Preprint
Article
Breathing Pattern Response after 6-Week of Inspiratory Muscle Training during Exercise
Altmetrics
Downloads
71
Views
27
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
23 November 2023
Posted:
07 December 2023
You are already at the latest version
Alerts
Abstract
(1) Background: During physical exercise, ventilation (VE) adapts to the intensity and characteris-tics of the effort. The breathing pattern is defined as the relationship between the tidal volume (VT) and breathing frequency (BF) for a given VE. The aim of this study was to evaluate whether inspiratory muscle training influenced the response of the breathing pattern during an incremen-tal effort in amateur cyclists. (2) Methods: 18 amateur cyclists completed an incremental test to exhaustion with gas analysis on a cycle ergometer and spirometry. Cyclists were randomly as-signed to two groups (IMTG=9; CON=9). The IMT group completed 6 weeks of inspiratory muscle training (IMT) using a PowerBreathe K3® device at 50% of the maximum inspiratory pressure (Pimax). The workload was adjusted weekly. After the 6-week intervention, the cyclists repeated the incremental exercise test with gas analysis and spirometry. The response of the breathing pat-tern was evaluated during the incremental exercise test. (3) Results: Pimax increased in the IMTG (p<0.05; +19.62%). Variables related to the breathing pattern response showed no differences be-tween groups after the intervention (EXPvsCON; p>0.05). Likewise, no differences were found in the IMTG after training (PREvsPOST; p>0.05). (4) Conclusions: IMT improved the strength of in-spiratory muscles and sports performance in amateur cyclists. These changes were not attributed to alterations in the response of the breathing pattern.
Keywords:
Subject: Medicine and Pharmacology - Pulmonary and Respiratory Medicine
1. Introduction
Breathing is an essential function to keep the body homeostatic [1]. The body's ability to obtain oxygen and remove carbon dioxide relies on the effectiveness of the respiratory muscles. These muscles, specifically the diaphragm and intercostal muscles, are fundamental for the respiration and gas exchange process. During exercise, breathing is related to exercise intensity, increasing the volume of gas inhaled-exhaled and breathing frequency as much as increases exercise demands. The breathing pattern is defined as the relationship between the tidal volume (Vt) and breathing frequency (BF) for a given ventilation (VE) [2]. In addition, VE can be decomposed in: (a) central inspiratory activity (driving) expressed as the relationship between Vt and inspiratory time (Vt/Ti) and (b) the inspiration-expiration alternation (timing) expressed by the relationship between inspiratory time (Ti) and the total duration of the breathing cycle (Ti/Ttot) [3,4]. These variables have been commonly used to evaluate the breathing pattern in humans, ranging from a mechanical perspective (VT, BF) to the central nervous system control (VT/Ti; Ti/Ttot).
There are different stimuli that can modify the breathing pattern response. Among them are: (a) hypoxia, (b) CO2 rebreathing, and (c) exercise. Concerning exercise, the study of the breathing pattern has been investigated as a possible adaptation to exercise in athletes. For example, Lucía et al. [5] found that the breathing pattern of two cyclist groups with different level (amateur vs professional) differed mainly in the professional cyclists, VE increased in this group at any exercise intensity as a result of increases in both VT and BF. However, endurance training did not change the breathing pattern response during a competitive season in professional road cyclists [6]. Similarly, the breathing pattern of world-class cyclists did not change over 3 competitive seasons in spite of changes in cycling performance [7]. In this regard, it could be established that endurance training has a residual effect on breathing pattern in highly trained athletes.
In this regard, inspiratory muscle training (IMT) has been shown an effective intervention to improve exercise performance in different situations. The mechanism proposed to explain the improvements reported after IMT are: (a) hypertrophy of the diaphragm [8], (b) an increase in blood flow to the locomotor muscles [9], (c) a reduction in subjective perception of fatigue and dyspnea ratings [8] and (d) a greater mechanical efficiency [10].
In the sports field, respiratory performance has been shown to be an effective intervention for increasing overall performance in different sports disciplines [11,12,13]. Understanding the interplay between inspiratory muscle performance and breathing pattern is important in numerous contexts, from pulmonary health to sports performance. However, from our knowledge, there are not previous studies that have investigated the relationship between inspiratory muscle performance and changes in breathing pattern. Therefore, this study aimed to evaluate the influence of 6 weeks of IMT on the breathing pattern of amateur cyclists. We hypothesized that IMT could modify the response of the breathing pattern.
2. Materials and Methods
2.1. Subjects
Eighteen physically active and healthy participants [n = 9 male (23.44±2.7 years; 180.22±3.5 cm; 78.2±5.5 kg; 48.39±7.28 ml·kg−1·min−1); n = 9 female (25.37±3.24 years; 168.75 ± 5.1 cm; 62.62.2±9.47 kg; 38.15±6.57 ml·kg−1·min−1)] were selected for the study. Before starting the study, written informed consent was obtained from each participant in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of the University of Innsbruck.
2.2. Design
Participants were randomly assigned to either an inspiratory muscle training group (IMTG) or a control group (CON). The IMTG performed two training sessions per day, 5 days per week during a period of 6-weeks. Each participant completed 30 inspiratory breaths against a restricted breathing flow device (PowerBreathe®, K3) at 50% of their individual Pimax. Inspiratory training load was adjusted weekly at 50% of the individual Pimax. Every training session was performed under expert supervision. The CON did not carry out any inspiratory training during the experimental period. Participants were advised not to change normal physical training habits during the experimental period.
2.3. Testing
Before (Pre) and after (Post) the experimental period, participants performed a spirometry (Schiller SP-1®, Switzerland) to assess the forced vital capacity (FVC), forced expiratory volume during the first second (FEV1), the ratio between forced expiratory capacity during the first second and vital capacity (FEV1/VC), the peak expiratory flow (PEF), and the peak inspiratory flow (PIF) (Table 1). The best attempt out of three tests was included in the analysis. Maximal peak inspiratory mouth pressure (Pimax) was determined with a portable device (PowerBreathe®, K3). During the Pimax test, participants had to inspire as fast as possible from a residual volume after a maximal expiration. Pimax was measured weekly using the same testing protocol.
In addition, participants performed an incremental exercise tests until exhaustion. During the tests, oxygen uptake (VO2), carbon dioxide output (VCO2), respiratory exchange ratio (RER), ventilation (VE), breathing frequency (BF), tidal volume (VT), driving (VT/Ti) and timing (Ti/Ttot) were measured breath by breath with a gas analyzer (Jaeger OxygenTM®, Germany). The system was calibrated prior to each test with gas mixtures of known concentration. Tests were carried out on a cycle ergometer (RBM Cyclus 2®, Germany) in the same place, same time and by the same researchers. After 4 min of warming up, participants started the test at 50 W and then the load was increased by 25 W each minute until volitional exhaustion. Achievement of maximum oxygen uptake (VO2max) was accepted when a plateau was found in the relationship between VO2 and power output or when three of the four criteria for VO2max were obtained (Howley et al., 1995). Participants were advised to avoid exhausting exercise 1 day before the tests and to take any ergogenic aids (e.g., caffeine).
2.4. Statistical analysis
The normal distribution of the data was checked by the Shapiro-Wilk test. The homogeneity of variance was evaluated by Levene’s test. To compare the values obtained for each variable during the test, paired sample T-test (PrevsPost) and independent samples T-test (IMTGvsCON) was applied. The level of significance was set at p<0.05 for each statistical analysis.
3. Results
Table 1 shows the analysis of spirometry variables and Pimax before and after IMT. Pimax increased significantly in IMTG (p<0.05). Table 2 compares the breathing pattern response during the incremental exercise test between groups before training. No significant differences were found before the intervention between groups (IMTGvsCON) in any variable analyzed in the study (p>0.05, Table 2). Figure 1 describes the VE, VT and BF response before and after training in IMTG. Figure 2 shows the evolution of VT/Ti (as VE increases) and Ti/Ttot (in relation to power output) before and after training in IMTG. In Figure 3 we can see the relationship between VT and BF compared with the nomogram of [14] for the CON and IMTG, both before and after training.
Table 1.
Spirometry variables measured before (pre) and after (post) the 6-week intervention period.
Table 1.
Spirometry variables measured before (pre) and after (post) the 6-week intervention period.
| IMTG | CON | |||
| Pre | Post | Pre | Post | |
| VC (l) | 5.53±0.9 | 5.17±1.14 | 5.24±1.1 | 5.11±1.06 |
| FVC (l) | 5.46±1 | 4.8±1.35 | 5.06±1.1 | 4.96±0.93 |
| FEV1 (l) | 4.64±0.92 | 4.19±0.80 | 4.31±0.85 | 4.06±0.79 |
| FEV1/VC (%) | 84.13±11.58 | 82.51±9.19 | 82.33±6.28 | 79.84±6.48 |
| PEF (l·min-1) | 9.27±2.23 | 8.20±1.53 | 8.90±2.47 | 8.73±2.40 |
| PIF (l·min-1) | 7.04±1.92 | 8.31±2.39 | 7.12±1.20 | 6.73±3.26 |
| Pimax (cmH2O) | 119.66±37.36 | 166.91±42.65* | 130.55±33.58 | 130.42±61.93 |
| Data are presented as Mean ± Standard deviation p indicates p-value * significant differences (p<0.05) | ||||
4. Discussion
The main finding of this study was that the inspiratory muscle training does not significantly modify the breathing pattern in healthy and active cyclists, despite producing an improvement in inspiratory muscle strength.
The traditional and commonly used method for evaluating breathing patterns in clinical applications is to analyze the VT and BF response [2,15]. Hey et al. [15] observed that VT increased linearly during exercise until the 50% of the FVC. Beyond this breakpoint, the increase in VE occurred due to changes in BF rather than VT. When this analysis was applied to healthy, trained, and untrained subjects during exercise, the relationship between VT and BF was found to be exponential, as it was described in a nomogram by [14]. Figure 3 illustrates the exponential response in CON and IMTG before and after training. In both groups, the exponential curve closely approaches the lower limit of the nomogram proposed by [14], indicating a normal and low-energy response. Based on the position of both curves on the nomogram after IMT, we could consider that the IMT intervention did not significantly influence the breathing pattern. This lack of influence is likely due to the initially highly efficient breathing pattern observed in the IMTG before training.
Milic-Emili & Grunstein [4] proposed a new analysis based on the central activity of breathing pattern using the VT/Ti and Ti/Ttot relationships. Figure 1 and Figure 2 compare breathing pattern before and after training in IMTG. No differences were found at any intensity after training in IMTG. In our healthy subjects, the central regulation of VE response to exercise intensity is primarily promoted by an increase in VT/Ti and a constant Ti/Ttot output (Figure 2), without changes in mechanical control (Figure 1). This similar behaviour has been reported after endurance training [5,6] and is considered the normal response to exercise in healthy subjects.
To our knowledge, only one study has investigated the relationship between IMT and breathing pattern response. Charususin et al. [16] evaluated the effect of IMT on breathing pattern in patients with chronic obstructive pulmonary disease (COPD). In these patients, the breathing pattern improved after 8-weeks of IMT. COPD patients usually develop a rapid and shallow breathing pattern, which is energetically opposite to the pattern required to minimize the work of breathing [17]. This inefficient breathing pattern is related to the restriction of VT expansion and might also be related to an imbalance between the load and capacity relationship of the inspiratory muscles [16]. On the other hand, our group reported that ventilator efficiency improved after IMT only in hypoxic conditions (FiO2=16.45%) but not in normoxia [18].
The lack of changes in the breathing pattern in our subjects after IMT could be explained by the initial efficient breathing pattern reported before training (Figure 3). As it has been described before, subjects with respiratory diseases and inefficient breathing pattern can significantly modify their ventilatory response after IMT. Therefore, we could consider that when there is not a stressful factor such as respiratory disease or altitude, the nervous system regulates the breathing pattern independently of inspiratory muscle strength.
The main limitation of this study could be the sample size. Although it was adequate for detecting changes in Pimax within the intervention group, it might have been insufficient to identify differences between CON and IMTG after the intervention period (Table 1). In fact, when we applied the Cohen’s d effect size analysis [19] for the PImax between groups after training, it was moderate (d = 0.68), indicating that with a larger sample size the change could have been significant.
5. Conclusions
In conclusion, IMT was not an effective intervention to improve the breathing pattern response during an incremental exercise test in healthy athletes. Our results, along with the evidence reported previously, indicate that the breathing pattern response is not related to fitness level or respiratory muscle performance. Driving and timing analysis suggest that the nervous system adjusts ventilation to exercise intensity independently of respiratory muscle strength. Our results not only provide valuable insights into the impact of IMT on breathing patterns but also offer practical implications for athletes and healthcare professionals involved in exercise prescription.
Author Contributions
Conceptualization, E.S-M.; methodology, E.S-M..; investigation, E.S-M.; writing—original draft preparation, E.S-M..; writing—review and editing, E.S-M.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board University of Innsbruck.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
The author acknowledges the support of the University of Innsbruck and expresses special gratitude to the Department of Sport Science.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brown, S.J.; Raman, A.; Schlader, Z.; Stannard, S.R. Ventilatory efficiency in juvenile elite cyclists. J Sci Med Sport 2013, 16, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Milic-Emili, G.; Cajani, F. [Frequency of breathing as a function of respiratory ventilation during rest]. Boll Soc Ital Biol Sper 1957, 33, 821–825. [Google Scholar] [PubMed]
- Milic-Emili, J. Recent advances in clinical assessment of control of breathing. 1982, 160, 1-17. [CrossRef]
- Milic-Emili, J.; Grunstein, M.M. Drive and timing components of ventilation. Chest 1976, 70, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Lucía, A.; Carvajal, A.; Calderón, F.J.; Alfonso, A.; Chicharro, J.L. Breathing pattern in highly competitive cyclists during incremental exercise. Eur J Appl Physiol Occup Physiol 1999, 79, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Lucía, A.; Hoyos, J.; Pardo, J.; Chicharro, J.L. Effects of endurance training on the breathing pattern of professional cyclists. Jpn J Physiol 2001, 51, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Martínez, E.; Terrados, N.; Burtscher, M.; Santalla, A.; Naranjo Orellana, J. Ventilatory efficiency and breathing pattern in world-class cyclists: A three-year observational study. Respir Physiol Neurobiol 2016, 229, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Downey, A.E.; Chenoweth, L.M.; Townsend, D.K.; Ranum, J.D.; Ferguson, C.S.; Harms, C.A. Effects of inspiratory muscle training on exercise responses in normoxia and hypoxia. Respir Physiol Neurobiol 2007, 156, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Harms, C.A.; Babcock, M.A.; McClaran, S.R.; Pegelow, D.F.; Nickele, G.A.; Nelson, W.B.; Dempsey, J.A. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol (1985) 1997, 82, 1573–1583. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.V.; Lima, W.L.; Nobre, A.; dos Santos, A.M.; Brito, L.M.; Costa Mdo, R. Inspiratory muscle training and respiratory exercises in children with asthma. J Bras Pneumol 2008, 34, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Illi, S.K.; Held, U.; Frank, I.; Spengler, C.M. Effect of respiratory muscle training on exercise performance in healthy individuals: a systematic review and meta-analysis. Sports Med 2012, 42, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Gallego-Gallego, D.; Corchete, L.A.; Fernández Zoppino, D.; González-Bernal, J.J.; García Gómez, B.; Mielgo-Ayuso, J. Inspiratory Muscle Training Program Using the PowerBreath(®): Does It Have Ergogenic Potential for Respiratory and/or Athletic Performance? A Systematic Review with Meta-Analysis. Int J Environ Res Public Health 2021, 18. [Google Scholar] [CrossRef] [PubMed]
- HajGhanbari, B.; Yamabayashi, C.; Buna, T.R.; Coelho, J.D.; Freedman, K.D.; Morton, T.A.; Palmer, S.A.; Toy, M.A.; Walsh, C.; Sheel, A.W.; et al. Effects of respiratory muscle training on performance in athletes: a systematic review with meta-analyses. J Strength Cond Res 2013, 27, 1643–1663. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, J.; Centeno, R.A.; Galiano, D.; Beaus, M. A nomogram for assessment of breathing patterns during treadmill exercise. Br J Sports Med 2005, 39, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Hey, E.N.; Lloyd, B.B.; Cunningham, D.J.; Jukes, M.G.; Bolton, D.P. Effects of various respiratory stimuli on the depth and frequency of breathing in man. Respir Physiol 1966, 1, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Charususin, N.; Gosselink, R.; McConnell, A.; Demeyer, H.; Topalovic, M.; Decramer, M.; Langer, D. Inspiratory muscle training improves breathing pattern during exercise in COPD patients. In Eur Respir J; England, 2016; Volume 47, pp. 1261-1264.
- Macklem, P.T. Therapeutic implications of the pathophysiology of COPD. Eur Respir J 2010, 35, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Martínez, E.; Gatterer, H.; Burtscher, M.; Naranjo Orellana, J.; Santalla, A. Influence of Inspiratory Muscle Training on Ventilatory Efficiency and Cycling Performance in Normoxia and Hypoxia. Front Physiol 2017, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical power analysis for the behavioral sciences; Lawrence Erlbaum Associates: United States of America, 1988. [Google Scholar]
Figure 1.
Breathing pattern analysis before and after IMT in the IMTG: ventilation (VE); tidal volumen (VT); breathing frecuency (BF).
Figure 1.
Breathing pattern analysis before and after IMT in the IMTG: ventilation (VE); tidal volumen (VT); breathing frecuency (BF).
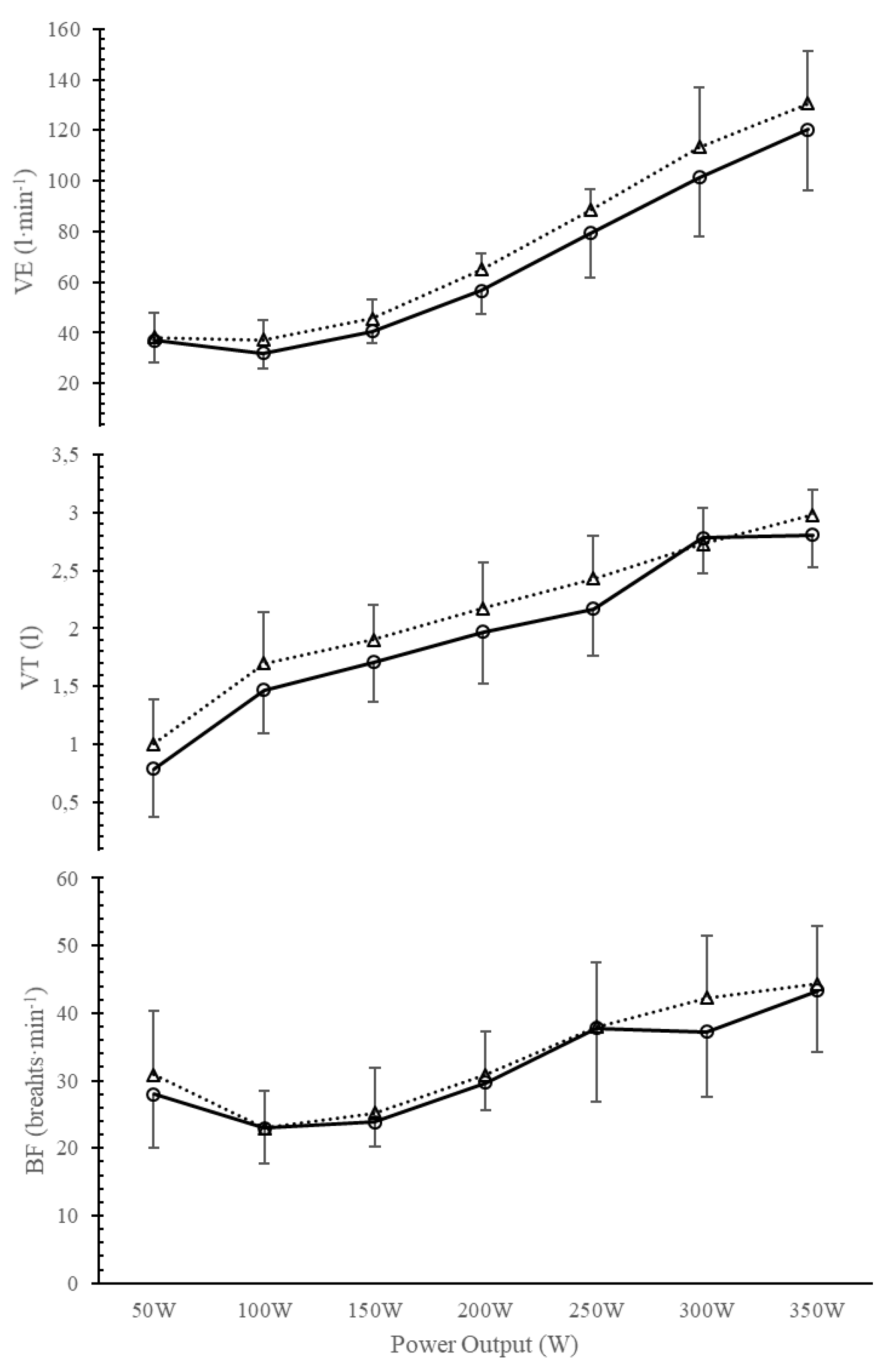
Figure 2.
Evolution of driving impulse (VT/Ti) and timing (Ti/Ttot) before and after training in IMTG.
Figure 2.
Evolution of driving impulse (VT/Ti) and timing (Ti/Ttot) before and after training in IMTG.
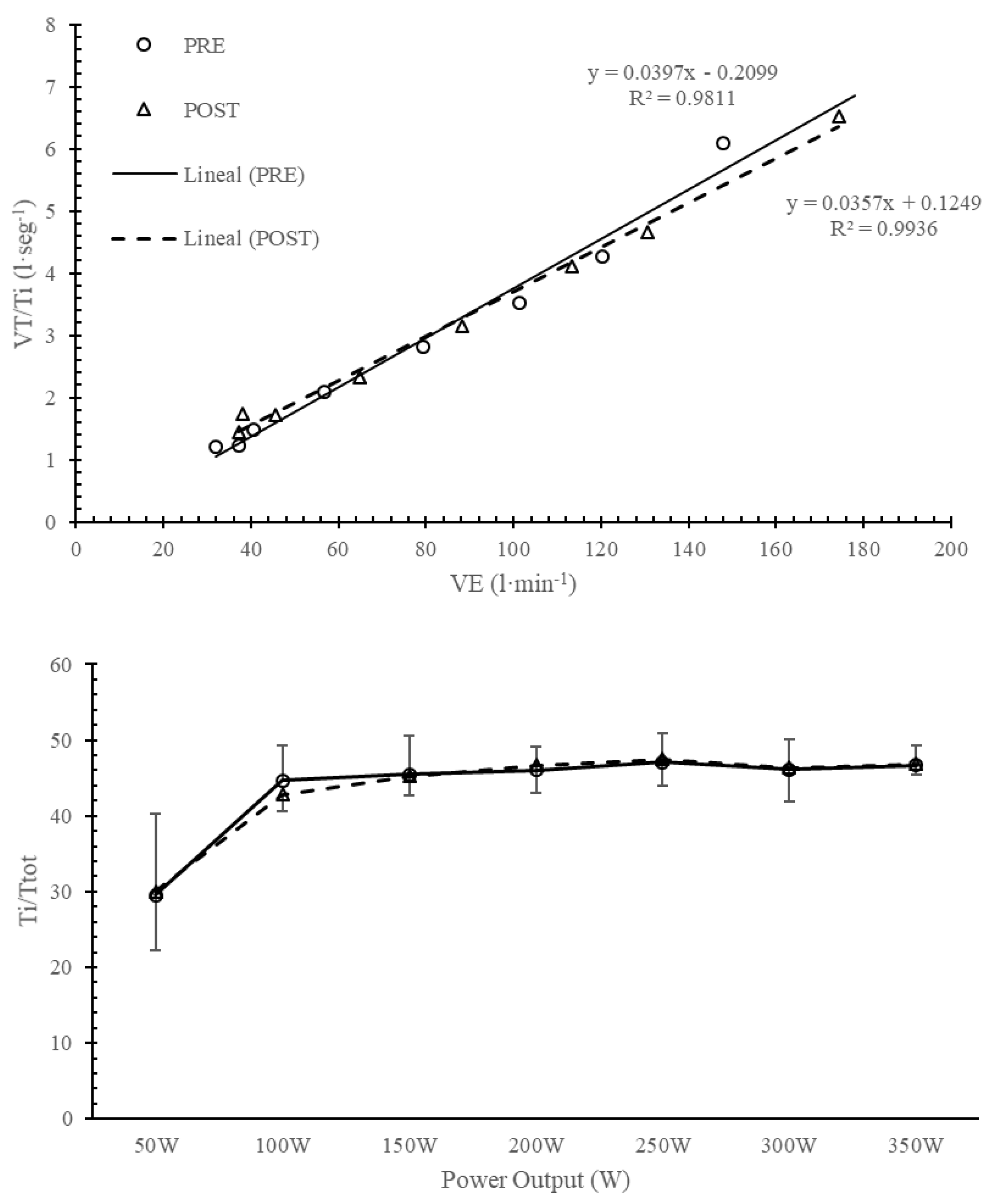
Figure 3.
Breathing pattern analysis in CON and IMTG before and after IMT based on Naranjo et al. nomogram [1].
Figure 3.
Breathing pattern analysis in CON and IMTG before and after IMT based on Naranjo et al. nomogram [1].
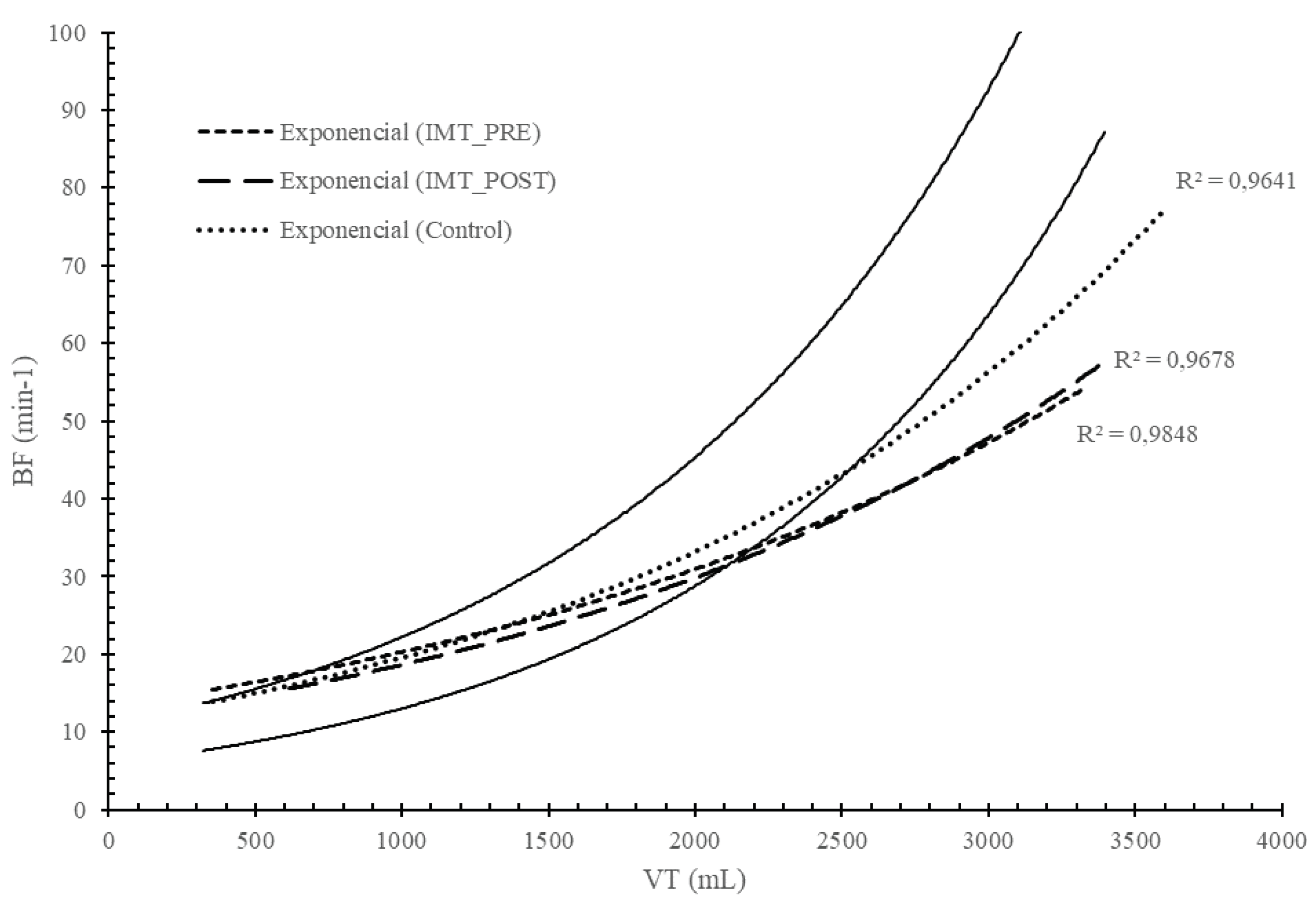
Table 2.
Comparison between groups (IMTGvsCON) before and after the 6-week IMT.
| Pre-IMT | |||||||||||||||
| VE (l·min-1) | BF (breahts·min-1) | VT (l) | VT/Ti (l·seg) | Ti/Ttot | |||||||||||
| IMTG | CON | p | IMTG | CON | p | IMTG | CON | p | IMTG | CON | p | IMTG | CON | p | |
| 50W | 35.11±13.97 | 33.89±13.91 | 0.855 | 28±7.98 | 30.11±8.57 | 0.596 | 0.79±0.41 | 0.77±0.41 | 0.930 | 1.23±0.36 | 1.26±0.3 | 0.894 | 29.56±10.6 | 30.11±9.9 | 0.911 |
| 100W | 31.89±6.11 | 30.11±9.98 | 0.655 | 23±5.24 | 23.89±10.64 | 0.825 | 1.47±0.38 | 1.62±0.52 | 0.807 | 1.21±0.47 | 1.09±0.4 | 0.500 | 44.67±4.6 | 47.11±5.5 | 0.328 |
| 150W | 40.56±4.42 | 41.22±14.52 | 0.897 | 23.89±3.69 | 25.78±9.86 | 0.598 | 1.71±0.34 | 1.91±0.52 | 0.329 | 1.48±0.42 | 1.64±0.4 | 0.317 | 45.44±5.0 | 44.67±4.4 | 0.745 |
| 200W | 56.67±9.3 | 56.33±16.53 | 0.959 | 29.67±4.12 | 31.11±7.2 | 0.609 | 1.97±0.45 | 2.03±0.48 | 0.796 | 2.1±0.49 | 2.2±0.49 | 0.610 | 46±3 | 45.33±3.6 | 0.682 |
| 250W | 79.44±17.76 | 83.33±31.85 | 0.753 | 37.78±10.88 | 37.11±8.84 | 0.888 | 2.17±0.41 | 2.43±0.55 | 0.271 | 2.82±0.88 | 3.08±0.8 | 0.473 | 47.11±3.8 | 47.33±3.2 | 0.895 |
| 300W | 101.29±23.44 | 107±36.7 | 0.726 | 37.29±9.62 | 44.89±12.21 | 0.242 | 2.78±0.3 | 2.42±0.53 | 0.134 | 3.52±1.06 | 4.01±1.1 | 0.298 | 46.14±3.8 | 45.89±4 | 0.910 |
| 350W | 120.17±24.1 | 124.6±31.19 | 0.796 | 43.33±9.14 | 49.6±6.35 | 0.253 | 2.81±0.28 | 2.56±0.64 | 0.402 | 4.27±1.2 | 4.44±1.2 | 0.782 | 46.67±2.6 | 47.8±2.2 | 0.473 |
| Post-IMT | |||||||||||||||
| VE (l·min-1) | BF (breahts·min-1) | VT (l) | VT/Ti (l·seg) | Ti/Ttot | |||||||||||
| IMTG | CON | p | IMTG | CON | p | IMTG | CON | p | IMTG | CON | p | IMTG | CON | p | |
| 50W | 38.11±9.81 | 31.11±6.15 | 0.089 | 30.88±9.53 | 29.44±4.30 | 0.684 | 1±0.38 | 0.77±0.16 | 0.135 | 1.73±0.78 | 1.37±0.3 | 0.209 | 30±7.71 | 28.55±7.98 | 0.701 |
| 100W | 37.22±7.64 | 30.77±6.49 | 0.072 | 23±5.5 | 21.44±4.63 | 0.526 | 1.69±0.44 | 1.53±0.37 | 0.424 | 1.45±0.26 | 1.36±0.5 | 0.667 | 42.77±2.1 | 42±10.07 | 0.824 |
| 150W | 45.66±7.33 | 46.44±7.76 | 0.830 | 25.22±6.74 | 25.88±6.33 | 0.832 | 1.9±0.3 | 1.81±0.35 | 0.574 | 1.72±0.33 | 1.71±0.2 | 0.970 | 45.22±2.48 | 44.11±4.75 | 0.543 |
| 200W | 64.88±6.62 | 58.11±8.23 | 0.072 | 30.88±6.37 | 28.77±6.33 | 0.491 | 2.17±0.39 | 2.06±0.44 | 0.587 | 2.33±0.31 | 2.17±0.3 | 0.301 | 46.66±3.6 | 44.44±5.19 | 0.308 |
| 250W | 88.33±8.42 | 81.5±13.52 | 0.224 | 38±9.55 | 34.12±8.11 | 0.385 | 2.25±0.26 | 2.35±0.52 | 0.741 | 3.15±0.35 | 2.79±0.7 | 0.200 | 46.75±2.91 | 47.12±6.72 | 0.902 |
| 300W | 113.25±23.83 | 116.1±5.82 | 0.771 | 42.25±9.19 | 50.37±10.41 | 0.120 | 2.8±0.23 | 2.42±0.48 | 0.111 | 4.1±0.63 | 4.08±0.6 | 0.973 | 45.16±3.25 | 48.25±2.31 | 0.307 |
| 350W | 130.5±20.72 | 139.5±11.38 | 0.456 | 44.33±8.64 | 50.25±12.76 | 0.187 | 3.01±0.25 | 2.95±0.64 | 0.917 | 4.66±0.8 | 4.7±0.6 | 0.929 | 47.25±1.5 | 49.33±3.78 | 0.180 |
| Data are presented as Mean ± Standard deviation p indicates p-value * significant differences (p<0.05) | |||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






