Submitted:
06 December 2023
Posted:
07 December 2023
You are already at the latest version
Abstract
Keywords:
1. Physiological Oxygen Sensing: The Carotid Body as an O2 sensor
2. Oxygen-Sensitive K+ Channels in the Carotid Body
2.1. Kv Channels and Oxygen-Sensitivity in the Carotid Body
2.2. BKCa Channels and Oxygen sensitivity in the Carotid Body
2.3. K2P Channels and Oxygen-Sensitivity in the Carotid Body
3. What we Know and What we Don’t About the Oxygen Sensors in Chemoreceptor Cells.
4. Coupling Mechanisms Between the Sensor and the K+ Channels.
5. Concluding Remarks in O2 Sensing in the Carotid Body
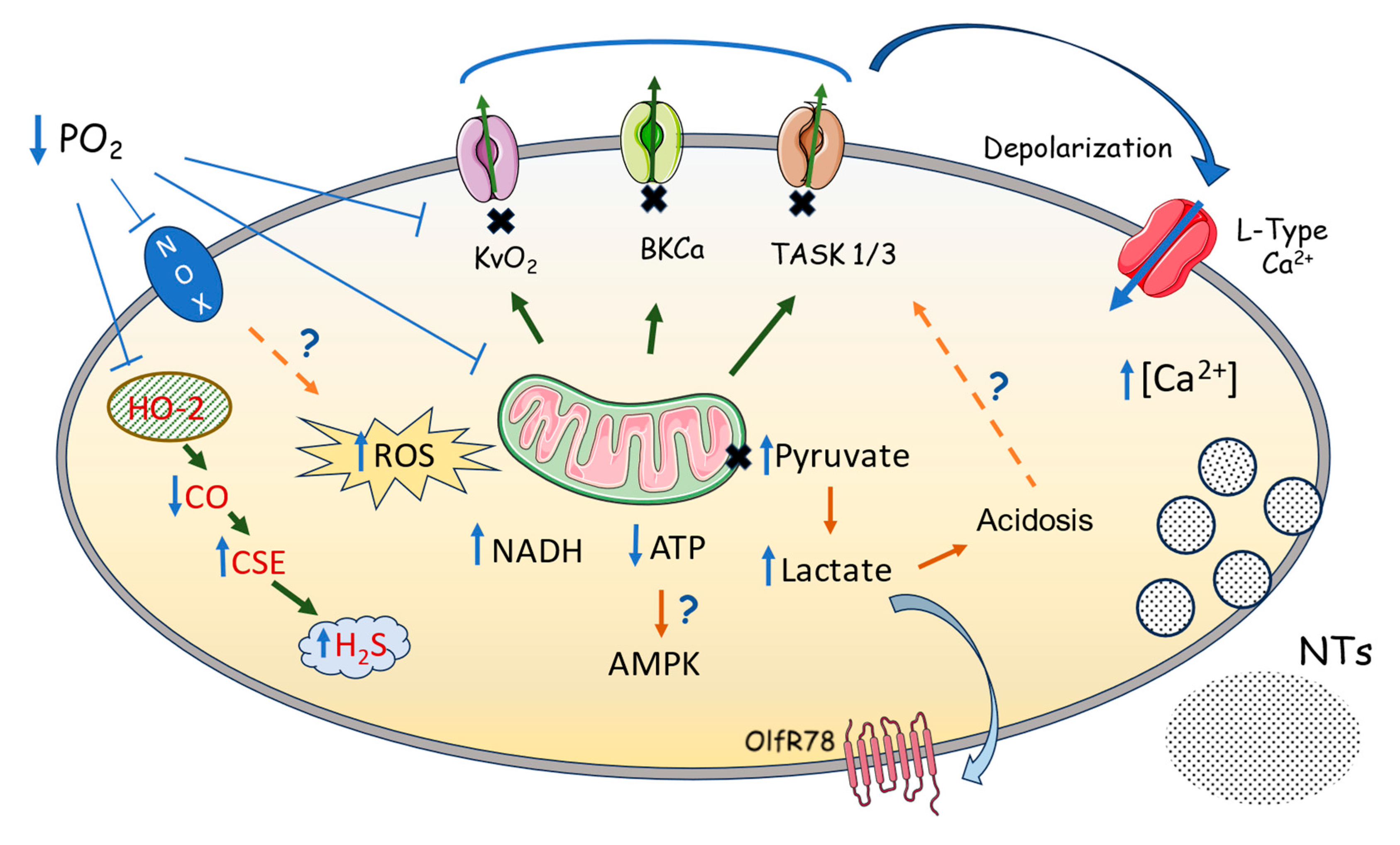
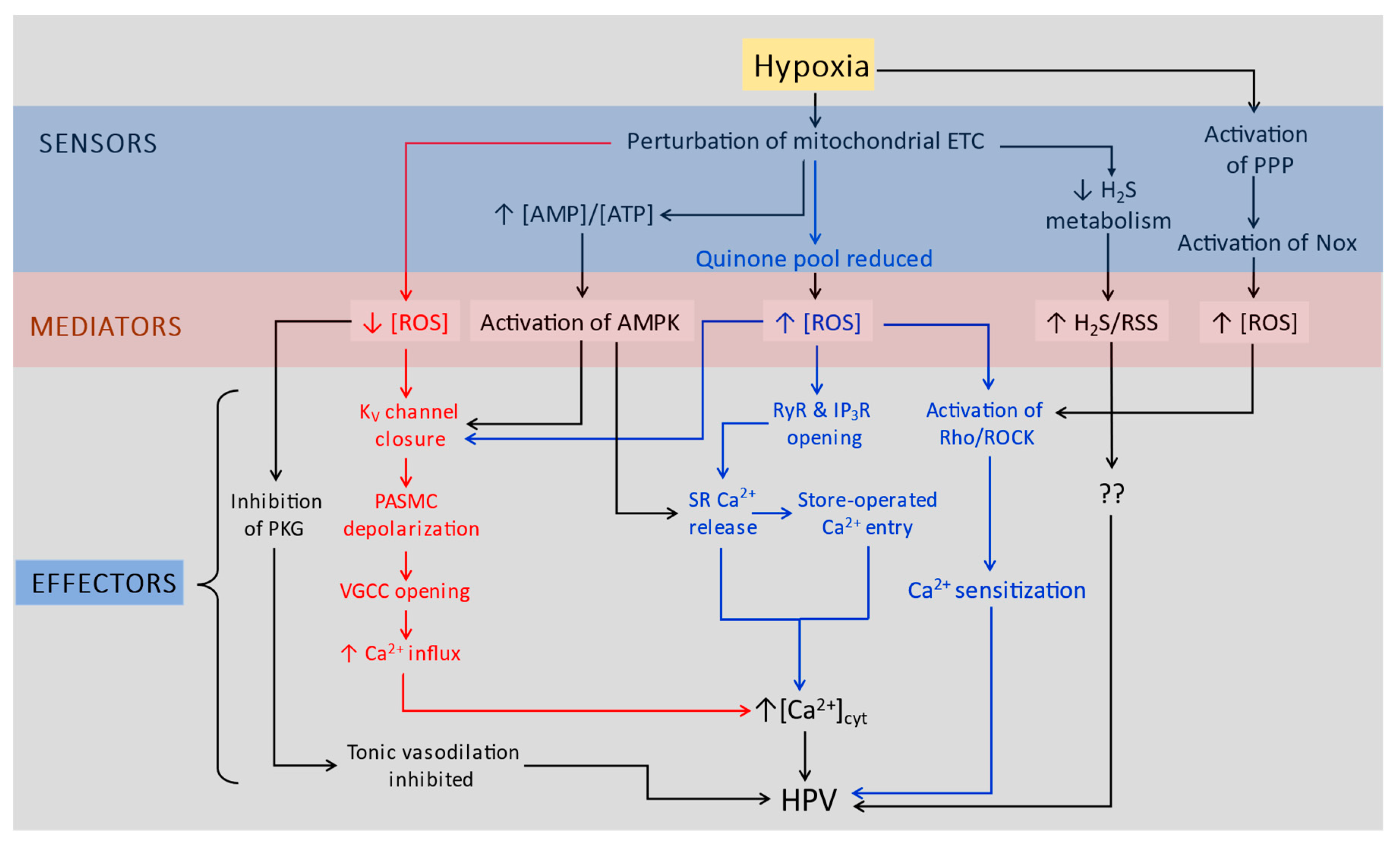
6. O2-Sensitive K+ Channels in the Pulmonary Vasculature
6.1. Kv Channels and O2 Sensing in PASMC
7. How Does PO2 Regulate K+ Channels in PASMC?
7.1. Evidence for KV channel regulation by cytoplasmic redox state
7.2. Evidence for TASK-1 channel regulation by cytoplasmic redox state
7.3. Other factors regulating PASMC K+ currents during hypoxia
8. Concluding Remarks: O2 sensing in CBCC versus PASMCs
Author Contributions
Funding
Conflicts of Interest
References
- Lopez-Barneo, J. , Lopez-Lopez, J.R., Urena, J., Gonzalez, C. Chemotransduction in the carotid body: K+ current modulated by PO2 in type I chemoreceptor cells. Science 1988, 241, 580–582. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C., Almaraz, L., Obeso, A., Rigual, R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994, 74(4), 829-898. [CrossRef]
- Marshall, B.E.; Marshall, C.; Frasch, F.; Hanson, C.W. (1994). Role of hypoxic pulmonary vasoconstriction in pulmonary gas exchange and blood flow distribution. 1. Physiologic concepts. Intensive Care Med 1994, 20, 291-297. [CrossRef]
- Peers, C.; Kemp, P.J. Acute oxygen sensing: diverse but convergent mechanisms in airway and arterial chemoreceptors. Respir Res 2001, 2, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, T.A.; Seidler, F.J. Adrenomedullary catecholamine release in the fetus and newborn: secretory mechanisms and their role in stress and survival. J Dev Physiol. 1988, 10(1),1-16.
- Pflüger, E. Ueber die ursacheder athembewegungen, sowie der dyspnoë und apnoë. Pflugers Arch. Gesamte Physiol. Meschen Tiere. 1868, 1, 61-106.
- Boycott, A.E.; Haldane, J.S. The effects of low atmospheric pressures on respiration. J Physiol 1908, 37, 355–377. [Google Scholar] [CrossRef] [PubMed]
- De Castro, F. Sur la structure et l'innervation du sinus carotidien de l'homme et des mammifères: Nouveaux faits sur l'innervation et la fonction du glomus caroticum. Trab Lab Invest Biol Univ Madrid 1928, 25, 330–380. [Google Scholar]
- Gonzalez, C.; Conde, S.V.; Gallego-Martín, T.; Olea, E.; Gonzalez-Obeso, E.; Ramirez, M.; Yubero, S.; Agapito, M.T.; Gomez-Nino, A.; Obeso, A.; Rigual, R.; Rocher, A. Fernando de Castro and the discovery of the arterial chemoreceptors. Front Neuroanat. 2014, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Anichkov, S.V.; Belen'kii, M.L. Pharmacology of the Carotid Body Chemoreceptors. McMillan, New York, 1963.
- Mills, E.; Jöbsis, F.F. Simultaneous measurement of cytochrome a3 reduction and chemoreceptor afferent activity in the carotid body. Nature 1970, 225, 1147–9. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; Jöbsis, F.F. Mitochondrial respiratory chain of carotid body and chemoreceptor response to changes in oxygen tension. J Neurophysiol. 1972, 35, 405–28. [Google Scholar] [CrossRef]
- Duchen, M.R.; Biscoe, T.J. Mitochondrial function in type I cells isolated from rabbit arterial chemoreceptors. J Physiol. 1992, 450, 13–31. [Google Scholar] [CrossRef]
- Duchen, M.R.; Biscoe, T.J. ; Relative mitochondrial membrane potential and [Ca2+]i in type I cells isolated from the rabbit carotid body. J Physiol. 1992, 450, 33–61. [Google Scholar] [CrossRef]
- Lahiri, S.; Rumsey, W.L.; Wilson, D.F.; Iturriaga, R. Contribution of in vivo microvascular PO2 in the cat carotid body chemotransduction. J Appl Physiol (1985), 1993, 75(3), 1035–1043. [Google Scholar] [CrossRef]
- Buckler, K.J.; Turner, P.J. Oxygen sensitivity of mitochondrial function in rat arterial chemoreceptor cells. J Physiol 2013, 591(14), 3549–63. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chien, M.S.; Kaleem, S.; Matsunami, H. Single cell transcriptome analysis of mouse carotid body glomus cells. J Physiol. 2016, 594(15), 4225–4251. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Bonilla-Henao, V.; García-Flores, P.; Arias-Mayenco, I.; Ortega- Sáenz, P.; López-Barneo, J. Gene expression analyses reveal metabolic specifications in acute O2-sensing chemoreceptor cells. J Physiol 2017, 595, 6091–6120. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Domínguez, A.; Ortega-Sáenz, P.; Gao, L.; Colinas, O.; García-Flores, P.; Bonilla-Henao, V.; Aragonés, J.; Hüttemann, M.; Grossman, L.I.; Weissmann, N.; Sommer, N.; López-Barneo, J. Acute O2 sensing through HIF2α-dependent expression of atypical cytochrome oxidase subunits in arterial chemoreceptors. Sci Signal. 2020, 13(615), eaay9452. [Google Scholar] [CrossRef] [PubMed]
- Almaraz, L.; Gonzalez, C.; Obeso, A. Effects of high potassium on the release of [3H] dopamine from the cat carotid body in vitro. J Physiol. 1986, 379, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Rocher, A.; Obeso, A.; Herreros, B.; Gonzalez, C. Activation of the release of dopamine in the carotid body by veratridine. Evidence for the presence of voltage-dependent Na+ channels in type I cells. Neurosci Lett 1988, 94, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, J.R.; Gonzalez, C.; Urena, J.; Lopez-Barneo, J. Low pO2 selectively inhibits K channel activity in chemoreceptor cells of the mammalian carotid body. J Gen Physiol 1989, 93, 1001–1015. [Google Scholar] [CrossRef]
- Peers, C. Hypoxic suppression of K+ currents in type I carotid body cells: selective effect on the Ca2(+)-activated K+ current. Neurosci Lett 1990, 119, 253–256. [Google Scholar] [CrossRef]
- Buckler, K.J. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J Physiol 1997, 498 (Pt 3), 649–662. [Google Scholar] [CrossRef]
- Buckler, K.J. Background leak K+ currents and oxygen sensing in carotid body type 1 cells. Respir Physiol. 1999, 115(2), 179–87. [Google Scholar] [CrossRef]
- Rocher, A.; Obeso, A.; Cachero, M.T.; Herreros, B.; Gonzalez, C. Participation of Na+ channels in the response of carotid body chemoreceptor cells to hypoxia. Am J Physiol 1994, 267, C738–C744. [Google Scholar] [CrossRef] [PubMed]
- Obeso, A.; Rocher, A.; Fidone, S.; Gonzalez, C. The role of dihydropyridine-sensitive Ca2+ channels in stimulus-evoked catecholamine release from chemoreceptor cells of the carotid body. Neuroscience 1992, 47(2), 463–72. [Google Scholar] [CrossRef] [PubMed]
- Buckler, K.J.; Vaughan-Jones, R.D. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J Physiol. 1994, 476(3), 423–8. [Google Scholar] [CrossRef] [PubMed]
- Stea, A.; Nurse C.A. Whole-cell and perforated-patch recordings from O2-sensitive rat carotid body cells grown in short- and long-term culture. Pflugers Arch. 1991, 418(1-2), 93-101. [CrossRef]
- Ureña, J.; Fernández-Chacón, R.; Benot, A.R.; Alvarez de Toledo, G.A.; López-Barneo, J. Hypoxia induces voltage-dependent Ca2+ entry and quantal dopamine secretion in carotid body glomus cells. Proc Natl Acad Sci U S A 1994, 91(21), 10208–10211. [Google Scholar] [CrossRef]
- Rocher, A.; Geijo-Barrientos, E.; Caceres, A.I.; Rigual, R.; Gonzalez, C.; Almaraz, L. Role of voltage-dependent calcium channels in stimulus-secretion coupling in rabbit carotid body chemoreceptor cells. J Physiol 2005, 562, 407–420. [Google Scholar] [CrossRef]
- Youngson, C.; Nurse, C.; Yeger, H.; Cutz, E. Oxygen sensing in airway chemoreceptors. Nature. 1993, 365(6442), 153–155. [Google Scholar] [CrossRef] [PubMed]
- Weir, E.K.; Archer, S.L. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. FASEB J 1995, 9(2), 183–189. [Google Scholar] [CrossRef] [PubMed]
- Peers, C.; Buckler, K.J. Transduction of chemostimuli by the type I carotid body cell. J Membr Biol. 1995, 144(1), 1–9. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, M.T.; Lopez-Lopez, J.R. Are Kv channels the essence of O2 sensing? Circ Res. 2000, 86(5), 490–491. [Google Scholar] [CrossRef]
- Patel, A.J.; Honore, E. Molecular physiology of oxygen-sensitive potassium channels. Eur Respir J. 2001, 8(1), 221–227. [Google Scholar] [CrossRef]
- Coppock, E.A.; Martens, J.R.; Tamkun, M.M. Molecular basis of hypoxia-induced pulmonary vasoconstriction: role of voltage-gated K+ channels. Am J Physiol Lung Cell Mol Physiol. 2001, 281(1), L1–12. [Google Scholar] [CrossRef] [PubMed]
- Kemp, P.J. Detecting acute changes in oxygen: will the real sensor please stand up? Exp Physiol. 2006, 91(5), 829–34. [Google Scholar] [CrossRef] [PubMed]
- Peers, C.; Wyatt, C.N.; Evans, A.M. Mechanisms for acute oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2010, 174(3), 292–8. [Google Scholar] [CrossRef] [PubMed]
- Jan, L.Y.; Jan, Y.N. Cloned potassium channels from eukaryotes and procaryotes. Annu Rev Neurosci 1997, 20, 91–123. [Google Scholar] [CrossRef] [PubMed]
- Coetzee, W.A.; Amarillo, Y.; Chiu, J.; Chow, A.; Lau, D.; McCormack, T.; Moreno, H.; Nadal, M.S.; Ozaita, A.; Pountney, D.; Saganich, M.; Vega-Saenz de Miera, E.; Rudy, B. Molecular diversity of K+ channels. Ann N Y Acad Sci. 1999, 868, 233–85. [Google Scholar] [CrossRef] [PubMed]
- Lotshaw, D.P. Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem Biophys. 2007, 47(2), 209–256. [Google Scholar] [CrossRef] [PubMed]
- Gutman, G.A.; Chandy, K.G.; Adelman, J.P.; Aiyar, J.; Bayliss, D.A.; Clapham, D.E.; Covarriubias, M.; Desir, G.V.; Furuichi, K.; Ganetzky, B.; Garcia, M.L.; Grissmer, S.; Jan, L.Y.; Karschin, A.; Kim, D.; Kuperschmidt, S.; Kurachi, Y.; Lazdunski, M.; Lesage, F.; Lester, H.A.; McKinnon, D.; Nichols, C.G.; O'Kelly, I.; Robbins, J.; Robertson, G.A.; Rudy, B.; Sanguinetti, M.; Seino, S.; Stuehmer, W.; Tamkun, M.M.; Vandenberg, C.A.; Wei, A.; Wulff, H.; Wymore, R.S. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev. 2003, 55(4), 583-6. [CrossRef]
- Bocksteins, E.; Snyders, D.J. Electrically silent Kv subunits: their molecular and functional characteristics. Physiology (Bethesda), 2012, 27(2), 73-84. [CrossRef]
- Martens, J.R.; Kwak, Y.G.; Tamkun, M.M. Modulation of Kv channel alpha/beta subunit interactions. Trends Cardiovasc Med. 1999, 9(8), 253–258. [Google Scholar] [CrossRef]
- Biggin, P.C.; Roosild, T.; Choe, S. Potassium channel structure: domain by domain. Curr Opin Struct Biol. 2000, 10(4), 456–61. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, M.T.; López-López, J.R.; González, C. Kvbeta1. 2 subunit coexpression in HEK293 cells confers O2 sensitivity to kv4.2 but not to Shaker channels. J Gen Physiol. 1999, 113(6), 897–907. [Google Scholar] [CrossRef]
- Coppock, E.A.; Tamkun, M.M. Differential expression of K(v) channel alpha- and beta-subunits in the bovine pulmonary arterial circulation. Am J Physiol Lung Cell Mol Physiol 2001, 281(6), L1350–1360. [Google Scholar] [CrossRef]
- Ganfornina, M.D.; López-Barneo, J. Gating of O2-sensitive K+ channels of arterial chemoreceptor cells and kinetic modifications induced by low PO2. J Gen Physiol. 1992, 100(3), 427–455. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, J.R.; Gonzalez, C. Time course of K+ current inhibition by low oxygen in chemoreceptor cells of adult rabbit carotid body: effects of carbon monoxide. FEBS Lett. 1992, 299, 251–254. [Google Scholar] [CrossRef]
- Perez-Garcia, M.T.; Lopez-Lopez, J.R.; Riesco, A.M.; Hoppe, U.; Gonzalez, C.; Marban, E.; Johns, D.C. Supression of transient outward K+ currents in chemoreceptor cells of the rabbit carotid body by viral gene transfer of inducible dominant negative Kv4.3 constructs. J Neurosci. 2000, 20, 5689–5695. [Google Scholar] [CrossRef]
- López-López, J.R.; Pérez-García, M.T.; Sanz-Alfayate, G.; Obeso, A.; Gonzalez, C. Functional identification of Kvalpha subunits contributing to the O2-sensitive K+ current in rabbit carotid body chemoreceptor cells. Adv Exp Med Biol. 2003, 536, 33–39. [Google Scholar]
- Sanchez, D.; López-López, J.R.; Pérez-García, M.T.; Sanz-Alfayate, G.; Obeso, A.; Ganfornina, M.D.; Gonzalez, C. Molecular identification of Kvalpha subunits that contribute to the oxygen-sensitive K+ current of chemoreceptor cells of the rabbit carotid body. J Physiol. 2002, 542(Pt 2), 369–382. [Google Scholar] [CrossRef]
- Pérez-García, M.T.; Colinas, O.; Miguel-Velado, E.; Moreno-Domínguez, A.; López-López, J.R. Characterization of the Kv channels of mouse carotid body chemoreceptor cells and their role in oxygen sensing. J Physiol. 2004, 557 Pt 2, 457–471. [Google Scholar] [CrossRef] [PubMed]
- López-López, J.R.; Pérez-García, M.T. Oxygen sensitive Kv channels in the carotid body. Respir Physiol Neurobiol. 2007, 157(1), 65–74. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.; Yuan, J.X. Hypoxic pulmonary vasoconstriction: role of voltage-gated potassium channels. Respir Res. 2000, 1(1), 40–48. [Google Scholar] [CrossRef]
- Sylvester, J.T.; Shimoda, L.A.; Aaronson, P.I.; Ward, J.P. Hypoxic pulmonary vasoconstriction. Physiol Rev 2012, 92(1), 367–520. [Google Scholar] [CrossRef]
- Lopez-Barneo, J.; Benot, A.R.; Urena, J. Oxygen Sensing and the Electrophysiology of Arterial Chemoreceptor Cells. NIPS 1993, 8, 191–195. [Google Scholar] [CrossRef]
- Montoro, R.J.; Urena, J.; Fernandez-Chacon, R.; Alvarez de Toledo, G.; López-Barneo, J. Oxygen sensing by ion channels and chemotransduction in single glomus cells. J. Gen. Physiol 1996, 107, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kim, D. Activation of voltage-dependent K+ channels strongly limits hypoxia-induced elevation of [Ca2+ ]i in rat carotid body glomus cells. J Physiol. 2018, 596(15), 3119–3136. [Google Scholar] [CrossRef]
- Latorre, R.; Oberhauser, A.; Labarca, P.; Alvarez, O. Varieties of calcium-activated potassium channels. Annu Rev Physiol. 1989, 51, 385–399. [Google Scholar] [CrossRef]
- Vergara, C.; Latorre, R.; Marrion, N.V.; Adelman, J.P. Calcium-activated potassium channels. Curr. Opin. Neurobiol. 1998, 8, 321–329. [Google Scholar] [CrossRef]
- Knaus, H.G.; Eberhart, A.; Glossmann, H.; Munujos, P.; Kaczorowski, G.J.; Garcia, M.L. Pharmacology and structure of high conductance calcium-activated potassium channels. Cell Signal. 1994, 6(8), 861–870. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowski, G.J.; Knaus, H.G.; Leonard, R.J.; McManus, O.B.; Garcia, M.L. High-conductance calcium-activated potassium channels; structure, pharmacology, and function. J Bioenerg Biomembr. 1996, 28(3), 255–267. [Google Scholar] [CrossRef]
- Butler, A.; Tsunoda, S.; McCobb, D.P.; Wei, A.; Salkoff, L. mSlo, a complex mouse gene encoding "maxi" calcium-activated potassium channel. Science 1993, 261, 221–224. [Google Scholar] [CrossRef]
- Schreiber, M.; Salkoff, L. A novel calcium-sensing domain in the BK channel. Biophys J. 1997, 73(3), 1355–63. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.H. Molecular determinants of voltage-gated potassium currents in vascular smooth muscle. Cell Biochem Biophys. 2005, 42(2), 167–95. [Google Scholar] [CrossRef]
- McManus, O.B.; Helms, L.M.; Pallanck, L.; Ganetzky, B.; Swanson, R.; Leonard, R.J. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron 1995, 14(3), 645–650. [Google Scholar] [CrossRef]
- Wyatt, C.N.; Peers, C. Ca(2+)-activated K+ channels in isolated type I cells of the neonatal rat carotid body. J Physiol. 1995, 483, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, C.N.; Wright, C.; Bee, D.; Peers, C. O2-sensitive K+ currents in carotid body chemoreceptor cells from normoxic and chronically hypoxic rats and their roles in hypoxic chemotransduction. Proc Natl Acad Sci U S A. 1995, 92(1), 295–299. [Google Scholar] [CrossRef] [PubMed]
- Pardal, R.; Ludewig, U.; Garcia-Hirschfeld, J.; Lopez-Barneo, J. Secretory responses of intact glomus cells in thin slices of rat carotid body to hypoxia and tetraethylammonium. Proc Natl Acad Sci U S A. 2000, 97(5), 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Riesco-Fagundo, A.M.; Pérez-García, M.T.; González, C.; López-López, J.R. O(2) modulates large-conductance Ca(2+)-dependent K(+) channels of rat chemoreceptor cells by a membrane-restricted and CO-sensitive mechanism. Circ Res 2001, 89(5), 430–436. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Castillo, K.; Carrasquel-Ursulaez, W.; Sepulveda, R.V.; Gonzalez-Nilo, F.; Gonzalez, C.; Alvarez, O. Molecular Determinants of BK Channel Functional Diversity and Functioning. Physiol Rev. 2017, 97(1), 39–87. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Roy, A.; Rozanov, C.; Mokashi, A. K current modulated by PO2 in type I cells in rat carotid body is not a chemosensor. Brain Res. 1998, 794(1), 162–165. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.F. Are oxygen dependent K+ channels essential for carotid body chemo-transduction? Respir Physiol. 1997, 110(2-3), 211-8. [CrossRef]
- Gomez-Niño, A.; Obeso, A.; Baranda, J.A; Santo-Domingo, J.; Lopez-Lopez, J.R.; Gonzalez, C. MaxiK potassium channels in the function of chemoreceptor cells of the rat carotid body. Am J Physiol Cell Physiol. 2009, 297(3), C715–722. [Google Scholar] [CrossRef] [PubMed]
- Hatton, C.J.; Carpenter, E.; Pepper, D.R.; Kumar, P.; Peers, C. Developmental changes in isolated rat type I carotid body cell K+ currents and their modulation by hypoxia. J Physiol. 1997, 501 Pt 1, 49–58. [Google Scholar] [CrossRef]
- Peers, C.; Wyatt, C.N. The role of maxiK channels in carotid body chemotransduction. Respir Physiol Neurobiol. 2007, 157(1), 75–82. [Google Scholar] [CrossRef]
- López-Barneo, J. All for one - O2 -sensitive K+ channels that mediate carotid body activation. J Physiol. 2018, 596(15), 2951–2952. [Google Scholar] [CrossRef]
- Eyzaguirre, C. Chemical and electric transmission in the carotid body chemoreceptor complex. Biol Res. 2005, 38(4), 341–345. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Heinemann, S.H.; Hoshi, T. Modulation of BKCa channel gating by endogenous signaling molecules. Physiology (Bethesda). 2009, 24, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kyle, B.D.; Braun, A.P. The regulation of BK channel activity by pre- and post-translational modifications. Front Physiol. 2014, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Bolotina, V.M.; Najibi, S.; Palacino, J.J.; Pagano, P.J.; Cohen, R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature 1994, 368, 850–853. [Google Scholar] [CrossRef]
- Jaggar, J.H.; Li, A.; Parfenova, H.; Liu, J.; Umstot, E.S.; Dopico, A.M.; Leffler, C.W. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005, 97(8), 805–812. [Google Scholar] [CrossRef]
- Williams, S.E.; Wootton, P.; Mason, H.S.; Bould, J.; Iles, D.E.; Riccardi, D.; Peers, C.; Kemp, P.J. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004, 306(5704), 2093–2097. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Dinerman, J.L.; Agani, F.H.; Snyder, S.H. Carbon monoxide: a role in carotid body chemoreception. Proc Natl Acad Sci U S A 1995, 92(6), 1994–7. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Sáenz, P.; Pascual, A.; Gómez-Díaz, R.; López-Barneo, J. Acute oxygen sensing in heme oxygenase-2 null mice. J Gen Physiol. 2006, 128(4), 405–11. [Google Scholar] [CrossRef]
- McCartney, C.E.; McClafferty, H.; Huibant, J.M.; Rowan, R.G.; Shipston, M.J.; Rowe, I.C.M. A cysteine-rich motif confers hypoxia sensitivity to mammalian large conductance voltage- and Ca-activated K (BK) channel alpha-subunits. Proc Natl Acad Sci U S A, 2005, 102(49), 17870-17876. [CrossRef]
- Ross, F.A.; Rafferty, J.N.; Dallas, M.L.; Ogunbayo, O.; Ikematsu, N.; McClafferty, H.; Tian, L.; Widmer, H.; Rowe, I.C.; Wyatt, C.N., Shipston MJ, Peers C, Hardie DG, Evans AM. Selective expression in carotid body type I cells of a single splice variant of the large conductance calcium- and voltage-activated potassium channel confers regulation by AMP-activated protein kinase. J Biol Chem. 2011, 286(14), 11929-11936. [CrossRef]
- Evans, A.M.; Mustard, K.J.; Wyatt, C.N.; Peers, C.; Dipp, M.; Kumar, P.; Kinnear, N.P.; Hardie, D.G. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells? J Biol Chem. 2005, 280(50), 41504–11. [Google Scholar] [CrossRef]
- Evans, A.M.; Hardie, D.G.; Peers, C.; Wyatt, C.N.; Viollet, B.; Kumar, P.; Dallas, M.L.; Ross, F.; Ikematsu, N.; Jordan, H.L.; Barr, B.L.; Rafferty, J.N.; Ogunbayo, O. Ion channel regulation by AMPK: the route of hypoxia-response coupling in thecarotid body and pulmonary artery. Ann N Y Acad Sci. 2009, 1177, 89–100. [Google Scholar] [CrossRef]
- Wyatt, C.N.; Mustard, K.J.; Pearson, S.A.; Dallas, M.L.; Atkinson, L.; Kumar, P.; Peers, C.; Hardie, D.G.; Evans, A.M. AMP-activated protein kinase mediates carotid body excitation by hypoxia. J Biol Chem. 2007, 282(11), 8092–8. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.D.; Lewis, S.; Juričić, L.; Udoh, U.A.; Hartmann, S.; Jansen, M.A.; Ogunbayo, O.A.; Puggioni, P.; Holmes, A.P.; Kumar, P.; Navarro-Dorado, J.; Foretz, M.; Viollet, B.; Dutia, M.B.; Marshall, I.; Evans, A.M. AMP-activated Protein Kinase Deficiency Blocks the Hypoxic Ventilatory Response and Thus Precipitates Hypoventilation and Apnea. Am J Respir Crit Care Med. 2016, 193(9), 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, S.; Holmes, A.P.; Dallas, M.L.; Mahmoud, A.D.; Shipston, M.J.; Peers, C.; Hardie, D.G.; Kumar, P.; Evans, A.M. LKB1 is the gatekeeper of carotid body chemosensing and the hypoxic ventilatory response. Commun Biol. 2022, 5(1), 642. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R.; Dombkowski, R.A.; Russell, M.J.; Doellman, M.M.; Head, S.K.; Whitfield, N.L.; Madden, J.A. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J Exp Biol. 2006, 209 Pt 20, 4011–23. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. Hydrogen sulfide and oxygen sensing: implications in cardiorespiratory control. J Exp Biol. 2008, 211(Pt 17), 2727–34. [Google Scholar] [CrossRef]
- Yuan, G.; Vasavda, C.; Peng, Y.J.; Makarenko, V.V.; Raghuraman, G.; Nanduri, J.; Gadalla, M.M.; Semenza, G.L.; Kumar, G.K. Snyder, S.H.; Prabhakar, N.R. Protein kinase G-regulated production of H2S governs oxygen sensing. Sci Signal. 2015, 8(373), ra37. [CrossRef]
- Peng, Y.J.; Nanduri, J.; Raghuraman, G.; Souvannakitti, D.; Gadalla, M.M.; Kumar, G.K.; Snyder, S.H.; Prabhakar, N.R. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci USA. 2010, 107(23), 10719–10724. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Sun, B.; Wang, X.; Jin, Z.; Zhou, Y.; Dong, L.; Jiang, L.H.; Rong, W. A crucial role for hydrogen sulfide in oxygen sensing via modulating large conductance calcium-activated potassium channels. Antioxid Redox Signal. 2010, 12(10), 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Telezhkin, V.; Brazier, S.P.; Cayzac, S.H.; Wilkinson, W.J.; Riccardi, D.; Kemp, P.J. Mechanism of inhibition by hydrogen sulfide of native and recombinant BKCa channels. Respir Physiol Neurobiol. 2010, 172(3), 169–78. [Google Scholar] [CrossRef]
- Kim, D.; Kim, I.; Wang, J.; White, C.; Carroll, J.L. Hydrogen sulfide and hypoxia-induced changes in TASK (K2P3/9) activity and intracellular Ca(2+) concentration in rat carotid body glomus cells. Respir Physiol Neurobiol. 2015, 215, 30–38. [Google Scholar] [CrossRef]
- Wang, J.; Hogan, J.O.; Wang, R.; White, C.; Kim, D. Role of cystathionine-γ-lyase in hypoxia-induced changes in TASK activity, intracellular [Ca2+] and ventilation in mice. Respir Physiol Neurobiol. 2017, 246, 98–106. [Google Scholar] [CrossRef]
- Olson, K.R. Are Reactive Sulfur Species the New Reactive Oxygen Species? Antioxid Redox Signal 2020, 33(16), 1125–1142. [Google Scholar] [CrossRef]
- Akaike, T.; Ida, T.; Wei, F.Y.; Nishida, M.; Kumagai, Y.; Alam, M.M.; Ihara, H.; Sawa, T.; Matsunaga, T.; Kasamatsu, S.; Nishimura, A.; Morita, M.; Tomizawa, K.; Nishimura, A.; Watanabe, S.; Inaba, K.; Shima, H.; Tanuma, N.; Jung, M.; Fujii, S.; Watanabe, Y.; Ohmuraya, M.; Nagy, P.; Feelisch, M.; Fukuto, J.M.; Motohashi, H. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat Commun. 2017, 8(1), 1177. [Google Scholar] [CrossRef] [PubMed]
- Olson, K.R. A Case for Hydrogen Sulfide Metabolism as an Oxygen Sensing Mechanism. Antioxidants (Basel). 2021, 10(11), 1650. [Google Scholar] [CrossRef] [PubMed]
- Buckler, K.J. Effects of exogenous hydrogen sulphide on calcium signalling, background (TASK) K channel activity and mitochondrial function in chemoreceptor cells. Pflugers Arch. 2012, 463(5), 743–54. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Coburger, I.; Langner, J.M.; Peter, N.; Hoshi, T.; Schonherr, R.; Heinemann, S.H. Modulation of K(+) channel N-type inactivation by sulfhydration through hydrogen sulfide and polysulfides. Pflugers Arch 2019, 471(4), 557–571. [Google Scholar] [CrossRef] [PubMed]
- Lesage, F.; Lazdunski, M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000, 279(5), F793–F801. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bang, H.; Kim, D. TBAK-1 and TASK-1, two-pore K(+) channel subunits: kinetic properties and expression in rat heart. Am J Physiol. 1999, 277(5), H1669–H1678. [Google Scholar] [CrossRef]
- Kim, Y.; Bang, H.; Kim, D. TASK-3, a new member of the tandem pore K(+) channel family. J Biol Chem. 2000, 275(13), 9340–9347. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Wischmeyer, E.; Xin Liu, G.; Preisig-Müller, R.; Daut, J.; Karschin, A.; Derst, C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel. An extracellular histiding as pH sensor. J Biol Chem. 2000, 275(22), 16650–7. [Google Scholar] [CrossRef]
- Ashmole, I.; Goodwin, P.A.; Stanfield, P.R. TASK-5, a novel member of the tandem pore K+ channel family. Pflugers Arch. 2001, 442(6), 828–833. [Google Scholar] [CrossRef]
- Decher, N.; Maier, M.; Dittrich, W.; Gassenhuber, J.; Brüggemann, A.; Busch, A.E.; Steinmeyer, K. Characterization of TASK-4, a novel member of the pH-sensitive, two-pore domain potassium channel family. FEBS Lett. 2001, 492(1-2), 84-9. [CrossRef]
- Medhurst, A.D.; Rennie, G.; Chapman, C.G.; Meadows, H.; Duckworth, M.D.; Kelsell, R.E.; Gloger, I.I.; Pangalos, M.N. Distribution analysis of human two pore domain potassium channels in tissues of the central nervous system and periphery. Brain Res Mol Brain Res. 2001, 86(1-2), 101-114. [CrossRef]
- Maingret, F.; Patel, A.J.; Lazdunski, M.; Honoré, E. The endocannabinoid anandamide is a direct and selective blocker of the background K(+) channel TASK-1. EMBO J. 2001, 20(1-2), 47-54. [CrossRef]
- Kindler CH, Yost CS, Gray AT. Local anesthetic inhibition of baseline potassium channels with two pore domains in tandem. Anesthesiology 1999, 90(4), 1092-1102. [CrossRef]
- Lesage, F. Pharmacology of neuronal background potassium channels. Neuropharmacology. 2003, 44(1), 1–7. [Google Scholar] [CrossRef] [PubMed]
- Buckler, K.J.; Williams, B.A.; Honore, E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J Physiol. 2000, 525 (Pt 1), 135–42. [Google Scholar] [CrossRef]
- Williams, B.A.; Buckler, K.J. Biophysical properties and metabolic regulation of a TASK-like potassium channel in rat carotid body type 1 cells. Am J Physiol Lung Cell Mol Physiol. 2004, 286(1), L221–230. [Google Scholar] [CrossRef] [PubMed]
- Buckler, K.J.; Williams, B.A.; Orozco, R.V.; Wyatt, C.N. The role of TASK-like K+ channels in oxygen sensing in the carotid body. Novartis Found Symp. 2006, 272, 73–94. [Google Scholar] [CrossRef]
- Czirják, G.; Enyedi, P. Formation of functional heterodimers between the TASK-1 and TASK-3 two-pore domain potassium channel subunits. J Biol Chem. 2002, 277(7), 5426–5432. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Han, J.; Talley, E.M.; Bayliss, D.A.; Kim, D. Functional expression of TASK-1/TASK-3 heteromers in cerebellar granule cells. J Physiol. 2004, 554(Pt1), 64–77. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kummer, W.; Atoji, Y.; Suzuki, Y. TASK-1, TASK-2, TASK-3 and TRAAK immunoreactivities in the rat carotid body. Brain Res. 2002, 950(1-2), 304-307. [CrossRef]
- Trapp, S.; Aller, M.I.; Wisden, W.; Gourine, A.V. A role for TASK-1 (KCNK3) channels in the chemosensory control of breathing. J Neurosci. 2008, 28(35), 8844–8850. [Google Scholar] [CrossRef]
- Ortega-Sáenz, P.; Levitsky, K.L.; Marcos-Almaraz, M.T.; Bonilla-Henao, V.; Pascual, A.; López-Barneo, J. Carotid body chemosensory responses in mice deficient of TASK channels. J Gen Physiol. 2010, 135(4), 379–92. [Google Scholar] [CrossRef]
- Varas, R.; Wyatt, C.N.; Buckler, K.J. Modulation of TASK-like background potassium channels in rat arterial chemoreceptor cells by intracellular ATP and other nucleotides. J Physiol. 2007, 583(Pt 2), 521–536. [Google Scholar] [CrossRef]
- Buckler, K.J. TASK channels in arterial chemoreceptors and their role in oxygen and acid sensing. Pflugers Arch. 2015, 467(5), 1013–25. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Wang, J.; Hogan, J.O.; Vennekens, R.; Freichel, M.; White, C.; Kim, D. Increase in cytosolic Ca2+ produced by hypoxia and other depolarizing stimuli activates a non-selective cation channel in chemoreceptor cells of rat carotid body. J Physiol. 2014, 592(9), 1975–1992. [Google Scholar] [CrossRef] [PubMed]
- Ganfornina, M.D.; López-Barneo, J. Single K+ channels in membrane patches of arterial chemoreceptor cells are modulated by O2 tension. Proc Natl Acad Sci U S A. 1991, 88(7), 2927–2930. [Google Scholar] [CrossRef]
- Lewis, A.; Peers, C.; Ashford, M.L.; Kemp, P.J. Hypoxia inhibits human recombinant large conductance, Ca(2+)-activated K(+) (maxi-K) channels by a mechanism which is membrane delimited and Ca(2+) sensitive. J Physiol. 2002, 540 Pt 3, 771–780. [Google Scholar] [CrossRef]
- Gonzalez, C.; Vaquero, L.M.; López-López, J.R.; Pérez-García, M.T. Oxygen-sensitive potassium channels in chemoreceptor cell physiology: making a virtue of necessity. Ann N Y Acad Sci. 2009, 1177, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Acker, H. Cellular oxygen sensors. Ann N Y Acad Sci. 1994, 718, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.R.; Henderson, L.; Jamesm, T.G.; Delpiano, A.; Hentschel, J.; Acker, H. Involvement of an NAD(P)H oxidase as a PO2 sensor protein in the rat carotid body. Biochem J. 1990, 272, 743–747. [Google Scholar] [CrossRef]
- Acker, H.; Bölling, B.; Delpiano, M.A.; Dufau, E.; Görlach, A.; Holtermann, G. The meaning of H2O2 generation in carotid body cells for PO2 chemoreception. J Auton Nerv Syst. 1992, 41(1-2), 41-51. [CrossRef]
- Obeso, A.; Gómez-Niño, A.; Gonzalez, C. NADPH oxidase inhibition does not interfere with low PO2 transduction in rat and rabbit CB chemoreceptor cells. Am J Physiol. 1999; 276(3), C593-601. [CrossRef]
- Hutchinson, D.S.; Csikasz, R.I.; Yamamoto, D.L.; Shabalina, I.G.; Wikstrom, P.; Wilcke, M.; Bengtsson, T. Diphenylene iodonium stimulates glucose uptake in skeletal muscle cells through mitochondrial complex I inhibition and activation of AMP-activated protein kinase. Cell Signal 2007, 19(7), 1610–1620. [Google Scholar] [CrossRef]
- He, L.; Dinger, B.; Sanders, K.; Hoidal, J.; Obeso, A.; Stensaas, L.; Fidone, S.; Gonzalez, C. Effect of p47phox gene deletion on ROS production and oxygen sensing in mouse carotid body chemoreceptor cells. Am J Physiol Lung Cell Mol Physiol. 2005, 289(6), L916–L924. [Google Scholar] [CrossRef]
- Dinger, B.; He, L.; Chen, J.; Liu, X.; Gonzalez, C.; Obeso, A.; Sanders, K.; Hoidal, J.; Stensaas, L.; Fidone, S. The role of NADPH oxidase in carotid body arterial chemoreceptors. Respir Physiol Neurobiol. 2007, 157(1), 45–54. [Google Scholar] [CrossRef]
- Gonzalez, C.; Sanz-Alyayate, G.; Agapito, M.T.; Obeso, A. Effects of reducing agents on glutathione metabolism and the function of carotid body chemoreceptor cells. Biol Chem. 2004, 385(3-4), 265-274. [CrossRef]
- Agapito, M.T.; Sanz-Alfayate, G.; Gomez-Niño, A.; Gonzalez C, Obeso A. General redox environment and carotid body chemoreceptor function. Am J Physiol Cell Physiol, 2009, 296(3), C620-631. [CrossRef]
- Mkrtchian, S.; Kahlin, J.; Ebberyd, A.; Gonzalez, C.; Sanchez, D.; Balbir, A.; Kostuk, E.W.; Shirahata, M.; Fagerlund, M. J.; Eriksson, L.I. The human carotid body transcriptome with focus on oxygen sensing and inflammation--a comparative analysis. J Physiol, 2012, 590(16), 3807-3819. [CrossRef]
- Yuan, G.; Khan, S.A.; Luo, W.; Nanduri, J.; Semenza, G.L.; Prabhakar, N.R. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol 2011, 226(11), 2925–2933. [Google Scholar] [CrossRef] [PubMed]
- Obeso, A.; Almaraz, L.; Gonzalez, C. Effects of cyanide and uncouplers on chemoreceptor activity and ATP content of the cat carotid body. Brain Res. 1989, 481(2), 250–7. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.F.; Mokashi, A.; Chugh, D.; Vinogradov, S.; Osanai, S.; Lahiri, S. The primary oxygen sensor of the cat carotid body is cytochrome a3 of the mitochondrial respiratory chain. FEBS Lett. 1994, 351(3), 370–4. [Google Scholar] [CrossRef] [PubMed]
- Mosqueira, M.; Iturriaga, R. Carotid body chemosensory excitation induced by nitric oxide: involvement of oxidative metabolism. Respir Physiol Neurobiol. 2002, 131(3), 175–187. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Sáenz, P.; Pardal, R.; García-Fernandez, M.; López-Barneo, J. Rotenone selectively occludes sensitivity to hypoxia in rat carotid body glomus cells. J Physiol. 2003, 548(Pt 3), 789–800. [Google Scholar] [CrossRef]
- Buckler, K.J.; Vaughan-Jones, R.D. Effects of mitochondrial uncouplers on intracellular calcium, pH and membrane potential in rat carotid body type I cells. J Physiol 1998, 513 (Pt 3), 819–833. [Google Scholar] [CrossRef]
- Wyatt, C.N.; Buckler, K.J. The effect of mitochondrial inhibitors on membrane currents in isolated neonatal rat carotid body type I cells. J Physiol. 2004, 556(Pt 1), 175–91. [Google Scholar] [CrossRef]
- Fernández-Agüera, M.C.; Gao, L.; González-Rodríguez, P.; Pintado, C.O.; Arias-Mayenco, I.; García-Flores, P.; García-Pergañeda, A.; Pascual, A.; Ortega-Sáenz, P.; López-Barneo, J. Oxygen Sensing by Arterial Chemoreceptors Depends on Mitochondrial Complex I Signaling. Cell Metab. 2015, 22(5), 825–37. [Google Scholar] [CrossRef] [PubMed]
- Arias-Mayenco, I.; González-Rodríguez, P.; Torres-Torrelo, H.; Gao, L.; Fernández-Agüera, M.C.; Bonilla-Henao, V.; Ortega-Sáenz, P.; López-Barneo, J. Acute O2 Sensing: Role of Coenzyme QH2/Q Ratio and Mitochondrial ROS Compartmentalization. Cell Metab. 2018, 28(1), 145–158. [Google Scholar] [CrossRef]
- Chang, A.J.; Ortega, F.E.; Riegler, J.; Madison, D.V.; Krasnow, M.A. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature 2015, 527(7577), 240–244. [Google Scholar] [CrossRef]
- Holmes, A.P.; Swiderska, A.; Nathanael, D.; Aldossary, H.S.; Ray, C.J.; Coney, A.M.; Kumar, P. Are Multiple Mitochondrial Related Signalling Pathways Involved in Carotid Body Oxygen Sensing? Fron. Physiol. 2022, 13, 908617. [Google Scholar] [CrossRef]
- Chandel, N.S.; Maltepe, E.; Goldwasser, E.; Mathieu, C.E.; Simon, M.C.; Schumacker, P.T. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998, 95(20), 11715–11720. [Google Scholar] [CrossRef] [PubMed]
- Duranteau, J.; Chandel, N.S.; Kulisz, A.; Shao, Z.; Schumacker, P.T. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem. 1998, 273(19), 11619–11624. [Google Scholar] [CrossRef]
- Chandel, N.S.; McClintock, D.S.; Feliciano, C.E.; Wood, T.M.; Melendez, J.A.; Rodriguez, A.M.; Schumacker, P.T. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000, 275(33), 25130–25138. [Google Scholar] [CrossRef]
- Waypa, G.B.; Chandel, N.S.; Schumacker, P.T. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res 2001, 88(12), 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Pell, V.R.; Gaude, E., Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; Eyassu, F.; Shirley, R.; Hu, C.H.; Dare, A.J.; James, A.M.; Rogatti, S.; Hartley, R.C.; Eaton, S.; Costa, A.S.H.; Brookes, P.S.; Davidson, S.M.; Duchen, M.R.; Saeb-Parsy, K.; Shattock, M.J.; Robinson, A.J.; Work, L.M.; Frezza, C.; Krieg, T.; Murphy, M.P. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature, 2014, 515(7527), 431-435. [CrossRef]
- Swiderska, A.; Coney, A.M.; Alzahran,i A. A.; Aldossary, H.S.; Batis, N.; Ray, C.J.; Kumar, P.; Holmes, A.P. Mitochondrial Succinate Metabolism and Reactive Oxygen Species Are Important but Not Essential for Eliciting Carotid Body and Ventilatory Responses to Hypoxia in the Rat. Antioxidants (Basel). 2021, 10(6), 840. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Sáenz. P.; Lopez-Barneo, J. Physiology of the Carotid Body: From Molecules to Disease. Annu. Rev. Physiol. 2020, 82, 127–149. [CrossRef]
- Peng, Y.J.; Makarenko, V.V.; Gridina, A.; Chupikova, I.; Zhang, X.; Kumar, G.K.; Fox, A.P.; Prabhakar, N.R. H2S mediates carotid body response to hypoxia but not anoxia. Respir Physiol Neurobiol. 2019, 259, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Torres-Torrelo, H.; Ortega-Sáenz, P.; Macías, D.; Omura, M.; Zhou, T.; Matsunami, H.; Johnson, R.S.; Mombaerts, P.; López-Barneo, J. The role of Olfr78 in the breathing circuit of mice. Nature 2018, 561(7724), E33–E40. [Google Scholar] [CrossRef] [PubMed]
- Delpiano, M.A; Acker, H. Extracellular pH changes in the superfused cat carotid body during hypoxia and hypercapnia. Brain Res. 1985, 342(2), 273–280. [Google Scholar] [CrossRef]
- Bernardini, A.; Wolf, A.; Brockmeier, U.; Riffkin, H.; Metzen, E.; Acker-Palmer, A.; Fandrey, J.; Acker, H. Carotid body type I cells engage flavoprotein and Pin1 for oxygen sensing. Am J Physiol Cell Physiol. 2020, 318(4), C719–C731. [Google Scholar] [CrossRef]
- Monti-Bloch, L.; Abudara, V.; Eyzaguirre C. Electrical communication between glomus cells of the rat carotid body.Brain Res. 1993, 622(1-2), 119-31. [CrossRef]
- Peng, Y.J.; Nanduri, J.; Wang, N.; Kumar, G.K.; Bindokas, V.; Paul, B.D.; Chen, X.; Fox, A.P.; Vignane, T.; Filipovic, M.R.; Prabhakar, N.R. Hypoxia sensing requires H2S-dependent persulfidation of olfactory receptor 78. Sci Adv. 2023, 9(27), eadf3026. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.P.; Ray, C.J.; Coney, A.M.; Kumar, P. Is Carotid Body Physiological O2 Sensitivity Determined by a Unique Mitochondrial Phenotype? Front Physiol. 2018, 9, 562. [Google Scholar] [CrossRef]
- Rakoczy, R.J.; Wyatt, C.N. Acute oxygen sensing by the carotid body: a rattlebag of molecular mechanisms. J Physiol. 2018, 596(15), 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Sanz-Alfayate, G.; Agapito, M.T.; Gomez-Niño, A.; Rocher, A.; Obeso, A. Significance of ROS in oxygen sensing in cell systems with sensitivity to physiological hypoxia. Respir Physiol Neurobiol. 2002, 132(1), 17–41. [Google Scholar] [CrossRef] [PubMed]
- Kilfoil, P.J.; Tipparaju, S.M.; Barski, O.A.; Bhatnagar, A. Regulation of ion channels by pyridine nucleotides. Circ Res. 2013, 112(4), 721–741. [Google Scholar] [CrossRef]
- Otsubo, T.; Kostuk, E.W.; Balbir, A.; Fujii, K.; Shirahata, M. Differential Expression of Large-Conductance Ca-Activated K Channels in the Carotid Body between DBA/2J and A/J Strains of Mice. Front Cell Neurosci. 2011, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Hartness, M.E.; Brazier, S.P.; Peers, C.; Bateson, A.N.; Ashford, M.L.; Kemp, P.J. Post-transcriptional control of human maxiK potassium channel activity and acute oxygen sensitivity by chronic hypoxia. J Biol Chem. 2003, 278(51), 51422–51432. [Google Scholar] [CrossRef] [PubMed]
- Chandel, N.S.; Schumacker, P.T. Cellular oxygen sensing by mitochondria: old questions, new insight. J Appl Physiol (1985) 2000, 88(5):1880-9. [CrossRef]
- Jamieson, D.; Chance, B.; Cadenas, E.; Boveris, A. The relation of free radical production to hyperoxia. Annu Rev Physiol. 1986, 48, 703–719. [Google Scholar] [CrossRef]
- Kumar, P. Sensing hypoxia in the carotid body: from stimulus to response. Essays Biochem. 2007, 43, 43–60. [Google Scholar] [CrossRef]
- Sanz-Alfayate, G.; Obeso, A.; Agapito, M.T.; González, C. Reduced to oxidized glutathione ratios and oxygen sensing in calf and rabbit carotid body chemoreceptor cells. J Physiol. 2001, 537(Pt 1), 209–20. [Google Scholar] [CrossRef]
- Yamamoto, Y.; König, P.; Henrich, M.; Dedio, J.; Kummer, W. Hypoxia induces production of nitric oxide and reactive oxygen species in glomus cells of rat carotid body. Cell Tissue Res. 2006, 325(1), 3–11. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Biological Production, Detection, and Fate of Hydrogen Peroxide. Antioxid Redox Signal. 2018, 29(6), 541–551. [Google Scholar] [CrossRef] [PubMed]
- Netto, L.E.; Antunes, F. The Roles of Peroxiredoxin and Thioredoxin in Hydrogen Peroxide Sensing and in Signal Transduction. Mol Cells. 2016, 39(1), 65–71. [Google Scholar] [CrossRef] [PubMed]
- Grayson, C.; Mailloux, R.J. J. Coenzyme Q10 and nicotinamide nucleotide transhydrogenase: Sentinels for mitochondrial hydrogen peroxide signaling. Free Radic Biol Med. 2023, 208, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.; Agapito, M.T.; Rocher, A.; Gomez-Niño, A.; Rigual, R.; Castañeda, J.; Conde, S.V.; Obeso, A. A revisit to O2 sensing and transduction in the carotid body chemoreceptors in the context of reactive oxygen species biology. Respir Physiol Neurobiol. 2010, 174(3), 317–30. [Google Scholar] [CrossRef] [PubMed]
- Papreck, J.R.; Martin, E.A.; Lazzarini, P.; Kang, D.; Kim, D. Modulation of K2P3.1 (TASK-1), K2P9.1 (TASK-3), and TASK-1/3 heteromer by reactive oxygen species. Pflugers Arch. 2012, 464(5), 471-480. [CrossRef]
- Rakoczy, R.J.; Schiebrel, C.M.; Wyatt, C.N. Acute Oxygen-Sensing via Mitochondria-Generated Temperature Transients in Rat Carotid Body Type I Cells. Front Physiol. 2022, 13, 874039. [Google Scholar] [CrossRef] [PubMed]
- Conrad, P.W.; Conforti, L.; Kobayashi, S.; Beitner-Johnson, D.; Rust, R.T.; Yuan, Y.; Kim, H.W.; Kim, R.H.; Seta, K.; Millhorn, D.E. The molecular basis of O2-sensing and hypoxia tolerance in pheochromocytoma cells. Comp Biochem Physiol B Biochem Mol Biol. 2001, 128(2), 187–204. [Google Scholar] [CrossRef] [PubMed]
- Robertson, T. P.; Hague, D.; Aaronson, P.I.; Ward, J.P. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol 2000, 525 (Pt 3), 669–680. [Google Scholar] [CrossRef]
- Weissmann, N.; Dietrich, A; Fuchs, B.; Kalwa, H.; Ay, M.; Dumitrascu, R.; Olschewski, A.; Storch, U.; Mederos-Schnitzler, M.; Ghofrani, H.A.; Schermuly, R.T.; Pinkenburg, O.; Seeger, W.; Grimminger, F.; Gudermann, T. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci U S A 2006, 103(50), 19093-19098. [CrossRef]
- Archer, S.L.; Wu, X.C.; Thebaud, B.; Nsair, A.; Bonnet, S.; Tyrrell, B.; McMurtry, M.S.; Hashimoto, K.; Harry, G.; Michelakis, E.D. Preferential expression and function of voltage-gated, O2-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells. Circ Res 2004, 95(3), 308–318. [Google Scholar] [CrossRef]
- Neo, B.H.; Patel, D.; Kandhi, S.; Wolin, M.S. Roles for cytosolic NADPH redox in regulating pulmonary artery relaxation by thiol oxidation-elicited subunit dimerization of protein kinase G1alpha. Am J Physiol Heart Circ Physiol 2013, 305(3), H330–343. [Google Scholar] [CrossRef]
- Rathore, R.; Zheng, Y. M.; Niu, C. F.; Liu, Q.H.; Korde, A.; Ho, Y. S.; Wang, Y. X. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKCepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 2008, 45(9), 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Gupte, R.S.; Rawat, D.K.; Chettimada, S.; Cioffi, D.L.; Wolin, M.S.; Gerthoffer, W.T.; McMurtry, I.F.; Gupte, S.A. Activation of glucose-6-phosphate dehydrogenase promotes acute hypoxic pulmonary artery contraction. J Biol Chem 2010, 285(25), 19561–19571. [Google Scholar] [CrossRef] [PubMed]
- Cogolludo, A.; Moreno, L.; Frazziano, G.; Moral-Sanz, J.; Menendez, C.; Castaneda, J.; Gonzalez, C.; Villamor, E.; Perez-Vizcaino, F. Activation of neutral sphingomyelinase is involved in acute hypoxic pulmonary vasoconstriction. Cardiovasc Res 2009, 82(2), 296–302. [Google Scholar] [CrossRef] [PubMed]
- Sommer, N.; Huttemann, M.; Pak, O;, Scheibe, S.; Knoepp, F.; Sinkler, C.; Malczyk, M.; Gierhardt, M.; Esfandiary, A.; Kraut, S.; Jonas, F.; Veith, C.; Aras, S.; Sydykov, A.; Alebrahimdehkordi, N.; Giehl, K.; Hecker, M.; Brandes, R.P.; Seeger, W.; Grimminger, F.; Ghofrani, H.A.; Schermuly, R.T.; Grossman, L.I.;Weissmann, N. Mitochondrial Complex IV Subunit 4 Isoform 2 Is Essential for Acute Pulmonary Oxygen Sensing. Circ Res 2017, 121(4), 424-438. [CrossRef]
- Olschewski, A.; Li, Y.; Tang, B.; Hanze, J.; Eul, B.; Bohle, R.M.; Wilhelm, J.; Morty, R.E.; Brau, M.E.; Weir, E.K.; Kwapiszewska, G.; Klepetko, W.; Seeger, W.; Olschewski, H. Impact of TASK-1 in human pulmonary artery smooth muscle cells. Circ Res 2006, 98(8), 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, C.; Tang, B.; Balint, Z.; Wygrecka, M.; Hrzenjak, A.; Kwapiszewska, G.; Stacher, E.; Lindenmann, J.; Weir, E. K.; Olschewski, H.; Olschewski, A. Src tyrosine kinase is crucial for potassium channel function in human pulmonary arteries. Eur Respir J 2013, 41(1), 85–95. [Google Scholar] [CrossRef]
- Sedivy, V.; Joshi, S.; Ghaly, Y.; Mizera, R.; Zaloudikova, M.; Brennan, S.; Novotna, J.; Herget, J.; Gurney, A.M. Role of Kv7 channels in responses of the pulmonary circulation to hypoxia. Am J Physiol Lung Cell Mol Physiol 2015, 308(1), L48–57. [Google Scholar] [CrossRef]
- Peng, W.; Karwande, S.V.; Hoidal, J.R.; Farrukh, I.S. Potassium currents in cultured human pulmonary arterial smooth muscle cells. J Appl Physiol (1985) 1996, 80(4), 1187–1196. [Google Scholar] [CrossRef]
- Yuan, X.J. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ Res 1995, 77(2), 370–378. [Google Scholar] [CrossRef]
- Wiener, C.M.; Banta, M.R.; Dowless, M.S.; Flavahan, N.A.; Sylvester, J.T. Mechanisms of hypoxic vasodilation in ferret pulmonary arteries. Am J Physio 1995, 269(3 Pt 1), L351–357. [Google Scholar] [CrossRef]
- Osipenko, O.N.; Evans, A.M.; Gurney, A.M. Regulation of the resting potential of rabbit pulmonary artery myocytes by a low threshold, O2-sensing potassium current. Br J Pharmacol 1997, 120(8), 1461–1470. [Google Scholar] [CrossRef]
- Shimoda, L.A.; Sylvester, J.T.; Sham, J.S. Chronic hypoxia alters effects of endothelin and angiotensin on K+ currents in pulmonary arterial myocytes. Am J Physiol 1999, 277(3), L431–439. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, C.; Li, Y.; Tang, B.; Bordag, N.; Guntur, D.; Enyedi, P.; Olschewski, H; Olschewski, A. Potassium Channels in the Transition from Fetal to the Neonatal Pulmonary Circulation. Int J Mol Sci 2022, 23, 9. [Google Scholar] [CrossRef]
- Post, J.M.; Hume, J.R.; Archer, S.L.; Weir, E.K. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992, 262(Pt1), C882–890. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, I.F.; Davidson, A.B.; Reeves, J.T.; Grover, R.F. Inhibition of hypoxic pulmonary vasoconstriction by calcium antagonists in isolated rat lungs. Circ Res 1976, 38(2), 99–104. [Google Scholar] [CrossRef] [PubMed]
- Harder, D.R. , Madden, J.A., Dawson, C. A membrane electrical mechanism for hypoxic vasoconstriction of small pulmonary arteries from cat. Chest 1985, 88(4), 233S–235S. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.J.; Goldman, W.F.; Tod, M.L.; Rubin, L.J.; Blaustein, M.P. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol 1993, 264(2 Pt 1), L116–123. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.J.; Goldman, W.F.; Tod, M.L.; Rubin, L.J.; Blaustein, M.P. Ionic currents in rat pulmonary and mesenteric arterial myocytes in primary culture and subculture. Am J Physiol 1993, 264(2 Pt 1), L107–115. [Google Scholar] [CrossRef]
- Post, J.; Gelband, C.H.; Hume, J.R. [Ca2+]i inhibition of K+ channels in canine pulmonary artery. Novel mechanism for hypoxia-induced membrane depolarization. Circ Res 1995, 77(1), 131-139. [CrossRef]
- Evans, A.M.; Osipenko, O.N.; Gurney, A.M. Properties of a novel K+ current that is active at resting potential in rabbit pulmonary artery smooth muscle cells. J Physiol 1996, 496 ( Pt 2), 407–420. [Google Scholar] [CrossRef]
- Patel, A.J.; Lazdunski, M.; Honore, E. Kv2.1/Kv9.3, a novel ATP-dependent delayed-rectifier K+ channel in oxygen-sensitive pulmonary artery myocytes. EMBO J 1997, 16(22), 6615–6625. [Google Scholar] [CrossRef]
- Archer, S.L.; Souil, E.; Dinh-Xuan, A.T.; Schremmer, B.; Mercier, J.C.; El Yaagoubi, A.; Nguyen-Huu, L.; Reeve, H.L.; Hampl, V. Molecular identification of the role of voltage-gated K+ channels, Kv1.5 and Kv2.1, in hypoxic pulmonary vasoconstriction and control of resting membrane potential in rat pulmonary artery myocytes. J Clin Invest 1998, 101(11), 2319–2330. [Google Scholar] [CrossRef]
- Archer, S.L.; London, B.; Hampl, V.; Wu, X.; Nsair, A.; Puttagunta, L.; Hashimoto, K.; Waite, R.E.; Michelakis, E.D. Impairment of hypoxic pulmonary vasoconstriction in mice lacking the voltage-gated potassium channel Kv1.5. FASEB J 2001, 15(10), 1801–1803. [Google Scholar] [CrossRef]
- London, B.; Guo, W.; Pan, X.; Lee, J.S.; Shusterman, V.; Rocco, C.J.; Logothetis, D.A.; Nerbonne, J.M.; Hill, J.A. Targeted replacement of KV1.5 in the mouse leads to loss of the 4-aminopyridine-sensitive component of I(K,slow) and resistance to drug-induced qt prolongation. Circ Res 2001, 88(9), 940–946. [Google Scholar] [CrossRef]
- Platoshyn, O.; Brevnova, E.E.; Burg, E.D.; Yu, Y.; Remillard, C.V.; Yuan, J.X. Acute hypoxia selectively inhibits KCNA5 channels in pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol 2006, 290(3), C907–916. [Google Scholar] [CrossRef] [PubMed]
- Hulme, J.T.; Coppock, E.A; Felipe, A.; Martens, J.R.; Tamkun, M.M. Oxygen sensitivity of cloned voltage-gated K(+) channels expressed in the pulmonary vasculature. Circ Res 1999, 85(6), 489–497. [Google Scholar] [CrossRef] [PubMed]
- Platoshyn, O.; Yu, Y.; Ko, E.A.; Remillard, C.V.; Yuan, J.X. Heterogeneity of hypoxia-mediated decrease in I(K(V)) and increase in [Ca2+](cyt) in pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2007, 293(2), L402–416. [Google Scholar] [CrossRef] [PubMed]
- Gurney, A. M; Osipenko, O.N.; MacMillan, D.; McFarlane, K.M.; Tate, R.J.; Kempsill, F.E. Two-pore domain K channel, TASK-1, in pulmonary artery smooth muscle cells. Circ Res 2003, 93(10), 957–964. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Sedivy, V.; Hodyc, D.; Herget, J.; Gurney, A. M. KCNQ modulators reveal a key role for KCNQ potassium channels in regulating the tone of rat pulmonary artery smooth muscle. J Pharmacol Exp Ther 2009, 329(1), 368–376. [Google Scholar] [CrossRef]
- Archer, S.L; Huang, J.; Henry, T.; Peterson, D.; Weir, E.K. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res 1993, 73(6), 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Firth, A. L.; Gordienko, D.V.; Yuill, K.H.; Smirnov, S.V. Cellular localization of mitochondria contributes to Kv channel-mediated regulation of cellular excitability in pulmonary but not mesenteric circulation. Am J Physiol Lung Cell Mol Physiol 2009, 296(3), L347–360. [Google Scholar] [CrossRef]
- Dunham-Snary, K.J.; Wu, D.; Potus, F.; Sykes, E.A.; Mewburn, J.D.; Charles, R.L.; Eaton, P.; Sultanian, R. A; Archer, S.L. Ndufs2, a Core Subunit of Mitochondrial Complex I, Is Essential for Acute Oxygen-Sensing and Hypoxic Pulmonary Vasoconstriction. Circ Res 2019, 124(12), 1727–1746. [Google Scholar] [CrossRef]
- Olschewski, A.; Hong, Z.; Peterson, D.A.; Nelson, D.P; Porter, V.A.; Weir, E.K. Opposite effects of redox status on membrane potential, cytosolic calcium, and tone in pulmonary arteries and ductus arteriosus. Am J Physiol Lung Cell Mol Physiol 2004, 286(1), L15–22. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Lee, S.H.; Ho, W.K.; Earm, Y.E. Redox agents as a link between hypoxia and the responses of ionic channels in rabbit pulmonary vascular smooth muscle. Exp Physiol 1995, 80(5), 835–842. [Google Scholar] [CrossRef]
- Park, M. K.; Bae, Y.M.; Lee, S.H.; Ho, W.K.; Earm, Y.E. Modulation of voltage-dependent K+ channel by redox potential in pulmonary and ear arterial smooth muscle cells of the rabbit. Pflugers Arch 1997, 434(6), 764–771. [Google Scholar] [CrossRef] [PubMed]
- Reeve, H.L.; Weir, E.K.; Nelson, D.P.; Peterson, D.A.; Archer, S.L. Opposing effects of oxidants and antioxidants on K+ channel activity and tone in rat vascular tissue. Exp Physiol 1995, 80(5), 825–834. [Google Scholar] [CrossRef] [PubMed]
- Schach, C.; Xu, M.; Platoshyn, O.; Keller, S.H.; Yuan, J.X. Thiol oxidation causes pulmonary vasodilation by activating K+ channels and inhibiting store operated Ca2+ channels. Am J Physiol Lung Cell Mol Physiol 2007, 292(3), L685–698. [Google Scholar] [CrossRef] [PubMed]
- Weir, E.K.; Hong, Z.; Porter, V.A.; Reeve, H.L. Redox signaling in oxygen sensing by vessels. Respir Physiol Neurobiol 2002, 132(1), 121–130. [Google Scholar] [CrossRef]
- Olschewski, A.; Weir, E.K. Redox regulation of ion channels in the pulmonary circulation. Antioxid Redox Signal 2015, 22(6), 465–485. [Google Scholar] [CrossRef]
- Frazziano, G.; Moreno, L.; Moral-Sanz, J.; Menendez, C.; Escolano, L.; Gonzalez, C.; Villamor, E.; Alvarez-Sala, J.L; Cogolludo, A.L.; Perez-Vizcaino, F. (2011). Neutral sphingomyelinase, NADPH oxidase and reactive oxygen species. Role in acute hypoxic pulmonary vasoconstriction. J Cell Physiol 2011, 226(10), 2633–2640. [Google Scholar] [CrossRef] [PubMed]
- Cogolludo, A.; Moreno, L.; Bosca, L.; Tamargo, J.; Perez-Vizcaino, F. Thromboxane A2-induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction: role of protein kinase Czeta. Circ Res 2003, 93(7), 656–663. [Google Scholar] [CrossRef]
- Cogolludo, A.; Frazziano, G.; Cobeno, L.; Moreno, L.; Lodi, F.; Villamor, E.; Tamargo, J.; & Perez-Vizcaino, F.; & Perez-Vizcaino, F. Role of reactive oxygen species in Kv channel inhibition and vasoconstriction induced by TP receptor activation in rat pulmonary arteries. Ann NY Acad Sci 2006, 1091, 41–51. [Google Scholar] [CrossRef]
- Moreno, L.; Frazziano, G.; Cogolludo, A,; Cobeno, L. ; Tamargo, J.; Perez-Vizcaino, F. Role of protein kinase Czeta and its adaptor protein p62 in voltage-gated potassium channel modulation in pulmonary arteries. Mol Pharmacol 2007, 72(5), 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Moral-Sanz, J.; Gonzalez, T.; Menendez, C.; David, M.; Moreno, L.; Macias, A.; Cortijo, J.; Valenzuela, C.; Perez-Vizcaino, F.; Cogolludo, A. Ceramide inhibits Kv currents and contributes to TP-receptor-induced vasoconstriction in rat and human pulmonary arteries. Am J Physiol Cell Physiol 2011, 301(1), C186–194. [Google Scholar] [CrossRef] [PubMed]
- Desireddi, J.R.; Farrow, K.N.; Marks, J.D.; Waypa, G.B.; Schumacker, P.T. Hypoxia increases ROS signaling and cytosolic Ca(2+) in pulmonary artery smooth muscle cells of mouse lungs slices. Antioxid Redox Signal 2010, 12(5), 595–602. [Google Scholar] [CrossRef] [PubMed]
- Moudgil, R.; Michelakis, E.D.; Archer, S.L. Hypoxic pulmonary vasoconstriction. J Appl Physiol (1985). 2005, 98(1), 390–403. [Google Scholar] [CrossRef] [PubMed]
- Caouette, D.; Dongmo, C.; Berube, J.; Fournier, D.; Daleau, P. Hydrogen peroxide modulates the Kv1.5 channel expressed in a mammalian cell line. Naunyn Schmiedebergs Arch Pharmacol 2003, 368(6), 479–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ma, J.H.; Zhang, P.H.; Zou, A.R.; Tu, D.N. Quercetin activates human Kv1.5 channels by a residue I502 in the S6 segment. Clin Exp Pharmacol Physiol 2009, 36(2), 154–161. [Google Scholar] [CrossRef] [PubMed]
- Duprat, F.; Guillemare, E.; Romey, G.; Fink, M.; Lesage, F.; Lazdunski, M.; Honore, E. Susceptibility of cloned K+ channels to reactive oxygen species. Proc Natl Acad Sci U S A 1995, 92(25), 11796–11800. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Noh, H.J.; Sung, D.J.; Kim, J.G.; Kim, J.M.; Ryu, S.Y.; Kang, K.; Kim, B.; Bae, Y.M.; Cho, H. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive Kv currents through S-glutathionylation. Pflugers Arch. 2015, 467(2), 285–97. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, L.K.; Reddie, K. G.; Zhang, L.; Vesely, E.D.; Williams, E.S.; Schumacher, S.M.; O'Connell, R.P.; Shaw, R.; Day, S.M.; Anumonwo, J.M.; Carroll, K.S.; Martens, J.R. Redox-sensitive sulfenic acid modification regulates surface expression of the cardiovascular voltage-gated potassium channel Kv1.5. Circ Res 2012, 111(7), 842–853. [Google Scholar] [CrossRef]
- Gamper, N.; Zaika, O.; Li, Y.; Martin, P.; Hernandez, C.C.; Perez, M.R.; Wang, A.Y.; Jaffe, D.B.; Shapiro, M.S. Oxidative modification of M-type K(+) channels as a mechanism of cytoprotective neuronal silencing. EMBO J 2006, 25(20), 4996–5004. [Google Scholar] [CrossRef]
- Yuan, X.J.; Wang, J.; Juhaszova, M.; Golovina, V.A.; Rubin, L.J. Molecular basis and function of voltage-gated K+ channels in pulmonary arterial smooth muscle cells. Am J Physiol 1998, 274(4), L621–635. [Google Scholar] [CrossRef] [PubMed]
- Bahring, R.; Milligan, C.J.; Vardanyan, V.; Engeland, B.; Young, B.A.; Dannenberg, J.; Waldschutz, R.; Edwards, J.P.; Wray, D; Pongs, O. Coupling of voltage-dependent potassium channel inactivation and oxidoreductase active site of Kvbeta subunits. J Biol Chem 2001, 276(25), 22923–22929. [Google Scholar] [CrossRef] [PubMed]
- Dwenger, M.M. ; Raph, S.M.; Reyzer, M.L.; Lisa Manier, M.; Riggs, D.W.; Wohl, Z.B.; Ohanyan, V.; Mack, G.; Pucci, T.; Moore, J.B.; Hill, B.G.; Chilian, W.M.; Caprioli, R.M.; Bhatnagar, A.; Nystoriak, M. A. Pyridine nucleotide redox potential in coronary smooth muscle couples myocardial blood flow to cardiac metabolism. Nat Commun, 2022, 13(1), 2051. [CrossRef]
- Raph, S.M.; Dwenger, M.M.; Hu, X.; Nystoriak, M.A. Basal NAD(H) redox state permits hydrogen peroxide-induced mesenteric artery dilatation. J Physiol 2023, 601(13), 2621–2634. [Google Scholar] [CrossRef] [PubMed]
- Platoshyn, O.; Remillard, C.V.; Fantozzi, I.; Mandegar, M.; Sison, T.T.; Zhang, S.; Burg, E.; Yuan, J.X. Diversity of voltage-dependent K+ channels in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2004, 287(1), L226–238. [Google Scholar] [CrossRef]
- Dwenger, M.M.; Raph, S.M.; Baba, S.P.; Moore, J.B.t.; Nystoriak, M.A. Diversification of Potassium Currents in Excitable Cells via Kvbeta Proteins. Cells 2022, 11(14). [CrossRef]
- Michelakis, E.D.; Hampl, V.; Nsair, A.; Wu, X.; Harry, G.; Haromy, A.; Gurtu, R.; Archer, S.L. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res 2002, 90(12), 1307–1315. [Google Scholar] [CrossRef]
- Michelakis, E.D.; Rebeyka, I.; Wu, X.; Nsair, A.; Thebaud, B.; Hashimoto, K.; Dyck, J.R., Haromy, A.; Harry, G.; Barr, A.; Archer, S.L. O2 sensing in the human ductus arteriosus: regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res 2002, 91(6), 478-486. [CrossRef]
- Kim, Y.; Lee, S.H.; Ho, W.K. Hydrogen peroxide selectively increases TREK-2 currents via myosin light chain kinases. Front Biosci 2007, 12, 1642–1650. [Google Scholar] [CrossRef]
- Lim, J.B.; Langford, T.F.; Huang, B.K.; Deen, W.M.; Sikes, H.D. A reaction-diffusion model of cytosolic hydrogen peroxide. Free Radic Biol Med. 2016, 90, 85–90. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, B.J.; Chun, Y.S.; So, I.; Choi, H.; Kim, M.S.; Park, J.W. NOX4 as an oxygen sensor to regulate TASK-1 activity. Cell Signal. 2006, 18(4), 499–507. [Google Scholar] [CrossRef] [PubMed]
- Nisimoto, Y.; Diebold, B.A.; Cosentino-Gomes, D.; Lambeth, J.D. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry. 2014, 53(31), 5111–20. [Google Scholar] [CrossRef]
- Stuart, J.A.; Fonseca, J.; Moradi, F.; Cunningham, C.; Seliman, B.; Worsfold, C.R.; Dolan, S.; Abando, J; Maddalena, L. A. How Supraphysiological Oxygen Levels in Standard Cell Culture Affect Oxygen-Consuming Reactions. Oxid Med Cell Longev. 2018, 2018, 8238459. [Google Scholar] [CrossRef]
- Lu, W; Wang, J.; Shimoda, L.A.; Sylvester, J.T. Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia. Am J Physiol Lung Cell Mol Physiol 2008, 295(1), L104-113. [CrossRef]
- Wang, J.; Shimoda, L.A.; Sylvester, J.T. Ca2+ responses of pulmonary arterial myocytes to acute hypoxia require release from ryanodine and inositol trisphosphate receptors in sarcoplasmic reticulum. Am J Physiol Lung Cell Mol Physiol 2012, 303(2), L161–168. [Google Scholar] [CrossRef]
- Chen, T.X.; Xu, X.Y.; Zhao, Z.; Zhao, F.Y.; Gao, Y.M.; Yan, X.H.; Wan, Y. Hydrogen peroxide is a critical regulator of the hypoxia-induced alterations of store-operated Ca(2+) entry into rat pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2017, 312(4), L477–L487. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.J.; Yang, X.R.; Cao, Y.N.; Sham, J.S. Hydrogen peroxide induced Ca2+ mobilization in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2007, 292(6), L1598–1608. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Song, T.; Joseph, L.; Mei, L.; Zheng, Y.M.; & Wang, Y.X. X. Important role of PLC-gamma1 in hypoxic increase in intracellular calcium in pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2013, 304(3), L143–151. [Google Scholar] [CrossRef] [PubMed]
- Moral-Sanz, J.; Mahmoud, A.D.; Ross, F.A.; Eldstrom, J.; Fedida, D.; Hardie, D.G.; Evans, A.M. AMP-activated protein kinase inhibits Kv1.5 channel currents of pulmonary arterial myocytes in response to hypoxia and inhibition of mitochondrial oxidative phosphorylation. J Physiol 2016, 594(17), 4901–4915. [Google Scholar] [CrossRef] [PubMed]
- Moral-Sanz, J.; Lewis, S.A.; MacMillan, S.; Ross, F.A.; Thomson, A.; Viollet, B.; Foretz, M.; Moran, C.; Hardie, D.G.; Evans, A.M. The LKB1-AMPK-alpha1 signaling pathway triggers hypoxic pulmonary vasoconstriction downstream of mitochondria. Sci Signal 2018, 11(550). [CrossRef]
- Del Gaudio, F.; Liu, D.; Lendahl, U. Notch signalling in healthy and diseased vasculature. Open Biol 2022, 12(4), 220004. [Google Scholar] [CrossRef]
- Smith, K.A.; Voiriot, G.; Tang, H.; Fraidenburg, D.R.; Song, S.; Yamamura, H.; Yamamura, A.; Guo, Q.; Wan, J.; Pohl, N.M.; Tauseef, M.; Bodmer, R.; Ocorr, K.; Thistlethwaite, P.A.; Haddad, G.G.; Powell, F.L.; Makino, A.; Mehta, D; Yuan, J.X. Notch Activation of Ca(2+) Signaling in the Development of Hypoxic Pulmonary Vasoconstriction and Pulmonary Hypertension. Am J Respir Cell Mol Biol 2015, 53(3), 355-367. [CrossRef]
- Jain, P.P.; Hosokawa, S.; Xiong, M.; Babicheva, A.; Zhao, T.; Rodriguez, M.; Rahimi, S; Pourhashemi, K.; Balistrieri, F.; Lai, N.; Malhotra, A.; Shyy, J.Y.; Valdez-Jasso, D.; Thistlethwaite, P.A.; Makino, A.; Yuan, J.X. Revisiting the mechanism of hypoxic pulmonary vasoconstriction using isolated perfused/ventilated mouse lung. Pulm Circ 2020, 10(4), 2045894020956592. [CrossRef]
- Song, S.; Babicheva, A.; Zhao, T.; Ayon, R.J.; Rodriguez, M.; Rahimi, S.; Balistrieri, F.; Harrington, A.; Shyy, J.Y.; Thistlethwaite, P.A.; Makino, A.; Yuan, J.X. Notch enhances Ca(2+) entry by activating calcium-sensing receptors and inhibiting voltage-gated K(+) channels. Am J Physiol Cell Physiol 2020, 318(5), C954–C968. [Google Scholar] [CrossRef]
- Knock, G.A.; Snetkov, V.A.; Shaifta, Y.; Drndarski, S.; Ward, J.P.; Aaronson, P.I. Role of src-family kinases in hypoxic vasoconstriction of rat pulmonary artery. Cardiovasc Res 2008, 80(3), 453–462. [Google Scholar] [CrossRef]
- MacKay, C.E.; Shaifta, Y.; Snetkov, V.V.; Francois, A.A.; Ward, J.P.T.; Knock, G.A. ROS-dependent activation of RhoA/Rho-kinase in pulmonary artery: Role of Src-family kinases and ARHGEF1. Free Radic Biol Med 2017, 110, 316–331. [Google Scholar] [CrossRef]
- Pak, O.; Nolte, A.; Knoepp, F.; Giordano, L.; Pecina, P.; Huttemann, M.; Grossman, L. I., Weissmann, N.; Sommer, N. Mitochondrial oxygen sensing of acute hypoxia in specialized cells - Is there a unifying mechanism? Biochim Biophys Acta Bioenerg, 2022, 1863(8), 148911. [CrossRef]
- Alruwaili, N.; Kandhi, S.; Sun, D.; Wolin, M. S. (2019, Oct 1). Metabolism and Redox in Pulmonary Vascular Physiology and Pathophysiology. Antioxid Redox Signal 2019, 31(10), 752–769. [Google Scholar] [CrossRef]
- Kemp, P.J.; Telezhkin, V. Oxygen sensing by the carotid body: is it all just rotten eggs? Antioxid Redox Signal. 2014, 20(5), 794–804. [Google Scholar] [CrossRef] [PubMed]
- Bilan, D.S.; Belousov, V.V. New tools for redox biology: From imaging to manipulation. Free Radic Biol Med 2017, 109, 167–188. [Google Scholar] [CrossRef] [PubMed]
- Lukyanov, K.A.; Belousov, V.V. Genetically encoded fluorescent redox sensors. Biochim Biophys Acta 2014, 1840(2), 745–756. [Google Scholar] [CrossRef] [PubMed]
- Pouvreau, S. Genetically encoded reactive oxygen species (ROS) and redox indicators. Biotechnol J 2014, 9(2), 282–293. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; Gems, D;, Kagan, V.E.; Kalyanaraman, B.; Larsson, N.G.; Milne, G.L.; Nystrom, T.; Poulsen, H.E.; Radi, R.; Van Remmen, H.; Schumacker, P.T.; Thornalley, P. J.; Toyokuni, S.; Winterbourn, C.C.; Yin, H.; Halliwell, B. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat Metab 2022, 4(6), 651-662. [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med 2016, 100, 14–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




