults: A clinical practice guideline. CMAJ 2020, 192, E875–E891.
- Andrade KAP, Rojas MAP. Abdominal circumference cut-off. [CrossRef]
- Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, et al. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. Journal of the American Society of Echocardiography 2019, 32, 1–64. [CrossRef]
- Eroğlu, S. How do we measure epicardial adipose tissue thickness by transthoracic echocardiography? Anadolu Kardiyoloji Dergisi. 2015, 15, 416–419. [Google Scholar]
- PERFIL REGIONAL DE SAÚDE REGIÃO CENTRO [Internet]. 2018. Available online: http://www.arscentro.min-saude.pt.
- Kuk JL, Lee S, Heymsfield SB, Ross R. Waist circumference and abdominal adipose tissue distribution: influence of age and sex 1-3 [Internet]. 2005. Available online: https://academic.oup.com/ajcn/article/81/6/1330/4648767.
- Stevens J, Katz EG, Huxley RR. Associations between gender, age and waist circumference. European Journal of Clinical Nutrition 2010, 64, 6–15. [CrossRef] [PubMed]
- Mahabadi AA, Massaro JM, Rosito GA, Levy D, Murabito JM, Wolf PA, et al. Association of pericardial fat, intrathoracic fat, and visceral abdominal fat with cardiovascular disease burden: The Framingham Heart Study. Eur Heart J 2009, 30, 850–856.
- Ni X, Jiao L, Zhang Y, Xu J, Zhang Y, Zhang X, et al. Correlation between the distribution of abdominal, pericardial and subcutaneous fat and muscle and age and gender in a middle-aged and elderly population. Diabetes, Metabolic Syndrome and Obesity 2021, 14, 2201–2208. [CrossRef] [PubMed]
- Rabkin SW. The relationship between epicardial fat and indices of obesity and the metabolic syndrome: A systematic review and meta-analysis. Metabolic Syndrome and Related Disorders 2014, 12, 31–42. [CrossRef] [PubMed]
- Zhu L, Gu S, Wang Q, Zhou X, Wang S, Fu C, et al. Left ventricular myocardial deformation: A study on diastolic function in the Chinese male population and its relationship with fat distribution. Quant Imaging Med Surg 2020, 10, 634–645. [CrossRef] [PubMed]
- Vaibhav Patel BB, Mori J, McLean BA, Basu R, Das SK, Ramprasath T, et al. ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. 2015.
- Iacobellis G, Leonetti F, Singh N, M Sharma A. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol 2007, 115, 272–273. [CrossRef] [PubMed]
- Kim JS, Kim SW, Lee JS, Lee SK, Abbott R, Lee KY, et al. Association of pericardial adipose tissue with left ventricular structure and function: a region-specific effect? Cardiovasc Diabetol 2021, 20.
- Fox CS, Gona P, Hoffmann U, Porter SA, Salton CJ, Massaro JM, et al. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function. Circulation 2009, 119, 1586–1591. [CrossRef] [PubMed]
- Mahajan R, Lau DH, Brooks AG, Shipp NJ, Manavis J, Wood JPM, et al. Electrophysiological, Electroanatomical, and Structural Remodeling of the Atria as Consequences of Sustained Obesity. J Am Coll Cardiol 2015, 66, 1–11. [CrossRef] [PubMed]
- Hahn RT, Badano LP, Bartko PE, Muraru D, Maisano F, Zamorano JL, et al. Tricuspid regurgitation: recent advances in understanding pathophysiology, severity grading and outcome. European Heart Journal Cardiovascular Imaging. Oxford University Press 2022, 23, 913–929.
- Cai A, Liu L, Zhou D, Zhou Y, Tang S, Feng Y. The patterns of left ventricular alteration by adipose tissue distribution: implication for heart failure prevention. ESC Heart Fail 2021, 8, 3093–3105. [CrossRef]
- Watanabe K, Kishino T, Sano J, Ariga T, Okuyama S, Mori H, et al. Relationship between epicardial adipose tissue thickness and early impairment of left ventricular systolic function in patients with preserved ejection fraction. Heart Vessels 2016, 31, 1010–1015. [CrossRef] [PubMed]
- van Woerden G, Gorter TM, Westenbrink BD, Willems TP, van Veldhuisen DJ, Rienstra M. Epicardial fat in heart failure patients with mid-range and preserved ejection fraction. Eur J Heart Fail 2018, 20, 1559–1566. [CrossRef] [PubMed]
- Obokata M, Reddy YNV, Borlaug BA. Diastolic Dysfunction and Heart Failure With Preserved Ejection Fraction: Understanding Mechanisms by Using Noninvasive Methods. JACC: Cardiovascular Imaging 2020, 13, 245–257.
- Cherian S, Lopaschuk GD, Carvalho E. Cellular cross-talk between epicardial adipose tissue and myocardium in relation to the pathogenesis of cardiovascular disease. Am J Physiol Endocrinol Metab 2012, 303, 937–949. Available online: http://www.ajpendo.org. [CrossRef] [PubMed]
- Van De Weijer T, Schrauwen-Hinderling VB, Schrauwen P. Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovascular Research 2011, 92, 10–18. [CrossRef] [PubMed]
- Ng ACT, Goo SY, Roche N, van der Geest RJ, Wang WYS. Epicardial Adipose Tissue Volume and Left Ventricular Myocardial Function Using 3-Dimensional Speckle Tracking Echocardiography. Canadian Journal of Cardiology 2016, 32, 1485–1492. [CrossRef] [PubMed]
- Arshi B, Aliahmad HA, Ikram MA, Bos D, Kavousi M. Epicardial Fat Volume, Cardiac Function, and Incident Heart Failure: The Rotterdam Study. J Am Heart Assoc 2023, 12.
- Okura H, Takada Y, Yamabe A, Kubo T, Asawa K, Ozaki T, et al. Age-and gender-specific changes in the left ventricular relaxation a doppler echocardiographic study in healthy individuals. Circ Cardiovasc Imaging 2009, 2, 41–46. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).