1. Introduction
Aging is a process of deterioration of the functional capacity of the organism, with a continuous, heterogeneous, universal, and irreversible development [
1]. During aging, there is a gradual reduction in homeostatic resilience, which is the ability to recover physiological parameters when these have been altered, and the diseases that develop are a consequence of it [
2]. These progressive changes are cumulative and increase the incidence of diseases such as diabetes, hypertension, and Alzheimer's. One of the most accepted theories to explain aging corresponds to the oxidative stress theory of aging, which postulates that this is the result of inadequate protection of the organism against damage induced by free radicals, also called reactive oxygen and nitrogen species (RONS) [
3]. This imbalance between the production of free radicals and the body's antioxidant defenses generates oxidative stress, the accumulation of which throughout life plays a fundamental role in the pathogenesis of many diseases and aging [
4]. In the world, the elderly population is constantly expanding, with a consequent increase in the prevalence of diseases related to aging. Therefore, bioactive compounds of natural origin with antioxidant capacity have gotten interest to reduce the development of several aging related diseases.
Brown algae (pheophytes) are a large and diverse group of organisms that comprise around 2000 species and are distributed in multiple marine ecosystems, presenting complex multicellularity and a wide morphological diversity among species [
5]. In Chile, there is the
Durvillaea incurvata seaweed, an endemic species that is known under the name of Cochayuyo, which is commonly collected for human consumption [
6].
Regarding extraction of bioactive compounds from natural sources such as cochayuyo, in recent years conventional extraction has been considered inefficient due to its long time, high cost, and degradation of sample quality, whereas other methods like ultrasonically assisted extraction turn out to be more efficient due to its low energy requirement, less time and solvent consumption. The ultrasound helps the solvent to penetrate the cells by destroying their cell walls, thus increasing the overall efficiency of the process [
7]. Although there is plenty of evidence on the effect of ultrasound in extraction processes, the study of the specific properties of these extracts is still limited for species such as cochayuyo, while research suggest the potential of brown seaweeds to obtain healthy bioactive extracts, specially related with phlorotannins, which in general have demonstrated several biological activities, like as antioxidant, anticancer, anti-inflammatory, antimicrobial, anti-diabetic, antiviral, and anti-allergic activities [
8,
9,
10,
19]. Thus, the objective of this research was to evaluate the ability of an ultrasound-assisted extract of
Durvillaea incurvata to inhibit key enzymes in the development of aging-related diseases, such as diabetes, Alzheimer's disease, and hypertension, providing the bases for further development of healthy food ingredients.
2. Materials and Methods
2.1. Optimization of ultrasound-assisted extraction
Seaweed cochayuyo (Durvillaea incurvata) was collected from "Palo Muerto" sector (Latitude: -39.8833 Longitude: -73.5167) Southern Chile, cleaned with seawater and transported to the lab in the same day. Once in the lab, the algae were washed with distilled water, cut into cubes of ~1 cm3, frozen at -80 ° C, lyophilized, ground to a size of ~0.05 mm, and finally stored at -80 ° C until extraction.
Response Surface Methodology (RSM) was used to optimize the ultrasound-assisted ethanolic extraction. Ethanol 70% v/v, and an ultrasonic processor (Sonics VCX series, 500 W, 20 kHz, Sonics & Materials Inc.) were used. A Box-Behnken experimental design was used (see
Table 1.), being the independent variables the extraction temperature (
X1; 30-50 °C), extraction time (
X2; 30-90 min), and ultrasound pulse cycle (
X3; 8-12 s), while the response variables were the total phenolic content (
YTPC), and the antioxidant activity (
YDPPH and
YORAC, for DPPH and ORAC, respectively). Immediately after extraction, each extract was filtered with a 0.45 µm cellulose syringe filter and stored at -80 °C until analysis. Quadratic models (excluding less significant effects) were used for each response. Multiple responses optimization was performed by using the “Desirability” function. All bioactivity related analyses were performed on this optimized extract.
As control, conventional extraction (CE) was performed by using ethanol 70% (v/v), temperature 30°C, agitation 60 rpm, and extraction time 12 hours. Extract was also filtered through a 0.45 μm cellulose syringe filter and stored at -80°C until analysis.
2.1.1. Total phenolic content
The total phenolic content was assessed by the Folin-Ciocalteau (FC) method, using gallic acid as a standard to construct the calibration curve (results expressed in mg·g
−1 of gallic acid equivalent, GAE) [
20]. In brief, 0.5 mL of the sample or solvent blank was diluted in 3.75 mL of distilled water. Afterward, 0.25 mL of the FC reagent was added and homogenized. Then, 0.5 mL of the sodium carbonate solution (10% w/v) was added, the resulting solution was homogenized and incubated for 1 h at room temperature in the darkness. The absorbance of the reaction product was measured at 765 nm (UV spectrophotometer 1240, Shimadzu, Kyoto, Japan). Analyses were performed in duplicate.
2.1.2. Antioxidant activity
The antioxidant activity was measured by two methods, DPPH and ORAC.
The anti-radical activity, 2,2-diphenyl-1-picrylhydracil (DPPH), was measured by using the method of Tierney et al. [
21]. First, a working solution of DPPH (0.048 mg/mL) was prepared by diluting a stock (0.238 mg/mL in methanol). For the analysis, 0.5 mL of DPPH solution was added to microtubes with 0.5 mL of the extract. After homogenizing, the tubes reacted for 30 min at room temperature, and the absorbance was measured at 520 nm on a UV 1240 spectrophotometer (Shimadzu, Kyoto, Japan). Trolox was used as the reference standard. The results were expressed in µmol equivalent of Trolox (ET)/g dry seaweed. Analyses were performed in duplicate.
As said before, the ORAC method was also used to measure the antioxidant activity. The reaction was carried out in a 75 mM phosphate buffer (pH 7.4), in a 96-well microplate. Forty-Five µL of the sample and 175 µL of fluorescein 108 mM were deposited. This mixture was incubated for 30 min at 37 °C; after that time, 50 µL of the AAPH solution 108 mM was added. The microplate was immediately placed in the dual-scan microplate spectrofluorometer (Gemini XPS, San Jose, CA, USA) for 60 min; fluorescence readings were recorded every 3 min (wavelengths of 485 nm excitation and 535 nm emission). The microplate was automatically shaken before and after each reading. For the calibration curve, Trolox was used at 6, 12, 18 and 24 M. All reactions were carried out in triplicate. The area under the curve (AUC) was calculated for each sample by integrating the relative fluorescence curve (r
2 > 0.99). The net AUC of the sample was calculated by subtracting the AUC of the blank. The regression equation between the net AUC and Trolox concentration was determined, and the ORAC values were expressed as mol Trolox equivalents/g of dry seaweed (ET/g) using the standard curve established previously [
22].
2.2. Inhibition of α-glucosidase and α-amylase enzymes.
The ability of the extracts to inhibit the α-glucosidase activity was measured using the method described by Nampoothiri et al. [
11], adapted by Lordan et al. [
12]. Briefly, 50 µL of 100 mM extract in sodium phosphate buffer (pH 6.9) and 50 µL of 5 mM p-nitrophenyl-α-D-glucopyranoside in phosphate buffer were mixed in a 96-well microplate and incubated at 37 °C for 5 min. Then, 100 µL phosphate buffer was added to each well, which contained 0.1 U/mL α-glucosidase. A microplate reader set at 37°C was used to record absorbance at 405 nm wavelength for 30 min. Blank (no enzyme) readings were subtracted from each well. The inhibitory effects of the extracts are expressed as IC
50 value, which is the concentration that inhibits 50% of the enzyme activity. The pharmacological inhibitor, acarbose, was included as a positive control. The activity of α-glucosidase was calculated as following:
where the control is the enzyme–substrate reaction in the absence of inhibitors.
The potential of the extracts to inhibit the activity of the α-amylase was measured using also the method described by Nampoothiri et al. (2011), adapted by Lordan et al. (2013) [
11,
12]. A volume of 100 µL of extract and 1% starch solution in 20 mM sodium phosphate buffer was taken (pH 6.9 with 6 mM sodium chloride), and kept in Eppendorf tubes at 25 °C. A 100 µL volume of porcine pancreatic α-amylase (0.5 mg/mL) was added to each tube and then incubated at 25 °C for 10 min. The reaction was stopped by adding 200 µL of dinitrosalicylic acid reagent and incubating the tubes at 100 °C for 5 min. Samples were cooled to room temperature and then 50 µL was taken from each tube and transferred to the wells of the 96-well microplate. The mixture was diluted by adding 200 µL of water to each well and the absorbance measured at 540 nm wavelength. Blank (no enzyme) readings were subtracted from each well. The inhibitory effects of the extracts are expressed as IC
50 value and acarbose was also included as a positive control. The α-amylase activity was also calculated using Eq. 1.
2.3. Inhibition of the acetylcholinesterase and butyrylcholinesterase enzymes.
The inhibitory activity of the extracts against cholinesterase enzymes was evaluated as described by Ellman [
13]. Briefly, 5-dithio-bis(2-nitrobenzoic) acid (DTNB) was dissolved in Tris-HCl buffer (pH 8.0) containing NaCl 0.1 M and MgCl
2 0.02 M. Then, filtered extract dissolved in deionized water (50 mL, 2 mg/mL) was mixed in a 96-well microplate with 125 mL of DTNB, acetylcholinesterase (AChE) or butyrylcholinesterase (BChE) solution (25 mL) dissolved in Tris-HCl buffer (pH 8.0), and incubated for 15 minutes at 25 °C. The reaction was started by the addition of acetylthiocholine iodide (ATCI) or butyrylthiocholine chloride (BTCl) (25 mL). In addition, a blank was prepared by adding the solution sample to all reagents without the enzyme solutions (AChE or BChE). After 10 minutes of reaction, the absorbance at 405 nm wavelength was measured. Finally, the IC
50 (µg/mL) was determined.
2.4. Inhibition of angiotensin-I converting enzyme.
The enzyme activity inhibition assay was carried out as described by Hou et al. (2003), modified by Jung et al. (2006) [
14,
15]. N-[3-(2-furyl)acryloyl]-Phe-Gly-Gly (FAPGG) (0.5 mM) and various concentrations of samples were completely dissolved in Tris-HCl buffer 50 mM (pH 7.5). Twenty µL of angiotensin-converting enzyme (ACE-I; 1 U/mL dissolved in 50 mM Tris-HCl buffer) was mixed with 200 µL of various concentrations of the samples, or with Tris-HCl buffer 50 mM (negative control). Then, 1 mL of FAPGG (0.5 mM) was added to the reaction mixture and the absorbance was measured at 345 nm wavelength, at 0, 5, 30, and 60 min. Captopril (antihypertensive agent) was used as a positive control. The inhibition value was calculated using the following equation:
3. Results
3.1. Optimization of ultrasound assisted extraction.
For ultrasound assisted ethanolic extraction optimization using RSM, the Box-Behnken experimental design was ranned and the results are shown in the
Table 1. For each independent variable (total phenolic content, and antioxidant activity assessed by two methods), polinomial equations were fitted by excluding less significant effects. Fitted equations, having the highest adjusted determination coefficient (R
2-adjusted), are shown in equations 3, 4, and 5. For Y
TPC, R
2 was 68.6 %, while R
2-adjusted was 51.1 %. For Y
DPPH values were R
2 = 72.1 % and R
2-adjusted = 51.17 %. Finally, for Y
ORAC, R
2 and R
2-adjusted were 37.3 % and 20.2 %, respectibely. These values show us how much are capable the models to explain the data variability.
Using multible optimization procedure, optimal conditions for maximize extraction were obtained (goal: maximize Y
TPC, Y
DPPH, and Y
ORAC). Such conditions and theorical optimal responses are shown also in
Table 1, while a comparison between experimental results obtained at optimal conditions and conventional ethanolic extract is shown in
Table 2. Results showed that extract obtained by ultrasound assisted ethanolic extraction at optimal conditions has similar content of phenolic compund than conventional extract, but with a higher antioxidant activity (p < 0.05).
3.2. Inhibition of α-glucosidase and α-amylase.
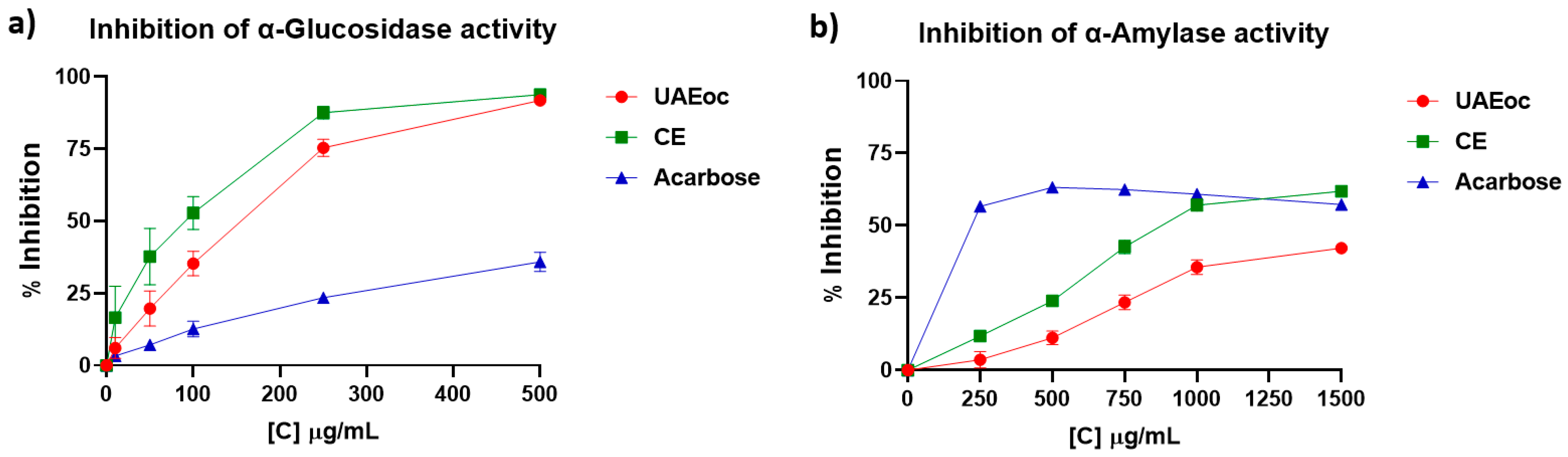
Figure 1 shows the activity of the enzymes α-glucosidase and α-amylase as affected by UAEoc, CE and acarbose.
Figure 1a shows that as the concentration of UAEoc, CE and acarbose increased (10 - 500 µg/mL), the inhibition of the activity of the α-glucosidase increased. At the highest concentration (500 µg/mL), UAEoc, CE, and acarbose generated 91.8 ± 1.0%, 93.8 ± 0.3%, and 35. 9 ± 3.3% of inhibition, respectively. The IC
50 values for the inhibition of α-glucosidase activity were: 155 ± 16, 94 ± 18, and 642 ± 58 µg/mL for UAEoc, CE, and acarbose, respectively. Outcomes (inhibition at highest concentration and IC
50) showed no differences between UAEoc and CE, while demonstrated that seaweed extracts are more efficient than acarbose (p<0.0001) for α-glucosidase inhibition.
On the other hand,
Figure 1b shows that, in the tested range (250 – 1500 µg/mL), acarbose inhibited the α-amylase at a constant level (~60%), while UAEoc and CE increased inhibition with concentration increasing, reaching 42.2 ± 1.4% and 61.9 ± 0.9% inhibition, respectively. Regarding IC
50 values for α-amylase, these were 1680 ± 71 µg/mL, 1048 ± 29 µg/mL, and 144 ± 2 µg/mL, for UAEoc, CE, and acarbose, respectively, being all values statistically different (p<0.0001), and meaning that acarbose has the highest inhibition capacity, followed by CE, and finally UAEoc.
3.3. Inhibition of the enzymes Acetylcholinesterase and Butyrylcholinesterase.
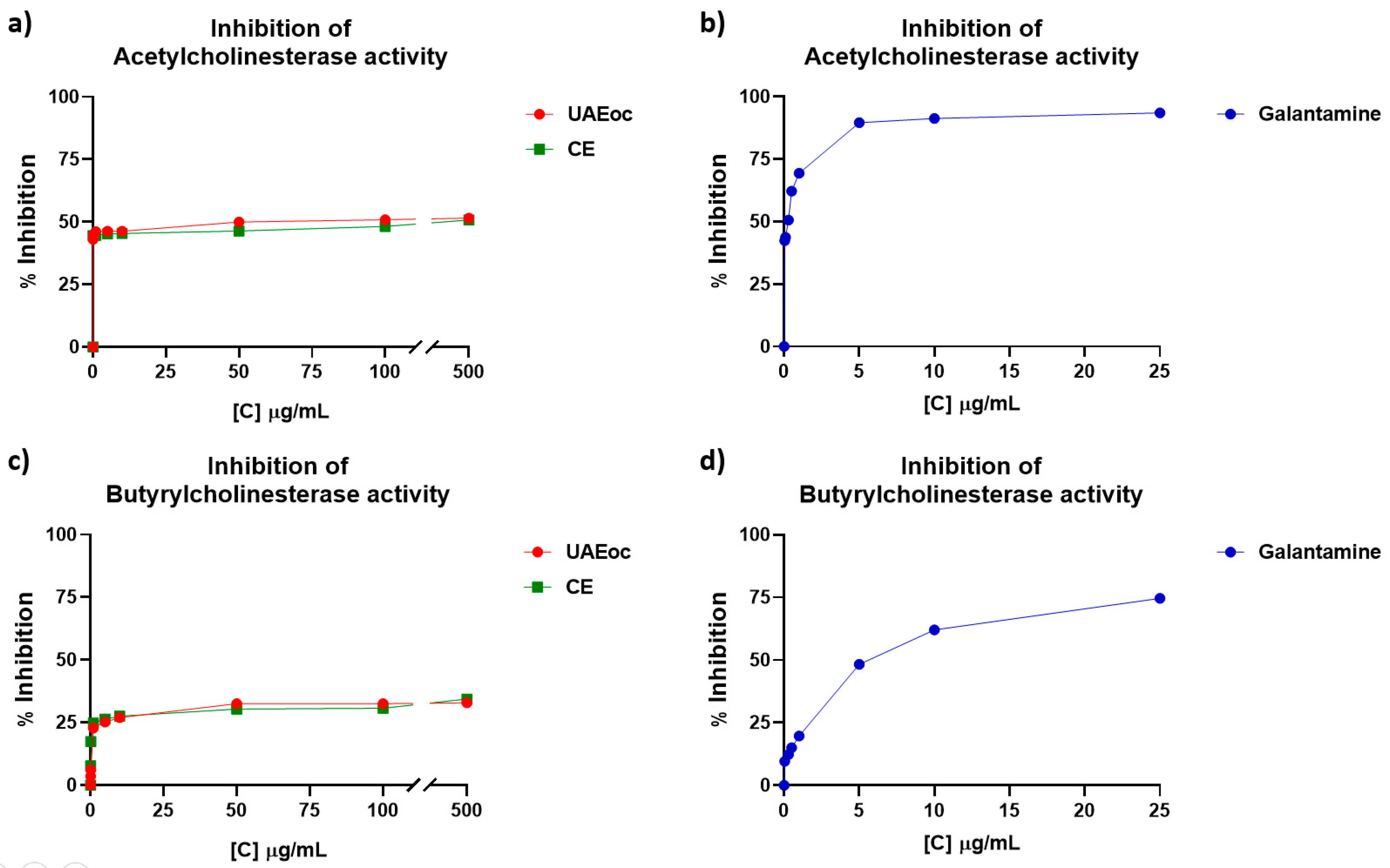
The
Figure 2 shows the inhibition of AChE and BChE enzymes in presence of UAEoc and CE, at increasing concentrations. Results showed that while CE and UAEoc increase (0.01 – 500 µg/mL), the inhibition of AChE increase from ~44% to ~51%, with no differences at any concentration (p>0.05) (
Figure 2a). The IC
50 values were 48.55 ± 0.021 µg/mL for UAEoc, and 153.15 ± 0.029 µg/mL for CE. Therefore, both extracts can inhibit the activity of AChE.
Regarding the effect on BChE,
Figure 2c shows that extracts are also capable to inhibit such enzyme, being this one depending on concentration, reaching approximately 34% inhibition at 500 µg/mL (highest tested concentration) for both extracts UAEoc and CE (p>0.05). IC
50 values were 87.58 ± 0.044 µg/mL for UAEoc, and 121.79 ± 0.071 µg/mL for CE. Galantamine, a commercial inhibitor of cholinesterase enzymes, and drug treatment for Alzheimer's, was used as positive control. IC
50 for this commercial inhibitor were 0.266 ± 0.029 µg/mL and 3.824 ± 0.025 µg/mL for AChE and BChE, respectively, which means that the standard drug galantamine is more efficient to inhibit these enzymes than cochayuyo extracts.
3.4. Inhibition of angiotensin-I-converting enzyme (ACE).
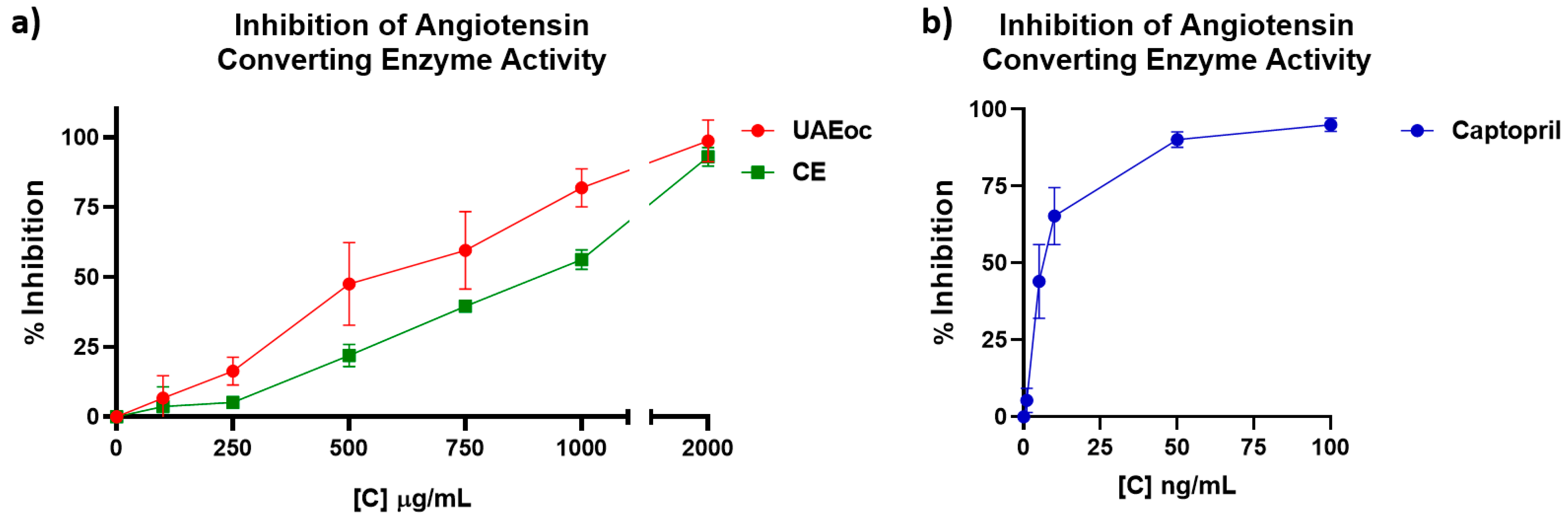
Activity of ACE as affected by cochayuyo extracts (100 -2000 µg/mL), and positive control Captopril (pharmacological inhibitor) is shown in
Figure 3 as inhibition percentage. Both extracts, UAEoc and CE, inhibited ACE in a concentration dependent way (
Figure 3a). At highest extract concentration (2000 µg/mL), UAEoc inhibited the enzyme activity until 98.7 ± 7.4%, while CE achieved 93.0 ± 3.4%. This inhibition capacity was lower than that generated by Captopril, which produced 95.0 ± 2.1% of inhibition at 100 ng/mL (
Figure 3b). IC
50 values were 613.951 ± 80.169 µg/mL, 901.219 ± 40.611 µg/mL, and 6.810 × 10ˉ³ ± 1.379 × 10ˉ³ µg/mL, for UAEoc, CE, and Captopril, respectively. In general, no statistically significant difference was observed between UAEoc and CE regarding inhibition at highest concentration and IC
50, however it was observed among the extracts and Captopril.
4. Discussion
4.1. Optimization of ultrasound assisted extraction.
Optimal condition of extraction were achieved by RSM, althoug determination coeficients (between 37.3 and 72.1%) were relatively lower than the obtained by other authors, such as Dang, et al. (7) (R
2 > 90%), Mohamed Ahmed, et al. (23) (R
2 > 80%), and Vuong, et al. (24) (R
2 beween 53 and 88%). This could mean that the variability of the process is high or that the “real” optimum condition of extraction is beyond the experimental range. Nevertheless, the optimal condition of extraction was more efficient than the conventional method, since the extract showed higher antioxidant activity (
Table 2). Given that, no differences were found regarding total phenolic compounds (despite antioxidant activity), there is a possibility to think that phenolic profiles could be different, or that ultrasound method is capable of extracting other compounds rather than phenolics, such as tocols (tocopherols and tocotrienols), which are abundant into cochayuyo and that also contribute with antioxidant activity (25).
4.2. Inhibition of α-glucosidase and α-amylase.
The results obtained are consistent with those described in previous investigations.
Regarding the inhibition capacity of the algae on the enzymes α-glucosidase and α-amylase, Erpel et al. (2021) showed that an extract of phlorotannins obtained from
Durvillaea incurvata from Niebla at a concentration of 500 µg/mL inhibited the activity of the α-glucosidase enzyme by approximately 80%, with an IC
50 of 245.1 ± 5.3 µg/mL and acarbose around 40%, with an IC
50 of 659.5 ± 36.7 µg/mL. Regarding α-glucosidase inhibition, the present extracts show a slightly higher percentage inhibition (at same concentration), and an IC
50 lower than that extract described by Erpal et al (2021), so they would be presenting a relatively higher inhibitory capacity. On the other hand, regarding α-amylase inhibition, authors reported no effect on the enzyme activity, while our extracts do demonstrate inhibition capacity. This may be since different extraction methods were used (pressurized hot liquid vs ultrasonic assisted), which may generate a different profile of bioactive compounds, having different inhibition capacities [
16]. Another study reported that ethanolic and acetone extracts of cochayuyo, at a concentration of 1000 µg/mL, inhibited α-glucosidase by 96.9 ± 0.4 and 99.3 ± 0.3%, showing an IC
50 of 473.4 ± 0.9 and 466.0 ± 1.3 µg/mL, respectively (acarbose 797.85 ± 1.1 µg/mL) [
8]. Based in the IC
50 values, present extracts appear be more efficient to inhibit the enzyme than those reported previously.
Regarding α-amylase, it has been reported that inhibitory effect of cochayuyo extract depends on the extraction method used, being more efficient the acetonic extract (43.4 ± 2.0% inhibition at 2000 µg/mL) than the ethanolic one (0% inhibition) [
8]. Present outcomes suggest that UAEoc (but also CE) is adequate to generate an antihyperglycemic ingredient, especially considering that a high inhibition of α-glucosidase joint with a moderate inhibition of α-amylase would be better since it could avoid some unwanted side effects related with excessive indigested starch getting to colon [
12], and also because it has been reported that a high α-amylase activity at oral level would be associated with improved glycemic homeostasis (lower glycemic response is achieved) following starch ingestion due to early insulin release [
18].
4.3. Inhibition of the enzymes Acetylcholinesterase and Butyrylcholinesterase.
Regarding the inhibition capacity of the algae on the AChE and BChE enzymes, Nho et al. (2020) previously evaluated the neuroprotective effects of a Phlorotannin-rich extract from
Ecklonia cava (PEEC), an edible brown alga. In such research, PEEC (1000 μg/mL) generated 95.4 y 74.7 % inhibition of AChE and BChE, respectively, which means that PEEC would have higher inhibitory capacity than our extracts (see
Figure 2), probably related with the different concentration and profile of phlorotannins [
9].
Another research evaluated the anticholinesterase potential of hydroethanolic extracts of some South African marine algae:
Ecklonia maxima (ECK),
Gelidium pristoides (GLD),
Gracilaria gracilis (GCL) y
Ulva lactuca (ULT) [
17]. At 500 μg/mL, the inhibition of the AChE was approximately 15% for ULT, 20% for GLD, and 25% for GCL and ECK, which is lower than inhibition generated by extracts in our study at the same concentration (see
Figure 2a). This lesser capacity may be due to the phlorotannins profile, or to the fact that the used extraction method was not optimized to maximize the polyphenols extraction, unlike that used by our group. On the other hands, at the same concentration (500 μg/mL), the inhibition of the BChE was approximately 20% for ULT, 25% for GLD, and 30% for GCL and ECK, which are like inhibition generated by our extracts UAEoc and CE (see
Figure 2b).
4.4. Inhibition of angiotensin-I-converting enzyme (ACE).
Previously, there has been observed potential of brown seaweed as antihypertensive agent, related with the capacity of inhibit ACE. For instance, Shih et al. (2022) analyzed the inhibition generated by extracts obtained by enzymatic extraction from
Durvillaea antarctica [
10]. Said extracts (1000 µg/mL), so-called Dur-A, Dur-B y Dur-C, generated an inhibition of ACE activity of 72.5 ± 1.4%, 80.7 ± 1.6% and 62.9 ± 0.6%, respectively. At the same concentration (1000 µg/mL), UAEoc generated an inhibition of ACE similar to Dur-B, while CE generated lesser inhibition than any of the three extracts (see
Figure 3a). These outcomes suggest the greater potential of UAEoc on ACE inhibition.
5. Conclusions
The results showed that ultrasound extraction is more efficient that conventional procedure, especially regarding the antioxidant activity of extract, maybe due to the different final compositions. Cochayuyo extracts (both UAEoc and CE) presented inhibitory activity on the α-glucosidase and α-amylase enzymes, even higher than the positive control used, showing potential to prevent postprandial hyperglycemia and the development of related diseases, as Diabetes. Extracts also showed inhibitory activity on AChE and BChE enzymes, at levels comparable with inhibitors obtained from other natural sources, exhibiting potential against Alzheimer's disease. Regarding antihypertensive potential, extracts showed inhibitory activity on ACE, an enzyme that plays a key role in regulating vascular tone and blood pressure, suggesting that these may help to prevent hypertension. So, outcomes show us that cochayuyo hydroethanolic extracts have potential as anti-aging related diseases edible ingredient, at least for diabetes, Alzheimer's disease, and hypertension. Further research is mandatory to study the incorporation of extracts in foods and corroborate its effect in vivo.
Author Contributions
Conceptualization, N.M. and J.P.; methodology, N.M., M.S. and J.P.; formal analysis, N.M. and R.M; writing—original draft preparation, N.M.; writing—review and editing, J.P.; supervision, J.P.; project administration, J.P.; funding acquisition, J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID-Chile through the grant FONDECYT Regular, grant number 1201670.
Data Availability Statement
The data used to support the findings of this study can be made available by the corresponding author upon request.
Acknowledgments
This research was funded by ANID-Chile through the grant FONDECYT Regular, grant number 1201670. We thank Sandy González for his technical support. We thank Jorge Rivas for his help with the cochayuyo samples collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- García, A. M. A., & Maya, Á. M. S. (2014). Análisis del concepto de envejecimiento. Archives of Environmental Health, 8(3), 458–458. [CrossRef]
- Sierra, F., & Pérez, V. (2009). ARTÍCULO ESPECIAL Biología del envejecimiento. Rev Méd Chile, 137, 296–302. [CrossRef]
- Rico-Rosillo, M. G., Oliva-Rico, D., & Vega-Robledo, G. B. (2018). Envejecimiento: algunas teorías y consideraciones genéticas, epigenéticas y ambientales. Revista Médica del Instituto Mexicano del Seguro Social, 56(3),287-294. [fecha de Consulta 9 de Mayo de 2023]. ISSN: 0443-5117. Recuperado de: https://www.redalyc.org/articulo.oa?id=457757174017 https://www.medigraphic.com/pdfs/imss/im-2018/im183l.pdf%0Ahttp://www.ncbi.nlm.nih.gov/pubmed/30394717.
- Luo, J., Mills, K., le Cessie, S., Noordam, R., & van Heemst, D. (2020). Ageing, age-related diseases and oxidative stress: What to do next? Ageing Research Reviews, 57, 100982. [CrossRef]
- Bringloe, T. T., Starko, S., Wade, R. M., Vieira, C., Kawai, H., De Clerck, O., Cock, J. M., Coelho, S. M., Destombe, C., Valero, M., Neiva, J., Pearson, G. A., Faugeron, S., Serrão, E. A., & Verbruggen, H. (2020). Phylogeny and Evolution of the Brown Algae. Critical Reviews in Plant Sciences, 39(4), 281–321. [CrossRef]
- Fraser, C. I., Velásquez, M., Nelson, W. A., Macaya, E. C., & Hay, C. H. (2020). The Biogeographic Importance of Buoyancy in Macroalgae: A Case Study of the Southern Bull-Kelp Genus Durvillaea (Phaeophyceae), Including Descriptions of Two New Species1. Journal of Phycology, 56(1), 23–36. [CrossRef]
- Dang, T. T., Van Vuong, Q., Schreider, M. J., Bowyer, M. C., Van Altena, I. A., & Scarlett, C. J. (2017). Optimisation of ultrasound-assisted extraction conditions for phenolic content and antioxidant activities of the alga Hormosira banksii using response surface methodology. Journal of Applied Phycology, 29(6), 3161–3173. [CrossRef]
- Pacheco, L. V., Parada, J., Pérez-Correa, J. R., Mariotti-Celis, M. S., Erpel, F., Zambrano, A., & Palacios, M. (2020). Bioactive Polyphenols from Southern Chile Seaweed as Inhibitors of Enzymes for Starch Digestion. Marine Drugs, 18(7), 1–12. [CrossRef]
- Nho, J. A., Shin, Y. S., Jeong, H. R., Cho, S., Heo, H. J., Kim, G. H., & Kim, D. O. (2020). Neuroprotective effects of phlorotannin-rich extract from brown seaweed ecklonia cava on neuronal PC-12 and SH-SY5Y cells with oxidative stress. Journal of Microbiology and Biotechnology, 30(3), 359–367. [CrossRef]
- Shih, M., Hou, C., Dong, C., Patel, A. K., & Tsai, Y. (2022). Production and Characterization of Durvillaea antarctica enzyme Extract for Antioxidant and Anti-Metabolic syndrome Effects. 1–17. [CrossRef]
- Nampoothiri, S. V., Prathapan, A., Cherian, O. L., Raghu, K. G., Venugopalan, V. V., & Sundaresan, A. (2011). In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food and Chemical Toxicology, 49(1), 125–131. [CrossRef]
- Lordan, S., Smyth, T. J., Soler-Vila, A., Stanton, C., & Paul Ross, R. (2013). The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chemistry, 141(3), 2170–2176. [CrossRef]
- Barrientos, R., Fernández-Galleguillos, C., Pastene, E., Simirgiotis, M., Romero-Parra, J., Ahmed, S., & Echeverría, J. (2020). Metabolomic Analysis, Fast Isolation of Phenolic Compounds, and Evaluation of Biological Activities of the Bark From Weinmannia trichosperma Cav. (Cunoniaceae). Frontiers in Pharmacology, 11(May), 1–13. [CrossRef]
- Hou, W. chi, Hen, Hs. J., & Lin, Y. H. (2003). Antioxidant Peptides with Angiotensin Converting Enzyme Inhibitory Activities and Applications for Angiotensin Converting Enzyme Purification. 1706–1709. [CrossRef]
- Jung, H. A., Hyun, S. K., Kim, H. R., & Choi, J. S. (2006). Angiotensin-converting enzyme I inhibitory activity of phlorotannins from Ecklonia stolonifera. Fisheries Science, 72(6), 1292–1299. [CrossRef]
- Erpel, F., Mariotti-Celis, M. S., Parada, J., Pedreschi, F., & Pérez-Correa, J. R. (2021). Pressurized hot liquid extraction with 15% v/v glycerol-water as an effective environment-friendly process to obtain durvillaea incurvata and lessonia spicata phlorotannin extracts with antioxidant and antihyperglycemic potential. Antioxidants, 10(7). [CrossRef]
- Olasehinde, T. A., Olaniran, A. O., & Okoh, A. I. (2019). Aqueous–ethanol extracts of some South African seaweeds inhibit beta-amyloid aggregation, cholinesterases, and beta-secretase activities in vitro. Journal of Food Biochemistry, 43(7), 1–10. [CrossRef]
- Javier Parada & Jose L. Santos (2016) Interactions between Starch, Lipids, and Proteins in Foods: Microstructure Control for Glycemic Response Modulation, Critical Reviews in Food Science and Nutrition, 56:14, 2362-2369. [CrossRef]
- Cassani, L., Gomez-Zavaglia, A., Jimenez-Lopez, C., Lourenço-Lopes, C., Prieto, M. A., & Simal-Gandara, J. (2020). Seaweed-based natural ingredients: Stability of phlorotannins during extraction, storage, passage through the gastrointestinal tract and potential incorporation into functional foods. Food research international (Ottawa, Ont.), 137, 109676. [CrossRef]
- Machu, L., Misurcova, L., Vavra Ambrozova, J., Orsavova, J., Mlcek, J., Sochor, J., & Jurikova, T. (2015). Phenolic Content and Antioxidant Capacity in Algal Food Products. Molecules, 20(1), 1118–1133. MDPI AG. [CrossRef]
- Tierney, M.S.; Smyth, T.J.; Hayes, M.; Soler-Vila, A.; Croft, A.K.; Brunton, N. Influence of pressurised liquid extraction and solid–liquid extraction methods on the phenolic content and antioxidant activities of Irish macroalgae. Int. J. Food Sci. Technol. 2013, 48, 860–869.
- Cao, G.; Prior, R. Measurement of Oxygen Radical Absorbance in Biological Samples. In Methods in Enzymology; Academic Press: Waltham, MA, USA, 1999; Volume 299, pp. 50–62.
- Mohamed Ahmed, I. A., Al-Juhaimi, F., Adisa, A. R., Adiamo, O. Q., Babiker, E. E., Osman, M. A., Gassem, M. A., Ghafoor, K., Alqah, H. A. S., & Elkareem, M. A. (2020). Optimization of ultrasound-assisted extraction of phenolic compounds and antioxidant activity from Argel (Solenostemma argel Hayne) leaves using response surface methodology (RSM). Journal of food science and technology, 57(8), 3071–3080. [CrossRef]
- Vuong, Q. V., Goldsmith, C. D., Dang, T. T., Nguyen, V. T., Bhuyan, D. J., Sadeqzadeh, E., Scarlett, C. J., & Bowyer, M. C. (2014). Optimisation of Ultrasound-Assisted Extraction Conditions for Phenolic Content and Antioxidant Capacity from euphorbia tirucalli Using Response Surface Methodology. Antioxidants (Basel, Switzerland), 3(3), 604–617. [CrossRef]
- Ortiz J., Romero N., Robert P., Araya J., Lopez-Hernández J., Bozzo C., Navarrete E., Osorio A., Rios A. Dietary Fiber, Amino Acid, Fatty Acid and Tocopherol Contents of the Edible Seaweeds Ulva Lactuca and Durvillaea Antarctica. Food Chem. 2006;99:98–104. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).